Significance
Invariant natural killer T (iNKT) cells react against CD1d antigen-presenting cells (APCs) in the absence of exogenous antigens, a feature defined as autoreactivity. How iNKT cell autoreactivity is fine-tuned to prevent autoimmunity remains enigmatic. Here, we show that iNKT cell activation is regulated by the lateral nanoscale organization of CD1d loaded with exogenous and endogenous antigens. Using a combination of advanced biophysical techniques, we show that CD1d molecules organize in nanoclusters on the membrane of APCs. We further discover that the actin cytoskeleton prevents enhanced CD1d nanoclustering by hindering physical encountering between CD1d diffusing nanoclusters, reducing basal iNKT cell activation. As such, regulation of CD1d nanoclustering through the actin cytoskeleton constitutes a novel mechanism to fine-tune peripheral iNKT cell autoreactivity.
Keywords: cell membrane compartmentalization, iNKT cell autoreactivity, protein nanoclustering, single-particle tracking, stimulated emission depletion nanoscopy
Abstract
Invariant natural killer T (iNKT) cells recognize endogenous and exogenous lipid antigens presented in the context of CD1d molecules. The ability of iNKT cells to recognize endogenous antigens represents a distinct immune recognition strategy, which underscores the constitutive memory phenotype of iNKT cells and their activation during inflammatory conditions. However, the mechanisms regulating such “tonic” activation of iNKT cells remain unclear. Here, we show that the spatiotemporal distribution of CD1d molecules on the surface of antigen-presenting cells (APCs) modulates activation of iNKT cells. By using superresolution microscopy, we show that CD1d molecules form nanoclusters at the cell surface of APCs, and their size and density are constrained by the actin cytoskeleton. Dual-color single-particle tracking revealed that diffusing CD1d nanoclusters are actively arrested by the actin cytoskeleton, preventing their further coalescence. Formation of larger nanoclusters occurs in the absence of interactions between CD1d cytosolic tail and the actin cytoskeleton and correlates with enhanced iNKT cell activation. Importantly and consistently with iNKT cell activation during inflammatory conditions, exposure of APCs to the Toll-like receptor 7/8 agonist R848 increases nanocluster density and iNKT cell activation. Overall, these results define a previously unidentified mechanism that modulates iNKT cell autoreactivity based on the tight control by the APC cytoskeleton of the sizes and densities of endogenous antigen-loaded CD1d nanoclusters.
It is well-established that different populations of T lymphocytes can recognize not only peptides in the context of major histocompatibility complex (MHC) class I (MHCI) and MHCII molecules but also, foreign and self-lipids in association with CD1 proteins (1), antigen-presenting molecules that share structural similarities with MHCI molecules. Of five CD1 isoforms, CD1d restricts the activity of a family of cells known as invariant natural killer T (iNKT) cells because of their semiinvariant T-cell receptor (TCR) use (1). To date, the exogenous glycolipid α-GalactosylCeramide (α-GalCer) represents the best characterized CD1d-restricted agonist for iNKT cells (2). Unlike conventional peptide-specific T cells, iNKT cells react against CD1d+ antigen-presenting cells (APCs) in the absence of exogenous antigens, a feature defined as autoreactivity (3). iNKT cell autoreactivity underpins the constitutive memory phenotype of iNKT cells and their ability to be activated during a variety of immune responses from infections to cancer and autoimmunity (1). Some of the endogenous antigens known to elicit iNKT cell autoreactivity belong to glycosphingolipid families, with a mix of α- and β-anomeric configurations (4–7). How iNKT cell autoreactivity is fine-tuned to prevent autoimmunity is subject of much investigation. Previous results have shown that exposure of APCs to Toll-like receptor (TLR) agonists enhances iNKT cell autoreactivity (8, 9), consistent with the proposed mechanism by which ligand availability is regulated by lysosomal glycosidases (4, 6).
The recent application of advanced optical techniques (10–13) in combination with substrate patterning and functionalization (14, 15) is providing detailed information on how the lateral organization of a variety of molecules located on both sides of the immunological synapse contributes to controlling T-cell activation. Specifically, single-molecule dynamic approaches and superresolution optical nanoscopy experiments have provided indisputable proof that many receptors on the cell membrane organize in small nanoclusters before ligand activation (16). Membrane nanodomains enriched in cholesterol and sphingolipids (17), protein–protein interactions (18), and interactions between transmembrane proteins and the cytoskeleton (19, 20) have been all implicated in regulating receptor dynamics and nanoclustering. An emerging concept attributes the actin cytoskeleton the ability of imposing barriers or fences on the cell membrane, restricting the lateral mobility of transmembrane proteins (19–21). This transient restriction would, in turn, increase the local concentration of transmembrane proteins, leading to protein nanoclusters. For instance, it has been shown that the actin cytoskeleton promotes the dimerization rate of EGF receptors and facilitates ligand binding and signaling activation (18, 22). Confinement of CD36 has also been observed as a result of its diffusion along linear channels dependent on the integrity of the cortical cytoskeleton (23). This constrained diffusion promotes CD36 clustering, influencing CD36-mediated signaling and internalization. A similar mechanism has been proposed for the maintenance of MHCI clusters on the cell membrane by the actin cytoskeleton, with loss of MHCI clustering resulting in a decreased CD8 T-cell activation (24, 25).
Recent confocal microscopy studies have revealed that the association between agonist-loaded CD1d molecules and lipid rafts might contribute to the regulation of iNKT cell activation (26). This elegant study for the first time, to our knowledge, linked the spatial organization of CD1d molecules on the cell membrane of APCs with the activation profile of iNKT cells. However, it remains unclear whether the results of these experiments obtained using mouse cells can be extended to human cells and whether additional insights can be obtained by using higher-resolution microscopy. Indeed, it is not yet known whether surface-expressed CD1d molecules exist as monomers or nanoclusters and whether the actin cytoskeleton might regulate CD1d lateral organization and iNKT cell activation. Interestingly, it has been recently reported that the actin cytoskeleton impairs antigen presentation by CD1d and that disruption of F actin or inhibition of the ρ-associated protein kinase enhances CD1d-mediated antigen presentation (27). These results suggest that the actin cytoskeleton might regulate, in a not yet known manner, antigen presentation by CD1d molecules.
Here, we combined dual-color single-molecule dynamic approaches with superresolution optical nanoscopy to characterize for the first time, to our knowledge, the spatiotemporal behavior of CD1d on living human myeloid cells. We find that α-GalCer–loaded human CD1d (hCD1d) molecules are organized in nanoclusters on the cell membrane of APCs. We report that the actin cytoskeleton prevents enhanced hCD1d nanoclustering by hindering physical encountering between hCD1d diffusing nanoclusters, thus reducing basal iNKT cell activation. Furthermore, we observed an increase in nanocluster density on activation of APCs with inflammatory stimuli, such as TLR stimulation, mirroring the increased iNKT cell stimulation. Notably, even during inflammation, the actin cytoskeleton retains an important role to limit hCD1d cluster size and iNKT cell activation. Overall, our results suggest that regulation of CD1d nanoclustering through the actin cytoskeleton represents a previously unidentified mechanism to fine-tune peripheral iNKT cell autoreactivity.
Results
The Actin Cytoskeleton Regulates the Mobility of α-GalCer–Loaded hCD1d Molecules.
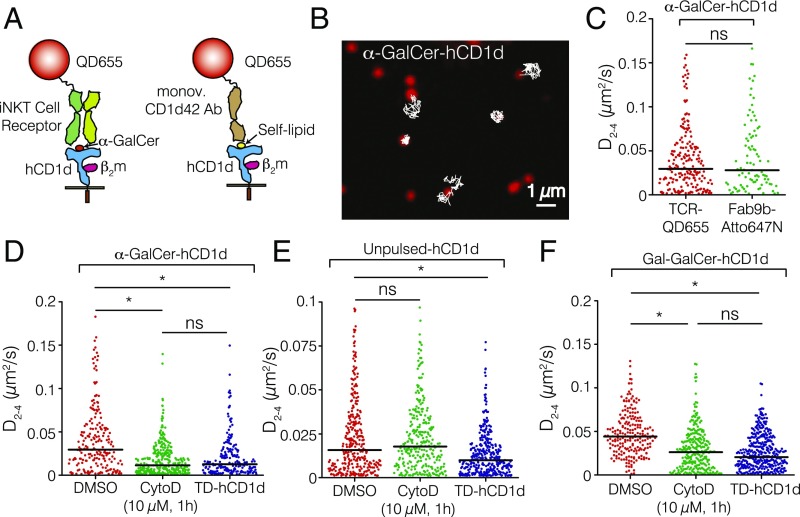
To study the lateral behavior of hCD1d on the cell membrane of APCs, we first performed high-speed single-particle tracking (SPT) on the human myelomonocytic cell line THP-1, transduced with lentiviral vectors encoding hCD1d molecules, and pulsed with the iNKT cell agonist α-GalCer. α-GalCer–loaded hCD1d molecules were labeled at low density using a conjugate consisting of a biotinylated iNKT-TCR (9, 28) and a streptavidin (SAV)-coated quantum dot (QD; QD655) (Fig. 1A). To ensure a single iNKT-TCR per QD, the iNKT-TCR-QD conjugate was prepared in an excess of free biotin to occlude SAV-QD excess binding sites. As another control to rule out any potential cross-linking effects induced by the SAV-QD multivalency, we also used the α-GalCer-hCD1d–specific Fab fragment (Fab9b) (9, 28, 29) covalently attached to the small dye Atto647N. As a third control for the iNKT-TCR probe, α-GalCer–loaded hCD1d molecules were labeled using a monovalent anti-CD1d Ab (CD1d42) conjugated to a QD under excess of free biotin. THP-1 cells were imaged on the ventral side using total internal reflection fluorescence microscopy (TIRFM). Individual features were detected and tracked to generate mobility trajectories (Fig. 1B). Individual trajectories were analyzed by deriving their instantaneous diffusion coefficients at short diffusion times (time lags 2–4; i.e., 20 ms) D2–4 (Fig. 1 C–F). The median D2–4 from multiple trajectories of α-GalCer–loaded hCD1d molecules labeled with the iNKT-TCR-QD conjugate on different cells was 0.029 μm2/s (Fig. 1C). Similar diffusion profiles and median diffusion coefficients were obtained with Fab9b-Atto647N (Fig. 1C) or when labeling α-GalCer–loaded hCD1d using the monovalent CD1d42-QD Ab (Fig. S1A). Altogether, these controls show that the lateral mobility of α-GalCer–loaded hCD1d molecules is not affected by the iNKT-TCR-QD conjugate used throughout this study.
Fig. 1.
The actin cytoskeleton affects the mobility of hCD1d molecules on the surface of APCs. (A) Schematic illustration of the labeling procedure. (Left) α-GalCer–loaded hCD1d molecules (blue) were labeled using a conjugate of an iNKT-TCR (light and dark green) and a QD (red). (Right) Unpulsed hCD1d molecules were labeled using a monovalent anti-CD1d42 Ab conjugated to a QD. Not to scale. (B) Selected TIRFM image of the ventral side of an α-GalCer–pulsed THP-1 cell displaying individual iNKT-TCR-QD–labeled hCD1d molecules (red) on the cell membrane. Representative 2D trajectories (white) are overlaid on the raw image (frame rate =100 Hz). (C) Distributions of the D2–4 values of α-GalCer–loaded hCD1d labeled with the iNKT-TCR-QD conjugate or Fab9b-Atto647N. (D–F) Distributions of the D2–4 values for (D) α-GalCer–pulsed, (E) unpulsed, and (F) Gal-GalCer–loaded hCD1d THP-1 cells treated with 10 µM CytoD or expressing TD-hCD1d mutant molecules. Note that the comparisons of D2–4 values are always made with respect to the basal situation (DMSO) and specifically, for each lipid-loaded condition, because the basal diffusion of hCD1d depends on the lipid. Data are representative of typically 200 trajectories per condition (90 trajectories in the case of hCD1d labeled with Fab9b-Atto647N) from at least 25 cells over eight different experiments. ns, Not significant (P > 0.05; one-way ANOVA test). *P < 0.0001 (one-way ANOVA test).
Fig. S1.
Different control experiments related to the mobility of α-GalCer–loaded CD1d. (A) The iNKT-TCR-QD conjugate does not influence the mobility of α-GalCer–loaded hCD1d on THP-1 cells. To rule out the possibility that the iNKT-TCR-QD conjugate influences the lateral mobility of α-GalCer–loaded hCD1d molecules, we performed an extra control experiment in addition to the Fab-Atto647N labeling experiments shown in Fig. 1C. Specifically, we labeled α-GalCer–loaded hCD1d molecules with a monovalent anti-CD1d42 Ab conjugated to a QD655 and found no difference in terms of hCD1d lateral mobility compared with iNKT-TCR-QD labeling. Moreover, equal reduction of lateral mobility was observed after treating the cells with CytoD using the two different probes, showing that the iNKT-TCR does not have any influence in the mobility of hCD1d. At least 189 trajectories on 14 cells over two different experiments per condition. ns, Not significant (P > 0.05; one-way ANOVA test). (B) Cell viability is not compromised on CytoD treatment. Cells were incubated with 10 µM CytoD for 1 h, and viability was assessed by flow cytometry after propidium iodide staining. Plots depict cell scatter [forward scatter light (FSC)] vs. propidium iodide (detected with a long pass filter at 670 nm, and excited at 488 nm, using the FL3 configuration of the flow cytometer). (i) Control, (ii) DMSO-treated, and (iii) CytoD-treated THP-1 cells (10 µM; 1 h). (C) Reduction of hCD1d lateral mobility on CytoD treatment is hCD1d-specific. To rule out the possibility that CytoD treatment (10 µM; 1 h) to THP-1 cells could induce an overall change on the cell membrane, affecting the mobility of all receptors, including hCD1d, we performed SPT of the transferrin receptor (CD71) with mobility that has been reported to increase on perturbation of the actin cytoskeleton (30). CD71 was labeled using an mAb against CD71 labeled with QD655. Changes in the lateral mobility of hCD1d on CytoD treatment are hCD1d-specific, because the lateral mobility of CD71 on the cell membrane of THP-1 cells is increased using the same CytoD treatment as opposed to the reduced mobility measured on α-GalCer–loaded hCD1d labeled with iNKT-TCR-QD. Data on CD71 are representative from at least 194 trajectories, nine cells, and two different experiments. *P < 0.0001 (one-way ANOVA test).
To address the potential role of the actin cytoskeleton in regulating the lateral mobility of hCD1d on the cell membrane of APCs, we treated THP-1 cells with the actin cytoskeleton-perturbing drug Cytochalasin D (CytoD) (20). CytoD treatment resulted in a nearly threefold reduction in the instantaneous mobility of α-GalCer–loaded hCD1d, with a median D2–4 of 0.011 μm2/s (Fig. 1D). Cell viability was not compromised under these conditions as shown by propidium iodide staining (Fig. S1B). Similar results were obtained when we followed the lateral mobility of α-GalCer–loaded hCD1d molecules on CytoD treatment using the monovalent CD1d42-QD Ab (Fig. S1A).
It has previously been shown that treatment of cells with a range of cytoskeleton-perturbing drugs increases the instantaneous mobility of receptors, such as the FcεRI receptor (19) and the B-cell receptor (20), which has been accounted for by the breaking of actin barriers that otherwise restrict the diffusion of receptors on the plasma membrane. To inquire whether the unexpected observed reduced mobility of hCD1d is specific or whether it results from an overall change of the membrane environment because of the CytoD treatment, we compared it with the mobility of CD71 molecules on THP-1 cells. In marked contrast to the behavior of hCD1d and consistent with the literature (30), treatment of THP-1 cells with a similar concentration of CytoD led to a significant increase of CD71 diffusion (Fig. S1C), indicating that the observed reduced mobility of hCD1d is specific. Finally, to rule out any other potential secondary effects induced by CytoD, we measured the mobility of a cytoplasmic tail-deleted hCD1d (TD-hCD1d) mutant, which lacks the final 10-aa residues, including the AP-2 and AP-3 internalization motif and the lysine residue, which is a target for ubiquitination (31). Similar to the results obtained with CytoD-treated THP-1 cells, the mobility of the α-GalCer–loaded TD-hCD1d mutant was threefold lower than that of α-GalCer–loaded WT hCD1d (Fig. 1D).
On biosynthesis and egress from the endoplasmatic reticulum (ER), CD1d molecules reach the cell surface and continuously recycle through the endolysosomal compartment, where they sample different pools of self- and foreign lipid antigens (32). Although presentation of complex exogenous antigens requires CD1d molecules to traffic deep into the lysosomes (33), autoreactivity of human iNKT cells has been shown to have different requirements for CD1d lysosomal trafficking, possibly as a result of the different APCs used in the experiments (28, 34). To test whether these different trafficking pathways have an influence on the lateral mobility of hCD1d, we imaged unpulsed THP-1 cells and tracked hCD1d labeled with anti–CD1d42-QD (Fig. 1A). The instantaneous diffusion of control unpulsed WT-hCD1d molecules was approximately a factor of two lower than α-GalCer–loaded molecules, with a median D2–4 value of 0.015 μm2/s (Fig. 1E). Interestingly, the lateral mobility of unpulsed WT-hCD1d was not affected by CytoD treatment (median D2–4 = 0.017 μm2/s), in strong contrast to the mobility reduction observed on α-GalCer–loaded molecules after CytoD treatment. Moreover, the mobility of the unpulsed TD-hCD1d mutant was modestly reduced by 1.5-fold compared with the WT counterpart (0.009 μm2/s) (Fig. 1E). As such, these results show that unpulsed CD1d molecules are less sensitive to disruption of actin cytoskeleton interactions. Importantly, these data also indicate that the mobility of hCD1d differs depending on whether we sample molecules that have just reached the cell surface from the ER (i.e., unpulsed and thus, presenting self-lipids) or whether they have been recycling through the early and late endosomes.
To further strengthen these observations, we analyzed the lateral mobility of WT-hCD1d and TD-hCD1d molecules loaded with Gal(α1→2)GalCer (hereafter referred to as Gal-GalCer), which requires cleavage of the terminal galactose by α-galactosidase A in the lysosome for recognition by the iNKT-TCR (33). Recognition of this pool of hCD1d molecules by the iNKT-TCR-QD, thus, identifies a cohort of hCD1d molecules that has exclusively trafficked through the endolysosomes and recycled back to the cell surface. These experiments indicated that the lateral mobility of Gal-GalCer–loaded hCD1d molecules was significantly reduced on CytoD treatment or deletion of the cytosolic tail (Fig. 1F), in analogy to the results obtained for α-GalCer–loaded hCD1d. Moreover, we also analyzed individual trajectories at longer times (over 250 ms) to obtain additional insight on the mobility of hCD1d molecules. Consistent with the reduction in the D2–4 values (instantaneous diffusion), the degree of anomalous diffusion at longer observation times increased for α-GalCer and the Gal-GalCer–loaded hCD1d molecules, whereas no changes were observed in the unpulsed case (Fig. S2). Altogether, these results show that, in the absence of interactions between the cytoplasmic tail of hCD1d and the actin cytoskeleton, the lateral mobility of hCD1d recycled through the endosomes is drastically reduced.
Fig. S2.
Single-molecule trajectory analysis of lipid-loaded CD1d molecules at longer observation times. Trajectories longer than 100 frames have been selected for the analysis. (A, D, and G) Representative MSD (black dots) plots of the first 250 ms (25 points) of individual trajectories of (A) α-GalCer–loaded, (D) unpulsed, and (G) Gal-GalCer–loaded hCD1d molecules. Individual MSD curves were fitted with the function MSD = 4Dtα (red line), where the exponent-α accounts for the degree of anomalous diffusion. (B, E, and H) Diffusion coefficients and (C, F, and I) α-values obtained from the fitting belonging to (B and C) α-GalCer–loaded, (E and F) unpulsed, and (H and I) Gal-GalCer–loaded hCD1d molecules. Significant reductions in the α-values were obtained on disruption of actin cytoskeleton interactions for the cases of exogenous lipid–loaded hCD1d molecules (α-GalCer and Gal-GalCer), whereas no changes in the α-values were observed for the unpulsed case. Data (same as Fig. 1) from typically 200 trajectories per condition from at least 25 cells over eight different experiments. ns, Not significant (P > 0.05; one-way ANOVA test). *P < 0.05 (one-way ANOVA test); **P < 0.0001 (one-way ANOVA test).
hCD1d Molecules Form Nanoclusters on the Surface of APCs with Properties That Depend on Actin Cytoskeleton Interactions.
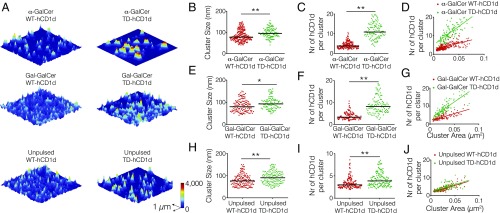
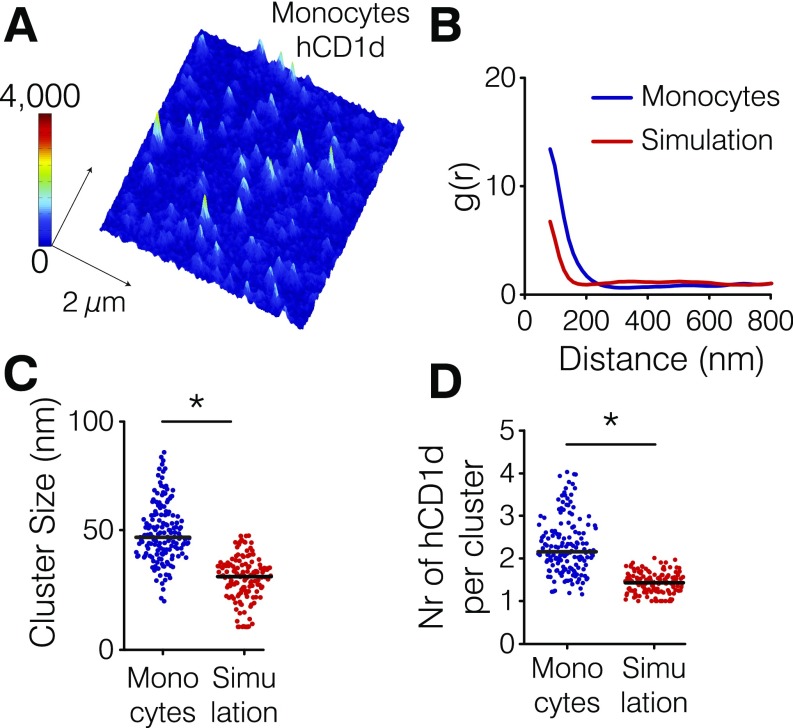
Previous reports indicate that the actin cytoskeleton regulates the lateral organization and nanoclustering of receptors on the cell membrane (23). Given the marked effect of the actin cytoskeleton and the cytoplasmic tail of hCD1d on the lateral mobility of hCD1d molecules that traffic through endosomes, we thought to visualize the nanoscale organization of hCD1d on the surface of THP-1 cells using stimulated emission depletion (STED) nanoscopy (35). STED images of anti-CD1d42–labeled hCD1d were performed on the dorsal side of fixed THP-1 cells. To avoid any potential artifacts related to CytoD treatment on the dorsal cell membrane morphology that could affect the quantification of STED images, we particularly focused on WT-hCD1d and the TD-hCD1d mutant, because SPT experiments showed similar lateral diffusion of exogenous lipid-loaded (α-GalCer and Gal-GalCer) TD-CD1d molecules and CytoD-treated samples. Images were analyzed using a custom-made algorithm based on Bayesian inference of the fluorescence intensity distribution, improving data quantification and increasing the effective resolution beyond that of STED raw images (Materials and Methods) (36). Interestingly, α-GalCer–loaded WT-hCD1d molecules formed small nanoclusters on the surface of THP-1 cells with a median cluster size of 78 nm containing 3.8 median number of hCD1d molecules per cluster (Fig. 2 A–C), resulting in a nanocluster density of around 75 hCD1d molecules per 1 μm2 (Fig. 2D). To verify that CD1d nanoclustering is real and not resulting from the enhanced expression of hCD1d on transduced THP-1 cells, we imaged hCD1d on blood-derived primary hCD14+ monocytes. Analysis of STED images confirmed the presence of hCD1d nanoclusters, albeit being smaller in size (49 nm as median cluster size) compared with THP-1 cells (Fig. S3). These data show that hCD1d forms nanoclusters on the plasma membrane of APCs.
Fig. 2.
hCD1d molecules form nanoclusters on the surface of APCs with properties that depend on actin cytoskeleton interactions. (A) STED images of (Top) α-GalCer–pulsed, (Middle) Gal-GalCer–pulsed, and (Bottom) unpulsed THP-1 cells transfected with (Left) WT-hCD1d or (Right) TD-hCD1d mutant. Cells were stretched onto poly-l-lysine–coated coverslips and labeled with anti-CD1d42 Ab. (B, E, and H) Cluster size distribution, (C, F, and I) distribution of the number (Nr) of hCD1d molecules per cluster, and (D, G, and J) scatterplots of the numbers (Nr) of hCD1d molecules per cluster vs. cluster area corresponding to the quantification of the STED images. STED nanoscopy data are representative of at least 43 different images per condition (3 × 3 μm in size from at least two different experiments). Horizontal bars correspond to median values. *P < 0.01 (one-way ANOVA test); **P < 0.0001 (one-way ANOVA test).
Fig. S3.
CD1d forms nanoclusters on the cell membrane of human monocytes. (A) Representative STED image of hCD1d on the cell membrane of monocytes. (B) Comparison between the correlation functions of the experimental data (blue) and corresponding Monte Carlo simulations (red) of hCD1d molecules randomly organized on the cell membrane using the same particle density as the experimental data. (C) Comparison between the experimental hCD1d cluster size (blue) and simulations of random organization (red). (D) Comparison between the number (Nr) of molecules per cluster obtained on the experimental data (blue) and the simulations (red). The larger values in cluster size and the number of molecules per cluster experimentally obtained compared with simulated data of random organization confirm that hCD1d is organized in nanoclusters on the surface of blood-derived primary hCD14+ monocytes. Experimental and simulated data are representative from at least 52 different STED images of 3 × 3 µm in size belonging to at least two different experiments. *P < 0.0001 (Student’s t test).
Remarkably, α-GalCer–loaded TD-hCD1d molecules, lacking the cytoplasmic tail, showed significantly larger and denser nanoclusters, with a molecular density nearly threefold higher (197 ± 17 hCD1d molecules per 1 μm2) than α-GalCer–loaded WT-hCD1d (Fig. 2 A–D). A similar trend was equally observed for Gal-GalCer–loaded TD-hCD1d. Indeed, larger nanoclusters (median cluster size of 93 nm; 8.2 median number of hCD1d molecules per cluster) and increased density (187 ± 12 hCD1d molecules per 1 μm2) were measured compared with their WT-hCD1d counterparts (median cluster size of 80 nm; 3.3 median number of hCD1d molecules per cluster; 80 ± 6 hCD1d molecules per 1 μm2) (Fig. 2 A and E–G). These results show that the mobility reduction measured on the exogenous-loaded TD-CD1d molecules (Fig. 1 D and F) is accompanied by an increased nanoclustering on the cell surface. In contrast, self-lipid–loaded TD-hCD1d molecules displayed only slightly larger nanoclusters in terms of size and number of molecules, with no significant changes in nanocluster densities compared with self-lipid–loaded WT-hCD1d molecules (Fig. 2 A and H–J). These small changes in nanoclustering degree on unpulsed TD-hCD1d compared with WT-hCD1d are also consistent with the minor changes observed on their lateral mobility. Altogether, these results indicate once more that the effect of the cytoplasmic tail on hCD1d organization is predominantly on the pool of molecules that has trafficked through the lysosome.
Perturbation of the Actin Cytoskeleton Increases Dynamic Encounters Between α-GalCer–Loaded hCD1d Nanoclusters.
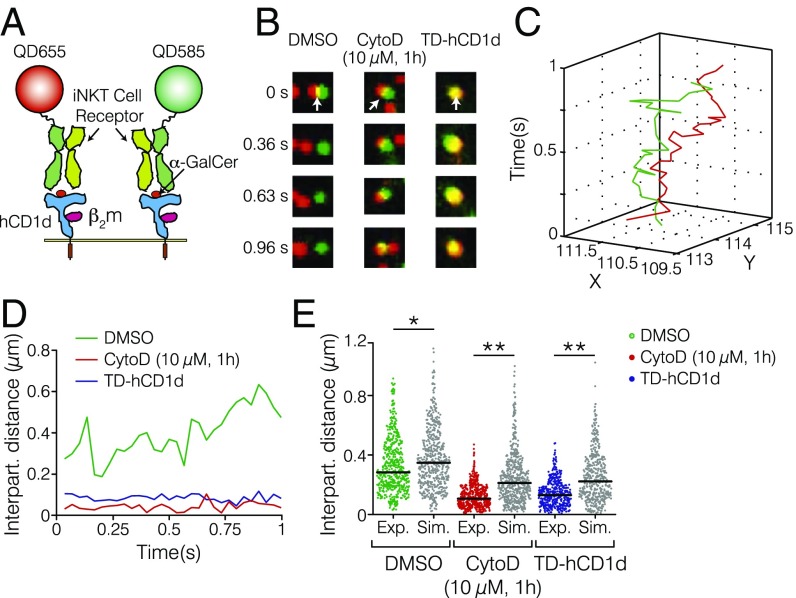
We hypothesized that the increased nanoclustering observed on α-GalCer–loaded TD-hCD1d might result from dynamic encounters between individual nanoclusters that could favorably lead to local enrichment of hCD1d molecules in the absence of cytoskeleton interactions. To test this possibility in living cells, we applied dual-color SPT. We labeled α-GalCer–loaded hCD1d using iNKT-TCRs coupled to two different QDs (Fig. 3A) at equimolar concentrations to increase the probability of detecting nanocluster interaction events (Fig. 3B and Movies S1–S3), while allowing for single-pair trajectory recording. 2D trajectories of spatially close QDs (red and green in Fig. 3C) were generated, and their separation distance was plotted vs. time (Fig. 3D). In the case of truly interacting nanoclusters, their separation distances should remain small (close to the nanocluster size) for periods longer than random encounter events (22, 37). As expected, CytoD treatment of α-GalCer–loaded WT-hCD1d and α-GalCer–loaded TD-hCD1d showed significantly shorter separation distances over time compared with the untreated pulsed WT-hCD1d control (Fig. 3 B, D, and E) (median interparticle distance of 106 nm for CytoD-treated cells, 130 nm for TD-hCD1d, and 284 nm for the control case). These results were further validated by Monte Carlo simulations of random encounters, while accounting for the respective diffusion coefficients in the three different cases. The shorter distances experimentally obtained compared with the simulations (Fig. 3E) confirm local enrichment of hCD1d nanoclusters on perturbation of actin cytoskeleton interactions after either CytoD treatment or removal of the hCD1d cytoplasmic tail. This sustained enrichment (lasting at least 1 s in our experiments) would effectively increase hCD1d nanoclustering in the absence of actin cytoskeleton interactions, consistent with the superresolution data.
Fig. 3.
The actin cytoskeleton regulates dynamic interactions between α-GalCer–loaded hCD1d nanoclusters. (A) Schematic illustration of two α-GalCer–loaded hCD1d molecules (blue) labeled with two different iNKT-TCR-QD conjugates (green and red) at equimolar concentrations. Not to scale. (B) Magnified dual-color TIRFM images (1.9 μm in size) displaying two diffusing hCD1d nanoclusters over time for (Left) the control case, (Center) CytoD-treated samples, and (Right) the TD-hCD1d mutant. White arrows indicate the starting times of the interactions. (C) Representative dual-color trajectories of two different hCD1d nanoclusters reconnected over a total observation time of 1 s (frame rate = 30 Hz). (D) Separation distances of two α-GalCer–loaded hCD1d nanoclusters over time for untreated WT-hCD1d (green), CytoD–treated WT-hCD1d (red), and the TD-hCD1d mutant (blue). (E) Distributions of the interparticle distances from experimental data (blue, green, and red dots) together with Monte Carlo simulations of random encounters (gray dots). Data are representative of at least 20 different trajectories on nine different cells per condition over six different experiments. *P < 0.05 (one-way ANOVA test); **P < 0.0001 (one-way ANOVA test).
The Actin Cytoskeleton Spatiotemporally Arrests Diffusing α-GalCer–Loaded hCD1d Nanoclusters on the Cell Membrane.
The data presented so far indicate that the actin cytoskeleton somehow hinders dynamic interactions between hCD1d nanoclusters, preventing their further coalescence into larger clusters. To directly visualize how actin might accomplish such a role, we performed dual-color TIRFM imaging of hCD1d and actin. We used hCD1d–transduced THP-1 cells expressing Lifeact-GFP, a fluorescent marker of F actin (38). We labeled individual α-GalCer–loaded hCD1d nanoclusters with the iNKT-TCR-QD conjugate and followed their lateral mobility with respect to actin as previously reported for other cell membrane receptors (19, 20) (Fig. 4A). Individual trajectories of diffusing WT-hCD1d nanoclusters were reconnected and superimposed on the actin image (Fig. 4 B and C and Movie S4). Interestingly, WT-hCD1d nanoclusters exhibited a highly restricted mobility in actin-rich regions (Fig. 4B). In contrast, WT-hCD1d mobility increased in actin-poor regions (Fig. 4C). These observations were substantiated by calculating the instantaneous mobility of a selected subpopulation of individual trajectories that were found to diffuse inside and outside actin-rich regions. Indeed, WT-hCD1d nanoclusters displayed a remarkably slow diffusion inside actin-rich regions (median D2–4 = 0.0074 μm2/s) compared with outside (median D2–4 = 0.025 μm2/s) (Fig. 4D), revealing that the actin cytoskeleton arrests mobile α-GalCer–loaded WT-hCD1d nanoclusters after they enter into actin-rich regions. In contrast, a similar analysis on a selection of α-GalCer–loaded TD-hCD1d trajectories that diffused outside and inside actin-rich regions showed no significant difference in terms of their instantaneous diffusion (Fig. 4D), consistent with the observations that the mobility of the TD mutant is not affected by the presence of the actin cytoskeleton (Fig. 1D).
Fig. 4.
The actin cytoskeleton actively arrests diffusing α-GalCer–loaded hCD1d nanoclusters on the cell membrane. (A) Snapshot of a dual-color TIRFM movie displaying Lifeact-GFP–labeled actin (green) and α-GalCer–loaded hCD1d nanoclusters (red) labeled with the iNKT-TCR-QD conjugate. (Scale bar: 2 µm.) (B and C) Representative magnified TIRFM images at two different time sequences with an example of a 2D trajectory of α-GalCer–loaded hCD1d diffusing inside (black line) and outside (white line) actin-rich regions, with the diffusing particle outlined with a red dashed circle. In B, hCD1d is inside actin, whereas C shows the time sequence when hCD1d is outside actin. (Scale bar: 500 nm.) (D) Distributions of the D2–4 values for α-GalCer–loaded WT-hCD1d and α-GalCer–loaded TD-hCD1d nanoclusters inside and outside high-actin regions. The D2–4 values reported here are somewhat different from the median values reported in Fig. 1, because a preselected number of trajectories that diffused outside and inside actin-rich regions was chosen for this analysis. ns, Not significant (Student’s t test). *P < 0.0001 (Student’s t test). (E and F) Cumulated cartography maps containing 8,000 localizations over a recording time of 20 s from (E) α-GalCer–loaded WT-hCD1d and (F) TD-hCD1d overlaid onto a Lifeact-GFP TIRFM image. The white dots correspond to the different spatial positions explored by hCD1d during the observation time. The pseudocolored image corresponds to the actin intensity, going from blue (actin-poor) to red (actin-rich). The black arrows point to hCD1d diffusion on actin-poor regions, whereas black-outlined arrows point to hCD1d diffusing close to actin-rich regions. (Scale bar: 1 µm.) (G) Fractional occurrence of hCD1d localization positions vs. changes in actin intensity: ∆actin for α-GalCer–loaded WT-hCD1d (black) and α-GalCer–loaded TD-hCD1d (red). Data were fitted with a straight line, and the slope was obtained. SPT data are from at least 87 trajectories on 30 different cells over five different experiments. Cartography maps data contain at least 8,000 localizations. Analysis has been performed on six different cells and three different experiments per condition.
To obtain a more robust quantification of the data, we generated cartography maps of α-GalCer–loaded WT-hCD1d and α-GalCer–loaded TD-hCD1d superimposed on actin fluorescence images. These maps provide the spatial positions of hCD1d nanoclusters as they dynamically explore the cell membrane in relation to actin, with a localization accuracy of 20 nm (37) (Fig. 4 E and F). Cartography maps of α-GalCer–loaded WT-hCD1d showed two different features: (i) more dispersed localization positions outside actin regions resulting from its dynamic exploration of the cell membrane and (ii) highly concentrated localization positions in actin-rich areas consistent with the arrest of the receptor in these regions (Fig. 4E). In contrast, no noticeable difference in terms of localization distribution with respect to actin was observed for α-GalCer–loaded TD-hCD1d (Fig. 4F), already suggesting that the dynamic exploration of α-GalCer–loaded TD-hCD1d is actin-independent. To quantify these differences, we applied a custom-made algorithm that calculates the relative fraction of hCD1d localization positions as a function of actin intensity changes in different regions, ∆actin (Materials and Methods and Fig. S4). Positive values of ∆actin represent actin-rich regions, whereas negative values reflect actin-poor regions; ∆actin = 0 corresponds to no actin intensity changes. Only THP-1 cells bearing similar expression levels of Lifeact-GFP were used for the analysis. WT-hCD1d displayed a clear linear relationship between the number of localization positions and the amount of actin, with a positive slope of 0.34 ± 0.03, indicating that the higher number of WT-hCD1d localization positions directly correlates with higher values of actin intensity (Fig. 4G). This increased number of spatial positions in actin-rich areas means that the receptor explores the same region multiple times, remaining spatiotemporally confined by actin. Together with the restricted mobility of WT-hCD1d observed on the actin-rich regions (Fig. 4D), these results prove that the actin cytoskeleton actively arrests WT-hCD1d nanoclusters on the cell membrane, preventing their additional aggregation. In marked contrast, the number of localizations positions for TD-hCD1d shows only a weak dependence on actin (slope of 0.07 ± 0.08) (Fig. 4G), showing no preferred interactions between TD-hCD1d and the actin cytoskeleton.
Fig. S4.
Quantification of cartography maps. At each given hCD1d localization position, two concentric circles of radii, R1 and R2, are drawn, and the numbers of localization positions and actin intensity signals in each circle are measured to derive the fractional occurrence of localization positions as a function of actin intensity changes between the two circles. (A) Representative magnified cartography map of the spatial localization positions explored by hCD1d with respect to actin. (B) Illustration of the methodology used for data quantification. Notice that, in this example, a large number of concentrated localizations are obtained on an actin-rich region delimited by the circle with radius R1, whereas more dispersed localizations are obtained within the circle of radius R2. (Scale bars: 1 μm.)
Perturbation of the Actin Cytoskeleton of APCs Results in Enhanced iNKT Cell Activation.
Given that perturbing actin cytoskeleton interactions resulted in enhanced α-GalCer–loaded hCD1d nanoclustering on APCs, we sought to address the consequences of such altered spatiotemporal organization on iNKT cell activation. APCs were pulsed with different α-GalCer concentrations and treated with CytoD. APCs were then fixed and incubated with iNKT cells. IFN-γ production on iNKT cell activation was measured by ELISA. Notably, CytoD-treated, hCD1d-tranduced THP-1 cells (Fig. 5A) and CytoD-treated immature dendritic cells (DCs) (Fig. 5B) elicited increased iNKT cell activation compared with APCs with an intact actin cytoskeleton. Moreover, this increase was more pronounced at low agonist concentrations. The increase in IFN-γ production was not caused by increased hCD1d expression on CytoD treatment (Fig. 5C). These results, thus, show that the actin cytoskeleton of APCs regulates the degree of iNKT cell activation. Interestingly, these data are fully in line with other reports, where nanoclustering of antigen-presenting proteins has been shown to more effectively enhance T-cell activation at low agonist densities (39).
Fig. 5.
Perturbation of the actin cytoskeleton on α-GalCer–pulsed APCs enhances iNKT cell activation. (A and B) IFN-γ production on iNKT cell activation by (A) hCD1d–transduced THP-1 cells or (B) immature dendritic cells (imDCs) pulsed with the given α-GalCer concentrations overnight before treatment with CytoD. (C) Expression levels of hCD1d as measured by FACS after treating THP-1 cells with CytoD (10 µM; 1 h). (D) IFN-γ production on iNKT cell activation by hCD1d THP-1 cells stimulated with 10 µg/mL TLR7/8 ligand R848 for 30 h before CytoD treatment. The unpulsed column refers to no lipid. ns, Not significant (P > 0.05; Student’s t test). *P < 0.05; •intensity value below the detection limit. Data are representative of three different experiments.
The Actin Cytoskeleton Regulates hCD1d Nanoclustering Under Inflammatory Conditions.
It has been described that innate stimuli, such as TLR stimulation, can trigger iNKT cell activation in a CD1d-dependent manner (8, 9, 28, 40). To test whether perturbation of the actin cytoskeleton of APCs exposed to inflammatory conditions also resulted in enhanced iNKT cell activation, we treated hCD1d-transduced THP-1 cells with the TLR7/8 ligand R848. After CytoD treatment and fixation, APCs were incubated with iNKT cells. Increased IFN-γ production by R848-matured, CytoD-treated WT-hCD1d THP-1 cells was observed, in line with the results with lipid-pulsed cells (Fig. 5D). Moreover, increased IFN-γ production was hCD1d-dependent, because blocking with an anti-CD1d Ab resulted in total abrogation of iNKT cell activation. Altogether, these results strongly indicate that the actin cytoskeleton of APCs controls the activation of iNKT cells under innate and adaptive stimuli.
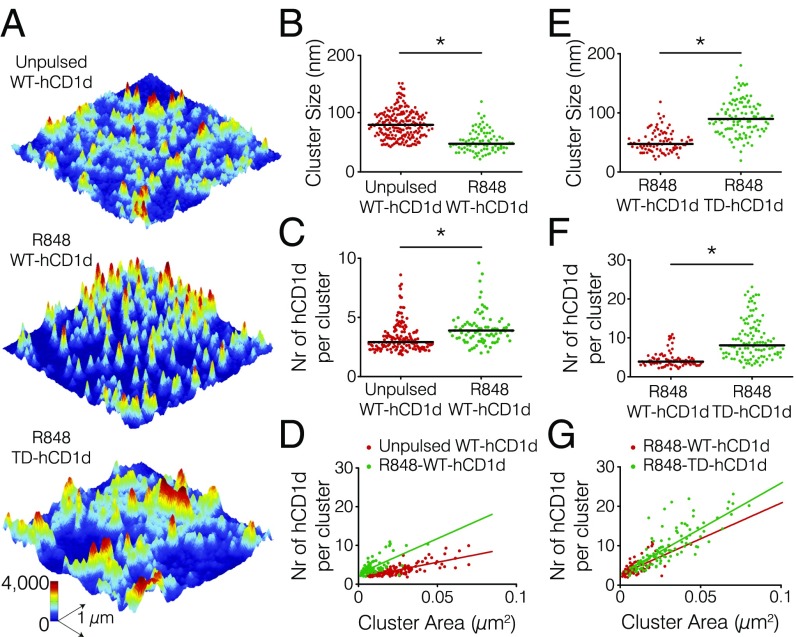
Because perturbation of the actin cytoskeleton of APCs resulted in enhanced iNKT cell activation on R848 treatment of THP-1 cells, we next addressed whether the interaction between the actin cytoskeleton and the cytoplasmic tail of hCD1d regulates hCD1d nanoclustering under inflammatory conditions, which we have shown for α-GalCer–loaded hCD1d. We stimulated both WT-hCD1d– and TD-hCD1d–transduced cells with the TLR7/8 ligand R848, performed STED nanoscopy (Fig. 6A), and analyzed hCD1d cluster size and composition. Interestingly, R848-stimulated WT-hCD1d formed smaller nanoclusters (47 nm as median cluster size) (Fig. 6B) than the unstimulated controls (77 nm as median cluster size). Moreover, those nanoclusters displayed a significantly higher number of hCD1d molecules (3.9 compared with 2.9 median number of unstimulated hCD1d molecules) (Fig. 6C), resulting in a strong increase of nanocluster density (180 ± 20 compared with 80 ± 5 hCD1d molecules per 1 μm2) of WT-hCD1d molecules under inflammatory conditions (Fig. 6D). Moreover, R848-stimulated TD-hCD1d nanoclusters had a larger size (90 compared with 47 nm) (Fig. 6E) and contained a higher number of molecules (8.1 compared with 3.9 hCD1d molecules) (Fig. 6F) than the WT counterpart, resulting in an increased nanocluster density of TD-hCD1d (231 ± 15 compared with 180 ± 20 hCD1d molecules per 1 μm2) (Fig. 6G) in analogy with the results obtained with α-GalCer-TD-hCD1d (Fig. 2 E–G).
Fig. 6.
The actin cytoskeleton regulates hDC1d nanoclustering under inflammatory conditions. (A) STED images of (Top) unpulsed WT-hCD1d, (Middle) R848-WT-hCD1d, and (Bottom) R848-TD-hCD1d on the dorsal cell membrane of THP-1 cells stretched on poly-l-lysine–coated glass coverslips. (B and E) Distributions of cluster size, (C and F) distribution of the number (Nr) of hCD1d molecules per cluster, and (D and G) scatterplots of the number (Nr) of hCD1d molecules per cluster vs. cluster area corresponding to the quantification of the STED images. STED nanoscopy analysis data from at least 30 different images of 3 × 3 μm in size from at least two different experiments. Horizontal bars correspond to median values. *P < 0.0001 (one-way ANOVA test).
These results highlight the important role of actin cytoskeleton interactions in controlling the extent of hCD1d nanoclustering on the cell membrane of APCs during inflammatory conditions. Moreover, because hCD1d undergoes lysosomal trafficking under inflammatory conditions in analogy to α-GalCer pulsing (28), these data provide additional support for the role of the interaction between the cytoplasmic tail of hCD1d and the actin cytoskeleton in modulating hCD1d nanoclustering after endosome recycling.
Discussion
We have for the first time, to our knowledge, characterized the spatiotemporal behavior of hCD1d on the surface of APCs at the nanoscale level. We found that hCD1d molecules organize in small nanoclusters on the cell membrane. Moreover, we observed that the actin cytoskeleton plays a major role in regulating the degree of hCD1d nanoclustering by segregating them away from each other. Treatment of THP-1 cells with CytoD or deletion of the hCD1d cytoplasmic tail led to larger nanoclusters and a concomitant reduction of hCD1d mobility. Furthermore, increased hCD1d nanoclustering directly correlated with enhanced iNKT cell activation. Notably, under inflammatory conditions, hCD1d formed denser nanoclusters, with organization that also depended on the actin cytoskeleton. These results are in remarkable analogy to the spatial organization of α-GalCer– and Gal-GalCer–loaded hCD1d molecules in THP-1 cells either treated with CytoD or expressing tail-deleted hCD1d molecules. Altogether, our results reveal a previously unidentified mechanism by which APCs regulate iNKT cell activation by fine-tuning the spatial organization of hCD1d on the cell membrane.
CD1d molecules are structurally related to the classical MHCI complex (41). Using superresolution near-field microscopy, clusters of MHCI from 70 to 600 nm in size were observed on the plasma membrane of fibroblasts (42). More recently, it was shown that such nanoclustering strongly depends on the actin cytoskeleton, which acts as a barrier to spatially concentrate MHCI molecules (24). Consistent with these observations, deletion of the cytoplasmic tail increased the lateral mobility of MHCI (43). Clustering of MHCII complexes on the plasma membrane of APCs has also been reported (44–46). Using SPT approaches, it was shown that MHCII displays hop diffusion on the cell membrane in a manner that it is dependent on the integrity of the actin cytoskeleton (47). Disruption of actin cytoskeleton interactions by either perturbing the actin network or truncating the MHCII cytoplasmic tail increased the mobility of MHCII (47, 48). In these experiments, the lateral organization of MHC molecules also had an impact on the extent of T-cell activation, with larger clusters enhancing peptide-specific T-cell effector functions, particularly at low antigen density (39). Notably, the role of actin on the spatiotemporal organization of MHCI and MHCII on the cell membrane of APCs is markedly different from the results reported here for hCD1d molecules. Although the actin cytoskeleton is essential to actively maintain the clustering of MHCI and MHCII, in the case of hCD1d molecules, perturbation of the actin cytoskeleton leads to enhanced aggregation of hCD1d molecules. Consistent with these findings and also, in contrast to MHCI and MHCII, disruption of the actin cytoskeleton or deletion of the hCD1d cytoplasmic tail results in reduced mobility of hCD1d nanoclusters. Altogether, our results indicate that hCD1d-mediated lipid antigen presentation is differently regulated at the cell surface of APCs compared with MHC-restricted peptide presentation. We speculate that the contrasting role played by the actin cytoskeleton in regulating MHC and hCD1d nanoclustering reflects an important functional difference between conventional peptide-specific T cells and innate-like lipid-specific T cells. Indeed, whereas peptide-specific MHCI- and MHCII-restricted T cells undergo extensive negative selection in the thymus (49) to eliminate the autoreactive peptide repertoire, peripheral iNKT cells retain the ability to react to self-lipids presented by CD1d molecules. This so-called “autoreactivity by design” (3) underpins the essential immunoregulatory role of iNKT cells.
Much research is being undertaken to understand how iNKT cell autoreactivity in the periphery is fine-tuned to prevent overt autoimmunity. Initial results suggested that β-anomeric self-lipids were the main self-ligands recognized by the iNKT cell autoreactive response (4) and that a combination of increased lipid biosynthesis, increased CD1d expression, and costimulatory cytokines was driving iNKT cell reactivity after APC activation (4, 8, 9). Costimulation by inflammatory cytokines is essential to achieve iNKT cell activation in these infectious settings, because extensive structural analysis revealed that the iNKT-TCRs interact with β-anomeric self-lipids CD1d complexes with low affinity (50). Recently, evidence has emerged for recognition of α-anomeric self-lipids bound to CD1d molecules (5, 6). Because the affinity of the iNKT-TCR for α-anomeric lipids is much higher than for β-anomeric CD1d–lipid complexes (50), an even tighter regulation of the availability of α-anomeric CD1d–lipid complexes is required. This tight regulation is accomplished, for example, through the concerted action of biosynthetic and catabolic pathways, which in turn, are modulated during inflammation (6). In addition to the affinity and CD1d occupancy of self-lipid antigens, our results underscore the importance of hCD1d clustering at the cell surface of APCs as an additional mechanism to fine-tune iNKT cell autoreactivity in peripheral tissues. Indeed, while at steady state, the actin network plays an important role in limiting basal iNKT cell autoreactivity, the observed increase in hCD1d nanocluster density upon TLR stimulation suggests that the actin cytoskeleton might also regulate iNKT cell activation under inflammatory conditions, increasing the overall avidity of self-lipid–loaded CD1d nanoclusters. This hypothesis is consistent with the previously reported increased staining of TLR-matured APCs with a soluble iNKT-TCR detecting self-lipid CD1d complexes (9).
Endogenous lipid-loaded CD1d molecules traffic from the ER to the cell surface by the Golgi system, where they acquire a variety of self-lipids (51). From the plasma membrane, CD1d molecules constitutively recycle through the endolysosomal compartment. A tyrosine-based internalization motif in the cytoplasmic tail initiates clathrin-dependent endocytosis, whereas association with the adaptor AP-2 and the Arf-like GTPase Arl8b controls lysosomal trafficking (52). It has been previously reported that the association of exogenous lipid-loaded CD1d with membrane lipid rafts on the plasma membrane of murine cells was dependent on the internal trafficking of CD1d molecules (26). Furthermore, it has also been shown that, on inflammatory stimuli, loading of self-lipids also requires trafficking of CD1d molecules through the lysosomal compartment (28). Our data extend these observations by showing that regulation of hCD1d nanoclustering by the actin cytoskeleton selectively differs between hCD1d molecules presenting either exogenous lipids, such as α-GalCer and Gal-GalCer, or self-lipids under inflammatory conditions and hCD1d molecules loaded with endogenous lipids hCD1d under resting conditions. These results are consistent with the hypothesis that regulation of hCD1d nanoclustering by the actin cytoskeleton selectively occurs on the pool of recycling hCD1d molecules that have trafficked backward and forward from the endolysosomal compartments to the cell surface rather than on the pool of hCD1d reaching the cell surface directly from the endoplasmic reticulum. It will be of interest to investigate how the spatiotemporal behavior of hCD1d might also be affected by the absence of lysosomal lipid transfer proteins, such as saposins, which have been shown to facilitate exogenous as well as self-lipid antigen presentation (28, 53).
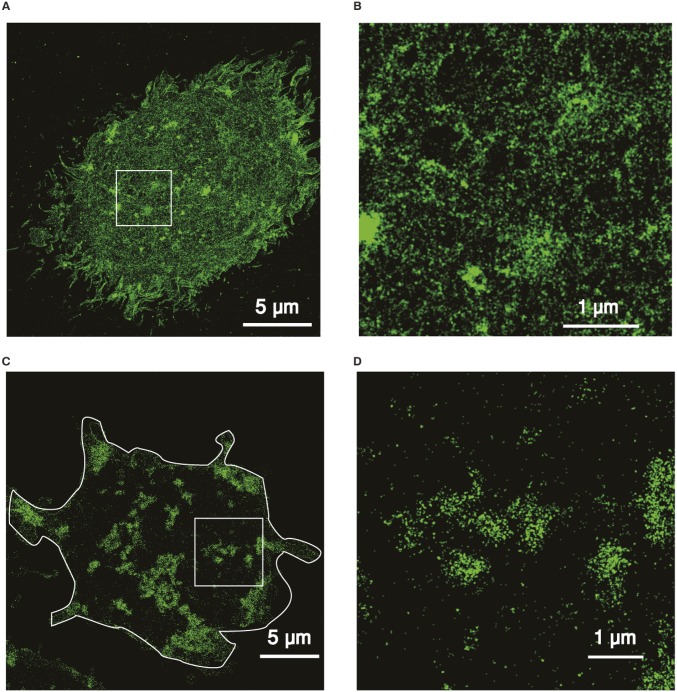
The general consensus on the emerging role of the actin cytoskeleton in regulating cell membrane organization is that it restricts the lateral diffusion of transmembrane proteins by creating temporal physical barriers close to the cell membrane (19). These barriers locally confine membrane receptors, increasing their local concentration and promoting clustering (23). In the case of hCD1d molecules, we observed similar arrest on its mobility in actin-rich regions. However, although hCD1d nanoclustering might be locally enhanced on certain hotspots of the cell membrane by actin, long-range hCD1d clustering is prevented by lowering the encountering probability of distant diffusing hCD1d nanoclusters. Thus, the actin cytoskeleton would effectively impose barriers and/or obstacles to the encountering of CD1d nanoclusters, limiting their aggregation state. Removal of these fences by actin disruption would then favor the encountering of CD1d nanoclusters, leading to larger clusters (Fig. 7). In support of this model, we have performed superresolution stochastic optical reconstruction microscopy (STORM) imaging of the actin cytoskeleton on THP-1 cells before and after CytoD treatment (Fig. S5). Although the intact actin cytoskeleton shows very fine structures with networks of tiny filaments and small compartments, treatment with CytoD led to a reduction of filament density and caused the formation of major actin aggregates separated by larger free spaces.
Fig. 7.
The actin cytoskeleton controls CD1d nanoclustering on the surface of APCs, fine-tuning iNKT activation. (A) Small basal nanoclusters of stimulating lipid-loaded CD1d molecules are present on the cell membrane of APCs. Barriers and/or obstacles by the actin cytoskeleton keep CD1d nanoclusters away from each other, limiting their aggregation state and regulating, in turn, iNKT cell activation, even under inflammatory conditions (iNKT cell autoreactivity). (B) Rearrangement of the actin cytoskeleton (achieved in our case by the use of CytoD treatment) increases the encountering rate between CD1d-1d nanoclusters, resulting in the formation of larger lipid-loaded CD1d nanoclusters and increased iNKT cell activation.
Fig. S5.
CytoD treatment strongly rearranges the actin cytoskeleton of THP-1 cells as revealed by direct (d)STORM. (A) Representative dSTORM superresolution image of the intact actin cytoskeleton of a THP-1 cell labeled with Alexa Fluor 647-Phalloidin. (B) Zoomed-in view of the region of the white box in A. (C) Representative dSTORM superresolution image of the actin cytoskeleton of a CytoD-treated (10 μM; 1 h) THP-1 cell. (D) Zoomed-in view of the region corresponding to the white box in C. The white contour lines in C delineate the boundaries of the cell.
These results might bring new insights on how the actin cytoskeleton fine-regulates the lateral behavior of membrane proteins at multiple temporal and spatial scales. Recent results have shown the importance of ezrin, radixin, and moesin (ERM) proteins in linking the transmembrane region of surface receptors, such as the B-cell receptor (BCR), to the actin cytoskeleton (20). Additional experiments are warranted to assess whether this family of proteins is also controlling the interaction between the CD1d cytosolic tail and the cortical actin cytoskeleton. Consistent with this possibility, it has recently been shown that ρ-kinase controls antigen presentation by CD1d molecules by prohibiting actin fiber depolymerization (27).
In conclusion, our results highlight a mechanism that APCs use to regulate lipid antigen presentation by CD1d molecules to modulate iNKT cell autoreactivity, a unique property that distinguishes this cell subset from conventional CD4+and CD8+ T cells. The observation that a similar actin-dependent mechanism regulates presentation of exogenous lipids and self-lipids on inflammatory stimuli further adds to the uniqueness of the CD1d-iNKT cell system in bridging innate and adaptive immune responses. Moreover, this study underscores the importance of emerging concepts, such as protein nanoclustering, in deepening our understanding of how leukocytes can fine-tune, at the molecular level, the outcome of an immune reaction. We predict that this type of studies will provide essential information for the future optimization of immune-based therapeutic strategies.
Materials and Methods
Antibodies and Reagents.
Monoclonal mouse anti-hCD1d (CD1d42) and monoclonal mouse anti-hCD71 (clone M-A712) antibodies were purchased from BD Pharmingen. Poly-l-lysine, CytoD, DMSO, Atto647N-NHS ester, and DTT were purchased from Sigma-Aldrich. Human fibronectin (FN) was purchased from Roche. SAV-coated QD655 and QD585, goat anti-mouse Alexa Fluor 488 Ab (A-11001), and d-biotin were purchased from Invitrogen. Maleimide-PEG2-Biotin and Slide-A-Lyzer MINI Dialysis Units were purchased from Thermo Scientific.
Cell Medium and Specific Reagents.
The complete medium (CM) used throughout this study was RPMI 1640 (Gibco) for THP-1 and IMDM (Gibco) for iNKT cells. CM was supplemented with 2 mM l-glutamine (Gibco), 1% nonessential amino acids (Gibco), 1% sodium pyruvate (Gibco), 1% pen/strep (Gibco), 5 × 10−5 2ME (Gibco) and serum [10% (vol/vol) FCS (Sigma-Aldrich) for THP-1 and 5% (vol/vol) Human AB Serum (Sigma-Aldrich) for iNKT cells]. Recombinant human IL-2 was produced in our laboratory as described (9).
Lipids.
α-GalCer and Gal-GalCer were synthesized using strategies described previously (9), and their structures were confirmed by MS. The dried lipids were dissolved at 10 mg/mL in a solution of chloroform:methanol:water (10:10:3; vol/vol/vol) followed by dilution in 150 mM NaCl and 0.5% Tween 20 (vehicle solution) at 100–200 μg/mL stock solution (depending on solubility). The solution was heated at 80 °C for 5 min followed by sonication for 5 min in an ultrasonic water bath.
Soluble iNKT-TCR heterodimers were generated as described (9).
Generation of iNKT Cells and DCs.
Blood was purchased from the UK National Blood Service. Human iNKT cells were isolated by cell sorting with CD1d-α-GalCer tetramers and/or Vα24 and Vβ11 antibodies (Immunotech) directly from peripheral blood mononuclear cells (PBMCs) or after expansion with autologous DCs pulsed with α-GalCer as described (28). iNKT cells were grown in CM [containing 5% (vol/vol) Human AB Serum instead of FCS] supplemented with 1,000 U/mL IL-2 and periodically restimulated. DCs were differentiated from magnetic-activated cell sorting (MACS)-purified CD14 monocytes from healthy blood donors as described (9).
Generation of THP-1 Cells Overexpressing hCD1d Constructs.
Full-length hCD1d was cloned in the pHR-SIN lentiviral vector. Lentivirus particles were made as described (28) and used to infect THP-1 cells (ATCC). TD-hCD1d, lacking the last 10 aa, was cloned in the pHR-SIN lentiviral vector using a strategy previously described (28). THP-1-CD1d cells were transduced with a lentivirus encoding Lifeact-GFP as previously described (9). GFP-positive cells were enriched by cell sorting.
iNKT Cell Stimulation Assays.
Immature DCs and THP-1-CD1d cells were plated at 50,000 cells per well in U-bottomed 96-well plates and pulsed overnight at the indicated concentration of lipids. In some experiments, cells were matured for 36 h with the TLR7/8 ligand R848 (5–10 μg/mL; Invivogen). Cells were extensively washed, treated for 1 h at 37 °C with CytoD used at 10 μM in HBSS (Gibco), and immediately fixed in glutaraldehyde (9). Fixed APCs were used to stimulate iNKT cells (20,000–30,000 cells per well in duplicate or triplicate). iNKT cell activation was assessed by IFN-γ ELISA (BD Pharmingen) on supernatants harvested after 36 h. When indicated, blocking anti-CD1d Ab (clone 42.1; BD Pharmingen) was added at 20 μg/mL 30 min before adding iNKT cells. Viability of APCs and hCD1d expression after CytoD treatment was assessed by flow cytometry on staining with anti-CD1d PE (BD Pharmingen) and propidium iodide (BD Pharmingen). Data were acquired on a Cyan DAKO Flow Cytometer and analyzed with Flowjo.
Labeling Conjugates for SPT Experiments in Living Cells.
Monovalent anti-hCD1d or -hCD71 Abs were prepared from CD1d42 and CD71 Ab by reduction with DTT following the manufacturer’s instructions. Reduced Abs were then biotinylated with Maleimide-PEG2-Biotin and quenched with Iodoacetamide. Unbound biotin was removed by overnight dialysis at 4 °C using Slide-A-Lyzer MINI Dialysis Units. To monitor each reaction step, a 4–12% (wt/vol) Bis-Tris gel under denaturing and nondenaturing conditions was performed.
Biotinylated iNKT-TCR or monovalent Abs were mixed with SAV-coated QD655 or QD585 in equimolar concentrations and stirred for at least 2 h at 4 °C. Excess of free biotin was added to the solution to ensure a single iNKT-TCR (10× excess biotin) or monovalent Ab (50× excess biotin) per QD.
Atto647N-NHS Ester labeling of the Fab9b (9, 29) was performed following the manufacturer’s instructions.
SPT.
hCD1d-transduced THP-1 cells were pulsed overnight with 50 ng/mL α-GalCer or 400 ng/mL Gal-GalCer. After 30 min of incubation on FN-coated coverslips at 37 °C and 5% (vol/vol) CO2, cells were labeled with 2 nM iNKT-TCR-QD655 conjugate for 5 min at room temperature. Cells were then washed with cell medium three times to remove unbound iNKT-TCR-QD655 conjugates. When labeling the cells with Ab-derived probes, 2% (vol/vol) human serum incubation was performed for 5 min at room temperature before adding monovalent antibodies or Fab fragments. Monovalent CD1d42-QD655 labeling was done at 0.2 nM concentration. Fab9b-Atto647N labeling was done at 10 nM concentration. On CytoD-treated samples, treatment (10 μM CytoD for 1 h) was performed before labeling on cells seeded on FN-coated coverslips for unpulsed hCD1d-THP-1 cells or after overnight pulsing with α-GalCer or Gal-GalCer. CytoD was maintained in the medium while imaging. CytoD controls were performed with DMSO diluted in the imaging medium with the same dilution used for CytoD experiments.
Fluorescence imaging was performed on the ventral side of the cell using a homemade single-molecule sensitive microscope working under internal reflection fluorescence geometry. Continuous excitation of the QDs was provided by the 488-nm line of an Ar+ laser (0.3 kW/cm2). Fluorescence was collected with a 1.4 N.A. oil immersion objective (Olympus) and guided into an electron-multiplying CCD camera (Hamamatsu) after suitable filtering. Movies S1–S4 were recorded at a frame rate of 100 Hz for single QDs and 10 Hz for Fab9b-Atto647N. For simultaneous dual-color measurements, the fluorescence-emitted light was split, selected with appropriate dichroic mirror and filters, and collected by the same electron-multiplying CCD camera (dual-color hCD1d imaging) or a second intensified CCD Pentamax Camera (dual-color hCD1d vs. Lifeact-GFP imaging). Images of multifluorophore fluorescent beads (0.2-µm Tetraspeck; Invitrogen), having emission spectrum covering the two spectral windows, were obtained to determine the spatial transformation leading to the overlay of the two spectral channels. To calculate the spatial transformation, at least 10 beads appearing on both channels were manually selected, and their centroid positions were calculated with subpixel accuracy and stored in two coordinate lists. The transformation matrix was inferred from the coordinate lists according to an affine transformation, correcting for displacement and small chromatic aberrations. For dual-color measurements of hCd1d, cells were labeled with an equimolar 2 nM concentration of two conjugates: iNKT-TCR-QD655 and iNKT-TCR-QD585. Simultaneous dual-color images of QDs were performed at a frame rate of 30Hz. Dual-color images of Lifeact-GFP and iNKT-TCR-QD655 were obtained on double-transduced Lifeact-GFP and WT-hCD1d or TD-hCD1d THP-1 cells that had been pulsed overnight with 50 ng/mL α-GalCer. Images were acquired at a frame rate of 60 Hz. Under these imaging conditions, no changes in the actin cytoskeleton were observed, and the actin intensity signal remained constant during the time of the experiments. Physiological conditions were maintained during the experiments using a temperature-controlled microincubation chamber (Warner Instruments) in combination with a constant 5% (vol/vol) CO2 supply (Okolab).
Single-Particle Trajectory Analysis.
Trajectories of individual QDs with at least 100 points were reconnected with Matlab routines based on an algorithm described in the work by Sergé et al. (54). Trajectories of individual Fab9b-Atto647N were manually reconnected with a minimum trajectory length of 19 points.
Individual trajectories were analyzed by calculating their mean squared displacement (MSD) according to the following equation (55):
| [1] |
where Δt is the time lag, N is the total number of frames of the trajectory, n represents the time increment, and x and y represent the 2D particle positions. Short-range diffusion coefficients were extracted from the linear fit to the second to fourth points of the MSD curve using the following equation:
| [2] |
where D2–4 is the instantaneous diffusion coefficient, and Δ0 is the MSD offset at zero time increment. The smallest detectable diffusion coefficient was obtained after imaging immobilized QDs or Atto647N dyes on a coverslip using TIRFM at the same frame rate as in the corresponding experiments: 100 and 10 Hz, respectively. Because in both cases, 95% of the immobile QDs displayed diffusion coefficients <1⋅10−3 µm2/s, this value was considered as the threshold for discriminating immobile vs. mobile trajectories. To avoid any overestimation of the immobile fraction caused by nonspecific interaction of QDs or Atto647N dyes with the coverslip, only trajectories classified as mobile were used for the trajectory analysis.
Dual-Color hCD1d Trajectory Analysis.
Two-color QD tracking of labeled hCD1d (WT and TD) nanoclusters was performed at 2 nM concentrations to increase the probability of finding nanocluster interaction events. 2D fluorescence trajectories of spatially close QDs (red, iNKT-TCR-QD655; green, iNKT-TCR-QD585) were generated, and the separation distance (interparticle distance) between QDs was determined from their dual-color pair trajectories by measuring the effective distance between the centroid positions of the diffusing QDs determined by fitting the point spread function (PSF) of the microscope with a Gaussian curve. Localization accuracy on the determination of the centroid position was 20 nm for both colors. The initial QDs separation distance considered was 350 nm, and interparticle distances were calculated at every frame for a total observation time of 1 s, yielding 30 data points per QD pair. Interparticle distances of all pairs at every frame were collected into a histogram. Monte Carlo simulations were performed to discriminate between random encounters of diffusing hCD1d nanoclusters or true enhanced interaction between hCD1d nanoclusters; 100 pairs of randomly diffusing nanoclusters were simulated, yielding 3,000 simulated interparticle distances per simulated pair. The instantaneous diffusion coefficient for each experimental condition was used for simulating the dual-color trajectories.
Dual-Color Trajectory Analysis of iNKT-TCR-QD–Labeled hCD1d on Lifeact-GFP–Labeled Actin.
A single Lifeact-GFP fluorescence image was bandpass-filtered in the frequency domain using standard ImageJ image processing. Only QDs that visited both low- and high-actin regions during the time course of an experiment (20 s) were manually tracked. The trajectories were then divided into 10 points segments, and the instantaneous diffusion coefficients of every segment were calculated as earlier described. The segments of the trajectories were visually classified to belong to either low- or high-actin regions after thresholding (>75%) the normalized Lifeact-GFP fluorescence images.
Generation of Time-Dependent Membrane Exploration Maps.
Time-dependent membrane exploration maps of iNKT-TCR-QD655–labeled hCD1d (WT and TD) with respect to actin were obtained as described in the work by Torreno-Pina et al. (37). In brief, we first obtained dual-color TIRFM images of iNKT-TCR-QD655 and Lifeact-GFP at a frame rate of 60 Hz for a total observation time of 1,200 frames. The position of each single QD with 20-nm localization accuracy was determined in every frame using a custom Matlab algorithm based on the work by Sergé et al. (54). The total number of QD localization positions in all of the frames was then collapsed in one single image and overlaid into one single Lifeact-GFP fluorescence image. Cells with an actin cytoskeleton that did not change over the time course of an experiment were carefully selected for generating cartography maps.
Quantification of hCD1d Localizations with Respect to Actin.
Quantification of the data was performed by generating two concentric circles with different radii, R1 and R2 (R1 = 225 nm and R2 = 1,035 nm), and having each time a given localization of the cartography map as the center for both circles (Fig. S4). The total number of localizations enclosed in each circle was quantified, with N1 being the total number of localizations enclosed by circle R1 and N2 = (no. of localizations in R2 − N1). Moreover, the mean raw fluorescence intensity value of actin (A1 and A2 from R1 and R2, respectively) enclosed by each radius was also extracted from the superimposition of the circles on top of the actin image. This procedure was repeated by positioning the center of the two concentric circles at each hCD1d localization value. The behavior of WT-CD1d and TD-CD1d molecules with respect to the actin cytoskeleton was extracted by plotting the relative fraction of hCD1d localizations (N1/N2) against (A1 − A2)/A1 = ∆actin. Only cells with a similar expression of Lifeact-GFP were selected for this analysis.
Sample Preparation for STED Nanoscopy.
To avoid any potential artifacts caused by CytoD treatment on membrane morphology that could affect the STED imaging, we exclusively focused on WT-hCD1d– or TD-hCD1d–transduced THP-1 cells. WT-hCD1d– or TD-hCD1d–transduced THP-1 cells were pulsed overnight with 100 ng/mL α-GalCer or 400 ng/mL Gal-GalCer. Alternatively, WT-hCD1d– or TD-hCD1d–transduced THP-1 cells were stimulated with 10 µg/mL R848 for 30 h. Cells were then stretched on poly-l-lysine–coated coverslips for 30 min at 37 °C with 5% (vol/vol) CO2. After cell fixation with 2% (wt/vol) paraformaldehyde, Fc receptors were blocked with 2% (vol/vol) human serum. CDd1 molecules on the cell membrane were labeled with 5 µg/mL anti-CD1d42 Ab. Fluorescent secondary Ab labeling was performed with a 5-µg/mL goat anti-mouse Alexa Fluor 488-labeled Ab. Isotype controls were performed to test the specificity of the labeling procedure.
STED Nanoscopy.
Confocal and STED images were obtained in a sequential manner using a 100× oil immersion objective (HCX PL APO 100×/1.4 N.A. Oil; Leica Microsystems) of a commercial CW-STED SP-7 Microscope (Leica Microsystems, Germany). The STED laser intensity at the focal plane on the upper cell membrane was 130 mW, and 1,024 × 1,024-pixel images were recorded at a scan speed of 1,400 Hz. In these conditions, no significant fluorescence photobleaching was observed. STED resolution was 85 nm as determined from the FWHM of sparse-labeled Abs on glass by fitting the PSF of the STED microscope using a Lorentzian curve.
Analysis of the STED Images.
STED images were analyzed by means of a previously published algorithm based on Bayesian inference (36). The algorithm essentially relies on the detection of fluorescence features and their fitting as a sum of different PSFs, with width and intensity distribution that are estimated from images of sparse markers. For each image, the localization positions of all of the retrieved PSFs were used to reconstruct an image that contains the molecular localizations belonging to each fluorescent feature of the raw STED image. The performance of such analysis has been previously tested on simulated images in a wide range of number densities, PSF widths, and signal-to-noise ratios (SI Text) (36). In our experimental conditions (number density = 5–50 μm−2; PSF width = 85 nm; signal-to-noise ratio = 15–20 dB), the algorithm is able to retrieve a large percentage (>80%) of particles and determine their location with high localization accuracy (∼20 nm). Cluster size and number of hCD1d molecules per cluster were then calculated from the reconstructed images using the pair correlation analysis (36). The pair correlation function g(r) was calculated on reconstructed images obtained by convoluting the particle localizations with 2D Gaussian functions having width equal to the localization accuracies. Each curve represents the average of at least 30 pair correlations obtained over 3 × 3-m2 regions of interest.
SI Text
Influence of the Bayesian Image Analysis on the Pair Correlation Functions.
Three main factors associated with Bayesian image analysis can affect the faithful image reconstruction and thus, indirectly, the estimation of parameters from the pair correlation function: the number of missed events, the false-positive identification rate, and the localization precision. Their effect has been extensively described in our previous publication (36). The number of missed events will depend on number density. In our experimental conditions (number density = 5–50 μm−2; PSF width = 85 nm; signal-to-noise ratio = 15–20 dB), the algorithm is able to retrieve a large percentage (>80%) of particles and determine their location with high localization accuracy (∼20 nm). Moreover, the analysis of STED images at different densities provided compatible values for the cluster sizes. As for the second factor, we have shown that our Bayesian algorithm provide a small and constant false-positive identification rate (36). Therefore, although it can have an impact on the absolute numbers, it does not largely affect relative changes between different conditions. The third factor is explicitly considered in the calculation of the pair correlation function and therefore, reflected in the fitting error.
Sample Preparation for Direct STORM Imaging of the Actin Cytoskeleton.
WT-hCD1d–expressing THP-1 cells were stretched on poly-l-lysine–coated coverslips. CytoD (Sigma-Aldrich) was added at a concentration of 10 µM and incubated for 1 h at 37 °C and 5% CO2. After cell fixation with 2% paraformaldehyde, THP-1 cells were permeabilized with 0.1% saponin (Sigma-Aldrich) at room temperature for 15 min. The actin cytoskeleton was stained by adding a 1:40 dilution of the Alexa Fluor 647-Phalloidin Stock Solution (A22287; Thermo Fisher Scientific) overnight at 4 °C in the dark. After thorough washing, the cells were kept in 2% paraformaldehyde.
Direct STORM Nanoscopy.
Imaging experiments were carried out with a commercial STORM microscope system from Nikon Instruments (N-STORM). Imaging was performed using a standard STORM imaging buffer containing 100 mM Cysteamine MEA, 0.5 mg/mL glucose oxidase, 40 μg/mL catalase, and 5% Glucose (all Sigma-Aldrich) in PBS. Laser light at 647 nm was used for exciting Alexa Fluor 647, and laser light at 405 nm was used for reactivating it. The emitted light was collected by an oil immersion objective (Apo internal reflection fluorescence 100×; 1.49 N.A.) filtered by an emission filter (ET705/72m) and imaged onto an electron-multiplying CCD camera at an exposure time of 15 ms per frame. STORM images were analyzed using custom-written software (Insight3; provided by Bo Huang, University of California, San Francisco, CA) by fitting the PSF of individual fluorophores with a simple Gaussian curve in every frame to determine the x and y coordinates.
Supplementary Material
Acknowledgments
We thank M. Rivas for technical assistance and M. Sixt for Lifeact-GFP plasmid. Stimulated emission depletion (STED) images were obtained at the Institut de Ciencies Fotoniques (ICFO)’s Super-Resolution Light Nanoscopy Facility (SLN@ICFO). Stochastic optical reconstruction microscopy (STORM) images were obtained at the Nikon Center of Excellence at the ICFO. This work was supported by Austrian Science Fund Grant FWF:J3045-B11 (to M.C.A.); Spanish Ministry of Economy and Competitiveness (Severo Ochoa Grant SEV-2015-0522 and Grant FIS2014-56107-R); Human Frontiers Science Program (HFSP) Grant GA RGP0027/2012; EC FP7-NANO-VISTA Grant GA 288263; LaserLab Europe Grant GA 284464; UK Medical Research Council, UK, Cancer Research UK Program Grant C399/A2291; and Wellcome Trust Grant 084923. G.S.B. acknowledges support from a Personal Research Chair from Mr. James Bardrick and Medical Research Council Grant MR/K012118/1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514530113/-/DCSupplemental.
References
- 1.Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014;32:323–366. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 2.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: Innate B and T lymphocytes. Nat Rev Immunol. 2001;1(3):177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 4.Brennan PJ, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12(12):1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan PJ, et al. Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction. Proc Natl Acad Sci USA. 2014;111(37):13433–13438. doi: 10.1073/pnas.1415357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kain L, et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian α-linked glycosylceramides. Immunity. 2014;41(4):543–554. doi: 10.1016/j.immuni.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou D, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306(5702):1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 8.Paget C, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27(4):597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Salio M, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci USA. 2007;104(51):20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhuri K, et al. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507(7490):118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dustin ML, Depoil D. New insights into the T cell synapse from single molecule techniques. Nat Rev Immunol. 2011;11(10):672–684. doi: 10.1038/nri3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vardhana S, Choudhuri K, Varma R, Dustin ML. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity. 2010;32(4):531–540. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson DJ, et al. Pre-existing clusters of the adaptor Lat do not participate in early T cell signaling events. Nat Immunol. 2011;12(7):655–662. doi: 10.1038/ni.2049. [DOI] [PubMed] [Google Scholar]
- 14.Manz BN, Jackson BL, Petit RS, Dustin ML, Groves J. T-cell triggering thresholds are modulated by the number of antigen within individual T-cell receptor clusters. Proc Natl Acad Sci USA. 2011;108(22):9089–9094. doi: 10.1073/pnas.1018771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310(5751):1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Parajo MF, Cambi A, Torreno-Pina JA, Thompson N, Jacobson K. Nanoclustering as a dominant feature of plasma membrane organization. J Cell Sci. 2014;127(Pt 23):4995–5005. doi: 10.1242/jcs.146340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 18.Chung I, et al. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464(7289):783–787. doi: 10.1038/nature08827. [DOI] [PubMed] [Google Scholar]
- 19.Andrews NL, et al. Actin restricts FcepsilonRI diffusion and facilitates antigen-induced receptor immobilization. Nat Cell Biol. 2008;10(8):955–963. doi: 10.1038/ncb1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treanor B, et al. The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity. 2010;32(2):187–199. doi: 10.1016/j.immuni.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morone N, et al. Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J Cell Biol. 2006;174(6):851–862. doi: 10.1083/jcb.200606007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low-Nam ST, et al. ErbB1 dimerization is promoted by domain co-confinement and stabilized by ligand binding. Nat Struct Mol Biol. 2011;18(11):1244–1249. doi: 10.1038/nsmb.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaqaman K, et al. Cytoskeletal control of CD36 diffusion promotes its receptor and signaling function. Cell. 2011;146(4):593–606. doi: 10.1016/j.cell.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavi Y, Gov N, Edidin M, Gheber LA. Lifetime of major histocompatibility complex class-I membrane clusters is controlled by the actin cytoskeleton. Biophys J. 2012;102(7):1543–1550. doi: 10.1016/j.bpj.2012.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segura J-M, et al. Increased mobility of major histocompatibility complex I-peptide complexes decreases the sensitivity of antigen recognition. J Biol Chem. 2008;283(35):24254–24263. doi: 10.1074/jbc.M803549200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Im JS, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30(6):888–898. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallo RM, et al. Regulation of the actin cytoskeleton by Rho kinase controls antigen presentation by CD1d. J Immunol. 2012;189(4):1689–1698. doi: 10.4049/jimmunol.1101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salio M, et al. Saposins modulate human invariant Natural Killer T cells self-reactivity and facilitate lipid exchange with CD1d molecules during antigen presentation. Proc Natl Acad Sci USA. 2013;110(49):E4753–E4761. doi: 10.1073/pnas.1310050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denkberg G, et al. Phage display-derived recombinant antibodies with TCR-like specificity against α-galactosylceramide and its analogues in complex with human CD1d molecules. Eur J Immunol. 2008;38(3):829–840. doi: 10.1002/eji.200737518. [DOI] [PubMed] [Google Scholar]
- 30.Di Rienzo C, Gratton E, Beltram F, Cardarelli F. Fast spatiotemporal correlation spectroscopy to determine protein lateral diffusion laws in live cell membranes. Proc Natl Acad Sci USA. 2013;110(30):12307–12312. doi: 10.1073/pnas.1222097110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugita M, et al. Failure of trafficking and antigen presentation by CD1 in AP-3-deficient cells. Immunity. 2002;16(5):697–706. doi: 10.1016/s1074-7613(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 32.Moody DB, Porcelli SA. Intracellular pathways of CD1 antigen presentation. Nat Rev Immunol. 2003;3(1):11–22. doi: 10.1038/nri979. [DOI] [PubMed] [Google Scholar]
- 33.Prigozy TI, et al. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291(5504):664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, et al. Distinct endosomal trafficking requirements for presentation of autoantigens and exogenous lipids by human CD1d molecules. J Immunol. 2007;178(10):6181–6190. doi: 10.4049/jimmunol.178.10.6181. [DOI] [PubMed] [Google Scholar]
- 35.Hell SW. Far-field optical nanoscopy. Science. 2007;316(5828):1153–1158. doi: 10.1126/science.1137395. [DOI] [PubMed] [Google Scholar]
- 36.Manzo C, et al. PSF decomposition of nanoscopy images via Bayesian analysis unravels distinct molecular organization of the cell membrane. Sci Rep. 2014;4:4354. doi: 10.1038/srep04354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torreno-Pina JA, et al. Enhanced receptor-clathrin interactions induced by N-glycan-mediated membrane micropatterning. Proc Natl Acad Sci USA. 2014;111(30):11037–11042. doi: 10.1073/pnas.1402041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riedl J, et al. Lifeact: A versatile marker to visualize F-actin. Nat Methods. 2008;5(7):605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson HA, Hiltbold EM, Roche PA. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol. 2000;1(2):156–162. doi: 10.1038/77842. [DOI] [PubMed] [Google Scholar]
- 40.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4(12):1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 41.Moody DB, Zajonc DM, Wilson IA. Anatomy of CD1-lipid antigen complexes. Nat Rev Immunol. 2005;5(5):387–399. doi: 10.1038/nri1605. [DOI] [PubMed] [Google Scholar]
- 42.Hwang J, Gheber LA, Margolis L, Edidin M. Domains in cell plasma membranes investigated by near-field scanning optical microscopy. Biophys J. 1998;74(5):2184–2190. doi: 10.1016/S0006-3495(98)77927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capps GG, Pine S, Edidin M, Zúñiga MC. Short class I major histocompatibility complex cytoplasmic tails differing in charge detect arbiters of lateral diffusion in the plasma membrane. Biophys J. 2004;86(5):2896–2909. doi: 10.1016/S0006-3495(04)74341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosch B, Heipertz EL, Drake JR, Roche PA. Major histocompatibility complex (MHC) class II-peptide complexes arrive at the plasma membrane in cholesterol-rich microclusters. J Biol Chem. 2013;288(19):13236–13242. doi: 10.1074/jbc.M112.442640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fooksman DR. Organizing MHC class II presentation. Front Immunol. 2014;5:158. doi: 10.3389/fimmu.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turley SJ, et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288(5465):522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 47.Umemura YM, et al. Both MHC class II and its GPI-anchored form undergo hop diffusion as observed by single-molecule tracking. Biophys J. 2008;95(1):435–450. doi: 10.1529/biophysj.107.123018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wade WF, Freed JH, Edidin M. Translational diffusion of class II major histocompatibility complex molecules is constrained by their cytoplasmic domains. J Cell Biol. 1989;109(6 Pt 2):3325–3331. doi: 10.1083/jcb.109.6.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: What thymocytes see (and don’t see) Nat Rev Immunol. 2014;14(6):377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12(12):845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fox LM, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7(10):e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barral DC, Brenner MB. CD1 antigen presentation: How it works. Nat Rev Immunol. 2007;7(12):929–941. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- 53.Kang S-J, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5(2):175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- 54.Sergé A, Bertaux N, Rigneault H, Marguet D. Dynamic multiple-target tracing to probe spatiotemporal cartography of cell membranes. Nat Methods. 2008;5(8):687–694. doi: 10.1038/nmeth.1233. [DOI] [PubMed] [Google Scholar]
- 55.Kusumi A, Sako Y, Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys J. 1993;65(5):2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.