Significance
Huntington’s disease is a devastating and incurable inherited neurodegenerative disease. Like at least eight other diseases, its primary genetic cause is the CAG repeat expansion in a specific gene. Mutant huntingtin protein undergoes misfolding and aggregation, causing degeneration of neurons through as-yet poorly understood mechanisms. Attempts to characterize the implicated protein deposits have until now had limited success. We present our structural studies of mutant huntingtin-derived protein deposits by advanced solid-state NMR spectroscopy. We determine the essential structural features of the fibrils’ rigid core, which is shown to feature intramolecular β-hairpins tied together via interdigitating extended side chains. These structural insights have direct implications for the mechanism by which the mutant protein misfolds and self-assembles.
Keywords: solid-state NMR, Huntington's disease, amyloid disease, protein aggregation, amyloid
Abstract
Polyglutamine expansion within the exon1 of huntingtin leads to protein misfolding, aggregation, and cytotoxicity in Huntington’s disease. This incurable neurodegenerative disease is the most prevalent member of a family of CAG repeat expansion disorders. Although mature exon1 fibrils are viable candidates for the toxic species, their molecular structure and how they form have remained poorly understood. Using advanced magic angle spinning solid-state NMR, we directly probe the structure of the rigid core that is at the heart of huntingtin exon1 fibrils and other polyglutamine aggregates, via measurements of long-range intramolecular and intermolecular contacts, backbone and side-chain torsion angles, relaxation measurements, and calculations of chemical shifts. These experiments reveal the presence of β-hairpin–containing β-sheets that are connected through interdigitating extended side chains. Despite dramatic differences in aggregation behavior, huntingtin exon1 fibrils and other polyglutamine-based aggregates contain identical β-strand–based cores. Prior structural models, derived from X-ray fiber diffraction and computational analyses, are shown to be inconsistent with the solid-state NMR results. Internally, the polyglutamine amyloid fibrils are coassembled from differently structured monomers, which we describe as a type of “intrinsic” polymorphism. A stochastic polyglutamine-specific aggregation mechanism is introduced to explain this phenomenon. We show that the aggregation of mutant huntingtin exon1 proceeds via an intramolecular collapse of the expanded polyglutamine domain and discuss the implications of this observation for our understanding of its misfolding and aggregation mechanisms.
The misfolding and aggregation of proteins is a common, but as-yet poorly understood, cause for human disease. One family of protein misfolding diseases involves the expansion of CAG repeats in specific genes (1). Beyond a threshold value, increasing CAG repeat lengths correlate to decreasing age of pathological onset and increasing toxicity. The most prevalent example is Huntington’s disease (HD), an incurable neurological disorder that impacts motor and cognitive abilities and is ultimately fatal. In HD, the expansion affects a polyglutamine (polyQ) domain near the N terminus of the huntingtin (htt) protein causing protein misfolding, N-terminal fragmentation, and aggregation. Much attention has focused on the N-terminal fragment coinciding with htt exon1 (Fig. 1A) because it is generated in vivo and induces HD-like disease pathology in mouse models (2, 3). Htt exon1 is known to misfold and self-assemble via a series of aggregated species, including spherical oligomers, protofibrils, mature amyloid fibrils, and large fibril clusters (3–6). Although some studies suggested that large inclusions are nontoxic and may be protective (7), recent work also revealed the presence in cells of smaller amyloid aggregates of exon1 that are not visible in normal fluorescence microscopy (8) and specific toxic cellular events triggered by inclusions (9). Despite their potential importance, only limited atomic resolution structural data are available on the fibrillar aggregates that are formed by htt exon1 or other polyQ-based proteins or peptides (10). Although it is generally accepted that the mature fibrils feature antiparallel β-sheets, there continue to be conflicting models not only for their fibrillar structure, but also for the specifics of the multistage pathway by which they are formed. For instance, intramolecular polyQ-based β-hairpins are both proposed to be either nuclei that initiate the rapid formation of β-hairpin–based fibrils (11) or semistable monomeric or oligomeric species that at least transiently resist progression to fibrils (12). Concrete structural data on the fibril’s internal structure are essential prerequisites for a truly molecular understanding of the way in which mutant htt exon1 and other polyQ disease proteins misfold and aggregate.
Fig. 1.
Huntingtin exon1 construct design and fibril formation. (A) Sequence of the used MBP-htt exon1 fusion protein. The exon1 sequence, factor Xa cleavage site, and location of the fibrils’ rigid amyloid core (16) are indicated. (B–E) Negatively stained TEM as a function of time after factor Xa release of unlabeled exon1. (B) Uncleaved htt exon1 MBP fusion protein. (C) Oligomers observed 1 h after cleavage. (D) By 3 h, fibrils have begun to form. (E) After 25 h, fibrils have grown and oligomers are no longer visible on the grid. (F) Mature [U-13C,15N]-labeled fibrils prepared for ssNMR. (Scale bars: 200 nm.)
Magic-angle-spinning (MAS) solid-state NMR (ssNMR) spectroscopy has developed into an essential tool for the determination of amyloid fibril structure (13, 14). We previously used MAS ssNMR to elucidate the domain structure of aggregated htt N-terminal fragments, in which we identified the rigid amyloid core (15, 16). This polyQ-based amyloid core was found to have an unusual spectral signature that is also shared by other polyQ aggregates (11, 15, 17). However, until now, no ssNMR-based structural constraints on the htt exon1 amyloid core were available. Here, we present structural ssNMR measurements on the exon1 amyloid core and compare it to polyQ peptide fibrils. We show that two kinds of β-strands make up the core assembly, where they engage in intimate intraprotein interactions. Interactions between β-sheets involve steric-zipper–like side-chain interdigitation (18), based on side-chain torsion angle measurements and other structural and dynamic constraints. Implications of the β-hairpin–based core structure for the misfolding and aggregation pathways followed by expanded polyQ domains in context of htt exon1 and beyond are discussed.
Results
The β-Sheet–Based Huntingtin exon1 Fibril Core.
Mutant htt exon1 with an expanded 44-residue polyQ domain was expressed as a maltose-binding protein (MBP) fusion construct (Fig. 1A) (5, 16). Cleavage with factor Xa releases exon1, which first forms oligomeric aggregates (Fig. 1C), and later amyloid-like fibrils (Fig. 1 D and E). The MAS ssNMR spectrum in Fig. 2A shows the Gln 13C signals of the rigid amyloid core of uniformly 13C and 15N (U-13C,15N) labeled exon1 fibrils, which have a width of ∼15 nm (Fig. 1F). Colored lines mark the Gln peaks of two types (“a” and “b”) of rigid core residues present in equal amounts. Signals from the partly mobile flanking domains outside the amyloid core (15, 16) are not marked. Despite reports of temperature-dependent polymorphism (19), no difference is seen between the polyQ core signals of fibrils formed at room temperature and 37 °C (SI Appendix, Fig. S1). A much smaller set of signals (type “c”) is observed for Gln outside the amyloid core (16, 17) (SI Appendix, Fig. S1). In “backbone walk” spectra, we correlate the 15N and 13C signals of sequential residues (Fig. 2 B and C). The intraresidue and interresidue correlation spectra are identical (Fig. 2D), which implies that each Gln is preceded and followed by residues of the same type. No evidence of direct a-b connections is observed. Thus, the exon1 fibril core must contain some combination of two structurally distinct types of uninterrupted polyQ tracts, each of which is exclusively comprised of one of two distinct Gln conformers that differ in their NMR signals (Fig. 2E).
Fig. 2.
MAS NMR on the polyQ core of mature huntingtin exon1 fibrils prepared at room temperature. (A) Two-dimensional 13C-13C spectrum shows the two sets of Gln peaks (type a and b) that account for the rigid amyloid core (red and blue lines). (B) An intraresidue NCACX spectrum connects 13C signals to their own backbone 15N. (C) An interresidue NCOCX spectrum connects the 13C signals to the 15N backbone shift of the next Gln. (D) Overlay of B and C. (E) The identical NCACX and NCOCX spectra show that connected Gln always have the exact same chemical shifts. No direct backbone connections between a and b are observed.
SI Appendix, Fig. S1 shows that these NMR signals are indistinguishable from those of “simple” polyQ fibrils without htt flanking domains (11, 15–17). Previous studies have noted that these chemical shifts are indicative of β-sheet rather than α-helical or random coil structure. However, some argue that polyQ aggregation may involve a nonstandard secondary structure, known as α-sheet, which may be indistinguishable from β-sheet by its NMR shifts (20, 21). To test this assertion, we did chemical shift-independent torsion angle measurements (22) and found unambiguous evidence for a β-sheet conformation. We used “NCCN” experiments that probe the relative orientation of Ni-Ciα and Ci’-Ni+1 dipolar coupling vectors and, thus, report on |ψ| (the magnitude of the ψ torsion angle; SI Appendix, Fig. S2) (23). We labeled two sequential Gln within a polyQ peptide, where both feature the amyloid core ssNMR signals (SI Appendix, Fig. S1 H and I). We obtained NCCN measurements for each of these signals (Fig. 3 A and B and SI Appendix, Fig. S2) and find that type-b Gln have |ψ| = 152 ± 2°. This result unambiguously contradicts an α-sheet–based assembly in which residues would occupy the α and Lα regions (Fig. 3C). The NCCN measurement and chemical shift analysis (24) agree on the β-strand conformation (Fig. 3C), similar to prior ssNMR studies of amyloid structure (25, 26). The type-a Gln have a different NCCN signal from type-b Gln (Fig. 3A). It also fits a β-sheet structure although, in this case, not uniquely so. We conclude that it reflects a β-strand structure based on agreement from chemical shift analysis (Fig. 3C) and the fact that the two conformers coassemble in the amyloid core (see below). Thus, the doubled ssNMR peaks of the htt exon1 fibril core and other polyQ amyloids are due to two equally populated, but structurally distinct β-strand types.
Fig. 3.
PolyQ amyloid structure and dynamics by MAS NMR. (A and B) Intersections (circled) of experimental NCCN data (horizontal lines) with the theoretical dependence on ψ (black line). Shaded areas indicate the SE. (C) Consensus of experimental backbone angles for polyQ amyloid, based on chemical shift analysis (diamonds) and ψ-angle measurements (colored lines and shaded areas) for conformers a (red) and b (blue). (D) Simulated HCCH curves for distinct χ2 side-chain angles. (E) Experimental χ2-sensitive HCCH data (Cβ/Cγ) for conformers a (red diamonds) and b (blue triangles), along with the theoretical χ2 = 180° curve (solid line). (F) χ1-sensitive HCCH data, with simulated curves for χ1 = −65° and 55° (lines). (G) R1ρ and R1 13C relaxation for backbone and side chains of a Gln in polyQ amyloid, measured at 60 kHz MAS. (H and I) Nitrogen-15 R1 and R1ρ values for Gln in polyQ amyloid (right) and in GB1 protein crystals (left), showing a striking difference for the side-chain Nε.
The htt exon1 Core β-Strands Form Intramolecular β-Hairpins.
We probed for interactions between these two types of β-strands by recording a 13C-13C spectrum with a longer 13C-13C polarization exchange time (Fig. 4 A and B). In this spectrum with 250-ms proton-driven spin diffusion (PDSD) mixing, we observe many strong cross-peaks between the two sets of Gln peaks. Both in the literature (27–29), and in reference experiments (SI Appendix, Fig. S3A), this mixing time allows for detectable signal transfer over up to ∼7 Å. Many of the “interform” (i.e., between a and b conformers) peaks are even visible at shorter PDSD mixing times (SI Appendix, Fig. S3D). We see extensive transfer between backbones, from backbone to side chains and vice versa, which implies that the two β-strand types are in intimate contact with each other and form a “composite” amyloid core (30).
Fig. 4.
Intramolecular and intermolecular β-strand–β-strand interactions within the htt exon1 core. (A) Extended (250 ms) 13C-13C mixing 2D spectrum on exon1 fibrils with complete 13C labeling. (B) Enlargement showing strong cross-peaks between the a and b Gln conformers. (C) Analogous 2D spectrum on diluted (26%) 13C-labeled fibrils (Materials and Methods), which also shows significant cross-peaks between the a and b signals. (D–F) One-dimensional slices extracted from the 2D spectra for polarization transferred from Cα, C′, and Cδ carbons of each Gln conformer (circled labels). Peaks due to transfer to the other β-strand type are marked (color-coded labels). These spectra were obtained at 800 MHz (1H) and with 13 kHz MAS. (G) Normalized volumes for cross peaks between the a and b strands in the mixed fibrils (C).
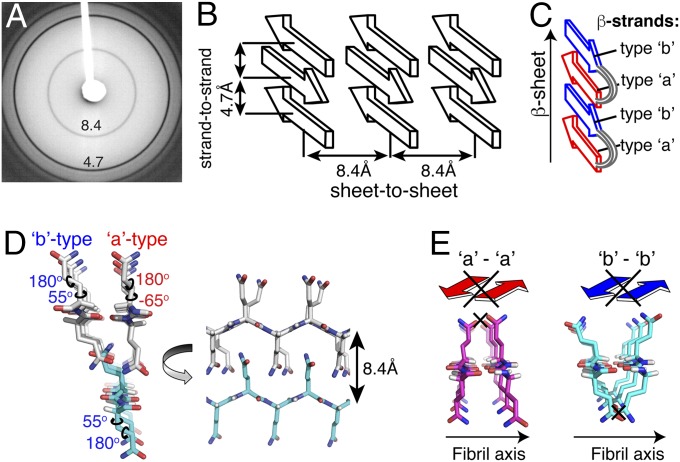
An in-depth structural analysis of these data is difficult, because in fully labeled amyloid fibrils (Fig. 4 A and B), the peaks reflect both interactions within a particular protein as well as those between different proteins. Thus, we prepared fibrils from a mix of 13C-only– and 15N-only–labeled exon1. Here, one expects that 13C-13C polarization transfer between 13C sites within each 13C-labeled monomer will be unchanged, but 13C-13C contacts between proteins will be suppressed. Using fibrils containing 26% 13C- and 74% 15N-labeled protein, we repeat the 13C-13C experiment (Fig. 4C). Even with the fourfold 13C dilution, cross-peaks between the a/b conformers are still observed. These peaks can be seen clearly in 1D slices (Fig. 4 D–F) comparing the fully labeled and mixed fibrils, both for polarization transfer from backbone (circled Cα and C′) or side-chain carbons (Cδ; circled). Peaks reflecting transfer across to the other β-strand (e.g., a to b or vice versa) are specifically marked. For more quantitative insights, we measured the 2D peak intensities and found the interform peaks to reflect 2–8% of the originating 13C signal (Fig. 4G and SI Appendix, Fig. S3B). In analogous data on a reference sample of known structure, such peak volumes correspond to distances up to 6.5 Å (SI Appendix, Fig. S3A). Based on the cross-β X-ray pattern of polyQ (Fig. 5 A and B and refs. 31 and 32), the backbones of β-sheets are separated by 8.4 Å. Then, the observed backbone-to-backbone interactions cannot be contacts between β-sheets, but must reflect interactions within a β-sheet. More precisely, they must occur between neighboring β-strands that are 4.7 Å apart (Fig. 5). Given that these are isotopically diluted fibrils, these neighboring a and b strands must be part of the same protein monomer (SI Appendix, Fig. S3A), forming an intramolecular β-hairpin (Fig. 5C).
Fig. 5.
PolyQ amyloid structure. (A) X-ray powder diffraction on hydrated K2Q31K2 fibrils shows the cross-β dimensions of polyQ amyloid. (B) The cross-β dimensions reflect repeat distances between β-strands (4.7 Å) and between β-sheets (8.4 Å). (C) The intraprotein 13C-13C contacts between a and b Gln backbones are too short to occur between sheets and are therefore between β-strands (color-coded by type) within a β-sheet. (D) β-strand structures that fulfill the torsion angle constraints, close proximity of side-chain Cδ to the backbone, and allow hydrogen bonding of both backbones and side chains. Extended side chains form a steric zipper interface to allow the 8.4-Å sheet-to-sheet distance. (E) The a and b strands are mutually compatible, but a–a or b–b interactions are not possible.
The similar cross-peak patterns in 13C-13C spectra of fully and mixed-labeled fibrils shows that the interactions within and between proteins must be similar. To selectively probe for the latter category of contacts, we looked for the dominant 13C-15N contacts in the 13C/15N mixed fibrils, using an “NHHC” experiment (SI Appendix, Fig. S4A; ref. 33). We found much stronger peaks for the side-chain nitrogens than for the backbones, which indicates that backbone-backbone contacts occur more commonly within proteins, whereas side chains mediate extensive interactions between proteins.
Interdigitation of the Extended and Rigid Gln Side Chains.
Among the dominant side-chain Nε contacts in the NHHC spectrum, we see close contact between one protein’s side-chain Nε and the backbone Cα of another protein (SI Appendix, Fig. S4A). The 13C-13C spectra also show strong peaks between the end of side chains (Cδ) and backbone carbons Cα and C′, in particular in the absence of isotopic dilution (Fig. 4 and SI Appendix, Fig. S3D). These data fit well to an interdigitated steric-zipper interface that places the side chain termini of one β-sheet close to the backbone of a neighboring β-sheet (18, 30). We tested for other characteristic features of steric zippers. First, we adapted a “HCCH” experiment previously used to measure a molecular torsion angle in rhodopsin’s retinal (34) to measure side-chain torsion angles in polyQ amyloid with one or two U-13C,15N Gln. We obtained χ2-sensitive HCCH data that were indistinguishable for the two β-strand types (Fig. 3E and SI Appendix, Fig. S5H) and show that χ2 = 180 ± 15° in both β-strands. This angle is inconsistent with bent side chains (SI Appendix, Fig. S6A; ref. 35) but fits a steric zipper (Fig. 5D). We also obtained χ1-dependent HCCH data, which were found to significantly differ between the a and b Gln (Fig. 3F and SI Appendix, Fig. S5G), showing that a and b β-strands differ in their side-chain structure. The best fitting χ1 angles are −65° for conformer a and 55° for conformer b, although reduced χ2 analysis cannot exclude other rotamers (SI Appendix, Fig. S5). We probed polyQ side-chain motion via 15N R1, 15N R1ρ, 13C R1, and 13C R1ρ relaxation measurements, in part enabled by ultrafast MAS (36, 37) (Fig. 3 G–I and SI Appendix, Fig. S5I). These results show the side chains to have a rigidity characteristic of their confinement in a steric zipper amyloid core (18, 30). Thus, the polyQ backbone in the amyloid core relaxes as slowly as the rigid backbone of the crystalline globular protein GB1 (e.g., Fig. 3 H and I). Remarkably, compared with Gln side chains in GB1 crystals, the side chains in the polyQ amyloid core exhibit relaxation similar to that of the backbone (Fig. 3 H and I), suggesting a lack of interaction with the solvent consistent with a “dry” interface. Altogether, the ssNMR data all point to an interdigitated steric-zipper–like structure, as illustrated in Fig. 5D.
Discussion
The htt exon1 polyQ Amyloid Core Features β-Sheets Interacting via Steric Zippers.
Using MAS ssNMR, we examined the structure of the polyQ amyloid core of htt exon1 fibrils and other polyQ aggregates. The exon1 amyloid core features equal populations of two specific types of Gln signals that are identically reproduced in amyloid fibrils formed by shorter N-terminal htt fragments and polyQ model peptides (SI Appendix, Fig. S1). MAS ssNMR torsion angle measurements showed that these doubled signals are not from α-sheets, but reflect two β-strand types with different backbone and side-chain χ1 torsion angles. They do share an identical χ2 angle (of 180°), having extended side chains. On the basis of polyQ’s cross-β parameters and various complementary ssNMR measurements, we found these side chains to interdigitate to form a steric-zipper interface between β-sheets.
The Antiparallel polyQ β-Sheets Contain Intramolecular β-Hairpins.
The 13C-13C spectra showed that the a- and b-type β-strands are in intimate contact, even when mixed with a majority of protein without 13C labels. Thus, the contacts represent interactions within a single protein, rather than between different proteins. These interactions include intimate (<6.5 Å) interactions between the backbones of the two β-strands, which cannot be between β-sheets (which are farther apart; Fig. 5 A and B). Thus, these intimate backbone-to-backbone distances must occur between neighboring polyQ β-strands in the same β-sheet. Given the lack of long loops or turns (see below), the only way to see such contacts within a single polypeptide chain is in the form of β-hairpins. To the best of our knowledge, this experiment presents the first direct evidence that β-hairpins are a prominent building block of the amyloid core of htt exon1 fibrils.
A β-hairpin–based structure implies that the fibrils must contain Gln in β-turns in addition to those forming β-strands. As noted above, the ssNMR signals of the exon1 fibril core are so strongly dominated by the β-sheet amyloid signals, that it is hard to analyze the ssNMR signals from the turn regions. This challenge is compounded by likely structural heterogeneity in these turns, as predicted by our mechanistic model introduced below and observed in polyQ with widely studied (11, 12, 38) β-hairpin–stabilizing mutations (SI Appendix, Fig. S7). The c-type Gln are the best candidates for the turn structure (16), and they constitute at most ∼10% of the total Gln signal. This observation is qualitatively similar for polyQ amyloid lacking htt’s flanking domains (17). If ∼90% of a 44-residue exon1 polyQ domain forms β-strands, and considering that β-turns usually span four or more residues (39), then the implication is that perhaps just a single turn region occurs per protein. Kinetic studies indicate that polyQ segments down to 26 residues in length aggregate via a β-hairpin–based monomeric nucleus, suggesting a minimal β-strand length of ∼11 residues (11, 40). Although 20-residue β-strands might seem unusually long, they fit easily within the exon1 fibril width (∼15 nm; Fig. 1F).
The polyQ Amyloid Core Building Block.
The obtained backbone torsion angles allow the construction of two β-strands with slightly different backbone conformations that are able to align with each other and form hydrogen bonds in an antiparallel fashion. Because residues within each β-strand have identical chemical shifts, it is most likely that all residues within each β-strand have the same torsion angles (in both backbone and side chain). The side-chain dihedral angles, relaxation, and 13C-13C correlation constraints were then used to construct the model shown in Fig. 5D. It features two different, but structurally compatible β-strands. The model uses the best-fit χ1 angles, which (as discussed above) are not unique solutions in the absence of other constraints. The obtained extended side chains are able to form an interdigitated interface between β-sheets consistent with the observed 13C-13C and 13C-15N contacts, as well as the (relatively short) β-sheet repeat distance of polyQ (Fig. 5A). The residues form a Gln “ladder” within each β-sheet, which was assumed to set the (as yet unconstrained) χ3 angle to allow hydrogen bonding between stacked side chains.
Chemical Shift Signature of the polyQ Amyloid Structure.
The ssNMR signals of polyQ amyloid are highly unusual and seemingly unique (11). A good structural model should rationalize this ssNMR signature. The most striking feature is that Cβ and Cγ carbons have highly unusual chemical shifts (11). Gln in a few globular proteins reproduce some of the shifts (17), but these residues are typically surface exposed, dynamic, and sample widely varying side-chain conformations. We submitted different polyQ models to ab initio 13C chemical shift calculations. The absence of side-chain motion (Fig. 3 G–I) and lack of aromatic residues render the polyQ amyloid core particularly amenable to this kind of analysis, at least for nonhydrogen-bonding carbons. We calculated 13C chemical shifts for reference compounds and different polyQ models (18, 32, 35, 41, 42) (SI Appendix, Tables S3–S4). The latter fail to reproduce the experimental ssNMR results (SI Appendix, Fig. S6), with the exception of our ssNMR-derived model (Fig. 5D). It predicts identical shifts throughout each β-strand, atypically small chemical shift differences between Cβ and Cγ in both β-strands, and Cβ/Cγ shifts for strand a that are several ppm higher than those of strand b. Thus, our model reproduces the shift patterns and rationalizes the presence of two distinct ssNMR signals at equal intensities.
PolyQ’s Intrinsic Peak Doubling Explained by a Stochastic Assembly Mechanism.
Despite the agreement between this model and our data, the model does not explain an intriguing feature of the polyQ signature: The peak doubling is also seen when just a single residue is labeled (Fig. 6A and SI Appendix, Fig. S1) (11, 15, 16). Thus, in half of the proteins in the sample, this Gln residue is present in an a-type β-strand, and in the other half it is part of a b-type β-strand. We propose that this intrinsic polymorphism is a universal feature of polyQ amyloids and reflects an aggregation mechanism that is stochastic during nucleation, fibril elongation, or both. The β-hairpin–based fibril structure implies that elongation must involve β-hairpin formation. During elongation, incoming proteins add to the exposed β-strands of the fibril ends. Fig. 6B schematically shows an exposed b-type β-strand. A section of the incoming polyQ domain then must form the other β-strand type (i.e., type a) as it binds, because same-to-same interactions are not allowed due to constraints on pairing of hydrogen bonding in the backbone and side chains (Fig. 5E). Although this step may thus appear deterministic, stochastic assembly arises from the degeneracy of the polyQ sequence: Different sections of the incoming polyQ domain are equally capable of being the initial point of interaction. This model is schematically visualized in Fig. 6B for a polyQ domain forming a single β-hairpin. A single labeled Gln near the N terminus randomly ends up in either of the two β-strand configurations, leading to that one residue showing both peaks at a 1:1 ratio, as observed experimentally.
Fig. 6.
Stochastic polyQ β-sheet assembly mechanism. (A) Peak doubling is seen for a single-labeled Gln in the amyloid core of a fibrillar htt N-terminal fragment (15). The specific Gln (Q19) is distributed between both β-strand types, shown with their schematic ssNMR spectra. (B) Schematic fibril core containing β-sheets with 2n β-strands (Top). Elongation maintains the alternating β-strand pattern, but can be initiated by different segments of the polyQ domain, which then causes the N-terminal labeled Gln (circles) to end up in both β-strand types (Bottom). (C) During nucleation, an a–b β-strand assembly can be formed in two ways, yielding related but distinct β-hairpin structures. The circles mark a single labeled Gln nearer the N terminus.
We hypothesize that even the formation of the initial elongation-capable structure could be stochastic. Mechanistic studies suggest β-hairpin formation to be critical in the nucleation process for long polyQ (11, 40). We propose that this could involve formation of not one specific β-hairpin structure, but rather the stochastic formation of structurally related, but nonetheless different, β-hairpins. They would be structurally related by always ending up with the complementary a and b β-strands, while being different in the ways that those strands are arranged. An N-terminal a-type β-strand can be combined with a more C-terminal b-type strand, or vice versa (Fig. 6C), with likely minimal energetic or kinetic differences.
Thus, we propose mutant htt exon1 and other polyQ peptides to follow a stochastic assembly mechanism that is a universal feature of polyQ, independent of aggregation kinetics and sequence context (SI Appendix, Fig. S1). A prior study (17) reported that D2Q15K2 fibrils showed the same two signals, but argued that individual Gln were present in either one or the other conformer, but not in both. We were intrigued by the potential implication that single-residue peak doubling would depend on the length of the polyQ segment and, thus, might point toward a structural or mechanistic rationale for the polyQ threshold phenomenon. When we measured D2Q15K2 fibrils with a single labeled Gln, however, we observed the doubled peak pattern (SI Appendix, Fig. S1F), showing no evidence of a change in the stochastic assembly process as a function of polyQ length.
Fibril Polymorphism in polyQ Amyloid.
Other work has argued for polymorphism in the structure of aggregated polyQ, with potential implications for aggregate toxicity (19). The ability for a single sequence to form polymorphic fibrils is common for amyloid-forming proteins, and ssNMR chemical shifts are the gold standard for detecting the underlying structural differences (14). However, polyQ fibrils always feature the same pattern of chemical shifts (SI Appendix, Fig. S1), reflecting the described combination of two β-strand configurations. One might expect that a purely Gln-based sequence could fulfill the fundamental architecture of an interdigitating β-hairpin–based assembly in different ways, but the data do not support this expectation. We hypothesize that the origins for this lack of variability must be in the nucleation process that initiates the amyloid formation. This event dictates the structure of the initial assembly that is faithfully extended and reproduced during elongation (Fig. 6). As examined in a recent molecular dynamics study, different β-strand–based polyQ structures have distinct propensities for initiating the aggregation process (43). We propose therefore that the particular structure that we observe in the polyQ amyloid core would be uniquely capable of nucleated elongation. It is not immediately obvious from the current data what makes this conformation so unique, but we hope that the structural insights enabled by ssNMR will facilitate computational and experimental explorations of this issue.
Conclusion
We have shown by ssNMR that the amyloid core of htt exon1 fibrils and other polyQ aggregates feature β-sheets that interact via interdigitation of side chains. We described how specific β-hairpin structures are present in the htt exon1 fibrils and a stochastic aggregation mechanism of expanded polyQ. These insights greatly enhance our understanding of polyQ misfolding and aggregation and provide support for mechanistic studies that have pinpointed β-hairpin formation to be a pivotal event in the aggregation process.
Materials and Methods
Fibril Sample Preparation.
Fibrillar htt exon1 with a 44-residue polyQ domain (Fig. 1A and SI Appendix, Table S1) was prepared following protocols similar to those reported (16), with several modifications described in SI Appendix, SI Materials and Methods. Site-specifically labeled peptide fibrils (SI Appendix, Table S1) were prepared according to reported protocols (11, 44), with more details provided in SI Appendix, SI Materials and Methods.
Transmission Electron Microscopy.
Transmission electron microscopy (TEM) was performed on mature U-13C,15N htt exon1 fibrils and on htt exon1 samples harvested during aggregation (SI Appendix, SI Materials and Methods). Samples were negatively stained with 1% (wt/vol) uranyl acetate. Imaging at 6,500- to 15,000-fold magnification was done by using a Technai T12spirit transmission electron microscope (FEI) operating at 120 kV and equipped with an UltraScan 1000 CCD camera (Gatan).
MAS ssNMR Spectroscopy.
MAS ssNMR experiments were performed by using Bruker spectrometers operating at 600 and 800 MHz 1H Larmor frequencies, using 1.3- and 3.2-mm MAS NMR probes. Assignments were performed by using 2D 13C-13C and 15N-13C assignment measurements. Distance constraints were obtained by using PDSD and NHHC experiments (33). Backbone and side-chain torsion angles were measured by using NCCN (23, 26) and HCCH-style dipolar recoupling measurements (34) (SI Appendix, Figs. S2 and S5). 15N R1ρ, 13C R1ρ, and 13C R1 relaxation rates were measured at 60 kHz MAS, and 15N R1 rates were measured at 20 kHz MAS (30, 36, 37). Further details are in SI Appendix, SI Materials and Methods.
X-Ray Powder Diffraction.
Hydrated K2Q31K2 amyloid fibrils sealed into a glass capillary were measured by X-ray powder diffraction at room temperature. Experimental details are given in SI Appendix, SI Materials and Methods.
Chemical Shift Calculations.
Ab initio calculations were carried out on different Gln-containing candidate structures, using the PQS program (45). Density functional GIAO shielding calculations were performed with the B3LYP functional and the Ahlrichs TZP basis set (46). Absolute shieldings were corrected as described (47). Reference calculations were carried out on an amyloidogenic peptide of known structure (18, 41). Additional details are given in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Mike Delk and Dr. Jochem Struppe for technical assistance, and Drs. James Conway and Alexander Makhov for access to the Department of Structural Biology’s EM facility. We acknowledge funding from the University of Pittsburgh and National Institutes of Health Grants R01 GM112678 and AG019322 (to P.C.A.v.d.W. and R.W.), R01 GM099718 (to R.W.), and T32 GM088119 (to C.L.H.); Biotechnology and Biological Sciences Research Council Grant BB/L022761/1 and Engineering and Physical Sciences Research Council (EPSRC) Grant EP/L025906/1 (to J.R.L.); an EPSRC Doctoral Training Grant (to J.M.L.); and National Center for Research Resources Grant UL1 RR024153. Molecular graphics were prepared with the Chimera software package, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by National Institute of General Medical Sciences Grant P41-GM103311).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1521933113/-/DCSupplemental.
References
- 1.Bates GP, Benn C. The polyglutamine diseases. In: Bates GP, Harper P, Jones L, editors. Huntington’s Disease. 3rd Ed. Oxford Univ Press; Oxford: 2002. pp. 429–472. [Google Scholar]
- 2.Mangiarini L, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87(3):493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 3.DiFiglia M, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277(5334):1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 4.Scherzinger E, et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90(3):549–558. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- 5.Poirier MA, et al. Huntingtin spheroids and protofibrils as precursors in polyglutamine fibrilization. J Biol Chem. 2002;277(43):41032–41037. doi: 10.1074/jbc.M205809200. [DOI] [PubMed] [Google Scholar]
- 6.Legleiter J, et al. Monoclonal antibodies recognize distinct conformational epitopes formed by polyglutamine in a mutant huntingtin fragment. J Biol Chem. 2009;284(32):21647–21658. doi: 10.1074/jbc.M109.016923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431(7010):805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 8.Duim WC, Jiang Y, Shen K, Frydman J, Moerner WE. Super-resolution fluorescence of huntingtin reveals growth of globular species into short fibers and coexistence of distinct aggregates. ACS Chem Biol. 2014;9(12):2767–2778. doi: 10.1021/cb500335w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu K-Y, et al. Disruption of the nuclear membrane by perinuclear inclusions of mutant huntingtin causes cell-cycle re-entry and striatal cell death in mouse and cell models of Huntington’s disease. Hum Mol Genet. 2015;24(6):1602–1616. doi: 10.1093/hmg/ddu574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wetzel R, Mishra R. Order, disorder, and conformational flux in the structural biology of Huntington’s Disease. In: Bates GP, Tabrizi S, Jones L, editors. Huntington’s Disease. 4th Ed Oxford Univ Press; Oxford: 2014. [Google Scholar]
- 11.Kar K, et al. β-hairpin-mediated nucleation of polyglutamine amyloid formation. J Mol Biol. 2013;425(7):1183–1197. doi: 10.1016/j.jmb.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang QC, et al. A compact beta model of huntingtin toxicity. J Biol Chem. 2011;286(10):8188–8196. doi: 10.1074/jbc.M110.192013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comellas G, Rienstra CM. Protein structure determination by magic-angle spinning solid-state NMR, and insights into the formation, structure, and stability of amyloid fibrils. Annu Rev Biophys. 2013;42(1):515–536. doi: 10.1146/annurev-biophys-083012-130356. [DOI] [PubMed] [Google Scholar]
- 14.Tycko R. Physical and structural basis for polymorphism in amyloid fibrils. Protein Sci. 2014;23(11):1528–1539. doi: 10.1002/pro.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivanandam VN, et al. The aggregation-enhancing huntingtin N-terminus is helical in amyloid fibrils. J Am Chem Soc. 2011;133(12):4558–4566. doi: 10.1021/ja110715f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoop CL, et al. Polyglutamine amyloid core boundaries and flanking domain dynamics in huntingtin fragment fibrils determined by solid-state nuclear magnetic resonance. Biochemistry. 2014;53(42):6653–6666. doi: 10.1021/bi501010q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider R, et al. Structural characterization of polyglutamine fibrils by solid-state NMR spectroscopy. J Mol Biol. 2011;412(1):121–136. doi: 10.1016/j.jmb.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 18.Sawaya MR, et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447(7143):453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 19.Nekooki-Machida Y, et al. Distinct conformations of in vitro and in vivo amyloids of huntingtin-exon1 show different cytotoxicity. Proc Natl Acad Sci USA. 2009;106(24):9679–9684. doi: 10.1073/pnas.0812083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armen RS, Bernard BM, Day R, Alonso DOV, Daggett V. Characterization of a possible amyloidogenic precursor in glutamine-repeat neurodegenerative diseases. Proc Natl Acad Sci USA. 2005;102(38):13433–13438. doi: 10.1073/pnas.0502068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayward S, Milner-White EJ. Simulation of the β- to α-sheet transition results in a twisted sheet for antiparallel and an α-nanotube for parallel strands: Implications for amyloid formation. Proteins. 2011;79(11):3193–3207. doi: 10.1002/prot.23154. [DOI] [PubMed] [Google Scholar]
- 22.Hong M. Torsion angle determination by solid-state NMR. In: Webb GA, editor. Modern Magnetic Resonance. Springer; Dordrecht, The Netherlands: 2006. pp. 723–729. [Google Scholar]
- 23.Costa PR, Gross JD, Hong M, Griffin RG. Solid-state NMR measurement of Psi in peptides: A NCCN 2Q-heteronuclear local field experiment. Chem Phys Lett. 1997;280(1–2):95–103. [Google Scholar]
- 24.Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: A hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44(4):213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaroniec CP, et al. High-resolution molecular structure of a peptide in an amyloid fibril determined by magic angle spinning NMR spectroscopy. Proc Natl Acad Sci USA. 2004;101(3):711–716. doi: 10.1073/pnas.0304849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Wel PCA, Lewandowski JR, Griffin RG. Structural characterization of GNNQQNY amyloid fibrils by magic angle spinning NMR. Biochemistry. 2010;49(44):9457–9469. doi: 10.1021/bi100077x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loquet A, et al. 3D structure determination of the Crh protein from highly ambiguous solid-state NMR restraints. J Am Chem Soc. 2008;130(11):3579–3589. doi: 10.1021/ja078014t. [DOI] [PubMed] [Google Scholar]
- 28.De Paëpe G, Lewandowski JR, Loquet A, Böckmann A, Griffin RG. Proton assisted recoupling and protein structure determination. J Chem Phys. 2008;129(24):245101. doi: 10.1063/1.3036928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Van der Wel PCA. Spinning-rate encoded chemical shift correlations from rotational resonance solid-state NMR experiments. J Magn Reson. 2013;230:117–124. doi: 10.1016/j.jmr.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewandowski JR, Van der Wel PCA, Rigney M, Grigorieff N, Griffin RG. Structural complexity of a composite amyloid fibril. J Am Chem Soc. 2011;133(37):14686–14698. doi: 10.1021/ja203736z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perutz MF, Pope BJ, Owen D, Wanker EE, Scherzinger E. Aggregation of proteins with expanded glutamine and alanine repeats of the glutamine-rich and asparagine-rich domains of Sup35 and of the amyloid beta-peptide of amyloid plaques. Proc Natl Acad Sci USA. 2002;99(8):5596–5600. doi: 10.1073/pnas.042681599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sikorski P, Atkins E. New model for crystalline polyglutamine assemblies and their connection with amyloid fibrils. Biomacromolecules. 2005;6(1):425–432. doi: 10.1021/bm0494388. [DOI] [PubMed] [Google Scholar]
- 33.Lange A, Luca S, Baldus M. Structural constraints from proton-mediated rare-spin correlation spectroscopy in rotating solids. J Am Chem Soc. 2002;124(33):9704–9705. doi: 10.1021/ja026691b. [DOI] [PubMed] [Google Scholar]
- 34.Feng X, et al. Direct determination of a molecular torsional angle in the membrane protein rhodopsin by solid-state NMR. J Am Chem Soc. 1997;119(29):6853–6857. [Google Scholar]
- 35.Sharma D, Shinchuk LM, Inouye H, Wetzel R, Kirschner DA. Polyglutamine homopolymers having 8-45 residues form slablike beta-crystallite assemblies. Proteins. 2005;61(2):398–411. doi: 10.1002/prot.20602. [DOI] [PubMed] [Google Scholar]
- 36.Lewandowski JR, et al. Measurement of site-specific 13C spin-lattice relaxation in a crystalline protein. J Am Chem Soc. 2010;132(24):8252–8254. doi: 10.1021/ja102744b. [DOI] [PubMed] [Google Scholar]
- 37.Lewandowski JR, Sass HJ, Grzesiek S, Blackledge M, Emsley L. Site-specific measurement of slow motions in proteins. J Am Chem Soc. 2011;133(42):16762–16765. doi: 10.1021/ja206815h. [DOI] [PubMed] [Google Scholar]
- 38.Thakur AK, Wetzel R. Mutational analysis of the structural organization of polyglutamine aggregates. Proc Natl Acad Sci USA. 2002;99(26):17014–17019. doi: 10.1073/pnas.252523899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou PY, Fasman GD. β-turns in proteins. J Mol Biol. 1977;115(2):135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- 40.Kar K, Jayaraman M, Sahoo B, Kodali R, Wetzel R. Critical nucleus size for disease-related polyglutamine aggregation is repeat-length dependent. Nat Struct Mol Biol. 2011;18(3):328–336. doi: 10.1038/nsmb.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van der Wel PCA, Lewandowski JR, Griffin RG. Solid-state NMR study of amyloid nanocrystals and fibrils formed by the peptide GNNQQNY from yeast prion protein Sup35p. J Am Chem Soc. 2007;129(16):5117–5130. doi: 10.1021/ja068633m. [DOI] [PubMed] [Google Scholar]
- 42.Perutz MF, Finch JT, Berriman J, Lesk A. Amyloid fibers are water-filled nanotubes. Proc Natl Acad Sci USA. 2002;99(8):5591–5595. doi: 10.1073/pnas.042681399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miettinen MS, Knecht V, Monticelli L, Ignatova Z. Assessing polyglutamine conformation in the nucleating event by molecular dynamics simulations. J Phys Chem B. 2012;116(34):10259–10265. doi: 10.1021/jp305065c. [DOI] [PubMed] [Google Scholar]
- 44.O’Nuallain B, et al. Kinetics and thermodynamics of amyloid assembly using a high-performance liquid chromatography-based sedimentation assay. Methods Enzymol. 2006;413:34–74. doi: 10.1016/S0076-6879(06)13003-7. [DOI] [PubMed] [Google Scholar]
- 45.Baker J, et al. Quantum chemistry in parallel with PQS. J Comput Chem. 2009;30(2):317–335. doi: 10.1002/jcc.21052. [DOI] [PubMed] [Google Scholar]
- 46.Schäfer A, Horn H, Ahlrichs R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J Chem Phys. 1992;97(4):2571–2577. [Google Scholar]
- 47.Magyarfalvi G, Pulay P. Assessment of density functional methods for nuclear magnetic resonance shielding calculations. J Chem Phys. 2003;119(3):1350–1357. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.