Significance
Excessive accumulation of oxidative stress is harmful to cancer cells. Our study demonstrates the important roles of a pentose phosphate pathway (PPP) enzyme, transketolase (TKT), in redox homeostasis in cancer development. We highlight the clinical relevance of TKT expression in cancers. We also show that TKT overexpression in cancer cells is a response of Nuclear Factor, Erythroid 2-Like 2 (NRF2) activation, a sensor to cellular oxidative stress. TKT locates at an important position that connects PPP with glycolysis to affect production of antioxidant NADPH. Our preclinical study shows that targeting TKT leads to elevation of oxidative stress, making cancer cells more vulnerable to therapeutic treatment, such as Sorafenib. Using TKT as an example, our study suggests that targeting enzymes for antioxidant production represents a direction for cancer treatment.
Keywords: TKT, HCC, ROS, metabolism, PPP
Abstract
Cancer cells experience an increase in oxidative stress. The pentose phosphate pathway (PPP) is a major biochemical pathway that generates antioxidant NADPH. Here, we show that transketolase (TKT), an enzyme in the PPP, is required for cancer growth because of its ability to affect the production of NAPDH to counteract oxidative stress. We show that TKT expression is tightly regulated by the Nuclear Factor, Erythroid 2-Like 2 (NRF2)/Kelch-Like ECH-Associated Protein 1 (KEAP1)/BTB and CNC Homolog 1 (BACH1) oxidative stress sensor pathway in cancers. Disturbing the redox homeostasis of cancer cells by genetic knockdown or pharmacologic inhibition of TKT sensitizes cancer cells to existing targeted therapy (Sorafenib). Our study strengthens the notion that antioxidants are beneficial to cancer growth and highlights the therapeutic benefits of targeting pathways that generate antioxidants.
Metabolic reprogramming has recently been recognized as a hallmark of cancer (1). Cancer cells preferentially use glycolysis instead of oxidative phosphorylation to generate energy even in the presence of oxygen (O2). This metabolic shift, named the Warburg Effect, channels glucose intermediates for macromolecule and antioxidant synthesis. A very important metabolic pathway that connects with glycolysis is the pentose phosphate pathway (PPP). The major goal of the PPP is the production of ribose-5-phosphate (R5P) and NADPH. R5P is the major backbone of RNA and is critical to nucleotide synthesis. NADPH is the major antioxidant that maintains the two major redox molecules, glutathione and thioredoxin, in the reduced state. NADPH therefore counteracts reactive oxygen species (ROS), enabling cancer cells to survive oxidative stress.
The PPP is composed of the oxidative and nonoxidative arms. The oxidative arm of the PPP produces NADPH and ribose by three irreversible steps. First, glucose-6-phosphate dehydrogenase (G6PD) converts glucose-6-phosphate (G6P) to 6-phospho-gluconolactone and NAPDH. Second, phosphogluconolactonase converts 6-phospho-gluconolactone to 6-phosphogluconate. Third, 6-phosphogluconate dehydrogenase converts 6-phosphogluconate to ribulose-5-phosphate (Ru5P) and NAPDH. Ru5P then enters the nonoxidative arm of the PPP. Ru5P is converted to xylulose-5-phosphate (X5P) and Ru5P by epimerase and isomerase, respectively. The transketolase (TKT) family [transketolase-like 1 (TKTL1) and TKTL2] transfers two-carbon groups from X5P to R5P to generate sedoheptulose-7-phosphate (S7P) to glyceraldehyde-3-phosphate (G3P). Transaldolase (TALDO) transfers three-carbon groups from S7P to G3P to generate erythrose-4-phosphate (E4P) and fructose-6-phosphate (F6P). Finally, TKT transfers two-carbon groups from X5P to E4P to generate G3P and F6P, which reenter glycolysis. All enzymes in the nonoxidative arm of the PPP are reversible, allowing cells to adapt to the dynamic metabolic demands. When cells experience high oxidative stress, metabolites from the nonoxidative arm are rechanneled into glycolysis to refill the oxidative arm for the synthesis of NAPDH. When cells are in need of nucleotides, the PPP produces ribose through the oxidative arm from G6P and the nonoxidative arm from F6P and G3P.
Although the PPP and glycolysis are equally important in the metabolism of cells, attention has been mostly drawn to the mechanisms by which glycolysis benefits tumor growth. Knowledge regarding the roles of the PPP in cancer cells is relatively scarce. Among all of the enzymes in the PPP, only the roles of G6PD and TKTL1 were briefly revealed in cancer. G6PD and TKTL1 were reported to be activated or overexpressed in cervical, lung, gastric, colorectal, and endometrial cancers (2–7). Suppressing TKTL1 in colorectal tumor cells reduced glucose uptake and lactate accumulation and enhanced sensitivity to oxidative stress (8).
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. It is the fifth most common cancer and the second leading cause of cancer deaths. The 5-y survival rate of HCC patients is less than 10% (9). Its high mortality rate is attributable to late symptom presentation and lack of promising curative therapy. Liver transplantation and tumor resection are so far the most effective HCC treatment. However, most HCC patients are not amenable to surgical treatments due to poor liver function or the presence of metastasis. Sorafenib, an oral multikinase inhibitor, is the only FDA-approved drug and extends the median survival of advanced HCC patients for about 3 mo. It is well known that HCC is one of the most rapidly growing solid cancers. Furthermore, the liver is known to be a metabolic hub that is responsible for major metabolic events in the body such as blood glucose homeostasis and glycogen metabolism. Nonetheless, the detailed metabolic alterations in HCC and the molecular mechanisms driving the metabolic adaptation in HCC remain largely unknown. Better understanding of the metabolic machineries of HCC will be beneficial for the development of therapeutic treatment. Here, using HCC as a cancer model, we demonstrate the imperative roles of the PPP in cancer development and illustrate an example that blocking a critical enzyme that connects glycolysis and the PPP, TKT, would be sufficient to impede HCC development and improve the efficiency of Sorafenib treatment in HCC.
Results
PPP Enzymes Are Frequently Up-Regulated in Cancer.
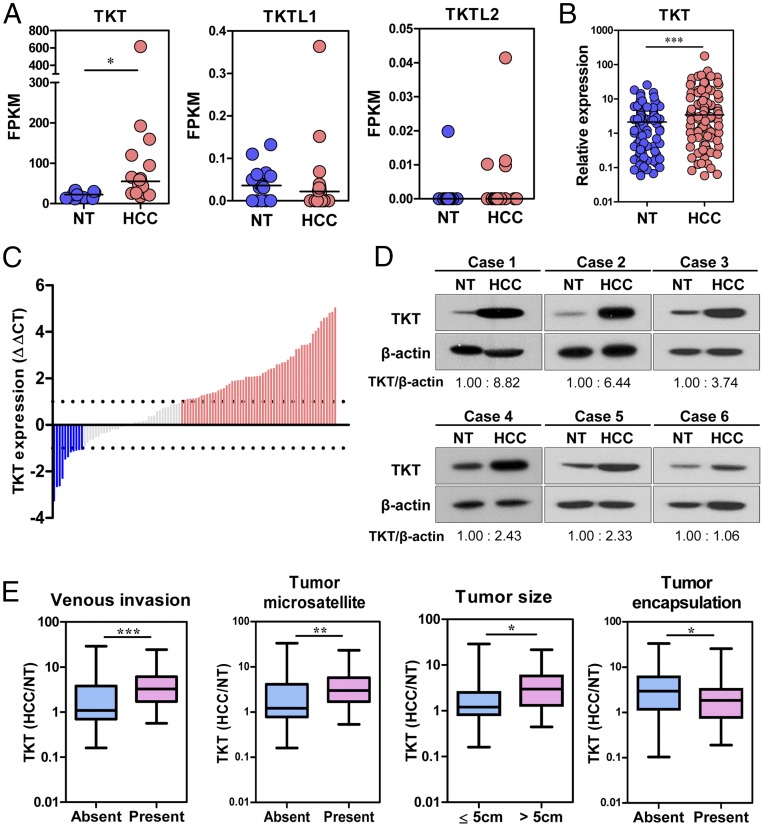
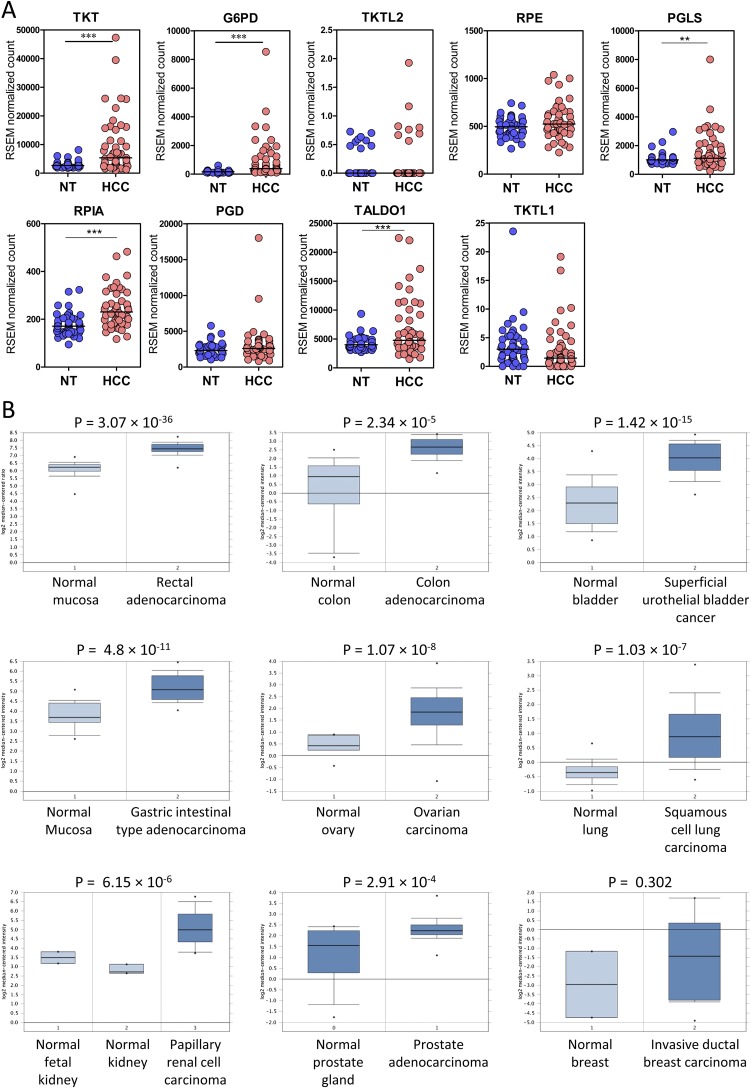
To gain insights into the clinical implications of the PPP in cancer development, we first compared the expression of all of the PPP enzymes in 16 pairs of human HCC and the corresponding nontumorous (NT) liver by transcriptome sequencing (Table 1). Intriguingly, most PPP enzymes were significantly up-regulated in human HCCs, suggesting that this pathway is deregulated in human HCC. Fragments per kilobase of transcript sequence per million mapped reads (FPKM) values suggested that TKT was the most abundantly expressed and most profoundly up-regulated PPP enzyme in HCC (Fig. 1A). Of note, two other members in the TKT family, TKTL1 and TKTL2, were barely detectable, suggesting that TKT is the predominant form in the context of liver. We further confirmed our observation in an expanded cohort of 103 HCC cases (Fig. 1B). Waterfall plot demonstrated that TKT was overexpressed by at least twofold in 54.4% (56/103) of the HCC patients (Fig. 1C). Western blotting further confirmed that TKT was also overexpressed at the protein level (Fig. 1D). Overexpression of TKT was also closely associated with aggressive clinicopathological HCC features, including the presence of venous invasion and tumor microsatellite formation, increased tumor size, and absence of tumor encapsulation (Fig. 1E and Table 2). Expression patterns of TKT and other PPP enzymes from The Cancer Genome Atlas (TCGA) database containing transcriptome sequencing data of 50 paired HCC samples also echo our own in-house data (Fig. S1A). Furthermore, the Oncomine database confirms that overexpression of TKT is not restricted to HCC but is also present in other cancer types such as colorectal, bladder, gastric, ovarian, lung, renal, prostate, and breast cancers (Fig. S1B).
Table 1.
Expression of PPP genes in clinical HCC samples
| Gene | Mean FPKM, HCC | Mean FPKM, NT | Fold change, HCC/NT | P value, t test |
| TKT | 101.722 | 22.797 | 4.462 | 0.0442 |
| G6PD | 7.807 | 2.080 | 3.753 | 0.0001 |
| TKTL2 | 0.005 | 0.001 | 3.659 | 0.4375 |
| RPE | 7.805 | 3.959 | 1.971 | 0.0004 |
| PGLS | 37.050 | 19.745 | 1.876 | 0.0007 |
| RPIA | 6.216 | 3.377 | 1.84 | 0.00006 |
| PGD | 34.922 | 20.841 | 1.676 | 0.0495 |
| TALDO1 | 93.148 | 61.181 | 1.522 | 0.0366 |
| TKTL1 | 0.047 | 0.041 | 1.146 | 0.4543 |
Expression of PPP genes in paired HCC and NT liver tissues were analyzed by transcriptome sequencing. P values were calculated by paired t test.
Fig. 1.
TKT was overexpressed in HCC samples, and overexpression correlated with aggressive clinicopathological features. (A) Transcriptome sequencing data showing the expression of TKT, TKTL1, and TKTL2 in 16 pairs of HCC and NT liver samples. Gene expression is represented as FPKM. (B) qRT-PCR analysis of mRNA levels of TKT in 103 pairs of HCC tumor and NT samples. HPRT was used as the internal control. (C) Waterfall plot shows that TKT was up-regulated in 54.4% (56/103) of human HCC samples by at least twofold. –ΔΔCT = –[(CTTKT – CTHPRT) of HCC – (CTTKT – CTHPRT) of NT]. (D) Protein expression of TKT in representative cases of HCC and their corresponding NT liver tissues was determined by Western blotting. Intensities of bands were analyzed by Image J and normalized to the corresponding NT samples. (E) TKT expression in HCC correlated with aggressive clinicopathological features of HCC, including venous invasion, tumor microsatellite formation, tumor size, and absence of tumor encapsulation. *P < 0.05, **P < 0.01, ***P < 0.001. A, paired t test; B, Wilcoxon signed rank test; E, Mann–Whitney test.
Table 2.
Clinicopathological correlation of TKT overexpression in human HCC
| Clinicopathological features | Status | Number of cases | Mean of −∆∆Ct, T-NT | P value |
| Gender | Male | 79 | 1.26 | 0.523 |
| Female | 22 | 0.98 | ||
| Venous invasion | Absent | 43 | 0.61 | 0.002** |
| Present | 55 | 1.69 | ||
| Tumor encapsulation | Absent | 64 | 1.51 | 0.025* |
| Present | 33 | 0.69 | ||
| Tumor microsatellite formation | Absent | 48 | 0.77 | 0.013* |
| Present | 49 | 1.62 | ||
| Hepatitis B surface antigen | Absent | 23 | 1.63 | 0.129 |
| Present | 71 | 1.00 | ||
| Direct liver invasion | Absent | 54 | 0.97 | 0.130 |
| Present | 34 | 1.50 | ||
| Cellular differentiation by Edmondson grading | I and II | 46 | 1.08 | 0.529 |
| III and IV | 51 | 1.30 | ||
| Tumor size | ≤5 cm | 36 | 0.70 | 0.028* |
| >5 cm | 61 | 1.49 | ||
| Cirrhosis | Absent | 46 | 1.35 | 0.483 |
| Present | 52 | 1.10 | ||
| Tumor stage | I and II | 35 | 1.73 | 0.066 |
| III and IV | 63 | 1.77 |
mRNA expression of TKT in paired HCC tumor and NT samples was measured by qRT-PCR. *P < 0.05, **P < 0.01, Student’s t test.
Fig. S1.
TKT was significantly overexpressed in human HCC and other cancers. (A) Transcriptome sequencing from TCGA dataset showing the mRNA expression of genes in the PPP (TKT, G6PD, TKTL2, RPE, PGLS, RPIA, PGD, TALDO1, TKTL1) in 50 cases of paired HCC and NT liver tissues. **P < 0.01, ***P < 0.001, paired t test. (B) Box and whisker plots of Oncomine data on TKT mRNA levels (expressed as the log2 median-centered ratio) in various normal and cancerous tissues. P values, Student’s t test.
NRF2 Competes with BACH1 to Activate TKT Expression.
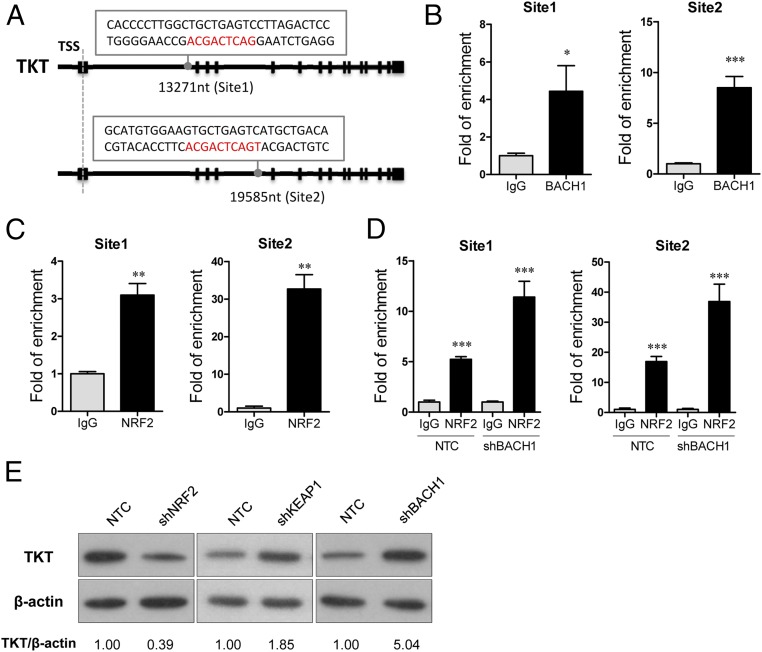
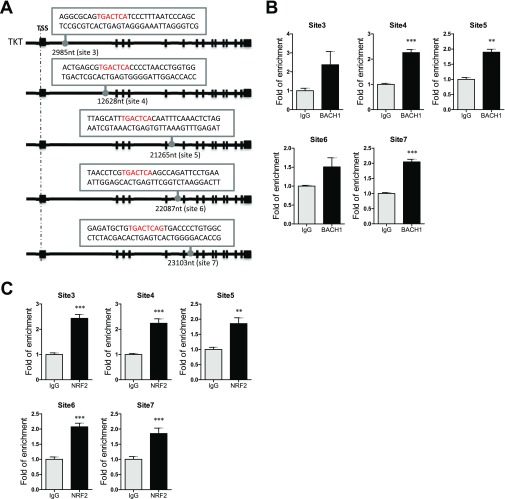
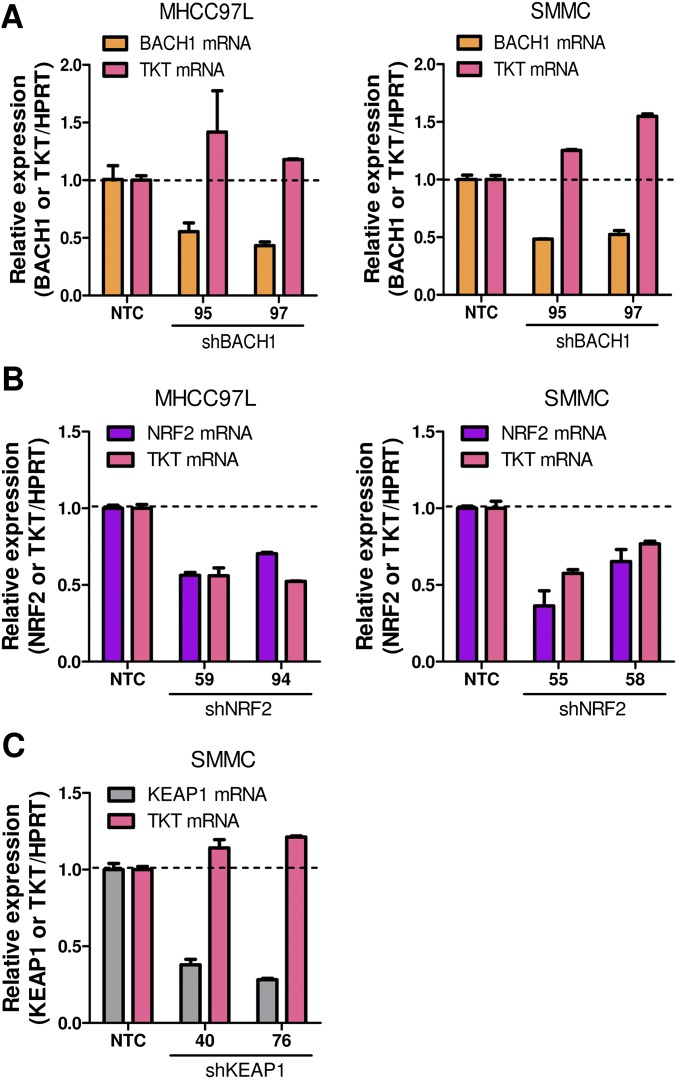
The next question we asked was how TKT was up-regulated in cancer. A chromatin immunoprecipitation sequencing (ChIP-seq) study revealed that a transcriptional repressor, BTB and CNC Homolog 1 (BACH1), can potentially bind to TKT (10). However, the precise binding location has not been validated. BACH1 is known to be an important regulator of oxidative stress through repressing heme oxygenase 1 (HMOX1), which degrades heme to form bilirubin, a strong ROS scavenger (11). BACH1 binds to the antioxidant response elements (AREs) of HMOX1 (11). Inspired from these findings, we performed in silico analysis and found seven putative AREs in TKT with consensus nucleotides, “-TGACTC-” (10) (Fig. 2A and Fig. S2A). ChIP assay was performed in a HCC cell line, MHCC97L, and we confirmed that BACH1 binds strongly to two of the seven putative AREs that are located in intron 1 and intron 4 of TKT (Fig. 2B). BACH1 has been shown to inhibit an antioxidant gene, NAD(P)H dehydrogenase, quinone 1 (NQO1), through competitive binding with a transcriptional activator, Nuclear Factor, Erythroid 2-Like 2 (NRF2) (12). Under normal conditions, NRF2 is ubiquitinated by KEAP1, leading to proteosomal degradation of NRF2. Oxidative stress inactivates KEAP1, thereby allowing NRF2 to enter the nucleus and initiate transcription of genes involved in counteracting oxidative stress. We therefore questioned if TKT, like other antioxidant genes, was regulated by the KEAP1/NRF2 pathway. By ChIP assay, we confirmed that NRF2 bound to the two strong BACH1-binding sites in intron 1 and in intron 4 of TKT (Fig. 2C). Of note, BACH1 and NRF2 also bound to the other five putative AREs at a weaker affinity, as reflected by the fold of enrichment values (Fig. S2 B and C). To validate that BACH1 and NRF2 antagonistically regulate TKT expression through competitive binding, we performed ChIP assay with the NRF2 antibody on BACH1 stable knockdown cells. Knockdown of BACH1 enhanced binding of NRF2 to TKT (Fig. 2D). Furthermore, stable knockdown of BACH1 and KEAP1 enhanced TKT expression, whereas stable knockdown of NRF2 suppressed TKT expression, suggesting that BACH1 and KEAP1 negatively regulated TKT expression, whereas NRF2 positively regulated TKT expression (Fig. 2E and Fig. S3). Coincidentally, the ARE in intron 4 of TKT was also recently found to be a binding site for NRF2 (13).
Fig. 2.
The NRF2/KEAP1/BACH1 pathway regulated TKT expression in HCC cells. (A) Positions and sequences of two NRF2 and BACH1 binding motifs in TKT. Positions refer to transcription start site (TSS). Exons are indicated as black blocks. (B and C) Recruitment of BACH1 (B) and NRF2 (C) to the two identified binding motifs in TKT. (D) Recruitment of NRF2 to the binding motifs in TKT in MHCC97L-NTC or -shBACH1 cells. ChIP assay was performed with antibodies against IgG, NRF2, or BACH1. Fold of enrichment was normalized to the according IgG controls. (E) Protein expression of TKT in MHCC97L-NTC, -shNRF2, -shKEAP1, and -shBACH1 subclones. Intensities of bands were analyzed by Image J and normalized to corresponding NTC. *P < 0.05, **P < 0.01, ***P < 0.001 versus the corresponding IgG, Student’s t test. Data are presented as means ± SD.
Fig. S2.
The NRF2/KEAP1/BACH1 pathway regulated TKT expression in HCC cells. (A) Five additional NRF2 and BACH1 binding motifs were found in TKT. Positions refer to TSS. (B) ChIP assay showing the binding of BACH1 to the five identified binding motifs. Fold of enrichment is normalized to the corresponding IgG. (C) ChIP assay showing the binding of NRF2 to the five identified binding motifs. Fold of enrichment is normalized to the corresponding IgG. **P < 0.01, ***P < 0.001 versus IgG, Student’s t test. Data are presented as means ± SD.
Fig. S3.
Knockdown of the NRF2/KEAP1/BACH1 pathway members altered TKT mRNA expression. (A) mRNA expression of TKT in MHCC97L and SMMC-NTC and -shBACH1 subclones. (B) mRNA expression of TKT in MHCC97L and SMMC-NTC and -shNRF2 subclones. (C) mRNA expression of TKT in SMMC-NTC and -shKEAP1 subclones. **P < 0.01, ***P < 0.001 versus BACH1, NRF2, or KEAP1 mRNA expression in NTC, ##P < 0.01, ###P < 0.001 versus TKT mRNA expression in NTC, Student’s t test. Data are presented as means ± SD.
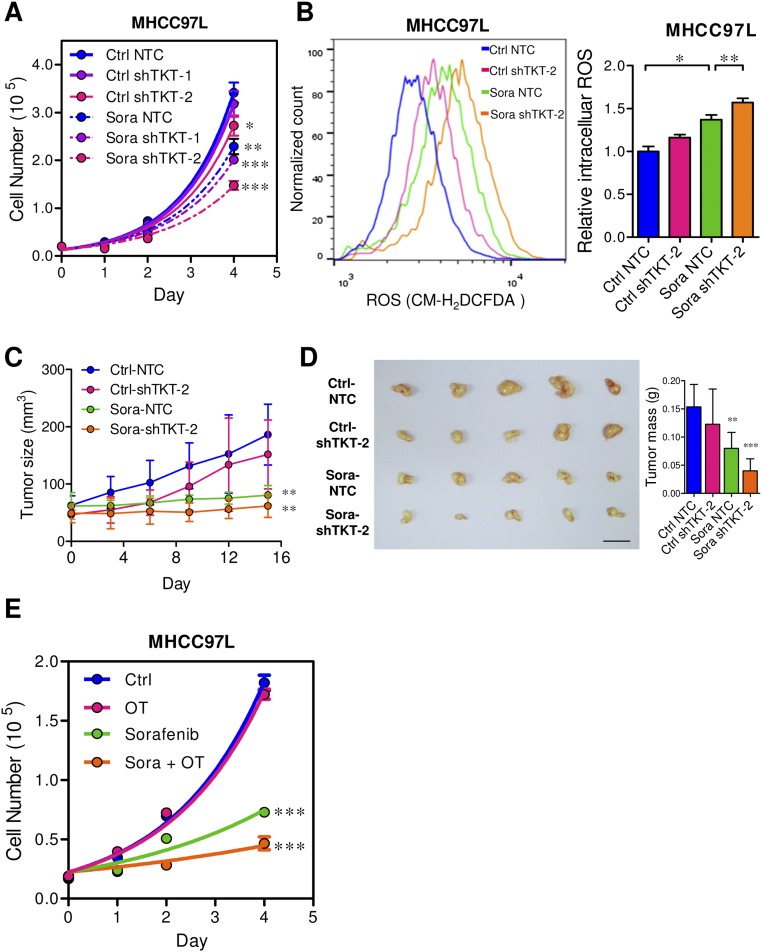
Knockdown of TKT Suppresses HCC Cell Proliferation by Inducing ROS Accumulation and ROS-Associated Cell-Cycle Delay.
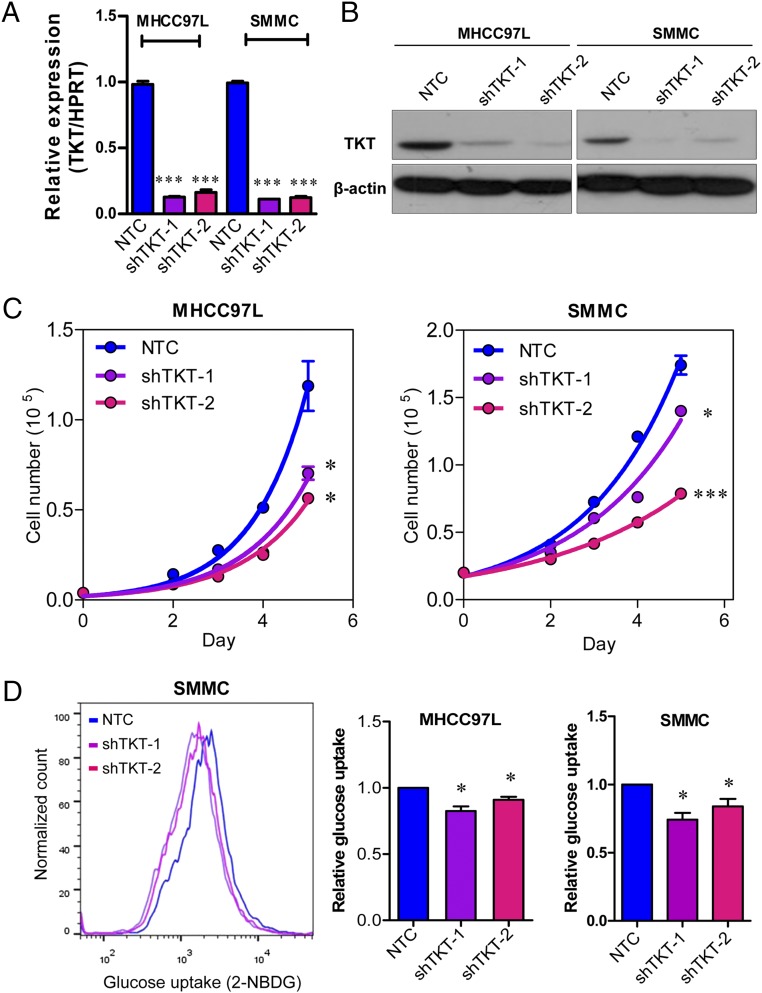
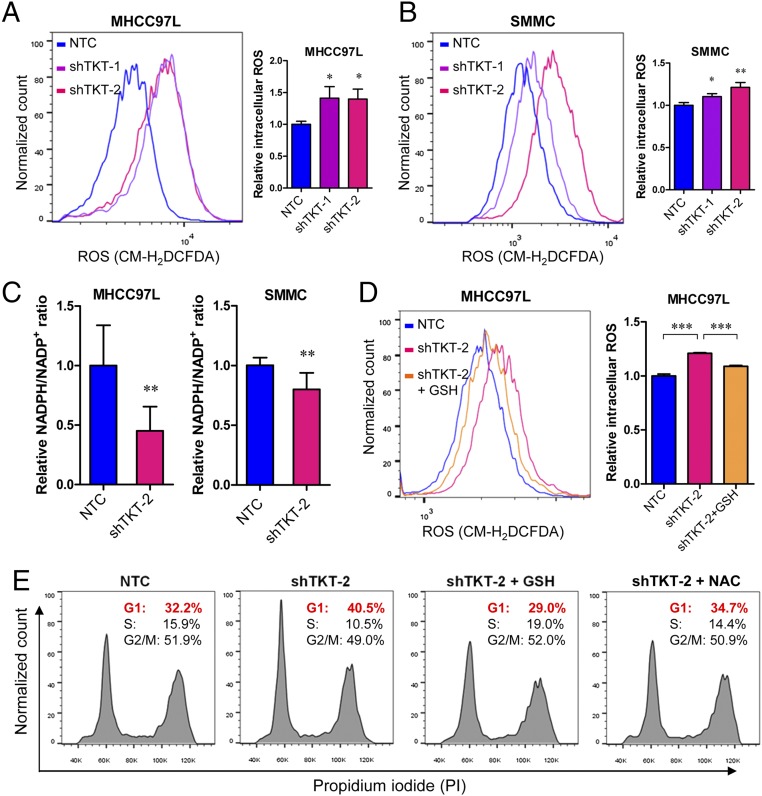
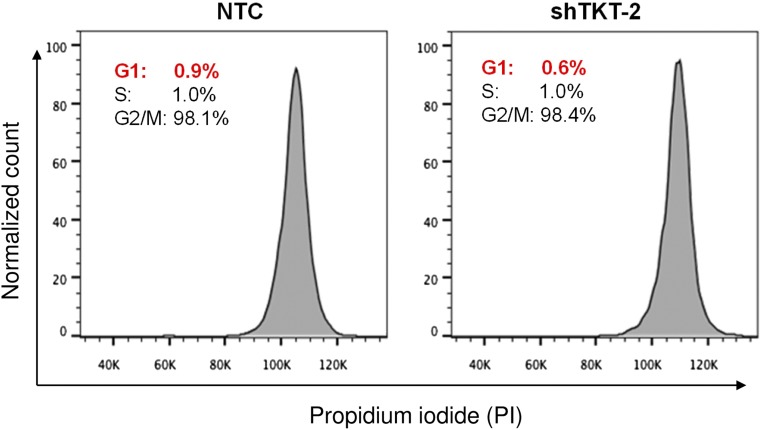
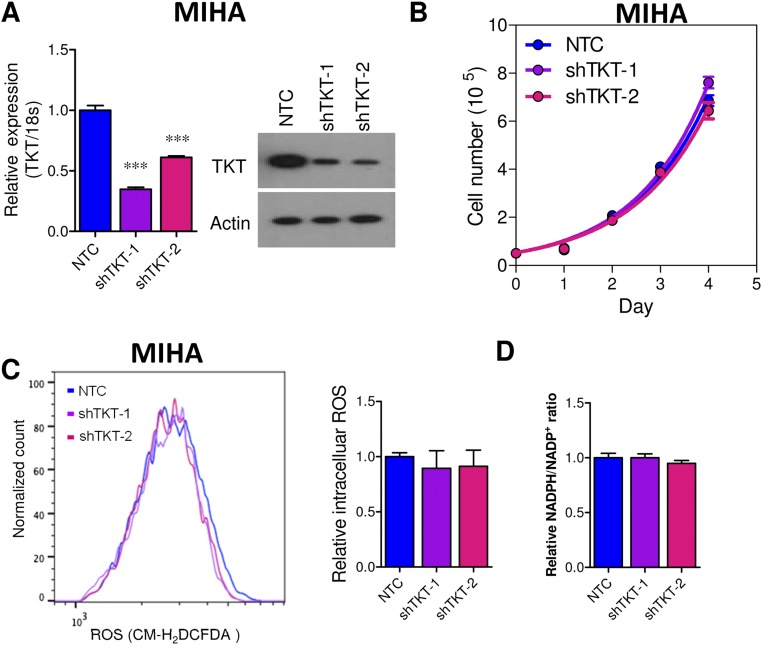
Given that TKT is located in a pivotal position connecting PPP and glycolysis, we postulated that TKT overexpression in cancer would substantially affect the metabolic machinery of cancer cells. To address this, we established TKT stable knockdown cells in two different HCC cell lines, MHCC97L and SMMC cells, by short-hairpin RNA (shRNA) (Fig. 3 A and B). Two independent shRNA sequences (shTKT-1 and shTKT-2) profoundly suppressed HCC cell proliferation in vitro (Fig. 3C). 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose (2-NBDG) staining confirmed that knockdown of TKT reduced glucose uptake in HCC cells (Fig. 3D). We also found that knockdown of TKT suppressed NAPDH production and increased ROS production in HCC cells (Fig. 4 A–C). ROS accumulation caused by TKT knockdown could be rescued by the antioxidant reduced glutathione (GSH) (Fig. 4D). Next, we asked if the ROS accumulation caused by TKT knockdown affects the cell-cycle profile of HCC cells. Nocodazole was used to synchronize all of the HCC subclones in the G2/M phase (Fig. S4). Cells were then released from nocodazole treatment for 9 h. Propidium iodide (PI) staining showed that more cells stayed in the G1 phase in TKT knockdown cells compared with nontarget control (NTC) cells (Fig. 4E). The addition of two mechanistically different antioxidants, GSH and N-acetyl-cysteine (NAC), allowed the TKT knockdown cells to reenter the S phase, suggesting that ROS accumulation caused by TKT knockdown resulted in cell-cycle delay, thereby inhibiting HCC cell proliferation (Fig. 4E). As TKT was more abundantly expressed in HCC compared with liver tissues, we postulated that knockdown of TKT would have a greater impact on HCC cell lines than on normal liver cell lines. We further established TKT knockdown stable cells in a normal liver cell line, MIHA (Fig. S5A). Knockdown of TKT did not affect the proliferative output, ROS, and NADPH levels in MIHA cells (Fig. S5 B–D).
Fig. 3.
Knockdown of TKT reduced HCC cell proliferation and glucose uptake in vitro. (A) mRNA expression of TKT in MHCC97L and SMMC-NTC, –shTKT-1, and –shTKT-2 subclones. HPRT was used as the internal control. (B) Protein expression of TKT in MHCC97L and SMMC-NTC, –shTKT-1, and –shTKT-2 subclones. β-actin was used as the loading control. (C) Cell proliferation assay in MHCC97L and SMMC-NTC, –shTKT-1, and –shTKT-2 subclones. (D) Glucose uptake assay was performed in MHCC97L and SMMC-NTC, –shTKT-1, and –shTKT-2 subclones. (Left) Representative flow cytometry analysis showing intensities of 2-NBDG in the indicated HCC subclones. (Right) Histograms summarize the 2-NBDG intensities (glucose uptake) in different HCC subclones. Relative values were calculated based on the according NTC subclones. *P < 0.05, ***P < 0.001 versus NTC. A, C, and D, Student’s t test. Data are presented as means ± SD.
Fig. 4.
Knockdown of TKT increased the intracellular ROS level and induced oxidative stress-associated G1 phase arrest. (A and B) Flow cytometry was performed to analyze the ROS levels in MHCC97L and SMMC-NTC, –shTKT-1, and –shTKT-2 subclones. (Left) Representative flow cytometry analysis showing intensities of ROS (CM-H2DCFDA) in the indicated subclones. (Right) Histograms summarize the ROS levels in different HCC subclones. Relative values were calculated based on the according NTC subclones. (C) Relative NADPH/NADP+ ratio in MHCC97L and SMMC-NTC and –shTKT-2 cells. The NADPH/NADP+ ratio was normalized to the corresponding NTC subclones. (D, Left) Representative flow cytometry analysis showing intensities of ROS (CM-H2DCFDA) in MHCC97L-NTC and –shTKT-2 cells with vehicle or 10 μM GSH. (Right) Histogram summarizes the ROS levels. Relative values were calculated based on the ROS level in the NTC. (E) Representative flow cytometry analysis showing intensities of PI in MHCC97L-NTC and –shTKT-2 cells treated with vehicle, 10 μM GSH, or 2.5 mM NAC. Cells were synchronized with nocodazole and released from nocodazole for 9 h for cell-cycle analysis. *P < 0.05, **P < 0.01, ***P < 0.001 versus NTC or as indicated by brackets, Student’s t test. Data are presented as means ± SD.
Fig. S4.
Synchronization of HCC cells in the G2/M phase. Representative flow cytometry analysis shows the intensities of PI in MHCC97L-NTC and –shTKT-2 cells right after nocodazole treatment. All cells were synchronized at the G2/M phase.
Fig. S5.
Knockdown of TKT in normal liver cell lines. (A, Left) mRNA expression of TKT in MIHA-NTC, –shTKT-1, and –shTKT-2 subclones. 18S was used as the internal normalizing control, and values were normalized to NTC. (Right) Protein expression of TKT in MIHA-NTC, –shTKT-1, and –shTKT-2 subclones. (B) Cell proliferation assay in MIHA-NTC, –shTKT-1, and –shTKT-2 subclones. (C) Flow cytometry was performed to analyze the ROS levels in MIHA-NTC, –shTKT-1, and –shTKT-2 subclones. (Left) Representative flow cytometry analysis showing intensities of CM-H2DCFDA (ROS) in the indicated subclones. (Right) Histograms summarize the ROS levels in different MIHA subclones. Values were normalized to NTC. (D) Relative NADPH/NADP+ ratio in MIHA-NTC, –shTKT-1, and –shTKT-2 cells. Values were normalized to NTC. ***P < 0.001 versus NTC, Student’s t test. Data are presented as means ± SD.
TKT Decreases Glucose Flux into Glycolysis and Increases Glutathione Synthesis.
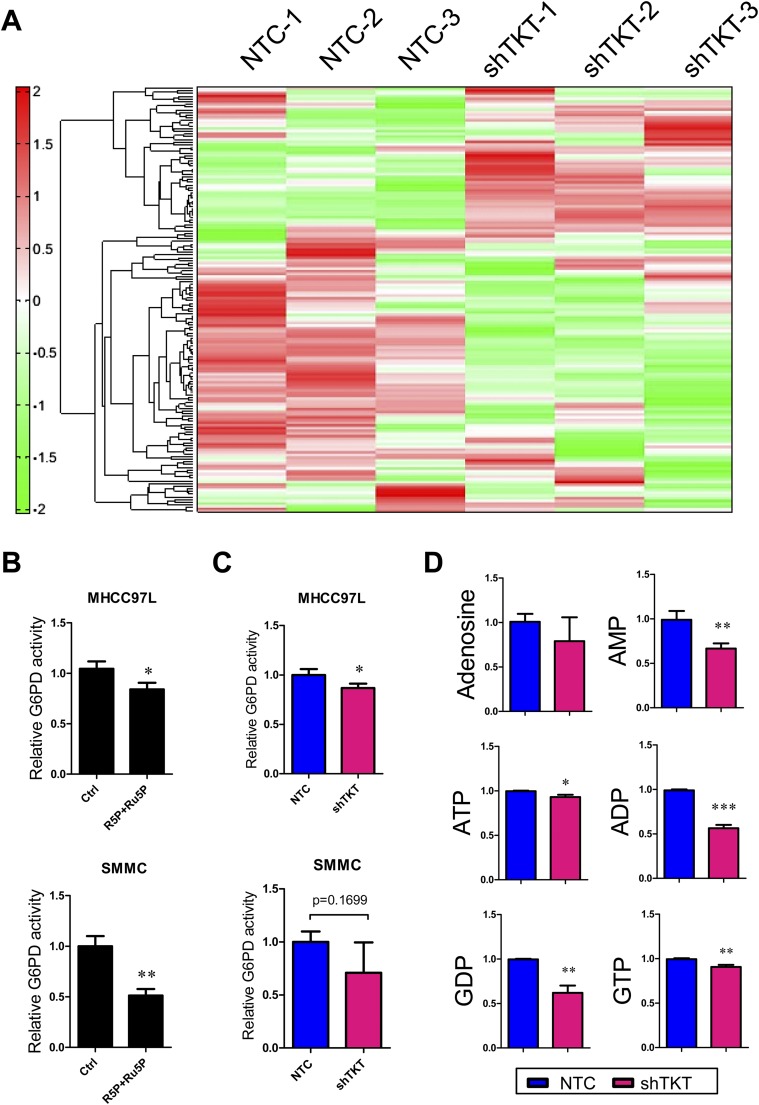
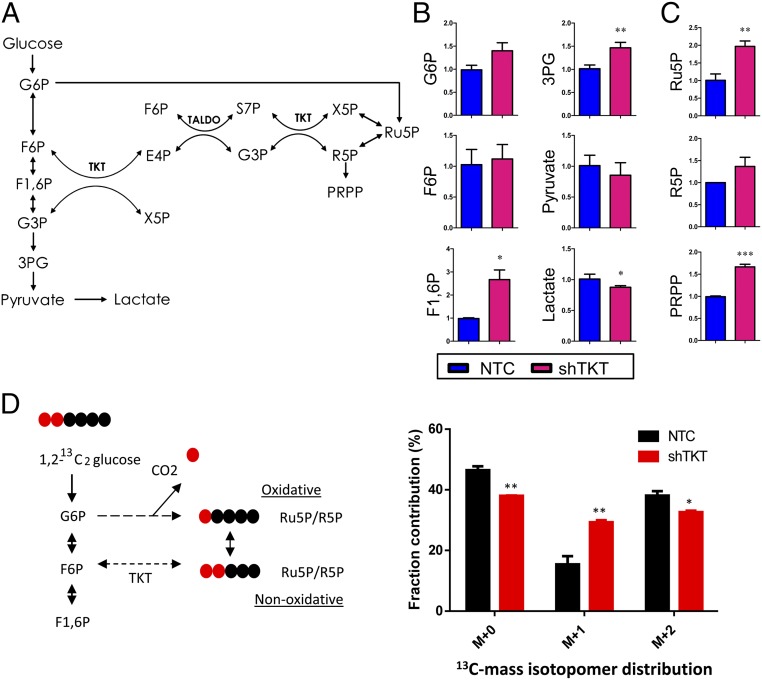
To comprehensively elucidate the impact of TKT on the metabolic pathways in cancer cells, we performed metabolomic profiling using capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS) in MHCC97L-NTC and -shTKT stable cells (Fig. S6A). As TKT recycles intermediates back into the oxidative arm through several steps in glycolysis (Fig. 5A), we found that those glycolytic intermediates including G6P, F6P, fructose-1,6-biphosphate (F1,6P), and 3PG accumulated in the TKT knockdown cells (Fig. 5B). Lactate, the final glycolytic product, was significantly decreased in TKT knockdown cells (Fig. 5B). These data were in line with those of our glucose uptake assay and suggested that glycolytic flux was decelerated in TKT knockdown cells. Meanwhile, the PPP intermediates, Ru5P and R5P, and R5P derivative, phosphoribosyl pyrophosphate (PRPP), were elevated (Fig. 5C), suggesting that TKT knockdown cells may fail to direct the intermediates back to the glycolysis, leading to the accumulation of nonoxidative arm intermediates. To confirm the direction of metabolic flux upon TKT knockdown, we performed a carbon tracing experiment with [1,2-13C2]-glucose and detected the amount of labeled Ru5P/R5P by liquid chromatography–mass tandem spectrometry (LC-MS). [1-13C1]-Ru5P/R5P ([M+1]) and [1,2-13C2]-Ru5P/R5P ([M+2]) indicate the amount of glucose entering the oxidative and nonoxidative arm, respectively (Fig. 5D). As expected, glucose was forced to enter the oxidative arm upon knockdown of TKT, which may explain the accumulation of Ru5P/R5P in the oxidative arm (Fig. 5D). Studies have shown that accumulation of Ru5P/R5P reduced the activity of enzymes in the oxidative arm (14, 15). We therefore examined the effect of Ru5P/R5P on the activity of G6PD, a key enzyme in the oxidative arm. We found that Ru5P and R5P together modestly reduced the activity of G6PD in HCC cells (Fig. S6B). Consistently, we found that the activity of G6PD was also slightly reduced upon TKT knockdown (Fig. S6C). Despite the accumulation of Ru5P/R5P, levels of purine metabolites including adenosine, AMP, ADP, ATP, GDP, and GTP were consistently reduced upon TKT knockdown (Fig. S6D). The pattern of our metabolomic study in TKT knockdown HCC cells is in agreement with the metabolomic study in NRF2 knockdown lung carcinoma cells reported by others (13), further reinforcing the regulatory role of NRF2 on TKT.
Fig. S6.
Profiles of metabolites in TKT knockdown cells. (A) Hierarchical clustering analysis of 108 metabolites in MHCC97L-NTC and -shTKT cells in triplicate is presented as a heat map. Each row represents one intracellular metabolite. Red–green color scale depicts the amount of metabolites relative to internal control in the CE-TOFMS analysis. Increasing levels of metabolites compared with internal control are represented as increasing intensity of red. Decreasing levels of metabolites compared with internal control are represented as increasing intensity of green. (B) G6PD enzymatic activity in MHCC97L and SMMC cells. Cell lysates of MHCC97L cells were incubated with 100 μM R5P and 100 μM Ru5P before measurement. Cell lysates of SMMC cells were incubated with 500 μM R5P and 500 μM Ru5P before measurement. (C) G6PD enzymatic activity in MHCC97L and SMMC-NTC and -shTKT cells. (D) Quantification of metabolic intermediates in the purine metabolism. ADP, adenosine diphosphate; AMP, adenosine monophosphate; ATP, adenosine triphosphate; GDP, guanosine diphosphate; GTP, guanosine triphosphate. *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t test. Data are presented as means ± SD.
Fig. 5.
Knockdown of TKT altered glucose metabolism and glutathione metabolism. (A) Metabolic intermediates and reactions in the glycolysis and PPP. (B and C) Quantification of metabolic intermediates in glycolysis (B) and the PPP (C). (D, Left) 1,2-13C2-glucose is converted to M+1 Ru5P/R5P through the oxidative arm and M+2 Ru5P/R5p through the nonoxidative arm. (Right) Quantification of mass isotopomer distribution of Ru5P/R5P in MHCC97L-NTC and -shTKT cells cultured in 1,2-13C2-glucose for 12 h. M+0 indicates unlabeled Ru5P/R5P. M+1 indicates one carbon-labeled Ru5P/R5P. M+2 indicates two carbon-labeled Ru5P/R5P. 3PG, 3-phosphoglyceric acid; E4P, erythrose 4-phosphate; F1,6P, fructose 1,6-bisphosphate; F6P, frutose-6-phosphate; G3P, glyceraldehyde 3-phosphate; G6P, glucose-6-phosphate; PRPP, phosphoribosyl pyrophosphate; R5P, ribose-5-phosphate; Ru5P, ribulose-5-phosphate; S7P, sedoheptulose-7-phosphate; X5P, xylulose-5-phosphate. *P < 0.05, **P < 0.01, ***P < 0.001 versus NTC, Student’s t test. Data are presented as means ± SD.
Knockdown of TKT Suppresses HCC Growth in Vivo.
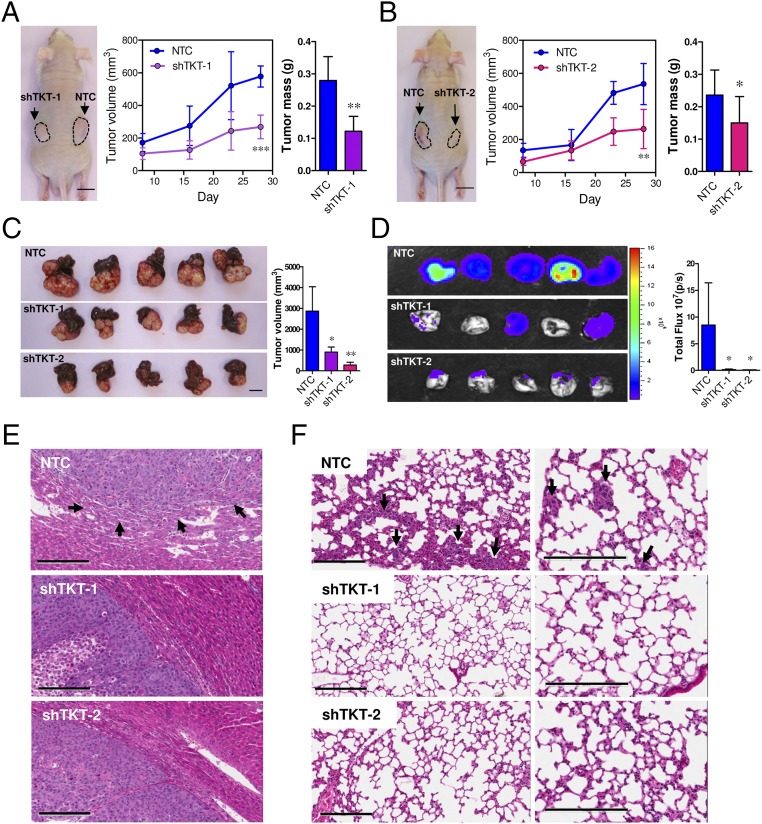
To confirm that the metabolic changes caused by knockdown of TKT would impact the cancer growth in vivo, we used two animal models: an s.c. tumor model and an orthotopic tumor model. In the s.c. tumor model, which allows easy monitoring of tumor growth, we injected SMMC-NTC, –shTKT-1, and –shTKT-2 cells into the flanks of nude mice and allowed the tumors to grow for 28 d. Knockdown of TKT significantly suppressed s.c. tumor growth (Fig. 6 A and B). In the orthotopic tumor model, which better mimics the tumor microenvironment of cancer, we injected luciferase-labeled MHCC97L-NTC, –shTKT-1, and –shTKT-2 cells into the left lobes of nude mice. Mice were killed 42 d after implantation. Knockdown of TKT profoundly suppressed the growth of the primary tumor in the livers of mice (Fig. 6C). Furthermore, knockdown of TKT drastically reduced the growth of metastatic lesions in the lungs of mice as reflected by Xenogen imaging (Fig. 6D). Histological analysis with hematoxylin and eosin (H&E)-stained HCC sections suggested that the HCC tumors derived from MHCC97L-NTC cells had more irregular tumor growth fronts and were more aggressive than those derived from MHCC97L-shTKT cells (Fig. 6E and Table 3). Metastatic foci in the mice implanted with MHCC97L-NTC cells were significantly larger (Fig. 6F).
Fig. 6.
Knockdown of TKT suppressed tumor growth and lung metastasis in vivo. (A and B) SMMC-NTC and –shTKT-1 cells (A) or SMMC-NTC and –shTKT-2 cells (B) were s.c. injected into flanks of nude mice. Tumor volumes were monitored for 28 d. (Left) Representative pictures of tumors formed in nude mice. (Scale bar, 1 cm.) (Middle) Growth curves of s.c. xenografts. (Right) Quantification of tumor mass on day 28. (C) Luciferase-labeled MHCC97L-NTC, –shTKT-1, or –shTKT-2 cells were orthotopically injected into left hepatic lobes of nude mice and allowed to grow for 42 d. (Left) Representative pictures of orthotopic xenografts. (Scale bar, 1 cm.) Right: quantification of tumor volume. (D, Left) Bioluminescent images of lung tissues. (Right) Quantification of bioluminescent intensities of lung tissues. (E) Representative pictures of H&E staining of tumor xenografts. Arrows indicate irregular growth front in the NTC group. (Scale bars, 200 μm.) (F) Representative pictures of H&E staining of lung tissues. Arrows indicate tumor cells found in the lung tissue. (Scale bars, 200 μm.) *P < 0.05, **P < 0.01 versus NTC, Student’s t test (N ≥ 5). Data are presented as means ± SD.
Table 3.
Results of orthotopic liver implantation from MHCC97L-Luc-NTC and -shTKT clones
| Experimental groups | Number of tumors with invasive growth front | Number of tumors with venous invasion | Number of tumors with microsatellite formation |
| NTC | 5/5 | 2/5 | 4/5 |
| shTKT-1 | 2/5 | 0/5 | 1/5 |
| shTKT-2 | 2/5 | 0/5 | 4/5 |
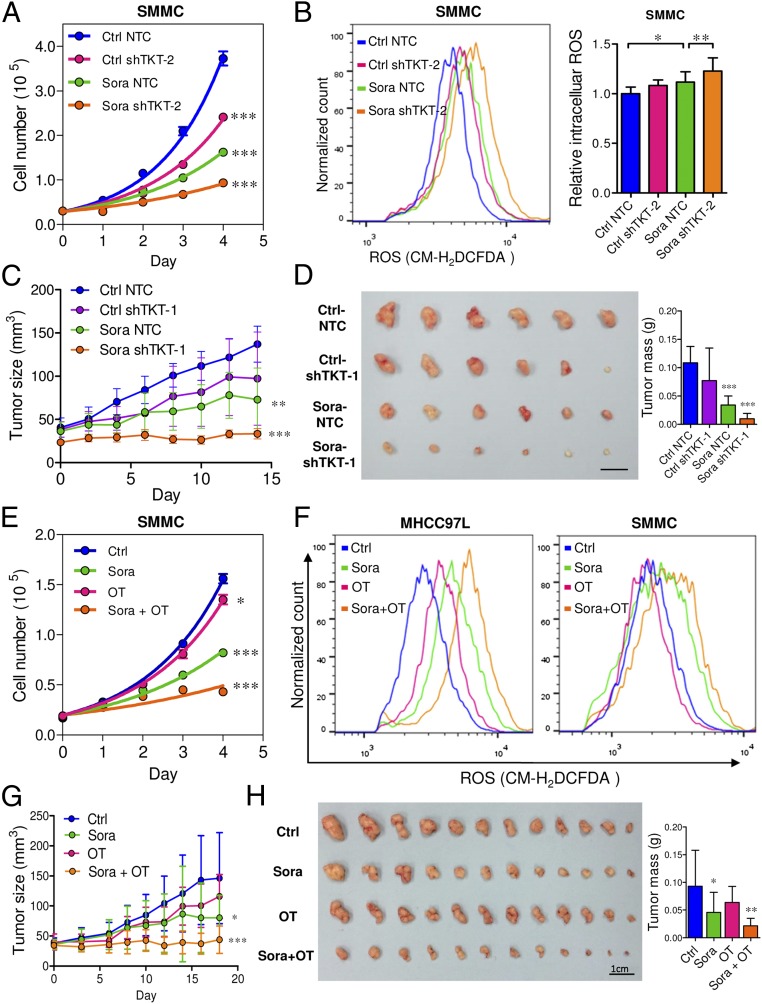
Inhibition of TKT Sensitizes Cancer Cells to Sorafenib Treatment.
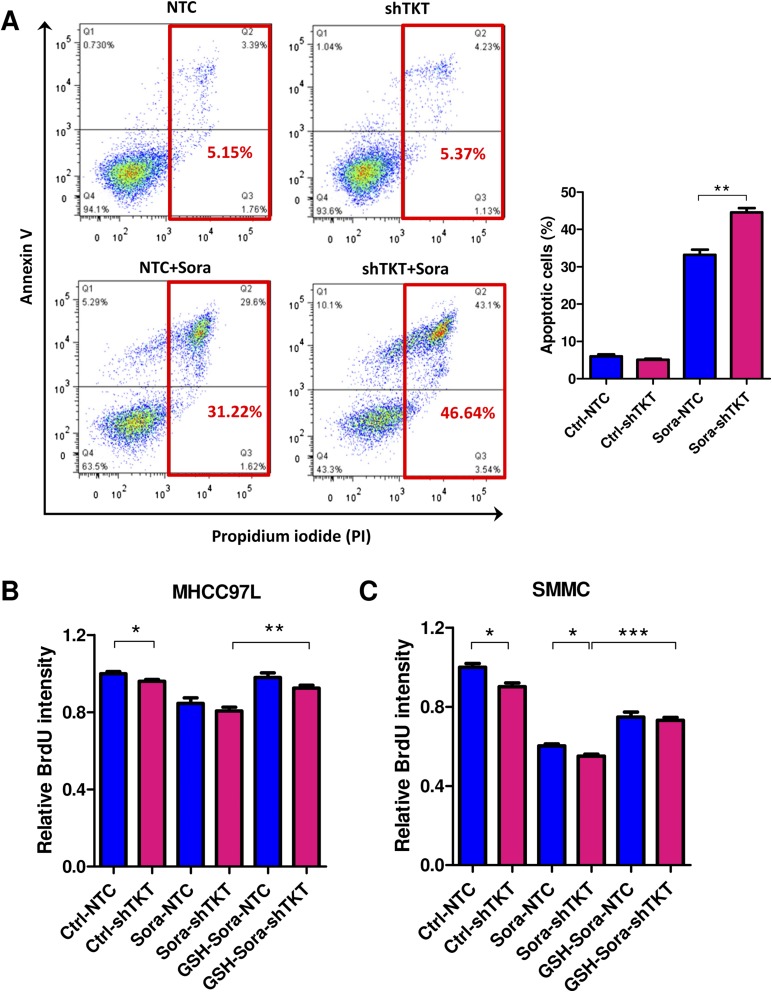
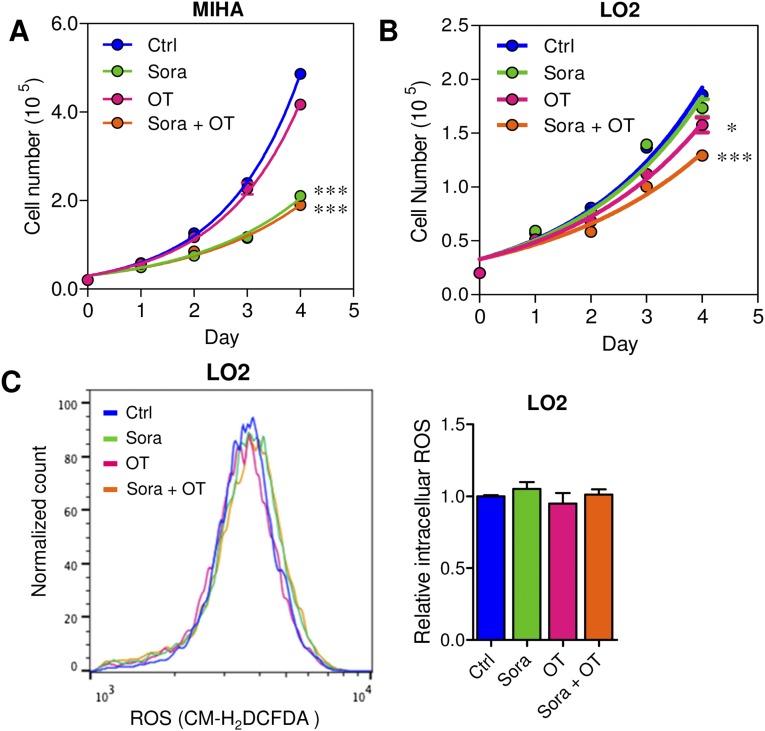
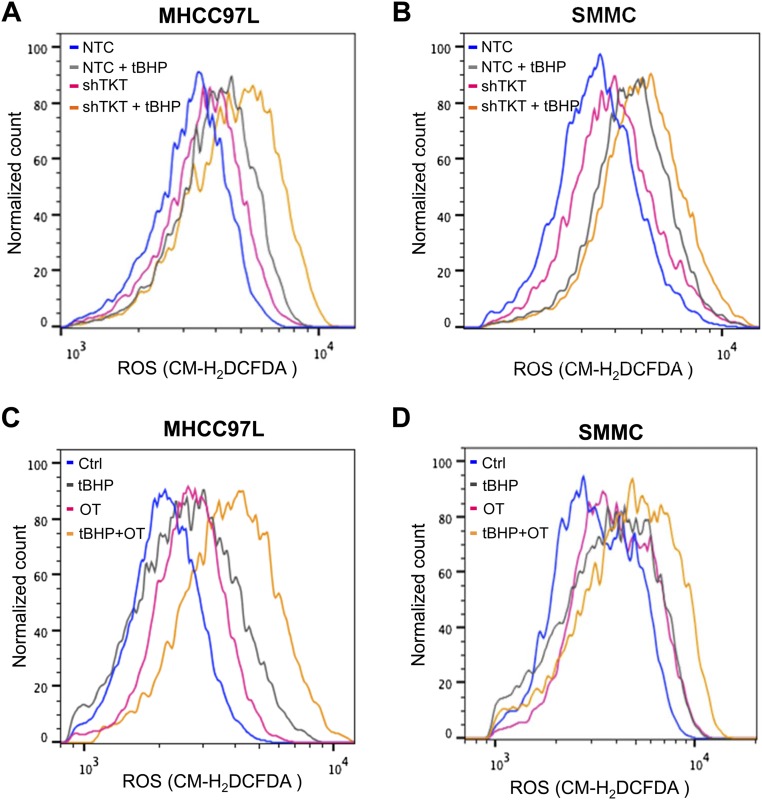
We demonstrated that TKT promoted cancer growth by attenuating oxidative stress. Increasing evidence has suggested that reduction of oxidative stress protects cancer cells from chemotherapy and radiation therapy. In light of this, we asked if inhibition of TKT would sensitize cancer cells to Sorafenib treatment, the only FDA-approved drug in HCC. Sorafenib partially reduced the cell proliferation in SMMC-NTC cells but completely blocked that in SMMC-shTKT cells (Fig. 7A). The levels of ROS in the SMMC-NTC and -shTKT cells treated with vehicle control and Sorafenib inversely correlated with their proliferative output (Fig. 7B). We implanted SMMC-NTC and –shTKT-1 subclones s.c. into nude mice for easy monitoring of the tumor growth. Consistent with our in vitro observation, Sorafenib partially repressed the growth of tumors derived from SMMC-NTC cells but completely retarded the growth of tumors derived from –shTKT-1 cells in vivo (Fig. 7 C and D). Similar results were observed in s.c. tumors derived from MHCC97L subclones (Fig. S7 A–D). We next examined the sensitization effect of the TKT inhibitor oxythiamine (OT), a thiamine antagonist, for Sorafenib. Cell proliferation assay revealed that OT drastically sensitized SMMC and MHCC97L cells to Sorafenib treatment in vitro (Fig. 7E and Fig. S7E) and increased ROS accumulation in the Sorafenib-treated HCC cells (Fig. 7F). Of note, we found that Sorafenib alone could induce ROS in both HCC cell lines. This observation has also been reported by Coriat et al. in some other HCC cell lines, such as Hepa1,6 and HepG2 (16), suggesting that the effect of Sorafenib in ROS induction is common and partially contributed to its suppressive effect in the growth of HCC. To confirm the mechanisms by which Sorafenib induces cell death, we performed Annexin V and PI staining to evaluate the percentage of apoptotic HCC cells. We found that Sorafenib induced apoptosis especially in the TKT knockdown HCC cells (Fig. S8A). A BrdU proliferation assay further confirmed that Sorafenib profoundly reduced cell growth, especially in the TKT knockdown HCC cells, which could be significantly rescued by GSH (Fig. S8 B and C). These data suggested that the sensitization effect upon TKT knockdown or inhibition is caused by ROS. Interestingly, the sensitization effect only holds true in HCC cells but not in normal liver cell lines, MIHA and LO2, suggesting a therapeutic window for combined treatment of OT and Sorafenib in HCC patients (Fig. S9 A–C). Concentrations of OT used in in vitro and in vivo experiments were based on the IC50 values and results of toxicity tests in other studies (17, 18). The s.c. tumor model further revealed that a combination treatment of OT and Sorafenib was most efficacious in suppressing tumor growth compared with the single treatment of either drug (Fig. 7 G and H). Apart from Sorafenib, we used another more well-characterized ROS inducer, tert-Butyl hydroperoxide (tBHP). Consistently, we found that knockdown of both TKT and OT could increase the ROS level of HCC cells together with tBHP (Fig. S10). This finding suggested that OT may also be used in combination with other existing drugs to raise the ROS level in cancer cells in a similar approach as Sorafenib.
Fig. 7.
Genetic knockdown and pharmacological inhibition of TKT sensitized HCC cells to Sorafenib treatment via enhancing oxidative stress. (A) Proliferation curves of SMMC-NTC and –shTKT-2 cells in the presence of vehicle DMSO (Ctrl) or 6 μM Sorafenib (Sora). (B) Representative flow cytometry histogram (Left) and quantification (Right) of ROS staining in SMMC-NTC and –shTKT-2 cells treated with vehicle (Ctrl) or 6 μM Sora for 24 h. (C and D) Growth curves of s.c. xenografts derived from SMMC-NTC or –shTKT-1 cells in nude mice that were administered the control vehicle (Ctrl) or 30 mg/kg/d Sora for 14 d. (D, Left) Representative picture of tumor xenografts. (Right) Quantification of tumor mass (n = 6). (Scale bar, 1 cm.) (E) Proliferation curves of SMMC cells treated with vehicles, 6 μM Sora, 5 mM OT, or 6 μM Sora and 5 mM OT (Sora + OT). (F) Representative flow cytometry histograms of ROS staining in MHCC97L (Left) and SMMC (Right) cells treated with vehicles, 6 μM Sora, 5 mM OT, or 6 μM Sora and 5 mM OT (Sora + OT) for 24 h. (G) Growth curves of s.c. xenografts derived from parental SMMC cells in mice that were administered the vehicles (Ctrl), 30 mg/kg/d Sora, 80 mg/kg/d OT, or 30 mg/kg/d Sora and 80 mg/kg/d OT (Sora + OT) for 18 d. (H, Left) Representative pictures of tumor xenografts. (Right) Quantification of tumor mass (n = 12). (Scale bar, 1 cm.) *P < 0.05, **P < 0.01, ***P < 0.001 versus Ctrl. Student’s t test. Data are presented as means ± SD.
Fig. S7.
Genetic knockdown and pharmacological inhibition of TKT sensitized MHCC97L cells to Sorafenib treatment via enhancing oxidative stress. (A) Proliferation curves of MHCC97L-NTC, –shTKT-1, or –shTKT-2 cells in the presence of control vehicle (Ctrl) or 2.5 μM Sorafenib (Sora). (B) Representative flow cytometry histogram (Left) and quantification (Right) of ROS staining in MHCC97L-NTC and –shTKT-2 cells in the presence of Ctrl or 10 μM Sora. (C) Growth curves of s.c. tumors derived from SMMC-NTC and –shTKT-2 cells in nude mice that were administered the vehicle control (Ctrl) or 30 mg/kg/d Sora for 14 d. (D, Left) Representative pictures of tumor xenografts from C. (Scale bar, 1 cm.) (Right) Quantification of tumor mass. (E) Proliferation curves of parental MHCC97L cells treated with DMSO (Ctrl), 2.5 μM Sora, 5 mM OT, or 2.5 μM Sora with 5 mM OT (Sora + OT). *P < 0.05, **P < 0.01, ***P < 0.001 versus Ctrl NTC or Ctrl, Student’s t test. Data are presented as means ± SD.
Fig. S8.
Effect of Sorafenib on cell death and cell proliferation in TKT knockdown cells. (A) Annexin V assay was performed to analyze the percentage of cell death in SMMC-NTC and –shTKT-2 cells after Sorafenib (Sora) treatment. Cells were incubated in serum-free DMEM-HG medium containing 10 μM Sorafenib for 24 h. (B) BrdU assay was performed to examine the cell proliferation of MHCC97L cells treated with vehicle (Ctrl) or 4 μM Sora with or without 10 μM GSH treatment. (C) BrdU assay was performed to examine cell proliferation of SMMC cells treated with vehicles (Ctrl) or 6 μM Sora with or without 10 μM GSH treatment. *P < 0.05, **P < 0.01, ***P < 0.001. Student’s t test.
Fig. S9.
Inhibition of TKT by OT in normal liver cell lines did not sensitize cells to Sorafenib treatment. (A) Proliferation curves of MIHA cells treated with vehicles (Ctrl), 2.5 μM Sorafenib (Sora), 5 mM OT, or 2.5 μM Sora and 5 mM OT (Sora + OT). (B) Proliferation curves of LO2 cells treated with vehicles (Ctrl), 2.5 μM Sorafenib (Sora), 5 mM OT, or 2.5 μM Sora and 5 mM OT (Sora + OT). (C) Flow cytometry was performed to analyze the ROS levels in LO2 cells treated with vehicles (Ctrl), 2.5 μM Sora, 5 mM OT, or 2.5 μM Sora and 5 mM OT (Sora + OT). *P < 0.05, **P < 0.01, ***P < 0.001. Student’s t test.
Fig. S10.
Effect of knockdown or inhibition of TKT on HCC cells upon tBHP treatment. (A) Representative flow cytometry histograms of ROS staining in MHCC97L-NTC or -shTKT cells treated with 1000 μM tert-Butyl hydroperoxide (tBHP). (B) Representative flow cytometry histograms of ROS staining in SMMC-NTC or -shTKT cells treated with 500 μM tBHP. (C) Representative flow cytometry histograms of ROS staining in MHCC97L cells treated with vehicle (Ctrl), 1,000 μM tBHP, 5 mM OT, and 1,000 μM tBHP with 5 mM OT (tBHP + OT). (D) Representative flow cytometry histograms of ROS staining in SMMC cells treated with vehicle (Ctrl), 500 μM tBHP, 5 mM OT, and 500 μM tBHP with 5 mM OT (tBHP + OT).
Discussion
There has been a revival of interest in metabolic reprogramming in cancers. In the past decade, tremendous efforts have been made to provide molecular explanations for the Warburg Effect, which was reported 60 y ago (19). Compelling evidence has suggested that aerobic glycolysis is achieved to maximize the production of macromolecules and antioxidants. In fact, aerobic glycolysis is accomplished together with changes in other metabolic pathways. Our study has provided evidence that a pivotal enzyme in the PPP, TKT, is indispensable to cancer development. The PPP produces two major products, ribose and NADPH, through the nonoxidative arm and oxidative arm, respectively. Surprisingly, we showed that knockdown of TKT drastically reduced tumor growth regardless of the accumulation of ribose intermediates, suggesting that ribose was not a limiting factor in cancer growth. Although our current study has not provided data to show whether ribose accumulation in the TKT knockdown cells is a result of decreased consumption or increased production, this remains an interesting question to be addressed in the future. On the other hand, TKT knockdown resulted in a decrease of NADPH and an increase of ROS, further suggesting that oxidative stress homeostasis is an important determining factor in cancer growth. Of note, one intriguing question that remains unanswered is why there was an increase of oxidative flux in the TKT knockdown cells but the level of NADPH, a metabolite from the oxidative arm, decreased. One speculation is that knockdown of TKT may transiently increase oxidative flux, but gradual accumulation of R5P and Ru5P would eventually block the oxidative arm of PPP. Our current study only showed that G6PD enzymatic activity was slightly reduced upon treatment of R5P and Ru5P. A more direct experimental approach to confirm our hypothesis in the future is to use [1-2H]-glucose to trace NADPH production in the TKT knockdown cells (20).
The PPP connects with glycolysis through G6PD and TKT, which directly metabolize several glycolytic metabolites. Our metabolomic profiling provided further evidence to support the intimate link between the PPP and glycolysis. We noticed that the end product of aerobic glycolysis, lactate, was reduced upon knockdown of TKT despite the levels of glycolytic intermediates including F1,6P were significantly elevated. This observation can be explained by the change of pyruvate kinase M2 (PKM2) activity, which is tightly controlled by F1,6P. PKM2 is one of the four pyruvate kinase isoforms that convert phosphoenolpyruvate (PEP) to pyruvate. PKM2, the cancer-specific isoform, has lower enzymatic activity compared with other pyruvate kinase isoforms (21). Low activity of pyruvate kinase reduces the entrance of pyruvate into the TCA cycle, thereby decelerating the metabolic flux of the TCA cycle and allowing TCA cycle intermediates to be synthesized into biomass, such as lipid (22). For its low enzymatic activity, PKM2 favors cancer growth. F1,6P allosterically activates PKM2 through inducing the tetramer formation of PKM2 (21). Therefore, F1,6P accumulation upon TKT knockdown may result in the activation of PKM2, leading to the decrease of aerobic glycolysis and a reduction of lactate accumulation. We have recently reported that PKM2 is the major isoform in HCC (23) and TKT might indirectly regulate PKM2 activity through F1,6P. Further knowledge on the interplay of TKT and PKM2 will help to devise combined therapies against the PPP and glycolysis for cancer patients.
The role of ROS in cancer development has been the subject of contentious debate. A wealth of studies have suggested that ROS drives mutations and activates signaling pathways that promote cell proliferation (24). Recently, more studies have provided evidence showing that excessive production of ROS is detrimental to cancer cells by triggering apoptosis and senescence (25–27). NRF2 is characterized as an oncogene for its ability to buffer ROS (28). Recently, NRF2 has been reported to transcriptionally activate some PPP genes, such as TKT, G6PD, and TALDO (13). Interestingly, p73 could also increase the metabolic flux in the PPP through initiating the transcription of G6PD (29). Although our study has showed the roles of TKT in redox homeostasis, further studies on other possible transcriptional and posttranscriptional regulation of TKT will yield important knowledge on how cancer cells overcome ROS.
Recent studies have highlighted the disadvantages of antioxidant intake in cancer patients. Antioxidants have been shown to confer cancer stem cells’ radio resistance (30) and promote anchorage-independent growth of cancer cells (31). Our study has illustrated an example that a pathway that synthesizes antioxidants, the PPP, is critical to tumor development. Our finding echoes a recent study that elegantly demonstrated the important roles of glutamate cysteine ligase, an enzyme that synthesizes glutathione, in cancer initiation (32). Intriguingly, Harris et al. demonstrated that cotreatment of inhibitors against the glutathione and thioredoxin antioxidant pathways could efficiently block tumor growth even when drugs were administered after tumor onset (32). Similarly, our study, using HCC as a cancer model, showed that blocking the PPP by the TKT inhibitor OT could synergize Sorafenib to halt cancer growth. The combined effect of OT with inhibitors against the glutathione and/or thioredoxin antioxidant pathways represents an exciting translational research direction. Furthermore, knowledge on the combined effects of these inhibitors with existing chemotherapy and targeted therapies will be important for the development of new therapeutic strategies for cancers, especially in HCC patients with very limited treatment options.
It is worth mentioning that patients with PPP deficiency, the G6PD deficiency that is an X chromosome-linked genetic disease in G6PD, have not been found to be protected against cancers (33). G6PD directly produces NADPH, which is the only source of GSH in red blood cells. G6PD deficiency increases oxidative stress in red blood cells, causing hemolysis, which destructs red blood cells and releases hemoglobin to plasma. G6PD deficiency has not been found to be protective against cancers, possibly because of the compensatory effects from other NADPH-producing enzymes (20, 34, 35). Although large-scale epidemiological studies on the protective roles of G6PD deficiency against cancers are awaited, the pathophysiology of G6PD deficiency suggested that systemic suppression of NADPH production may adversely cause side effects similar to the symptoms of G6PD deficiency. Careful examination of the therapeutic window of drugs that target antioxidant production in cancer treatment should be performed before clinical trials. In conclusion, results from this study underscore the protumorigenic roles of antioxidants in cancer development and support the notion that antioxidant intake may not be definitely beneficial to cancer patients.
Materials and Methods
Patients.
Use of human clinical specimens in this study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority of Hong Kong (HKU/HA HKW IRB; ref. no. UW 09–185). The patients were explained, and signed consent forms to acknowledge, the use of their resected tissues for research purposes.
Ethical Statement.
Animal experiments were performed following UK Coordinating Committee on Cancer Research (CCCR) PMID: 9459138 Guidelines for the Welfare of Animals in Experimental Neoplasia (36) to ensure minimal suffering of animals. The experimental procedures of the animal studies were approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong.
HCC Patient Samples and HCC Cell Lines.
Clinical HCC and corresponding adjacent NT liver tissue were obtained from patients at Queen Mary Hospital, Pamela Youde Nethersole Eastern Hospital, and Queen Elizabeth Hospital in Hong Kong. Tissue specimens were snap-frozen in liquid nitrogen and stored at –80 °C for RNA or protein extraction. Human HCC cell lines SMMC and MHCC97L were gifts from the Shanghai Institute of Cell Biology (Chinese Academy of Sciences) and Fudan University of Shanghai, respectively.
RNA Extraction, Reverse Transcription PCR, and Quantitative Real-Time PCR.
RNA was extracted from clinical specimens or HCC cell lines by TRIzol reagent (Life Technologies) and reverse-transcribed by the GeneAmp PCR Reagent Kit (Applied Biosystems). SYBR Green qPCR Master Mix (Applied Biosystems) and corresponding primers (Table S1) were used for quantitative real-time PCR (qRT-PCR).
Table S1.
Primer sequences used in qRT-PCR analysis
| Target gene | Sequence |
| Human TKT | Forward, GAAGATCAGCTCCGACTTGG |
| Reverse, GTCGAAGTATTTGCCGGTGT | |
| Human HPRT | Forward, CTTTGCTGACCTGCTGGATT |
| Reverse, CTGCATTGTTTTCGGAGTGT | |
| Human NRF2 | Forward, GGTTGCCCACATTCCCAAAT |
| Reverse, AGCAATGAAGACTGGGCTCT | |
| Human BACH1 | Forward, TAGTGTGGAGCGAGAAGTGG |
| Reverse, ACCTAACCACGGACACTCAG | |
| Human KEAP1 | Forward, CCTTCAGCTACACCCTGGAG |
| Reverse, AACATGGCCTTCAAAACAGG | |
| Human TKT site 1, ChIP | Forward, CTTCACAAGTGGGCAGTGTC |
| Reverse, AACCCAGTTCCCACCTAGTG | |
| Human TKT site 2, ChIP | Forward, GAGGCCACAGAAGAGCAGTA |
| Reverse, GCAGGAAAAGGCCCTCAAC | |
| Human TKT site 3, ChIP | Forward, TGAGGCCAGGAGTTCAAGAC |
| Reverse, CAAAGTGCTGGGATTAAAGGG | |
| Human TKT site 4, ChIP | Forward, GTGTGCGAGAATGTTGGTACT |
| Reverse, CTCCAATCAGCGCTCCCA | |
| Human TKT site 5, ChIP | Forward, AGCACCATCTAAAGGTCCCT |
| Reverse, CCTGGCCCACATATCCTGAG | |
| Human TKT site 6, ChIP | Forward, AGAGTACAACCCCAGCAGC |
| Reverse, CCACGCCACAAAGCAAGTTA | |
| Human TKT site 7, ChIP | Forward, GAAGGGGAGCGGGAGATG |
| Reverse, TCTCCTCCTGCCTGTACATG |
Transcriptome Sequencing.
Transcriptome sequencing was performed in 16 cases of HCC tissues and paired NT liver tissues. TruSeq Standard mRNA Sample Prep Kit (Illumina) was used to prepare the polyA + mRNA library. We performed 100-bp paired-end sequencing in Illumina HiSeq2000 by Axeq Technologies. Data were analyzed by the TopHat-Cufflinks pipeline (37), and values were indicated by FPKM.
TCGA and Oncomine Data.
Transcriptome sequencing data of 50 cases of paired HCC and NT tissues were retrieved from TCGA via the Broad Institute (gdac.broadinstitute.org/). mRNA expression levels of the PPP genes in different cancer types were retrieved from the Oncomine database (https://www.oncomine.org//resource/login.html).
Clinicopathological Correlation Analysis.
Clinicopathological features of HCC patients including venous invasion, tumor encapsulation, tumor microsatellite formation, hepatitis B surface antigen, direct liver invasion, cellular differentiation, tumor size, and pathological tumor–node–metastasis (pTNM) tumor stage were graded and analyzed by a pathologist as previously described (38). Clinicopathological correlation was performed with SPSS20.0 software.
ChIP Assay.
Cells were cross-linked with formaldehyde and sonicated. Sheared DNA was incubated with antibodies against NRF2 (Abcam), BACH1 (Santa Cruz), or IgG (Santa Cruz). DNA–protein–antibody complexes were incubated with ChIP beads of protein A/G (Merck Millipore). Beads were washed with gradients of salt buffer and eluted in 1% SDS/NaHCO3. ChIP DNA was analyzed by qRT-PCR with primers amplifying the putative regions (Table S1).
Establishment of Stable Knockdown Clones.
pLKO.1-puro vectors encompassing shRNA targeting TKT, NRF2, KEAP1, BACH1 (Table S2), or a nontarget control (Sigma Aldrich) were stably transfected into HCC cell lines by a lentiviral-mediated approach, as we previously described (39). HCC cells that stably expressed the vectors were selected by puromycin.
Table S2.
Target regions of shRNA constructs
| shRNA clones | GenBank | Target nucleotides |
| shTKT-1 | NM_001064.1 | 1795–1815 |
| shTKT-2 | NM_001064.1 | 1432–1452 |
| shNRF2-55 | NM_006164.2 | 1987–2007 |
| shNRF2-58 | NM_006164.2 | 1144–1164 |
| shNRF2-59 | NM_006164.2 | 1862–1882 |
| shNRF2-94 | NM_006164.3 | 1113–1133 |
| shBACH1-95 | NM_001186.2 | 1777–1797 |
| shBACH1-97 | NM_001186.2 | 1590–1610 |
| shKEAP1-40 | NM_012289.3 | 2117–2137 |
| shKEAP1-76 | NM_012289.3 | 1644–1664 |
Cell Proliferation Assays.
For cell counting assays, an equal number of cells was seeded in a 12-well plate 24 h before the proliferation assay. The cell number in each well was counted daily by using the Z1 Particle Counter (Beckman Coulter) for 4–5 d. The BrdU assay (Roche) was performed based on the manufacturer’s instructions. Data were from three independent experiments and are presented as means ± SD.
Protein Extraction and Immunoblotting.
Protein was extracted from human HCC samples or HCC cells using radio-immunoprecipiation assay (RIPA) lysis buffer in the presence of PhosSTOP phosphatase inhibitor mixture (Roche) and protease inhibitor mixture (Roche). Equal amounts of protein were subjected to electrophoresis using 10% (vol/vol) SDS/PAGE. Antibodies against TKT (Abcam) and β-actin (Sigma) were used for Western blotting.
ROS, NADPH, Glucose Uptake, and G6PD Enzymatic Assays.
For ROS assays, an equal number of cells were stained with 2 μM chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) (Life Technologies) and analyzed by BD FACSCanto II Analyzer. NADPH/NADP+ ratios were measured by the NADPH Colorimetric Assay Kit (Biovision) according to the manufacturer’s instructions. In brief, HCC cells were lysed by five repeated freeze/thaw cycles in extraction buffer. A portion of the samples were heated at 60 °C for 30 min for NADP decomposition. A portion of the samples remained unheated. Heated and unheated samples were incubated with NADP cycling buffer for 5 min and mixed with NADPH developer. NADPH and NADP intensities were measured using a plate reader at OD450. NADPH/NADP+ was calculated by the following equation: (intensity of heated)/(intensity of unheated – heated samples). For glucose uptake assays, cells were stained with 100 μM 2-NBDG (Life Technologies) and analyzed by BD FACSCanto II Analyzer. Enzymatic activity of G6PD was measured by the G6PD activity colorimetric assay kit (Biovision) according to the manufacturer’s instructions. For R5P/Ru5P treatment, cell lysates were incubated with R5P (Sigma) and Ru5P (Sigma) for 30 min at room temperature before addition of the substrate mix.
Cell-Cycle Analysis.
Cell-cycle distribution was analyzed by PI (Calbiochem) staining. Cells were synchronized in the G2/M phase by 18 h of Nocodazole (200 ng/mL) treatment. Synchronized cells were released from Nocodazole for 9 h and harvested. Cells were fixed with 75% (vol/vol) ethanol for at least 1 h and incubated with 50 μg/mL PI for 30 min. The PI signal was measured by BD FACSCanto II Analyzer.
Apoptosis Analysis.
Cells were seeded for 24 h and incubated with serum-free culture medium containing Sorafenib or an equal amount of DMSO. After 24 h, cells were trypsinized and suspended in Annexin V binding buffer (BD Science) containing Annexin V-FITC (MBL International) and PI (BD Science) for 15 min at room temperature based on the manufacturers’ instructions. Samples were immediately analyzed by the BD FACSCanto II Analyzer.
Metabolomics.
To extract intracellular metabolites, MHCC97L-NTC or MHCC97L-shTKT cells were washed with 5% (wt/wt) mannitol and extracted with methanol in the presence of 10 μM of internal control solution (compound C1 with the m/z at 182.048 and compound A1 with the m/z at 231.070), which was provided by Human Metabolome Technologies. Extracted solution was collected and centrifuged. Supernatants were filtered by a centrifuge filter unit (Human Metabolome Technologies). Filtered extracts were dried up by a centrifugal evaporator. Extracted metabolites were analyzed by CE-TOFMS by Human Metabolome Technologies.
Metabolic Flux Analysis.
The 1.5 × 106 MHCC97L-NTC and -shTKT cells were cultured in DMEM (Sigma, D5030) with 25 mM [1,2-13C2]-glucose (Cambridge Isotope Laboratories) and 4 mM l-glutamine in 10% dialyzed FBS (HyClone) in 10-cm plates for 12 h. After cells were quenched with HPLC-grade methanol and dehydrated completely, samples were sparked with 10 µL of compound A1 (2 ppm) as the internal standard. The samples were then briefly sonicated, vortexed, and centrifuged at 15,133 × g for 20 min. The supernatants were collected and dehydrated and dissolved in 200 µL of 80% methanol. The samples were vortexed and centrifuged at 15,133 × g for 20 min, and the supernatants were collected for ultra performance liquid chromatography (UPLC)-MS/MS analysis. Analysis was performed on a TSQ Quantiva triple quadrupole mass spectrometer (Thermo Fisher Scientific) via an electrospray interface, operating in negative ionization mode and configuring in selective reaction monitoring mode. The metabolite separation was performed using a SeQuant ZIC-HILIC column (3.5 μm, 100 × 2.1 mm) (Merck). The mobile phases consisted of acetonitrile (A) and 20 mM ammonium acetate in water (B). The gradient elution program initiated from 90% (vol/vol) A and held for 1 min, decreased to 60% (vol/vol) A in 7 min, and held for 5 min, then increased to 90% (vol/vol) for re-equilibrium for 5 min with a flow rate of 0.2 mL/min. Mass spectrometric conditions were optimized for each metabolite by using the reference standard. Spray voltage, vaporizer temperature, sheath gas, auxiliary gas, and capillary temperature were set at –2,300 V, 350 °C, 35 arb, 10 arb, and 320 °C, respectively. The LC-MS/MS data were acquired and processed with LCquanTM software version 2.5.6 (Thermo Fisher Scientific). The selective reaction monitoring transitions of R5P, [1-13C1]-R5P, [1,2-13C2]-R5P, and compound A1 were set at 229/97, 230/97, 231/97, and 231/80, respectively. The collision energies were also set at 16 eV and 35 eV for R5P and compound A1, respectively.
Animal Experiments.
For the s.c. tumor model, 1 × 106 HCC cells were suspended in 100 μL PBS and s.c. injected into BALB/cAnN-nu (nude) mice. Tumor volume was measured by electronic caliper. For the orthotopic tumor model, 1 × 106 luciferase-labeled MHCC97L cells were suspended in 100% Matrigel (BD Bioscience) and orthotopically injected into the left lobes of the liver of nude mice. At 42 d after implantation, mice were injected with 100 mg/kg d-luciferin (PerkinElmer), and bioluminescence was measured by Xenogen IVIS100. Tumors and lungs were harvested for ex vivo imaging and histology. Tissues were fixed in 10% formalin and stained with H&E for histological analysis.
Drug Treatment.
Sorafenib (LC laboratories) was dissolved in DMSO. OT (Sigma), GSH (Sigma), and N-acetyl-l-cysteine (Sigma) were dissolved in H2O. For in vitro experiments, Sorafenib, OT, GSH, N-acetyl-l-cysteine, and vehicles were added to cells for proliferation assay, ROS assays, or cell-cycle analysis. For in vivo experiments, nude mice were administered orally with Sorafenib, and intraperitoneally with OT and vehicle (0.9% NaCl) based on the therapeutic regimens shown in Fig. 7.
Acknowledgments
We thank Dr. Sandy Leung-Kuen Au for her technical support and advice in the animal experiments. We also thank the Faculty Core Facility, University of Hong Kong Li Ka Shing Faculty of Medicine for its support in the flow cytometry analysis and Xenogen imaging. This work was supported by the Health and Medical Research Fund (HMRF-02132696), SK Yee Medical Research Fund 2011, University Development Fund of the University of Hong Kong, Lee Shiu Family Foundation, National Natural Science Foundation of China (NSFC-21377106), and Hong Kong Research Grant Council Collaborative Research Fund (HKBU5/CRF/10). I.O.-L.N. is Loke Yew Professor in Pathology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508779113/-/DCSupplemental.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, et al. Overexpression of transketolase-like gene 1 is associated with cell proliferation in uterine cervix cancer. J Exp Clin Cancer Res. 2009;28:43. doi: 10.1186/1756-9966-28-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz-Moralli S, Tarrado-Castellarnau M, Alenda C, Castells A, Cascante M. Transketolase-like 1 expression is modulated during colorectal cancer progression and metastasis formation. PLoS One. 2011;6(9):e25323. doi: 10.1371/journal.pone.0025323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang P, Du W, Yang X. A critical role of glucose-6-phosphate dehydrogenase in TAp73-mediated cell proliferation. Cell Cycle. 2013;12(24):3720–3726. doi: 10.4161/cc.27267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krockenberger M, et al. Expression of transketolase-like 1 protein (TKTL1) in human endometrial cancer. Anticancer Res. 2010;30(5):1653–1659. [PubMed] [Google Scholar]
- 6.Schultz H, et al. TKTL1 is overexpressed in a large portion of non-small cell lung cancer specimens. Diagn Pathol. 2008;3:35. doi: 10.1186/1746-1596-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staiger WI, et al. Expression of the mutated transketolase TKTL1, a molecular marker in gastric cancer. Oncol Rep. 2006;16(4):657–661. [PubMed] [Google Scholar]
- 8.Xu X, Zur Hausen A, Coy JF, Lochelt M. Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int J Cancer. 2009;124(6):1330–1337. doi: 10.1002/ijc.24078. [DOI] [PubMed] [Google Scholar]
- 9.Hertl M, Cosimi AB. Liver transplantation for malignancy. Oncologist. 2005;10(4):269–281. doi: 10.1634/theoncologist.10-4-269. [DOI] [PubMed] [Google Scholar]
- 10.Warnatz HJ, et al. The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in control of the cell cycle. J Biol Chem. 2011;286(26):23521–23532. doi: 10.1074/jbc.M111.220178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21(19):5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280(17):16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 13.Mitsuishi Y, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22(1):66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Berdis AJ, Cook PF. Overall kinetic mechanism of 6-phosphogluconate dehydrogenase from Candida utilis. Biochemistry. 1993;32(8):2036–2040. doi: 10.1021/bi00059a021. [DOI] [PubMed] [Google Scholar]
- 15.Perl A, Hanczko R, Telarico T, Oaks Z, Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol Med. 2011;17(7):395–403. doi: 10.1016/j.molmed.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coriat R, et al. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol Cancer Ther. 2012;11(10):2284–2293. doi: 10.1158/1535-7163.MCT-12-0093. [DOI] [PubMed] [Google Scholar]
- 17.Ramos-Montoya A, et al. Pentose phosphate cycle oxidative and nonoxidative balance: A new vulnerable target for overcoming drug resistance in cancer. Int J Cancer. 2006;119(12):2733–2741. doi: 10.1002/ijc.22227. [DOI] [PubMed] [Google Scholar]
- 18.Zhao F, et al. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1alpha-induced metabolic reprograming. Oncogene. 2010;29(20):2962–2972. doi: 10.1038/onc.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 20.Fan J, et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510(7504):298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452(7184):181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 22.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 23.Wong CC, et al. Switching of pyruvate kinase isoform L to M2 promotes metabolic reprogramming in hepatocarcinogenesis. PLoS One. 2014;9(12):e115036. doi: 10.1371/journal.pone.0115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tochhawng L, Deng S, Pervaiz S, Yap CT. Redox regulation of cancer cell migration and invasion. Mitochondrion. 2013;13(3):246–253. doi: 10.1016/j.mito.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 26.Hampton MB, Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: Implications for apoptosis. FEBS Lett. 1997;414(3):552–556. doi: 10.1016/s0014-5793(97)01068-5. [DOI] [PubMed] [Google Scholar]
- 27.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeNicola GM, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du W, et al. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat Cell Biol. 2013;15(8):991–1000. doi: 10.1038/ncb2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diehn M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schafer ZT, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461(7260):109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris IS, et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27(2):211–222. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Cocco P. Does G6PD deficiency protect against cancer? A critical review. J Epidemiol Community Health. 1987;41(2):89–93. doi: 10.1136/jech.41.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med. 2010;16(9):387–397. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 35.DeBerardinis RJ, et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. UKCCCR (1998) United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) guidelines for the welfare of animals in experimental neoplasia (Second Edition). Br J Cancer 77(1):1–10. [DOI] [PMC free article] [PubMed]
- 37.Trapnell C, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng IO, Lai EC, Fan ST, Ng MM, So MK. Prognostic significance of pathologic features of hepatocellular carcinoma. A multivariate analysis of 278 patients. Cancer. 1995;76(12):2443–2448. doi: 10.1002/1097-0142(19951215)76:12<2443::aid-cncr2820761207>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 39.Wong CC, et al. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology. 2014;60(5):1645–1658. doi: 10.1002/hep.27320. [DOI] [PubMed] [Google Scholar]