Significance
Regulated activation of the NF-κB family of transcription factors is important for normal development, immune cell function, and inflammatory responses. NEMO, the NF-κB essential modulator, controls activation of the canonical IKK complex and NF-κB–mediated cellular responses, but details of how this is achieved are not fully known. Our results show that C-terminal mutations in NEMO can cause hyperactivation of inflammatory responses to Toll-like receptor and TNF ligands through impaired recruitment of the negative NF-κB regulator A20/TNFAIP3. Our results help to explain the inflammatory symptoms in patients harboring these NEMO mutations. Furthermore, our findings suggest that targeting this molecular interaction by enhancing A20 expression or its recruitment to the NEMO C-terminus may be a therapeutic strategy for human inflammatory disease.
Keywords: NF-κB, autoinflammatory disease, A20, HED-ID, TLR
Abstract
Receptor-induced NF-κB activation is controlled by NEMO, the NF-κB essential modulator. Hypomorphic NEMO mutations result in X-linked ectodermal dysplasia with anhidrosis and immunodeficiency, also referred to as NEMO syndrome. Here we describe a distinct group of patients with NEMO C-terminal deletion (ΔCT-NEMO) mutations. Individuals harboring these mutations develop inflammatory skin and intestinal disease in addition to ectodermal dysplasia with anhidrosis and immunodeficiency. Both primary cells from these patients, as well as reconstituted cell lines with this deletion, exhibited increased IκB kinase (IKK) activity and production of proinflammatory cytokines. Unlike previously described loss-of-function mutations, ΔCT-NEMO mutants promoted increased NF-κB activation in response to TNF and Toll-like receptor stimulation. Investigation of the underlying mechanisms revealed impaired interactions with A20, a negative regulator of NF-κB activation, leading to prolonged accumulation of K63-ubiquitinated RIP within the TNFR1 signaling complex. Recruitment of A20 to the C-terminal domain of NEMO represents a novel mechanism limiting NF-κB activation by NEMO, and its absence results in autoinflammatory disease.
Activation of the NF-κB family of transcription factors is required for normal development, innate and adaptive immunity, and the inflammatory response (1, 2). NF-κB–induced transcription of proinflammatory cytokines and chemokines amplifies immune-response programs and stimulates recruitment of inflammatory cells. Transcriptional activity of the classic NF-κB p65/p50 complex is regulated by the inhibitor of NF-κB kinase (IKK), consisting of the α- and β-catalytic subunits and the NEMO (NF-κB essential modulator, IKKγ) regulatory subunit. IKK activity leads to phosphorylation and K48-linked polyubiquitination of IκBα, the inhibitor of NF-κB. Ubiquitinated IκBα is then rapidly degraded, allowing nuclear translocation of NF-κB subunits and gene transactivation. NEMO functions as a scaffold within the IKK complex that is required for canonical IKK enzymatic activity and NF-κB activation (3).
Activation of TNF, IL-1R, and Toll-like receptor (TLR) family receptors leads to K63 and linear (also termed M1) ubiquitination of cytosolic adapter proteins, such as receptor interacting protein 1 (RIP1). This in turn promotes recruitment of the IKK complex to the receptor signaling complex (4–8). A well-known negative regulator of NF-κB activation is A20 (encoded by the gene TNFAIP3). In the TNF-R1 complex, A20 removes K63-linked ubiquitin modifications on RIP1 through its deubiquitinase activity and converts these to K48-linked polyubiquitin chains through its E3 ligase activity (9). Editing of polyubiquitin linkages at the receptor complex in this manner results in rapid degradation of RIP1 and other signaling proteins, such as TRAF6, TRAF2, cIAP1, and cIAP2 (9, 10), promoting the termination of receptor-induced NF-κB activation. In addition, A20 directly inhibits IKK activity independently of its deubiquitinase activity in a manner that depends upon binding to NEMO (11). A20-deficient cells demonstrate prolonged NF-κB activation and elevated production of inflammatory cytokines in response to TNF and TLR ligands. A20-deficient mice are hypersensitive to TNF and LPS-induced septic shock and spontaneously develop multiorgan inflammation and cachexia that results in premature mortality (12, 13).
The importance of regulated NF-κB activation in humans is illustrated by the effect of hypomorphic NEMO mutations, which result in a combination of immune and developmental defects. Ectodermal dysplasia with anhidrosis and immunodeficiency (EDA-ID) is a pleomorphic X-linked disorder affecting the development of ectodermally derived structures (eccrine glands, hair follicles, and teeth), as well as innate and adaptive immunity (14). Immunodeficiency, marked by the frequency and severity of bacterial, viral, or fungal infections, is the salient clinical feature among most individuals diagnosed with EDA-ID. This clinical phenotype can be traced to defects in NF-κB signaling through a number of different receptors families, including TLR, TNF, and antigen receptors. However, inflammatory disease phenotypes, including arthritis, colitis, and graft versus host disease (GVHD)-like dermatitis have also been observed in some patients with NEMO syndrome (15–17). Inflammatory disorders mediated predominantly by cells and molecules of the innate immune system, without evidence of autoantibodies or autoreactive T cells, have been broadly termed “autoinflammatory” (18).
Autoinflammatory manifestations in the NEMO syndrome could result from loss of NF-κB activation and increased cell death, as has been seen in intestinal epithelial cells lacking NEMO (19). NEMO mutations resulting in gain-of-function features may also drive inflammation, although to date none have been described. Here, we report that males harboring mutations leading to truncation of the NEMO C terminus (ΔCT-NEMO) develop a distinct syndrome with autoinflammatory disease manifestations, including dermatitis, colitis, arthritis, and macrophage activation syndrome. Remarkably, these ΔCT-NEMO mutations enhance NF-κB activation both in the resting state and via increased canonical IKK activity in response to receptor activation. We find that these C-terminal NEMO truncations uniquely fail to recruit the A20-negative regulator of NF-κB, leading to stabilization of K63-ubiquitinated RIP in the TNFR1 signaling complex and enhanced IKK kinase activity. These results represent a novel mechanism of disease pathogenesis, where failure to recruit a negative regulator of inflammatory cytokine signaling contributes to the development of autoinflammatory symptoms in what would otherwise be a primary immunodeficiency syndrome.
Results
NEMO C-Terminal Truncation Mutations Associated with Inflammatory Disease Lead to Increased NF-κB–Dependent Responses to Innate Immune Stimuli.
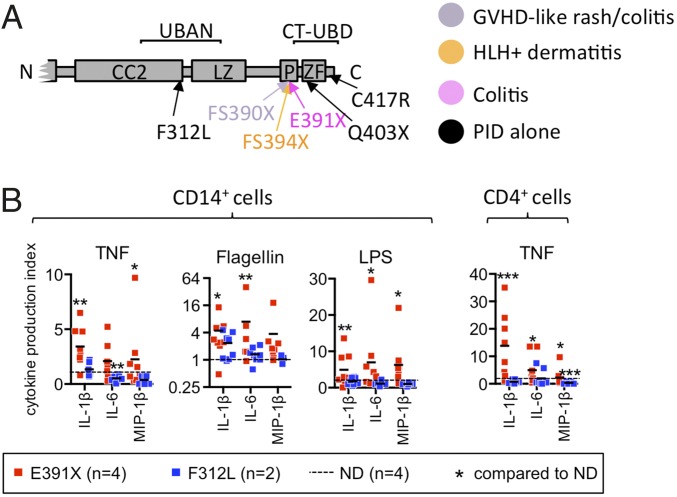
In a genotype-phenotype correlation study, we previously found two “hotspots” of NEMO mutations associated with inflammatory symptoms (20). More detailed analysis of mutations in this region indicated that only nonsense mutations that truncate the C-terminal zinc finger (ZF) domain and adjacent proline-rich sequence, but not missense mutations resulting in single amino acid changes, were associated with inflammatory disease phenotypes (Fig. 1A and Table S1). The concordance between the presence of a NEMO truncation and an autoinflammatory phenotype in multiple unrelated individuals suggests that these particular mutations in NEMO, rather than other background genetic or environmental factors, are responsible for the inflammatory disease in these patients. In one large kindred harboring a NEMO C-terminal truncation mutation (E391X), nine individuals, including two females, were affected (16), (Fig. S1A, Table S2, and clinical description in SI Materials and Methods). In contrast, a kindred with a NEMO-F312L mutation that impairs the ubiquitin binding in ABIN and NEMO (UBAN) domain suffered from typical EDA-ID immunodeficiency symptoms characterized by infection with pneumococcal meningitis, poor antibody response to vaccination, and skin infection with atypical mycobacteria (Fig. S1A, Table S2, and clinical description in SI Materials and Methods).
Fig. 1.
NEMO C-terminal truncation is associated with inflammatory disease and results in enhanced stimulation-induced proinflammatory gene expression and cytokine production in patient monocytes and T cells. (A) NEMO syndrome inflammatory disease phenotypes affecting the C terminus. Color-coded protein mutations depict disease phenotypes. Mutations not associated with inflammatory disease but included in this study are shown in black. (B) Healthy control and patient-purified CD14+ and CD4+ cells were cultured in media alone or with TNF or TLR ligands for 24 h and secreted cytokines were measured by capture assay. Four individual patients with the NEMO-E391X mutation and two individual patients with the NEMO-F312L mutation were studied. The mean value of three independent experiments (E391X) or two experiments (F312L) is shown by the horizontal black bar. Statistical significance is determined in relation to normal donor. (See Dataset S1 for absolute cytokine production values from patient and healthy control CD14+ monocytes and CD4+ T-cell experiments.) GVHD, graft versus host disease; HLH, hemophagocytic lymphohistiocytosis; PID, primary immunodeficiency.
Table S1.
Known NEMO C-terminus mutations and inflammatory disease phenotypes
 |
The predicted protein sequences of C-terminus NEMO mutations, which result from missense mutation, nonsense mutation, or frame-shift mutation are depicted. The proline-rich sequence and zinc finger domains are depicted by yellow and green shading, respectively. Mutations associated with inflammatory disease are bounded by a red box. Amino acid residues, which differ from wild-type NEMO, are shown in boldface.
Fig. S1.
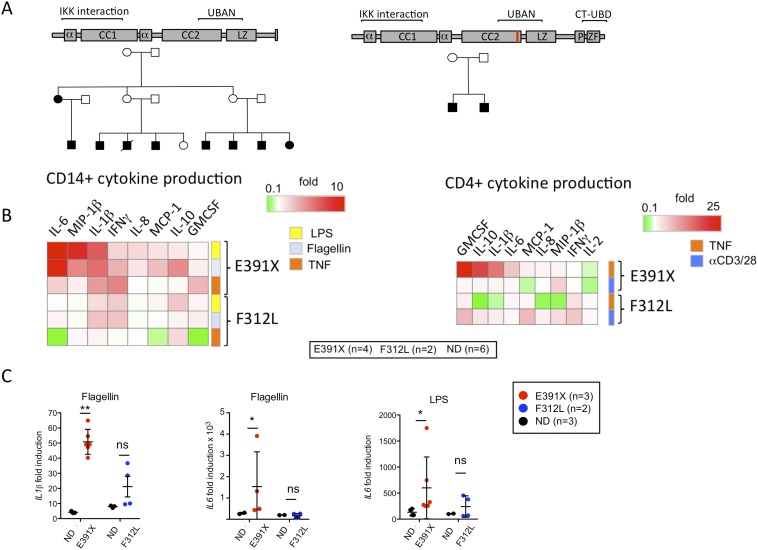
Pedigrees, gene expression, and cytokine production in patient and healthy control cells. (A) Pedigrees of kindreds affected by ΔCT-NEMO and UBAN-NEMO mutants used in this study. (B) Expanded array of cytokine production indices from cells of patients harboring E391X-NEMO, F312L-NEMO, and healthy controls, as described in Fig. 1B. Cytokines measured by capture assay from cultured cells are depicted in columns, and individual stimuli are in rows. Data shown are mean values from six individual patients (four with E391X-NEMO and two with F312L-NEMO) normalized to six paired healthy-donor controls. The CPI is depicted by intensity of the shaded box. Results are mean values from four independent experiments. (C) E391X and F312L patient-derived PBMC were cultured in media alone or media supplemented with Flagellin or LPS for 3 h. Fold-induction represents the ratio of gene expression in stimulated cells to that in cells cultured in media alone. The mean value of three independent experiments (E391X) or two experiments (F312L) is shown by the horizontal black bar. Statistical significance is determined in relation to normal donor.
Table S2.
Summary of clinical phenotypes in patients with NEMO-UBAN domain missense mutation and NEMO C-terminus truncation mutation
| Phenotype | E391X | F312L |
| Immunodeficiency | Sepsis, encapsulated bacteria | Atypical mycobacteria, peritoneal abscess |
| Inflammatory disease | colitis, dermatitis | Occasional fevers |
| Ectodermal dysplasia | Present | Mild |
To determine the functional consequences of ΔCT-NEMO in immune cells, we measured stimulation-induced production of proinflammatory cytokines and chemokines from purified CD14+ monocytes and CD4+ T cells isolated from patients and healthy controls. TNF and TLR agonists induced excess IL-1β, IL-6, and MIP-1β in monocytes and CD4+ T cells with the NEMO-E391X mutation (Fig. 1B and Fig. S1B). In contrast, cells with the known hypomorphic NEMO-F312L mutation produced similar or reduced cytokines in response to these stimuli compared with healthy donor cells. These changes in cytokine and chemokine production were a result of changes in gene transcription, because TLR stimulation of peripheral blood mononuclear cells (PBMC) from individuals with the NEMO-E391X mutation resulted in greater induction of IL1β and IL6 mRNA compared with healthy donor control samples (Fig. S1C). As with protein production, the hyperactive response to TLR stimulation was unique to cells from patients with the NEMO C-terminal domain truncation, because NEMO-F312L PBMC exhibited normal IL1β and IL6 expression following stimulation with Flagellin and LPS. These data indicate that unlike all previously described NEMO mutations, the NEMO-E391X mutation confers increased responsiveness to innate immune stimuli.
NEMO ΔCT Mutations Potentiate TNFR- and TLR-Induced NF-κB Activity.
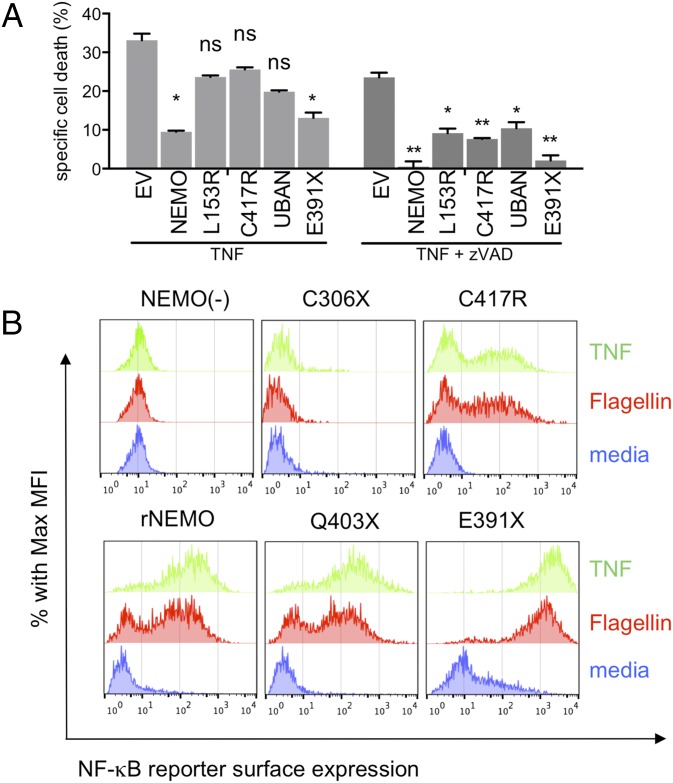
The results obtained using primary immune cells ex vivo from patients with NEMO mutations could have been influenced by their clinical status or genetic background. To determine how NEMO-E391X and other ΔCT truncations affect NF-κB signaling in a system independent of the effects of EDA-ID, we reconstituted a NEMO-deficient Jurkat T-cell line with physiological levels of wild-type NEMO, ΔCT-NEMO, or hypomorphic NEMO mutants using retroviral transduction (20, 21) (Fig. S2A). Cells harboring NEMO mutations that fail to activate NF-κB in response to TNF have a characteristic phenotype in which TNF induces apoptosis because of failure to up-regulate NF-κB–dependent antiapoptotic genes, such as A20 and c-FLIP (20, 22). As previously described, NEMO-deficient Jurkat cells were sensitive to TNF-induced cell death, with both a caspase-dependent and independent component (Fig. 2A) (21, 23). Reconstitution of these cells with wild-type NEMO reduced cell death, with the residual cell death being almost completely caspase-dependent, whereas loss of function NEMO mutations affecting the first coiled-coil domain (L153R), the C-terminal zinc finger (C417R), or the UBAN domain (E319AE320A) all failed to prevent cell death in response to TNF (Fig. 2A). However, the NEMO-E391X mutant was able to rescue cell death nearly as effectively as wild-type NEMO. These data show that the NEMO-E391X mutant retains ability to promote cell survival following TNF stimulation, suggesting that its NF-κB activating function may be intact.
Fig. S2.
Stable expression of NEMO mutants in NEMO-deficient cell lines and patient samples. (A) NEMO intracellular stain as previously described of parental Jurkat cell line (3T8), NEMO-deficient (8321), and NEMO-reconstituted 8321 cells (rNEMO). (B) Cartoon of wild-type NEMO, NEMO syndrome mutants, and mutant forms of NEMO expressed in the NEMO-deficient Jurkat cell lines used in this study. (C) Western blot of cell lysates from patient and healthy control blasting T cells were probed with antibody specific for NEMO, IKKβ, and actin as a loading control.
Fig. 2.
NEMO-truncation leads to increased NF-κB activity upon TNF-R1 or TLR5 stimulation and prevents TNF-induced apoptosis to the same degree as wild-type NEMO. (A) NEMO-deficient and reconstituted cell lines were cultured in media alone, TNF, or TNF + zVAD for 18 h. Apoptosis was measured by positivity for Annexin-V surface staining and mean percent-specific cell death was calculated as described in Materials and Methods, (n = 3). (B) Surface protein expression of the NF-κB reporter, rat Thy-1, in NEMO-deficient [NEMO(−)] or reconstituted Jurkat cells was detected by flow cytometry following stimulation in media alone (blue), TNF (green), or Flagellin (red) and quantified (n = 3) (Fig. S3A).
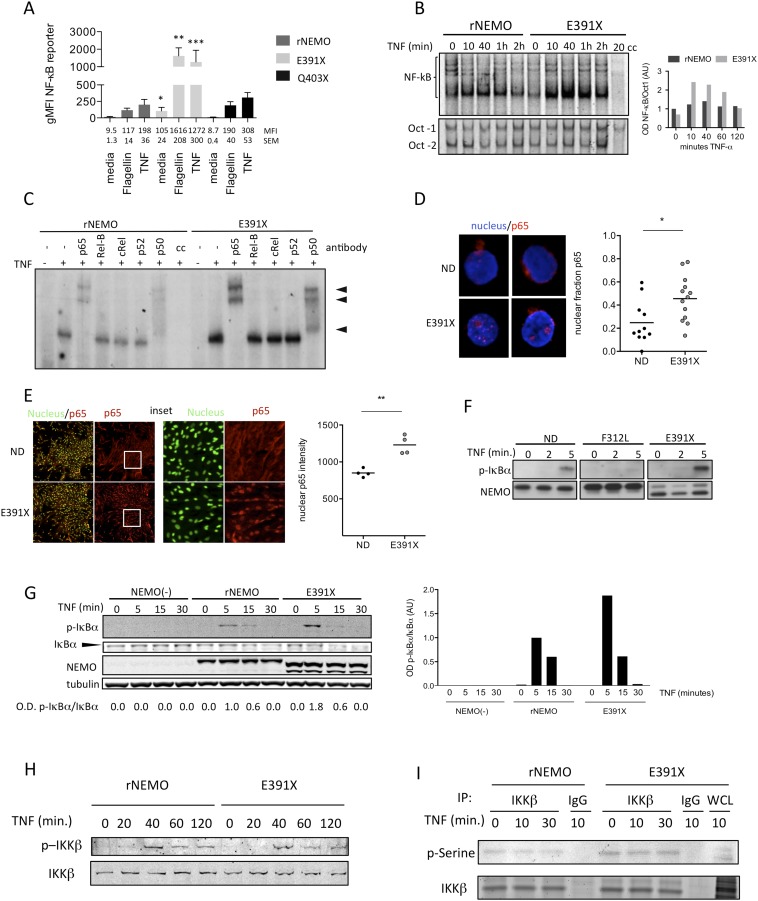
To more directly measure NF-κB activation induced by ΔCT-NEMO mutants, we analyzed reconstituted Jurkat cells by flow cytometry using a stably incorporated NF-κB reporter construct encoding Thy-1. Cells reconstituted with NEMO-C306X, a mutant lacking both ubiquitin-binding domains, were completely unresponsive to either TNF or Flagellin stimulation, similar to NEMO-deficient cells (Fig. 2B). As expected, reconstitution with the previously described patient-derived hypomorphic NEMO C417R led to reduced responsiveness compared with cells reconstituted with wild-type NEMO. In contrast, Jurkat cells expressing the partial ΔCT-NEMO mutant Q403X exhibited intact NF-κB responses to TNF and Flagellin, and the full ΔCT-NEMO mutant E391X showed substantially increased basal levels of the NF-κB reporter in addition to increased responsiveness compared with cells reconstituted with wild-type NEMO (Fig. 2B, Lower, and Fig. S3A). These results demonstrate ΔCT-NEMO mutations preserve NF-κB activity, and in the case of the E391X mutant result in enhanced NF-κB activity compared with full-length NEMO, both in the resting state and in response to TNF or TLR ligands. Taken together, these data show that NEMO-deficient Jurkat T cells reconstituted with ΔCT-NEMO mutants recapitulate the increased responsiveness to innate immune stimuli that characterized primary immune cells with these mutants.
Fig. S3.
The canonical NF-κB pathway is activated in NEMO and ΔCT-mutant reconstituted cells following TNF stimulation. (A) Quantitation of surface protein expression (n = 3) of Thy1.1 NF-κB reporter as done in Fig. 2B. Average geometric mean fluorescence intensity (gMFI) and SEM appear beneath each condition. (B) Nuclear extracts were incubated with labeled NF-κB consensus sequence oligonucleotide or control (Oct-1) sequence and visualized by EMSA. Nuclear extracts loaded in lane labeled “cc” were preincubated with 100-fold excess of unlabeled consensus NF-κB or Oct-1 oligonucleotide before incubation with labeled oligonucleotide. Densitometry of the NF-κB/DNA complexes normalized to Oct-1 from a representative experiment (n = 3). (C) Reconstituted Jurkat T cells were stimulated with TNF and specificity of nuclear NF-κB complexes was determined by antibody-induced supershift of DNA-protein complexes. (D) Healthy control and E391X-NEMO patient PBMC were isolated. Nuclei were stained followed by intracellular p65 staining on cells following fixation. Images of two representative unstimulated cells are shown. Quantitation indicates nuclear fraction of p65 determined from images obtained by confocal microscopy at 63× magnification of ∼10 cells per condition. (E) Healthy control and E391X-NEMO patient iPS-derived fibroblast-like mesenchymal stem cells in culture. Nuclear and intracellular p65 staining was performed on fixed cells. Images at 4× magnification of a representative area of unstimulated cells are shown. The area bounded by the white box is shown enlarged at 10× magnification in panels on the right. For quantitation, each circle represents a single experimental replicate and each replicate consists of images containing ∼2,000 cells (n = 2). (F) Activated NEMO-E391X and NEMO-F312L patient T cells were stimulated with TNF. Western blot of whole cell lysates was probed with antibody specific to phosphorylated serine residues 32 and 36 of IκBα, NEMO was probed as a loading control. The result shown is representative of three independent experiments. (G) Reconstituted Jurkat T cells were stimulated with TNF for the indicated times and detection of p-IkBa and IkBa was performed by Western blot (n = 2); optical densitometry indicating the ratio of p-IkBa/IkBa was done (Right). (H) Reconstituted Jurkat T cells were stimulated with TNF and detection of IKK-b and IKK-a serine phosphorylation was performed by specific antibody following Western blot. (I) Reconstituted Jurkat T cells were stimulated with TNF followed by immunoprecipitation of IKK-β and subsequent detection of total phospho-serine using a specific antibody.
NEMO ΔCT Mutants Lead to Sustained IKK Activity and Increased NF-κB Nuclear Translocation.
To determine how ΔCT mutants directly affected NF-κB transcription factor activity, we measured NF-κB binding by EMSA following TNF stimulation in NEMO-deficient Jurkat cells reconstituted with wild-type or NEMO-E391X. Extracts from cells expressing NEMO-E391X showed increased binding to NF-κB target sequences following TNF stimulation at all time points (Fig. S3B). Densitometric analysis of NF-κB binding normalized to control Oct-1 binding confirmed this result (Fig. S3B, Right). The E391X mutant specifically activated the canonical NF-κB pathway, as supershift assays showed increased signal shifted with p65 and p50 antibodies but not with RelB, c-Rel or p100 antibodies (Fig. S3C). Increased spontaneous nuclear localization of the p65 subunit of NF-κB was observed in peripheral blood leukocytes from a patient with the E391X-NEMO mutation (Fig. S3D). Similar to what we had observed in the primary peripheral blood cells, mesenchymal stem cell-like fibroblasts generated from patient induced pluripotent stem cells (iPS) displayed constitutive enhanced nuclear NF-κB translocation (Fig. S3E). Taken together, these data show that the NEMO-E391X mutant acts to enhance NF-κB nuclear translocation and DNA binding, both ex vivo from patient cells and in reconstituted cell lines.
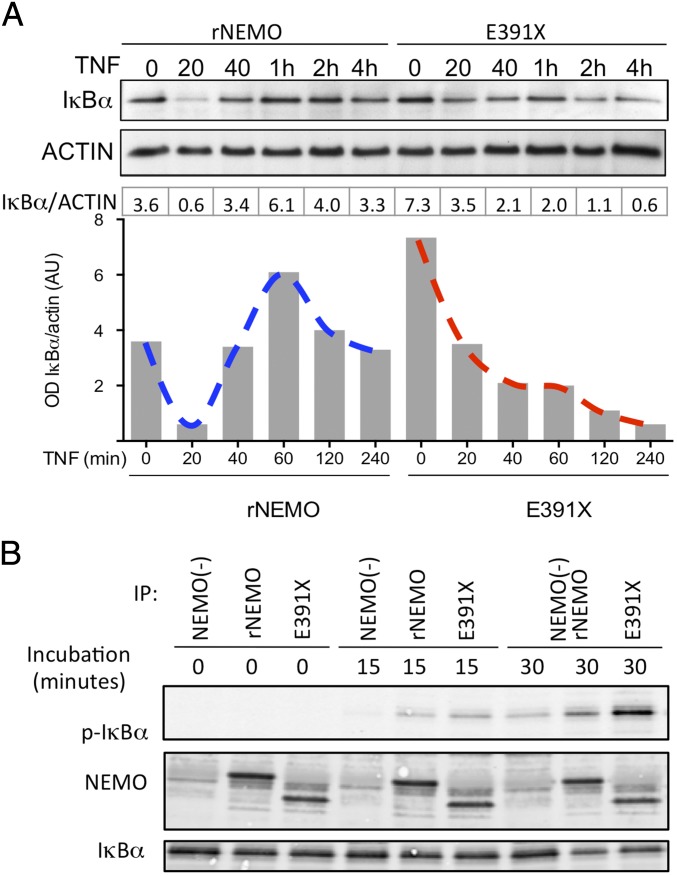
To evaluate the mechanisms of enhanced NF-κB signaling in cells expressing ΔCT-NEMO mutations in more detail, we used Jurkat cells reconstituted with full-length NEMO and NEMO-E391X. Steady-state levels of IκBα were higher in the E391X mutant compared with wild-type NEMO (Fig. 3A). However, IκBα degradation in the 30 min following TNF stimulation in cells expressing NEMO-E391X appeared equivalent to that in cells with wild-type NEMO (Fig. 3A). Importantly, at longer time points, the IκBα in cells with the E391X-NEMO mutation failed to maintain baseline levels as it does in cells expressing wild-type NEMO, suggesting that sustained IKK kinase activity may be preventing IκBα from reaccumulating. To determine whether C-terminal NEMO truncations increase IKK complex kinase activity, we stimulated activated T cells from EDA-ID patients harboring the ΔCT-NEMO-E391X mutation, the NEMO-F312L mutation, which is a loss-of-function missense mutation affecting the UBAN domain, and healthy controls. As expected, TNF induced IKK activity, as measured by phosphorylation of IκBα, was reduced in T cells from an individual with NEMO-F312L loss-of-function mutation (Fig. S3F). Strikingly, there was enhanced phosphorylation of IκBα in T cells from an individual with the E391X mutation compared with a healthy donor control (Fig. S3F), a finding that was reproduced in NEMO-deficient cells reconstituted with full-length NEMO or E391X-NEMO (Fig. S3G), suggesting that this mutation may confer gain-of-function properties to the canonical IKK kinase.
Fig. 3.
NEMO-truncation leads to increased TNF-induced IKK complex activity. (A) Western blot of IκBα following TNF stimulation with actin blot as a loading control. Quantitation of IκBα, normalized to actin, was performed by optical densitometry, below. (B) NEMO-deficient and reconstituted Jurkat cell lines were treated with TNF and an in vitro IKK kinase assay (KA) was performed using immunoprecipitated IKK complexes in the presence of ATP and substrate and Western blot analysis of coimmunoprecipitated phospho-IκBα; results are representative of three independent experiments.
To investigate the effect of ΔCT-NEMO on IKK activity in the absence of proteasome-mediated effects on IκBα, we isolated the IKK complex after TNF stimulation and incubated NEMO immunoprecipitates with recombinant GST-IκBα. Kinase activity, as measured by detection of phosphorylation of GST-IκBα, was elevated in IKK immune complexes containing NEMO-E391X compared with those containing wild-type NEMO (Fig. 3B). Taken together, these results strongly suggest that the E391X-NEMO mutation enhances intrinsic IKK activity and transactivation of NF-κB after stimulation with TNF. In general, it is thought that activation of canonical IKK activity occurs via phosphorylation of T-loop serines on IKK-α and IKK-β by upstream kinases (24). To determine whether ΔCT-NEMO forms enhanced activation of the canonical IKK by this mechanism, we stimulated reconstituted Jurkat cells with TNF and measured specific phosphorylation of T-loop serines by Western blot. We observed equivalent phosphorylation of both T-loop serines and total phosphoserine of IKKβ in cells that express E391X-NEMO (Fig. S3 H and I) compared with full-length NEMO. This finding suggests that enhanced kinase activity in ΔCT-NEMO forms is not a result of enhanced activation by upstream kinases, but instead, results from altered function or interaction with a signaling partner “downstream” of IKK T-loop activation.
ΔCT-NEMO Mutants Fail to Interact with A20.
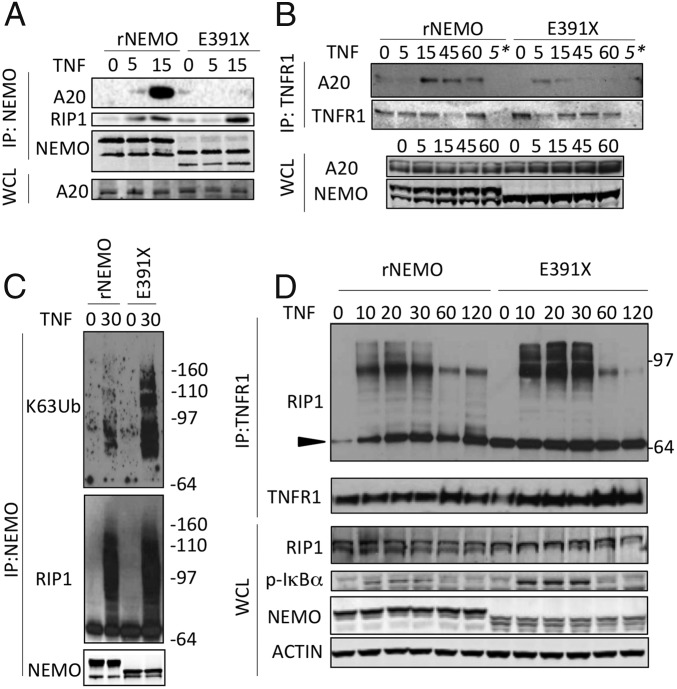
One way by which absence of the NEMO C terminus could lead to enhanced activation of NF-κB would be if ΔCT-NEMO mutants fail to interact with a negative regulator of NF-κB signaling. A20, the product of the gene TNFAIP3, is a ubiquitin-editing enzyme that has a well-recognized role as a negative regulator of NF-κB activation (9, 25, 26). We stimulated Jurkat cells reconstituted with wild-type or E391X-NEMO with TNF and examined NEMO and TNFR1-associated protein complexes through immunoprecipitation. Whereas A20 is recruited to full-length NEMO following TNF stimulation, A20 failed to inducibly associate with E391X-NEMO (Fig. 4A). Impaired recruitment of A20 by E391X is specific, as ΔCT-NEMO was able to associate with CYLD in response to TNF stimulation (Fig. S4 A and B). Analysis of the TNFR1 signaling complex following TNF stimulation of these Jurkat lines revealed deficient recruitment of A20 to the TNFR1 receptor complex in NEMO-E391X cells (Fig. 4B). A20 within the TNFR complex facilitates the conversion of K63-linked to K48-linked RIP1, leading to subsequent degradation of RIP1 (9). We therefore investigated whether there was increased high molecular-weight RIP1 in association with the IKK complex in cells that express E391X-NEMO. Indeed, coimmunoprecipitation of RIP1 with NEMO indicated that 30 min after TNF stimulation, high molecular-weight RIP1 could be detected in association with both full-length and E391X-NEMO. Moreover, probing this immunoprecipitate with antibody specific for K63 linkage indicates increased K63-polyubiquitination of proteins associated with NEMO lacking the C terminus (Fig. 4C, Upper, compare lanes 2 and 4). In cells reconstituted with NEMO-E391X, we observed the persistence of high molecular-weight forms corresponding to ubiquitinated RIP1 in the TNFR1 signaling complex compared with cells expressing wild-type NEMO (Fig. 4D and Fig. S4C), reminiscent of the effect of A20 deficiency on RIP1 in the TNFR1 signaling complex (9). Taken together with the increased K63-ubiquitnated protein in the NEMO IP (Fig. 4C), these data most likely represent K63-ubiquitin modified RIP1 that fails to become deubiquitinated because of reduced recruitment of A20 and its K63-deubiquitination activity. These results provide a molecular mechanism for the enhanced NF-κB activation in cells that express ΔCT-NEMO forms by stabilization of polyubiquitinated RIP1 at the TNFR1 signaling complex, and enhanced IKK complex activity resulting from failure to recruit A20 by the NEMO C terminus.
Fig. 4.
Impaired recruitment of A20 to a NEMO C terminus truncation mutant is associated with increased K63-polyubiquitinated RIP1 associated with NEMO and the TNFR. (A) Reconstituted Jurkat T Cells were stimulated with TNF for the indicated times, and NEMO was isolated by immunoprecipitation to detect NEMO/A20 association. NEMO was probed as an immunoprecipitation control. (B) Following TNF stimulation for the indicated times, the TNFR1 was immunoprecipitated (IP) and the associated A20 was detected by Western blot; TNFR1 was probed as a control. The asterisk denotes IP with IgG as a control. (C) Following TNF stimulation, NEMO was immunoprecipitated and the associated RIP1 was detected in addition to specific K63 ubiquitin linkages using K63-linkage–specific antibody, NEMO was probed as an IP control. (D) Following TNF stimulation, TNF-R1 was immunoprecipitated and the associated RIP1 was detected by Western blot; unmodified RIP1 is indicated by the black triangle. RIP1, phospho-IκBα, NEMO, and actin were detected in corresponding whole-cell lysates. Experiments were performed in NEMO-deficient Jurkat cells reconstituted with either wild-type or E391X NEMO.
Fig. S4.
The DUB CYLD can associate with DCT-NEMO in response to TNF. (A) 293 cells were transfected with CYLD, full-length NEMO, or E391X-NEMO vectors and treated with TNF for 10 min before immunoprecipitation of cell lysates with anti-NEMO followed by Western blot for CYLD and NEMO in IP and whole-cell lysates. (B) Reconstituted Jurkats were stimulated with TNF for 10 min, lysed, and CYLD was immunoprecipitated with anti-CYLD. The upper band in the NEMO blot of the IP is a combination of mouse heavy chain and full-length NEMO. High molecular-weight RIP1 is stabilized at the TNFR in cells that express E391X-NEMO. (C) Quantitation by densitometry of Western blot bands corresponding to RIP1 (unmodified and ubiquitinated) coimmunoprecipitated with TNFR1 as performed in Fig. 4D, (n = 3). The effect of genotype (NEMO or E391X) was determined by two-way ANOVA and was highly significant (P < 0.0001).
SI Materials and Methods
Cell Isolation.
For patient and normal donor-derived peripheral blood samples, informed consent was obtained in accordance with an NIH Institutional Review Board-approved protocol. PBMC were isolated by Ficoll-Paque (Amersham Biosciences) gradient centrifugation and used immediately for gene-expression studies or CD14+ and CD4+ cell purification. Cell activation and gene expression are described below.
Co-IP and Western Blots.
Cells were lysed in 20 mM Tris⋅HCl, 150 mM NaCl, 5 mM MgCl2, 1% (wt/vol) TritonX-100, 1× Complete protease inhibitor (Roche), 250 mM β-glycerophosphate, 10 mM Na-orthovanadate, 50 mM Na-pyrophosphate, 500 mM NaF, 10 mM Na-molybdate, 20 mM EGTA, 5 mM N-ethyl maleimide (NEM), and 5 mM iodoacetamide (IAA), and cleared of cell membranes by centrifugation. IP was performed by incubating lysates with appropriate antibodies for 3 h at 4 °C and followed by addition of magnetic G protein beads (Invitrogen). Immunoblotting of lysates was performed as previously described (20). Antibodies used for IP and Western blot: p-IκBa (#9246, Cell Signaling), NEMO (#611306, BD), NEMO (sc-8330, Santa Cruz), β-tubulin (#6046, Abcam), IKKβ (#2678, Cell Signaling), Ubiquitin (#3936, Cell Signaling), RIP1 (#3493, Cell Signaling), actin (#3280, Abcam), TNFR1 (sc-8436, Santa Cruz), K63 (#14-6077, eBioscience), A20 (#5630S, Cell Signaling). Densitometry quantitation was performed using ImageJ software (rsbweb.nih.gov/ij/).
Cell Activation.
T-cell blasts (activated T cells) were generated by stimulation with Concanavalin-A (Sigma) 5 μg/mL, followed 48 h later by culture in IL-2 (National Cancer Institute repository). Monocytes were isolated from fresh PBMC by positive selection using anti–CD14-PE and anti-PE microbeads (Miltenyi Biotec) on the AutoMACS magnetic separation system (Miltenyi Biotec). The CD14+ cell-depleted fraction was collected for purification of CD4+ T-cells by staining with anti–CD4-FITC and anti-FITC microbeads, followed by magnetic separation as described above. Purity of the isolated fractions was greater than 98%, as determined by flow cytometry, by gating on PE+ cells for CD14+ monocytes and FITC for CD4+ cells. PBMCs and isolated CD14+ monocytes and CD4+ T-cells were added to 96-well plates at a density of 2.50 × 105 per well in 250 μL RPMI-1640 medium (Cellgro) containing 10% (vol/vol) FCS (HyClone). Cells were incubated in media alone or stimulated with TNF (20 ng/mL; R&D Systems), Flagellin (100 ng/mL; InvivoGen), and LPS (100 ng/mL; Enzo Life Sciences). For each experiment, a normal donor sample was run in parallel for purposes of normalization.
Quantitative RT-PCR for Measurement of Gene Expression.
Total RNA was isolated from PBMCs using RNeasy Mini Kit (Qiagen). Quantitative RT-PCR was performed on a CFX96 Real-Time System (Bio-Rad) with the use of TaqMan Universal PCR Master Mix (Applied Biosystems) with iScript RT Supermix (Bio-Rad) or qScript RT SuperMix (Quanta). Predesigned primer/probe sets for IL-1β (Hs01555410_m1), IL-6 (Hs00985639_m1), and MIP-1β (Hs99999148_m1), were from Applied Biosystems. Probes specific for b2-microglobulin (Hs00187842_m1-FAM probe or 4310886E-VIC probe) were used as endogenous controls. Amplification efficiency was calculated with LinRegPCR software and mean normalized expression of each cytokine (ΔΔCt) was calculated with the Excel-based software Q-Gene (www.gene-quantification.de).
Cytokine Production Analysis.
Following 24- or 48-h stimulation, cells were spun down and supernatants were frozen until subsequent analysis. The concentrations of cytokines in the supernatant of CD4+ and CD14+ cells were determined using the Bio-Plex Pro Human Cytokine 17-plex immunoassay kit or combination of single-plex sets (Bio-Rad) according to the manufacturer’s protocol. The Bio-Plex Pro Human Cytokine standard group I was used as a standard for the assay. Each sample was assessed in duplicate. The Cytokine production index (CPI) is the ratio of the concentration of a particular cytokine (IL-1β, IL-6, and so forth) in the supernatant of stimulated cells divided by the concentration of that same cytokine in the supernatant from unstimulated cells normalized to the healthy control’s cytokine production in response to the same stimulus. Statistical significance was determined by unpaired t test.
Cell Lines.
Mutant NEMO cDNA were generated by site-directed mutagenesis and used to reconstitute the NEMO-deficient Jurkat T-cell line 8321, provided by A. Ting, Mount Sinai Hospital, New York. cDNA encoding wild-type NEMO in pCDNA3 served as a template which was mutated by PCR amplification of the coding sequence using primers designed to introduce single amino acid change, resulting in patient-specific mutants (E391X, E390RfsX4, Q403X, C417R, L153R, and NEMO-PRS containing a E391A/P392A mutation). All mutants were packaged into a Migr1 retroviral plasmid that also encodes GFP and allows sorting of reconstituted lines. The 8321 line contains a stably integrated NF-κB reporter construct consisting of the rat Thy-1 gene preceded by four concatamers of synthetic NF-κB sites. Reconstitution and properties of the 8321 line was previously described (20). Reconstituted clones were matched for GFP expression and equivalent expression of NEMO was determined by Western blot and intracellular staining followed by flow cytometry. Patient and healthy control iPSCs were derived from PBMC using episomal vectors. Fibroblast-like MSC were obtained following iPS culture in E6 media (StemCell) with a TGF-β inhibitor (SB-431542).
Nuclear Fractionation and EMSA.
Cells were lysed in hypotonic solution: 10 mM Hepes, 10 mM KCl, 1 nM EGTA, 1 nM EDTA, 1 mM DTT, complete protease inhibitor, and 0.3% (wt/vol) Nonidet P-40. Nuclear pellets and cytosolic supernatants were separated by centrifugation 30 s at 13,000 × g. Nuclei were lysed in 20 mM Hepes, 160 mM NaCl, 1 nM EGTA, 1 nM EDTA, complete protease inhibitor, and 2 mM DTT. For supershift experiments, nuclear extracts were incubated with antibodies to p65 and p50, p52, c-Rel, and RelB antibody for 1 h before incubation with labeled DNA in 5 mM MgCl2, poly dI/dC, BSA, and 1.25% (wt/vol) Nonidet P-40. Extracts were then incubated with labeled DNA specific for NF-κB promoter consensus sequence, and subsequently separated by nondenaturing polyacrylamide gel electrophoresis. Consensus NF-κB oligonucleotide sequence 5′-AGT TGA GGG GAC TTT CCC AGG C-3′ or OCT1 sequence 5′-TGT CGA ATG CAA ATC ACT AGA A-3′ was end-labeled with 32P or IrDye700 - 82907924 (IDT) or unlabeled “cold” competition control for binding specificity.
Statistical Analysis.
Error bars indicate the SD of the mean for patient cell-derived studies of gene expression and cytokine production, and the SEM for data derived from reconstituted cell lines; unpaired t test was used to determine statistical significance. To indicate statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Clinical description of autoinflammatory disease linked to ΔCT-NEMO.
The majority of nonsense mutations arise a result of mutations in the same region because of insertion or deletion of one or more nucleotides within a string of seven consecutive cytosines. These lead to expression of a mutant form of NEMO lacking the final 29 amino acids of the protein. The inflammatory disease associated with ΔCT NEMO manifests as a diffuse skin and gut disease that initially presents as a malabsorption syndrome. Biopsy reveals colitis, which is generally described as acute and is clinically responsive to enteric steroids (15, 16, 20, 46–50). Erythroderma appears at birth and is characterized as eczematous or sebhorreic (15, 16, 46, 49). Inflammatory cell infiltrates on biopsy of lesional skin indicate the presence of a combination of lymphocytes, activated macrophages, neutrophils, and eosinophils with proliferation of keratinocytes and edema. White blood cell counts and eosinophils are frequently elevated in peripheral blood (15–17, 20, 48). Frequently by several months of age, individuals have experienced several episodes of bacterial infection requiring intravenous antibiotics. ΔCT-NEMO mutations appear in seven related individuals (with the E391X mutation) and an additional six unrelated individuals with similar C-terminus truncation following the insertion of missense amino acids Arg-Gly-Ala-Thr. In contrast to NEMO truncation mutations, 10 individuals with missense mutations in the C-terminal region have frequent and severe infections, but no inflammatory disease phenotype (14, 17, 20, 51–57).
Clinical description of NEMO-UBAN primary immunodeficiency disease.
Patient 1 developed fevers of unknown origin at 2 y of age and was subsequently diagnosed with cutaneous atypical mycobacterial infection. Patient 2 was born full term without complications, and at 4 mo of age developed pneumococcal meningitis affecting hearing. He was subsequently found to be not reactive to pneumococcal vaccination. At the age of 6 y, the patient developed skin lesions on the face that were identified as Mycobacterium avium intracellulare that responded to a prolonged course of antimicrobial therapy. Both patients 1 and 2 had evidence of abnormal ectodermal development in that primary teeth were conical in shape; however, secondary dentition, hair, and ability to sweat in patient 1 appear normal, whereas patient 2 does not sweat normally.
Discussion
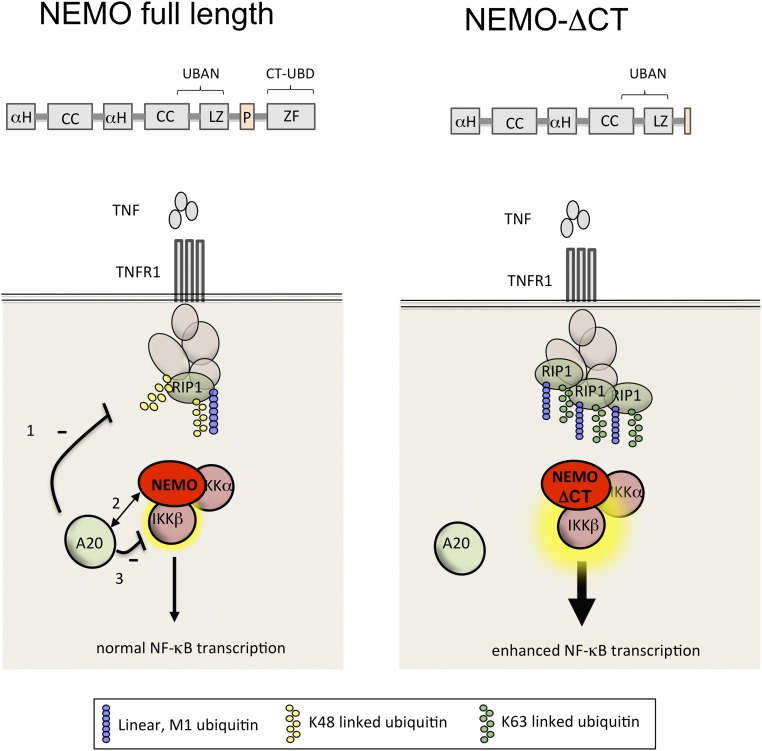
In this study, we have described clinically relevant gain-of-function NEMO mutations, and show that the NEMO C terminus plays an essential regulatory role in NF-κB signaling to prevent inflammatory disease in humans. The impaired ability of these NEMO mutants to interact with a negative regulator of NF-κB, A20, combined with preserved ability to activate the canonical IKK kinase, leads to increased responsiveness to TNF and TLR agonists through a number of mechanisms (Fig. S5). Reduced levels of A20 within the TNFR1 signaling complex results in failure to convert K63 linkages on A20 substrates, such as RIP1 to K48-linked ubiquitin, preserving K63-linked ubiquitin-modified RIP1 and prolonging the recruitment of the IKK signalosome (9).
Fig. S5.
NEMO ubiquitin binding specificity and its effect on A20 recruitment and function in NF-κB activation and regulation. (Left) Full-length NEMO enables TLR and TNF-R signaling via combined K63- and M1-linked polyubiquitin recognition and recruitment of A20 that modifies RIP1, leading to its degradation. (Right) Loss of the C terminus leads to a decrease in K63 polyubiquitin binding, impaired association with A20, and enhanced TNF-induced IKK activity, in part because of enhanced stabilization of signaling components at the receptor, such as RIP1. Similarly, TLR stimulation leads to canonical IKK complex activation with absent recruitment of A20 and subsequently increased proinflammatory cytokine production.
Our results suggest that the NEMO C terminus plays an essential role in the recruitment and stabilization of A20 to the TNFR. A20 recruitment is in part K63-polyubiquitin–mediated, as the A20 ZnF4 and ZnF7 domains required for inducible recruitment to the TNFR and NEMO, respectively, are K63 ubiquitin binding domains (11, 27, 28). Previous work showed that the NEMO N terminus is required for the formation of the tripartite complex including K63-linked polyubiquitin and A20 (11). Our results demonstrate the essential contribution made by the C terminus of NEMO to A20 recruitment and is consistent with previous work that demonstrates the preferential recognition of K63 linked polyubuiquitin by the NEMO C-terminus ubiquitin binding domain (29). Further work needs to be done to understand the details of the biochemical chain of events that lead to A20 recruitment and complex disassembly.
Because ΔCT-NEMO mutants continue to associate with RIP1 (Fig. 4C), our results suggest that other domains within NEMO, such as the UBAN domain that preferentially recognizes linear polyubiquitin, may have increased ability to bind linear ubiquitin in the absence of C-terminus regulation. This could also be mediated through enhanced stabilization of the linear ubiquitin assembly chain complex (LUBAC) complex, which is impaired by the A20 ZF7 domain (25). In addition to preserving RIP1 ubiquitination, failure of A20 recruitment by ΔCT-NEMO forms may also directly enhance canonical IKK activation through loss of the inhibitory effects of A20 following IL-1β, LPS, or TNF stimulation (11, 12). Our results suggest that the C terminus of NEMO is neither required for canonical IKK complex recruitment to the TNFR nor for canonical IKK activation in response to TLR or TNF stimulation. We found that A20 destabilization at the TNFR and impaired recruitment of A20 to ΔCT-NEMO leads to a constitutively active canonical IKK complex that exhibits enhanced kinase activity upon stimulation. These findings are in agreement with prior studies in which disrupting the abovementioned A20 ZnF4 or ZnF7 ubiquitin binding domains or the ZnF1 RIP1 interaction domain leads to increased canonical IKK activity (11, 27, 28).
The gain-of-function by ΔCT-NEMO mutant forms is in sharp contrast to other NEMO hypomorphic mutations, which result in impaired ability to activate NF-κB (17, 20, 30, 31). Some IKBKG mutations can result in the lack of NEMO protein expression; however, impaired canonical IKK activity and NF-κB activation can also be seen in association with mutations with intact protein expression (14, 31). In contrast, ΔCT-NEMO is detectable as a truncated protein in whole-cell extracts, and can form a functional canonical IKK complex (Fig. 3B and Figs. S2C and S3 F and G). A number of mechanisms, including enhanced cell death, failure of regulatory immune mechanisms, and infectious triggers may underlie inflammatory disease in the setting of primary immunodeficiency. In patients with ΔCT-NEMO mutations, enhanced NF-κB activation leads to proinflammatory cytokine production in response to innate immune stimuli by both CD14+ monocytes and CD4+ T cells, which likely accounts for the inflammatory features distinguishing this subset of patients from others with NEMO mutations and EDA-ID.
In addition to the inflammatory disease seen in patients with ΔCT-NEMO mutations, some features of immunodeficiency and ectodermal dysplasia persist. Impaired cellular functions leading to immunodeficiency and ectodermal development could stem from requirements by specific receptors, such as antigen receptors or the EDA receptor for utilization of the C terminus of NEMO to fully activate NF-κB. Similarly, certain specific cell types may require an intact NEMO C terminus to function. Such cell-type and receptor-specific defects have previously been described for the immunodeficiency Mendelian susceptibility to mycobacterial disease (MSMD), that is due to NEMO mutation affecting the UBAN domain and autoinflammatory disease as a result of impaired LUBAC signaling (32, 33). The inflammatory symptoms seen in patients harboring ΔCT-NEMO forms are reminiscent of the inflammatory pathology seen in A20-deficient mice that experience arthritis, colitis, and dermatitis (34–36). TNFAIP3 SNPs that in some cases function to reduce A20 expression levels have been identified that confer susceptibility to rheumatoid arthritis, systemic lupus erythematosus, psoriasis, and Behçets disease (37–40). A recently described familial syndrome with reduced A20 function as a result of haploinsufficiency exhibits inflammatory disease phenotypes that are similar to those with ΔCT-NEMO mutation (41). Therefore, our findings underscore the functional importance of A20 in preventing inflammatory disease in humans.
The pathogenesis of inflammatory symptoms in patients with ΔCT-NEMO mutations appears to be distinct from the inflammation that can develop in the setting of deficient NF-κB signaling. Mice in which IKKβ or NEMO was disrupted in colonic epithelial cells develop colitis, presumably because of impaired NF-κB–dependent epithelial cell function and survival during normal homeostasis and infection (19, 42). Similar mechanisms may underlie inflammatory bowel disease in patients with hypomorphic NEMO mutations. LUBAC has recently emerged as an important signaling platform that mediates NF-κB activation under a variety of stimuli. Spontaneous mutation in LUBAC components leads to impaired NF-κB activation, causing chronic dermatitis in mice characterized by inflammatory skin lesions and transient immune cell infiltration of the gut (43, 44). Recently, two kindreds with mutations in HOIL-1 (a LUBAC component) leading to absent protein expression were described (33). Primary fibroblasts from these individuals stimulated with TNF or IL-1β demonstrate impaired NF-κB activation. In contrast, cells harboring ΔCT-NEMO mutants have intact activation of NF-κB signaling and transcriptional responses, and are rescued from TNF-induced apoptosis. Unlike LUBAC-, NEMO-, IKKβ-deficient cells, or cells from the sharpincpdm mutant mouse, inflammation in patients with mutant ΔCT NEMO would appear not to result from excessive cell death, but rather selective gain-of-function in activation of the canonical IKK complex. As would be predicted from mouse models in which inflammation resulting from excessive cell death is not confined to the hematopoietic compartment, individuals that express loss-of-function NEMO mutants have experienced residual colitis following hematopoietic stem-cell transplantation (45). In contrast, inflammatory disease symptoms have generally tended to resolve after transplantation in patients with ΔCT-NEMO mutations (46), supporting a hematopoietic-cell intrinsic role of immune cell hyperactivation.
The molecular basis of the autoinflammatory phenotype in patients with C-terminal mutations in NEMO represents a novel molecular paradigm. Impaired interaction of NEMO with A20 or other negative regulators of NF-κB may apply to a subset of more common polygenic inflammatory diseases in which NEMO and A20 interaction may be disrupted by other means, such as by altered posttranslational modification of A20 that would impair recruitment to NEMO or decreased expression of A20.
Materials and Methods
Informed Consent.
For patient and normal donor-derived peripheral blood samples, informed consent was obtained in accordance with an NIH Institutional Review Board-approved protocol.
NF-κB Activation and Programmed Cell Death in Reconstituted Jurkat Cells.
Cells were incubated in the presence of TNF 10 ng/mL for 10 h to determine susceptibility to apoptosis, which was evaluated by 7-AAD and AnnexinV-Cy5.5 staining. Parallel cultures were incubated with TNF 10 ng/mL or Flagellin 2 μg/mL, and NF-κB–dependent Thy-1 gene transcription was determined by surface staining and flow cytometry (anti-rat Thy1; BD 554898).
Coimmunoprecipitation and Western Blots.
Cells were lysed in 1% (wt/vol) TritonX-100 containing deubiquitinase inhibitors, and the relevant proteins were detected using specific antibodies. Details can be found in SI Materials and Methods.
Kinase Assay.
Immunoprecipitated NEMO forms were washed in 20 mM Mops pH 7.5, 1 mM EDTA, 5% (wt/vol) glycerol, 0.1% β-mercaptoethanol, 1 mg/mL BSA. Kinase reaction was performed in 8 mM Mops pH 7.0 and 0.2 mM EDTA with 1 μg GST-IκBα (Abcam) in the presence of ATP (Sigma) at 32 °C.
Supplementary Material
Acknowledgments
The authors thank the patients and their families for their participation in this study; and Diane Wara, Jennifer Puck, and Mica Muskat (University of California, San Francisco), and April Brundidge (National Institute of Arthritis and Musculoskeletal and Skin Diseases) for their role in patient care and handling of patient samples.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518163113/-/DCSupplemental.
References
- 1.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruland J. Return to homeostasis: Downregulation of NF-κB responses. Nat Immunol. 2011;12(8):709–714. doi: 10.1038/ni.2055. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph D, et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14(7):854–862. [PMC free article] [PubMed] [Google Scholar]
- 4.Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD. Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and Toll-like receptor-mediated NF-kappaB activation. Mol Cell Biol. 2008;28(10):3538–3547. doi: 10.1128/MCB.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8(4):398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 6.Rahighi S, et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell. 2009;136(6):1098–1109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Hadian K, et al. NF-κB essential modulator (NEMO) interaction with linear and lys-63 ubiquitin chains contributes to NF-κB activation. J Biol Chem. 2011;286(29):26107–26117. doi: 10.1074/jbc.M111.233163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas TL, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36(5):831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Wertz IE, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430(7000):694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 10.Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327(5969):1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skaug B, et al. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol Cell. 2011;44(4):559–571. doi: 10.1016/j.molcel.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boone DL, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5(10):1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 13.Lee EG, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289(5488):2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Döffinger R, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27(3):277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 15.Mancini AJ, Lawley LP, Uzel G. X-linked ectodermal dysplasia with immunodeficiency caused by NEMO mutation: Early recognition and diagnosis. Arch Dermatol. 2008;144(3):342–346. doi: 10.1001/archderm.144.3.342. [DOI] [PubMed] [Google Scholar]
- 16.Cheng LE, et al. Persistent systemic inflammation and atypical enterocolitis in patients with NEMO syndrome. Clin Immunol. 2009;132(1):124–131. doi: 10.1016/j.clim.2009.03.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orange JS, et al. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. J Allergy Clin Immunol. 2004;113(4):725–733. doi: 10.1016/j.jaci.2004.01.762. [DOI] [PubMed] [Google Scholar]
- 18.Kastner DL, Aksentijevich I, Goldbach-Mansky R. Autoinflammatory disease reloaded: A clinical perspective. Cell. 2010;140(6):784–790. doi: 10.1016/j.cell.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446(7135):557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 20.Hanson EP, et al. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122(6):1169–1177.e16. doi: 10.1016/j.jaci.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He KL, Ting AT. A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Mol Cell Biol. 2002;22(17):6034–6045. doi: 10.1128/MCB.22.17.6034-6045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell MA, Hase H, Legarda D, Ting AT. NEMO inhibits programmed necrosis in an NFκB-independent manner by restraining RIP1. PLoS One. 2012;7(7):e41238. doi: 10.1371/journal.pone.0041238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Israël A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2010;2(3):a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verhelst K, et al. A20 inhibits LUBAC-mediated NF-κB activation by binding linear polyubiquitin chains via its zinc finger 7. EMBO J. 2012;31(19):3845–3855. doi: 10.1038/emboj.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity. 2000;12(3):301–311. doi: 10.1016/s1074-7613(00)80183-1. [DOI] [PubMed] [Google Scholar]
- 27.Bosanac I, et al. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-κB signaling. Mol Cell. 2010;40(4):548–557. doi: 10.1016/j.molcel.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Lu TT, et al. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity. 2013;38(5):896–905. doi: 10.1016/j.immuni.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laplantine E, et al. NEMO specifically recognizes K63-linked poly-ubiquitin chains through a new bipartite ubiquitin-binding domain. EMBO J. 2009;28(19):2885–2895. doi: 10.1038/emboj.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siggs OM, et al. A mutation of Ikbkg causes immune deficiency without impairing degradation of IkappaB alpha. Proc Natl Acad Sci USA. 2010;107(7):3046–3051. doi: 10.1073/pnas.0915098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ørstavik KH, et al. Novel splicing mutation in the NEMO (IKK-gamma) gene with severe immunodeficiency and heterogeneity of X-chromosome inactivation. Am J Med Genet A. 2006;140(1):31–39. doi: 10.1002/ajmg.a.31026. [DOI] [PubMed] [Google Scholar]
- 32.Filipe-Santos O, et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J Exp Med. 2006;203(7):1745–1759. doi: 10.1084/jem.20060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boisson B, et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat Immunol. 2012;13(12):1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vereecke L, et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207(7):1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matmati M, et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet. 2011;43(9):908–912. doi: 10.1038/ng.874. [DOI] [PubMed] [Google Scholar]
- 36.Nagamachi A, et al. Acquired deficiency of A20 results in rapid apoptosis, systemic inflammation, and abnormal hematopoietic stem cell function. PLoS One. 2014;9(1):e87425. doi: 10.1371/journal.pone.0087425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair RP, et al. Collaborative Association Study of Psoriasis Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41(2):199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, et al. TNFAIP3 gene polymorphisms confer risk for Behçet’s disease in a Chinese Han population. Hum Genet. 2013;132(3):293–300. doi: 10.1007/s00439-012-1250-7. [DOI] [PubMed] [Google Scholar]
- 39.Mele A, Cervantes JR, Chien V, Friedman D, Ferran C. Single nucleotide polymorphisms at the TNFAIP3/A20 locus and susceptibility/resistance to inflammatory and autoimmune diseases. Adv Exp Med Biol. 2014;809:163–183. doi: 10.1007/978-1-4939-0398-6_10. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Wen F, Wiley GB, Kinter MT, Gaffney PM. An enhancer element harboring variants associated with systemic lupus erythematosus engages the TNFAIP3 promoter to influence A20 expression. PLoS Genet. 2013;9(9):e1003750. doi: 10.1371/journal.pgen.1003750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou Q, et al. (2016) Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Gen 48(1)67–73. [DOI] [PMC free article] [PubMed]
- 42.Zaph C, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446(7135):552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 43.Seymour R, Shirley BJ, Hogenesch H, Shultz LD, Sundberg JP. Loss of function of the mouse Sharpin gene results in Peyer’s patch regression. PLoS One. 2013;8(2):e55224. doi: 10.1371/journal.pone.0055224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerlach B, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471(7340):591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 45.Pai SY, et al. Allogeneic transplantation successfully corrects immune defects, but not susceptibility to colitis, in a patient with nuclear factor-kappaB essential modulator deficiency. J Allergy Clin Immunol. 2008;122(6):1113–1118.e1. doi: 10.1016/j.jaci.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Permaul P, Narla A, Hornick JL, Pai SY. Allogeneic hematopoietic stem cell transplantation for X-linked ectodermal dysplasia and immunodeficiency: Case report and review of outcomes. Immunol Res. 2009;44(1-3):89–98. doi: 10.1007/s12026-008-8085-2. [DOI] [PubMed] [Google Scholar]
- 47.Zonana J, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67(6):1555–1562. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aradhya S, et al. Atypical forms of incontinentia pigmenti in male individuals result from mutations of a cytosine tract in exon 10 of NEMO (IKK-gamma) Am J Hum Genet. 2001;68(3):765–771. doi: 10.1086/318806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pachlopnik Schmid JM, et al. Transient hemophagocytosis with deficient cellular cytotoxicity, monoclonal immunoglobulin M gammopathy, increased T-cell numbers, and hypomorphic NEMO mutation. Pediatrics. 2006;117(5):e1049–e1056. doi: 10.1542/peds.2005-2062. [DOI] [PubMed] [Google Scholar]
- 50.Tono C, et al. Correction of immunodeficiency associated with NEMO mutation by umbilical cord blood transplantation using a reduced-intensity conditioning regimen. Bone Marrow Transplant. 2007;39(12):801–804. doi: 10.1038/sj.bmt.1705658. [DOI] [PubMed] [Google Scholar]
- 51.Orange JS, et al. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J Clin Invest. 2002;109(11):1501–1509. doi: 10.1172/JCI14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orange JS, et al. Human nuclear factor kappa B essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J Allergy Clin Immunol. 2004;114(3):650–656. doi: 10.1016/j.jaci.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 53.Orange JS, Levy O, Geha RS. Human disease resulting from gene mutations that interfere with appropriate nuclear factor-kappaB activation. Immunol Rev. 2005;203:21–37. doi: 10.1111/j.0105-2896.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 54.Jain A, et al. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2(3):223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 55.Jain A, et al. Specific NEMO mutations impair CD40-mediated c-Rel activation and B cell terminal differentiation. J Clin Invest. 2004;114(11):1593–1602. doi: 10.1172/JCI21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holland SM, et al. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N Engl J Med. 1994;330(19):1348–1355. doi: 10.1056/NEJM199405123301904. [DOI] [PubMed] [Google Scholar]
- 57.Hubeau M, et al. New mechanism of X-linked anhidrotic ectodermal dysplasia with immunodeficiency: Impairment of ubiquitin binding despite normal folding of NEMO protein. Blood. 2011;118(4):926–935. doi: 10.1182/blood-2010-10-315234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.