Significance
Characterizing the abnormalities in sleep and activity that are associated with bipolar disorder (BP) and identifying their causation are key milestones in unraveling the biological underpinnings of this severe and highly prevalent disorder. We have conducted the first systematic evaluation of sleep and activity phenotypes in pedigrees that include multiple BP-affected members. By delineating specific sleep and activity measures that are significantly heritable in these families, and those whose variation correlated with the BP status of their members, and by determining the chromosomal position of loci contributing to many of these traits, we have taken the first step toward discovery of causative genetic variants. These variants, in turn, could provide clues to new approaches for both preventing and treating BP.
Keywords: bipolar disorder, endophenotypes, circadian rhythms, actigraphy, behavior
Abstract
Abnormalities in sleep and circadian rhythms are central features of bipolar disorder (BP), often persisting between episodes. We report here, to our knowledge, the first systematic analysis of circadian rhythm activity in pedigrees segregating severe BP (BP-I). By analyzing actigraphy data obtained from members of 26 Costa Rican and Colombian pedigrees [136 euthymic (i.e., interepisode) BP-I individuals and 422 non–BP-I relatives], we delineated 73 phenotypes, of which 49 demonstrated significant heritability and 13 showed significant trait-like association with BP-I. All BP-I–associated traits related to activity level, with BP-I individuals consistently demonstrating lower activity levels than their non–BP-I relatives. We analyzed all 49 heritable phenotypes using genetic linkage analysis, with special emphasis on phenotypes judged to have the strongest impact on the biology underlying BP. We identified a locus for interdaily stability of activity, at a threshold exceeding genome-wide significance, on chromosome 12pter, a region that also showed pleiotropic linkage to two additional activity phenotypes.
Quantitative sleep and activity measures are hypothesized to be endophenotypes for bipolar disorder (BP). Disturbance of sleep and circadian activity typically precedes and may precipitate the initial onset of BP (1, 2). Decreased sleep and increased activity occur before and during manic and hypomanic episodes. Conversely, increased sleep and decreased activity characterize BP–depression. Extreme diurnal variation in mood features prominently in both mania and depression, whereas shifts in circadian phase (the time within the daily activity cycle at which periodic phenomena such as bed time or awakening occur) can induce mania and ameliorate symptoms of BP–depression (3).
Twin studies have identified multiple heritable sleep and activity phenotypes, including sleep duration, sleep quality, phase of activity preference and sleep pattern, and sleep architecture variables [e.g., the amount of slow wave and rapid eye movement (REM) sleep (4) and polysomnography profiles during non-REM sleep (5)]. Euthymic BP individuals, compared with healthy controls, display trait-like alterations in several such phenotypes—for example, sleep time and time in bed, sleep onset latency, and periods of being awake after sleep onset (6). However, no prior investigations have assayed the heritability of such phenotypes in BP individuals and their relatives.
We report here the delineation of sleep and activity BP endophenotypes through investigations of 26 pedigrees (n = 558) ascertained for severe BP (BP-I), from the genetically related populations of the Central Valley of Costa Rica (CR) and Antioquia, Colombia (CO) (7–9). Pedigrees ascertained for multiple cases of severe BP (BP-I) should be enriched for extreme values of quantitative traits that are BP endophenotypes, enhancing their utility for genetic mapping studies of such phenotypes. Additionally, such pedigrees derived from recently expanded founder populations are likely to show increased frequencies for many deleterious alleles—another feature that will enhance their utility for mapping these traits (10, 11). We previously described, in these pedigrees, multiple heritable and BP-associated phenotypes from the domains of temperament, neurocognition, and neuroanatomy (10).
Actigraphy (activity measurement using wrist-worn accelerometers) can be conducted over prolonged periods without impinging on an individual’s usual activities, enabling assessment of sleep and activity on a scale sufficient for genetic investigations. Actigraphy data on sleep quality and duration correlate strongly with those obtained through polysomnography, the gold standard for sleep research (12). Using actigraphy, one can estimate the main circadian activity parameters, which follow a sinusoidal waveform with a ∼24-h period: phase, amplitude (the strength of circadian rhythms, as measured by the difference in the amount of activity between active and inactive moments), and coherence of the rhythm (the degree of consolidation and stability of activity, rest, and sleep). Finally, actigraphy enables quantification of BP-associated features, such as fragmentation of rest and activity within and between days, that do not fit a sinusoidal waveform and cannot be analyzed parametrically (13).
We recorded activity in euthymic BP-I individuals and their non–BP-I relatives from the CR and CO pedigrees for, on average, 14 consecutive days. For each actigraphy phenotype, we evaluated association with BP-I, assessed the heritability of each trait, and performed genome-wide genetic linkage analyses on all significantly heritable traits.
Results
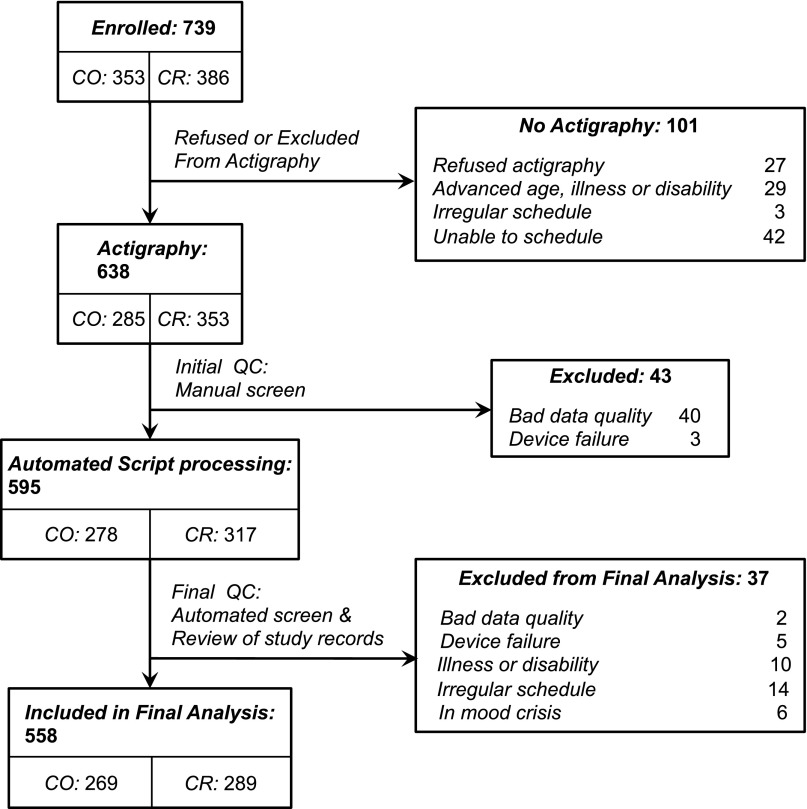
Through actigraphy, we obtained activity recordings (illustrated in Fig. S1) from 638 members of 26 CR and CO pedigrees. After applying quality control (QC) procedures (SI Methods) that led to the exclusion of 80 recordings (Fig. S2), we analyzed activity data from 558 individuals, including 136 BP-I individuals and 422 of their non–BP-I relatives (Table 1). A series of algorithms obtained from published sources (14–17) were then applied to the activity data to obtain 116 quantitative sleep and activity phenotypes. These phenotypes can be classified into six broad domains that quantified patterns of activity and sleep during the major rest period of the day (i) and during the awake period (ii), the fragmentation or consolidation of activity (iii), overall activity levels (iv), and the fit of daily activity patterns to curves based on sine and cosine functions (using two different approaches) (v and vi). Details on the construction of phenotypes that fall into each domain are provided in SI Methods.
Fig. S1.
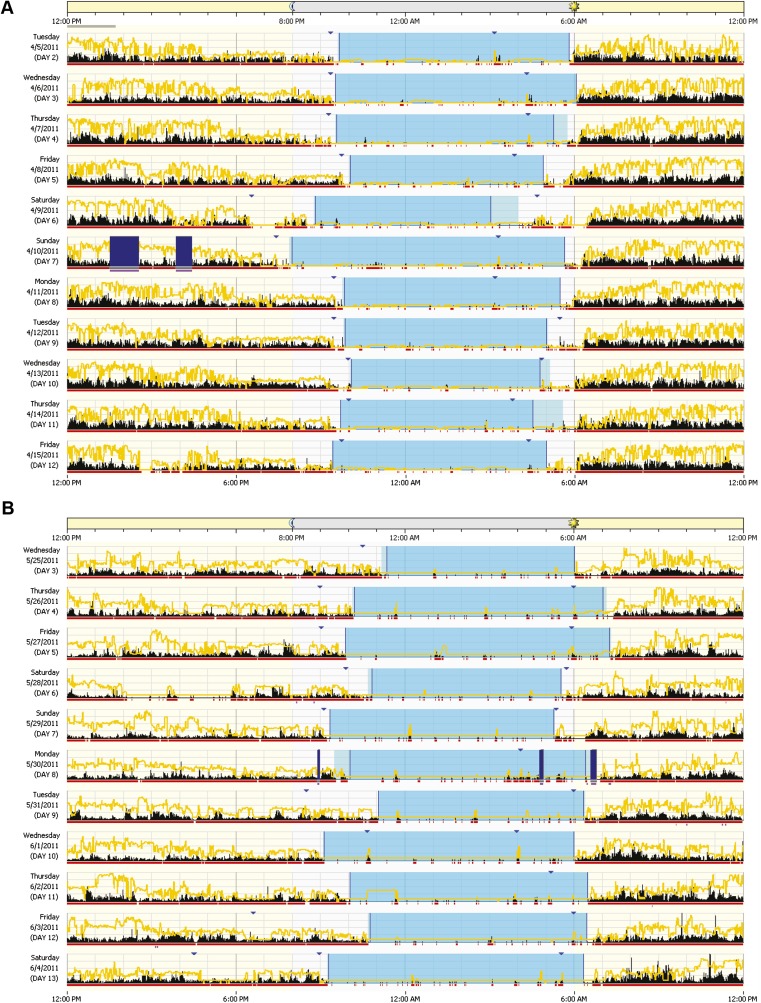
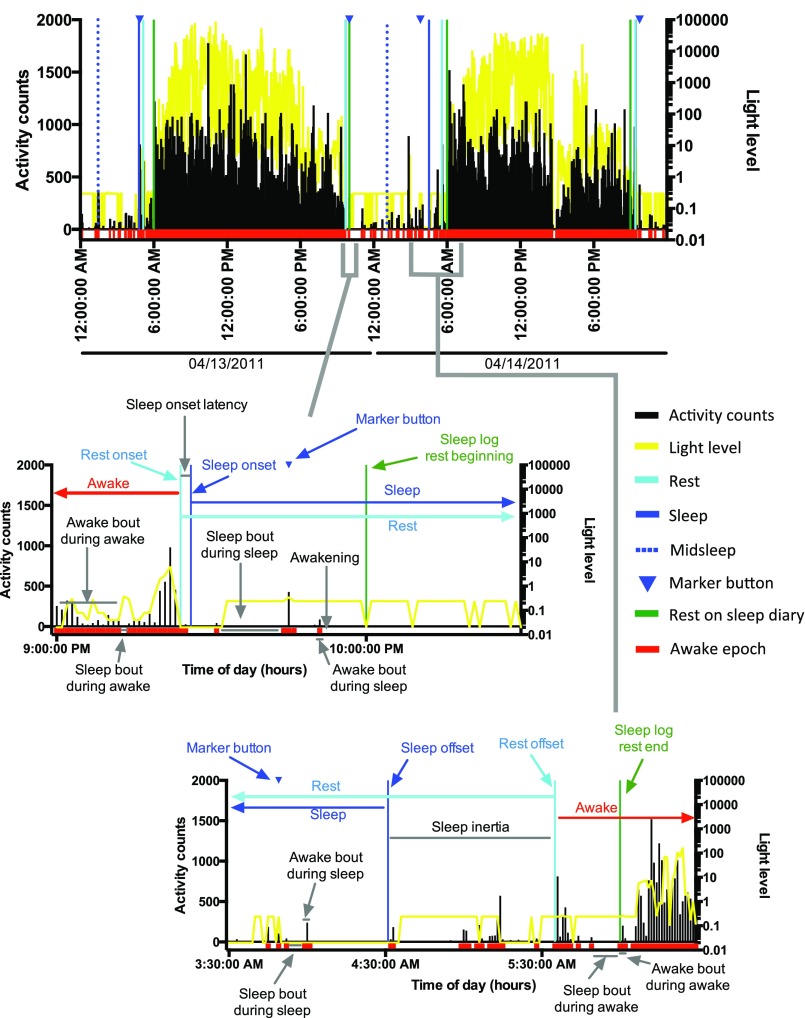
Example of actigraphies from one non–BP-I and one BP-I participant, from the same family. The activity level is indicated in black. The yellow line represents light levels. Dark blue bars indicate when the watch was off-wrist. Red squares below activity indicate the epochs that are scored as awake. Blue triangles indicate when the subject pressed the marker button (ideally at rest onset and rest offset). Light blue periods represent the rest period; darker light blue periods within the rest period indicate the sleep period. Rest and sleep periods were calculated by R algorithms. (A) Activity of one female non–BP-I volunteer, 59 y old, from a CO family. (B) Activity of one female BP-I volunteer, 58 y old, from the same family as A.
Fig. S2.
Flowchart representing the included and excluded subjects.
Table 1.
Sample characteristics and recording days by country and family
| Family | n (BP-I cases) | Female | Mean age (SD), range | Mean recorded days (SD), range |
| CO All | 269 (66) | 58% | 46.3 (16.6), 18–83 | 15.1 (2.1), 7–24 |
| CR All | 290 (70) | 53% | 49.2 (16.0), 17–88 | 15.8 (2.9), 6–27 |
| CO4 | 33 (7) | 61% | 42.0 (17.2), 18–76 | 15.1 (2.3), 13–24 |
| CO7 | 96 (23) | 53% | 44.8 (17.0), 18–80 | 14.8 (1.6), 7–21 |
| CO8 | 5 (2) | 60% | 40.8 (12.8), 24–54 | 14 (4.9), 7–21 |
| CO10 | 22 (5) | 73% | 53.4 (15.4), 32–77 | 15.4 (2), 14–21 |
| CO13 | 14 (4) | 57% | 42.1 (14.7), 18–66 | 17.1 (3.1), 14–21 |
| CO14 | 13 (4) | 46% | 43.8 (15.4), 20–74 | 14.1 (2.4), 10–21 |
| CO15 | 19 (4) | 58% | 41.7 (15.3), 19–73 | 14.5 (1.6), 12–20 |
| CO18 | 20 (6) | 55% | 59.3 (14.2), 34–77 | 15.2 (1.9), 14–20 |
| CO23 | 21 (6) | 67% | 45.0 (15.2), 18–83 | 15.3 (1.9), 14–20 |
| CO25 | 8 (2) | 63% | 56.5 (13.5), 45–82 | 15.6 (2.6), 14–21 |
| CO27 | 18 (3) | 67% | 46.7 (16.8), 18–74 | 15.4 (1.8), 14–21 |
| CR001 | 5 (1) | 60% | 56.0 (11.3), 44–68 | 19.6 (2.6), 15–21 |
| CR004 | 37 (7) | 51% | 55.6 (13.5), 30–84 | 15 (1.9), 12–21 |
| CR006 | 7 (2) | 29% | 52.7 (11.8), 38–66 | 13.9 (4.1), 6–20 |
| CR007 | 4 (2) | 50% | 47.0 (6.6), 39–55 | 13.8 (0.5), 13–14 |
| CR008 | 9 (3) | 44% | 43.7 (16.1), 21–68 | 15.7 (2.5), 14–20 |
| CR009 | 25 (6) | 60% | 40.6 (14.4), 20–74 | 15.6 (2.8), 12–22 |
| CR010 | 12 (3) | 58% | 43.9 (15.9), 21–74 | 14.2 (2.2), 11–19 |
| CR011 | 9 (2) | 56% | 48.8 (23.2), 21–86 | 18.2 (3), 14–21 |
| CR012 | 19 (5) | 68% | 40.8 (15.3), 20–68 | 17 (3.2), 14–21 |
| CR013 | 5 (2) | 80% | 52.2 (19.3), 35–74 | 15.4 (2.2), 14–19 |
| CR014 | 2 (1) | 50% | 45.0 (2.8), 43–47 | 14.5 (0.7), 14–15 |
| CR015 | 8 (1) | 63% | 51.6 (14.2), 38–71 | 18.4 (2.2), 16–21 |
| CR016 | 13 (4) | 38% | 50.1 (14.5), 19–66 | 16.8 (2.6), 13–21 |
| CR201 | 125 (28) | 51% | 50.6 (16.2), 17–88 | 15.8 (3.1), 7–27 |
| CR277 | 9 (3) | 56% | 49.3 (11.6), 37–71 | 14.1 (0.3), 14–15 |
| Grand total | 558 (136) | 56% | 47.8 (16.3), 17–88 | 15.4 (2.6), 6–27 |
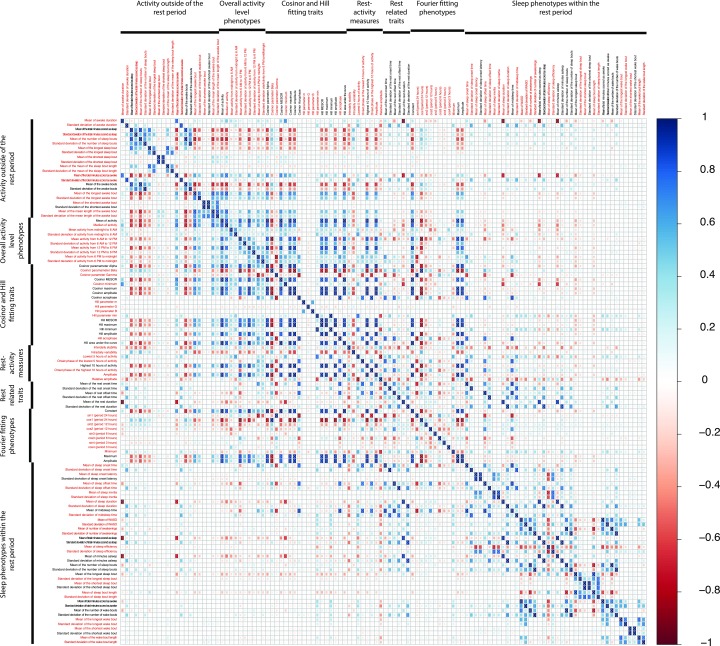
To reduce the multiple testing burden and eliminate completely redundant variables, we calculated pair-wise correlations among all 116 phenotypes (Fig. S3) and performed a hierarchical clustering analysis using 1 – correlation as the distance metric. We then selected one representative phenotype from each cluster, yielding 73 phenotypes (Fig. S3), which we analyzed further as described below. The 43 phenotypes excluded at this stage were very highly correlated with other phenotypes, all with r > 0.90 and most with r > 0.99.
Fig. S3.
Correlation matrix among 116 variables. Darker shades of blue indicate stronger positive correlation, whereas darker shades of red indicate higher negative correlation between two variables. The 73 variables in red text were selected for further study.
Heritability of Phenotypes and Their Association to BP-I.
Estimating the familial aggregation of the 73 phenotypes (an indicator of heritability) and their relationship to BP-I allowed us to determine which phenotypes have a significant genetic component, to proceed with analyses to identify genes contributing to phenotypes that are potentially important in the etiology of BP-I. We subjected each phenotype to an inverse-normal transformation, and to control for covariates, we regressed [in the software SOLAR (Sequential Oligogenic Linkage Analysis Routines) (18)] the transformed phenotypes on age, gender, and country. The residuals from this regression were assessed for heritability and for a mean difference between BP-I individuals and their non–BP-I relatives.
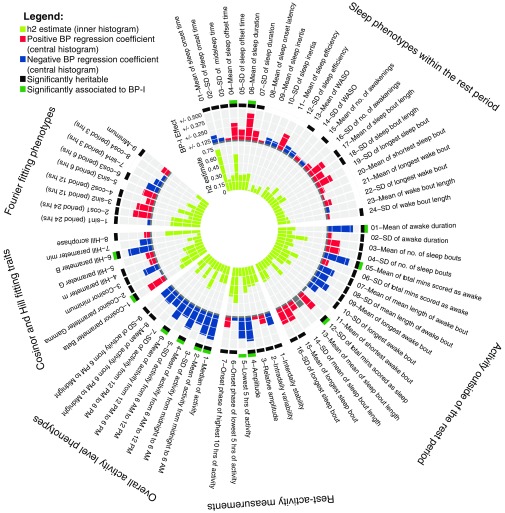
Of the 73 phenotypes, 49 (67%) demonstrated significant heritability. Heritable phenotypes included measures related to sleep and activity duration, timing, fragmentation, and consolidation; activity levels and variability; and the timing and periodicity of mean daily activity (Fig. 1).
Fig. 1.
Polar histogram of heritability traits and association to BP-I. The inner histogram represents the heritability estimate (h2) in yellow–green. The middle histogram represents the association to BP-I; positive associations with BP-I are presented in red (i.e., the trait has a higher value in those with BP-I), whereas negative associations are presented in blue (indicating those with BP-I have lower values on the trait). The outer histogram summarizes the heritable traits (black) and the phenotypes associated with BP-I (green). hrs, hours; mins, minutes; no., number.
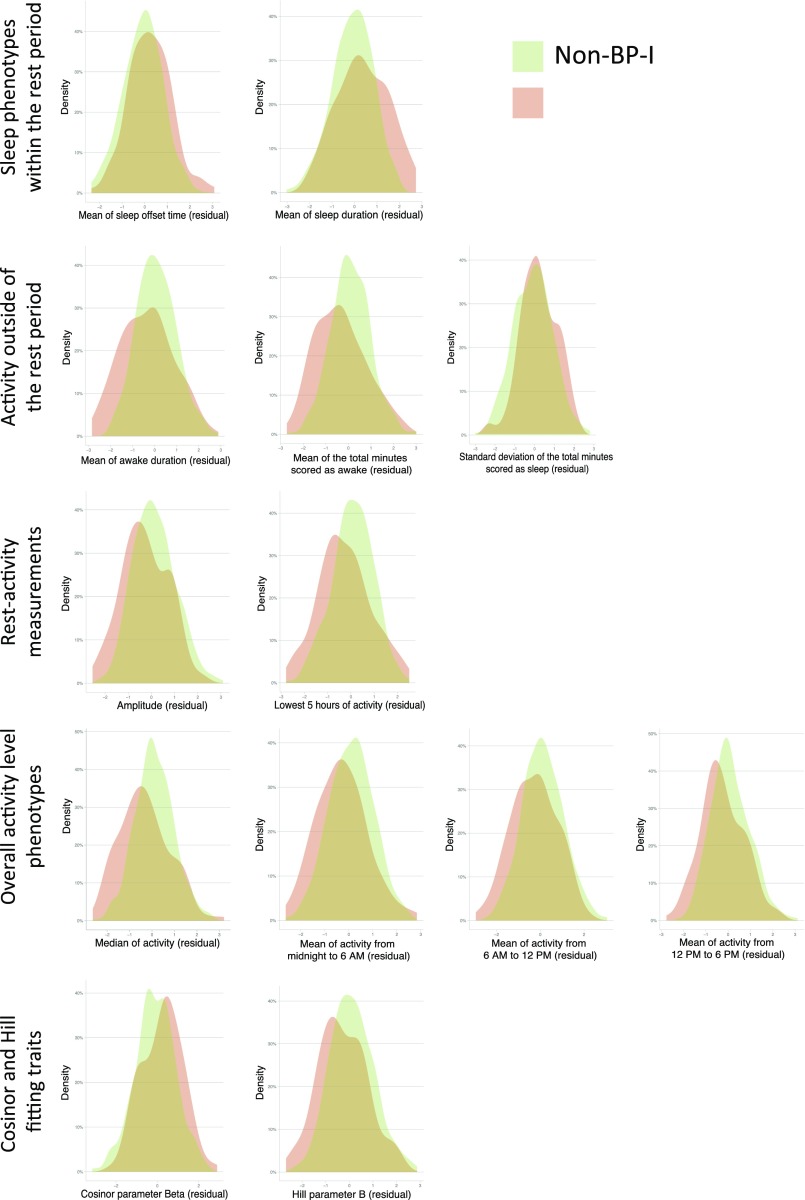
Thirteen phenotypes (18%) were significantly associated with BP-I, of which 12 (92%) were also heritable (Fig. 1 and Fig. S4). BP-I subjects awoke later and slept longer than non–BP-I subjects (phenotypes, mean of sleep offset time and mean sleep duration). Outside of the rest period, BP-I individuals were, on average, awake fewer minutes than non–BP-I individuals (phenotypes, mean of awake duration and mean of total minutes scores as awake) and had more variability in the time during the awake period scored as sleeping (phenotype, SD of the total minutes scored as sleep). Similar to previous studies (16, 19), we found that euthymic BP-I individuals display lower activity levels than non–BP-I individuals, by multiple measures: amplitude; L5, 5-h period of minimal activity; median activity; mean activity in 6-h windows; and two parameters related to the amplitude and phase of curve fits to mean daily activity (parameter B from the fit of the Hill transformation to the daily activity profile and Cosinor parameter Beta).
Fig. S4.
Density distribution of the phenotypes found different between BP-I and non–BP-I subjects.
Linkage Analysis of Selected Heritable Traits.
To identify genetic loci with the largest impact on sleep and activity phenotypes in the BP-I pedigrees, we conducted genome-wide linkage analysis using a dense set of SNPs. Our primary analyses focused on 13 phenotypes that we considered most relevant to BP. These phenotypes included traits with prior evidence of BP association, traits that showed strong BP-I association in our pedigrees, and traits biologically relevant to fragmentation or consolidation of circadian activity or with biological relevance to phase (Table 2).
Table 2.
Thirteen phenotypes chosen for the primary linkage analysis
| Phenotype | Trait | Importance |
| Amplitude | Relative amplitude | BP-I associated (19) |
| Amplitude | Association with BP-I in the present study | |
| Median of activity level | Association with BP-I in the present study | |
| Phase | Time of sleep onset | BP-I associated (17) |
| Time of sleep offset | BP-I associated (17) | |
| Hill acrophase | Biologically relevant for phase | |
| Fragmentation/ consolidation | IV, Intradaily Variability in activity | BP-I associated (17) |
| IS, Interdaily Stability in activity | BP-I associated (17) | |
| Mean of the number of sleep bouts during the awake period | Biologically relevant to fragmentation/consolidation | |
| Mean of the length of sleep bouts during the sleep period | Biologically relevant to fragmentation/consolidation | |
| Efficiency of sleep | WASO, total minutes in awake bouts after sleep onset | BP-I associated (24) |
| Mean of awake duration | Association with BP-I in the present study | |
| Mean total minutes scored as awake during the awake period | Association with BP-I in the present study |
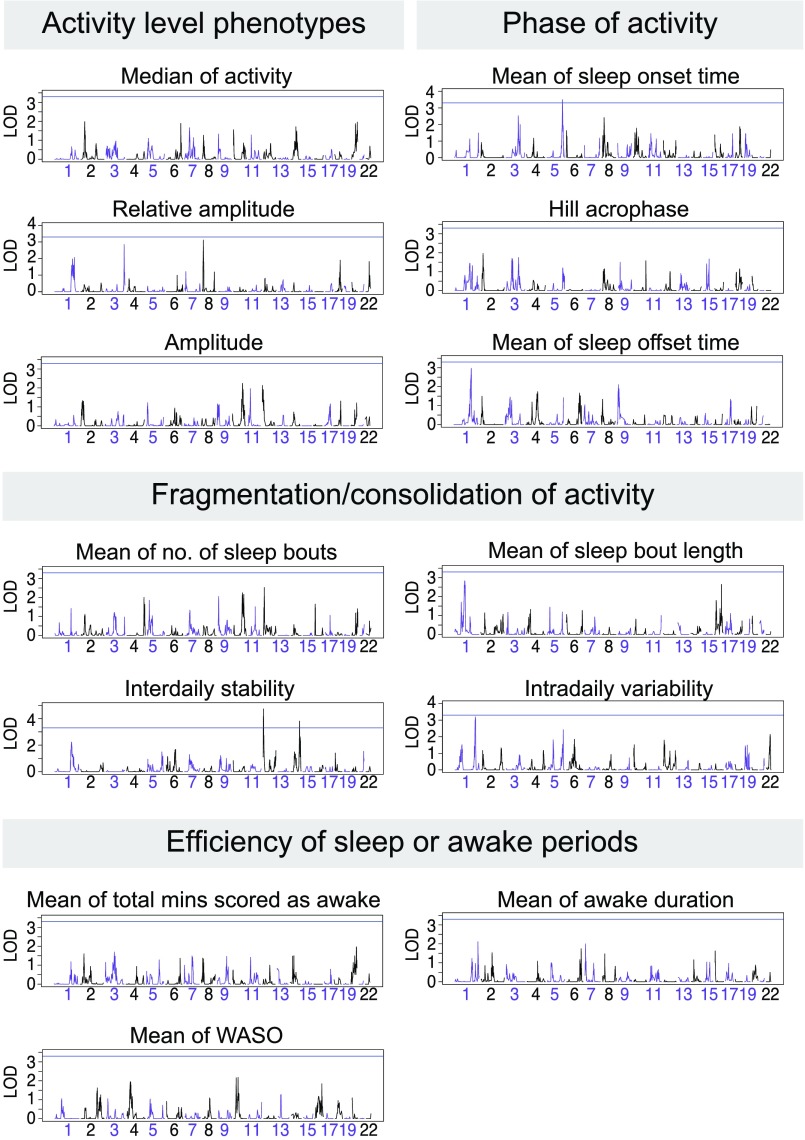
Using SOLAR, we performed multipoint linkage analysis on these 13 phenotypes. The strongest linkage was for the rest–activity phenotype Interdaily Stability (IS), that represents the degree of variability of activity level on an hourly basis from day-to-day (Fig. 2), for which we observed a maximum LOD [logarithm (base 10) of odds] score (4.73, 4 Mb from chromosome 12pter), exceeding the traditional genome-wide significance threshold (P < 10−4). Interdaily stability linkage remains genome-wide significant at the 0.05 alpha level, after correcting for the 13 phenotypes (empirical P values associated to the highest LOD score and to the smallest Simes’ P value are 0.03 and 0.02, respectively). The linkage evidence for interdaily stability diminishes only slightly when including BP-I status as a covariate (LOD = 4.65; Table S1).
Fig. 2.
Representation of the multipoint LOD scores on different chromosomes (which are depicted in alternating violet and black) for 13 traits that we considered of highest biological significance. Blue line indicates an LOD score of 3.3, corresponding to a P value of 5 × 10−5. Mins, minutes; no., number.
Table S1.
Linkage results for the 13 traits of higher biological interest, with and without BP status as a covariate
| Without BP as covariate | With BP as covariate | |||||
| Phenotype | maxLOD | chr | cM | maxLOD | chr | cM |
| Mean of awake duration | 2.11 | 1 | 237 | 2.09 | 7 | 6 |
| Amplitude | 2.24 | 10 | 105 | 2.14 | 10 | 105 |
| Hill acrophase | 1.97 | 2 | 21 | 1.84 | 2 | 21 |
| Interdaily stability | 4.73 | 12 | 4 | 4.66 | 12 | 4 |
| Interdaily variability | 3.20 | 1 | 210 | 2.98 | 1 | 210 |
| Median activity | 1.98 | 2 | 40 | 1.98 | 20 | 57 |
| Relative amplitude | 3.12 | 8 | 12 | 2.97 | 8 | 12 |
| Mean length of sleep bouts during the sleep period | 2.83 | 1 | 102 | 3.21 | 16 | 72 |
| Mean number sleep bouts during awake period | 2.53 | 12 | 8 | 2.20 | 12 | 8 |
| Time of sleep offset | 2.97 | 1 | 180 | 2.43 | 1 | 180 |
| Time of sleep onset | 3.50 | 5 | 161 | 3.64 | 5 | 161 |
| Mean total minutes scored awake | 1.97 | 20 | 56 | 2.24 | 20 | 57 |
| WASO, total minutes in awake bouts after sleep onset | 2.18 | 10 | 45 | 2.09 | 10 | 45 |
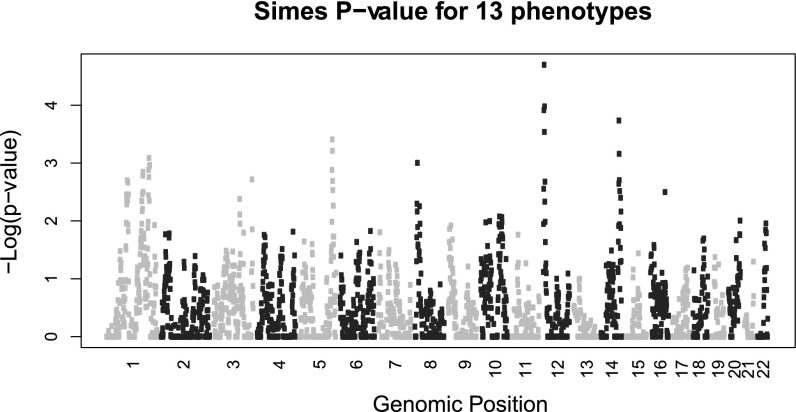
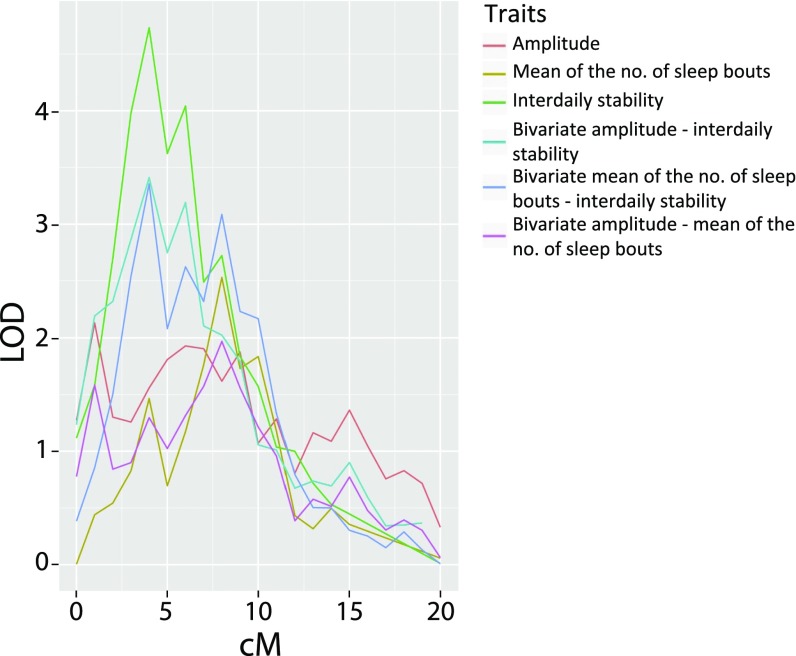
Fig. 3 presents a summary of the significance of linkage results considering all 49 heritable phenotypes (20). Two linkage peaks stand out. The largest peak includes the interdaily stability linkage on chromosome 12pter, having a Simes P value < 0.0001. In the same region of linkage to interdaily stability, we observed suggestive linkage for two additional phenotypes (Fig. 4): the mean number of sleep bouts in the awake period, and amplitude (the difference in activity between the 5 most active hours and the 10 least active hours), with peak LOD scores, respectively, of 2.53 (8 Mb from pter) and 2.13 (1 Mb from pter). Interdaily stability and mean number of sleep bouts show moderately negative phenotypic correlation (rhop = –0.51) but strongly negative genetic correlation (rhog = –0.93); joint multipoint linkage analysis suggests complete pleiotropy, indicating a common genetic component to both phenotypes, centered 4 Mb from 12pter (LOD = 3.35); lower interdaily stability indicates a weaker rhythm, while a lower value of mean number of sleep bouts is associated to a more consolidated rhythm, leading to a negative correlation between these variables. Amplitude and interdaily stability display phenotypic and genotypic correlation of a similar magnitude, albeit in a positive direction (rhop = 0.51 and rhog = 0.92); their joint linkage analysis (LOD = 3.41) suggests near-complete pleiotropy between these phenotypes, at the same location.
Fig. 3.
Multipoint linkage analysis across all 49 heritable phenotypes. Chromosomes are represented in alternating colors (light gray–dark gray).
Fig. 4.
Multipoint linkage analysis of the most biological significant phenotypes; depiction of the region of pleiotropic linkage on chromosome 12p. no., number.
A second (nonsignificant) linkage peak on chromosome 1q displayed LOD > 3.0 for four correlated phenotypes related to the SD of sleep onset and activity (SD of the time of sleep onset, LOD 3.9 at 183 Mb; SD of the active phase, LOD 3.6 at 185 Mb; SD of the time of midsleep, LOD 4.3 at 186 Mb; SD of the total minutes awake, LOD 3.4 at 186 Mb). These phenotypes all have a pair-wise genetic correlation >0.85, as is reflected in joint linkage results suggesting complete pleiotropy among them (joint LOD scores range from 3.1 to 4.2 at 185 Mb; Table S2).
Table S2.
Bivariate analysis of phenotypes linked to chromosome 1
| Phenotype | AlphaSD | MidsleepSD | SleepOnsetSD | TotalMinAwakeSD |
| (A) | ||||
| AlphaSD | 1 | 0.98 | 0.98 | |
| MidsleepSD | 0.74 | 0.97 | 1 | |
| SleepOnsetSD | 0.65 | 0.7 | 0.85 | |
| TotalMinAwakeSD | 0.75 | 0.54 | 0.5 | |
| (B) | ||||
| AlphaSD | 185 | 185 | 185 | |
| MidsleepSD | 3.52 | 185 | 186 | |
| SleepOnsetSD | 3.96 | 4.06 | 185 | |
| TotalMinAwakeSD | 3.13 | 4.1 | 4.2 |
Alpha is the duration of the awake period. (A) Top above the diagonal, rhoG; bottom below the diagonal, rhoE. (B) Top above the diagonal, location maximum LOD score (Mb); bottom below the diagonal, maximum bivariate LOD score.
SI Methods
Activity Recording Calibrations.
At the time of initial purchase of each Actiwatch, and after each battery replacement, we performed two independent procedures, as described below, to calibrate each device and to minimize the effect of interdevice variability on our results.
Rocker test calibrations.
Configuration of devices included setting epoch length at 1 min, setting the log mode to “Activity,” and enabling collection of red, green, blue, and photopic light levels. We strapped the Actiwatches to a laboratory rocker (ArmaLab Rock 100) in a consistent conformation (on the left edge of the rocker platform 5.5 inches from the rear edge of the platform), set the rocker to 60 rpm, and let it run for at least 7 min. We recorded both the start and end times of data collection. At the end of the collection period, we downloaded data and examined activity measures for each minute, excluding the first and last minutes of the collection period. The range of activity considered acceptable was 270–350 cpm, with consistent (identical) measurement of activity units for at least three 1-min periods during the 5 min of data examined. After recording this consistent number, we repeated the test at least two more times. If three tests resulted in the same number of activity units, the Actiwatch met criteria for rocker test calibration; watches that did not meet these criteria were returned to the manufacturer for servicing.
Wrist test calibrations.
Volunteers wore two Actiwatches simultaneously for ∼24 h. To ensure consistent time settings, we configured the two Actiwatches on the same computer, with the same settings as in the rocker tests. The volunteers wore both devices on the nondominant arm (usually left). The device closest to the wrist (distal) was worn face down (watch face on the inner surface of the wrist), and the other device (proximal) was worn face up, as when using a normal watch. Actiwatches were worn continuously both overnight and part of the next day. We instructed the volunteers to press the marker button on each device when they lay down to sleep and when they woke up in the morning. After 24 h, project staff checked the display times on the two devices for consistency and downloaded and compared the data from the two devices. Devices were considered acceptably calibrated when the number of minutes of immobility recorded by the two devices was equivalent or showed <10 min of difference and the difference in minutes of sleep recorded by the two devices was in the 15–25-min range.
Inclusion and Exclusion Criteria for Activity Monitoring and Individual Recordings.
We excluded pedigree members from the actigraphy studies based on several variables that we judged would interfere with the interpretation of the data. These exclusion criteria included the following: (i) Individual was not in a euthymic state (Hamilton Depression Rating Scale score >14 or Young Mania Rating Scale score >14) at the time scheduled for recording, and recording could not be rescheduled; (ii) individual had known irregular activity patterns—for example, due to shift or night work schedules based on self-report in daily sleep logs or on a questionnaire collecting background information relevant to the data collection period (e.g., “Will you work this week?”; “Which of the following schedules best describes your work schedule for the coming week?”); and (iii) individuals were subject to other factors that could interfere with interpretation of data, including medical illness (e.g., poorly controlled epilepsy or multiple sclerosis), pain due to recent surgery, or caring for a sick child, also based on self-reported responses to the background questionnaire (“Did anything happen in the past two weeks that was outside of your normal routine?”).
We discarded recordings where a low battery led to minimum activity levels never falling below 40 activity units and/or constant high light level readings over a 24-h period, where Actiwatch readings (“off-wrist” flags) indicated a device was removed or too loosely placed on the wrist for more than 6 h over the total collection period or where participants left the watch in place for fewer than 6 d of recording with less than 45 min of total off-wrist time during any one day.
Delineation of the Rest Period.
We first delineated the rest period for each 24 h (the interval from the time an individual gets into bed until he/she gets out of bed), combining actigraphy data with information from sleep logs, when available, using an algorithm written in R (36). The rest period for a given day was defined as the first moment (after 6:00 PM) when the participant lay down in bed attempting to sleep. An activity threshold (median of overall activity, ≤not exceeding 50 counts per epoch) identified consistent epochs of low activity. The algorithm identified the epoch that, according to the rest onset indicated in the sleep log, was preceded by consistent activity and followed by ≥at least 15 epochs of activity below the threshold. Similarly, the definition of rest offset, according to the time indicated in the sleep log, was the epoch that is followed by consistent activity and preceded by ≥at least 15 min of activity below the threshold.
Description of Phenotypes and Phenotypic Domains.
Rest period phenotypes.
The majority of actigraphy phenotypes describe, for each day of monitoring, characteristics relative to the rest period (Fig. S5): the sleep period (the time, within the rest period, from when an individual starts to sleep until he/she awakens) and the awake period (the time between rest offset in the morning, and rest onset in the evening). Within the overall sleep and awake periods, sleep and awake bouts are shorter periods scored as asleep or awake. For each day of recording, we summarize sleep and awake bouts by their number and length.
Fig. S5.
Example of phenotypes extracted during rest, sleep, and activity. Dark columns are activity epochs; yellow curve represents light counts. Red represents epochs scored as awake. Blue triangles indicate the epoch when the marker button was pressed by the subject to indicate bed time (rest onset and offset). Green bars represent the time that the subject indicated in the sleep log for rest onset or offset. Rest onset and offset (light blue bars), sleep onset and offset (blue bars), and midsleep time (dashed blue bars) were computed by a script in R, as described in Methods. Sleep onset latency is the time between rest onset and sleep onset. Sleep inertia is the time between sleep offset and rest offset. Examples of measurements of phase are the time of rest and sleep onset and offset and midsleep. Examples of measurement of entrainment, or fragmentation, are the sleep and awake bouts.
Activity fragmentation or consolidation phenotypes.
Eight standard measures relating to the fragmentation or consolidation of activity derive from analysis of the curve of average daily activity data. Measures reflecting maximal and minimal periods of activity include the 10-h period of maximal activity (M10), the 5-h period of minimal activity (L5), the onset of each of these periods, amplitude (estimated by M10 – L5), and relative amplitude ([M10 – L5]/[M10 + L5]). Interdaily stability, the degree of variability of activity day-by-day, ranges between 0 (activity does not display a circadian rhythm) and 1 (a perfectly stable rhythm). We estimate IS as the ratio of the average squared difference between hourly means (over all days of measurement) and the overall activity mean to the average squared difference between each hour’s activity and the overall activity mean. Interdaily variability quantifies the fragmentation of circadian rhythm, ranging between 0 and 2; higher values indicate more consolidated daytime activity and nighttime sleep. We estimate IV as the ratio of the mean squared difference in activity between successive hours of measurement and the variance of activity (13, 17, 41, 42). To analyze circadian characteristics of the rest–activity cycles, we performed additional analysis, as previously described (43).
Overall activity level phenotypes.
Ten phenotypes, indexing overall activity levels across the circadian cycle, were derived from the raw mean and median of activity over 24 h, and mean and SD of activity in four 6-h time windows, beginning at midnight.
Curve fitting phenotypes.
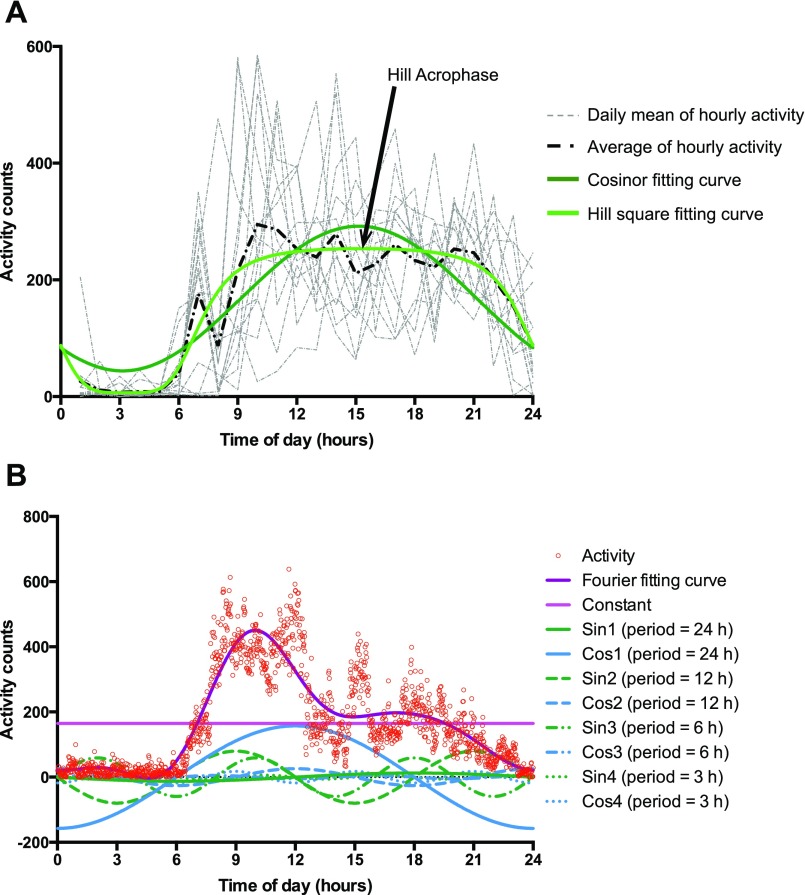
We fit the curve of the average of activity over the 24-h cycle to a cosine function (44) and a sigmoidally transformed cosine function (Hill transformation) that may more accurately represent human activity (the Hill transformation) (14) (Fig. S6A); the Cosinor equation can be represented as α + γ*sin(wt) + β*cos(wt), where t = time, w = 2*π/T, T = period of the zeitgeber (24 h). Estimates of α (Alpha), γ (Gamma), and β(Beta) for each individual were used as endophenotypes. The Hill Transformation can be represented as MIN+B*((1 + cos((t – φ)w))G)/((mG) + (1 + cos((t – φ)w))G), where φ = acrophase, t = time, w = 2*π/T, and T = period of the zeitgeber (24 h). Estimates of Min, B, G, φ, and m for each individual were used as endophenotypes. We obtained an additional 12 phenotypes by fitting a curve to the activity data with a nine-parameter Fourier transformation, which describes a cyclic signal as a combination of sines and cosines and which provides information complementary to that obtained from the other curve-fitting approaches (15) (Fig. S6B). To perform the Fourier fitting curve analysis, we created a time series of activity, averaging the n-th activity minutes over all of the complete days. The time series were fitted, using the R package “Actigraphy” (15) to the smooth Fourier expansion of nine basic functions for the following equation: B1 + B2*sin(wt) + B3*cos(wt) + B4*sin(2wt) + B5*cos(2wt) + B6*sin(3wt) + B7*cos(3wt) + B8*sin(4wt) + B9*cos(4wt). Estimates of the regression coefficients B1–B9 for each person were used as endophenotypes. The parameters B1–B9 associated with the Fourier transformation represent the contribution of activity at different periods to the daily activity curve, ranging from a period of once a day to a period of six times a day.
Fig. S6.
Activity fitting curves. (A) Cosinor and Hill transformation fitting curve. The gray curves represent the average of the hourly activity in different days of recording. The black dashed curve is the mean of the hourly average curves. Dark green solid curve represents the cosinor fitting curve on the mean of the hourly average of activity, whereas the light green solid curve is the square transformation of the cosine curve Hill transformation. The estimate of Hill Acrophase is indicated by the arrow. (B) Fourier fitting curve. Circles are the average activity of the n-th minute. Dark purple curve is the Fourier fitting curve on the activity. The curve is given by the sum of nine functions: constant (light purple), four sine curves (1–4, in green, with period of 24 h, 12 h, 6 h, and 3 h respectively), and four cosine curves (1–4, in blue, with period of 24 h, 12 h, 6 h, and 3 h, respectively).
In all three of these approaches for fitting curves to the average of daily activity, we assessed phenotypes from (i) the parameters related to the terms of the fitted curves; (ii) the values of maximum, minimum, and average of the fitted curve; (iii) amplitude of the curve; and (iv) as a measure of phase, the time of the peak of the fitted curve. Additionally, for the Hill transformation, we derived an additional phenotype from the area under the curve, which is another measure of overall activity.
Covariates.
We evaluated the effect of several variables hypothesized to influence activity levels; those variables with significant effects on the phenotypes would be included as covariates in subsequent analyses. We initially considered six possible covariates: age; gender, type of employment, household (i.e., effect of living in the same house), country (CR compared with CO), and Actiwatch device. We identified no significant phenotypic associations for either type of employment or household and therefore did not include them as covariates in the analyses reported here. In these initial analyses, we observed that participants from CO displayed higher median levels of activity than participants from CR; as no devices were used in both countries, we decided that including “device” as a covariate could obscure true differences in activity between the countries. The covariates included in the analyses described below included age (associated with 41 phenotypes), gender (associated with 20 phenotypes), and country (associated with 10 phenotypes).
Genome-Wide Genotyping and Quantitative Trait Linkage Analysis.
Genotyping.
Genotyping of 856 individuals used the Illumina Omni 2.5 chip and was done in three batches. A subset of samples was repeated in each batch to enable concordance checks between batches. Eighteen individuals were excluded from final analysis because of sample mixup/contamination (12 individuals) or because they were married-in individuals whose children were not recruited into the study (six individuals). Among the 838 subjects who passed QC and were used in analysis, genotyping completeness was good, averaging 99.78%. Genotyping completeness was <95% for only one individual; as this person carried a BP-I diagnosis and was missing only 5.7% of genotypes, we retained his data in the study.
A total of 2,026,257 SNPs were polymorphic and passed all QC procedures; 1,054,197 of these SNPs had a minor allele frequency (MAF) > 10%. SNPs were excluded in the QC process due to discordance among the three batches in replicated individuals (8,280 SNPs), missing >5% of data (97,158 SNPs), gross violation of Hardy–Weinberg Equilibrium (79 SNPs), and presence of >4 errors in Mendelian inheritance among fully typed trios (2,976 samples). After excluding markers with >4 errors in Mendelian inheritance, the Mendel error rate among fully typed trios was 0.01%, and all further sporadic errors were set to missing in the entire trio. For linkage mapping, we used 99,446 SNPs with an MAF > 0.35 that had been LD-pruned to have an r2 < 0.5. All allele frequency calculations, calculations of Hardy–Weinberg Equilibrium, and estimates of LD were performed using only unrelated (founder) individuals. Further pedigree-wide Mendel checks were performed on the set of 99,446 SNPs, 0.7% of markers had one or more errors in Mendelian inheritance in these additional checks, and all data for the marker in the family that generated the error were set to missing.
Multipoint linkage analysis.
Whole genome multipoint linkage analysis was implemented in the software SOLAR, which uses a variance component approach to partition the genetic covariance between relatives for each trait into locus-specific heritability (h2q) and residual genetic heritability (h2r). The software package Loki (39, 40), which implements Markov Chain Monte Carlo, was used to estimate the MIBD allele-sharing among family members from genotype data, using a ∼30K (29,454) subset of the 99,446 SNP set, that had been further LD pruned. Linkage analysis was performed at 1-cM intervals using the likelihood ratio statistic.
Discussion
We report here, to our knowledge, the first large-scale delineation of sleep and activity phenotypes in BP-affected individuals and their relatives. More generally, it is the first genetic investigation of such a comprehensive set of sleep and circadian measures in any human study.
Phenotypes significantly associated to BP-I paint a consistent picture; activity is lower in euthymic BP-I individuals than in their non–BP-I relatives, reflecting a longer sleep duration, with a later time of sleep offset and rest offset, resulting in a shorter duration of the active phase. Furthermore, during the active phase, BP-I individuals have fewer total minutes scored as awake and more variability in the total minutes scored as asleep. Such individuals also display lower amplitude, mainly due to their lower activity level during the least active hours of the day.
As the BP-I individuals who participated in the study were all euthymic at the time of recording, the phenotypes that we observed could be considered representative of the remission phase of the disorder. Previous studies have suggested that disturbances in sleep and circadian activity are early signs of manic episodes, particularly in individuals affected with rapid-cycling forms of BP (1, 2). We did not observe a high rate of subsequent mania among those BP-I individuals in whom we detected the most extreme sleep and activity phenotypes; however, it is possible that we may have missed such a relationship given that we did not systematically monitor clinical state after the 2-wk recording period.
Our observation of longer sleep duration in BP-I individuals accords with the results of a meta-analysis conducted in euthymic BP cases compared with controls (6). However, although the meta-analysis observed a difference between cases and controls in sleep onset latency, we did not observe such a difference between BP-I individuals and their non–BP-I relatives. Our study differs from the previous investigation in two ways: First, whereas it compared BP cases (defined broadly) to normal controls, we compared BP cases (defined narrowly) with participants defined only by the absence of BP-I. Second, our comparisons involved close relatives rather than independent participants.
We evaluated the possible effects of medication use on the association of activity phenotypes to BP-I. Twelve variables, including several measuring mean activity levels, were lower for BP-I subjects on neuroleptics than for those not prescribed these agents. When subjects receiving neuroleptics were removed from the analysis, however, activity levels remained significantly lower in BP-I subjects for the majority of variables (Table S1). Antidepressant medication and lithium treatment appeared to have no significant effect on the group differences except for one variable, mean activity from midnight to 6:00 AM, which was lower in subjects taking lithium. The finding of decreased activity in BP-I subjects also remained significant, when lithium-treated patients were removed from the analysis.
The size and composition of the pedigree set enabled us to identify significant heritability for most measures that we evaluated. This finding encouraged us to conduct, to our knowledge, the first genome-wide mapping study of quantitative traits representing the most important features of human circadian behavior: phase, amplitude, and rhythm coherence or robustness.
Previous studies of rare, autosomal dominant circadian rhythm disorders have implicated genes [including PER2 (PERIOD2), CK1δ (Casein kinase 1 delta), and DEC2 (BHLHE41, basic helix-loop-helix family member e41)] already known to function in the regulation of the circadian clock (21–23). In contrast, the linkage regions identified here for quantitative activity traits do not include any known clock genes. Similarly, prior work has found little evidence that such genes play a role in quantitative circadian activity phenotypes in mice (24).
Our most striking genetic finding was the genome-wide significant linkage for interdaily stability, a measure of day-to-day variability of the waveform of activity, near chromosome 12pter. The suggestive linkage peaks in this region for two additional phenotypes, amplitude and mean number of sleep bouts, which show pleiotropy with interdaily stability in bivariate linkage analysis, underline its importance for the regulation of circadian activity. Several genes in this region could plausibly influence activity-related behaviors, including the histone lysine demethylase JARID1a (KMD5A) and calcium channel subunit 1C (CACNA1C). JARID1a forms a complex with the core clock proteins CLOCK and BMAL1, thereby recruiting them to the Per2 promoter. On this promoter, JARID1a enhances Per2 transcription through a demethylase-independent mechanism. Depletion of JARID1a, in mammalian cells in culture, shortens the circadian period (25).
CACNA1C demonstrates a circadian expression pattern. Its loss affects the ability to phase advance wheel running behavior in mouse after a light pulse and impairs the induction of Per2 and Per1 expression (26). In multiple GWASs (genome-wide association studies), CACNA1C has shown genome-wide significant associations to BP (27–29) as well as to other psychiatric disorders (27). Studies in two cohorts have suggested a role of variants in CACNA1C in sleep habits and insomnia (30, 31), and CACNA1C knockout mice display lower EEG spectral power and impaired REM sleep recovery (32). These diverse GWAS findings, together with the pleiotropic effects that we observed, and the moderate association in our dataset between interdaily stability and BP-I, suggest that variants in this region could have a complex phenotypic impact beyond BP; however, SNPs in CACNA1C known to be associated to BP (30, 31) are not associated to interdaily stability and when included as covariates in our linkage analysis do not decrease our evidence for linkage to chromosome 12. Whole-genome sequencing underway in these pedigrees will enable us to evaluate, in relation to 12pter-linked sleep and activity phenotypes, variants in the genes noted above as well as other genes in this region.
Although the study demonstrates the feasibility of large-scale genetic investigation of human circadian activity, its limitations reflect the imprecision of actigraphy as a representation of circadian rhythm. By conducting the recordings while individuals were carrying out their usual activities, we were unable to exclude the possibility that various masking and confounding factors, such as social and natural/artificial light entrainment, could have influenced our results. An alternative study design might have been to analyze sleep and circadian parameters in traditional laboratory conditions, such as constant routine or forced desynchrony (33). We did not, however, consider such a design feasible. Not only were we concerned that exposure of BP-I–affected individuals to such conditions might trigger an acute episode, but such laboratory studies are extremely expensive and labor-intensive, making the recording and analysis of circadian activity and sleep patterns in such a big cohort virtually impossible.
To counter the limitations noted above, future investigations of circadian rhythms in these pedigrees will use molecular assays that record circadian rhythms in skin fibroblasts, from affected and unaffected individuals, in real time for several days. From this analysis, we will obtain information on the period length of the cells as well as parameters such as amplitude, phase, and entrainment. Compared with behavioral phenotypes, circadian phenotypes resulting from this analysis will more directly reflect the underlying genetic properties of the clock (34, 35).
In summary, this is the first large-scale analysis of activity phenotypes in pedigrees ascertained for BP. We demonstrate lower activity in euthymic BP-I individuals compared with their non–BP-I relatives and heritability for phenotypes assaying multiple facets of sleep and activity. The genome-wide significant linkage to interdaily stability, a phenotype associated with BP in case-control studies (17), provides an opportunity to identify sequence variants contributing to the biological underpinnings of this disorder.
Methods
Activity Recording Procedures.
We used the Actiwatch Spectrum (Philips Respironics) to record activity count (in 1-min epochs) and ambient light level. At the time of purchase and after annual servicing and battery replacement, we performed two independent procedures, to calibrate devices and minimize interdevice variability (SI Methods). Project staff in CR and CO provided calibrated Actiwatches to all participants, whom we ascertained as reported previously (10) and who provided informed consent, as approved by US and local Institutional Review Boards (University of California–Los Angeles Medical Institutional Review Board, the Ethics Committees of the University of Costa Rica, the Ethics Committees of the University of Antioquia, and University of Texas Southwestern Medical Center Institutional Review Board). As close as possible to the time of other phenotypic assessments, we placed the Actiwatch on the nondominant wrist and instructed participants to not remove it for 14 d, press the marker button when they lay down to sleep and when they got out of bed, and keep a sleep log, registering bed times and nap times.
Activity Data Analysis.
Two research assistants visually inspected activity recordings (Fig. S1) for gross abnormalities or deficiencies in data collection that would exclude them from analyses (SI Methods). For acceptable recordings, we first delineated the rest period for each 24 h (the interval from the time an individual gets into bed until he/she gets out of bed), combining actigraphy data with information from sleep logs using an algorithm written in R (36) (SI Methods and Figs. S5 and S6). We analyzed sleep parameters using a script written in R, based on published Respironics definitions and algorithms (14–17). R scripts are available upon request.
Analysis of Heritability and Association to BP-I.
As in the previous endophenotyope analyses of these pedigrees (10), we analyzed heritability and association to BP-I in SOLAR (18), which implements a variance component method to estimate the proportion of phenotypic variance due to additive genetic factors (narrow sense heritability). Under the null hypothesis that the value of the additive genetic variance is zero, testing significance of the estimate of genetic variance compared with the null value is a one-sided test.
Variance components analysis is sensitive to outliers and nonnormal trait distributions. To guard against statistical artifacts induced by skewed distributions, before analyses we used, in SOLAR, a standard rank-based procedure (37) to inverse-normal transform all phenotypes and thereby avoid correlations between relatives or inflated heritability estimates (38).
We regressed all phenotypes on three covariates [sex, age, and country (CR vs. CO), also used in all analyses described below]; of six potential covariates that we evaluated, only these three significantly affected phenotype values (SI Methods). We initially considered household effects as a potential source of phenotypic variation; however, we found that “household” was not a significant component of variation for any phenotype and therefore did not include it as a variable in further models. We implemented regressions in SOLAR, using pedigree structures, using residuals from these models in all further analyses. We tested for BP-I association (difference in trait means between individuals with and without this diagnosis), using SOLAR to account for dependencies among relatives in a two-sided test of the null hypothesis of no association. As the non–BP-I category includes individuals diagnosed with other psychiatric disorders, this is not a case-control comparison (and likely underestimates the degree of BP-I association of each measure). We controlled family-wise error rate at the 0.05 level, using a Bonferroni-corrected threshold for each test (heritability and BP-I association; P < 6.7 × 10−4).
Genome-Wide Genotyping and Quantitative Trait Linkage Analysis.
Genotyping of 856 individuals, performed in three batches, used the Illumina Omni 2.5 chip (for QC details, see SI Methods). We implemented genome-wide multipoint linkage analysis in SOLAR, which uses a variance component approach to partition the genetic covariance between relatives for each trait into locus-specific heritability (h2q) and residual genetic heritability (h2r). The software package Loki (39, 40), which implements Markov Chain Monte Carlo, provided estimated multipoint identical by decent (MIBD) allele-sharing among family members from genotype data, using a linkage disequilibrium (LD)-pruned subset of markers that passed QC procedures (SI Methods). We performed linkage analysis at 1-cM intervals focusing primarily on phenotypes that we considered most relevant to BP; for these phenotypes, we also performed a secondary genome-wide linkage analysis including BP status as a covariate. There were 558 individuals with genotype and phenotype data for all analyses (136 BP-I and 422 non–BP-I). Power analysis in SOLAR, using simulated data, indicated that this sample size provided >80% power to detect a LOD score of 3, provided the estimate of locus-specific heritability was ≥35%.
To evaluate the significance of the strongest linkage finding while appropriately accounting for multiple comparisons among phenotypes most relevant to BP, we used simulations. Specifically, we used gene dropping to generate genotypes consistent with the relationships between the pedigree members, independent from the recorded phenotypic values. This approach allowed us to simulate null datasets that maintain the specific dependency structure existing across these 13 phenotypes. We used gene-drop simulations to construct a null distribution of LOD scores, rather than permuting phenotypic values, because individuals in pedigrees are not exchangeable; phenotypic permutation would likely render most of our phenotypes nonheritable, therefore biasing the null distribution toward zero LOD scores. For each of 100 datasets so generated, we carried out linkage analysis for each phenotype, recording both the highest LOD score obtained and the most significant P value for the hypothesis of no linkage to any phenotype (Simes P value).
Acknowledgments
We thank the members of CR and CO families for participating in this study and John Blangero and Thomas Dyer (Texas Biomedical Research Institute and University of Texas Health Science Center) for calculating MIBDs. This research was supported by National Institute of Health Grants R01MH075007, R01MH095454, and P30NS062691 (to N.B.F.), T32MH073526 (to P.A.S.C.), K23MH074644-01 (to C.E.B.), and K08MH086786 (to S.C.F.), the Colciencias and Codi-University of Antioquia (to C.L.-J.), and the Joanne and George Miller Family Endowed Term Chair (to C.E.B.). J.S.T. is an investigator in the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 1477.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513525113/-/DCSupplemental.
References
- 1.Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. J Affect Disord. 2003;74(3):209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- 2.Leibenluft E, Albert PS, Rosenthal NE, Wehr TA. Relationship between sleep and mood in patients with rapid-cycling bipolar disorder. Psychiatry Res. 1996;63(2-3):161–168. doi: 10.1016/0165-1781(96)02854-5. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti F, Barbini B, Colombo C, Smeraldi E. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11(6):509–522. doi: 10.1016/j.smrv.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Linkowski P. EEG sleep patterns in twins. J Sleep Res. 1999;8(Suppl 1):11–13. doi: 10.1046/j.1365-2869.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 5.De Gennaro L, et al. The electroencephalographic fingerprint of sleep is genetically determined: A twin study. Ann Neurol. 2008;64(4):455–460. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- 6.Ng TH, et al. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: A systematic review and meta-analysis. Sleep Med Rev. 2014;20:46–58. doi: 10.1016/j.smrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Carvajal-Carmona LG, et al. Genetic demography of Antioquia (Colombia) and the Central Valley of Costa Rica. Hum Genet. 2003;112(5-6):534–541. doi: 10.1007/s00439-002-0899-8. [DOI] [PubMed] [Google Scholar]
- 8.Service S, et al. Results of a SNP genome screen in a large Costa Rican pedigree segregating for severe bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(4):367–373. doi: 10.1002/ajmg.b.30323. [DOI] [PubMed] [Google Scholar]
- 9.Reich D, et al. Reconstructing Native American population history. Nature. 2012;488(7411):370–374. doi: 10.1038/nature11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fears SC, et al. Multisystem component phenotypes of bipolar disorder for genetic investigations of extended pedigrees. JAMA Psychiatry. 2014;71(4):375–387. doi: 10.1001/jamapsychiatry.2013.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoll G, et al. Deletion of TOP3β, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat Neurosci. 2013;16(9):1228–1237. doi: 10.1038/nn.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12(1):23–30, viii. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27(6):563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 14.Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: A mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25(22):3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, et al. Measuring the impact of apnea and obesity on circadian activity patterns using functional linear modeling of actigraphy data. J Circadian Rhythms. 2011;9(1):11. doi: 10.1186/1740-3391-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvatore P, et al. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008;10(2):256–265. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 17.Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7(2):176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 18.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey AG, Schmidt DA, Scarnà A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162(1):50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- 20.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73(3):751–754. [Google Scholar]
- 21.Xu Y, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434(7033):640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, et al. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128(1):59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, et al. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325(5942):866–870. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimomura K, et al. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001;11(6):959–980. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- 25.DiTacchio L, et al. Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science. 2011;333(6051):1881–1885. doi: 10.1126/science.1206022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmutz I, et al. A specific role for the REV-ERBα-controlled L-Type Voltage-Gated Calcium Channel CaV1.2 in resetting the circadian clock in the late night. J Biol Rhythms. 2014;29(4):288–298. doi: 10.1177/0748730414540453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cross-Disorder Group of the Psychiatric Genomics Consortium Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira MA, et al. Wellcome Trust Case Control Consortium Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40(9):1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Psychiatric GCBDWG. Psychiatric GWAS Consortium Bipolar Disorder Working Group Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43(10):977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byrne EM, et al. A genome-wide association study of sleep habits and insomnia. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(5):439–451. doi: 10.1002/ajmg.b.32168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons MJ, et al. Replication of genome-wide association studies (GWAS) loci for sleep in the British G1219 cohort. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(5):431–438. doi: 10.1002/ajmg.b.32106. [DOI] [PubMed] [Google Scholar]
- 32.Kumar D, et al. Ca1.2 modulates electroencephalographic rhythm and rapid eye movement sleep recovery. Sleep. 2015;38(9):1371–1380. doi: 10.5665/sleep.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nováková M, Sumová A. New methods to assess circadian clocks in humans. Indian J Exp Biol. 2014;52(5):404–412. [PubMed] [Google Scholar]
- 34.Pagani L, et al. The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PLoS One. 2010;5(10):e13376. doi: 10.1371/journal.pone.0013376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S, Van Dongen HP, Wang K, Berrettini W, Bućan M. Assessment of circadian function in fibroblasts of patients with bipolar disorder. Mol Psychiatry. 2009;14(2):143–155. doi: 10.1038/mp.2008.10. [DOI] [PubMed] [Google Scholar]
- 36.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- 37.van der Waerden BL. Order tests for the two-sample problem and their power. Indag Math. 1952;14:453–458. [Google Scholar]
- 38.Pilia G, et al. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2006;2(8):e132. doi: 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heath SC. Markov chain Monte Carlo segregation and linkage analysis for oligogenic models. Am J Hum Genet. 1997;61(3):748–760. doi: 10.1086/515506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heath SC, Snow GL, Thompson EA, Tseng C, Wijsman EM. MCMC segregation and linkage analysis. Genet Epidemiol. 1997;14(6):1011–1016. doi: 10.1002/(SICI)1098-2272(1997)14:6<1011::AID-GEPI75>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 41.Jones SH, Tai S, Evershed K, Knowles R, Bentall R. Early detection of bipolar disorder: A pilot familial high-risk study of parents with bipolar disorder and their adolescent children. Bipolar Disord. 2006;8(4):362–372. doi: 10.1111/j.1399-5618.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- 42.Ankers D, Jones SH. Objective assessment of circadian activity and sleep patterns in individuals at behavioural risk of hypomania. J Clin Psychol. 2009;65(10):1071–1086. doi: 10.1002/jclp.20608. [DOI] [PubMed] [Google Scholar]
- 43.Van Someren EJ, et al. Bright light therapy: Improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505–518. doi: 10.3109/07420529908998724. [DOI] [PubMed] [Google Scholar]
- 44.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6(4):305–323. [PubMed] [Google Scholar]