Significance
Bacteria can produce β-lactamases, enzymes that destroy β-lactam antibiotics and thereby resist these potent antibiotics that target cell wall synthesis. Production of β-lactamases is often controlled by β-lactam-induced perturbations in the cell wall. Here, we have identified a new mechanism controlling β-lactamase production. We found a signaling system in which a membrane-associated histidine kinase directly binds β-lactams, triggering the expression of a β-lactamase and resistance to β-lactam antibiotics. Direct sensing of β-lactam antibiotics may enable sufficiently rapid induction of β-lactamase to degrade β-lactam antibiotics before the integrity of the cell wall is disturbed.
Keywords: Vibrio, histidine kinase, β-lactam receptor, β-lactamase
Abstract
β-Lactams disrupt bacterial cell wall synthesis, and these agents are the most widely used antibiotics. One of the principle mechanisms by which bacteria resist the action of β-lactams is by producing β-lactamases, enzymes that degrade β-lactams. In Gram-negative bacteria, production of β-lactamases is often induced in response to the antibiotic-associated damage to the cell wall. Here, we have identified a previously unidentified mechanism that governs β-lactamase production. In the Gram-negative enteric pathogen Vibrio parahaemolyticus, we found a histidine kinase/response regulator pair (VbrK/VbrR) that controls expression of a β-lactamase. Mutants lacking either VbrK or VbrR do not produce the β-lactamase and are no longer resistant to β-lactam antibiotics. Notably, VbrK autophosphorylation is activated by β-lactam antibiotics, but not by other lactams. However, single amino acid substitutions in the putative periplasmic binding pocket of VbrK leads its phosphorylation in response to both β-lactam and other lactams, suggesting that this kinase is a β-lactam receptor that can directly detect β-lactam antibiotics instead of detecting the damage to cell wall resulting from β-lactams. In strong support of this idea, we found that purified periplasmic sensor domain of VbrK binds penicillin, and that such binding is critical for VbrK autophosphorylation and β-lactamase production. Direct recognition of β-lactam antibiotics by a histidine kinase receptor may represent an evolutionarily favorable mechanism to defend against β-lactam antibiotics.
The β-lactams are the most widely used and among the most valuable classes of antibiotics (1). These agents contain a β-lactam ring in their structures and inhibit the activity of transpeptidases or penicillin binding proteins (PBPs), the essential enzymes for the biosynthesis of peptidoglycan (PG) (2, 3), thereby damaging the integrity of bacterial cell wall. One of the principal mechanisms by which bacteria develop resistance to β-lactam antibiotics is the production of intrinsic or horizontally acquired β-lactamases that can degrade and inactivate β-lactams (4).
In many bacterial species, β-lactamase expression is inducible in response to β-lactam antibiotic treatment. For example, in Gram-positive bacteria, β-lactamase can be induced directly by β-lactam antibiotics or indirectly by muropeptides that are released from PG after β-lactam treatment (5). In Gram-negative bacteria (e.g., Enterobacteriaceae), muropeptide produced after β-lactam treatment can also induce the expression β-lactamase (6, 7). In addition to β-lactam treatment, β-lactamase expression in Gram-negative bacteria can also be induced by the changes of growth rate (8, 9). The direct role of β-lactam in inducing the expression of β-lactamase in Gram-negative bacteria has not been reported.
Two-component systems (TCSs), which are typically composed of a sensor histidine kinase (HK) and a response regulator (RR), are widely present in many bacterial species (10, 11). Typically, environmental signals are sensed by histidine kinase, leading to its autophosphorylation and subsequent phosphoryl transfer to its cognate response regulator (12, 13). Upon phosphorylation, a response regulator usually controls the expression of genes for adaptation to changing environment. Certain TCSs have been shown to contribute to antibiotic (e.g., glycopeptide) resistance. For example, VanS, the histidine kinase of VanSR TCS, directly binds to vancomycin, leading to the expression of genes that are required for the synthesis of alternate peptidoglycan precursors that have low affinity for vancomycin (14–16). Histidine kinases also contribute to the resistance to the antimicrobial peptides, e.g., LL-37, which activates the histidine kinase PhoQ by directly binding to the acidic surface of the PhoQ periplasmic domain (17). TCSs (e.g., BlrAB in Aeromonas or CreBC in Escherichia coli and Pseudomonas) have also been shown to be involved in the expression of β-lactamase and thus are important for β-lactam resistance (3, 9, 18). The response regulator of BlrAB or CreBC triggers the expression of β-lactamase by recognizing the signature sequences (cre/blr-tag: TTCACnnnnnnTTCAC) located in the promoter of β-lactamase gene (3, 9, 18). Despite the evidence that TCS plays an important role in the induction of β-lactamase expression, the identity of the cues that are recognized and transmitted by TCS to control β-lactamase expression remain completely unknown (19).
Here, we reported a previously unidentified mechanism that governs β-lactamase production in Gram-negative bacterium Vibrio parahaemolyticus, the leading cause of seafood-borne diarrheal disease worldwide. Most isolates of V. parahaemolyticus from both clinical and environmental settings exhibit resistance to β-lactam antibiotics, thereby limiting treatment options. At the time we initiated these studies, there was minimal knowledge of the mechanisms underlying V. parahaemolyticus resistance to β-lactam antibiotics. However, a recent report revealed that essentially all V. parahaemolyticus isolates encode a class A chromosomal carbenicillin-hydrolyzing (CARB) β-lactamase (blaV110) (20). We independently identified this chromosome II-encoded enzyme as part of the regulon of a novel TCS (VbrK/VbrR) that controls V. parahaemolyticus resistance to β-lactam antibiotics. Notably, we show that the sensor histidine kinase, VbrK, in this system detects β-lactam antibiotics via direct binding and transmits the signal to the response regulator, VbrR, to control the expression of this CARB β-lactamase gene.
Results
Identification of a Histidine Kinase That Is Essential for V. parahaemolyticus Resistance to β-Lactams.
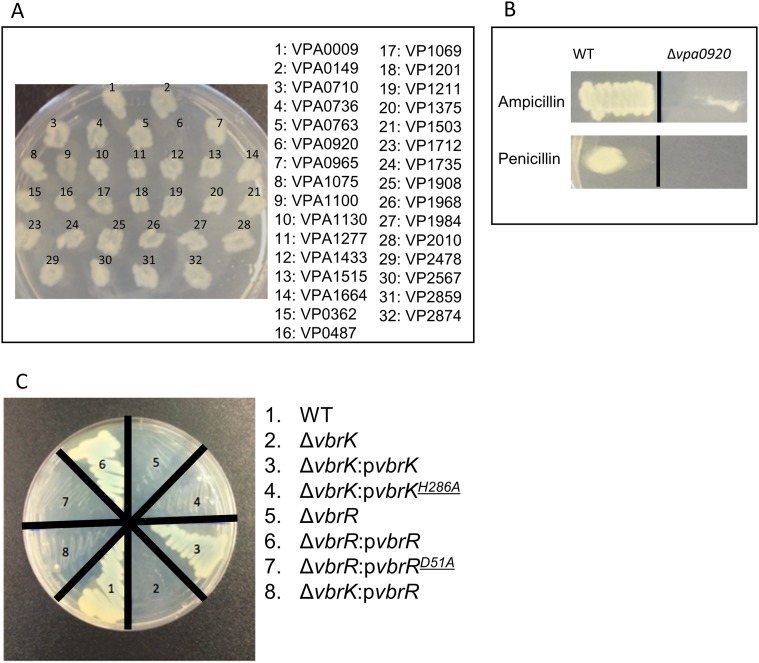
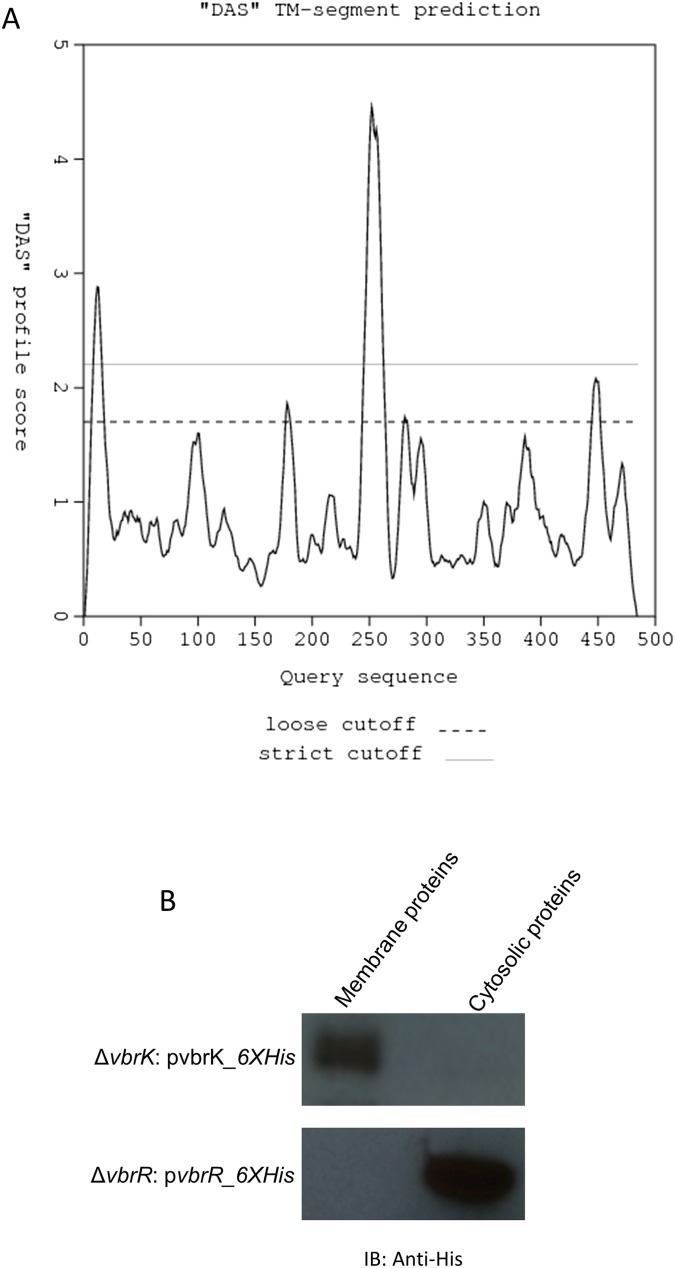
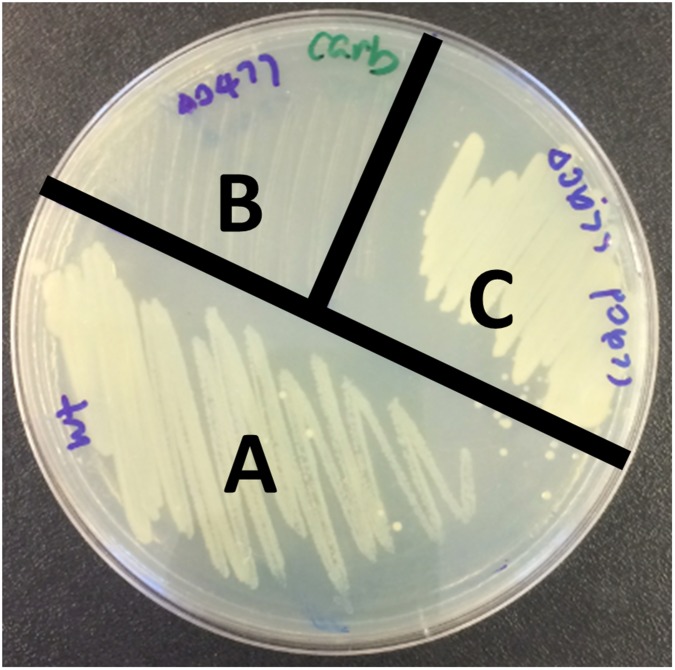
V. parahaemolyticus isolated from both environmental and clinical settings are routinely resistant to β-lactam antibiotics. As part of an ongoing project to characterize the roles of V. parahaemolyticus’ TCSs, we attempted to delete all 32 of the predicted histidine kinases encoded in the pathogen’s genome. We succeeded in creating 31 mutants, each containing a deletion of an individual ORF encoding a putative histidine kinase. Notably, we found that one of the mutants, harboring a deletion of vpa920, could not grow on LB agar plates containing carbenicillin (Fig. S1A), ampicillin, or penicillin (Fig. S1B). Complementation of the ∆vpa0920 mutant with a wild-type vpa0920 in trans restored resistance to carbenicillin (Fig. S1C), indicating that vpa0920 is important for carbenicillin resistance. These results indicate that the predicted histidine sensor kinase vpa0920 (now designated as VbrK for Vibrio β-lactam resistance sensor kinase) is essential for V. parahaemolyticus β-lactam resistance. VbrK is composed of 484 aa, of which amino acids 14–275 contain the domain of DUF3404 with unknown functions. The region encompassing amino acids 274–332 is predicted to be the histidine kinase (HisKA) domain, and the region from amino acids 390–475 is the ATPase domain. VbrK is present in all Vibrio species with 60–90% sequence identity across the species. VbrK is predicted to be a membrane protein with two potential transmembrane regions located at amino acids 9–16 and amino acids 240–260, respectively (Fig. S2A). Amino acids 25–240 are predicted to be located extracellularly, whereas amino acids 261–484 are predicted to be located intracellularly. We expressed VbrK with a C-terminal 6xHis tag in ∆vbrK, and our results showed that VbrK_6xHis was exclusively present in the membrane extracts (Fig. S2B), experimentally demonstrating that VbrK is a membrane protein. In the downstream of vbrK, there is an ORF (vpa0919) encoding predicted response regulator (designated as vbrR for Vibrio β-lactam resistance response regulator). As expected, VbrR_6xHis is a cytosolic protein (Fig. S2B).
Fig. S1.
Identification of a histidine kinase that is essential for the resistance of V. parahaemolyticus to β-lactam antibiotics. (A) Mutants with deletion of predicted histidine kinases were streaked on LB agar plate containing 50 µg/mL carbenicillin. (B) ORF for the predicted histidine kinase that was deleted was shown. Deletion of vpa0920 abolished the ability of V. parahaemolyticus to resist ampicillin and penicillin. (C) Vpa0920 was designated as VbrK. Complementation of ∆vbrK with WT vbrK, but not mutant vbrK with point mutation H286A, restored carbenicillin resistance. Deletion of vbrR (vpa0919) abolished the ability of V. parahaemolyticus to resist carbenicillin. Complementation of ∆vbrR with WT vbrR, but not mutant vbrK with point mutation D51A, restored carbenicillin resistance. Expression of vbrR in trans in a plasmid cannot restore the carbenicillin resistance of ∆vbrK.
Fig. S2.
VbrK is predicted to be a membrane protein. (A) The transmembrane region is predicted to be located at amino acids 9–16 and amino acids 240–260 (www.sbc.su.se/∼miklos/DAS/). Amino acids 25–240 are extracellular, whereas amino acids 261–484 are intracellular. (B) VbrK was expressed exclusively on the membrane, whereas VbrR is expressed as cytosolic protein.
Identification of Genes That Are Regulated by VbrK.
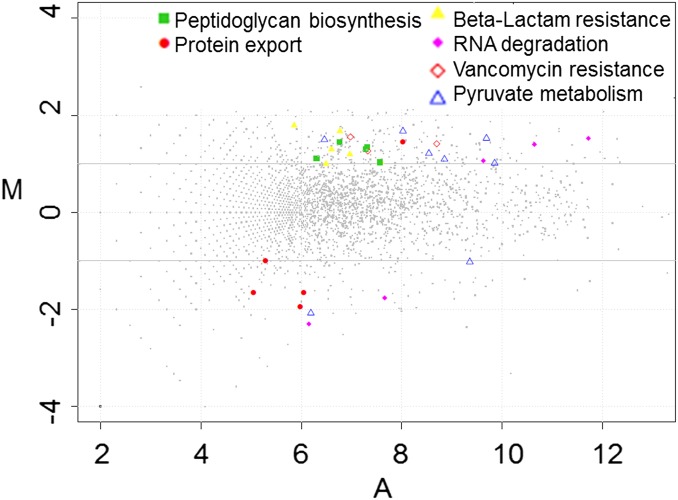
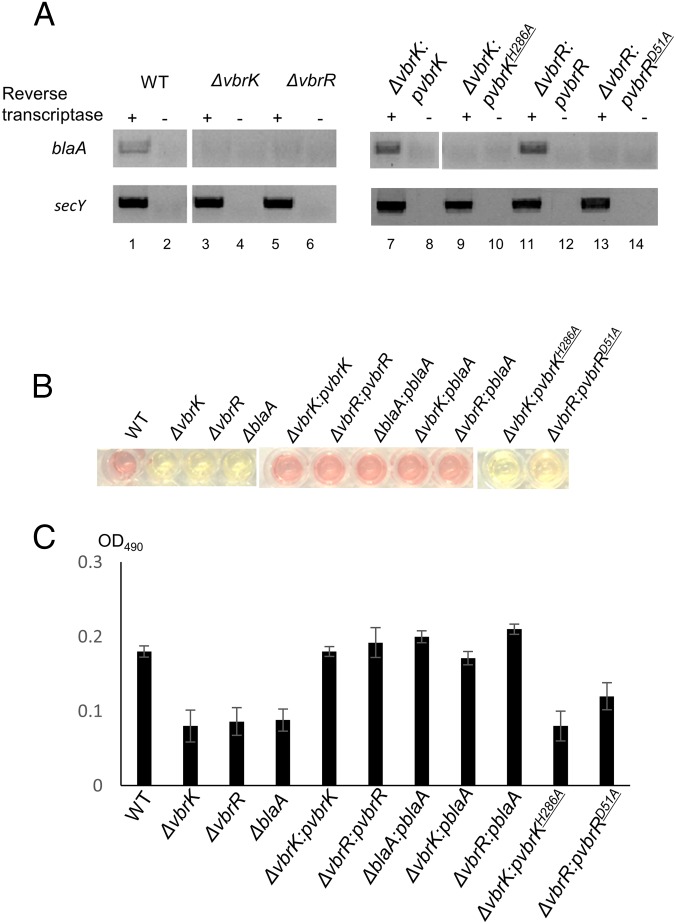
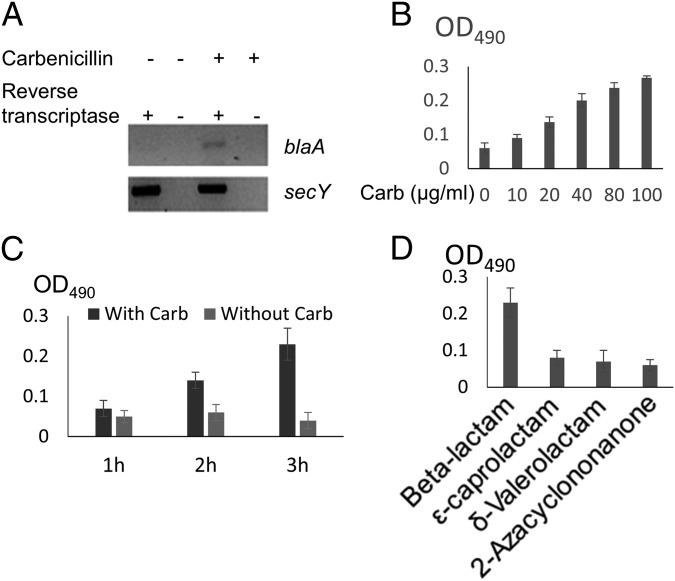
We carried out RNA sequencing (RNA-seq) experiments to identify the VbrK regulon, with the ultimate goal of elucidating the mechanisms by which this histidine kinase regulates β-lactam resistance. In these experiments, we compared the transcriptomes of wild-type and ∆vbrK V. parahaemolyticus grown in LB. We found that 230 (4.9%) and 235 (5.0%) of the 4,868 annotated V. parahaemolyticus ORFs were significantly up- and down-regulated (more than threefold), respectively, in the ∆vbrK strain compared with WT. To gain insight into the pathways that are regulated by VbrK, we grouped the differentially expressed genes based on their GO annotations. This analysis revealed that several GO categories were significantly reduced (more than twofold) in the ∆vbrK mutant compared with the WT, including those involving peptidoglycan biosynthesis, vancomycin resistance, pyruvate metabolism, and β-lactam resistance (Fig. 1 and Table S1). Genes in the β-lactam resistance category include vp0040 encoding TetR transcriptional factor, vp0039 encoding HlyD membrane fusion protein, vp0454 encoding penicillin binding protein 3, vp0545 encoding β-hexosaminidase, vp2751 encoding penicillin binding protein 1A, and vpa0477 encoding a class A β-lactamase. The latter gene had the largest fold change (∼3.5 folds) between the WT and ∆vbrK mutant, and encodes a class A CARB β-lactamase (blaA) with 98.36% identity in nucleotide sequence and 98.59% identity in amino acid sequences to the recently described blaV110 (20). We performed RT-PCR analyses to verify that blaA is regulated by VbrK. Notably, expression of blaA was dependent on VbrK; we observed blaA expression in the WT strain (Fig. 2A, lanes 1 and 2), but not in the ∆vbrK strain (Fig. 2A, lanes 3 and 4). Complementation of ∆vbrK with the WT vbrK gene in trans restored the expression of blaA (Fig. 2A, lanes 7 and 8), indicating that VbrK is essential for the expression of blaA.
Fig. 1.
RNA-seq analysis of genes that are regulated by VbrK. MA plots of relative transcript abundance between WT and ∆vbrK. The log2 of the ratio of abundances of each transcript between the two strains (M in y axis) is plotted against the average log2 of abundance of that transcript in both strains (A in x axis).
Table S1.
Members of GO groups significantly overrepresented among up- and down-regulated genes in ∆vbrK strain (compared with WT)
| GO term | No. of genes | P value |
| Peptidoglycan biosynthesis | 10 | 1.15E-05 |
| Protein export | 5 | 0.014736 |
| β-Lactam resistance | 6 | 0.016001 |
| RNA degradation | 5 | 0.020946 |
| Vancomycin resistance | 3 | 0.023682 |
| Pyruvate metabolism | 8 | 0.047559 |
P < 0.05 was considered significantly different.
Fig. 2.
The role of VbrK/VbrR in the expression of β-lactamase. Indicated strains were cultured in LB. (A) RT-PCR analysis of blaA expression (Upper) with secY as an internal control (Lower). (B) β-lactamase activity was measured by mixing V. parahaemolyticus whole-cell lysate with the β-lactamase substrate (nitrocefin). Once hydrolyzed, the degraded nitrocefin compound rapidly changes color from yellow to red. (C) OD490 was measured to reflect the relative amount of β-lactamase produced by each individual bacterial strain.
VbrK Is Essential for V. parahaemolyticus β-Lactamase Activity.
Because VbrK is critical for the expression of blaA (Fig. 2A), and blaA is predicted to encode a β-lactamase, we hypothesized that VbrK is also essential for β-lactamase activity. β-Lactamase activity was determined by measuring the ability of bacterial cell lysate to hydrolyze the substrate nitrocefin, which rapidly changes color from yellow to red upon degradation. As seen in Fig. 2B, lysates from WT hydrolyzed nitrocefin yielding a red color (Fig. 2B) detectable as an increase in OD490 (Fig. 2C), whereas the lysate from the ∆vbrK mutant did not hydrolyze nitrocefin (Fig. 2B), yielding a baseline OD490 indistinguishable from a ∆blaA mutant (Fig. 2C). Complementation of the ∆vbrK mutant with a WT vbrK gene in trans restored the strain’s ability to hydrolyze nitrocefin (Fig. 2 B and C) demonstrating that VbrK is essential for V. parahaemolyticus β-lactamase activity. Furthermore, deletion of blaA abolished β-lactamase activity (Fig. 2 B and C) and β-lactam resistance (Fig. S3). However, expression of blaA in the ∆vbrK mutant restored the strain’s ability to produce β-lactamase and resist β-lactam antibiotics (Fig. 2 B and C). Thus, these observations strongly suggest that VbrK controls β-lactam resistance via regulating the expression of blaA that encodes a functional β-lactamase.
Fig. S3.
blaA is required for β-lactam resistance. (A) WT is resistant to carbenicillin. Deletion of blaA abolished carbenicillin resistance (B), whereas complementation of ΔblaA with a wild-type blaA restored carbenicillin resistance (C).
Transcription of blaA and Production of β-Lactamase Require β-Lactam Treatment.
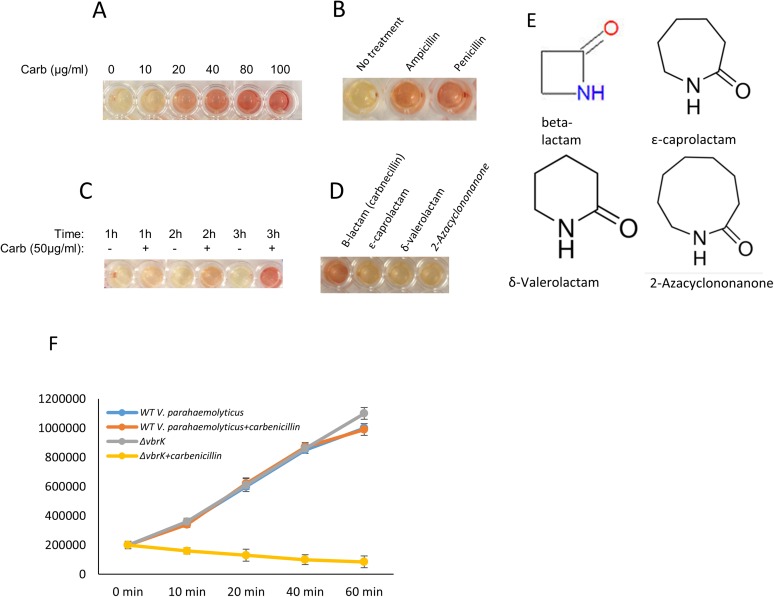
We have shown that blaA is expressed in a VbrK-dependent fashion when V. parahaemolyticus is grown in LB containing carbenicillin (Fig. 2). We hypothesized that blaA transcription is regulated by β-lactams. To eliminate the confounding factors in LB, we grew WT in minimum (M9) medium with or without carbenicillin and determined the expression of blaA by RT-PCR. The results showed that blaA was transcribed in the presence of carbenicillin, whereas it was not transcribed in the absence of carbenicillin (Fig. 3A). We further showed that β-lactamase activity in WT was controlled by carbenicillin in a dose-dependent manner (Fig. 3B and Fig. S4A). In addition to carbenicillin, β-lactamase activity can also be induced by other β-lactams, including ampicillin and penicillin (Fig. S4B). We used 50 µg/mL carbenicillin in subsequent studies. β-Lactamase activity was gradually increased from 0 to 3 h after β-lactam was supplied (Fig. 3C and Fig. S4C), indicating that β-lactamase activity was controlled by carbenicillin in a time-dependent manner. Although β-lactamase activity is only slightly increased at 1 h after carbenicillin was added, such an increase was sufficient to trigger β-lactam resistance, because the growth curve of WT in the presence of carbenicillin was similar to that in the absence of carbenicillin (Fig. S4F), whereas cfu of ∆vbrK was decreased in the presence of carbenicillin (Fig. S4F) in the first hour of growth. These results suggest that carbenicillin triggers VbrK-dependent β-lactam resistance quickly (as early as 10 min after carbenicillin was added). β-Lactams contain a four-ring atom structure, whereas other lactams contain five-ring atoms (γ-lactam), six-ring atoms (δ-lactam), seven-ring atoms (ε-lactam), and nine-ring atoms (Fig. S4E). We found that β-lactamase activity was only triggered by the β-lactam, but not other lactams (Fig. 3D and Fig. S4D).
Fig. 3.
Regulation of blaA transcription and β-lactamase activity by β-lactam. (A) WT V. parahaemolyticus was cultured in M9. RT-PCR analysis of the blaA transcription in the presence or absence of carbenicillin. (B) The activity of β-lactamase activity in WT V. parahaemolyticus treated with increasing dose of carbenicillin. (C) β-Lactamase activity was measured at different time points after carbenicillin was added. (D) β-Lactamase activity in WT V. parahaemolyticus treated with β-lactam and other lactams including ε-caprolactam, δ-valerolactam, and 2-azacyclononanone.
Fig. S4.
Regulation of β-lactamase activity by VbrK/VbrR and carbenicillin. (A) β-Lactamase as indicated by the color change from yellow to red in WT V. parahaemolyticus treated with different doses of carbenicillin. (B) β-Lactamase activity in WT V. parahaemolyticus treated with ampicillin and penicillin. (C) β-Lactamase was activated by carbenicillin in a time-dependent manner. (D) β-Lactamase was activated only by β-lactam, and not by other lactams. (E) The structures of β-lactam, ε-caprolactam, δ-valerolactam, and 2-azacyclononanone are shown. (F) Growth curve of WT and ∆vbrK in LB in the presence or absence of carbenicillin.
VbrK Can Be Phosphorylated on Its Conserved Histidine Residue in Vivo.
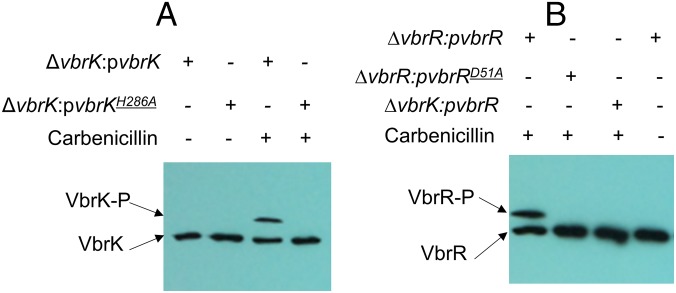
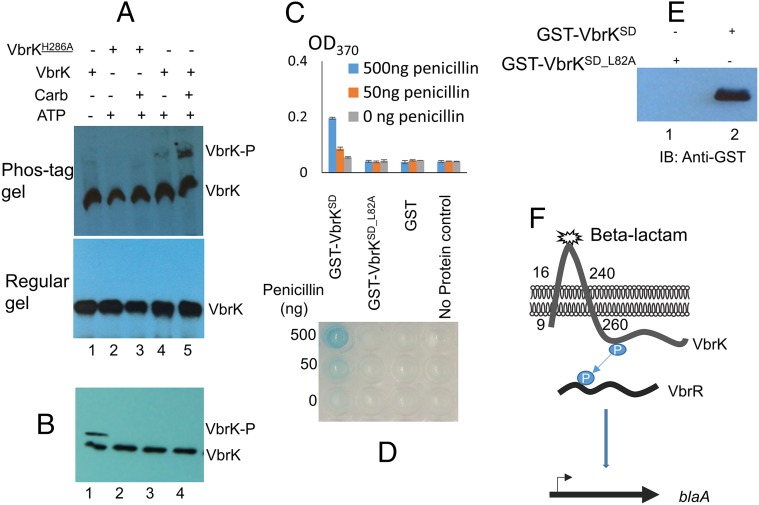
Histidine kinases usually contain a conserved histidine residue that can be phosphorylated, and such phosphorylation is essential for its signal transducing function. We determined VbrK phosphorylation using Phos-tag, which preferentially binds phosphorylated protein, retarding migration in SDS/PAGE gels (21). In this experiment, we grew bacteria in LB with or without carbenicillin, and whole-cell lysate was obtained for SDS/PAGE and Western blot. In the presence of carbenicillin, a shifted band (VbrK-P) of VbrK_6xHis was detected when proteins were separated in Phos-tag gel (Fig. 4A), indicating that VbrK was phosphorylated. When the conserved histidine residue at the 286th amino acid of VbrK was substituted with alanine (H286A), VbrK phosphorylation was abolished (Fig. 4A), indicating that VbrK phosphorylation is dependent on its conserved histidine residue. In the absence of carbenicillin, phosphorylation of VbrK was not detected (Fig. 4A). Furthermore, the substitution of histidine with alanine in BsrK abolished blaA expression (Fig. 2A, lanes 9 and 10), β-lactamase activity (Fig. 2 B and C), and β-lactam resistance (Fig. S1C), indicating that VbrK phosphorylation is functionally important for V. parahaemolyticus β-lactam resistance.
Fig. 4.
The role of β-lactam in the phosphorylation of VbrK and VbrR when V. parahaemolyticus was cultured in LB. (A) Phosphorylation analysis of VbrK or VbrKH286A in the indicated strains in the presence or absence of carbenicillin. (B) Phosphorylation analysis of VbrR or VbrRD51A in the indicated strains in the presence or absence of carbenicillin.
VbrK Forms a TCS with Its Response Regulator VbrR.
Deletion of vbrR, the putative response regulator located adjacent to vbrK on the chromosome, abolished blaA gene expression (Fig. 2A, lanes 5 and 6), β-lactamase activity (Fig. 2 B and C), and β-lactam resistance (Fig. S1C). Complementation of ∆vbrR with a WT vbrR gene in trans restored the blaA gene transcription (Fig. 2A, lanes 11 and 12), β-lactamase activity (Fig. 2 B and C), and β-lactam resistance (Fig. S1C), indicating that VbrR is essential for β-lactam resistance. Together, these observations strongly suggest that VbrK and VbrR form a TCS that controls cellular production of β-lactamase. To determine if VbrR can be phosphorylated, we expressed VbrR_6xHis in ∆vbrR, and phosphorylation was assessed using the Phos-tag assay. A shifted band of VbrR was observed when bacteria were cultured in the presence of carbenicillin (Fig. 4B); in contrast, the shifted band was not observed in the absence of carbenicillin (Fig. 4B), indicating that VbrR was phosphorylated in response to carbenicillin. More importantly, when VbrR was expressed in ∆vbrK, the shifted band of VbrR was not observed (Fig. 4B), indicating that VbrR phosphorylation requires VbrK, and VbrK and VbrR form a functional TCS regulating β-lactam resistance. To determine if phosphorylation of VbrR is important for its function to induce β-lactamase expression, we substituted the conserved aspartate residue at the 51st amino acid of VbrR (a predicted phosphorylation site) with alanine and expressed the VbrRD51A protein in the ∆vbrR strain. As expected, D51A substitution completely eliminated the phosphorylation of VbrR (Fig. 4B). Furthermore, there was no blaA expression (Fig. 2A, lanes 13 and 14), β-lactamase activity (Fig. 2 B and C), and β-lactam resistance (Fig. S1C) in the ∆vbrR:pVbrRD51A strain, indicating that VbrR phosphorylation by VbrK is essential for β-lactam resistance.
Mutation in the Extracellular Region of VbrK Alters Its Recognition Specificity for Lactams.
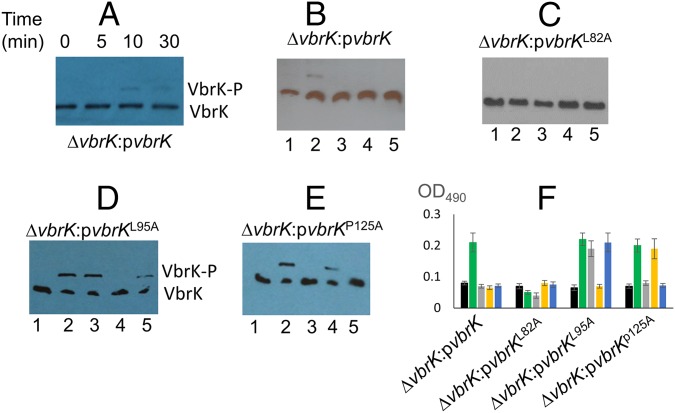
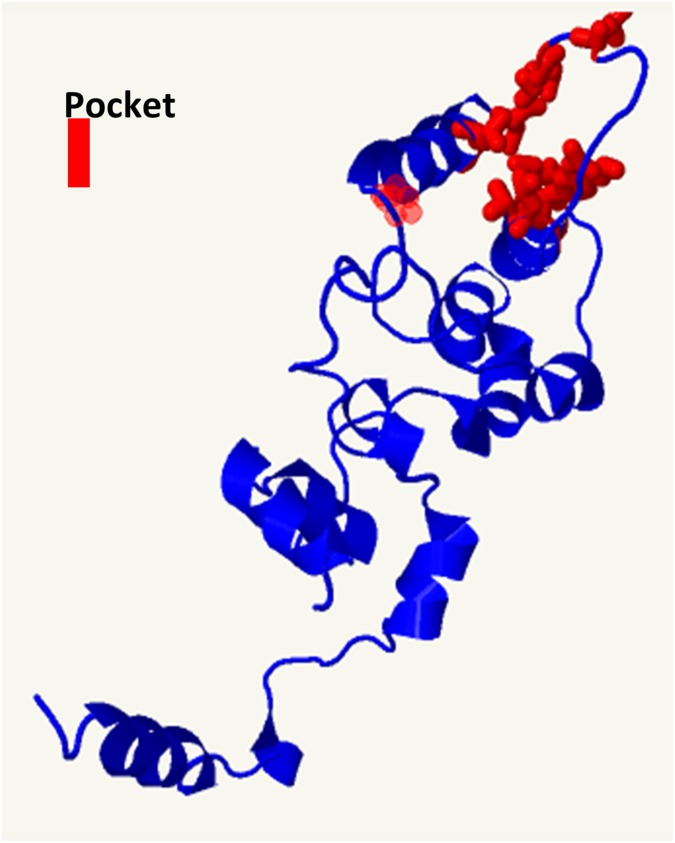
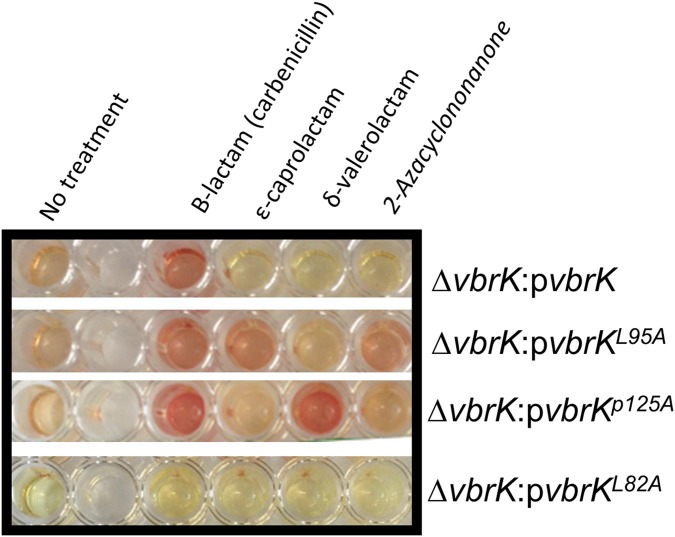
Because β-lactamase activity was triggered only by β-lactams (Fig. 3D and Fig. S4D), we determined VbrK phosphorylation under these conditions. In these experiments, we obtained whole-cell lysates of bacteria grown in M9 medium supplemented with different lactams. Phosphorylation of VbrK was observed as early as 10 min after carbenicillin was supplied (Fig. 5A). In the subsequent studies, we harvested proteins 3 h after carbenicillin was added to get more phosphorylated proteins. VbrK was phosphorylated when V. parahaemolyticus was treated with β-lactam, but not with other lactams (Fig. 5B), indicating that β-lactam triggers β-lactamase expression via phosphorylation of VbrK. VbrK phosphorylation upon treatment with β-lactam does not necessary indicate that VbrK is directly responding to β-lactam; it is possible, e.g., that β-lactam treatment produces peptidoglycan breakdown products (i.e., muropeptides), which serve as the trigger for VbrK phosphorylation. To test the hypothesis that VbrK is a β-lactam receptor that directly detects β-lactam, we mutated the conserved amino acids in the extracellular region predicted to form a pocket (Fig. S5) to serve as the signal recognition site. Single-mutation L82A in VbrK completely abolished the ability to produce β-lactamase (Fig. 5F and Fig. S6). Furthermore, VbrK with L82A mutation cannot be phosphorylated upon treatment with β-lactam or other lactams (Fig. 5C), suggesting that L82 is critical for β-lactam recognition. Notably, the mutant harboring VbrKL95A phosphorylated VbrK (Fig. 5D) and yielded expression of β-lactamase (Fig. 5F and Fig. S6) in response to ε-caprolactam and 2-azacyclononanone in addition to carbenicillin. Similarly, the mutant harboring VbrKP125A phosphorylated VbrK and expression of β-lactamase (Fig. 5 E and F and Fig. S6) in response to δ-valerolactam as well as carbenicillin. Because ε-caprolactam, 2-azacyclononanone, and δ-valerolactam do not produce peptidoglycan breakdown products, but have the capacity to induce phosphorylation of VbrK containing amino acid substitutions in its periplasmic pocket domain (Fig. 5 D and E), it is unlikely that peptidoglycan breakdown products activate VbrK phosphorylation. Taken together, these findings strongly suggest that VbrK is a bona fide β-lactam receptor.
Fig. 5.
Identification of VbrK amino acid residues essential for specific recognition of different lactams. Indicated strains were cultured in M9. (A) VbrK phosphorylation was measured at different time points after carbenicillin was added to the culture. Phosphorylation of VbrK (B), VbrKL82A (C), VbrKL95A (D), and VbrKP125A (E) in V. parahaemolyticus in the presence of carbenicillin (lane 2), ε-caprolactam (lane 3), δ-valerolactam (lane 4), and 2-azacyclononanone (lane 5) or in the absence of any lactams (lane 1). (F) Indicated strains were untreated (black bar) or treated with carbenicillin (green bar), ε-caprolactam (gray bar), δ-valerolactam (yellow bar), and 2-azacyclononanone (blue bar), and OD490 was measured to indicate β-lactamase activity.
Fig. S5.
The extracellular portion of VbrK (amino acids 25–240) was predicted by Phyre2 (www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index) to have a pocket domain. The amino acids that form the pocket are highlighted in red. These amino acids highlighted in red include Y71, D75, I76, Q78, L79, L82, L95, I96, T97, P99, L100, and P125.
Fig. S6.
β-Lactamase activity of various strains (shown on right) of V. parahaemolyticus in response to the treatment of β-lactam (carbenicillin) and other lactams. The color change from yellow to red indicates the ability of whole-cell lysate to hydrolyze nitrocefin.
VbrK Is Phosphorylated in Vitro.
To further investigate whether VbrK is sufficient to interact with and respond (via autophosphorylation) to β-lactams, we isolated membrane extracts from an E. coli strain expressing VbrK. The membrane extracts were treated with both carbenicillin and ATP in vitro and VbrK phosphorylation was assessed with the Phos-tag assay. A shifted band of VbrK was observed when the membrane extract was treated with both carbenicillin and ATP; the intensity of such a shifted band was much lower without carbenicillin treatment (Fig. 6A, lanes 4 and 5), indicating that carbenicillin directly triggers the phosphorylation of VbrK. Carbenicillin-dependent phosphorylation requires the presence of ATP (Fig. 6A, lane 1). Furthermore, carbenicillin did not trigger phosphorylation of a similar membrane extract from an E. coli strain expressing VbrKH286A (Fig. 6A, lanes 2 and 3), strongly suggesting that the conserved histidine residue in VbrK is required for carbenicillin to trigger the protein’s autophosphorylation. Treatment with other lactams did not induce the phosphorylation of VbrK (Fig. 6B). Taken together, these results reveal that VbrK phosphorylation is directly triggered by β-lactam, but not by other lactams.
Fig. 6.
In vitro analysis of VbrK phosphorylation and interaction between VbrK and β-lactam. (A) Membrane extracts from E. coli expressing 6xHis-tagged VbrK (lanes 4 and 5) or VbrKH286A (lanes 2 and 3) were treated with carbenicillin (lanes 3 and 5) or left without treatment of carbenicillin (lanes 2 and 4). No addition of ATP was used as a negative control (lane 1). Following treatment, each sample was resolved in the Phos-tag gel (Upper) or regular gel (Lower) and blotted with anti-His antibody. (B) In vitro phosphorylation assay for VbrK treated with carbenicillin (lane 1), ε-caprolactam (lane 2), δ-valerolactam (lane 3), and 2-azacyclononanone (lane 4). (C and D) Analysis of binding between the VbrK sensor domain (VbrKSD) and penicillin. Microtiter plate was precoated with GST-VbrKSD, GST-VbrKSD_L82A, or GST, or without protein coating. Penicillin (50 ng or 500 ng) was added to the precoated plate. Following sufficient washing, anti-penicillin antibody and HRP secondary antibody were added. Blue color formation was observed (D) and OD370 was measured (C) to reflect the relative amount of penicillin that binds to the coated proteins. (E) Pulldown assay was performed by addition of equal amount of GST-VbrKSD (lane 2) or GST-VbrKSD_L82A (lane 1) into the protein G Sepharose immobilized with penicillin and anti-penicillin antibody. (E) After sufficient washing, protein elution was probed with anti-GST antibody. (F) Schematic model for VbrK/VbrR-mediated β-lactamase expression.
Extracellular Sensor Domain of VbrK Directly Binds Penicillin.
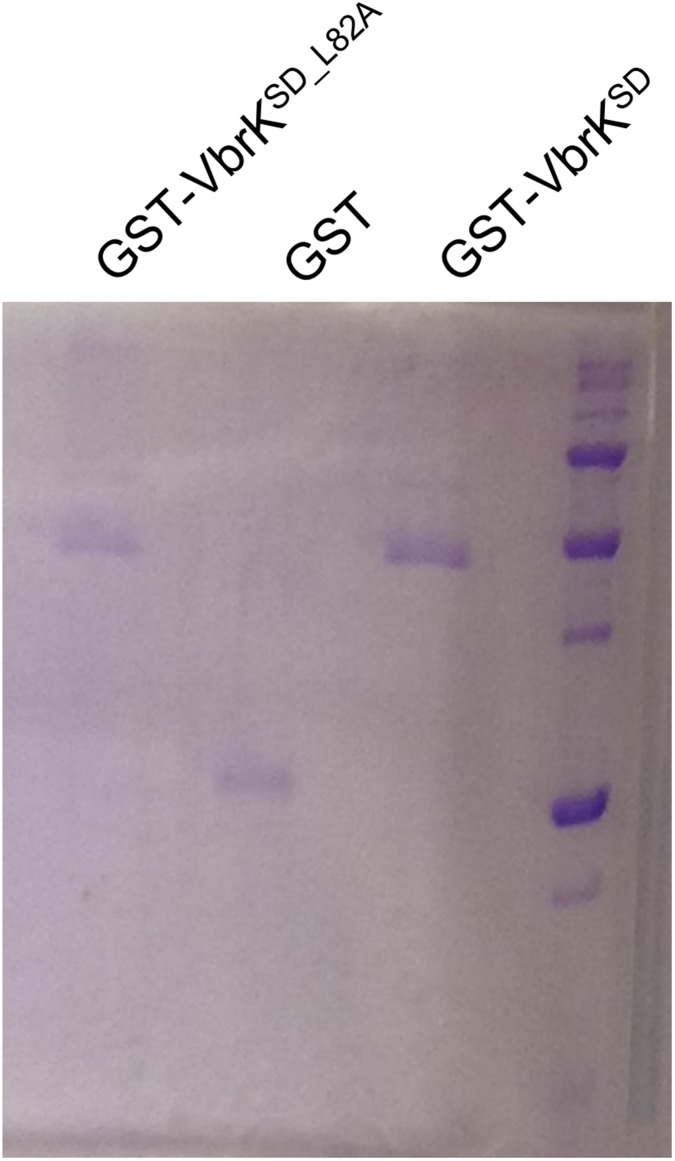
To further elucidate the mechanisms by which β-lactam triggers the phosphorylation of VbrK, we determined if penicillin can directly bind to the periplasmic sensor domain of VbrK (amino acids 25–240, VbrKSD). We purified GST-VbrKSD, GST, and GSTSD_L82A to homogeneity as shown by Coomassie blue staining (Fig. S7). A microtiter plate was subsequently coated with these purified proteins, and penicillin binding to the precoated microtiter plate was measured using anti-penicillin antibody and HRP-conjugated secondary antibody. Blue color formation and increases of OD370 indicate that penicillin binds to the microtiter plate. Addition of 50 ng penicillin to the wells precoated with GST-VbrKSD results in slight blue color formation and approximately twofold increase of OD370 compared with the wells without addition of penicillin (Fig. 6 C and D). More significant blue color and approximately fourfold increase in OD370 was observed when 500 ng penicillin was added (Fig. 6 C and D), indicating that penicillin directly binds the VbrK sensor domain in a concentration-dependent manner. Such binding was specific because addition of penicillin to the wells precoated with purified GST alone did not result in a significant blue color and increase of OD370 (Fig. 6 C and D). Furthermore, addition of penicillin to the wells precoated with GST-VbrKSD_L82A did not result in an increase in OD370 (Fig. 6 C and D), indicating that L82A mutation in the sensor domain of VbrK abolished its binding with penicillin. To further determine the binding between penicillin and VbrKSD, we performed a pull-down assay in which penicillin and anti-penicillin antibody were immobilized into a protein G Sepharose followed by addition of GST-VbrKSD or GST-VbrKSD_L82A. The results showed that the elution contained GST-VbrKSD but not GST-VbrKSD_L82A, as detected by anti-GST antibody (Fig. 6E), further verifying that the sensor domain of VbrK binds penicillin, and L82 is important for such binding. Coupled with the results that L82A mutation abolished VbrK phosphorylation and β-lactamase production in response to β-lactam treatment (Fig. 5 C and F), we concluded that β-lactam triggers the phosphorylation of VbrK by directly binding to the sensor domain of VbrK.
Fig. S7.
Purification of VbrK sensor domain. E. coli strains containing the vectors that express GST-VbrKSD, GST-VbrKSD_L82A, or GST alone were subcultured in LB for 1 h, followed by addition of 1 mM IPTG for protein expression. The protein was purified and resolved in SDS/PAGE and stained by Coomassie blue. An equal amount of each protein was used to coat the microtiter plate.
Discussion
In this study, we show that histidine kinase VbrK is a β-lactam receptor that triggers the expression of β-lactamase in response to β-lactam antibiotics in a Gram-negative bacterium V. parahaemolyticus. This conclusion was supported by the results that (i) deletion of VbrK or its response regulator VbrR greatly reduced the expression of β-lactamase and abolished the β-lactam resistance; (ii) VbrK activation is specifically triggered by β-lactam antibiotics, but not other lactam; and (iii) single amino acid substitution in the predicted sensor domain of VbrK alters its specificity to lactams. Based on these results, we propose a model in which the membrane-associated histidine kinase VbrK directly binds β-lactam antibiotics, leading to the VbrK phosphorylation, phosphoryl transfer to VbrR, and, ultimately, the expression of β-lactamase (Fig. 6F).
In Gram-positive bacteria (e.g., Staphylococcus aureus and Bacillus lichenformis), β-lactam resistance is mediated by the expression of β-lactamase (blaZ) or the production penicillin binding protein 2a (PBP2a or MecA), which has low affinity for β-lactam antibiotics (22–25). Expression of blaZ is initiated upon the direct interaction of β-lactam with the membrane-associated β-lactam receptor (BlaR1). Here, we show that Gram-negative bacterium, V. parahaemolyticus, uses a novel β-lactam receptor VbrK to induce the expression of β-lactamase. Although both BlaR1 and VbrK are membrane associated and recognize β-lactam via direct binding, the subsequent signaling pathway for β-lactamase expression is different. Upon binding to the β-lactam, BlaR1 becomes irreversibly acylated, which results in the autoproteolytic cleavage within the cytoplasmic domain of BlaR1. The cleaved form of BlaR1 is an active metalloprotease and can inactivate the repressor BlaI, leading to the dissociation of BlaI from the promoter of blaZ and consequent expression of blaZ (26, 27). In contrast, VbrK becomes autophosphorylated upon binding to β-lactams. The phosphoryl group is subsequently transferred to the response regulator VbrR to control the expression of β-lactamase. When we screened for VbrK mutants that can detect other lactams, we identified P125A and L95A. These two substitutions may modify the β-lactam binding pocket to allow for the recognition of other lactams, leading to the subsequent VbrK phosphorylation, phosphoryl transfer to the VbrR, and production of β-lactamase. We also identified L82A mutation that abolished the ability of VbrK to bind to β-lactam and induce the expression of β-lactamase, suggesting that L82 is critical for VbrK binding to β-lactam. Alternatively, L82A mutation may alter appropriate protein folding, leading to an inactive VbrK. β-Lactam binding to the pocket of VbrK may result in a conformational change that is transmitted to the HisKA domain, leading to a closer association with the ATPase domain and consequent phosphorylation of the histidine residue.
β-Lactamase in Gram-negative bacteria is often induced by the β-lactam-associated perturbation of cell wall integrity (6). In Enterobacteriaceae, this induction mechanism is complex and involves multiple proteins and steps (6, 7). β-Lactam antibiotics disrupt the murein biosynthesis, leading to the accumulation of muropeptides in the periplasm. These muropeptides can be transported through the inner membrane channel created by a membrane protein AmpG. Within the cytoplasm, muropeptides bind the transcriptional factor AmpR, and such binding activates AmpR’s DNA binding activity, leading to the transcription and expression of AmpC β-lactamase (28). It is conceivable that such multistep defensive mechanisms occur at the cost of damages in the cell wall integrity. Here, we provide evidence that Gram-negative bacterium V. parahaemolyticus can use a histidine kinase VbrK to directly sense β-lactam antibiotics, leading to the production of β-lactamase. Direct sensing of β-lactam antibiotic to induce the expression of β-lactamase could occur potentially in a rapid fashion and at no cost of damages in the cell wall integrity, which may represent a novel mechanism to defend against β-lactam antibiotics.
Although TCSs are present in a wide range of bacterial species, and their function in the recognition and transduction of environmental signals has been well documented, very few physiological signals for the histidine kinases are known. Particularly, there is only one antibiotic, vancomycin, that has been reported to be the signal molecule for the histidine kinase VanS (15, 16). Because of the lack of the defined signals that histidine kinase can recognize, structural analyses of ligand–histidine kinase interactions are very limited (11, 29, 30). Defining the ligand, β-lactam, as the signal molecule for the histidine kinase VbrK provides molecular tools to study the structure–activity relationship of VbrK and the mechanisms by which histidine kinase is phosphorylated and activated. Importantly, VbrK is present in almost all Vibrio species, and the residues (L82, L95, and P125) that are responsible for specific recognition of lactams are conserved in different Vibrio species (Fig. S8). The gene encoding β-lactamase was also found in many Vibrio species, including non-O1/non-O139 Vibrio cholerae, Vibrio harveyi, and Vibrio alginolyticus (31, 32). These results suggest that direct recognition of β-lactam antibiotic by VbrK is a well-conserved mechanism to induce β-lactamase gene expression in Vibrio species. Application of β-lactamase inhibitors could potentially reduce the prevalence of β-lactam resistance (33, 34). However, the inhibitors exhibit variable affinity to different β-lactamases, and the efficacy may be compromised by the overwhelming quantity of β-lactamases they produced. Defining the VbrK/VbrR regulatory pathway that controls the expression of β-lactamase would provide a promising target to inhibit β-lactamase production and β-lactam resistance. Lead compounds that can inhibit the activity of histidine kinase have been identified for a number of TCSs (35–38). There is future promise in rationally designing VbrK inhibitors to enhance the efficacy of β-lactam antibiotics in treating infections caused by Vibrio species.
Fig. S8.
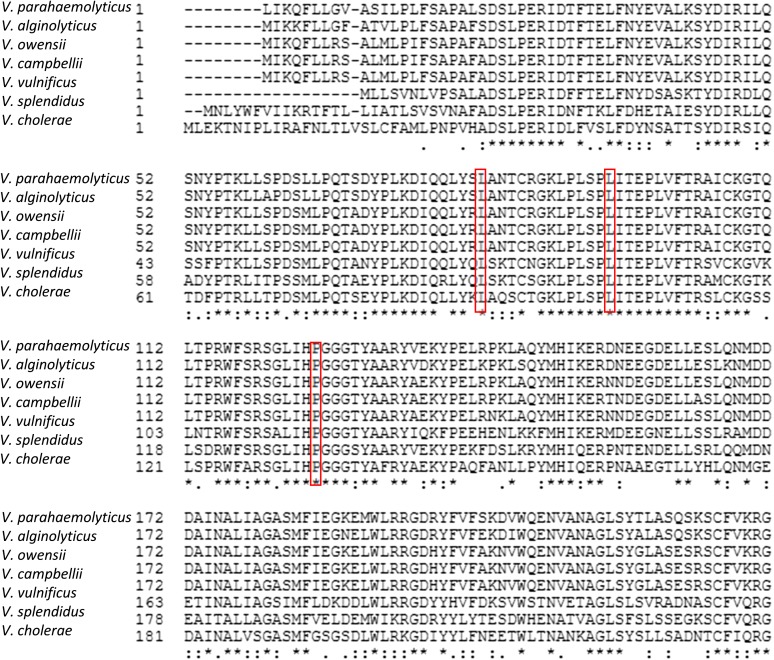
Alignment of the sensor domain of VbrK (amino acids 1–240) from different Vibrio species. Amino acids in the red boxes are these that govern the specificity of sensing β-lactams. Amino acids with an asterisk (*) at the bottom are identical across different Vibrio species.
Materials and Methods
The strains and primers used in this study are shown in Tables S2 and S3. The detailed protocols for construction of mutants, complemented strains, RNA-seq, RT-PCR, β-lactamase assay, in vitro and in vivo phosphorylation assay, and penicillin binding assay are provided in SI Materials and Methods.
Table S2.
Strains and plasmids used in this study
| Strains and plasmids | Relevant genotype/description | Source |
| Strains | ||
| V. parahaemolyticus RIMD 2210633 | Clinical isolate | 18 |
| ΔVbrK | RIMD 2210633 Δvpa0920 | This work |
| ΔVbrR | RIMD 2210633 Δvpa0919 | This work |
| ΔblaA | RIMD 2210633 Δvpa0477 | This work |
| ΔVbrK:pVbrK | ΔVbrK complemented with vbrK gene located in pMMB207 | This work |
| ΔVbrR:pVbrR | ΔVbrR complemented with VbrR gene located in pMMB207 | This work |
| ΔblaA:pblaA | ΔblaA complemented with blaA gene located in pMMB207 | This work |
| ΔVbrK:pblaA | ΔVbrK containing blaA gene located in pMMB207 | This work |
| ΔVbrK:pVbrR | ΔVbrK containing VbrR gene located in pMMB207 | This work |
| ΔVbrK:pVbrKH286A | ΔVbrK complemented with mutated vbrK gene(H286A) located in pMMB207 | This work |
| ΔVbrR:pVbrRD51A | ΔVbrR complemented with mutated VbrR gene(D51A) located in pMMB207 | This work |
| E. coli SM10λpir | KmR, thi-1, thr, leu, tonA, lacY, supE, recA::RP4-2-Tc::Mu, λpir | This work |
| E. coli DH5αλpir | supE44, ΔlacU169(ΦlacZΔM15), recA1, endA1, hsdR17, thi-1, gyrA96, relA1, λpir | This work |
| Plasmids | ||
| pDM4-19 | Plasmid for deletion of vpa0919 | This work |
| pDM4-20 | Plasmid for deletion of vpa0920 | This work |
| pDM4-0477 | Plasmid for deletion of vpa0477 | This work |
| pDM4-HK1-32 | Plasmids for deletion of histidine kinases shown in Fig. S1A | This work |
| pMMB207-19 | RBS and vpa0919 sequences cloned into pMMB207 | This work |
| pMMB207-20 | vpa0920 sequence cloned into pMMB207 | This work |
| pMMB207-0477 | RBS and vpa0477 sequences cloned into pMMB207 | This work |
| pMMB207-20-H286A | vpa0920 sequences with a point mutation in histidine 286 and sequences for 6His amino acid cloned into pMMB207 | This work |
| pMMB207-19-D51A | vpa0919 sequences with a point mutation in aspartic acid 51 and sequences for 6His amino acid cloned into pMMB207 | This work |
| pGEX-0920SD | Sequences of vpa0920 sensor domain cloned into pGEX | This work |
| pGEX-0920-L82A | Sequences of vpa0920 with a point mutation in leucine 82 cloned into pGEX | This work |
| pGEX-0920-L95A | Sequences of vpa0920 with a point mutation in leucine 95 cloned into pGEX | This work |
| pGEX-0920-P125A | Sequences of vpa0920 with a point mutation in proline 125 cloned into pGEX | This work |
Table S3.
Primers used in this study
| Primer na+G5:H94me | Sequence (5′–3′) |
| VPA0009_1F | AGGATAAACTCGAGCGGCATTAAGTTTGGGTAGTC |
| VPA0009_1R | AGTTAGGAATTCATGAAATCACTCCGATAATCTTG |
| VPA0009_2F | AGTTAGGAATTCGTAACTTGAAATTGATCAAGTTAAG |
| VPA0009_2R | AGTTAGAGATCTGCGCCTGCAGTTTTCGC |
| VPA0149_1F | GCTTATCGATACCGTCGACCCTCGATGAAAGCGCGCTGCAGTAC |
| VPA0149_1R | CGACTTGAACTGCGATGCCTGGCGCCCACAGATTCTAAATAAC |
| VPA0149_2F | CAGGCATCGCAGTTCAAGTCGCGGGTAACAATGAAGTGAACTCG |
| VPA0149_2R | CACTAGTGACGCGTACTCGAAGAAACGTTCACGGTTACGGC |
| VPA0710_1F | GCTTATCGATACCGTCGACCCTCGACTTCTTCTTCGCTGATCAAAC |
| VPA0710_1R | CGACTTGAACTGCGATGCCTGACTATCTAATTTGTCTACTCCGC |
| VPA0710_2F | CAGGCATCGCAGTTCAAGTCGTGGATTTAAAACAGAGCTCAGG |
| VPA0710_2R | CACTAGTGACGCGTACTCGACACTTGAGGTAATTAAAGCGGC |
| VPA0736_1F | GCTTATCGATACCGTCGACCCTCGAACGAACCCAGGAGAAGGTTG |
| VPA0736_1R | CGACTTGAACTGCGATGCCTGAAATGCTTCCTCTAATTGGAAAG |
| VPA0736_2F | CAGGCATCGCAGTTCAAGTCGTGTGATGGTGATTGATGACC |
| VPA0736_2R | CACTAGTGACGCGTACTCGACAAGCACCTCTCCACTTTG |
| VPA0763_1F | GCTTATCGATACCGTCGACCCTCGAGCAGCTGCAATCATCCAAATC |
| VPA0763_1R | CGACTTGAACTGCGATGCCTGGATAGGATTCCAAGAAATGACTTG |
| VPA0763_2F | CAGGCATCGCAGTTCAAGTCGTAAAAGTAGTCATAGTGGAAGATG |
| VPA0763_2R | CACTAGTGACGCGTACTCGACGGAACATAAAAGGATCTAAGC |
| VPA0920_1F | GCTTATCGATACCGTCGACCCTCGAATGGTCTAGTAGTTCATCACG |
| VPA0920_1R | CGACTTGAACTGCGATGCCTGGGACGATAAAAACTTGGCTG |
| VPA0920_2F | CAGGCATCGCAGTTCAAGTCGACTGTTACCGTTACTTGTTTC |
| VPA0920_2R | CACTAGTGACGCGTACTCGATCTGCCTGGTCACATGAACG |
| VPA0965_1F | GCTTATCGATACCGTCGACCCTCGAAAGCACGCACAGGCTTGC |
| VPA0965_1R | CGACTTGAACTGCGATGCCTGTAAAAGGACTCTTGAGAGAATAG |
| VPA0965_2F | CAGGCATCGCAGTTCAAGTCGCTCAAGATGTTTGGATTTTTACG |
| VPA0965_2R | CACTAGTGACGCGTACTCGAATAGGGATCAGAGCGCCAC |
| VPA1075_1F | GCTTATCGATACCGTCGACCCTCGACAGATACCAAGGAACACGAG |
| VPA1075_1R | CGACTTGAACTGCGATGCCTGCGTCCAAATTTGCCAATGAAG |
| VPA1075_2F | CAGGCATCGCAGTTCAAGTCGGAATAGTCTCTAATCGTGATTC |
| VPA1075_2R | CACTAGTGACGCGTACTCGACTGGCGTTGTTGGAAGATAAC |
| VPA1100_1F | GCTTATCGATACCGTCGACCCTCGATTTTCATGCGCCATGTGGTG |
| VPA1100_1R | CGACTTGAACTGCGATGCCTGAGTGGCTGTTCGCTCATTTC |
| VPA1100_2F | CAGGCATCGCAGTTCAAGTCGTTTCCAGCACCTCTAAAATTAC |
| VPA1100_2R | CACTAGTGACGCGTACTCGAGTTTAAACGCGCTGGAAACC |
| VPA1130_1F | GCTTATCGATACCGTCGACCCTCGAATACGCTGTTTGATATGGGATG |
| VPA1130_1R | CGACTTGAACTGCGATGCCTGTGCTTTTGTGAATCGTACTTGG |
| VPA1130_2F | CAGGCATCGCAGTTCAAGTCGCGCCCACTTATCTCGAAATG |
| VPA1130_2R | CACTAGTGACGCGTACTCGATATTACAGGGATTATTGTGGCG |
| VPA1277_1F | GCTTATCGATACCGTCGACCCTCGAAGATTTGATGCTGCCGGGC |
| VPA1277_1R | CGACTTGAACTGCGATGCCTGTTATGCGTTCCATGCATCCG |
| VPA1277_2F | CAGGCATCGCAGTTCAAGTCGCGGGCGAACAACGATAAAAG |
| VPA1277_2R | CACTAGTGACGCGTACTCGACGAGAGCTTGTGTTTTGGTG |
| VPA1433_1F | GCTTATCGATACCGTCGACCCTCGAAGTTTTCCCGGCTTGAGAATC |
| VPA1433_1R | CGACTTGAACTGCGATGCCTGGTTACAAAGTTTTAGTCGTGGAC |
| VPA1433_2F | CAGGCATCGCAGTTCAAGTCGCCAAAATTCGATGCAGAAAC |
| VPA1433_2R | CACTAGTGACGCGTACTCGATTGATCATGGACGAAGCAAC |
| VPA1515_1F | GCTTATCGATACCGTCGACCCTCGATGCCGGACTACATGGCTTTC |
| VPA1515_1R | CGACTTGAACTGCGATGCCTGGAAGTGTGAATCAGACAGATC |
| VPA1515_2F | CAGGCATCGCAGTTCAAGTCGCCTATCGCCTCCTCACAC |
| VPA1515_2R | CACTAGTGACGCGTACTCGATTGCGTCAACGGCAAGATTG |
| VPA1664_1F | GCTTATCGATACCGTCGACCCTCGATTTTGCTCAGCAAGCTCCATG |
| VPA1664_1R | CGACTTGAACTGCGATGCCTGGACTATGAACAAACTGGCATTC |
| VPA1664_2F | CAGGCATCGCAGTTCAAGTCGAAGGGAGGGTTTGAAAGAAAG |
| VPA1664_2R | CACTAGTGACGCGTACTCGACGATGCCGACCCGTTTGAAC |
| VP0362_1F | GCTTATCGATACCGTCGACCCTCGAATTATGTTGCCTAAACTCGATGG |
| VP0362_1R | CGACTTGAACTGCGATGCCTGGACTCTCTTCGAGTTTGTAGC |
| VP0362_2F | CAGGCATCGCAGTTCAAGTCGAAGGCAAACCTTGGCTTGCC |
| VP0362_2R | CACTAGTGACGCGTACTCGATTTGAAAGCAGTGGCCTGGC |
| VP0487_1F | GCTTATCGATACCGTCGACCCTCGATTCTTCAGACTGCGTGGCTC |
| VP0487_1R | CGACTTGAACTGCGATGCCTGTCACTACTCCATGGCACAAG |
| VP0487_2F | CAGGCATCGCAGTTCAAGTCGCATGAAGAAACTTGCACCCG |
| VP0487_2R | CACTAGTGACGCGTACTCGATGGTGACTTAGAAGACTAATTC |
| VP1069_1F | GCTTATCGATACCGTCGACCCTCGAATCGTACAACATGGTAAGAGAG |
| VP1069_1R | CGACTTGAACTGCGATGCCTGATCTGTAGACGAATCATTCCC |
| VP1069_2F | CAGGCATCGCAGTTCAAGTCGTATACAGGTAAAAAAGCAGTCC |
| VP1069_2R | CACTAGTGACGCGTACTCGACGGCATGACGAATACAAAATG |
| VP1201_1F | GCTTATCGATACCGTCGACCCTCGAGCAGCAGCATCCACTATTAC |
| VP1201_1R | CGACTTGAACTGCGATGCCTGCACCTACATCAAATGTTGTCC |
| VP1201_2F | CAGGCATCGCAGTTCAAGTCGTCTTTGTCCACTTTATATCGTTG |
| VP1201_2R | CACTAGTGACGCGTACTCGAATAGTGCGAAGCGAAACACAC |
| VP1211_1F | GCTTATCGATACCGTCGACCCTCGATTCAGGCTTGTCCATTTGG |
| VP1211_1R | CGACTTGAACTGCGATGCCTGAACGAATCACTAAAGACTCTTTTTC |
| VP1211_2F | CAGGCATCGCAGTTCAAGTCGTCAGTTGATACCCTAAGCC |
| VP1211_2R | CACTAGTGACGCGTACTCGAGACGCGATTGTGCTCGAC |
| VP1375_1F | GCTTATCGATACCGTCGACCCTCGATCGCCTTATGTGGTGCTATTC |
| VP1375_1R | CGACTTGAACTGCGATGCCTGTACATGTATTACCCAATATGCC |
| VP1375_2F | CAGGCATCGCAGTTCAAGTCGAGAACCGTTGAATAAATGGCTG |
| VP1375_2R | CACTAGTGACGCGTACTCGACGAAACAGAACGTGGCTGTG |
| VP1503_1F | GCTTATCGATACCGTCGACCCTCGATACCGTTTAAACGTTCTGAAAATTG |
| VP1503_1R | CGACTTGAACTGCGATGCCTGATCCCTCATCCTCAGAATCC |
| VP1503_2F | CAGGCATCGCAGTTCAAGTCGCGTAAACCAACGCCAGTTCC |
| VP1503_2R | CACTAGTGACGCGTACTCGATCATAAAGGTTGATTTACCACAAC |
| VP1712_1F | GCTTATCGATACCGTCGACCCTCGAGCTTCACCAACAAACAAGTCG |
| VP1712_1R | CGACTTGAACTGCGATGCCTGACATGGCAACGCGAGTGATG |
| VP1712_2F | CAGGCATCGCAGTTCAAGTCGGGTACATCCTTACCGTTAACC |
| VP1712_2R | CACTAGTGACGCGTACTCGACCAAATTGCCTGACTTCAATG |
| VP1735_1F | GCTTATCGATACCGTCGACCCTCGAGCGTTCACTGCCGTAATTTTG |
| VP1735_1R | CGACTTGAACTGCGATGCCTGGAATGTTTTTGATGGCGACG |
| VP1735_2F | CAGGCATCGCAGTTCAAGTCGCGAAAAAAGACGACTAAAAAGG |
| VP1735_2R | CACTAGTGACGCGTACTCGACAAGAATCCATAGCCCAGCG |
| VP1908_1F | GCTTATCGATACCGTCGACCCTCGACGTCGCGATTTTCTTACTGC |
| VP1908_1R | CGACTTGAACTGCGATGCCTGTGTGGTGATCGTCGATGATC |
| VP1908_2F | CAGGCATCGCAGTTCAAGTCGCCTTCCCTCCATGAAACGC |
| VP1908_2R | CACTAGTGACGCGTACTCGAGCGTTTACGTATACAGGCGG |
| VP1968_1F | GCTTATCGATACCGTCGACCCTCGAGGTATGAGATTTTGCCCTTG |
| VP1968_1R | CGACTTGAACTGCGATGCCTGGTTCATTCACCTGCGTATTTTG |
| VP1968_2F | CAGGCATCGCAGTTCAAGTCGTTGGCATTAAAACTGCTTAACTG |
| VP1968_2R | CACTAGTGACGCGTACTCGAGTGAATAGCTCAAGGGATTTG |
| VP1984_1F | GCTTATCGATACCGTCGACCCTCGACATGCTGCCGCGCATGG |
| VP1984_1R | CGACTTGAACTGCGATGCCTGGCACCCGGCTCCAAGC |
| VP1984_2F | CAGGCATCGCAGTTCAAGTCGTTGCGATGCCGATTCCGACT |
| VP1984_2R | CACTAGTGACGCGTACTCGATATTGTTGAGCAGATGGTGG |
| VP2010_1F | GCTTATCGATACCGTCGACCCTCGAATTGCAATGCACAGCTCATCC |
| VP2010_1R | CGACTTGAACTGCGATGCCTGCTAATCGGTATTTAGGAGCAG |
| VP2010_2F | CAGGCATCGCAGTTCAAGTCGTCCTTGCAGATATTCTTGTTCAG |
| VP2010_2R | CACTAGTGACGCGTACTCGAATTGGCGCTTGGTTTTCAATAC |
| VP2478_1F | GCTTATCGATACCGTCGACCCTCGAACGGTTTGTCTGGTCGCG |
| VP2478_1R | CGACTTGAACTGCGATGCCTG GGATAAGATTACAACTCGTAAAG |
| VP2478_2F | CAGGCATCGCAGTTCAAGTCG ACCTCTCATAAAATACAAGCCTC |
| VP2478_2R | CACTAGTGACGCGTACTCGA GCGCTGAAAGCGTTAATTAATC |
| VP2567_1F | GCTTATCGATACCGTCGACCCTCGAGTCAATTGTTGGTCATAATTGAG |
| VP2567_1R | CGACTTGAACTGCGATGCCTGGTTATCTAATTACATCGGAGTC |
| VP2567_2F | CAGGCATCGCAGTTCAAGTCGGCATTCGGACTAAACTTCATAC |
| VP2567_2R | CACTAGTGACGCGTACTCGAAGTCTGTGGCAGAGATGAAAG |
| VP2859_1F | GCTTATCGATACCGTCGACCCTCGAGAAACGCTGAAGCGTCTGC |
| VP2859_1R | CGACTTGAACTGCGATGCCTGTAATCCTCCTCAAGCAGCATATA |
| VP2859_2F | CAGGCATCGCAGTTCAAGTCGCTCTACAAGATTTCAGTTGATAATG |
| VP2859_2R | CACTAGTGACGCGTACTCGAGTCTTTGAACTTTTCGAAGTG |
| VP2874_1F | GcttatcgataccgtcgaccctcgaCGGTTATTCCTGAAGCCGC |
| VP2874_1R | CGACTTGAACTGCGATGCCTGCTTAGGTAAGTTGAACGCATG |
| VP2874_2F | CAGGCATCGCAGTTCAAGTCGCCGATATCCTTTCTAGCTTTTC |
| VP2874_2R | CACTAGTGACGCGTACTCGAGCGCACGCTGTTTATTGTTG |
| VPA0919_1F | GCTTATCGATACCGTCGACCCTCGACAATTGGCGGAGGCAAGTAAAGA |
| VPA0919_1R | CGACTTGAACTGCGATGCCTGCTGCTTGTTCCAGGCTTAC |
| VPA0919_2F | CAGGCATCGCAGTTCAAGTCGACTGTAGATACCCACGTACTTC |
| VPA0919_2R | CACTAGTGACGCGTACTCGAACTCATCAACCCAACGACG |
| VPA 0477_1F | GCTTATCGATACCGTCGACCCTCGACACGGCGGCAATTATCATCC |
| VPA 0477_1R | CGACTTGAACTGCGATGCCTGGCGTGGTGTTCTTCGTTCTT |
| VPA 0477_2F | CAGGCATCGCAGTTCAAGTCGGAACTCGAGCAACCAAGCC |
| VPA 0477_2R | CACTAGTGACGCGTACTCGAACAGTATCGAGCACCTGCTG |
| VPA0920_pMMB_F | AGCTCGGTACCCGGGGATCCTCTAGTTGATTAAACAGTTTTTGCTCGG |
| VPA0920_pMMB_R | TCTCATCCGCCAAAACAGCCAAGCTTCAACGAGAAGCAGTGTCTGTT |
| VPA0919_pMMB_F | GGTACCCGGGGATCCTCTAGTAAGGAGGTAGGATAATAGTGAAACAGACACTGCTTC |
| VPA0919_pMMB_R | TCTCATCCGCCAAAACAGCCAAGCTTTATGCTTTCATCTTGTAGCCTA |
| vpa0477_RBS_pMMB_F | GGTACCCGGGGATCCTCTAGTAAGGAGGTAGGATAATAATGAAAAAGTTATTCCTGTTGG |
| vpa0477_pMMB_R | TCCGCCAAAACAGCCAAGCTTTAACTTTCTTTGTAGTGCTCTA |
| VPA0920_pMMB_His_R | TCTCATCCGCCAAAACAGCCAAGCTTCAATGGTGGTGGTGATGATGTACGAGAAGCAGTGTCTGTT |
| VPA0919_pMMB_His_R | TCTCATCCGCCAAAACAGCCAAGCTTTAATGGTGGTGGTGATGATGATGTGCTTTCATCTTGTAGCCTA |
| VPA0920H286A_1R | AGCCGTTAAGATCTGTAAGAC |
| VPA0920H286A_2F | GTCTTACAGATCTTAACGCTGAGCTTCGAACGCC |
| VPA0919D51A_1R | AGCTAGGATGACAAGATCCG |
| VPA0919D51A_2F | CGGATCTTGTCATCCTAGCTCGCCAGCTACCTG |
| VPA0477_RT_F | GCGACATGGACAGCGGCAA |
| VPA0477_RT_R | AATCGGTCAAGTCGCGTTGC |
| secY_RT_F | GGCATCATGCCGTATATTTCG |
| secY_RT_R | GGCAATCCAGCAACAATACC |
| 920SD_GST_FBamHI | AACGGATCCCTACCAGAACGCATAGATACG |
| 920SD_GST_RXhoI | AACCTCGAGGTGATCTTCTACATCCCAAC |
| 920L82A_1R | CAGCGCAGCGAATACATGTC |
| 920L82A_2F | GACATGTATTCGCTGCGCTG |
| 920P125A_1R | CACGCAGGTGGCGGTACTTAC |
| 920P125A_2F | GTAAGTACCGCCACCTGCGTG |
| 920L95A_1R | CCTGCAATTACTGAACCTCTAG |
| 920L95A_2F | CTAGAGGTTCAGTAATTGCAGG |
SI Materials and Methods
Strains and Plasmids.
All E. coli strains were cultured in LB at 37 °C. WT V. parahaemolyticus RIMD2210633 (18) was grown in LB containing 1% NaCl. All deletion mutants were generated by allelic exchange using the suicide vector pDM4 as described previously (39). Complementation was conducted by cloning the respective genes in the low-copy (approximately seven copies per cell) vector pMMB207 as described previously (39). The strains and vectors used in this study are listed in Table S2. Primers used in this study are listed in Table S3.
RNA-seq and RT-PCR.
RNA-seq was performed as described previously (40). Bacteria were grown in LB supplemented with 50 µg/mL carbenicillin for 3 h. Total RNA was isolated by RNEasy kit (Qiagen) according to the manufacturer’s instruction. RNA samples were treated with DNase, followed by the removal of rRNA using the RiboZero kit for Gram-negative bacteria (Epicentre). Bacterial mRNA was fragmented to yield fragments in the range of 60–200 nt. The fragmented RNA and random primers were used for the synthesis of first-strand cDNA. Second-strand cDNA was synthesized subsequently. Libraries for Illumina sequencing were generated by adding Illumina-specific adaptors to cDNA. Reads from Illumina sequencing were aligned to chromosomes I and II of V. parahaemolyticus. The total number of reads that can be aligned to the genome was calculated. Subsequently, total reads per gene were normalized by RPKM [(reads/kb of gene)/(million reads aligning to genome)] (41). DEseq, a program that was developed to analyze the statistical differences in gene expression (42), was used to identify differentially expressed genes between WT and ΔVbrK strains. RT-PCR was performed using blaA-specific primers (Table S3). and secY was used as an internal control.
β-Lactamase Activity Assay.
β-Lactamase activity was quantified based on hydrolysis of nitrocefin. Bacteria were subcultured in LB for 3 h in the presence of carbenicillin (50 µg/mL), and an equal amount of bacterial cells were pelleted, resuspended in PBS, and sonicated. Cell lysate was incubated with 50 µg/mL nitrocefin for 30 min at room temperature before OD490 was measured. To determine the effect of β-lactam on β-lactamase activity, bacteria were subcultured in M9 medium containing different concentrations of carbenicillin, and β-lactamase activity was quantified at different time points after carbenicillin was added.
Phosphorylation Assay.
Both in vivo and in vitro phosphorylation were performed using a Phos-tag assay. For in vivo phosphorylation, bacterial strains harboring VbrK or VbrR vectors were grown in LB or M9 with or without treatment of carbenicillin (50 µg/mL). Following 3 h of treatment, whole-cell lysate was obtaining by sonication and resolved in 12% (wt/vol) SDS gels (29:1) containing 50 µM Phos-tag acrylamide (Wako) and 100 µM MnCl2. Proteins in the Phos-tag gels were transferred onto a nitrocellulose membrane, and protein mobility shift was determined by Western blot using anti-His antibody. For in vitro phosphorylation, E. coli DH5α harboring plasmids encoding WT or mutant VbrK constructs were grown in LB at 37 °C, and protein expression was induced by 1 mM IPTG for 3 h with shaking. Following centrifugation (5,000 × g) for 5 min, the cell pellet was resuspended in lysis buffer [50 mM Tris (pH 8.0), 200 mM NaCl, and 5 mM MgCl2], and lysed by sonication. Cell lysates were centrifuged for 30 min at 9,300 × g to remove the cell debris, and the cleared supernatant was ultracentrifuged at 180,000 × g for 1 h. Kinase buffer containing 50 mM Tris (pH 8.0), 100 mM KCl, 5 mM MgCl2, and 10% (vol/vol) glycerol was then used to resuspend the membrane pellets. The membrane extracts containing WT VbrK or mutant VbrK were treated with carbenicillin (50 µg/mL) or left without treatment of carbenicillin. Subsequently, samples were resolved in Phos-tag gels, and protein mobility shift was determined. In vitro phosphorylation was also performed for VbrK membrane extracts treated with other lactams, including ε-caprolactam, δ-valerolactam, and 2-azacyclononanone.
Penicillin Binding Assay.
E. coli strains harboring plasmids encoding GST-tagged VbrK sensor domain (GST-VbrKSD), GST-VbrKSD_L82A, or GST alone were grown in LB at 37 °C for 2 h before 1 mM IPTG was added to the culture to induce protein expression. Following 1.5-h induction, GST-tagged proteins were purified by glutathione–agarose (Sigma-Aldrich) as per manufacturer’s instructions. Purified proteins were visualized by SDS/PAGE and Coomassie blue stain. For penicillin binding assay, purified proteins were coated to PVC microtiter plate by mixing with bicarbonate/carbonate buffer (100 mM, pH 9.6) for 2 h, followed by incubation with or without penicillin in phosphate buffer (20 mM potassium phosphate, 40 mM sodium chloride, pH 7.5) for 1 h. Mouse monoclonal anti-penicillin antibody (Fisher Scientific) and HRP-conjugated anti-mouse IgG secondary antibody (diluted in 1% BSA) were then added and incubated with protein for 1 h, sequentially. PBC was manifested by adding HRP substrate (TMB) to each well and measuring OD370. All incubations were carried out at room temperature and were followed by decanting and washing with PBS containing 0.05% Tween-20 four times before subsequent procedures. Pull-down assay was performed by immobilizing the penicillin and anti-penicillin antibody into protein G Sepharose beads. Subsequently, GST-VbrKSD or GST-VbrKSD_L82A were added onto the beads and incubated for 2 h to allow the binding between VbrK and penicillin. After sufficient washing to remove nonspecific binding, proteins were eluted and Western blot using anti-GST was performed for the elution.
Construction of Plasmids.
Plasmids pDM4-HK1-32.
The up- and downstream regions flanking 31 potential histidine kinase genes (the ORF number is shown in Fig. S1) were amplified using primer pairs (Table S3). V. parahaemolyticus RIMD 2210633 chromosomal DNA was used as a template. The resulting product was inserted into XhoI-digested pDM4, resulting in plasmids of pDM4-HK1-32 (Table S2). These plasmids were used to construct the deletion mutants lacking 31 histidine kinases shown in Fig. S1A.
Plasmid pDM4-19.
The up- and downstream regions flanking vpa0919 (vbrR) were amplified using primer pairs VPA0919_1F/VPA0919_1R and VPA0919_2F/VPA0919_2R, respectively. The resulting product was inserted into XhoI-digested pDM4, resulting in plasmid pDM4-19 (Table S2). This plasmid was used to generate vbrR deletion mutant.
Plasmid pDM4-0477.
The up- and downstream regions flanking vpa0477 (blaA) were amplified using primer pairs VPA0477_1F/VPA0477_1R and VPA0477_2F/VPA0477_2R, respectively. The resulting product was inserted into XhoI-digested pDM4, resulting in plasmid pDM4-0477 (Table S2). This plasmid was used to generate the blaA deletion mutant
Plasmid pMMB207-19.
The vbrR gene was amplified using primers VPA0919_pMMB_F and VPA0919_pMMB_R. A 6xHis tag was added at the C terminus. The PCR product was inserted into BamHI, XbaI double-digested pMMB207, resulting in plasmid pMMB207-19 (Table S2). This plasmid was used to complement ∆vbrR and protein expression for phosphorylation assay.
Plasmid pMMB207-20.
The vbrK gene was PCR amplified using primers VPA0920_pMMB_F and VPA0920_pMMB_R. A 6xHis tag was added at the C terminus. The PCR product was inserted into BamHI, XbaI double-digested pMMB207, resulting in plasmid pMMB207-20 (Table S2). This plasmid was used to complement ∆vbrK and protein expression for phosphorylation assay.
Plasmid pMMB207-20-H286A.
Point mutation H286A was introduced in the coding sequence of vbrK by PCR using primer pairs VPA0920_pMMB_F/VPA0920H286A_1R and VPA0920H286A_2F/VPA0920 pMMB_R, respectively. A 6xHis tag was added at the C terminus. The PCR product was inserted into BamHI, XbaI double-digested pMMB207, resulting in plasmid pMMB207-20-H286A (Table S2). This plasmid was used to express VbrKH286A protein.
Plasmid pMMB207-19-D51A.
Point mutation D51A was introduced in coding sequence of vbrR by PCR amplification from V. parahaemolyticus using primer pairs VPA0919_pMMB_F/VPA0919D51A_1R and VPA0919D51A_2F/VPA0919_pMMB_R, respectively. A 6xHis tag was added at the C terminus. The PCR product was inserted into BamHI, XbaI double-digested pMMB207, resulting in plasmid pMMB207-19-D51A. This plasmid was used to express VbrRD51A protein.
Plasmid pMMB207-0477.
The gene for blaA was PCR amplified from V. parahaemolyticus using primers VPA0477_RBS_pMMb_F and VPA0477_pMMB_R. The PCR product was inserted into BamHI, XbaI double-digested pMMB207, resulting in plasmid pMMB207-0477 (Table S2). This plasmid was used to complement ∆blaA.
Plasmid pGEX-0920SD.
The coding sequence for hypothetical sensor domain (amino acids 25–240) of VbrK was PCR amplified from V. parahaemolyticus using primers 920SD_GST_FBamHI and 920SD_GST_RXhoI. The resulting PCR product was digested with BamHI or XhoI, accordingly, and inserted into the equivalent site of plasmid pGEX, resulting in plasmid pGEX-0920SD (Table S2). This plasmid was used to express the GST-tagged sensor domain of VbrK.
Plasmids pGEX-0920-L82A, pGEX-0920-L95A, and pGEX-0920-P125A.
The point mutation L82A/L95A/P125A was introduced into VbrK by PCR amplification using primer pairs 920SD_GST_FBamHI/920L82A_1R and 920SD_GST_RXhoI/920L82A_2F, 920SD_GST_FBamHI/920L95A_1R and 920SD_GST_RXhoI/920L95A_2F, and 920SD_GST_FBamHI/920P125A_1R and 920SD_GST_RXhoI/920P125A_2F, respectively (Table S3). A 6xHis tag was added at the C terminus of each mutant. The resulting PCR products were digested with BamHI or XhoI, accordingly, and inserted into the equivalent site of plasmid pGEX, resulting in plasmids pGEX-0920-L82A, pGEX-0920-L95A, and pGEX-0920-P125A (Table S2). These plasmids were used to express GST-VbrKL82A, GST-VbrKL95A, and GST-VbrKP125A.
Acknowledgments
We are grateful to Matthew Waldor for insightful comments on the manuscript. This work was supported by the start-up fund from the University of Connecticut, the National Institute of Food and Agriculture, US Department of Agriculture Grant CONS00935 (to X.Z.), and National Natural Science Foundation of China Grants 31372560 and 41376128 (to Q.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520300113/-/DCSupplemental.
References
- 1.Elander RP. Industrial production of beta-lactam antibiotics. Appl Microbiol Biotechnol. 2003;61(5-6):385–392. doi: 10.1007/s00253-003-1274-y. [DOI] [PubMed] [Google Scholar]
- 2.Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to beta-lactam antibiotics: Compelling opportunism, compelling opportunity. Chem Rev. 2005;105(2):395–424. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 3.Meroueh SO, Minasov G, Lee W, Shoichet BK, Mobashery S. Structural aspects for evolution of beta-lactamases from penicillin-binding proteins. J Am Chem Soc. 2003;125(32):9612–9618. [Google Scholar]
- 4.Wilke MS, Lovering AL, Strynadka NC. Beta-lactam antibiotic resistance: A current structural perspective. Curr Opin Microbiol. 2005;8(5):525–533. doi: 10.1016/j.mib.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Amoroso A, et al. A peptidoglycan fragment triggers β-lactam resistance in Bacillus licheniformis. PLoS Pathog. 2012;8(3):e1002571. doi: 10.1371/journal.ppat.1002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs C, Frère JM, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible beta-lactam resistance in gram-negative bacteria. Cell. 1997;88(6):823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs C, Huang LJ, Bartowsky E, Normark S, Park JT. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 1994;13(19):4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergström S, Olsson O, Normark S. Common evolutionary origin of chromosomal beta-lactamase genes in enterobacteria. J Bacteriol. 1982;150(2):528–534. doi: 10.1128/jb.150.2.528-534.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaurin B, Grundström T, Edlund T, Normark S. The E. coli beta-lactamase attenuator mediates growth rate-dependent regulation. Nature. 1981;290(5803):221–225. doi: 10.1038/290221a0. [DOI] [PubMed] [Google Scholar]
- 10.Cock PJ, Whitworth DE. Evolution of prokaryotic two-component system signaling pathways: Gene fusions and fissions. Mol Biol Evol. 2007;24(11):2355–2357. doi: 10.1093/molbev/msm170. [DOI] [PubMed] [Google Scholar]
- 11.Krell T, et al. Bacterial sensor kinases: Diversity in the recognition of environmental signals. Annu Rev Microbiol. 2010;64:539–559. doi: 10.1146/annurev.micro.112408.134054. [DOI] [PubMed] [Google Scholar]
- 12.Capra EJ, Laub MT. Evolution of two-component signal transduction systems. Annu Rev Microbiol. 2012;66:325–347. doi: 10.1146/annurev-micro-092611-150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 14.Arthur M, Molinas C, Courvalin P. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1992;174(8):2582–2591. doi: 10.1128/jb.174.8.2582-2591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchings MI, Hong HJ, Buttner MJ. The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Mol Microbiol. 2006;59(3):923–935. doi: 10.1111/j.1365-2958.2005.04953.x. [DOI] [PubMed] [Google Scholar]
- 16.Koteva K, et al. A vancomycin photoprobe identifies the histidine kinase VanSsc as a vancomycin receptor. Nat Chem Biol. 2010;6(5):327–329. doi: 10.1038/nchembio.350. [DOI] [PubMed] [Google Scholar]
- 17.Bader MW, et al. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122(3):461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Makino K, et al. Genome sequence of Vibrio parahaemolyticus: A pathogenic mechanism distinct from that of V cholerae. Lancet. 2003;361(9359):743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 19.Zeng X, Lin J. Beta-lactamase induction and cell wall metabolism in Gram-negative bacteria. Front Microbiol. 2013;4:128. doi: 10.3389/fmicb.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiou J, Li R, Chen S. CARB-17 family of β-lactamases mediates intrinsic resistance to penicillins in Vibrio parahaemolyticus. Antimicrob Agents Chemother. 2015;59(6):3593–3595. doi: 10.1128/AAC.00047-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics. 2006;5(4):749–757. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Clarke SR, Dyke KG. The signal transducer (BlaRI) and the repressor (BlaI) of the Staphylococcus aureus beta-lactamase operon are inducible. Microbiology. 2001;147(Pt 4):803–810. doi: 10.1099/00221287-147-4-803. [DOI] [PubMed] [Google Scholar]
- 23.Geronimus LH, Cohen S. Induction of staphylococcal penicillinase. J Bacteriol. 1957;73(1):28–34. doi: 10.1128/jb.73.1.28-34.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geronimus LH, Cohen S. Further evidence for inducibility of staphylococcal penicillinase. J Bacteriol. 1958;76(1):117–118. doi: 10.1128/jb.76.1.117-118.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiramatsu K, Asada K, Suzuki E, Okonogi K, Yokota T. Molecular cloning and nucleotide sequence determination of the regulator region of mecA gene in methicillin-resistant Staphylococcus aureus (MRSA) FEBS Lett. 1992;298(2-3):133–136. doi: 10.1016/0014-5793(92)80039-j. [DOI] [PubMed] [Google Scholar]
- 26.Fuda CC, Fisher JF, Mobashery S. Beta-lactam resistance in Staphylococcus aureus: The adaptive resistance of a plastic genome. Cell Mol Life Sci. 2005;62(22):2617–2633. doi: 10.1007/s00018-005-5148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang HZ, Hackbarth CJ, Chansky KM, Chambers HF. A proteolytic transmembrane signaling pathway and resistance to beta-lactams in staphylococci. Science. 2001;291(5510):1962–1965. doi: 10.1126/science.1055144. [DOI] [PubMed] [Google Scholar]
- 28.Lindquist S, Lindberg F, Normark S. Binding of the Citrobacter freundii AmpR regulator to a single DNA site provides both autoregulation and activation of the inducible ampC beta-lactamase gene. J Bacteriol. 1989;171(7):3746–3753. doi: 10.1128/jb.171.7.3746-3753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung J, Hendrickson WA. Structural analysis of ligand stimulation of the histidine kinase NarX. Structure. 2009;17(2):190–201. doi: 10.1016/j.str.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neiditch MB, et al. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126(6):1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choury D, et al. Characterization and nucleotide sequence of CARB-6, a new carbenicillin-hydrolyzing beta-lactamase from Vibrio cholerae. Antimicrob Agents Chemother. 1999;43(2):297–301. doi: 10.1128/aac.43.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ottaviani D, et al. Antimicrobial susceptibility of potentially pathogenic halophilic vibrios isolated from seafood. Int J Antimicrob Agents. 2001;18(2):135–140. doi: 10.1016/s0924-8579(01)00358-2. [DOI] [PubMed] [Google Scholar]
- 33.Bush K, Macielag MJ. New β-lactam antibiotics and β-lactamase inhibitors. Expert Opin Ther Pat. 2010;20(10):1277–1293. doi: 10.1517/13543776.2010.515588. [DOI] [PubMed] [Google Scholar]
- 34.Harris PN, Ferguson JK. Antibiotic therapy for inducible AmpC β-lactamase-producing Gram-negative bacilli: What are the alternatives to carbapenems, quinolones and aminoglycosides? Int J Antimicrob Agents. 2012;40(4):297–305. doi: 10.1016/j.ijantimicag.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Gilmour R, et al. New class of competitive inhibitor of bacterial histidine kinases. J Bacteriol. 2005;187(23):8196–8200. doi: 10.1128/JB.187.23.8196-8200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasko DA, et al. Targeting QseC signaling and virulence for antibiotic development. Science. 2008;321(5892):1078–1080. doi: 10.1126/science.1160354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9(2):117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 38.Wilke KE, Francis S, Carlson EE. Inactivation of multiple bacterial histidine kinases by targeting the ATP-binding domain. ACS Chem Biol. 2015;10(1):328–335. doi: 10.1021/cb5008019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Shah DH, Konkel ME, Call DR. Type III secretion system 1 genes in Vibrio parahaemolyticus are positively regulated by ExsA and negatively regulated by ExsD. Mol Microbiol. 2008;69(3):747–764. doi: 10.1111/j.1365-2958.2008.06326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livny J, et al. Comparative RNA-Seq based dissection of the regulatory networks and environmental stimuli underlying Vibrio parahaemolyticus gene expression during infection. Nucleic Acids Res. 2014;42(19):12212–12223. doi: 10.1093/nar/gku891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner GP, Kin K, Lynch VJ. A model based criterion for gene expression calls using RNA-seq data. Theory Biosci. 2013;132(3):159–164. doi: 10.1007/s12064-013-0178-3. [DOI] [PubMed] [Google Scholar]
- 42.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]