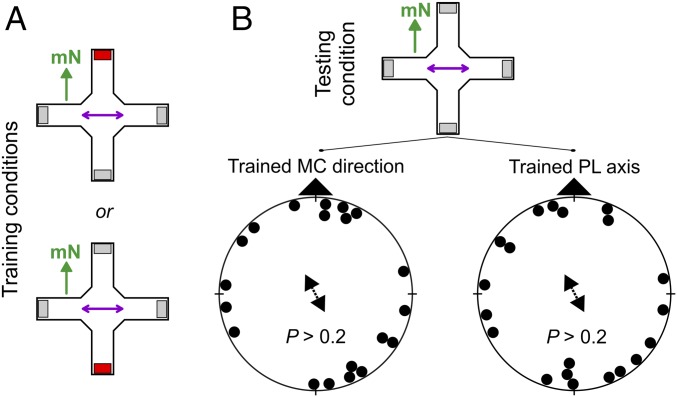
Significance
Birds have a light-dependent magnetic compass that is suggested to be mediated by light-induced, biochemical reactions in specialized magnetoreceptor molecules in the avian retina. Natural skylight reaching these receptors is always directional and to some degree polarized, which has largely been neglected in biophysical models and behavioral experiments on the magnetic compass sense. Training zebra finches in a spatial orientation assay, we show that overhead polarized light modulates radical pair-based magnetic compass orientation. The magnetic compass is only operational when overhead polarized light is aligned parallel, but not perpendicular, to the magnetic field. These findings reveal fundamentally new properties of the light-dependent magnetoreceptor that significantly advance our understanding of how birds, and animals in general, perceive the Earth’s magnetic field.
Keywords: magnetoreception, magnetic compass, orientation, skylight polarization, radical-pair process
Abstract
Magnetoreception of the light-dependent magnetic compass in birds is suggested to be mediated by a radical-pair mechanism taking place in the avian retina. Biophysical models on magnetic field effects on radical pairs generally assume that the light activating the magnetoreceptor molecules is nondirectional and unpolarized, and that light absorption is isotropic. However, natural skylight enters the avian retina unidirectionally, through the cornea and the lens, and is often partially polarized. In addition, cryptochromes, the putative magnetoreceptor molecules, absorb light anisotropically, i.e., they preferentially absorb light of a specific direction and polarization, implying that the light-dependent magnetic compass is intrinsically polarization sensitive. To test putative interactions between the avian magnetic compass and polarized light, we developed a spatial orientation assay and trained zebra finches to magnetic and/or overhead polarized light cues in a four-arm “plus” maze. The birds did not use overhead polarized light near the zenith for sky compass orientation. Instead, overhead polarized light modulated light-dependent magnetic compass orientation, i.e., how the birds perceive the magnetic field. Birds were well oriented when tested with the polarized light axis aligned parallel to the magnetic field. When the polarized light axis was aligned perpendicular to the magnetic field, the birds became disoriented. These findings are the first behavioral evidence to our knowledge for a direct interaction between polarized light and the light-dependent magnetic compass in an animal. They reveal a fundamentally new property of the radical pair-based magnetoreceptor with key implications for how birds and other animals perceive the Earth’s magnetic field.
A range of animals, including birds, use directional information from the Earth’s magnetic field for orientation and navigation (1–6). There is growing evidence that this ability is by far not restricted to migratory animals, but that it is likely an omnipresent capability of the majority of organisms, playing a fundamental role in the animals’ daily routines in all stages of life (7). Behavioral and physiological studies on taxonomically diverse animals suggest the presence of two fundamentally different, independent magnetoreception mechanisms that detect different parameters of the Earth’s magnetic field (1, 8–11): A light-dependent magnetic compass detects the axial alignment of the magnetic field, and an iron mineral-based mechanism provides positional magnetic map information.
Magnetoreception of the light-dependent magnetic compass is suggested to be mediated by light-induced, biochemical reactions taking place in specialized photoreceptors (8, 10–12). Upon light excitation, the photopigment molecules form magnetically sensitive radical-pair intermediates. The magnetic field then alters the ratio of the spin states of the radical pairs (i.e., singlet vs. triplet state) and, thereby, affects the photopigments’ response to light. Such magneto-sensitive photoreceptors arranged in an ordered array in the avian retina would show increased or decreased sensitivity to light, depending on their alignment to the magnetic field (8, 10, 12, 13). The animals would perceive the magnetic field as a magnetic modulation pattern centered on the magnetic field lines, either superimposed on the visual field or mediated by a separate channel (12). Cryptochromes have been proposed as putative candidate receptor molecules (8, 14) and found to be expressed in the retinas of birds exhibiting magnetic orientation behavior (15–17). Biophysical models on magnetic field effects on radical pairs generally assume that light reaching the magnetoreceptor molecules is nondirectional and unpolarized, and that light absorption is isotropic, i.e., that the probability of excitation by light is equal for all receptor molecules (8, 10, 12). Likewise, behavioral experiments testing the mechanisms of magnetic compass orientation in migratory birds have typically been carried out under depolarized light (e.g., refs. 1 and 18–22). As recently pointed out by Lau et al. (13), traditional radical-pair models thus do not take into consideration that natural skylight always enters the eyes directionally through the cornea and lens and that the magnetoreceptor molecules absorb light anisotropically, i.e., they preferentially absorb light of a specific direction and polarization. The probability of photoexcitation and, thereby, formation of radical pairs will therefore differ between magnetoreceptors across the retina and result in a photoselected population of magnetoreceptors that the magnetic field can act upon (13, 23, 24). The ratio of singlet and triplet states and, thereby, the magnetic field effect, will in turn depend on the relative alignment of the radical pair to the magnetic field (8, 10). Such a photoselection effect has been shown to be strong enough to allow for a functional magnetic compass without major rotational restrictions of the receptor molecules (13).
Natural skylight is also always to some degree polarized, with the exception of totally overcast or foggy conditions (25–27). The degree of polarization and the alignment of the polarization axis vary across the sky with respect to the position of the sun and depend on weather conditions (25–27). Magnetoreceptor molecules in different parts of the avian retina will therefore receive light of different degrees and angles of polarization, depending on their location in the retina. Cryptochromes, like all photopigments, are linearly dichroic and, thereby, intrinsically polarization sensitive. The isoalloxazine ring of the light-responsive chromophore in cryptochromes, the flavin adenine dinucleotide, preferentially absorbs light polarized parallel to the ring formation (13, 28). Consequently, the population of cryptochrome receptors with their transition dipoles aligned parallel to the e-vector of light will be preferentially excited, i.e., polarization selected (13, 23, 24). Recent models suggest that partially or fully polarized light enhances the photoselection effects on the radical-pair magnetoreceptors described above (13). Assuming that the lens, ocular media, or retinal tissue in the avian eye do not significantly depolarize the incoming light before reaching the magnetoreceptor molecules, the light-dependent magnetic compass is therefore expected to be based on a photo- and polarization-selected population of magnetoreceptors whose signaling state depends on the relative alignment of the receptor molecules with polarized light and the magnetic field (13, 23, 24). Magnetic compass orientation in birds and other animals is, therefore, expected to be influenced by polarized light aligned at different angles to the magnetic field. Alternatively, or additionally, a cryptochrome-based reception system could be involved in polarized light reception and mediate polarized light information (29, 30). Sensitivity to polarized light is well understood in invertebrates (31), but little is known about how vertebrates, including birds, sense polarized light (30, 31). There are no obvious anatomical structures in the avian retina specialized for polarized light reception, and no viable theory exists on how birds, and most other vertebrates, can perceive polarized light (32). The functional and physiological prerequisites for a radical pair-based magnetic compass could in principle also apply for a polarized light receptor, which suggests that polarized light and magnetic compass reception could be based on a similar, or possibly the same, receptor mechanism in at least some responses to these two types of stimuli.
To investigate putative interactions between polarized light and the magnetic compass, we developed a spatial orientation assay and trained zebra finches (Taeniopygia guttata) to locate a hidden food reward in a four-arm plus maze by using magnetic compass and/or overhead linearly polarized light cues (Fig. 1A and Methods).
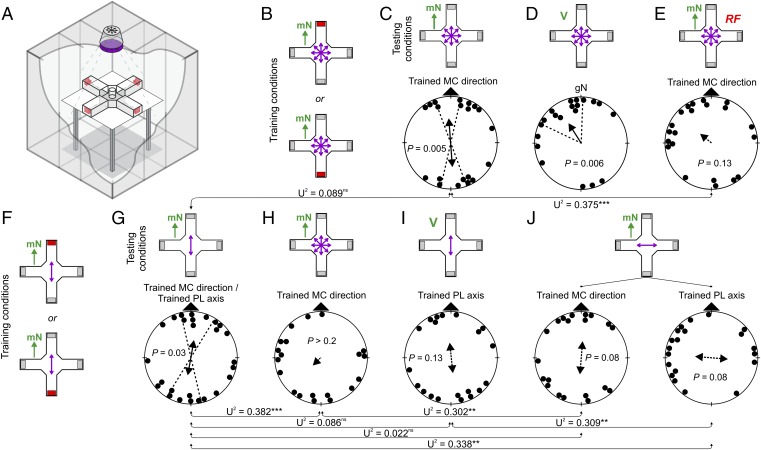
Fig. 1.
(A) Illustration of the experimental setup. The plus maze is centered on a wooden table inside the magnetic coil (Merritt design). At the end of each of the four arms, the food reward (during training trials only) is hidden in one of the four red trays. The cylinder in the center of the maze shows the release device from where the birds are remotely released to explore the maze. The unpolarized light source is centered above the maze. Linear polarizers and/or depolarizers can be inserted in a filter holder just below the light source. (B–E) Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using magnetic compass cues. (B) Individual birds were trained to mN (Top) or mS (Bottom) under unpolarized light. Orientation of birds tested under the training condition (C), in a vertical magnetic field (V) without any directional magnetic information (D), and in the presence of a 1.4 MHz RF field at 80 nT (RF) (E). (F–J) Orientation of zebra finches trained to relocate a food reward using magnetic compass cues in the presence of overhead polarized light aligned parallel to the magnetic field. (F) Individual birds were trained to either mN or mS, with the e-vector of overhead polarized light (violet double-headed arrow) aligned parallel to the magnetic field. Orientation of birds tested in the presence of both cues (training condition) (G), in the presence of magnetic compass cues only (H), in a vertical magnetic field with access to polarized light only (I), and with magnetic compass and polarized light cues aligned perpendicular to each other (J). Maze illustrations: Green arrows with mN symbolize training or testing in the presence of an Earth-strength magnetic field. The gray boxes at the end of the maze indicate the food trays, with the red tray containing the reward (training only). The four violet double arrows in the center of the maze indicate unpolarized light. Circular diagrams: Each dot represents the orientation of one bird; the arrows give the mean orientation of the group of birds; solid arrows with two dotted lines indicate significantly orientated groups (P < 0.05, Rayleigh test) with 95% CI; dashed arrows indicate nonsignificant distributions; P values of the Rayleigh test are given inside each circular diagram. MC, magnetic compass; PL, polarized light. Watson U2 test statistics are given for comparisons between experimental groups: ***P < 0.001; **P < 0.01; *P < 0.05; ns, P ≥ 0.05. For detailed statistics, see Tables S1–S3.
Results and Discussion
Zebra Finches Use a Radical Pair-Based Magnetic Compass for Spatial Orientation.
Zebra finches trained to relocate a food reward in the spatial orientation assay using directional magnetic compass information learned the task rapidly, in only three to four training trials (Fig. 1B and Table S1) (33). Birds trained to find the reward at either magnetic north (mN) or magnetic south (mS) were significantly oriented along the trained magnetic compass axis when subsequently tested in the presence of a magnetic field aligned in one of four topographic directions [P < 0.05, confidence interval (CI) test; Fig. 1C; see SI Results and Discussion for discussion of axial orientation]. When tested in a vertical magnetic field, the birds oriented toward topographic northwest (Fig. 1D) thus likely resorted to a fixed topographic response in the absence of trained directional cues. To determine which magnetoreception mechanism was involved in this learned response, we tested the zebra finches in the presence of a weak radio-frequency (RF) electromagnetic field. The use of low-intensity, oscillating RF fields at 0.1–10 MHz (intensity <1 μT) is the most powerful tool to test for an involvement of a radical-pair mechanism in the primary magnetoreception process (19, 34). RF fields have been shown to influence the interconversion between the singlet and triplet excited states of the radical pairs and, thereby, alter or eliminate the effects produced by the Earth’s magnetic field (19, 34). Consistent with predictions from the radical-pair theory, zebra finches tested in the presence of such RF fields at the Larmor frequency (1.4 MHz; intensity 80 nT) were disoriented (Fig. 1E). The failure to exhibit consistent orientation to the magnetic field in the presence of the RF field agrees with previous studies (19, 20, 35, 36) and provides the critical evidence that a radical-pair mechanism is involved in the trained magnetic orientation response shown by the zebra finches in the spatial orientation assay.
Table S1.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using magnetic compass cues under unpolarized light
| Birds tested for magnetic compass orientation (Fig. 1C) | Birds tested in a vertical magnetic field (Fig. 1D) | Birds tested for magnetic compass orientation in a RF field (Fig. 1E) | |||||
| gN | mN | Trained MC direction | gN | gN | mN | Trained MC direction | |
| n | 20 | 20 | 20 | 20 | 21 | 21 | 21 |
| Mean direction | 258° | 356°/176° | 356°/176° | 328° | 75° | 265° | 309° |
| Mean vector length | 0.155 | 0.506 | 0.506 | 0.496 | 0.270 | 0.321 | 0.311 |
| Rayleigh test (P) | 0.626 | 0.005 | 0.005 | 0.006 | 0.218 | 0.129 | 0.130 |
Orientation statistics per group relative to geographic North (gN), magnetic North (mN), and relative to the trained magnetic compass (MC) direction. Sample size (n), mean direction, mean vector length as measure of scatter, and the P value for the Rayleigh test are given for each group. See Fig. 1.
Interaction Between Magnetic Compass and Polarized Light Cues.
To investigate putative interactions between polarized light and magnetic compass reception, we trained zebra finches to magnetic compass cues in the presence of overhead polarized light aligned parallel to the horizontal component of the magnetic field (Fig. 1F and Tables S2 and S3). Birds tested under the training condition were significantly oriented along the trained magnetic compass/polarized light axis (P < 0.05, CI test; Fig. 1G), comparable to the responses of birds trained and tested to magnetic compass cues under unpolarized light (Fig. 1C). In contrast, birds trained in the presence of both cues were not significantly oriented when tested under either cue presented alone during the probe trial (Fig. 1 H and I). The orientation of the birds tested for magnetic compass orientation under unpolarized light (Fig. 1H) was significantly different from the control group (Fig. 1G), suggesting that the birds were not able to orient with the magnetic compass anymore. Birds tested in the presence of the overhead polarized light axis in a vertical magnetic field (Fig. 1I) showed a tendency to orient along the trained polarized light axis, similar to the control group (Fig. 1G). Interestingly, when tested with the two cues aligned perpendicularly to each other, the birds tended to orient along the trained magnetic compass axis, but perpendicular to the polarized light axis (Fig. 1J). The difficulty of the birds trained to magnetic compass cues in the presence of overhead polarized light aligned parallel to the magnetic field to orient with access to only one of the two cues was surprising. It suggests one of the following explanations: (i) magnetic compass and overhead polarized light cues are two independent compass cues that have to be recalled together when learned together, (ii) changes in the polarization state of the overhead light alter the visual perception of the maze to such a degree that the birds become confused or create secondary cues that the birds could use for orientation, or (iii) overhead polarized light directly interacts with the magnetic compass and modulates how the birds perceive the magnetic field.
Table S2.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using magnetic compass cues with overhead polarized light aligned parallel to the magnetic field
| Birds tested in the presence of magnetic compass and polarized light cues (Fig. 1G) | Birds tested for magnetic compass orientation under unpolarized light (Fig. 1H) | ||||||
| gN | mN | Trained PL axis | Trained MC direction | gN | mN | Trained MC direction | |
| n | 26 | 26 | 26 | 26 | 20 | 20 | 20 |
| Mean direction | 18° | 11°/191° | 11°/191° | 11°/191° | 56°/236° | 70° | 226° |
| Mean vector length | 0.149 | 0.361 | 0.361 | 0.361 | 0.195 | 0.242 | 0.224 |
| Rayleigh test (P) | 0.567 | 0.032 | 0.032 | 0.032 | 0.471 | 0.314 | 0.371 |
Table S3.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using magnetic compass cues with overhead polarized light aligned parallel to the magnetic field
| Birds tested in a vertical magnetic field with access to polarized light (Fig. 1I) | Birds tested for magnetic compass orientation with polarized light aligned perpendicular to the magnetic field (Fig. 1J) | |||||
| gN | Trained PL axis | gN | mN | Trained PL axis | Trained MC direction | |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean direction | 237° | 174°/354° | 336° | 5°/185° | 95°/275° | 5°/185° |
| Mean vector length | 0.344 | 0.321 | 0.382 | 0.358 | 0.358 | 0.358 |
| Rayleigh test (P) | 0.093 | 0.127 | 0.052 | 0.075 | 0.075 | 0.075 |
No Evidence for Overhead Polarized Light as Independent Compass Cue.
If the birds learned the spatial orientation task using two independent directional cues that both had to be available to solve the task (explanation 1), we would expect both the magnetic field and overhead polarized light to be independent compass cues. The avian magnetic compass is clearly an independent compass and functional without polarized light. Our zebra finches were able to learn to orient to magnetic compass cues in the maze under unpolarized light (Fig. 1C; see also Fig. 3 B and F below). This finding is further supported by numerous experiments testing magnetic compass orientation in birds under fully depolarized light (e.g., refs. 1 and 18–22). It is unclear, however, whether birds use overhead polarized light as an independent compass cue. There is convincing evidence that birds use directional information from the skylight polarization pattern for orientation and compass calibration (reviewed by refs. 30 and 37). It has been suggested that polarized light cues from different parts of the sky have different functions (30, 38, 39). We have shown that migratory songbirds calibrate their magnetic compass exclusively with information from the vertically aligned e-vector near the horizon at sunrise and sunset, and not with polarized light information from the zenith (40, 41). Sunrise/sunset polarized light cues from the lower half of the sky are suggested to be used as compass cues to determine the departure direction (42, 43). It is not known, however, whether birds use overhead polarized light information near the zenith for sky compass orientation and whether they can perceive polarized light at times of the day other than sunrise and sunset (25, 26, 30).
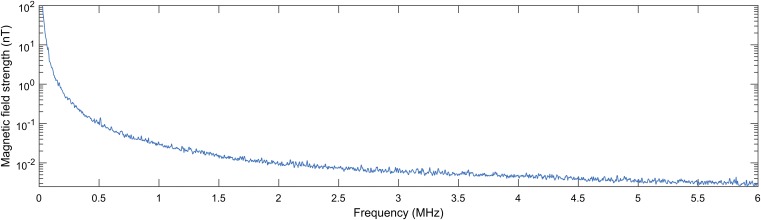
Fig. S3.
Baseline RF field in the experimental area measured with a magnetic field probe connected to a spectrum analyzer (see SI Methods for specifications). The magnetic coil, arena lights, and camera were switched on during the measurement.
To test whether birds can use an overhead polarized light axis as an independent orientation cue, we trained zebra finches to find a food reward in the two trays along the e-vector axis of overhead polarized light (Fig. 2A and Table S4). Despite repeated training, however, the birds did not consistently orient with respect of the trained polarized light axis (Fig. 2B). They seemed to be unable to use the e-vector alignment for orientation. It is therefore unlikely that polarized light cues near the zenith provide independent directional information for compass orientation. The skylight polarization compass and calibration reference sense(s) thus appear to respond uniquely to e-vectors of light from the region of sky at intermediate elevations and/or to vertical e-vectors near the horizon. The disorientation of the zebra finches trained to magnetic compass cues in the presence of polarized light aligned parallel to the magnetic field and tested under either one of the two cues separately (Fig. 1 H and I) can therefore not be explained by two independent compass cues that have to be recalled together when learned together (explanation 1).
Fig. 2.
Orientation of zebra finches trained to relocate a food reward using overhead polarized light (A and B) or magnetic compass cues (C and D). (A) Individual birds were trained in a vertical magnetic field to 100% overhead polarized light. Both maze arms along the e-vector axis contained rewards. (B) Orientation of birds tested under the training condition. (C) Individual birds were trained to mN (Top) or mS (Bottom) under unpolarized light. (D) Orientation of birds tested in a vertical magnetic field in the presence of polarized light. For explanations, see Fig. 1; for detailed statistics, see Tables S4 and S5.
Table S4.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using overhead polarized light
| Birds tested for polarized light orientation in a vertical magnetic field (Fig. 2B) | ||
| gN | Trained PL axis | |
| n | 14 | 14 |
| Mean direction | 33°/213° | 102°/282° |
| Mean vector length | 0.253 | 0.049 |
| Rayleigh test (P) | 0.416 | 0.968 |
No Innate Orientation Response to Polarized Light or Change in Visual Perception of Maze by Polarized Light.
If the overhead polarized light axis does not provide compass information, could it change the visual environment in the maze or introduce light artifacts instead (explanation 2)? The weak alignment along the polarization axis shown by the birds trained to magnetic compass and polarized light cues and tested under polarized light cues in a vertical magnetic field (Fig. 1I) may be explained by an innate alignment response along the polarization axis or by light artifacts produced by differential reflection of the polarized light on the maze. However, the zebra finches did not show any innate preference for a specific polarization axis, as demonstrated by the random orientation of birds trained to magnetic compass cues (Fig. 2C and Table S5) and then tested in the presence of overhead polarized light in a vertical magnetic field (Fig. 2D). The total lack of orientation suggests that the birds did not use any light intensity artifacts to orient in the maze, or that the polarized light generated a response pattern that the birds mistook for that generated by the magnetic field. The finding that the birds tested with the two cues aligned perpendicular to each other (Fig. 1J) tended to orient perpendicular to the polarized light axis further argues against an experimental artifact. The weak alignment of the birds along the polarization axis in Fig. 1I could instead be due to a calibration or transfer of information during training between the magnetic compass and polarized light cues in the lower part of the surroundings close to the maze arms.
Table S5.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using magnetic compass cues
| Birds tested for polarized light orientation in a vertical magnetic field (Fig. 2D) | ||
| gN | PL axis | |
| n | 20 | 20 |
| Mean direction | 73°/253° | 101°/281° |
| Mean vector length | 0.187 | 0.148 |
| Rayleigh test (P) | 0.503 | 0.650 |
There is to date no evidence that birds have true polarization vision (44, 45), i.e., that they can differentiate the angle of the e-vector of polarized light independently. We were unable to train zebra finches to polarized light stimuli presented on modified liquid crystal display screens in a two-choice conditioning experiment (45). Also, in control experiments where we trained zebra finches to color cues in the maze, we found no significant differences between birds tested under different alignments of overhead polarized light (Fig. S1 and Tables S6 and S7). Thus, it is unlikely that the changes in the polarization state of the overhead light altered the birds’ visual perception of the maze to such a degree that the birds became disoriented or created secondary cues that they could use for orientation (explanation 2).
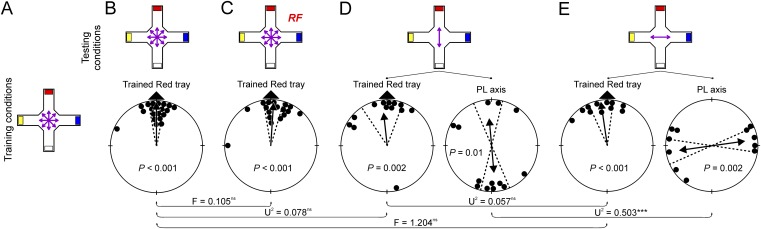
Fig. S1.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using color cues. (A) Individual birds were trained in the local geomagnetic field to the red tray under unpolarized light. Orientation of birds tested under the training condition (B), in the presence of a 1.4 MHz RF-field at 80 nT (C), in the presence of overhead polarized light aligned parallel to the red tray (D), and with polarized light aligned perpendicular to the red tray (E). Test statistics for the Watson U2 or Watson–William F test are given for comparisons between experimental groups: ***P < 0.001; ns, P > 0.05. For detailed statistics, see Tables S6 and S7.
Table S6.
Orientation of zebra finches trained to relocate a food reward in a colored tray
| Birds tested for color cue (Fig. S1B) | Birds tested for color cue in the presence of an RF field (Fig. S1C) | Birds tested for color cue in the presence of overhead polarized light aligned toward the red tray (Fig. S1D) | |||||
| gN | Trained red tray | gN | Trained red tray | gN | Trained red tray | PL axis | |
| n | 19 | 19 | 17 | 17 | 13 | 13 | 13 |
| Mean direction | 177°/357° | 360° | 99°/279° | 3° | 144°/324° | 353° | 176°/356° |
| Mean vector length | 0.164 | 0.943 | 0.345 | 0.893 | 0.322 | 0.718 | 0.565 |
| Rayleigh test (P) | 0.607 | 7.18e-08 | 0.132 | 4.67e-07 | 0.265 | 5.09e-4 | 0.013 |
Table S7.
Orientation of zebra finches trained to find a food reward in a colored tray
| Birds tested for color cue in the presence of overhead polarized light aligned perpendicular to the red tray (Fig. S1E) | |||
| gN | Trained red tray | PL axis | |
| n | 11 | 11 | 11 |
| Mean direction | 284° | 351° | 82°/262° |
| Mean vector length | 0.156 | 0.920 | 0.709 |
| Rayleigh test (P) | 0.774 | <1e-12 | 0.002 |
Direct Interaction of Polarized Light with the Primary Magnetic Compass Receptor.
If overhead polarized light does not provide directional information (explanation 1) and the behavior of the birds in Fig. 1 can be explained by neither an innate preference for a polarization axis nor by artifacts (explanation 2), could polarized light instead play a role in radical pair-based magnetoreception (explanation 3)? To test this hypothesis, we used zebra finches that had never been trained to polarized light. We trained these birds to magnetic compass cues under unpolarized light (Fig. 3A and Tables S8 and S9) and tested them in the presence of overhead polarized light aligned either parallel or perpendicular to the magnetic field (Fig. 3 B–D). The behavior of the birds in the different testing conditions was strikingly different. The control group that was tested for magnetic compass cues under unpolarized light was oriented along the trained magnetic field axis (Fig. 3B; P < 0.05, CI test), comparable to the control group in Fig. 1C (P > 0.2, U2 = 0.07, Watson U2 test). Birds tested with polarized light aligned parallel to the magnetic field were likewise well oriented along the trained magnetic compass/polarized light axis (Fig. 3C; P < 0.05, CI test), suggesting that the parallel alignment was not affecting magnetic compass reception. However, the group of birds tested in the presence of perpendicularly aligned polarized light showed no significant pattern of orientation (Fig. 3D), indicating that the perception of the magnetic field had changed to such a degree that made magnetic compass information unreadable or uninterpretable.
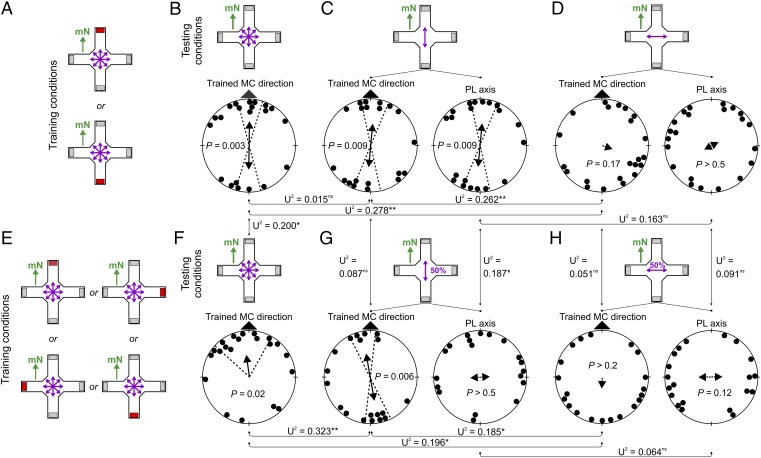
Fig. 3.
Orientation of zebra finches trained to relocate a food reward by using magnetic compass cues. (A) Individual birds were trained to mN (Top) or mS (Bottom) under unpolarized light. Orientation of birds tested under the training condition (B), in the presence of 100% overhead polarized light aligned parallel to the magnetic field (C), and with 100% overhead polarized light aligned perpendicular to the magnetic field (D). (E) Individual birds were trained to mN, mS, mE, or mW under unpolarized light. Orientation of birds tested under the training condition (F), in the presence of overhead 50% polarized light aligned parallel to the magnetic field (G), and with 50% overhead polarized light aligned perpendicular to the magnetic field (H). For explanations see Fig. 1; for detailed statistics, see Tables S8–S11.
Table S8.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using magnetic compass cues under unpolarized light
| Birds tested for magnetic compass orientation under unpolarized light (Fig. 3B) | Birds tested for magnetic compass orientation in the presence of overhead polarized light aligned parallel to the magnetic field (Fig. 3C) | ||||||
| gN | mN | Trained MC direction | gN | mN | PL axis | Trained MC direction | |
| n | 21 | 21 | 21 | 20 | 20 | 20 | 20 |
| Mean direction | 31° | 1°/181° | 1°/181° | 7° | 5°/185° | 5°/185° | 5°/185° |
| Mean vector length | 0.296 | 0.512 | 0.512 | 0.374 | 0.478 | 0.478 | 0.478 |
| Rayleigh test (P) | 0.159 | 0.003 | 0.003 | 0.059 | 0.009 | 0.009 | 0.009 |
Table S9.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using magnetic compass cues under unpolarized light
| Birds tested for magnetic compass orientation with polarized light aligned perpendicular to the magnetic field (Fig. 3D) | ||||
| gN | mN | PL axis | Trained MC direction | |
| n | 22 | 22 | 22 | 22 |
| Mean direction | 16° | 317° | 63°/243° | 111° |
| Mean vector length | 0.167 | 0.130 | 0.023 | 0.286 |
| Rayleigh test (P) | 0.548 | 0.696 | 0.989 | 0.165 |
Under natural conditions, the degree of polarized skylight is generally less than 80% (25–27). To test whether the observed effects of polarized light on the magnetic compass were present also at lower degrees of polarization, we repeated the experiments by using 50% instead of 100% polarized light. We trained birds under unpolarized light to relocate the food reward by using magnetic compass information at mN, mS, magnetic east (mE), or magnetic west (mW) (Fig. 3E and Tables S10 and S11). Birds subsequently tested under the training condition were significantly oriented toward the trained magnetic direction (Fig. 3F; P < 0.05, CI test; see SI Text for discussion of unimodal response). Birds tested in the presence of 50% polarized light aligned parallel to the magnetic field were significantly oriented along the trained magnetic compass axis (Fig. 3G; P < 0.05, CI test), comparable to the response observed under 100% polarized light (Fig. 3C). When tested with the axis of 50% polarized light aligned perpendicular to the magnetic field, the birds were not able to orient anymore (Fig. 3H), as has been observed under 100% polarized light (Fig. 3D).
Table S10.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using magnetic compass cues under unpolarized light
| Birds tested for magnetic compass orientation under unpolarized light (Fig. 3F) | Birds tested for magnetic compass orientation in the presence of overhead 50% polarized light aligned parallel to the magnetic field (Fig. 3G) | ||||||
| gN | mN | Trained MC direction | gN | mN | PL axis | Trained MC direction | |
| n | 21 | 21 | 21 | 19 | 19 | 19 | 19 |
| Mean direction | 249° | 44°/224° | 351° | 151° | 115° | 86°/266° | 170°/350° |
| Mean vector length | 0.232 | 0.310 | 0.421 | 0.225 | 0.201 | 0.149 | 0.510 |
| Rayleigh test (P) | 0.326 | 0.132 | 0.022 | 0.388 | 0.470 | 0.662 | 0.006 |
Table S11.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using magnetic compass cues under unpolarized light
| Birds tested for magnetic compass orientation with 50% polarized light aligned perpendicular to the magnetic field (Fig. 3H) | ||||
| gN | mN | PL axis | Trained MC direction | |
| n | 20 | 20 | 20 | 20 |
| Mean direction | 133/313° | 358°/178° | 88°/268° | 182° |
| Mean vector length | 0.404 | 0.328 | 0.328 | 0.196 |
| Rayleigh test (P) | 0.036 | 0.116 | 0.116 | 0.469 |
Polarized Light-Sensitive Magnetic Compass.
The differential magnetic compass response shown by zebra finches tested in the presence of polarized light aligned parallel (Fig. 3 B and C) or perpendicular (Fig. 3 F and G) to the magnetic field demonstrates that overhead polarized light affects light-dependent magnetic compass orientation and, thereby, changes the birds’ perception of the magnetic field. The birds were equally well oriented under unpolarized as under fully or partially polarized light with the e-vector axis aligned parallel to the horizontal component of the magnetic field (Figs. 1C and 3 B, C, F, and G). However, birds tested with the two cues aligned perpendicularly to each other did not seem to be able to properly read the magnetic compass information (Figs. 1J and 3 D and H). Thus, polarized light aligned perpendicular to the magnetic field appears to directly interfere with the primary magnetoreception of the light-dependent magnetic compass. If true, we would expect birds not to be able to learn to orient by using their magnetic compass when trained with overhead polarized light aligned perpendicular to the magnetic field. Indeed, zebra finches trained and tested for magnetic compass orientation in the presence of polarized light aligned perpendicular to the magnetic field were totally disoriented (Fig. 4 and Table S12).
Fig. 4.
Orientation of zebra finches trained to relocate a food reward by using magnetic compass cues in the presence of overhead polarized light aligned perpendicular to the magnetic field. (A) Individual birds were trained to mN (Top) or mS (Bottom) with overhead polarized light aligned perpendicular to the magnetic field. (B) Orientation of birds tested under the training condition. For explanations, see Fig. 1; for detailed statistics, see Table S12.
Table S12.
Orientation of zebra finches trained to relocate a food reward in the spatial orientation assay using magnetic compass cues with overhead polarized light aligned perpendicular to the magnetic field
| Birds tested for magnetic compass orientation with polarized light aligned perpendicular to the magnetic field (Fig. 4B) | |||||
| gN | mN | PL axis | Trained PL axis | Trained MC direction | |
| n | 20 | 20 | 20 | 20 | 20 |
| Mean direction | 171°/351° | 158°/338° | 68°/248° | 158°/338° | 158°/338° |
| Mean vector length | 0.181 | 0.240 | 0.240 | 0.240 | 0.240 |
| Rayleigh test (P) | 0.526 | 0.319 | 0.319 | 0.319 | 0.319 |
Magnetic compass orientation was affected in our zebra finches by overhead polarized light not only when polarization was maximal (100%), but also when only 50% of the light was polarized. Thus, the magnetic compass might be affected by overhead polarized light also under natural conditions. However, the degree of skylight polarization is ≥50% only under optimal weather conditions and close to sunrise and sunset when the e-vector is aligned roughly along the geographic north–south axis (25–27). An effect would therefore only arise in geographic areas with magnetic declinations diverging significantly from 0° and/or at high latitudes close to summer and winter solstice, when the sun rises and sets close to geographic north/south. If magnetic compass orientation is impaired by perpendicularly aligned polarized light of <50%, it would affect animals primarily near solar noon, when the e-vector near the zenith is aligned along the geographic east–west axis. In theory, the interaction of polarized light with the magnetic compass could be a mechanism to weaken the magnetic modulation pattern and, thereby, minimize a potential interference with the visual system during times of day when vision is crucial for other tasks, like e.g., foraging or predator detection. Natural selection is likely to have influenced the design of the magnetic compass to reduce the confounding effects of the varying e-vector alignments of overhead polarization to a minimum, while using the advantages of the photo- and polarization selection effects (see below; ref. 13).
The effects of overhead polarized light on magnetic compass orientation demonstrated here reveal a fundamentally new property of the light-dependent, radical pair-based magnetic compass that has hitherto largely been neglected. Our findings provide convincing evidence that the primary magnetoreceptor is photo- and polarization selective, and thereby provide the foundation for a magnetic compass based on light-induced rotational order, as suggested by recent biophysical models (13, 24). This property relaxes the requirement for an intrinsic rotational order of the receptor molecules (as long as rotational motion is restricted) and opens for putative cryptochrome magnetoreceptors distributed in any, also nonrandomly oriented, cells in the avian retina (13). Similar effects are expected to occur also in other organisms orienting with a light-dependent magnetic compass based on radical-pair reactions. Our findings thereby add a new dimension to the understanding of how not only birds, but animals in general, perceive the Earth’s magnetic field.
SI Results and Discussion
Axial vs. Unimodal Control Directions.
Axial orientation along the trained axis in birds trained to magnetic compass cues and tested for magnetic compass orientation (Figs. 1 C and G and 3B) instead of the expected unimodal orientation relative to the reward is commonly observed in magnetic training assays (35, 47). It may (i) be related to the unnatural situation not allowing the distinction between the correct side of the magnetic field axis, or (ii) be the effect of anthropogenic noise in the experimental setup (48), making the magnetic modulation pattern difficult to see. In our case, electromagnetic noise from the control switches in the box regulating the power to the magnetic coils was likely the problem, because after exchanging some of them with new ones, the orientation of the birds became unimodal (Fig. 3F).
Control Experiments: Training to Color Cues.
We carried out control experiments not involving magnetic or polarized light cues to test whether RF fields or overhead polarized light had any effects on the birds’ orientation not related to the magnetic compass sense. The birds were trained in the spatial orientation assay in the local geomagnetic field to locate a food reward in a red tray from four trays of different colors that were always arranged in the same relative order (red, blue, white, and yellow, in a clockwise order; Fig. S1A and Tables S6 and S7). Training and testing procedures were identical to those in the magnetic/polarized light assay, with the exception that total training quality = 3 was considered a valid training, because most birds learned this task easily. Birds that had been successfully trained in at least three different arms were included in the analysis, whereas birds that spent >540 frames in the center were excluded for statistical reasons (see SI Methods).
The birds readily learned this task and were highly significantly oriented toward the red tray in the probe trial (Fig. S1B). Their orientation was affected by neither the RF field nor the addition of overhead polarized light (Fig. S1 C–E). These results demonstrate that RF fields and overhead polarized light per se have no effects on the birds’ spatial orientation abilities that are not related to the magnetic compass sense.
Methods
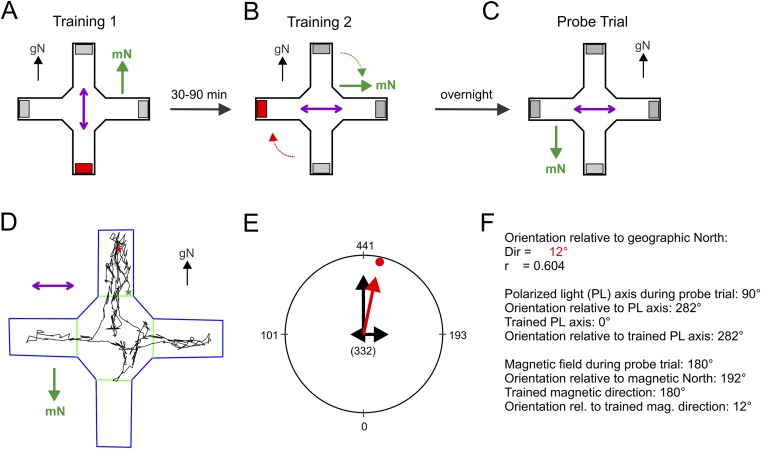
We trained female and male zebra finches, at least 6 mo of age, to use directional magnetic compass and/or polarized light cues to locate a hidden food reward in a visually symmetric four-arm (plus) maze (Fig. 1A). The maze was centered in a magnetic coil (Merritt design), thus the magnetic field could be directed toward any of the four maze arms [mN at geographic north (gN), geographic south (gS), geographic east (gE), or geographic west (gW)]. Individual birds were trained to find a food reward at the end of one of the maze arms under one of three training conditions (for example, see Fig. S2): (i) birds were trained to either mN or mS (Figs. 1B, 2C, and 3A) or to mN, mS, mE, or mW (Fig. 3E) under unpolarized light; (ii) birds were trained to either mN or mS in the presence of overhead polarized light aligned parallel (Fig. 1F) or perpendicular (Fig. 4A) to the horizontal component of the magnetic field; or (iii) birds were trained along an overhead polarized light axis, i.e., they were rewarded in the two trays on either side of the polarized light axis, in a vertical magnetic field (Fig. 2A). Birds that successfully passed the training trails were tested in a probe trial without food reward. They were allowed to search the maze for 90 s, and their movement was tracked with a custom-made video tracking program that automatically counted the number of frames that the bird spent in each of the four arms. The orientation of an individual bird was calculated from the time (number of frames) spent in each of the four maze arms during the 90-s trial. The individual mean orientations for birds tested in the presence of a magnetic field were then recalculated relative to mN (0°, taking into consideration that different individuals were tested in one of four magnetic fields), and relative to the trained magnetic compass direction (correcting for whether a bird was trained to mN, mS, mE, or mW; Fig. S2). Similarly, the individual mean orientation of birds tested in the presence of polarized light was recalculated relative to the alignment of the polarized light axis during the probe trial, and relative to the trained polarized light axis (where applicable), whereby the north and east end of the polarization axis was set to 0°.
Fig. S2.
Illustration of the training and testing procedure and the data analysis used in the spatial orientation assay. The figure is an example of a training to magnetic compass cues in the presence of overhead polarized light aligned parallel to the magnetic field, followed by a probe trial for magnetic compass orientation in the presence of overhead polarized light aligned perpendicular to the magnetic field. The bird is trained to find the food reward at mS and along the polarized light axis. (A) In training trial 1, the reward (red rectangle) is located in the maze arm at gS and the magnetic field (green arrow with mN) points toward gN, with the overhead polarized light axis (pink double arrow) aligned along the gN/gS axis. (B) In training trial 2, the food reward is moved 90° clockwise to the gW arm and the magnetic field is shifted accordingly. (C) In the probe trial the day after training, the bird is tested (without reward) for magnetic compass orientation in the presence of overhead polarized light aligned perpendicular to the magnetic field that is pointed in either one of four geographic directions (mN at gN, gS, gE, or gW; in the example mN = gS). (D) Original path of the bird in the maze as tracked by the computer program. The green star gives the start of the track when the bird enters the first arm; the red star gives the end of the track after 90 s. (E) Calculation of the individual mean orientation. The number of frames in each arm (displayed outside of the circle) are automatically determined by the computer program and summed by vector addition to give the individual mean orientation and mean vector length (in red). The number in brackets in the center of the circle gives the number of frames the bird spent in the center of the maze. (F) Recalculation of the mean orientation relative to gN to the orientation relative to the polarized light axis, with the east end of the polarized light axis defined as 0°, and relative to the trained polarized light axis, with the north end of the axis defined as 0°. Recalculation of the mean orientation relative to gN to the orientation relative to mN and the trained magnetic direction.
For each experimental condition, the mean orientation of the group of birds was calculated by using vector addition of the individual mean directions, disregarding the individual mean vector lengths. For all groups, we compared whether a unimodal or axial distribution best fitted the orientation data by calculating the mean vector length for the two distributions. To calculate the axial distributions, we doubled the individual mean angles (46). For the distribution that best described the data, i.e., the one with the larger mean vector length, the Rayleigh test was performed to test for significance (46). We used the CI test to examine whether the groups of birds were oriented relative to the trained magnetic direction or polarized light axis, i.e., whether the trained direction was included in the 95% CI of the distributions of birds in significantly oriented groups (46). Watson U2 tests were used to test for differences between experimental groups (46). See SI Methods, Fig. S3, and Table S13 for more details. All experiments were carried out in accordance with ethical permission from the Malmö-Lund Animal Ethics Committee, permits M 176–08, M 158–11 and M 423–12.
Table S13.
Numbers of excluded probe trials
| Fig. | >50% of time (540 frames) in center |
| 1C | 0 |
| 1D | 0 |
| 1E | 1 |
| 1G | 3 |
| 1H | 0 |
| 1I | 1 |
| 1J | 0 |
| 2B | 0 |
| 2D | 1 |
| 3B | 3 |
| 3C | 1 |
| 3D | 2 |
| 3F | 1 |
| 3G | 0 |
| 3H | 0 |
| 4B | 0 |
| S1B | 0 |
| S1C | 1 |
| S1D | 6 |
| S1E | 6 |
SI Methods
Study Site and Model Species.
All behavioral experiments were carried out between 2011 and 2015 at Stensoffa Ecological Field Station (55° 42′ N, 13° 25′ E) near Lund, Sweden. We used zebra finches as model species, both females and males at least 6 mo of age from our breeding colony. The birds were housed in a wooden building, and trained and tested in a separate testing building made entirely of wood and nonmagnetic materials. The testing building was divided into the main testing area with the experimental setup and a small booth for the experimenter with a video screen to observe the birds, switches for the arena lights, and the opening mechanism for the release device (see below).
Experimental Setup.
The birds were trained to use directional magnetic compass and/or polarized light cues to locate a hidden food reward in a visually symmetrical four-arm plus maze. The maze (1.2 × 1.2 m; Fig. 1A) was made of transparent Plexiglas and covered with plastic mesh on top. A red tray was located at the end of each arm. During the trainings, the food reward (millet seeds) was placed in the tray, where it was invisible to the birds until they jumped onto the tray. In the center of the maze, a release device (Plexiglas cylinder with a mesh top) could be remotely lifted to release the bird into the maze.
The maze was centered in a magnetic coil (Merritt design; ref. 49; 2 × 2 × 2 m), which was aligned along the cardinal magnetic directions. The coil had two wraps along the north–south (NS) and one wrap along the east–west (EW) axis. One of the wraps along the NS axis was used to compensate the horizontal component of the natural magnetic field, which resulted in a vertical magnetic field without directional information (total intensity of ∼47,550 nT; remaining horizontal component <100 nT). The remaining two wraps, one each along the NS and EW axis, were used to create the testing fields. The testing fields had the same magnetic properties as the natural magnetic field (total intensity ∼50,600 nT, inclination = 69.8°), but could be directed toward any of the four maze arms (mN at gN, gS, gE, or gW). Before each training/testing session, the alignment of the magnetic field was confirmed with a magnetic compass, and on a regular basis, the intensities of the magnetic field components were checked with a high-speed three-axis digital fluxgate magnetometer (Applied Physics Systems; Model 693). This magnetic coil setup is comparable to doubly wrapped coils (49), because during any experimental condition, with the exception of the vertical magnetic field condition, two coil wraps carry a current, eliminating any potential biases produced by the coil (49). We used 12-V car batteries with custom-made regulators to power the magnetic coil to avoid RF contamination. Baseline RF fields in the experimental area were measured with a magnetic field probe (Probe 901 from E & H Near Field set 7405; ETS Lindgren) connected to a spectrum analyzer (Agilent N9340B). The spectrum analyzer measured the magnetic component of the electromagnetic field between 10 kHz and 6 MHz for a period of 10 min at 3-kHz resolution bandwidth (1,850 measurement points). The magnetic coil, arena lights, and camera were switched on during the measurements. The measured fields were below 0.1 nT (Fig. S3), thus well below the values known to disturb the orientation of birds (19, 20, 48).
All light reaching the maze came from a central light source (ø 0.3 m) located 1 m above the maze (Fig. 1A). The full-spectrum white light produced by halogen and/or light-emitting diode lights was led through at least one translucent Plexiglas diffuser before reaching the maze. The overhead, linearly polarized light was produced by a circular sheet of a linear polarizer (ø 0.3 m; P500, 3Dlens Cooperation; transmittance 43%, polarizing efficiency 99.9%, spectral range 380–700 nm) inserted just below the overhead light source. To produce the 100% polarized light, we used one sheet of the polarizer, covered with one layer of wax paper on one side that fully depolarized the light. Thereby, the filter could be flipped to either side to be used as either polarizer or depolarizer, keeping light irradiance constant between conditions. To produce 50% linearly polarized light, we added five sheets of transparent mylar/polyester to the sheet of polarizer, with their polarization axes aligned at various angles relative to each other. The exact degree of linear polarization was determined with a spectrophotometer (QE65000; Ocean Optics) with a mounted linear polarizer (Glan-Thompson Calcite Polarizer; GTH5M-A) and a quarter wave retarder (Fresnel Rhomb Retarder, FR600QM; both from Thorlabs) and by photo-polarimetric methods as described in Horváth and Varjú (50). To minimize potential brightness cues due to reflection of polarized light, we covered the roof and walls of the magnetic coil with matte white fabric. Light measurements with a radiometer (IL 1400 with detector SHD033; International Light Technologies) confirmed that there were no detectable differences between different alignments of polarized light. Light irradiance levels in the center of the maze varied slightly between testing years (2011: 0.1–1.8 µW/m2; 2012: 9.4 µW/m2; 2013: 9.4–16.5 µW/m2; 2014: 9.4–16.5 µW/m2; 2015: 3.75 µW/m2). However, they were consistent within a bird’s experimental series. We found no differences in orientation between birds tested at different light irradiance levels.
Training and Testing Procedures.
Individual birds were trained to find the food reward under one of three training conditions (Fig. S2 A and B): (i) birds were trained under unpolarized light to either mN or mS (Figs. 1B, 2C, and 3A) or mN, mS, mE, or mW (Fig. 3E); (ii) birds were trained to either mN or mS in the presence of overhead polarized light aligned parallel (Fig. 1F) or perpendicular (Fig. 4A) to the horizontal component of the magnetic field; (iii) birds were trained along an overhead polarized light axis, i.e., they were rewarded in the two trays on either side of the polarized light axis, in a vertical magnetic field (Fig. 2A). For each experimental condition, we balanced the number of birds trained to the different magnetic training directions as much as possible. Within an experimental series (Figs. 1 B–E and F–J and 3 A–D and E–H), individual birds were repeatedly trained under the same training condition and tested in probe trials under different experimental conditions (see below). The order of the different experimental conditions was pseudorandomized as much as possible between individuals to avoid biases or effects of testing order. We found no differences in orientation between birds tested in different orders, thus there is no indication that the birds’ performance improved or worsened over time. The birds in Figs. 2 and 4 were only tested under one experimental condition. Within each experimental condition, only the first valid probe trial (see below) of an individual bird was included, thus each individual bird is only represented once in each group. We balanced the number of birds tested in either one of the four magnetic testing fields and/or the two polarized light axes in each experimental condition.
Training trials.
Training trials took place in the late afternoons (Fig. S2 A and B). Individual birds selected for training were captured from the holding cages and transferred to smaller cages holding 2–3 birds to reduce stress from capture immediately before training and testing. The birds were trained one by one. Each bird was placed in a dark bird bag and transported to the testing hut (approximately 20 m) where all lights were switched off. In complete darkness, the bird was placed inside the central release device (Fig. 1A), facing a random direction. Once the experimenter was seated in the booth, a timer was set and the arena lights were switched on. Thirty seconds later, the experimenter elevated the release device and the bird was free to explore the arena. Each time a bird hopped onto a wrong, empty tray, it was punished by 5–10 s of darkness before allowed to proceed. Once the bird found the food, it was allowed to eat for 30–60 s, before being removed from the maze and returned to the cage. A training trial was considered successful when a bird was able to find the food reward without help from the experimenter by entering no more than 8–10 arms. Birds that did not pass this threshold were excluded from further training the same afternoon.
Birds that successfully found the reward were trained once again 30–90 min after the first training. Between the two training sessions, the location of the rewarded arm was rotated by ±90° together with the alignment of magnetic compass and/or polarized light cues, so that the birds learned that not the absolute (geographic) cues, but instead the magnetic compass/polarized light cues lead to the reward. Learning acquisition with this procedure is extremely rapid (33)—the birds learn the task in only 2–4 training trials. This fast learning is in contrast to traditional conditioning assays where the learning curves of individuals are important measures. In our assay, repeated training does not lead to better performance; however, individual birds can be repeatedly trained and tested under the same or different experimental conditions.
Two successful training trials during the same afternoon, taking no longer than 8 min in total, and a minimum total training quality were necessary to qualify for a probe trial. Training quality (TQ) was defined as follows: birds that found the reward in the first arm they visited received TQ = 1.5; birds that visited at least one arm before finding the reward received TQ = 2. The total TQ was defined as the sum of the TQ from the first training and the second training. Birds that were trained in three arms (i.e., trained at least three times, each time in a different arm) needed a total TQ ≥ 4; birds that had been trained in ≥4 arms needed a minimum total TQ of 3.5. One exception to this rule were birds trained to a polarized light axis (Fig. 2A). Because the chance of successfully finding the reward was 50%, a minimal total TQ = 3 was considered a valid training for birds trained at least four times.
Probe trials.
Birds that successfully passed both training trails were tested in a probe trial in the morning, occasionally in the afternoon of the day after training, or on rare occasions in the morning two days after training. Testing procedures were identical to the training procedures, with the exception that there was no food reward in any of the trays during the probe trials. The birds were allowed to search the maze for 90 s (starting when the bird entered the first arm) without any interference by the tester. At the end of each probe trial, a tray with millet was placed either at the end of the correct arm (if the probe trial conditions were identical to the training conditions) to reinforce training, or in the center of the maze (all other conditions) to neutrally reward the birds, but preventing them from learning anything.
Probe trials in the presence of an RF field: The use of low-intensity, oscillating electromagnetic RF fields in the lower MHz range (<10 MHz, <0.1% of the Earth’s magnetic field intensity) is a unique diagnostic tool to test the radical-pair mechanism, because magnetite-based magnetoreceptors are not affected by RF fields (19, 34). We tested birds under a vertically aligned, single-frequency RF field of 1.4 MHz (Larmor frequency for the testing site) at 80 nT intensity. The RF field was produced by a RF loop antenna (ø 120 cm) constructed from a coaxial cable with the center 2 cm of the shielding removed. It was attached horizontally underneath the testing table and powered by a function/arbitrary waveform generator (Agilent; 33210A 10 MHz) with a broadband amplifier (Toellner TOE 7607, DC to 5 MHz, Toellner Electronic Instrumente). The field was regularly checked with a magnetic field probe (Probe 901 from E & H Near Field set 7405; EST Lindgren) connected to a spectrum analyzer (Agilent N9340B).
Data Analysis.
All probe trials were recorded for subsequent analysis with a CCD camera connected to a digital video recorder. The camera was located above the maze in the center of the overhead light source (Fig. 1A). The current (12 VDC) to the camera was filtered with an RF filter (1JX2454) from Dearborn Electronics). The movies were reduced to 12 frames per second, and the position of each bird was automatically tracked with a video tracking program written in MATLAB 2013b (The MathWorks Inc.). The program provided a complete track of the movements of each bird and automatically determined the start of the track, which was when the bird entered the first arm (Fig. S2C). The program then automatically counted the number of frames that the bird spent in each of the four arms during the 90-s trial, ensuring that the person analyzing the videos had no influence on the final outcome. Preliminary tests showed that the first arm choice did not provide a reliable measure of orientation. Instead, the orientation of an individual bird was calculated from the number of frames the bird spent in each of the four maze arms. We did not distinguish where exactly in the arm the bird was located, but grouped all frames in the north arm as 0°, in the east arm as 90°, in the south arm as 180° and in the west arm as 270°. The number of frames in each arm direction was summed up by vector addition that resulted in an individual mean direction relative to gN and an individual mean vector length for each track as illustrated in Fig. S2D. To guarantee that the mean vectors were based on comparable numbers of frames between animals, we excluded probe trials from subsequent analyses when a bird spent less than half of the time (<45 s or <540 frames) in one of the arms (for numbers of excluded experiments per condition, see Table S13).
The individual mean orientation of birds tested in the presence of a magnetic field was recalculated (Fig. S2E) relative to mN (0°, taking into consideration that different individuals were tested in one of four magnetic fields), and/or relative to the trained magnetic compass direction (correcting for whether a bird was trained to mN or mS). Similarly, the individual mean orientation of birds tested in the presence of polarized light was recalculated (Fig. S2E) relative to the polarized light axis and/or relative to the trained polarized light axis, whereby the north and east end of the polarized light axis was defined as 0°. The trained polarized light direction was defined as 0° for training along the polarized light axis (Figs. 1F and 2A), or 90° for training perpendicular to the polarized light axis (Fig. 4A).
Statistics.
For each experimental condition, the mean orientation of the group of birds was calculated by using vector addition of the individual mean directions, disregarding the individual mean vector lengths. We always calculated the mean orientation relative to topographic North (Tables S1–S12) and relative to the last trained arm (all P > 0.1), which both act as important controls for topographic biases. For all groups, we compared whether a unimodal or axial distribution best fitted the orientation data by calculating the mean vector length for the two distributions. To calculate the axial distributions, we doubled the individual mean angles as described in Batschelet (46). For the distribution that best described the data, i.e., the one with the larger mean vector length, the Rayleigh test was performed to test for significance (46). We used the CI test to examine whether significantly oriented groups of birds were oriented relative to the trained magnetic direction or polarized light axis, i.e., whether the trained direction was included in the 95% CI of the distributions of birds in the different experimental groups (46). Watson U2 tests were used to test for differences in the mean direction and/or spread between significantly oriented groups (46). In cases where two groups with mean vector lengths r > 0.75 were compared (Fig. S1), we used a Watson–William F test. All circular statistics were carried out either with the Matlab Toolbox “CircStat” by Philipp Berens and Marc J. Velasco or Oriana Version 3 (Kovach Computing Services).
Acknowledgments
We thank John B. Phillips for invaluable input to the development of the behavioral assay and critical discussions and comments on the manuscript; Johan Bäckman from the Centre of Animal Movement Research (CAnMove) for help with the experimental setup; Adam Egri and James Foster for help with polarized light measurements; and Brian E. Dalton, Lukas Landler, Miriam Liedvogel, and two anonymous reviewers for comments on the manuscript. This work was financially supported by Swedish Research Council Grants 2007-5700 and 2011-4765, Crafoord Society Grants 2010-1001 and 2013-0737, and the Royal Physiographical Society in Lund (all to R.M.). R.M. is a principal investigator in the Centre of Animal Movement Research financed by Linnaeus Grant 349-2007-8690 from the Swedish Research Council and Lund University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513391113/-/DCSupplemental.
References
- 1.Wiltschko R, Wiltschko W. Magnetic Orientation in Animals. Springer; Berlin: 1995. [Google Scholar]
- 2.Cain SD, Boles LC, Wang JH, Lohmann KJ. Magnetic orientation and navigation in marine turtles, lobsters, and molluscs: Concepts and conundrums. Integr Comp Biol. 2005;45(3):539–546. doi: 10.1093/icb/45.3.539. [DOI] [PubMed] [Google Scholar]
- 3.Muheim R, Edgar NM, Sloan KA, Phillips JB. Magnetic compass orientation in C57BL/6J mice. Learn Behav. 2006;34(4):366–373. doi: 10.3758/bf03193201. [DOI] [PubMed] [Google Scholar]
- 4.Phillips JB, Jorge PE, Muheim R. Light-dependent magnetic compass orientation in amphibians and insects: Candidate receptors and candidate molecular mechanisms. J R Soc Interface. 2010;7(Suppl 2):S241–S256. doi: 10.1098/rsif.2009.0459.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begall S, Malkemper EP, Červený J, Němec P, Burda H. Magnetic alignment in mammals and other animals. Mamm Biol - Z Saugetierkd. 2013;78(1):10–20. [Google Scholar]
- 6.Painter MS, Dommer DH, Altizer WW, Muheim R, Phillips JB. Spontaneous magnetic orientation in larval Drosophila shares properties with learned magnetic compass responses in adult flies and mice. J Exp Biol. 2013;216(Pt 7):1307–1316. doi: 10.1242/jeb.077404. [DOI] [PubMed] [Google Scholar]
- 7.Phillips JB, Muheim R, Jorge PE. A behavioral perspective on the biophysics of the light-dependent magnetic compass: A link between directional and spatial perception? J Exp Biol. 2010;213(Pt 19):3247–3255. doi: 10.1242/jeb.020792. [DOI] [PubMed] [Google Scholar]
- 8.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78(2):707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnsen S, Lohmann KJ. The physics and neurobiology of magnetoreception. Nat Rev Neurosci. 2005;6(9):703–712. doi: 10.1038/nrn1745. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers CT, Hore PJ. Chemical magnetoreception in birds: The radical pair mechanism. Proc Natl Acad Sci USA. 2009;106(2):353–360. doi: 10.1073/pnas.0711968106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouritsen H, Hore PJ. The magnetic retina: Light-dependent and trigeminal magnetoreception in migratory birds. Curr Opin Neurobiol. 2012;22(2):343–352. doi: 10.1016/j.conb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Solov’yov IA, Mouritsen H, Schulten K. Acuity of a cryptochrome and vision-based magnetoreception system in birds. Biophys J. 2010;99(1):40–49. doi: 10.1016/j.bpj.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau JCS, Rodgers CT, Hore PJ. Compass magnetoreception in birds arising from photo-induced radical pairs in rotationally disordered cryptochromes. J R Soc Interface. 2012;9(77):3329–3337. doi: 10.1098/rsif.2012.0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liedvogel M, Mouritsen H. Cryptochromes--a potential magnetoreceptor: What do we know and what do we want to know? J R Soc Interface. 2010;7(Suppl 2):S147–S162. doi: 10.1098/rsif.2009.0411.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouritsen H, et al. Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. Proc Natl Acad Sci USA. 2004;101(39):14294–14299. doi: 10.1073/pnas.0405968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niessner C, et al. Avian ultraviolet/violet cones identified as probable magnetoreceptors. PLoS One. 2011;6(5):e20091. doi: 10.1371/journal.pone.0020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nießner C, et al. Magnetoreception: Activated cryptochrome 1a concurs with magnetic orientation in birds. J R Soc Interface. 2013;10(88):20130638. doi: 10.1098/rsif.2013.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muheim R, Bäckman J, Åkesson S. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J Exp Biol. 2002;205(Pt 24):3845–3856. doi: 10.1242/jeb.205.24.3845. [DOI] [PubMed] [Google Scholar]
- 19.Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature. 2004;429(6988):177–180. doi: 10.1038/nature02534. [DOI] [PubMed] [Google Scholar]
- 20.Ritz T, et al. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys J. 2009;96(8):3451–3457. doi: 10.1016/j.bpj.2008.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stapput K, Güntürkün O, Hoffmann K-P, Wiltschko R, Wiltschko W. Magnetoreception of directional information in birds requires nondegraded vision. Curr Biol. 2010;20(14):1259–1262. doi: 10.1016/j.cub.2010.05.070. [DOI] [PubMed] [Google Scholar]
- 22.Wiltschko R, Stapput K, Thalau P, Wiltschko W. Directional orientation of birds by the magnetic field under different light conditions. J R Soc Interface. 2010;7(Suppl 2):S163–S177. doi: 10.1098/rsif.2009.0367.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda K, et al. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc Natl Acad Sci USA. 2012;109(13):4774–4779. doi: 10.1073/pnas.1118959109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoneham AM, Gauger EM, Porfyrakis K, Benjamin SC, Lovett BW. A new type of radical-pair-based model for magnetoreception. Biophys J. 2012;102(5):961–968. doi: 10.1016/j.bpj.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brines ML, Gould JL. Skylight polarization patterns and animal orientation. J Exp Biol. 1982;96(1):69–91. [Google Scholar]
- 26.Cronin TW, Warrant EJ, Greiner B. Celestial polarization patterns during twilight. Appl Opt. 2006;45(22):5582–5589. doi: 10.1364/ao.45.005582. [DOI] [PubMed] [Google Scholar]
- 27.Horváth G, Barta A, Hegedüs R. In: Polarization of the Sky. Polarized Light and Polarization Vision in Animal Sciences, Springer Series in Vision Research. Horváth G, editor. Springer; Berlin: 2014. pp. 367–406. [Google Scholar]
- 28.Johansson LBA, Davidsson A, Lindblom G, Naqvi KR. Electronic transitions in the isoalloxazine ring and orientation of flavins in model membranes studied by polarized light spectroscopy. Biochemistry. 1979;18(19):4249–4253. doi: 10.1021/bi00586a033. [DOI] [PubMed] [Google Scholar]
- 29.Phillips JB, Deutschlander ME, Freake MJ, Borland SC. The role of extraocular photoreceptors in newt magnetic compass orientation: Parallels between light-dependent magnetoreception and polarized light detection in vertebrates. J Exp Biol. 2001;204(Pt 14):2543–2552. doi: 10.1242/jeb.204.14.2543. [DOI] [PubMed] [Google Scholar]
- 30.Muheim R. Behavioural and physiological mechanisms of polarized light sensitivity in birds. Philos Trans R Soc Lond B Biol Sci. 2011;366(1565):763–771. doi: 10.1098/rstb.2010.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horváth G. Polarized Light and Polarization Vision in Animal Sciences. Springer; Berlin: 2014. [Google Scholar]
- 32.Roberts NW, Porter ML, Cronin TW. The molecular basis of mechanisms underlying polarization vision. Philos Trans R Soc Lond B Biol Sci. 2011;366(1565):627–637. doi: 10.1098/rstb.2010.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips JB, et al. Rapid learning of magnetic compass direction by C57BL/6 mice in a 4-armed ‘plus’ water maze. PLoS One. 2013;8(8):e73112. doi: 10.1371/journal.pone.0073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henbest KB, Kukura P, Rodgers CT, Hore PJ, Timmel CR. Radio frequency magnetic field effects on a radical recombination reaction: A diagnostic test for the radical pair mechanism. J Am Chem Soc. 2004;126(26):8102–8103. doi: 10.1021/ja048220q. [DOI] [PubMed] [Google Scholar]
- 35.Voss J, Keary N, Bischof H-J. The use of the geomagnetic field for short distance orientation in zebra finches. Neuroreport. 2007;18(10):1053–1057. doi: 10.1097/WNR.0b013e32818b2a21. [DOI] [PubMed] [Google Scholar]
- 36.Keary N, et al. Oscillating magnetic field disrupts magnetic orientation in Zebra finches, Taeniopygia guttata. Front Zool. 2009;6(1):25. doi: 10.1186/1742-9994-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Åkesson S. In: The Ecology of Polarisation Vision in Birds. Polarized Light and Polarization Vision in Animal Sciences, Springer Series in Vision Research. Horváth G, editor. Springer; Berlin: 2014. pp. 275–292. [Google Scholar]
- 38.Muheim R, Moore FR, Phillips JB. Calibration of magnetic and celestial compass cues in migratory birds--a review of cue-conflict experiments. J Exp Biol. 2006;209(Pt 1):2–17. doi: 10.1242/jeb.01960. [DOI] [PubMed] [Google Scholar]
- 39.Phillips JB, Waldvogel JA. Celestial polarized light patterns as a calibration reference for sun compass of homing pigeons. J Theor Biol. 1988;131(1):55–67. [Google Scholar]
- 40.Muheim R, Phillips JB, Åkesson S. Polarized light cues underlie compass calibration in migratory songbirds. Science. 2006;313(5788):837–839. doi: 10.1126/science.1129709. [DOI] [PubMed] [Google Scholar]
- 41.Muheim R, Phillips JB, Deutschlander ME. White-throated sparrows calibrate their magnetic compass by polarized light cues during both autumn and spring migration. J Exp Biol. 2009;212(Pt 21):3466–3472. doi: 10.1242/jeb.032771. [DOI] [PubMed] [Google Scholar]
- 42.Able KP. Skylight polarization patterns at dusk influence migratory orientation in birds. Nature. 1982;299(5883):550–551. [Google Scholar]
- 43.Able KP. Skylight polarization patterns and the orientation of migratory birds. J Exp Biol. 1989;141(1):241–256. [Google Scholar]
- 44.Greenwood VJ, Smith EL, Church SC, Partridge JC. Behavioural investigation of polarisation sensitivity in the Japanese quail (Coturnix coturnix japonica) and the European starling (Sturnus vulgaris) J Exp Biol. 2003;206(Pt 18):3201–3210. doi: 10.1242/jeb.00537. [DOI] [PubMed] [Google Scholar]
- 45.Melgar J, Lind O, Muheim R. No response to linear polarization cues in operant conditioning experiments with zebra finches. J Exp Biol. 2015;218(Pt 13):2049–2054. doi: 10.1242/jeb.122309. [DOI] [PubMed] [Google Scholar]
- 46.Batschelet E. Circular Statistics in Biology. Academic; London: 1981. [Google Scholar]
- 47.Freire R, Munro UH, Rogers LJ, Wiltschko R, Wiltschko W. Chickens orient using a magnetic compass. Curr Biol. 2005;15(16):R620–R621. doi: 10.1016/j.cub.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Engels S, et al. Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature. 2014;509(7500):353–356. doi: 10.1038/nature13290. [DOI] [PubMed] [Google Scholar]
- 49.Kirschvink JL. Uniform magnetic fields and double-wrapped coil systems: Improved techniques for the design of bioelectromagnetic experiments. Bioelectromagnetics. 1992;13(5):401–411. doi: 10.1002/bem.2250130507. [DOI] [PubMed] [Google Scholar]
- 50.Horváth G, Varju D. Polarization pattern of freshwater habitats recorded by video polarimetry in red, green and blue spectral ranges and its relevance for water detection by aquatic insects. J Exp Biol. 1997;200(7):1155–1163. doi: 10.1242/jeb.200.7.1155. [DOI] [PubMed] [Google Scholar]