Significance
The Polycomb protein (PC) is well known for its role in transcriptional silencing and binding to trimethylated histone H3 Lys27 (H3K27me3). We report here that PC inhibits the histone acetyltransferase (HAT) activity of CREB-binding protein (CBP). PC interacts directly with the CBP HAT domain, binding to its autoregulatory loop, whose autoacetylation greatly enhances enzyme activity. PC binding inhibits histone H3 acetylation. Interestingly, CBP autoacetylation impairs PC binding in vitro, and PC is preferentially associated with unacetylated CBP in vivo. Altering PC levels in vivo alters the acetylated H3K27 (H3K27ac) level in a predictable manner. PC inhibition of CBP HAT activity at enhancers and promoters with paused RNA polymerase II may affect regulation of both repressed and active genes.
Keywords: Polycomb, CBP, acetylation, histone H3K27, Drosophila
Abstract
Drosophila Polycomb (PC), a subunit of Polycomb repressive complex 1 (PRC1), is well known for its role in maintaining repression of the homeotic genes and many others and for its binding to trimethylated histone H3 on Lys 27 (H3K27me3) via its chromodomain. Here, we identify a novel activity of PC: inhibition of the histone acetylation activity of CREB-binding protein (CBP). We show that PC and its mammalian CBX orthologs interact directly with the histone acetyltransferase (HAT) domain of CBP, binding to the previously identified autoregulatory loop, whose autoacetylation greatly enhances HAT activity. We identify a conserved PC motif adjacent to the chromodomain required for CBP binding and show that PC binding inhibits acetylation of histone H3. CBP autoacetylation impairs PC binding in vitro, and PC is preferentially associated with unacetylated CBP in vivo. PC knockdown elevates the acetylated H3K27 (H3K27ac) level globally and at promoter regions of some genes that are bound by both PC and CBP. Conversely, PC overexpression decreases the H3K27ac level in vivo and also suppresses CBP-dependent Polycomb phenotypes caused by overexpression of Trithorax, an antagonist of Polycomb silencing. We find that PC is physically associated with the initiating form of RNA polymerase II (Pol II) and that many promoters co-occupied by PC and CBP are associated with paused Pol II, suggesting that PC may play a role in Pol II pausing. These results suggest that PC/PRC1 inhibition of CBP HAT activity plays a role in regulating transcription of both repressed and active PC-regulated genes.
The Polycomb group (PcG) and Trithorax group (TrxG) proteins are well known for their mutually antagonistic roles in maintaining, respectively, stable heritable repression and activation of genes that specify the different cell identities comprising the body plans of multicellular organisms. Two principal types of PcG-containing complexes, termed Polycomb repressive complex 1 (PRC1) and PRC2, have been identified in Drosophila and in mammals (1). PRC1 and PRC2 are recruited to their target genes by specialized “Polycomb response elements” (PREs) in Drosophila (2, 3) and by unmethylated CpG islands in mammals (4). The discovery of enzyme activities associated with PRC2 and PRC1 has provided important insights into their functions. PRC2 trimethylates histone H3 on Lys27 (H3K27me3), and the genome-wide distribution of its H3K27me3 product is highly correlated with transcriptionally silent genes (5). Moreover, Drosophila harboring a histone H3K27R or H3K27A point mutation fails to silence PcG target genes, indicating that this modification is essential for silencing (6, 7). The repressive effect of H3K27 methylation by PRC2 is thought to be due, in part, to direct blocking of H3K27 acetylation (H3K27ac) (8), a mark of active enhancers and promoters, because methyl- and acetyl modifications of the Lys ε-amino group are mutually exclusive.
Biochemical studies have shown that PRC1, composed of core subunits Polycomb (PC), PH, PSC, and RING/Sex combs extra (SCE), can exert a repressive effect on transcription from chromatin templates in vitro by inhibiting nucleosome remodeling (9, 10) and transcription initiation (11) and by promoting chromatin compaction (12, 13). The RING and PSC subunits of PRC1 have been shown to mediate ubiquitylation of histone H2AK118 (K119 in mammals). This modification has been reported to be linked to Polycomb silencing in mammalian ES cells (14, 15) but is dispensable for silencing in Drosophila (16) and for mouse embryogenesis (13, 17). The PC subunit contains a conserved N-terminal chromodomain (18) that binds specifically to the H3K27me3 mark deposited by PRC2 (19, 20), thereby targeting the chromatin compaction and other activities of PRC1 to H3K27me3-containing nucleosomes. Consistent with this observation, the genome-wide binding pattern of PRC1 is highly correlated with inactive genes marked by H3K27me3. However, several recent studies have revealed that PRC1 is also bound at promoters of many active genes that contain little or no H3K27me3 (21, 22), suggesting that PRC1 can be targeted independent of the PC chromodomain and may also negatively modulate transcription of active genes.
The repressive effects of PRC1 and PRC2 are antagonized by TrxG proteins, which include histone modifying and chromatin remodeling enzymes. Prominent among the antagonistic activities associated with TrxG proteins is H3K27 acetylation, which is catalyzed by the acetyltransferase CREB-binding protein (CBP) in Drosophila and by the closely related CBP and p300 proteins in mammals (8). We previously found that some H3K27ac is dependent on the Trithorax protein (TRX) (8), a well-known antagonist of Polycomb silencing, and reflects a direct interaction of TRX with CBP (23). Moreover, both the elevated H3K27ac level and impaired Polycomb silencing phenotypes caused by overexpression of TRX in vivo are suppressed by reducing the CBP level (23).
CBP and p300 play important roles as transcriptional coactivators. Their histone acetyltransferase (HAT) activity is required for CBP/p300-dependent transcription from chromatin in vitro and in vivo (24, 25). They are recruited by hundreds of different transcription factors (26, 27) to specific genomic sites, notably enhancers, where they acetylate multiple Lys residues in their histone and nonhistone substrates, including many transcription factors (28). Sequence-specific targeting of p300 catalytic domain to specific enhancers and promoters, using a Cas9-p300 fusion protein, is sufficient to induce H3K27 acetylation and robust transcription of the associated genes (29).
CBP and p300 are large modular proteins with multiple conserved domains, including KIX, Cys/His-rich region 1 (CH1), bromodomain (BD), CH2 (composed of a discontinuous PHD finger domain interrupted by a RING finger domain), HAT, and CH3 domains. Their tandemly arranged BD, PHD, RING, and HAT domains are required for robust acetyltransferase activity in vitro and in vivo. A recent p300 crystal structure (30) revealed that these domains form a single compact structural module referred to as the “catalytic core.” The RING domain is positioned over the substrate-binding pocket of the HAT domain, with which it makes multiple contacts, partially occluding it, suggesting that the RING domain may play a role in regulating substrate binding and/or HAT activity of the native enzyme (30).
The p300 HAT activity toward its histone and nonhistone substrates is activated by autoacetylation and inactivated by deacetylation by SIRT2 (31). Autoacetylation occurs in trans, predominantly on multiple conserved Lys residues within a flexible “autoinhibitory loop” (AIL) (also known as an autoacetylation loop) and greatly stimulates HAT activity (32). It induces conformational changes within the HAT domain (33) that are thought to allow access to the active site by histones and other substrates (30). Autoacetylation of the AIL thus acts like a switch to potentiate robust acetylation activity.
Here, we show that PC binds directly to the CBP HAT domain via a major contact with the CBP AIL. We identify a short PC motif with a Lys Arg Gly (KRG) core that is required for this interaction and is conserved in all five mammalian PC orthologs (CBX2, CBX4, CBX6, CBX7, and CBX8). We show that PC competes with histone H3 for CBP binding and inhibits H3 acetylation. PC binding to the AIL in vitro is impaired by CBP autoacetylation, and PC is preferentially associated with unacetylated CBP in vivo, further suggesting that PC binding negatively regulates CBP HAT activity on chromatin. Knockdown of PC elevates the bulk H3K27ac level and the H3K27ac level at promoter regions of some genes targeted by both PC and CBP. Overexpression of PC also reduces the elevated H3K27ac level and the Polycomb phenotypes caused by TRX overexpression. Our findings thus identify a previously unknown conserved activity of PC in addition to the well-known binding of its chromodomain to H3K27me3-marked nucleosomes. We propose that PC inhibition of CBP HAT activity plays a role in establishing and maintaining transcriptionally inactive chromatin states of PcG-regulated genes as well as modulating transcription of some active PcG-regulated genes.
Results
PC Is Associated with Drosophila CBP in Vivo.
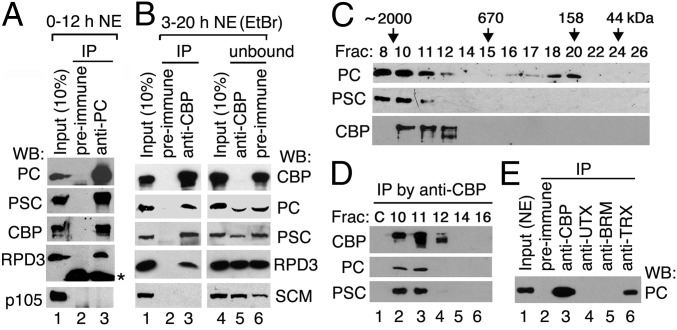
To determine whether PC and CBP are physically associated in vivo, we performed immunoprecipitation (IP) from embryo nuclear extract (NE). CBP was present in immunoprecipitates obtained with anti-PC Abs (Fig. 1A, lane 3). In reciprocal IP with anti-CBP Abs, PC and the PRC1 subunit PSC [but not Sex comb on midleg (SCM)] were coimmunoprecipitated with CBP (Fig. 1B, lane 3). In the residual unbound material, small but noticeable amounts of PC and PSC were depleted by anti-CBP Abs (Fig. 1B, compare lane 5 with lane 6), suggesting that a portion of PC (and likely PRC1) is stably associated with CBP in vivo. Interestingly, RPD3, which associates with PRC1 and PRC2 and is required for deacetylation of H3K27ac (8), coimmunoprecipitated not only with PC (Fig. 1A, lane 3) as previously reported (34) but also with CBP (Fig. 1B, lane 3), suggesting that a small portion of RPD3 may be associated with the CBP/PC complex.
Fig. 1.
PC is physically associated with Drosophila CBP. (A and B) Immunoblots of CBP and PRC1 subunits after IP from embryo NEs with anti-PC and anti-CBP Abs (lane 3). NEs were treated with ethidium bromide (EtBr) to eliminate DNA-mediated protein associations. An asterisk next to the RPD3 blot in A indicates the heavy chain of IgG. (C) Fractionation of NE on a Superose 6 (size exclusion) column. Proteins in fractions were analyzed by Western blots (WBs). The size standards and fraction numbers are indicated at the top. (D) IP from fractions 10, 11, 12, 14, and 16 in C with anti-CBP Abs. Mock IP from NE without Abs serves as a negative control (lane C). (E) PC is also associated with TRX, but not with UTX or BRM. IPs were performed with anti-CBP (positive control), anti-UTX, anti-BRM, and anti-TRX Abs (lanes 3–6). Preimmune serum (lane 2 in A, B, and E) serves as a negative control.
To confirm the association of PC and CBP further, we fractionated NEs on a size exclusion column. PRC1 subunits PC and PSC are present in fractions 8–11 and are cofractionated with CBP in fractions 10 and 11 but not in fraction 8 (∼2 MDa) (Fig. 1C). Furthermore, both PC and PSC could be coimmunoprecipitated with CBP from fractions 10 and 11 (Fig. 1D), suggesting that PRC1 may be associated with CBP in a 1.5- to 1.8-MDa complex.
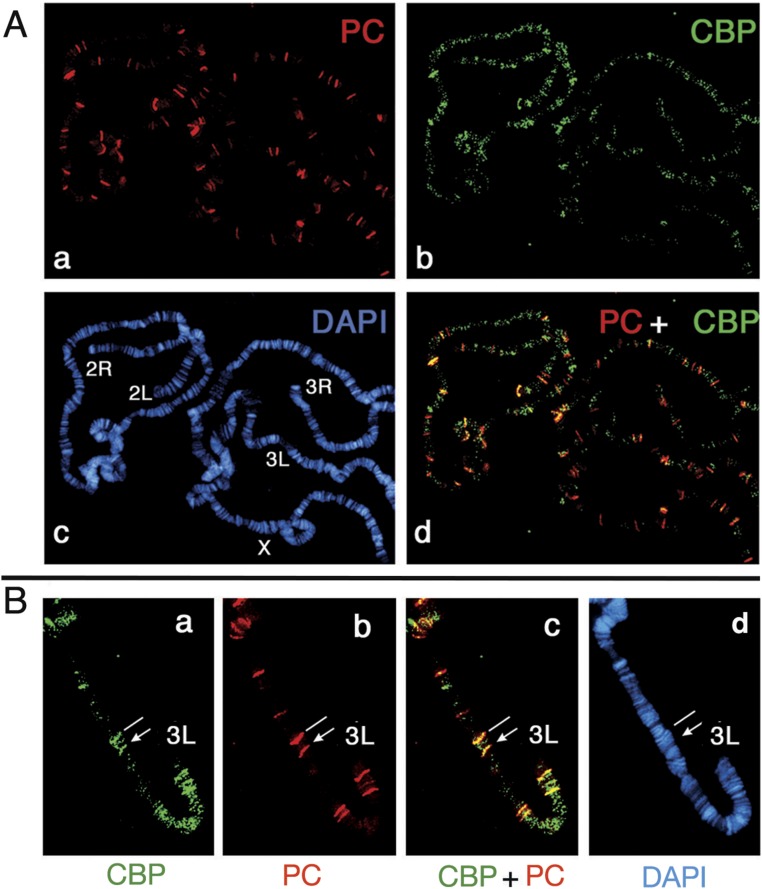
We also examined the colocalization of PC and CBP on salivary gland polytene chromosomes by double immunostaining. CBP can be recruited to the insertion site of a transgene containing a PRE (35). More than one-third of identifiable PC-bound sites colocalized with CBP, and both are recruited to the insertion site of a transgene containing a 670-bp Ubx PRE (Fig. S1 A and B). This result is consistent with our previous observation from ChIP-chip data that PC and CBP co-occupy many genomic sites (36) with active or repressed marks (37).
Fig. S1.
Partial colocalization of PC and CBP on salivary gland polytene chromosomes. (A) PC- and CBP-binding sites on chromosomes were detected with affinity-purified rabbit anti-PC (a) and chicken anti-CBP Abs (b). DAPI-stained DNA and merged images of PC and CBP are shown in c and d. (B) Chromosomal-binding sites of PC and CBP on the distal portion of chromosome arm 3L are shown in a transformant containing the 670-bp PstI-NdeI fragment of the Ubx PRE inserted at 65B. A landmark site at 65D, which is bound by PC and CBP as well as other PcG proteins, is indicated by a white line. Note that both PC and CBP were recruited to this PRE insertion site (indicated by arrows).
We previously showed that CBP is physically associated with several TrxG proteins in Drosophila embryos, including the H3K4 methyltransferase TRX (23), the H3K27 demethylase UTX, and the chromatin remodeler BRM (36). We found that PC was absent from IPs obtained with either anti-UTX or anti-BRM Abs (Fig. 1E, lanes 4 and 5), suggesting that CBP/PC complexes are distinct from CBP complexes containing UTX or BRM. However, PC was coimmunoprecipitated with TRX (lane 6), suggesting that PC may be associated with a CBP/TRX complex (23, 35). Consistent with this possibility, PC and TRX co-occupy many PcG-target genes genome-wide (21, 38).
PC Interacts with the CBP HAT Domain.
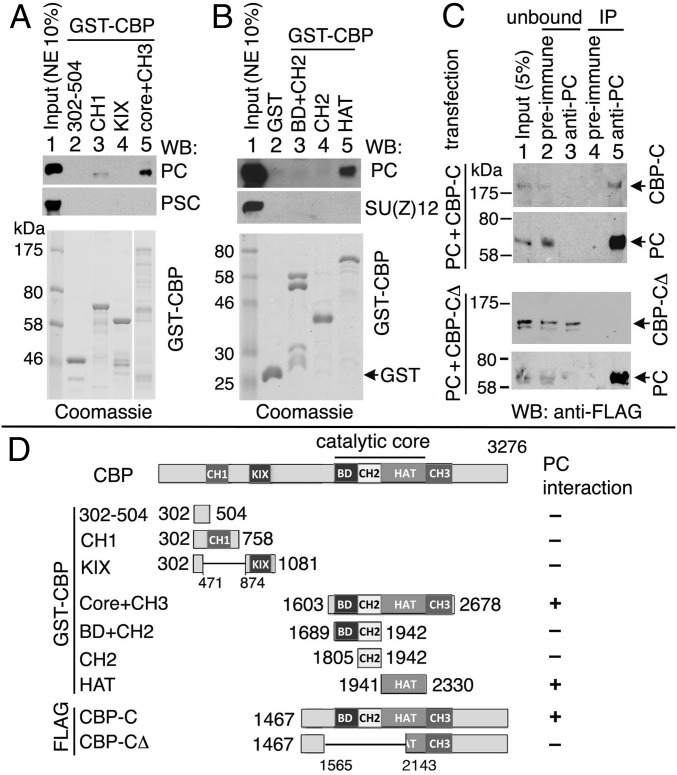
We mapped the region of CBP that interacts with PC by GST pull-down assays, initially by using a panel of GST-CBP fusion proteins to pull down endogenous PC from NE. The GST-CBP fusion proteins cover all of the conserved domains of CBP: CH1 (residues 524–594); the KIX domain (residues 938–1,018); CH3 (residues 2,333–2,470); the catalytic core (residues 1,698–2,330), which includes the BD (residues 1,699–1,806); the CH2 region (PHD and RING domains) (residues 1,807–1,940); and the HAT domain (residues 1,941–2,330) (Fig. 2D). GST-CBP1603-2678 (including catalytic core + CH3) pulled down PC (Fig. 2A, lane 5), but CH1 and KIX did not (Fig. 2A, lanes 3 and 4). Further mapping of the PC-binding region within CBP revealed that PC interacts with the HAT domain (Fig. 2B, lane 5).
Fig. 2.
CBP HAT domain interacts with PC. (A and B) Purified GST-CBP fragments (Bottom, Coomassie blue staining; constructs are shown in D) and embryo NE were used in pull-down assays. PC, PSC, and SU(Z)12 were analyzed by WBs. (C) Transiently coexpressed FLAG-PC and FLAG-CBP-C or FLAG-PC and FLAG-CBP-CΔ (deletion of the central region of CBP-C) in S2 cells were immunoprecipitated with anti-PC Abs (lane 5) and analyzed by WBs with anti-FLAG mAbs. Preimmune serum (lane 4) was used as a negative control. Unbound proteins are shown in lanes 2 and 3. (D) Schematic summary of GST-CBP and FLAG-CBP constructs and results of PC binding to CBP.
To confirm this interaction in vivo, we cotransfected Drosophila S2 cells and performed IP. When FLAG-PC and FLAG-CBP-C (including catalytic core to C terminus) were coexpressed in S2 cells, both FLAG-PC and FLAG-CBP-C were immunoprecipitated from cell extracts with anti-PC Abs (Fig. 2C, Top, lane 5) but not with preimmune serum (Fig. 2C, Top, lane 4), indicating a specific association of PC with CBP-C in vivo. However, when FLAG-PC was coexpressed with FLAG-CBP-CΔ containing a deletion of BD, CH2, and a part of HAT (Fig. 2D), only FLAG-PC, but not CBP-CΔ, was immunoprecipitated with anti-PC (Fig. 2C, Bottom). All CBP constructs and PC interaction results are summarized in Fig. 2D.
PC Binds Directly to the AIL Within the CBP HAT Domain.
We used recombinant PC and GST-CBP purified from Escherichia coli to determine whether PC interacts directly with CBP in vitro. We found that PC was pulled down by GST-CBP fusion proteins containing the entire catalytic core or just the HAT domain (Fig. 3A, Top, lanes 3 and 6), but not by GST-CBP fusion proteins containing just the BD and CH2 domains (Fig. 3A, Top, lanes 4 and 5), indicating direct interaction between PC and CBP HAT. By further examining N- and C-terminal halves of the HAT domain, we found that HAT-C (CBP2132–2330), which includes the ∼70-residue AIL, retained strong PC-binding ability, whereas HAT-N (CBP1941–2138) mediates a weaker interaction with PC (Fig. 3A, Top, lanes 7 and 8).
Fig. 3.
PC75–228 binds directly to CBP HAT and AIL. (A and B) Purified GST-CBP fragments (Bottom, Coomassie blue staining; constructs are shown in C) and purified PC (full-length and fragments) were used in pull-down assays. PC was detected by WB with anti-His Ab. (C) Schematic summary of GST-CBP constructs tested and results of PC binding. (Top) Conserved chromodomain (CD) and conserved C-terminal motif (c-box) are indicated in black in the schematic of PC. Note that CD-deleted PC and PC75–228 are sufficient for interaction of CBP HAT-C1 (residues 2,132–2,247, including AIL).
To map the region of PC that is responsible for its direct binding to CBP, we generated recombinant PC fragments and tested their binding to GST-CBP fusions to catalytic core fragments. PC1–86, containing the chromodomain (residues 25–74), did not bind to CBP, whereas the complementary PC75–390 missing the chromodomain retained binding to GST-CBP HAT (Fig. 3A, panels 2 and 3). PC75–228, missing both the chromodomain and the conserved C-terminal RING/SCE-binding motif (39, 40), is sufficient for the PC interaction with CBP HAT, whereas PC222–390 only binds weakly (Fig. 3A, panels 4 and 5).
In tests of subfragments of HAT-C, HAT-C1 (CBP2132–2247, containing the AIL) is sufficient for strong PC binding (Fig. 3B, lane 4), whereas HAT-C2 (CBP2132–2193, which lacks the AIL) binds weakly to full-length PC but not to PC75–228 (Fig. 3B, panels 1 and 2, lane 5). Furthermore, GST-AIL (CBP2175–2247) also pulled down PC (full-length) and PC75–228 (Fig. 3B, lane 6), although their signals are not as strong as HAT-C1 (Fig. 3B, lane 4). PC222–390 barely binds to the AIL but binds the region preceding the AIL (HAT-C1 and HAT-C2) (Fig. 3B, panel 3). Deletion of two poly-His stretches in the central region of PC did not affect its binding (Fig. 3B, panel 4). All tested GST-CBP constructs and PC-binding results are summarized in Fig. 3C. We conclude that the AIL-containing HAT-C1 is sufficient for a robust PC interaction. PC75–228 binds directly to the CBP AIL, and PC222–390 binds weakly to the region preceding the AIL.
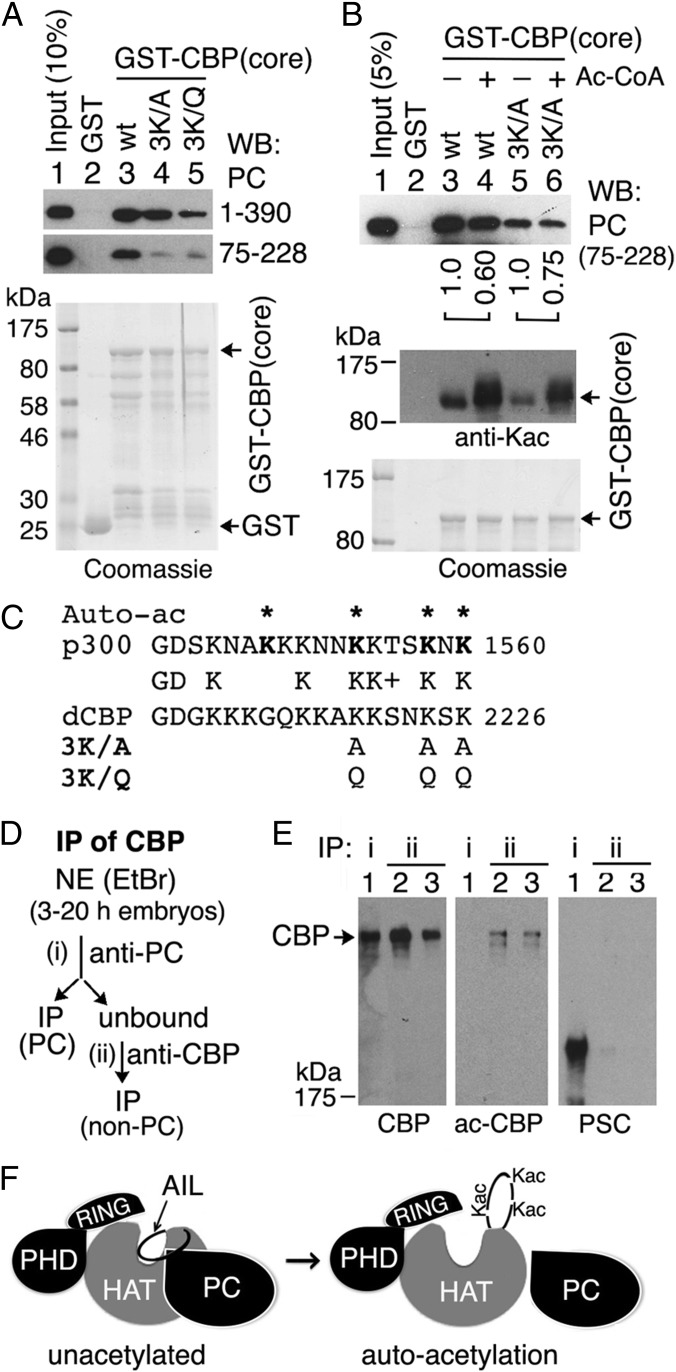
Autoacetylation of CBP Impairs Its Interaction with PC.
The AIL becomes highly acetylated by autoacetylation (32). We mutated a cluster of three highly conserved Lys residues in the Drosophila CBP AIL (indicated in boldface: KKxxKxK 2226) that are autoacetylated in mammalian p300 (32) to Ala or Gln (3K/A and 3K/Q in Fig. 4C) and tested them in GST pull-downs. Compared with the wild-type (wt) GST-CBP catalytic core (Fig. 4A, lane 3), the presence of the 3K/A mutation in the AIL slightly weakened the interaction with full-length PC (Fig. 4A, lane 4), whereas the 3K/Q mutation, which mimics Lys acetylation, greatly impaired PC binding (Fig. 4A, lane 5), suggesting that acetylation of one or more of these Lys may impair the interaction of the AIL with full-length PC. However, both the 3K/A and 3K/Q mutations almost abolished the interaction between GST-CBP catalytic core and PC75–228 (Fig. 4A, panel 2, lanes 4 and 5), suggesting that one or more of these Lys are also particularly important for the interaction of the CBP AIL with this central region of PC that binds most strongly to the AIL. This result also suggests that the weaker effect of the 3K/A mutation on binding of the CBP core to full-length PC is due to additional CBP core contacts with PC sequences that lie outside of the PC75–228 polypeptide.
Fig. 4.
CBP autoacetylation impairs PC binding. (A) GST-CBP pull-down using GST-CBP catalytic core wt and mutants in which three conserved Lys residues in the AIL are mutated to Ala (3K/A) or Gln (3K/Q) (sequence is shown in C). (B) GST-CBP wt and 3K/A mutant, with (+) or without (−) preincubation with acetyl-CoA (Ac-CoA) to allow in vitro autoacetylation, were used to pull down PC75–228. Relative PC75–228 levels (lane 4/lane3, lane 6/lane 5), quantified by a GE Typhoon TRIO Imager, are shown. (Middle) Autoacetylated GST-CBP core proteins (wt and mutants) were detected by an antiacetylated Lys (Kac) Ab. GST and GST-CBP core (wt and mutations) were stained with Coomassie blue (A, Bottom and B, Bottom). (C) Partial sequences of human p300 and Drosophila CBP AIL are shown. Four autoacetylation sites in p300 are marked by asterisks above the sequence. The 3K/A and 3K/Q mutations are shown below sequences. (D and E) PC is associated with unacetylated CBP in vivo. (D) Schematic for IPs of CBP: (i) IP of CBP by anti-PC from embryo NE treated with EtBr and (ii) IP of CBP by anti-CBP Abs from the unbound by anti-PC IP. (E) Western blots were performed for CBP, acetylated CBP (ac-CBP), and PSC. IP of CBP by anti-CBP Abs (lanes 2 and 3) was diluted to the similar amount of CBP IP by anti-PC Ab (lane 1). (F) Model of PC interaction with CBP HAT. PC binds to unacetylated (or hypoacetylated) AIL and HAT domain (Left) but is unassociated with hyperacetylated AIL and HAT (Right) after CBP autoacetylation, which may induce conformational changes within the HAT domain (33).
The GST-CBP catalytic core becomes partially autoacetylated when expressed in E. coli, and the presence of the 3K/A mutation resulted in reduced autoacetylation in E. coli (Fig. 4B, Middle, compare lane 5 with lane 3), suggesting that one or more of these Lys residues are also autoacetylation sites in Drosophila CBP. To test whether autoacetylation affects the binding of CBP to PC, the wt and 3K/A mutant GST-CBP catalytic core proteins were both further autoacetylated in vitro by incubating them with acetyl-CoA (Fig. 4B, Middle, compare lane 4 with lane 3 and lane 6 with lane 5). This hyperacetylation impaired PC binding in pull-down assays (Fig. 4B, Top, compare lane 4 with lane 3 and lane 6 with lane 5), suggesting that PC binds preferentially to the hypo- or unacetylated CBP HAT in vivo. To test this possibility, we compared the acetylation status of the CBP present in anti-PC immunoprecipitates from embryo NE with the acetylation status of the CBP remaining in the residual unbound soluble fraction after anti-PC IP, which was then recovered by anti-CBP IP (Fig. 4D). CBP, acetylated CBP, and PSC were determined by Western blots (Fig. 4E). Both CBP and PSC were readily detected in anti-PC immunoprecipitates (Fig. 4E, lane 1), but acetylated CBP was not (Fig. 4E, Middle, lane 1). Acetylated CBP, assayed with a “pan–acetyl-Lys” Ab, was only detected in the unbound fraction (i.e., not associated with PC) (Fig. 4E, lanes 2 and 3), indicating that PC is preferentially associated with unacetylated or hypoacetylated CBP in vivo (Fig. 4F).
PC Binding to the CBP HAT Domain Is Conserved in Mammals.
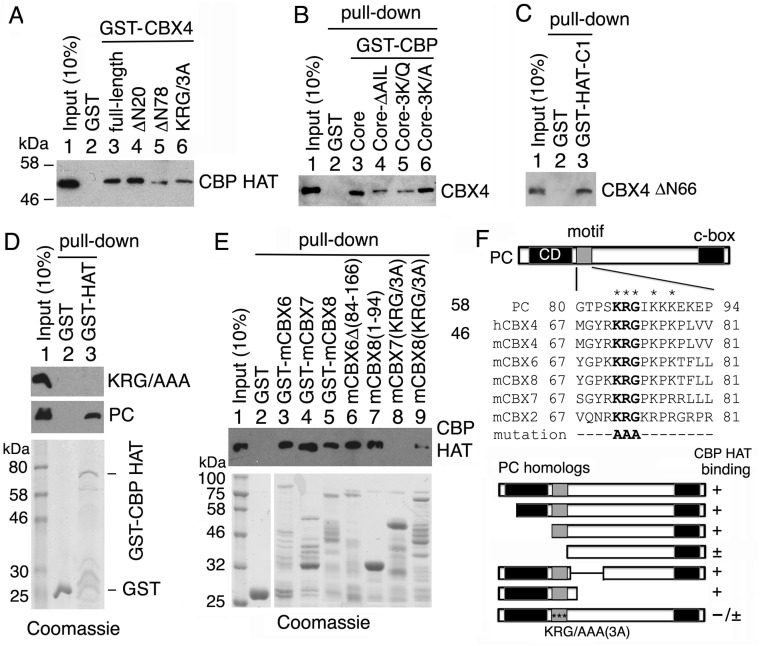
To determine whether there is a conserved CBP-binding motif in PC and its mammalian orthologs, we aligned their sequences and found a short conserved region just past the chromodomain. This region is rich in basic amino acid residues, has a KRG core (Fig. S2F), and is present only in PC orthologs (CBX2, CBX4, CBX6, CBX7, and CBX8) and not in the related HP1 class of chromodomain proteins (CBX1, CBX3, and CXB5). We performed GST pull-down assays using purified CBX4 (also known as human PC2). GST-CBX4 (full-length) and a CBX4 with a deletion of the N-terminal 20 residues (ΔN20), including part of its chromodomain, pulled down CBP HAT (Fig. S2A, lanes 3 and 4), whereas deletion of the N-terminal 78 residues (ΔN78) or substitution of KRG73 with three Ala's (AAA) severely impaired CBP HAT binding (Fig. S2A, lanes 5 and 6). In reciprocal pull-down assays, GST-CBP core and GST-CBP-C1 pulled down CBX4 and its chromodomain-deleted form CBX4ΔN66 (Fig. S2 B and C, lane 3). Furthermore, the GST-CBP core containing either the AIL deletion or the 3K/Q mutation impaired CBX4 binding (Fig. S2B, lanes 4 and 5). Similarly, substitution of the KRG core of PC75–228 with AAA abrogates its strong binding to GST-CBP HAT domain (Fig. S2D, panel 1, lane 3).
Fig. S2.
Human PC2 (CBX4) and mouse orthologs of PC bind directly to the CBP HAT domain. (A) GST-CBX4 fusion proteins (full-length, N-terminal residue deletions ΔN20 and ΔN78 and the mutation KRG73/AAA) were used to pull down purified CBP HAT domain in vitro. (B) GST-CBP core (wt, AIL-deletion, and 3K/Q or 3K/A mutations as indicated above lanes 3–6) were used to pull down purified CBX4. (C) GST-CBP-C1 (residues 2,132–2,247) was used to pull down CBX4ΔN66. All pull-down proteins (with a His-tag at the C terminus) were detected by Western blots with anti-His mAb. (D) GST and GST-CBP HAT domain were used to pull down purified PC75–228 with the KRG/AAA mutation (Top) and wt PC (Middle). (E) Pull-down of the CBP HAT domain using various forms of GST-mCBX6, GST-mCBX7, and GST-mCBX8. (D and E, Bottom) Purified GST and GST fusion proteins were stained with Coomassie blue. (F, Top) Sequence alignments of KRG motifs of PC and its mammalian CBX orthologs at the top. In addition to the conserved chromodomain (CD) at the N terminus and conserved C-terminal motif (c-box) at the C terminus, a motif with a KRG core (gray box) adjacent to the CD is highly conserved in human (h) and mouse (m) orthologs of Drosophila PC, which is absent in CBX1, CBX3, and CBX5 (orthologs of Drosophila HP-1). (F, Bottom) Results of GST pull-down of CBP HAT by mammalian orthologs of PC are summarized. Three asterisks within a gray box represent the KRG/AAA mutation.
We also conducted GST pull-down assays with the mouse PC orthologs mCBX6, mCBX7, and mCBX8, and found that all of them bind to the CBP HAT domain (Fig. S2E, lanes 3–5). Furthermore, the KRG/AAA mutation in mCBX7 and mCBX8 severely impaired their binding to the CBP HAT (Fig. S2E, lanes 8 and 9); CBX6 containing a deletion that removes its central region, but not its KRG motif, retained its binding ability, and mCBX8 containing a deletion of its C terminus, but not its KRG motif, also retained its binding ability (Fig. S2E, lanes 6 and 7), further suggesting that KRG motif is critical for CBP HAT binding. The results of these binding assays are summarized in Fig. S2F. We conclude that PC binding to the CBP HAT domain is conserved in mammals, strongly suggesting that it is functionally important in vivo.
PC Inhibits CBP HAT Activity in Vitro.
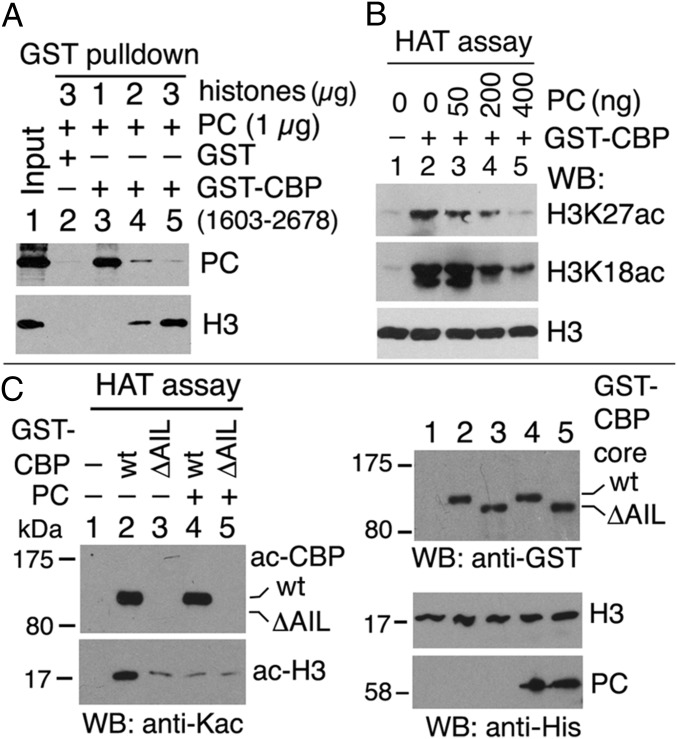
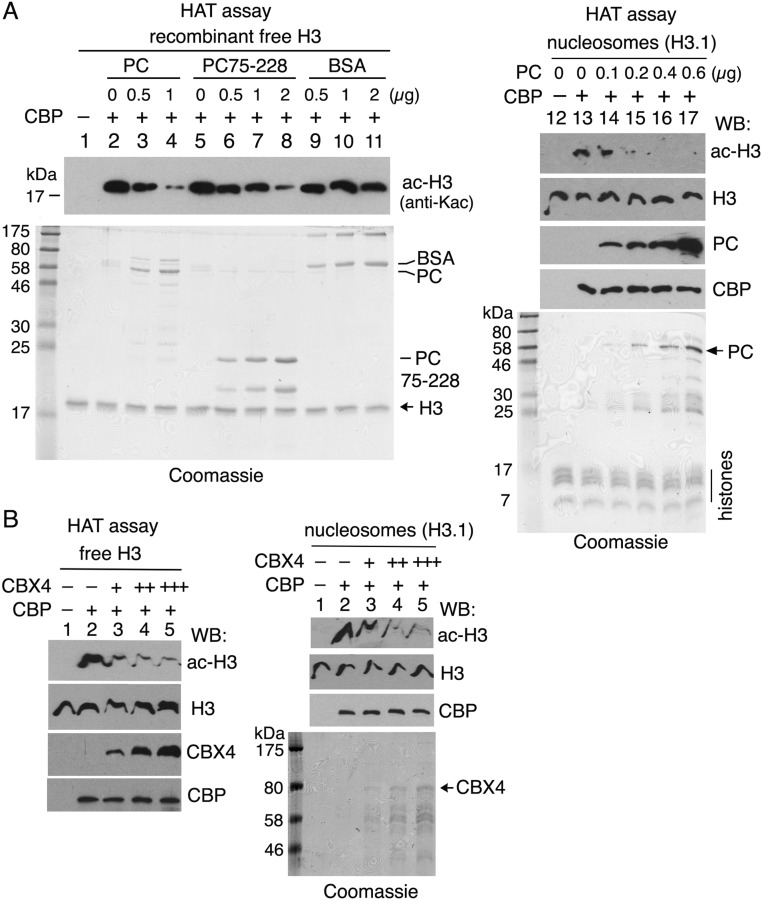
The conserved interaction between PC and the CBP HAT domain and the preferential association of PC with unacetylated CBP in vivo suggested that PC may have an effect on CBP HAT activity. We first tested whether PC affects the interaction between the HAT domain and its histone H3 substrate in vitro in a GST-CBP pull-down assay. As shown in Fig. 5A, PC binding to GST-CBP1603–2678 (lanes 3–5) was inversely proportional to the amount of core histones added, suggesting that PC competes with histone H3 for binding to the CBP HAT domain. Using an in vitro HAT assay with purified GST-CBP1603–2678 and recombinant histone H3 as a substrate, we detected decreased yields of H3K27ac and H3K18ac when increasing amounts of PC were added to the reaction (Fig. 5B, lanes 3–5). This result suggests that PC inhibits H3 acetylation by CBP. PC had a similar inhibitory effect when using purified GST-CBP1689–2330 (CBP core) in the HAT assay (Fig. S3A, lanes 3 and 4) and recombinant human nucleosomes as substrates (Fig. S3A, lanes 12–17). Furthermore, PC75–228 also inhibited H3 acetylation by CBP (Fig. S3A, lanes 6–8), although to a somewhat lesser extent than full-length PC, indicating that inhibition does not require the PC chromodomain or the conserved C-terminal motif (c-box) that binds the RING subunit of PRC1 (39, 40).
Fig. 5.
PC inhibits histone H3 acetylation by CBP in vitro. (A) Competition between PC and histone H3 for binding to CBP. PC (total of 1 μg) and histone H3 (1–3 μg) were pulled down by GST-CBP1603–2678 and were analyzed by WBs. (B) HAT activity of recombinant GST-CBP1603–2678 on free histone H3 in the absence or presence of PC. WBs were performed with anti-H3K27ac, anti-H3K18ac, and anti-H3 Abs. (C) PC inhibits CBP HAT activity regulated by the CBP AIL. The wt GST-CBP core (lanes 2 and 4) and its AIL-deleted (ΔAIL, lanes 3 and 5) were used in HAT assays in the absence (lanes 2 and 3) or presence (lanes 4 and 5) of PC. (Left) Autoacetylated GST-CBP core (ac-CBP) and acetylated histone H3 (ac-H3) were detected by WBs with anti-Kac Ab. (Right) PC and recombinant H3 were detected by anti-His Ab. GST-CBP core was detected by anti-GST Ab.
Fig. S3.
PC, PC75–228, and CBX4 inhibit histone H3 acetylation by CBP. (A) In vitro HAT assays were performed similarly as in Fig. 5B using GST-CBP core (residues 1,689–2,330) and recombinant free histone H3 (lanes 1–11, 2 μg) and human nucleosomes (H3.1) (lanes 12–17, 1.5 μg) as substrates in the presence of PC (lanes 3–4 and lanes 14–17), PC75–228 (lanes 6–8), and BSA (lanes 9–11), with total protein amounts indicated at the top of each lane. Note that both PC and PC75–228 inhibit histone H3 acetylation but PC is more efficient than PC75–228 (compare lanes 3–4 with lanes 6–8). Acetylated H3 (ac-H3) was detected by an anti-acetylated Lys (Kac) that does not recognizes H3 purified from E. coli (lane 1, negative control). (B) In vitro HAT assays were performed in the absence or presence of human CBX4 as in A. (A and B, Bottom) Coomassie blue staining gels are shown. WB, Western blot.
To determine whether the inhibitory effect of PC on CBP HAT activity has been evolutionarily conserved, we tested whether human PC2 (CBX4) also inhibits CBP HAT activity. Preincubating CBP with purified human CBX4 before addition of acetyl-CoA to an in vitro HAT assay severely impaired acetylation of free histone H3 and recombinant human nucleosomes (Fig. S3B, Left and Right, lanes 3–5). This result suggests that inhibition of CBP HAT activity by PC is conserved in mammals.
CBP HAT activity is greatly enhanced by autoacetylation, predominantly on multiple Lys in the AIL (32). We generated a GST-CBP catalytic core containing a deletion of just the AIL (ΔK2184-G2242 or ΔAIL) and compared its HAT activity with the HAT activity of the wt construct. As shown in Fig. 5C, although wt CBP core exhibited strong autoacetylation and H3 acetylation activities (Fig. 5C, Left, lane 2), CBP core ΔAIL exhibited undetectable autoacetylation and very weak H3 acetylation (Fig. 5C, Left, lane 3). Preincubation of wt CBP core with PC before addition of acetyl-CoA resulted in strong inhibition of H3 acetylation, reducing it to a level similar to the level observed with the CBP core ΔAIL in the absence of PC (Fig. 5C, Bottom Left, compare lane 4 with lane 3). Similar preincubation of the CBP core ΔAIL with PC had little or no additional effect on its weak H3 acetylation activity (Fig. 5C, Bottom Left, lane 5). These results, together with the direct binding of PC to the unacetylated AIL, support the conclusion that the AIL mediates the inhibition of CBP HAT activity by PC. They also strongly suggest that the acetylated AIL is itself required for robust H3 acetylation. In summary, PC inhibits CBP HAT activity by binding to the AIL and competing with histone H3 for binding to the CBP HAT domain, presumably directly or indirectly preventing the interaction of H3 with the active site of the enzyme.
Altering PC Levels in Vivo Affects the H3K27ac Level.
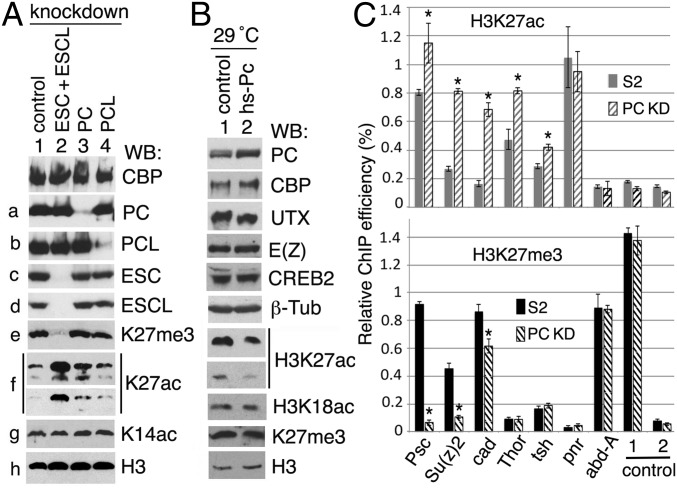
To explore the effect of PC on CBP HAT activity in vivo, we altered PC levels by RNAi knockdown in S2 cells. We assayed protein levels and histone modifications by Western blots. For comparison, we also performed double knockdown of the PcG proteins ESC and ESC-like (ESCL) and single knockdown of PCL. ESC and ESCL are alternative core subunits of PRC2 that are essential for PRC2 methyltransferase activity (41), whereas PCL is present in a larger PRC2 complex (42, 43) and required for the high levels of H3K27me3 at some Polycomb target genes (43). As we previously showed, simultaneous knockdown of ESC and ESCL abolished H3K27me3 (8, 41) and resulted in a very substantial increase of the alternative H3K27ac modification (8) (Fig. 6A, e and f, compare lane 2 with lane 1). Knockdown of PCL caused little change in bulk H3K27me3 and H3K27ac levels (Fig. 6A, e and f, compare lane 4 with lane 1).
Fig. 6.
Altering PC levels in vivo affects histone acetylation by CBP. (A) Immunoblots of S2 cells after knockdown of GFP (control), ESC + ESCL, PC, and PCL as indicated at the top of lanes 1–4. Two different exposures of one WB of H3K27ac are shown in f. (B) Immunoblots of extracts from Oregon-R (control) and hs-Pc adult male flies raised at 29 °C. PC level in hs-Pc flies was increased 75–80% in comparison to wt control at 29 °C. The H3K27ac level is shown in two separate WBs. Note that the H3K27ac level was decreased but the CBP level was not reduced in hs-Pc flies. (C) ChIP-qPCR analysis. IPs were performed with anti-H3K27ac (Top) and anti-H3K27me3 (Bottom) Abs. The qPCR assays were performed with primers at promoter regions of seven genes [abd-A, cad, pnr, Psc, Su(z)2, Thor, and tsh]. Primer sets for a region 3′ of Thor gene (control 1) and for a region 5′ of Su(z)2 gene (control 2) were used for controls. Rabbit preimmune serum was also used for control IP from S2 cells (knockdown with GFP dsRNA) to estimate background. The average ChIP-qPCR signal in these controls was 0.1% at promoters of the seven genes, and signals below 0.2% were considered to be background. In comparing the relative ChIP efficiency from control and PC knockdown (KD) S2 cells, the significant increases of the H3K27ac level at Psc, Su(z)2, cad, Thor, and tsh and the significant decreases of the H3K27me3 level at Psc, Su(z)2, and cad are marked by asterisks. Note that H3K27me3 levels at promoters of two active genes Thor and tsh are at background in PC KD and S2 cells.
In contrast to knockdown of PRC2 core subunits (ESC + ESCL), knockdown of PC caused no detectable effect on the bulk H3K27me3 level (Fig. 6A, e, lane 3) but resulted in approximately a twofold increase in the total H3K27ac level (Fig. 6A, f, compare lane 3 with lane 1). The effect of PC knockdown is thus also distinct from the effect of PCL knockdown. The levels of CBP and H3K14ac (which is acetylated by GCN5 in Drosophila) were also barely changed after PC knockdown (Fig. 6A, panels 1 and g), suggesting that the effect is specific to CBP HAT activity.
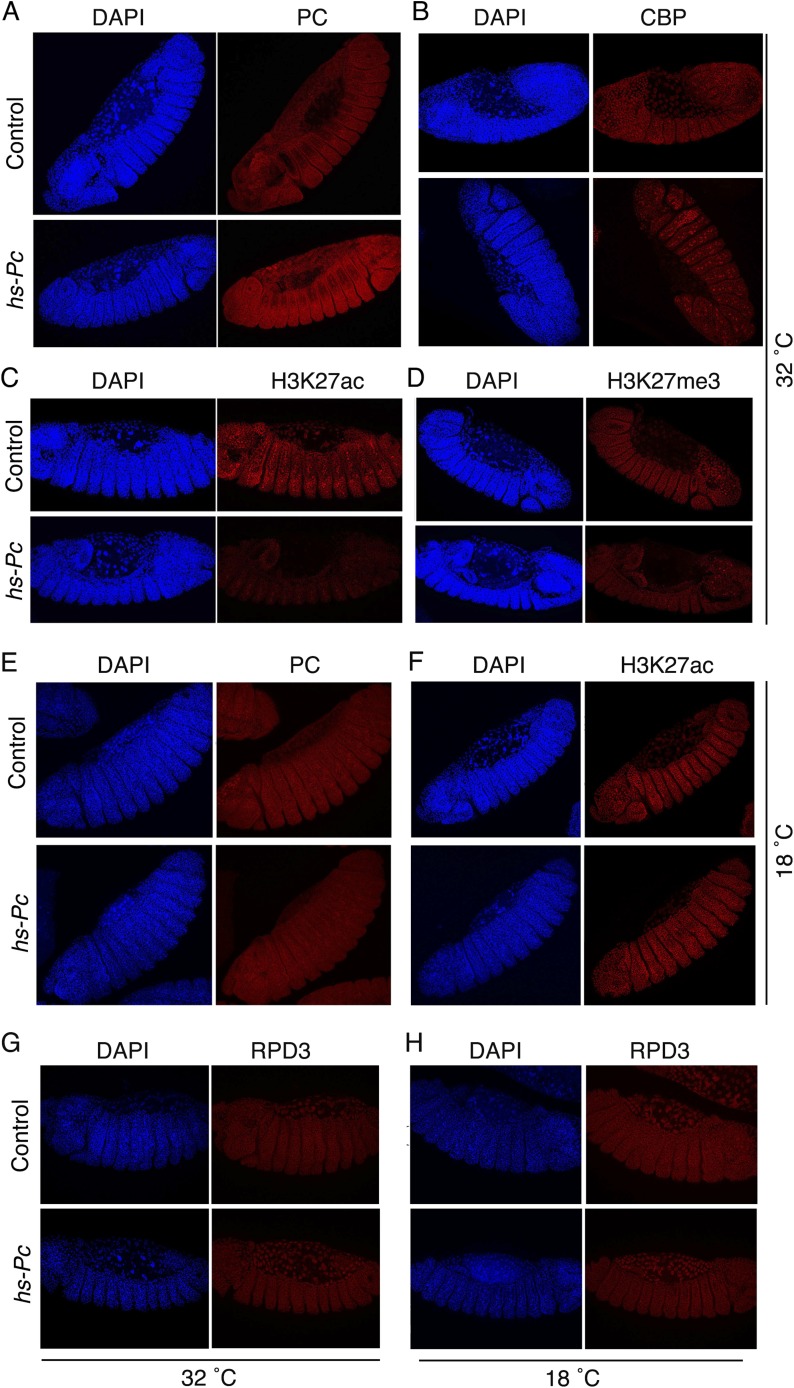
Conversely, moderate constitutive overexpression of PC from a hs-Pc transgene by raising homozygotes continuously at 29 °C (a subheat shock temperature that promotes substantial leaky expression of the Hsp70 promoter) resulted in a decrease in the bulk H3K27ac level in adult male flies but little detectable change in the bulk H3K27me3 level (Fig. 6B, lane 2). We also examined the effect of PC overexpression (Fig. S4A) on the H3K27ac level by immunostaining hs-Pc and wt embryos raised at 32 °C. Whereas the CBP level appeared unchanged (Fig. S4B), the H3K27ac level was substantially reduced (Fig. S4C). This result is unlikely to be due to increased deacetylation, because the level of the H3K27ac deacetylase RPD3 in wt control embryos and hs-Pc embryos is indistinguishable (Fig. S4 G and H). These results of PC knockdown and overexpression are consistent with the in vitro results shown in Fig. 5 and suggest that PC inhibits CBP HAT activity in vivo. The lack of a detectable change in total H3K27me3 when PC is knocked down or overexpressed does not rule out the possibility that some individual genes may have altered H3K27me3 levels (discussed below). However, it does suggest that the concomitant substantial changes in the H3K27ac level may be occurring predominantly at transcriptionally active PC-regulated genes, where an increase in H3K27ac would not have to occur at the expense of H3K27me3, as is the case at silent (H3K27me3-marked) PC-regulated genes, due to the mutually exclusive nature of these two modifications.
Fig. S4.
PC overexpression in embryos results in a decrease of the H3K27ac level. Oregon-R (control) and hs-Pc flies were kept at 32 °C (A–D and G) and 18 °C (E, F, and H) for 4 d before collection of embryos. Embryos were stained by DAPI and immunostained by various Abs (PC, CBP, H3K27ac, H3K27me3, and RPD3) as indicated. Images were collected using a Leica TCS-SP8 Confocal Microscope. Note that PC and H3K27ac levels are significantly changed in the hs-Pc embryos maintained at 32 °C (A and C) but little changed or unchanged in the embryos maintained at 18 °C (E and F). (D) H3K27me3 level is slightly reduced. (G and H) Levels of the H3K27ac deacetylase RPD3 in wt control embryos (Top) and hs-Pc embryos (Bottom) are indistinguishable, suggesting that the reduced H3K27ac is not due to deacetylation.
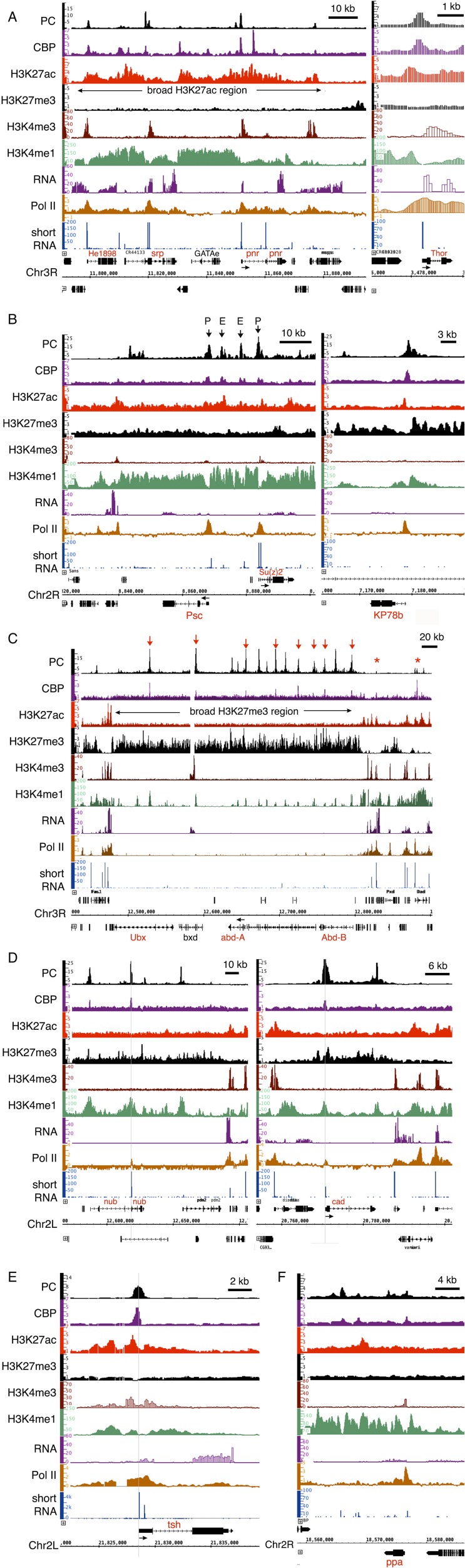
To determine if the changes in the bulk H3K27ac level after PC knockdown reflect changes at individual genes, particularly active genes, we also examined the effect of PC RNAi knockdown on the H3K27ac and H3K27me3 levels at promoter regions of seven individual genes representing four classes of genes co-occupied by PC and CBP in ChIP-chip (36) and ChIP-sequencing (seq) datasets (21, 44, 45) (Fig. S5 and Table S1) and distinguished by histone marks, RNA levels, RNA polymerase II (Pol II) occupancy, and short RNAs [sRNAs; an indicator of Pol II pausing (45)]. Among seven PC-regulated genes examined [abd-A, cad, pnr, Psc, Su(z)2, Thor, and tsh], Thor, tsh, and pnr belong to the “active” class (marked by H3K27ac and expressed), but only pnr is embedded within a broad H3K27ac region that covers the entire gene (Fig. S5A). abd-A, a homeotic/HOX gene in the Bithorax complex, belongs to the “repressed” class, which is inactive, lacking Pol II and sRNA, and embedded within a broad H3K27me3 region (Fig. S5C). cad is in the “poised” class, marked by H3K27me3 and not expressed, but containing Pol II and sRNA (paused Pol II) at promoter regions (Fig. S5D). Psc and Su(z)2 were included because they are distinctive. They encode related alternative core subunits of PRC1 and must be expressed in all cells, but are themselves PcG-regulated. They have been placed in the so-called “balanced” (38) class because they appear to contain both H3K27me3 and H3K27ac over their promoter and gene body regions (Fig. S5B). This pattern has been hypothesized to reflect dynamic regulation or oscillating on/off expression resulting from their negative autoregulation (46). Some genes in the poised and balanced classes [including cad, Psc, and Su(z)2] and in the active class (including tsh) have been previously reported to be up-regulated upon knockdown of PC and PH in Kc cells (47). We previously showed that the Thor gene, which is expressed in S2 cells, was expressed at a higher level after knockdown of PC (48). We used PC knockdown and control S2 cells to perform chromatin IP with anti-H3K27ac and anti-H3K27me3 Abs coupled with real-time quantitative PCR (ChIP-qPCR) to examine changes in the H3K27ac/H3K27me3 levels at these seven genes.
Fig. S5.
Four classes of genes marked by PC and CBP. Representative plots of nine factors (PC, CBP, H3K27ac, H3K27me3, H3K4me3, H3K4me1, RNA, Pol II, and sRNA) from ChIP-chip data (36) and published ChIP-seq data (21, 44, 45) in S2 cells. (A) Active class. All PcG-target genes in this active region are marked with strong peaks of Pol II and RNA. (Left) Strong CBP peaks but weak PC peaks are present at the enhancer and promoter, whereas strong and broad H3K27ac (absence of H3K27me3) and H3K4me1 peaks are present at the region. The active Thor gene (Right) is similar to active genes (Left) but without a broad H3K27ac peak. The sRNA and H3K4me3 at the promoter suggest that paused Pol II is released into elongation. (B) Balanced class. Genes [Psc, Su(z)2, and KP78b] are expressed at a low level (RNA) and are marked by PC, CBP, and H3K27ac at promoter regions but by H3K37me3 at gene bodies. Pol II, H3K4me3 (weak), and sRNA are present at the promoter region, suggesting that Pol II (Ser5-P form) is paused and a small portion of Pol II (Ser2-P form) is released into elongation. Arrows at the top indicate enhancers (E) and promoters (P) of Psc and Su(z)2. (C) Repressed class. All genes in this domain, the Bithorax complex (Ubx, abd-A, and Abd-B), are repressed without Pol II, expression (RNA), and sRNA (paused Pol II). Strong and broad PC and H3K27me3 peaks are present across the region (over the 300 kb for the Bithorax complex). H3K27ac is absent, although CBP is colocalized with PC. Note that arrow-indicated CBP peaks co-occupy with strong peaks of PC and H3K37me3, whereas asterisk-indicated CBP peaks co-occupy with weak peaks of PC and strong peaks of H3K27ac, which is consistent with PC inhibition of CBP HAT activity. The homeotic genes in the Antennapedia complex (laboratory, pb, Scr, and Antp) belong to this class. (D) Poised class. Genes (cad and nub) are repressed (H3K27me3 marked, no RNA) with a weak Pol II peak and sRNA but without H3K27ac and H3K4me3 at the promoter (indicated by a vertical gray line), suggesting that Pol II (Ser5-P form) is paused for elongation. (E and F) Active class without broad H3K27ac peaks. Active tsh and ppa genes are shown. The ChIP-chip data of Pol II in S2 cells were downloaded from modENCODE (accession no. modEncode_329). The genome version of D.melanogaster_Apr_2006 was used.
Table S1.
Four classes of genes targeted by PC and CBP
| Factors | PC | CBP | H3K27ac | H3K27me3 | H3K4me3 | H3K4me1 | RNA | Pol II | sRNA |
| Active | + | ++ | +++ | − | ++ | +++ | ++ | +++ | + |
| Balanced | ++ | ++ | ++ | + | + | ++ | + | ++ | + |
| Poised | +++ | ++ | − | ++ | − | ++ | − | + | + |
| Repressed | ++++ | + | − | +++ | − | + | − | − | − |
Genes co-occupied by PC and CBP are divided into four classes according to the presence (+) or absence (−) of histone marks (H3K27ac/me3, H3K4me1/me3), RNA expression, Pol II, and sRNA: (i) the active class (H3K27ac and RNA), (ii) the balanced class (both H3K27ac and H3K27me3, sRNA, and low-level RNA), (iii) the poised class (H3K27me3, Pol II, and sRNA, but no RNA), and (iv) the repressed class (absence of H3K27ac, Pol II, RNA, and sRNA). Pol II is present not only at promoter but also at the body of gene in the active class, whereas it is only present at the promoter in the poised and balanced classes.
As shown in Fig. 6C, five of the seven genes examined exhibited significantly elevated H3K27ac levels after PC knockdown. It did not alter the robust H3K27me3 signal at the promoter region of the repressed abd-A gene, which remained devoid of H3K27ac, as would be expected if the H3K27me3 mark already occupies all of the H3K27 substrates there. In contrast, the H3K27ac levels were significantly increased at the promoter regions of the already active Thor and tsh genes, which contain little or no H3K27me3. Interestingly, the active pnr gene, with its already very strong and broad H3K27ac signal encompassing the entire gene, did not exhibit any increase of H3K27ac in its promoter region after PC knockdown. The inactive cad gene, with a strong H3K27me3 signal and paused Pol II, exhibits a very substantial increase in H3K27ac after PC knockdown and a moderate drop in its H3K27me3 signal. The adjacent and divergently transcribed Psc and Su(z)2 genes, which exhibit substantial broad H3K27ac and H3K27me3 signals and low expression, show very substantial increases in H3K27ac and loss of H3K27me3. H3K27ac levels at two control regions, one downstream of Thor gene (with a high H3K27me3 level) (control 1) and another upstream of Su(z)2 (control 2), were unchanged after PC knockdown (Fig. 6C), suggesting that changes of H3K27ac levels observed above are specific to promoter regions of these PC-regulated genes. These ChIP-qPCR results reveal a variety of behaviors of different classes of CBP-occupied PcG-regulated promoter regions in response to RNAi knockdown of PC. The elevated H3K27ac levels observed for the already active Thor and tsh genes are consistent with the observed in vitro inhibition of CBP by PC and the hypothesis that PC inhibition of CBP modulates H3K27ac levels at active genes. The unchanged high H3K27ac level at the active pnr promoter region may reflect a lack of PC inhibition of CBP there and already saturating acetylation of all available H3K27 in its promoter region. The substantial increase in H3K27ac at both Psc and Su(z)2 is consistent with PC inhibition of CBP at their promoters, but this interpretation is complicated by the concomitant drop in H3K27me3, which could reflect an indirect effect, or, as suggested previously, two different populations of S2 cells where Psc and Su(z)2 are either active or repressed. An important goal of future studies will be to investigate genome-wide changes of H3K27ac and H3K27me3 at CBP and PC co-occupied genes by ChIP-seq after PC knockdown.
Overexpression of PC Suppresses CBP-Dependent Perturbation of Polycomb Silencing Caused by TRX Overexpression.
We also examined the phenotypic effect of the reduced H3K27ac caused by PC overexpression in another genetic assay. We previously showed that CBP interacts directly with TRX (23) and that TRX overexpression in vivo increases the level of H3K27ac in bulk histones and antagonizes Polycomb silencing (8). TRX overexpression also results in phenotypes characteristic of Pc mutants, including the appearance of sex combs on second and third thoracic (T2 and T3) legs due to derepression of the Scr gene, which is normally expressed only in T1. Moderate RNAi knockdown of CBP suppresses both the elevated H3K27ac level and the impaired silencing phenotypes caused by TRX overexpression, indicating that the phenotypic manifestations of this antagonistic effect of TRX overexpression on Polycomb silencing requires CBP (23). Given that PC overexpression also decreases the H3K27ac level, we reasoned that it may suppress these CBP-dependent phenotypes caused by TRX overexpression, thus providing additional evidence for PC inhibition of CBP activity in vivo.
We found that the same moderate overexpression of PC from the hs-Pc transgene (29 °C) partially suppresses the impaired Polycomb silencing induced by constitutive TRX overexpression from a GAL4-inducible trx+ allele (Materials and Methods). As shown in Table 1, the frequencies of sex comb transformations from T2 to T1 (41%) or from T3 to T1 (12%) in TRX overexpressers were decreased to 27% and 7%, respectively, by simultaneous overexpression of PC. This phenotypic suppression further suggests that PC overexpression also inhibits CBP in vivo.
Table 1.
PC overexpression suppresses the Pc phenotypes of TRX overexpressors
| Genotype (male progeny) | T2 to T1 leg transformation | T3 to T1 leg transformation |
| Act-Gal4/+;trxEP3541/+ | 32/78 (41%) | 10/78 (12%) |
| hs-Pc/Y;Act-Gal4/+;trxEP3541/+ | 19/70 (27%) | 5/70 (7%) |
Crosses were done at a constant 29 °C. Adult males were scored for the presence of extra sex combs on T2 and T3 legs. hs-Pc, heat-inducible Pc transgene; trxEP3541, GAL4-inducible trx allele.
PC Is Physically Associated with Paused Pol II in Vivo.
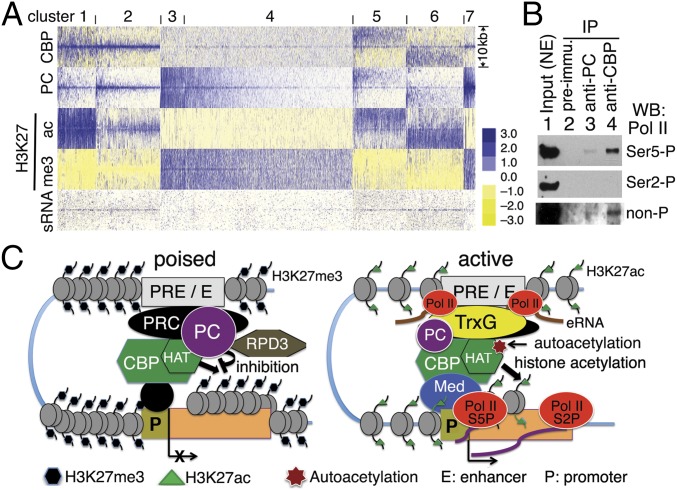
Genome-wide chromatin occupancy patterns of PC and CBP in cultured S2 cells have revealed that PC and CBP cobind many sites, primarily PREs, enhancers, and promoters of many active and repressed genes (23, 36), and that PC is associated with many active promoters that exhibit Pol II pausing (21, 22). To examine the genome-wide correlation between PC, CBP, CBP HAT activity (H3K27ac), and paused Pol II, we generated a heat map using ChIP-chip data for CBP, PC, H3K27ac, and H3K27me3 (36), and sRNAs produced by promoter-proximal (i.e., paused) Pol II (45). All PC peaks on all chromosomes were clustered with the other four factors into seven groups (Fig. 7A). CBP is present with PC in all clusters except cluster 3. Although CBP peaks are strong in both clusters 1 and 2, H3K27ac levels are different: In cluster 1, H3K27ac is strong and broad, whereas PC is present at a moderate level; in cluster 2, where PC peaks are stronger, H3K27ac peaks are weaker, suggesting that CBP HAT activity is greater in cluster 1 and attenuated in cluster 2, possibly due to inhibition by PC. As expected, there is no detectable H3K27ac associated with the weaker CBP peaks in clusters 7 and 4, where PC and broad H3K27me3 peaks are present. H3K27ac is present in clusters 5 and 6, where medium-height CBP peaks are associated with low PC peaks, suggesting that CBP is enzymatically active at these sites (Fig. S5 E and F). Interestingly, paused Pol II (indicated by peaks of sRNAs) is strongly correlated with CBP and PC in clusters 1, 2, 5, and 6 and weakly correlated with CBP and PC in cluster 7 (which include PREs, enhancers. and promoters), suggesting that inhibition of CBP HAT activity by PC is associated with Pol II pausing in both active and repressed states.
Fig. 7.
PC and CBP are associated with paused Pol II in vivo. (A) Heat map of all PC peaks correlated with peaks of CBP, H3K27ac, H3K27me3, and sRNA in S2 cells, forming seven clusters. The 5 kb on each side of peak midpoints is shown. Note that PC and CBP peaks are correlated with sRNA (paused Pol II) in clusters 1, 2, 5, 6, and 7. (B) Immunoblots of Pol II (Ser5-P, Ser2-P, and non-P forms) from IP by rabbit anti-PC (lane 3) and anti-CBP (lane 4) Abs from embryo NE. Lane 1 (input) is 5–10% of NE. Rabbit preimmune serum was used in lane 2 for a negative control. Note that the non-P and Ser5-P forms of Pol II were detected in IPs by anti-CBP and anti-PC Abs. (C) Model of PC function in inhibition of CBP HAT activity. (Left) In the repressive state, chromatin is condensed. CBP, PC, and PRC complexes are present at poised enhancer and PRE (marked by H3K27me3 and low-level H3K4me1). PC is associated with unacetylated CBP. CBP HAT activity to histones (and nonhistone proteins) is likely inhibited by PC. Furthermore, PC- and CBP-associated RPD3 may maintain H3K27 in a deacetylated state. (Right) In the active state, chromatin is relaxed. H3K27 acetylation occurs, but its level is inversely proportional to PC level (cluster 2 in A). Autoacetylated CBP, Pol II, and TrxG proteins, and a relatively lower level of PC are present at active enhancers (marked by H3K27ac and high-level H3K4me1) that are transcribed into enhancer RNA (eRNA). Mediator (Med), binding to transcription factors, CBP and Pol II, is involved in looping of active enhancer and promoter. Paused Pol II in the Ser5-P (S5P) form and sRNA are present at the proximal transcription start site (TSS). Hyperautoacetylated CBP and H3K27ac near the TSS facilitate TrxG-mediated transcription elongation (RNA product) by Ser2-P (S2P) Pol II.
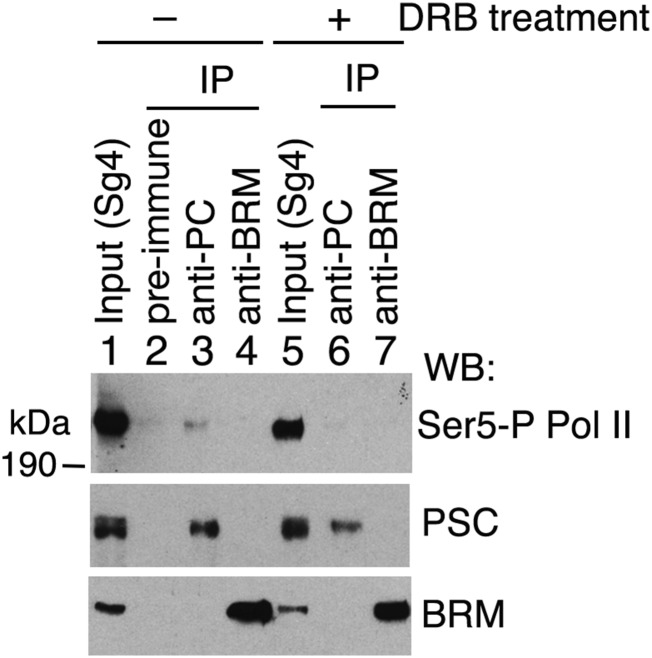
We further examined the physical association of PC and paused Pol II by co-IP. CBP/p300 is present in a Pol II complex (49) that preferentially contains a non- or hypophosphorylated form of Pol II (50–52). As shown in Fig. 7B for IP from embryo NE, the unphosphorylated (non-P) form and Ser5-phosphorylated (Ser5-P) form, but not the Ser2-phosphorylated (Ser2-P) form, of Pol II were found in association with Drosophila CBP (Fig. 7B, lane 4), similar to what was found for human p300 (50). Moreover, a small portion of Pol II with non-P and Ser5-P forms (but not the Ser2-P form) is also associated with PC (Fig. 7B, lane 3), suggesting that PC may be transiently associated with these forms of Pol II at promoters and active enhancers (53). Ser5-P Pol II also coimmunoprecipitated with PC from Sg4 cell extracts (Fig. S6). Treatment of Sg4 cells with the RNA elongation inhibitor 5,6-dichlorobenzimidazole 1-β-d-ribofuranoside appeared to impair the association of Ser5-P Pol II with PC (Fig. S6, compare lane 6 with lane 3). This result is consistent with the previous finding that PC/PRC1 is associated with some general transcription factors (54, 55). This result suggests that PC inhibition of CBP HAT activity may play a role in Pol II pausing. This view is supported by recent evidence that RNAi knockdown of PC results in the appearance of greater amounts of Pol II on the bodies of actively transcribed genes (22).
Fig. S6.
PC is associated with Ser5-P Pol II in Sg4 cells. Immunoblots of Pol II (Ser5-P form), PSC, and BRM from IPs by anti-PC and anti-BRM in Sg4 cells without (lanes 1–4) or with (lanes 5–7) overnight treatment with 50 μM RNA synthesis inhibitor 5,6-dichlorobenzimidazole 1-β-D-ribofuranoside (DRB) from Sigma. Lanes 1 and 5 are 5% inputs of cell extracts. Rabbit preimmune serum was used in lane 2 for a negative control. Note that PSC remained coimmunoprecipitated with PC in DRB-treated Sg4 cells but Ser5-P Pol II co-IP with PC was impaired in DRB treated cells (compare lane 6 with lane 3).
Discussion
The results presented above reveal a previously unidentified evolutionarily conserved function of the PC subunit of PRC1: inhibition of CBP acetyltransferase activity. PC binds directly to the CBP HAT domain, making a major contact with its previously identified AIL. The multiple Lys of the AIL are the predominant targets of CBP autoacetylation, which acts like a switch to enhance greatly the weak HAT activity of unacetylated CBP and enable robust acetylation of histones and other CBP substrates. The AIL is required for strong binding of PC to CBP and for PC inhibition of histone acetylation. To our knowledge, PC is the first protein shown to bind to the AIL. PC inhibition of H3 acetylation might simply be due to the observed competition between PC and H3 for binding to the CBP HAT domain. However, because PC binds to the AIL, it might also reduce CBP catalytic activity by inhibiting autoacetylation of the AIL. Consistent with this possibility, PC is preferentially associated with unacetylated CBP in vivo, whereas autoacetylation of CBP in vitro impairs its binding to PC.
PC binding to the CBP HAT domain does not require the PC chromodomain, but it requires an adjacent short motif with a KRG core that is conserved in mammalian PC orthologs, which also bind to CBP HAT domain and inhibit its histone acetylation activity. The conservation of CBP HAT binding, the KRG-binding motif, and in vitro inhibition of CBP HAT activity by mammalian PC orthologs further suggests that PC plays a conserved role in the regulation of CBP/p300 activity in vivo. Interestingly, despite its intrinsic autoacetylation activity, the bulk of the p300 in mammalian cell extracts, as well as recombinant p300 purified from insect cells, is unacetylated or hypoacetylated (56), indicating that its acetylation state is tightly regulated. SIRT2 has been identified as the principal p300/CBP deacetylase in mammalian cells (31). The p300 that is unacetylated on the AIL has been shown to be the form that is preferentially recruited by transcription factors to chromatin, where it subsequently becomes activated by autoacetylation in situ (56). The results reported here, including the genome-wide colocalization of PC and CBP at many active as well as repressed genes, PC inhibition of H3 binding and acetylation by CBP in vitro, and the preferential association of PC with unacetylated CBP in vivo, suggest that PC also plays a role in inhibiting or modulating CBP activity in situ on chromatin. The elevated H3K27ac level caused by PC RNAi knockdown, both in bulk histones and on five of seven PC-regulated genes analyzed, and the reduced H3K27ac level caused by PC overexpression provide support for this conclusion, as does the ability of PC overexpression to suppress the CBP-dependent effects of TRX overexpression, including both the elevated H3K27ac level and the homeotic phenotypes due to impaired Polycomb silencing. An important future goal will be to determine the genome-wide locations of acetylated (active) and unacetylated (inactive) CBP as well as the elevated H3K27ac level caused by loss of PC and whether they are consistent with genome-wide PC inhibition of CBP activity at active as well as repressed genes.
What are the roles of PC inhibition of CBP activity? CBP is recruited to specific sites on chromatin, notably enhancers and promoters, by a multitude of different transcription factors. CBP catalytic activity plays many roles in transcription regulation, including acetylation of histones at active enhancers and promoters, and acetylation of many transcription factors, including the Pol II C-terminal domain, which is essential for stable association of Pol II with some promoters (57). PC inhibition of CBP HAT activity could have biological consequences in any of the contexts in which PC and CBP become physically associated. Enhancers, PREs, and promoters figure prominently as candidates, given the genome-wide pattern of PC and CBP cobinding and the effects of PC knockdown and overexpression on global H3K27ac levels. The requirement of CBP for long-distance enhancer/promoter interactions (58) also suggests that PC inhibition of CBP might regulate gene expression by affecting local chromatin structure.
CBP/p300 occupancy is a key feature of the chromatin signature of enhancers, along with the H3K4me1 modification and clusters of bound transcription factors. CBP/p300 is responsible for the H3K27ac mark that distinguishes active enhancers from poised enhancers, some of which are marked by PRC2 with H3K27me3, a hallmark of Polycomb silencing. Although the presence of H3K27me3 at cis-regulatory regions of silent Polycomb target genes is sufficient to block H3K27ac and to facilitate recruitment of PC/PRC1 for chromatin compaction and other activities, binding of PC to H3K27me3 and its inhibition of CBP at these enhancers could act as an important backup mechanism to prevent unscheduled acetylation of H3K27 in the event of its adventitious demethylation. Furthermore, inhibiting acetylation of other CBP histone and nonhistone substrates at these enhancers, including transcriptional activators, may also be important for maintaining their poised or repressed state and preventing unscheduled activation. PC inhibition of CBP HAT activity may also play a role in switching from active to silent states, where it may be required in conjunction with the removal of CBP-dependent active modifications by deacetylases to maintain the deacetylated state and allow time for acquisition of the H3K27me3 mark and maturation of a silent chromatin state. PC inhibition of CBP at enhancers of PC-regulated genes may also regulate the onset of enhancer transcription, which has been reported to be impaired by p300 knockdown (59). Recent genome-wide temporal profiling of enhancer transcription revealed that a rapid burst of it, accompanied by enrichment of the H3K27ac mark on enhancers, occurs just before transcription from the promoters of their associated genes and is the most common initial transcription event genome-wide (60).
PC is also found associated with the promoters of both repressed and active Polycomb-regulated genes. At the former, H3K27me3 binding by the PC chromodomain may facilitate local inhibitory interactions of PC/PRC1 with CBP. Active Polycomb-regulated genes, which lack the H3K27me3 mark, are enriched for paused Pol II. The presence of PC/PRC1 at these genes suggests that PC/PRC1 is recruited to them by a mechanism that is independent of H3K27me3 and that PC inhibition of CBP HAT activity may also modulate the transcription of these active genes at one or more of the multiple steps leading to mature mRNA production. The changes we observed in global H3K27ac levels in response to PC overexpression and knockdown, which occur without detectable changes in H3K27me3, suggest that these changes may be occurring predominantly at active genes, where an increase of H3K27ac need not occur at the expense of H3K27me3. This possibility is further supported by the observation of increased H3K27ac levels at promoter regions of the active PC-regulated genes Thor and tsh, measured by ChIP-qPCR after PC knockdown in S2 cells (Fig. 6C). Recent evidence suggests that cohesin, which interacts physically with PRC1 (61), is required for the recruitment of PRC1 to promoters of some active genes, many of which exhibit Pol II pausing (22). Loss of PC or other PRC1 components has been shown to lead to an increase in Pol II on the bodies of these genes, suggesting that PRC1 may influence Pol II pausing and/or elongation (22). The association of PC with the initiating form of Pol II (Ser5-P) that we observed may point to such a role.
In summary (Fig. 7C), we have shown that the PC protein, presumably in the context of PRC1, binds directly to the CBP HAT and AIL and inhibits histone acetylation activity, and competes with histone H3 for binding to the CBP catalytic domain. Moreover, PC is associated with unacetylated CBP in vivo and negatively regulates the total H3K27ac level and the H3K27ac level at individual genes in vivo. Taken together, these results point to a new mechanism by which PC/PRC1 exerts a repressive effect on transcription.
Materials and Methods
Abs.
Rabbit anti-PC Abs were raised against recombinant full-length PC (with a 6× His-tag at the C terminus). Affinity purification of anti-PC Abs on PC-coupled Sepharose beads was performed as previously described (42). Guinea pig and rabbit anti-CBP Abs and other Abs were described previously (8). Goat anti-H3 (ab12079), rabbit anti-H3K27ac (ab4729), and anti-H3K18ac (ab1191) Abs were from Abcam. Rabbit anti-H3K27me3 (07-449) and anti-H3K14ac (06-911) Abs were from Millipore. Anti-FLAG mAb was from Sigma. Rabbit anti-acetyl Lys (9441) Ab was from Cell Signaling. The mAbs anti-Pol II (8WG16), phosphoserine 5 (Ser5-P) Pol II (H14), and phosphoserine 2 (Ser2-P) Pol II (H5) were from Covance. Goat anti-GST Ab was from GE Healthcare.
Constructs.
An EST clone (RE66837) with the full length of PC coding sequence was obtained from the Berkeley Drosophila Genome Center. Fragments encoding PC amino acid residues 1–390 (full-length), 1–86, 75–390, 75–228, and 222–390 were generated by PCR and inserted into a modified pET-11 vector (cut by Nde I and Nsi I). CBP constructs were previously described (8, 36). Other CBP constructs were made by inserting PCR fragments into the pGEX-2T vector using the In-Fusion HD Cloning Kit (Clontech). PCΔ134–167, PC75–228(KRG/AAA), CBP 3K/A and 3K/Q mutations, and ΔAIL were generated with In-Fusion HD Cloning Plus (Clontech). Construct pGEX-5X-CBX4 (human) was a gift from Jinke Cheng (Shanghai Jiao Tong University, Shanghai, China). pGEX-5X-CBX4ΔN20 was made from pGEX-5X-CBX4 by deleting a BamHI-BamHI fragment. Constructs pGEX-2T-CBX4ΔN78, pGEX-2T-CBX4(KRG/AAA), pET-CBX4-H6, and pET-CBX4ΔN66-H6 were generated by inserting a PCR fragment into pGEX-2T and pET vectors, respectively. Mouse CBX6, CBX7, and CBX8 constructs were generated similarly.
In Vitro Acetylation Assay.
The acetylation assay was performed in 30-μL volumes of total reaction buffer containing 5 mM sodium butyrate and 1 mM PMSF for 30–90 min at 30 °C (8). Recombinant GST-CBP1603–2678 or GST-CBP1689–2330 (∼50 ng) and free histone H3 (1–2 μg) (with a 6× His-tag at the C terminus) or recombinant human nucleosomes containing histone H3.1 (1.5 μg) (active motif) as a substrate were used in these assays. The reaction was quenched by adding 30 μL of 2× SDS sample buffer. For the inhibition assay, PC, CBX4, or BSA (0.1–1 μg) was mixed with GST-CBP for 40–60 min at 4 °C before acetylation assay.
Cell Culture.
Drosophila S2 and Sg4 cells (from the Drosophila Genomics Resource Center) were cultured in Schneider’s medium supplemented with 10% (vol/vol) FBS at 25 °C. S2 cells were cotransfected for transient expression of FLAG-PC and FLAG-CBPΔN as previously described (8, 62). Cells extracts were used for IP.
Protein Extracts and Fractionation.
Embryo NE (0.2 mL) was fractionated on a Superose 6 column (10/300 mm; GE Healthcare) as previously described (63) and collected at a rate of 0.5 mL per tube, with the void volume ending with fraction 6.
Protein and Histone Extracts from Adult Flies Overexpressing PC.
Embryos were collected from wt (Oregon-R) and homozygous hs-Pc transgenic flies and raised continuously at 29 °C for 2 wk. One hundred male flies were placed in a 1.5-mL tube and frozen in liquid nitrogen and then ground into power. Proteins were extracted with 0.2 mL of nuclear extraction buffer (plus proteinase and phosphatase inhibitors and 10 mM Na butyrate) from ground flies, and histones were extracted from nuclear pellets with 0.4 mL of 0.2 M H2SO4 as previously described (8).
IP.
A 1:1 mixture of Protein A and G beads (30 μL; GE Healthcare) was preincubated with binding buffer containing 1 mg/mL BSA (64), incubated with ∼20 μL of Abs or preimmune serum in the presence of 0.3 mL of binding buffer for 1–2 h at 4 °C, and then washed four times with buffer containing 0.5 M NaCl. Beads were resuspended in 0.2 mL of binding buffer. NE (0.1 mL) or samples (0.2 mL) from Superose 6 column fractions were incubated with the prepared Protein A/G beads for 2 h at 4 °C on a rotator. Beads were washed four times with buffer containing 0.3 M NaCl and once with buffer containing 0.1 M NaCl. Bound proteins on beads were eluted by addition of 30–40 μL of 2× SDS sample buffer. The samples were boiled, and 10-μL aliquots were used for Western blots.
GST Pull-Down Assay.
GST and GST fusion proteins immobilized on glutathione beads (15–30 μL) were incubated with protein extracts or purified protein (∼1 μg), core histones (total of 2–4 μg of calf thymus histones) in 0.2–0.3 mL of binding buffer (containing 1 mg/mL BSA) for 90 min at 4 °C. The beads were then washed four times with washing buffer [50 mM Hepes, 0.25 or 0.3 M NaCl, 0.2% Nonidet P-40, 5% (vol/vol) glycerol] and once with washing buffer containing 0.1 M NaCl. Bound proteins were eluted with 20–30 μL of 2× SDS sample buffer for Western blots.
ChIP-qPCR Analysis.
S2 (Drosophila Genomics Resource Center) cells were treated with dsRNA for 7 d and then cross-linked with 1% formaldehyde for 10 min at room temperature at a concentration of 2 × 107 cells per milliliter. Each chromatin sample was prepared from 2.5 × 106 cells after sonication (size of DNA fragments: 0.3–1 kb) and was incubated with 0.1 mL of Protein G for 1–2 h at 4 °C for precleaning. IP using 3–5 μg of anti-H3K27ac Ab (Abcam) or anti-H3K27me3 Ab (Millipore) and qPCR (100- to 120-bp size fragments) were performed as previously described (8) in duplicate. Information about qPCR primers for abd-A, cad, pnr, Psc, Su(z)2, Thor, and tsh and two controls is provided in Table S2.
Table S2.
Information of primers for qPCR
| Name | Chr | Length, bp | Location | Primer sequence |
| abd-A | 3R | 107 | 116 bp 5′ of TSS | 5′-GCGCTCCTCTCCACGCTG-3′ |
| 5′-GAGTCGTTTGGTTAATGGCTAG-3′ | ||||
| cad | 2L | 100 | 377 bp 5′ of TSS | 5′-AGTGAGTGCCGACTGCGG-3′ |
| 5′-GTAGAGCAAGACTCGACTGA-5′ | ||||
| pnr | 3R | 108 | 8,847 bp 5′ of TSS | 5′-ACGCGCGTGTGTGACCGT-3′ |
| 5′-CGACGGGGACTCCTTGTC-3′ | ||||
| Psc | 2R | 105 | 1,049 bp 5′ of TSS | 5′-GAGGCCTGCTGGCAATTTC-3′ |
| 5′-GGGCACACAGAATATGGAAAA-3′ | ||||
| Su(z)2 | 2R | 119 | 1,818 bp 5′ of TSS | 5′-GGTGTGCAAGTGCGCGCC-3′ |
| 5′-CCCACGTTTGATGACGCTCT-3′ | ||||
| Thor | 2L | 114 | 334 bp 5′ of TSS | 5′-CGCTACACCCCTTATCATCT-3′ |
| 5′-GACAAACCGGCTGGTTCTTG-3′ | ||||
| tsh | 2L | 101 | 1,233 bp 5′ of TSS | 5′-TCCAAGCGTTCACTTGCCAG-3′ |
| 5′-CCCGAAACCGATGGGTAGC-3′ | ||||
| cont 1 | 2L | 120 | 4 kb 3′ of Thor TSS | 5′-CACAGATCCCCCTTTCGC-3′ |
| 5′-TCGGGCACCAAGGAGGG-3′ | ||||
| cont 2 | 2R | 100 | 2,657 bp 5′ of Su(z)2 TSS | 5′-CGAGTGCGAGAGGGAGAG-3′ |
| 5′-TCTTCTGTCACTTCAGCTGC-3′ |
Chr, chromosome; cont, control; TSS, transcription start site.
Heat Map Generation from ChIP-chip and ChIP-seq Data.
ChIP-chip (36) (genetics.case.edu/Harte_ChIP-chip/) and sRNA-seq (GSM463298) (45) data from S2 cells were loaded on the Integrated Genome Browser for Drosophila chromosomes 2L, 2R, 3L, 3R, 4, and X. Peaks were called by value (1.5 for PC) or percentile (98% for CBP) and saved in a BED (Browser Extensible Data) file. The sgr (simcity 4 graphics rules) files from ChiP-chip were converted to WIG (wiggle) files based on a standard peak finding protocol involving BWA (Burrows-Wheeler Aligner), samtools, and MACS (Model-based Analysis of ChIP-Seq). The z score for peaks from all data (PC, CBP, H3K27Ac, H3K27me3, and sRNAs) was calculated based on the BED file and corresponding WIG files (Perl code kindly provided by Alina Saiakhova, Case Western Reserve University, Cleveland). Based on the z scores, peaks were clustered by genes (fix cluster number = 7) using Euclidean distance in Cluster 3.0 (65). The png (portable network graphics) tree file was then opened using Java TreeView to generate the heat map.
Genetic Crosses.
To test whether overexpression of PC suppresses the Pc mutant-like phenotypes caused by TRX overexpression, males of the genotype Act-Gal4/Act-Gal4;trxEP3541/trxEP3541, which carry a GAL4-inducible trx+ allele (trxEP3541) and the constitutive and nearly ubiquitous Act4-GAL4 driver, were crossed to females carrying an X chromosome insertion of a hs-Pc transgene (66) composed of a Pc cDNA under the control of the Hsp70 promoter. Adult male progeny of the genotype hs-Pc/Y;Act-Gal4/+;trxEP3541/+ (experimental) were scored for homeotic transformations of T2 and T3 legs to T1 legs based on the appearance of sex comb teeth on T2 and T3. As a control Act-Gal4/Act-Gal4;trxEP3541/trxEP3541 females were crossed (GE Healthcare:) to the Oregon-R wt strain, and the male progeny of the genotype Act-Gal4/+;trxEP354/+ were similarly scored. The hs-Pc transgene itself causes no homeotic transformations or other obvious morphological phenotypes. All of the crosses were done at 29 °C. The trxEP3541 and hs-Pc lines were obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537).
Immunostaining of polytene chromosomes and embryos and RNAi knockdown in S2 cells with 15 μg/mL dsRNA for 7 d were performed as previously described (8). The dsRNAs of Pc and Pcl are correspondent to 676 bp of the Pc coding region (base pairs 223–898) and 705 bp of the Pcl coding region (base pairs 1,267–1,971), respectively.
Acknowledgments
We thank Dr. Sarah Smolik for chicken anti-CBP Ab; Dr. Jeffrey Simon for anti-SCM Ab; Dr. Jinke Cheng for human CBX4 construct; Dr. Danny Reinberg for cDNA of mouse CBX genes; Dr. Jayashree Prasad-Sinha for polytene chromosome staining; Alina Saiakhova for Perl code; and Patrick Wu, Emily Bentley, and Brendan Mullen for assistance in plasmid construction. This work was supported by NIH Grant R01GM39255 (to P.J.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515465113/-/DCSupplemental.
References
- 1.Simon JA, Kingston RE. Occupying chromatin: Polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Mol Cell. 2013;49(5):808–824. doi: 10.1016/j.molcel.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller J, Kassis JA. Polycomb response elements and targeting of Polycomb group proteins in Drosophila. Curr Opin Genet Dev. 2006;16(5):476–484. doi: 10.1016/j.gde.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Kassis JA, Brown JL. Polycomb group response elements in Drosophila and vertebrates. Adv Genet. 2013;81:83–118. doi: 10.1016/B978-0-12-407677-8.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch MD, et al. An interspecies analysis reveals a key role for unmethylated CpG dinucleotides in vertebrate Polycomb complex recruitment. EMBO J. 2012;31(2):317–329. doi: 10.1038/emboj.2011.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz YB, et al. Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat Genet. 2006;38(6):700–705. doi: 10.1038/ng1817. [DOI] [PubMed] [Google Scholar]
- 6.Pengelly AR, Copur Ö, Jäckle H, Herzig A, Müller J. A histone mutant reproduces the phenotype caused by loss of histone-modifying factor Polycomb. Science. 2013;339(6120):698–699. doi: 10.1126/science.1231382. [DOI] [PubMed] [Google Scholar]
- 7.McKay DJ, et al. Interrogating the function of metazoan histones using engineered gene clusters. Dev Cell. 2015;32(3):373–386. doi: 10.1016/j.devcel.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tie F, et al. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development. 2009;136(18):3131–3141. doi: 10.1242/dev.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao Z, et al. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell. 1999;98(1):37–46. doi: 10.1016/S0092-8674(00)80604-2. [DOI] [PubMed] [Google Scholar]
- 10.Francis NJ, Saurin AJ, Shao Z, Kingston RE. Reconstitution of a functional core polycomb repressive complex. Mol Cell. 2001;8(3):545–556. doi: 10.1016/s1097-2765(01)00316-1. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann L, et al. Polycomb repressive complex 1 (PRC1) disassembles RNA polymerase II preinitiation complexes. J Biol Chem. 2012;287(43):35784–35794. doi: 10.1074/jbc.M112.397430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis NJ, Kingston RE, Woodcock CL. Chromatin compaction by a polycomb group protein complex. Science. 2004;306(5701):1574–1577. doi: 10.1126/science.1100576. [DOI] [PubMed] [Google Scholar]
- 13.Eskeland R, et al. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell. 2010;38(3):452–464. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431(7010):873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- 15.Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20(6):845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Pengelly AR, Kalb R, Finkl K, Müller J. Transcriptional repression by PRC1 in the absence of H2A monoubiquitylation. Genes Dev. 2015;29(14):1487–1492. doi: 10.1101/gad.265439.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illingworth RS, et al. The E3 ubiquitin ligase activity of RING1B is not essential for early mouse development. Genes Dev. 2015;29(18):1897–1902. doi: 10.1101/gad.268151.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paro R, Hogness DS. The Polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc Natl Acad Sci USA. 1991;88(1):263–267. doi: 10.1073/pnas.88.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischle W, et al. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev. 2003;17(15):1870–1881. doi: 10.1101/gad.1110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min J, Zhang Y, Xu RM. Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev. 2003;17(15):1823–1828. doi: 10.1101/gad.269603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enderle D, et al. Polycomb preferentially targets stalled promoters of coding and noncoding transcripts. Genome Res. 2011;21(2):216–226. doi: 10.1101/gr.114348.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaaf CA, et al. Cohesin and polycomb proteins functionally interact to control transcription at silenced and active genes. PLoS Genet. 2013;9(6):e1003560. doi: 10.1371/journal.pgen.1003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tie F, et al. Trithorax monomethylates histone H3K4 and interacts directly with CBP to promote H3K27 acetylation and antagonize Polycomb silencing. Development. 2014;141(5):1129–1139. doi: 10.1242/dev.102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kundu TK, et al. Activator-dependent transcription from chromatin in vitro involving targeted histone acetylation by p300. Mol Cell. 2000;6(3):551–561. doi: 10.1016/s1097-2765(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 25.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 26.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14(13):1553–1577. [PubMed] [Google Scholar]
- 27.Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114(Pt 13):2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- 28.Thompson PR, Kurooka H, Nakatani Y, Cole PA. Transcriptional coactivator protein p300. Kinetic characterization of its histone acetyltransferase activity. J Biol Chem. 2001;276(36):33721–33729. doi: 10.1074/jbc.M104736200. [DOI] [PubMed] [Google Scholar]
- 29.Hilton IB, et al. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol. 2015;33(5):510–517. doi: 10.1038/nbt.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delvecchio M, Gaucher J, Aguilar-Gurrieri C, Ortega E, Panne D. Structure of the p300 catalytic core and implications for chromatin targeting and HAT regulation. Nat Struct Mol Biol. 2013;20(9):1040–1046. doi: 10.1038/nsmb.2642. [DOI] [PubMed] [Google Scholar]
- 31.Black JC, Mosley A, Kitada T, Washburn M, Carey M. The SIRT2 deacetylase regulates autoacetylation of p300. Mol Cell. 2008;32(3):449–455. doi: 10.1016/j.molcel.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson PR, et al. Regulation of the p300 HAT domain via a novel activation loop. Nat Struct Mol Biol. 2004;11(4):308–315. doi: 10.1038/nsmb740. [DOI] [PubMed] [Google Scholar]
- 33.Arif M, Kumar GV, Narayana C, Kundu TK. Autoacetylation induced specific structural changes in histone acetyltransferase domain of p300: Probed by surface enhanced Raman spectroscopy. J Phys Chem B. 2007;111(41):11877–11879. doi: 10.1021/jp0762931. [DOI] [PubMed] [Google Scholar]
- 34.Chang YL, et al. Essential role of Drosophila Hdac1 in homeotic gene silencing. Proc Natl Acad Sci USA. 2001;98(17):9730–9735. doi: 10.1073/pnas.171325498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petruk S, et al. Trithorax and dCBP acting in a complex to maintain expression of a homeotic gene. Science. 2001;294(5545):1331–1334. doi: 10.1126/science.1065683. [DOI] [PubMed] [Google Scholar]
- 36.Tie F, Banerjee R, Conrad PA, Scacheri PC, Harte PJ. Histone demethylase UTX and chromatin remodeler BRM bind directly to CBP and modulate acetylation of histone H3 lysine 27. Mol Cell Biol. 2012;32(12):2323–2334. doi: 10.1128/MCB.06392-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmqvist PH, Mannervik M. Genomic occupancy of the transcriptional co-activators p300 and CBP. Transcription. 2013;4(1):18–23. doi: 10.4161/trns.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz YB, et al. Alternative epigenetic chromatin states of polycomb target genes. PLoS Genet. 2010;6(1):e1000805. doi: 10.1371/journal.pgen.1000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoorlemmer J, et al. Ring1A is a transcriptional repressor that interacts with the Polycomb-M33 protein and is expressed at rhombomere boundaries in the mouse hindbrain. EMBO J. 1997;16(19):5930–5942. doi: 10.1093/emboj/16.19.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorfinkiel N, et al. The Drosophila Polycomb group gene Sex combs extra encodes the ortholog of mammalian Ring1 proteins. Mech Dev. 2004;121(5):449–462. doi: 10.1016/j.mod.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Kurzhals RL, Tie F, Stratton CA, Harte PJ. Drosophila ESC-like can substitute for ESC and becomes required for Polycomb silencing if ESC is absent. Dev Biol. 2008;313(1):293–306. doi: 10.1016/j.ydbio.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tie F, Prasad-Sinha J, Birve A, Rasmuson-Lestander A, Harte PJ. A 1-megadalton ESC/E(Z) complex from Drosophila that contains polycomblike and RPD3. Mol Cell Biol. 2003;23(9):3352–3362. doi: 10.1128/MCB.23.9.3352-3362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nekrasov M, et al. Pcl-PRC2 is needed to generate high levels of H3-K27 trimethylation at Polycomb target genes. EMBO J. 2007;26(18):4078–4088. doi: 10.1038/sj.emboj.7601837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herz HM, et al. Enhancer-associated H3K4 monomethylation by Trithorax-related, the Drosophila homolog of mammalian Mll3/Mll4. Genes Dev. 2012;26(23):2604–2620. doi: 10.1101/gad.201327.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nechaev S, et al. Global analysis of short RNAs reveals widespread promoter-proximal stalling and arrest of Pol II in Drosophila. Science. 2010;327(5963):335–338. doi: 10.1126/science.1181421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park SY, Schwartz YB, Kahn TG, Asker D, Pirrotta V. Regulation of Polycomb group genes Psc and Su(z)2 in Drosophila melanogaster. Mech Dev. 2012;128(11-12):536–547. doi: 10.1016/j.mod.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Fang M, et al. Drosophila ptip is essential for anterior/posterior patterning in development and interacts with the PcG and trxG pathways. Development. 2009;136(11):1929–1938. doi: 10.1242/dev.026559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mason-Suares H, Tie F, Yan CM, Harte PJ. Polycomb silencing of the Drosophila 4E-BP gene regulates imaginal disc cell growth. Dev Biol. 2013;380(1):111–124. doi: 10.1016/j.ydbio.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kee BL, Arias J, Montminy MR. Adaptor-mediated recruitment of RNA polymerase II to a signal-dependent activator. J Biol Chem. 1996;271(5):2373–2375. doi: 10.1074/jbc.271.5.2373. [DOI] [PubMed] [Google Scholar]
- 50.Cho H, et al. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol Cell Biol. 1998;18(9):5355–5363. doi: 10.1128/mcb.18.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Mikecz A, Zhang S, Montminy M, Tan EM, Hemmerich P. CREB-binding protein (CBP)/p300 and RNA polymerase II colocalize in transcriptionally active domains in the nucleus. J Cell Biol. 2000;150(1):265–273. doi: 10.1083/jcb.150.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byun JS, et al. Dynamic bookmarking of primary response genes by p300 and RNA polymerase II complexes. Proc Natl Acad Sci USA. 2009;106(46):19286–19291. doi: 10.1073/pnas.0905469106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim TK, Shiekhattar R. Architectural and Functional Commonalities between Enhancers and Promoters. Cell. 2015;162(5):948–959. doi: 10.1016/j.cell.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breiling A, Turner BM, Bianchi ME, Orlando V. General transcription factors bind promoters repressed by Polycomb group proteins. Nature. 2001;412(6847):651–655. doi: 10.1038/35088090. [DOI] [PubMed] [Google Scholar]
- 55.Saurin AJ, Shao Z, Erdjument-Bromage H, Tempst P, Kingston RE. A Drosophila Polycomb group complex includes Zeste and dTAFII proteins. Nature. 2001;412(6847):655–660. doi: 10.1038/35088096. [DOI] [PubMed] [Google Scholar]
- 56.Black JC, Choi JE, Lombardo SR, Carey M. A mechanism for coordinating chromatin modification and preinitiation complex assembly. Mol Cell. 2006;23(6):809–818. doi: 10.1016/j.molcel.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 57.Schröder S, et al. Acetylation of RNA polymerase II regulates growth-factor-induced gene transcription in mammalian cells. Mol Cell. 2013;52(3):314–324. doi: 10.1016/j.molcel.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kellner WA, Ramos E, Van Bortle K, Takenaka N, Corces VG. Genome-wide phosphoacetylation of histone H3 at Drosophila enhancers and promoters. Genome Res. 2012;22(6):1081–1088. doi: 10.1101/gr.136929.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tresser J, et al. doublesex/mab3 related-1 (dmrt1) is essential for development of anterior neural plate derivatives in Ciona. Development. 2010;137(13):2197–2203. doi: 10.1242/dev.045302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arner E, et al. FANTOM Consortium Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science. 2015;347(6225):1010–1014. doi: 10.1126/science.1259418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strübbe G, et al. Polycomb purification by in vivo biotinylation tagging reveals cohesin and Trithorax group proteins as interaction partners. Proc Natl Acad Sci USA. 2011;108(14):5572–5577. doi: 10.1073/pnas.1007916108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tie F, Stratton CA, Kurzhals RL, Harte PJ. The N terminus of Drosophila ESC binds directly to histone H3 and is required for E(Z)-dependent trimethylation of H3 lysine 27. Mol Cell Biol. 2007;27(6):2014–2026. doi: 10.1128/MCB.01822-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tie F, Furuyama T, Prasad-Sinha J, Jane E, Harte PJ. The Drosophila Polycomb Group proteins ESC and E(Z) are present in a complex containing the histone-binding protein p55 and the histone deacetylase RPD3. Development. 2001;128(2):275–286. doi: 10.1242/dev.128.2.275. [DOI] [PubMed] [Google Scholar]
- 64.Tie F, Furuyama T, Harte PJ. The Drosophila Polycomb Group proteins ESC and E(Z) bind directly to each other and co-localize at multiple chromosomal sites. Development. 1998;125(17):3483–3496. doi: 10.1242/dev.125.17.3483. [DOI] [PubMed] [Google Scholar]
- 65.de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20(9):1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 66.Fauvarque MO, Zuber V, Dura JM. Regulation of polyhomeotic transcription may involve local changes in chromatin activity in Drosophila. Mech Dev. 1995;52(2-3):343–355. doi: 10.1016/0925-4773(95)00412-t. [DOI] [PubMed] [Google Scholar]