Significance
To our knowledge, this article is the first report explaining how cFLIP, an inhibitor of apoptosis, regulates apoptosis in vivo. Although the antiapoptotic role of cFLIP was proposed based on in vitro studies and the early embryonic lethality of cFLIP-deficient mice, the specific role of cFLIPL (long) and cFLIPR (short) isoforms is poorly understood. In this study, we describe a previously unidentified allele of caspase 8- and FADD-like apoptosis regulator (Cflar) (encoding cFLIP) that makes mice of MSM strain resistant to Fas-mediated lethality. The mutant allele affects the ratio of cFLIPL:cFLIPR, leading to high levels of long FLIP in MSM. As a result, the abundant cFLIPL forms enzymatically active heterodimers with caspase 8 (CASP8) in MSMs, which prevents formation of proapoptotic CASP8 p10/p20 and cleaves receptor interacting protein kinase 1 (RIP1), thus setting up a higher threshold for CD95-mediated apoptosis and RIP1-mediated necroptosis.
Keywords: apoptosis, liver failure, Fas-receptor, caspase 8, cFLIP
Abstract
cFLIP, an inhibitor of apoptosis, is a crucial regulator of cellular death by apoptosis and necroptosis; its importance in development is exemplified by the embryonic lethality in cFLIP–deficient animals. A homolog of caspase 8 (CASP8), cFLIP exists in two main isoforms: cFLIPL (long) and cFLIPR (short). Although both splice variants regulate death receptor (DR)-induced apoptosis by CASP8, the specific role of each isoform is poorly understood. Here, we report a previously unidentified model of resistance to Fas receptor-mediated liver failure in the wild-derived MSM strain, compared with susceptibility in C57BL/6 (B6) mice. Linkage analysis in F2 intercross (B6 x MSM) progeny identified several MSM loci controlling resistance to Fas-mediated death, including the caspase 8- and FADD-like apoptosis regulator (Cflar) locus encoding cFLIP. Furthermore, we identified a 21-bp insertion in the 3′ UTR of the fifth exon of Cflar in MSM that influences differential splicing of cFLIP mRNA. Intriguingly, we observed that MSM liver cells predominantly express the FLIPL variant, in contrast to B6 liver cells, which have higher levels of cFLIPR. In keeping with this finding, genome-wide RNA sequencing revealed a relative abundance of FLIPL transcripts in MSM hepatocytes whereas B6 liver cells had significantly more FLIPR mRNA. Importantly, we show that, in the MSM liver, CASP8 is present exclusively as its cleaved p43 product, bound to cFLIPL. Because of partial enzymatic activity of the heterodimer, it might prevent necroptosis. On the other hand, it prevents cleavage of CASP8 to p10/20 necessary for cleavage of caspase 3 and, thus, apoptosis induction. Therefore, MSM hepatocytes are predisposed for protection from DR-mediated cell death.
The Fas receptor [also called cluster of differentiation 95 (CD95), APO-1, or TNFRSF6] is a death receptor family member constitutively expressed by most tissues, including the liver (1), where ligation of ubiquitously expressed CD95 leads to potentially lethal hepatitis and liver failure. Although CD95L (Fas ligand) is the only known physiological ligand of CD95 (2), the agonistic antibody Jo2 has been used extensively to ligate CD95 and model CD95-mediated hepatotoxicity and mortality in mice (3). In contrast to the ability of tumor necrosis factor receptor (TNFR)-mediated signaling to lead to profound inflammatory responses in addition to cell death (4), CD95 is predominantly used in apoptosis and necrosis and therefore engages a limited number of downstream components (5). Specifically, ligand binding induces CD95 oligomerization and binding of Fas-associated death domain (FADD) via its death domain (DD), which then recruits caspase 8 (CASP8) via a death effector domain (DED), forming the death-inducing signaling complex (DISK) comprising receptor interacting protein kinase 1 (RIP1), FADD, and CASP8 (6).
Interactions of caspase 8 (CASP8) with its enzymatically inactive homolog cFLIPL further complicate the regulation of CD95-mediated signaling (7): CASP8 forms partially enzymatically active heterodimers with long splice variant cFLIPL in which CASP8 is partially cleaved into its p43 form from its pro-caspase p55 form through transcleavage via other CASP8 molecules (8). These heterodimers are more stable than CASP8 homodimers, thereby preventing processing of CASP8 into the fully active p18 and p10 products that can cleave caspase 3 and inhibit apoptosis. Nevertheless, the cFLIP (an inhibitor of apoptosis)-p43CASP8 heterodimer is still able to cleave the kinase domain of full-length RIP1 (6, 9), thereby preventing necroptosis induction. Similar to cFLIPL, the short variant of cFLIP, cFLIPR, stabilizes pro-CASP8 and makes it available for execution of apoptosis (10). There are three major cFLIP isoforms in the literature: one long (cFLIPL) and two short (cFLIPR and cFLIPS). Only cFLIPL and cFLIPR have been shown to be present in mice (11) whereas all three are found in humans. Precisely how the cFLIP isoforms regulate apoptotic signaling in vivo is poorly understood in part because cFLIP deficiency is embryonically lethal (12, 13). RIP3 is able to rescue cFLIP deficiency but only in the absence of FADD (13). Furthermore, RIP3 or CASP8 deficiency is embryonically lethal, but RIP3/CASP8 double knockout mice are viable (14), demonstrating the complex interplay among the components of CD95-mediated signaling.
In terms of CD95-mediated apoptosis, cells can be broadly categorized as type I (mitochondria-independent) or type II (mitochondria-dependent) (1, 15). Type II cells, including hepatocytes, require synergistic activation of the mitochondria-dependent pathway, most likely to amplify an initially weaker death signal. Differences in oligomerization of the DISK components downstream of Jo2 vs. CD95L may determine the overall apoptotic effect (16).
Here, we report a previously unidentified model of resistance to CD95-mediated liver failure in which mice of the wild-derived MSM strain survived injection of the Jo2 agonistic antibody to CD95 (17, 18) at doses lethal to wild-type controls [C57BL6 (B6)]. This resistance was tissue-specific because MSM thymocytes were susceptible to Jo2-mediated toxicity. Furthermore, this resistance could be overcome by multimeric Fas Ligand (MegaFasL). F1 hybrids (B6 × MSM) were partially resistant to Jo2, allowing us to pursue this phenotype via classical genetic analysis using F2 intercross (B6 × MSM) progeny and identify the Casp8/caspase 8- and FADD-like apoptosis regulator (Cflar) locus as one of the loci linked to Jo2 resistance in MSM.
Further analysis revealed that CASP8 in MSM livers is predominantly present in its cleaved p43 isoform whereas cFLIP is present predominantly in its FLIPL form. In contrast, B6 mice exhibit higher levels of cFLIPR relative to the FLIPL form. Concordant with the ratio of short and long FLIPs, B6 have higher levels of p55 CASP8 in their livers compared with MSM mice.
We sequenced cFLIP cDNA from MSM and identified a 21-bp insertion in the 3′ UTR of the fifth exon of Cflar that was also conserved in other wild-derived strains, including MOLF/Ei and SPRET/Ei mice that are similarly resistant to death receptor-mediated lethality (19), as well as in Rat (Rattus norvegicus). The insertion was identical to the U2snRNP binding region (20), and this finding may provide insight into why the ratio of FLIP isoforms is skewed toward cFLIPR in MSM mice. Indeed, genome-wide RNA sequencing revealed a relative abundance of the long FLIP transcript in MSM mice and significantly more FLIPR mRNA in B6 mice, thus suggesting that splicing of FLIP in MSM/Ms is preferentially skewed toward spliced FLIPL mRNA. These data support a model according to which, in MSM mice, CASP8 is predominantly bound to FLIPL, producing an enzymatically active heterodimer that cleaves RIP1, which might prevent necroptosis, and processes CASP8 to p43, thus increasing the threshold for CD95-mediated initiation of apoptosis. We propose that the ratio of cFLIP isoforms in a given cell type may be a critical component of detecting signal strength.
Results
MSM Mice Are Resistant to Jo2-Induced Liver Injury.
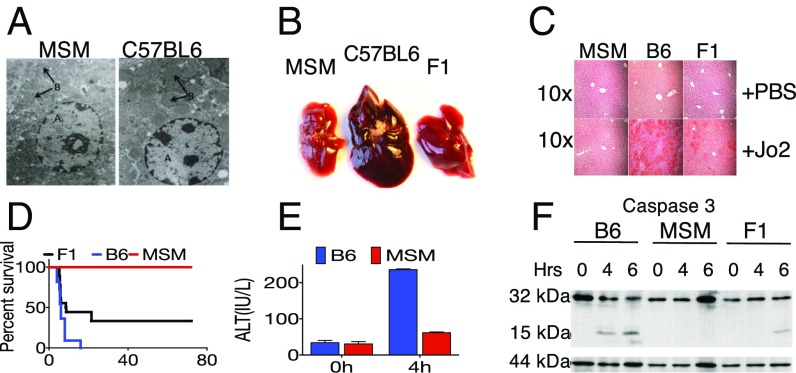
In a genetic screen for novel innate immune phenotypes, we observed mice of the wild-derived strain MSM to be extremely resistant to LPS-induced lethality, which correlated with reported resistance of other wild-derived strains to septic shock (19, 21). Because LPS-induced lethality is mediated in part through TNFα (22), we decided to investigate whether this resistance extended to other death receptor-mediated signaling. CD95 is a member of the tumor necrosis factor superfamily of receptors that has been extensively characterized for its role in apoptotic signaling (23). Thus, we decided to examine MSM responses to Jo2, an agonistic mAb that ligates CD95 and induces lethality characterized by hepatocyte death and liver hemorrhage. Compared with B6, MSM mice showed resistance to lethal i.p. doses of Jo2 (Fig. 1 A–D). Electron microscopy of the livers of B6, but not MSM, mice injected with Jo2 showed nuclear fragmentation and mitochondrial swelling (Fig. 1A), accompanied by substantial hemorrhaging (Fig. 1B), whereas MSM livers appeared grossly normal, and F1 (B6 × MSM) livers showed intermediate damage (Fig. 1B). Similarly, H&E staining of livers (Fig. 1C) after Jo2 injection showed severe tissue damage and leukocyte infiltration in B6 livers, but MSM livers appeared unaffected. Again, the livers of F1 hybrids exhibited intermediate pathology (Fig. 1C). In keeping with these pathology findings, F1 mice showed increased resistance to Jo2-induced lethality compared with B6 mice whereas MSM mice were completely resistant (Fig. 1D). High levels of ALT (a marker of liver injury) (23) in B6, but not MSM, serum indicated liver failure as the main cause of death in B6 mice. To confirm apoptosis in the livers of Jo2-injected mice, we probed liver lysates for cleaved caspase 3 (CASP3), the so-called executioner caspase of the death receptor pathway. In B6 livers, injection of Jo2 induced cleavage of CASP3, resulting in the appearance of the p17 band after 4.5 h whereas F1 livers exhibited delayed CASP3 cleavage after 6 h. No band was observed in MSM livers, suggesting that their hepatocytes are specifically resistant to Jo2-induced apoptosis.
Fig. 1.
MSM/Ms are resistant to Fas-mediated liver mortality and hepatic injury. (A) Electron micrograph of hepatocytes from MSM/Ms and B6 after injection of 10 μg Jo2 showing nuclei (site A) and mitochondria (site B). (B) Gross morphology of livers from MSM/Ms, B6, and F1 (MSM/Ms × B6) 4.5 h post-Jo2 i.p. injection. (C) H&E staining of liver sections from B. (D) Kaplan-Meier curve showing lethality data for MSM/Ms (n = 10), B6 (n = 11), and F1 (n = 18) mice after i.p. injections of 10 μg of Jo2. (E) A time course for the ALT levels in mice injected with Jo2. (F) Western blot analysis of a time course of caspase 3 activation in the livers of stimulated with Jo2 B6 (B6), MSM/Ms (MSM), or F1 (B6 × MSM) mice. GSTP (glutathione S-transferase P, 44 kDa) is shown as a loading control.
The CD95 Signaling Pathway Is Functional in MSM Mice.
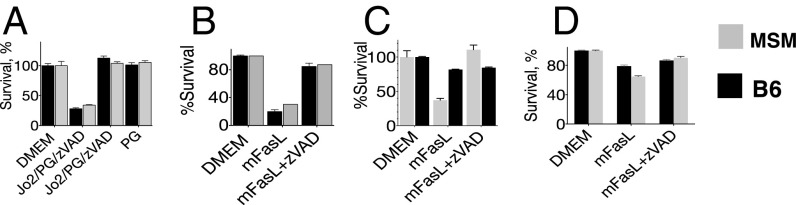
Genome-wide gene expression analysis by means of RNA and exome sequencing revealed multiple polymorphisms in MSM genes encoding components of the CD95 signaling pathway, including CASP8, FADD, RIP1, and CD95 itself. To dismiss the possibility of defective signaling due to one of these polymorphisms, we compared in vivo responses to Jo2 in B6 and MSM in livers (predominantly made up of hepatocytes, which are type II cells) with Jo2 responses in thymocytes in vitro (24), which are type I cells and thus have less stringent requirements for execution of CD95-mediated apoptosis. To mimic the natural cross-linking that is observed in in vivo injections of antibodies, we added protein G to facilitate cross-linking of the monoclonal Jo2 antibodies (18). In contrast to the in vivo liver data, there was a substantially cytotoxic effect of Jo2 on freshly isolated MSM thymocytes (Fig. 2A), comparable with the effect of Jo2 on B6 thymocytes. Addition of the pan-caspase inhibitor zVAD completely reversed this cytotoxicity, confirming apoptosis as the underlying route of thymocyte death. To address the possibility of protein G possibly confounding our results, we also tested thymocytes with mFasL (25) and observed similar responses in thymocytes to mFasL as were seen in response to Jo2 (Fig. 2B). To further confirm the functionality of CD95-mediated signaling in cells other than thymocytes, we examined the responses of MSM and BL/6 mouse embryonic fibroblasts (MEFs) (Fig. 2D) and peritoneal macrophages (Fig. 2C) to mFasL and found similar susceptibility to CD95-mediated death.
Fig. 2.
The resistance to Jo2-induced apoptosis is tissue-specific phenotype in MSM. (A) Thymocytes from both B6 and MSM/Ms are susceptible to Jo2-mediated lethality. Thymocytes were cultured in triplicate plus Jo2 plus protein G (PG) plus zVAD for 8 h, and an ATP assay for viability was carried out for the various stimulations at the end of the assay. (B) Susceptibility of C57BL6 or MSM/Ms thymocytes to membrane-bound FasL (mFasL). (C) Susceptibility of peritoneal macrophages to Jo2. (D) MEFs from MSM/Ms and B6 are susceptible to Jo2. For A–D, the viability was measured using the ATP assay (CelltiterGlo), and survival was made relative to values from the untreated group (media only). All data are representative of three independent experiments.
MSM Mice Are Susceptible to MegaFasL-Induced Liver Injury.
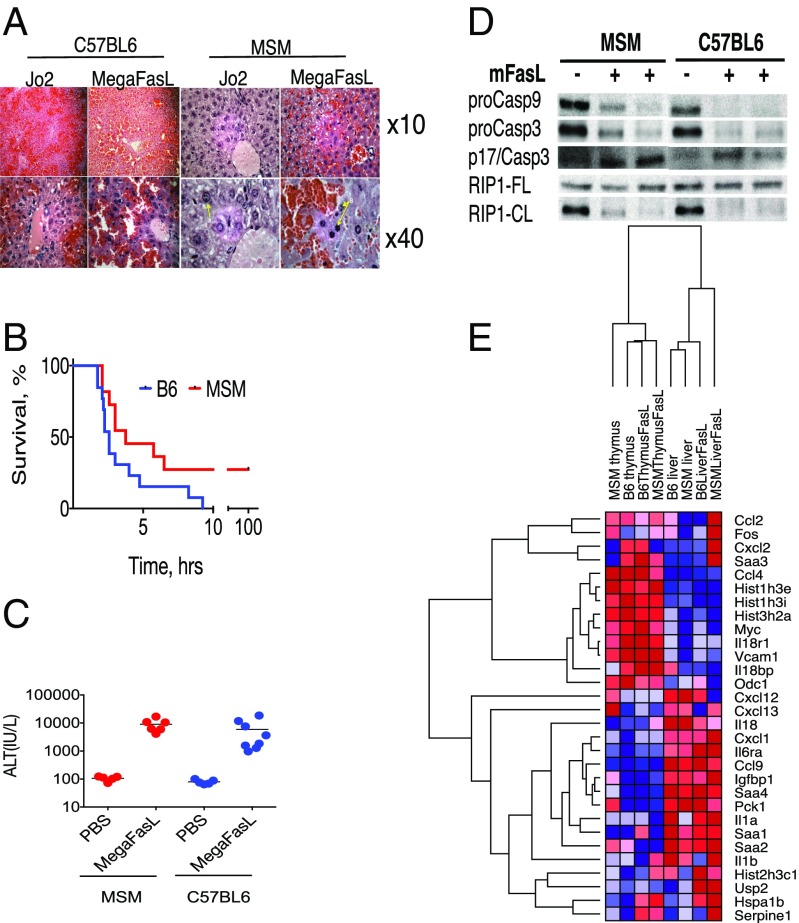
Hepatocytes are type II cells (1) that require activation of the mitochondrial loop to synergize with the CASP8 pathway to induce apoptosis whereas thymocytes are type I cells in which CD95 ligation is more efficient and is sufficient for apoptotic signaling. Because MSM hepatocytes, but not thymocytes, were resistant to Jo2, we hypothesized that Jo2 may not provide strong enough ligation of CD95 in MSM livers, rendering them resistant to death. Accordingly, we next examined the effect of multimeric FasL (MegaFasL), which stimulates more efficient CD95 ligation, on MSM mice. We observed that MSM mice were, similarly to B6 mice, sensitive to MegaFasL-induced lethality (Fig. 3B) whereas histological analysis (Fig. 3A) and elevated ALT levels (Fig. 3C) after injection indicated severe liver injury. Thus, more efficient ligation of CD95 in MSM livers seems capable of overcoming their resistance to apoptosis, resulting in liver failure and death. These data confirmed that MSM mice have a higher threshold for CD95 ligation and activation than B6 mice, which is overcome by the more potent CD95 agonist, MegaFasL. This finding is in agreement with the reported ability of MegaFasL to overcome resistance from exogenously expressed cFLIP in a dose-dependent manner (10, 26).
Fig. 3.
MSM/Ms are susceptible to MegaFasL-induced liver damage. (A) H&E of liver sections from MSM/Ms injected with either 10 μg of Jo2 (Left) or 2.4 μg of MegaFasL (Right). (B) Kaplan–Meier curve showing mouse survival after an i.p. injection of 1.5 μg of MegaFasL. (C) ALT levels in mice 2 h postinjection with either PBS or MegaFasL (1.2 μg per mouse). (D) Western blot analysis from liver lysates looking at activation of apoptotic proteins caspase-9, caspase-3, and RIP1 from mice either uninjected (−) or injected (+) with MegaFasL 2 h postinjection. (E) Cladogram showing how gene profiles between livers and thymi change after MegaFasL injection in MSM and B6 mice; alignment of RNA-sequencing data was done using GenePattern software.
To further characterize the apoptotic cascade in the livers of B6 and MSM mice injected with MegaFasL, we looked at the proteolytic cleavage of caspases 9 and 3 (Fig. 3D). Specifically, we observed a decrease in pro-caspase9 and pro-caspase3, as well as a modest increase in the active subunit of caspase3 (p17) upon injection with MegaFasL. Interestingly, we also observed a decrease in the levels of cleaved RIP1 after MegaFasL injection—suggesting a breakdown in the regulation of necroptosis via caspases after MegaFasL injection.
Genome-Wide Analysis Suggests That Resistance to Apoptosis Preexists in MSM.
To further investigate the resistance phenotype in MSM at the molecular level, we performed genome-wide analysis of mRNA levels in the liver and thymus of MSM and B6 mice in response to MegaFasL. First, we compared expression levels of typical genetic markers of liver injury (27), such as the Saa-group of genes, histones, and some cytokines (Fig. 3E). Based on the heat map and relation between the samples in the cladogram in Fig. 3E, the samples divided into two groups: one associated with the liver-specific expression and the other associated with the thymus. The levels of mRNAs for these groups were not dramatically different within the liver or thymus but were strikingly different between the tissues. Furthermore, CD95 ligation did not significantly affect the expression of these genes: If they were highly expressed in resting liver cells, expression remained high in MegaFasL-treated hepatocytes (such as the top half of the compared genes, Fig. 3E). Nevertheless, based on this gene pattern, MSM thymus and MegaFasL-activated MSM liver were positioned at the most distal branches of the cladogram, suggesting unique differences for MSM in their genetic makeup. Genome-wide comparison of all genes in unstimulated and stimulated tissues confirmed that MSM thymus, in its gene-expression pattern, is significantly different from other tissues (Fig. 3E). Based on these data, we hypothesized that resistance to apoptosis in MSMs is not necessarily conferred by response to CD95 ligation, but, rather, conditions for such resistance preexist in MSM before activation of the Fas pathway.
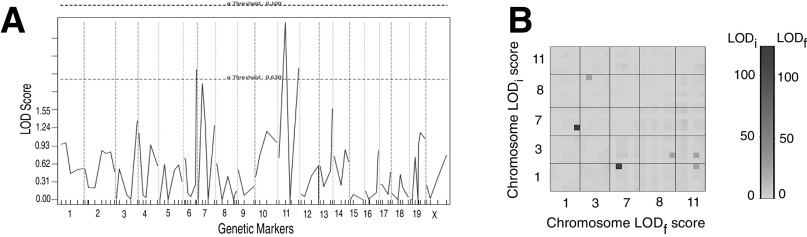
Linkage and Sequencing Analysis Identifies Cflar as One of the Loci Conferring the Trait.
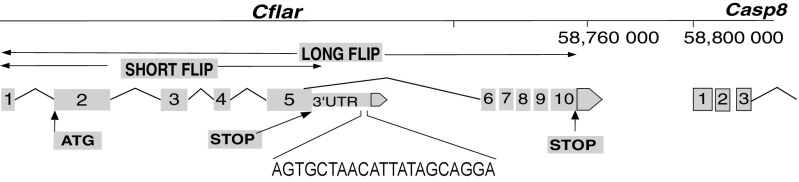
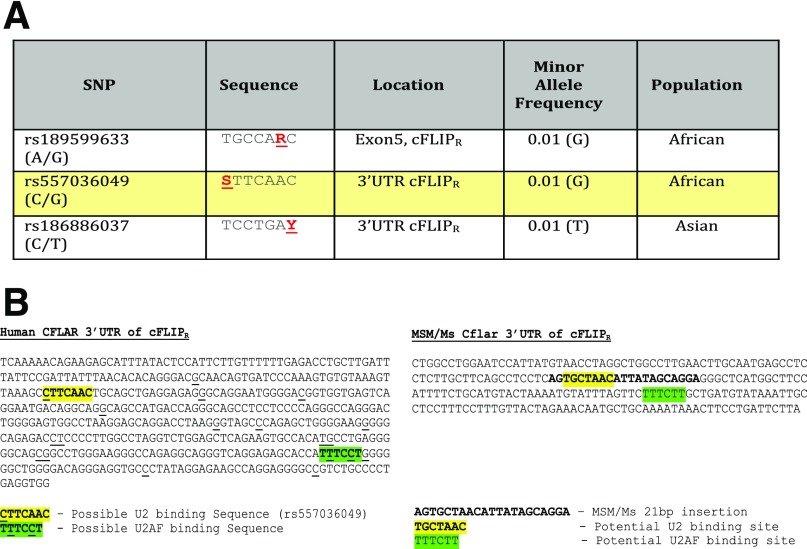
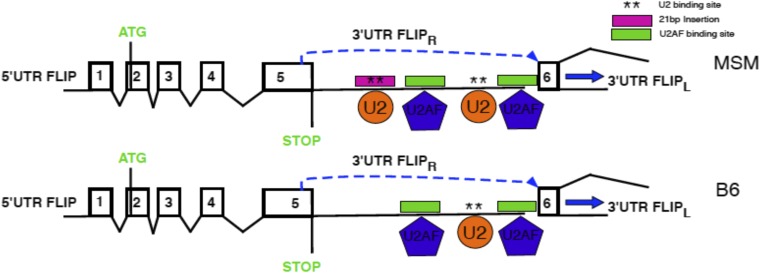
As was shown earlier (Fig. 1 B–D), the resistance of MSM to Jo2-induced lethality was transmitted at the F1 hybrid level and therefore constituted a genetic trait that could be pursued by classical genetic analysis. Accordingly, we produced second-generation intercross F2 mice and injected them with a dose of Jo2, which is lethal for B6 mice, and monitored the survival. Next, using linkage analysis QTL software (28), we looked for association between the survival and the genomic loci in the F2 panel of mice. We identified several loci that may contribute to resistance to Jo2, in an F2 panel using QTL analyses (Fig. S1). A 2D analysis, which tries to identify whether particular genotypes on different chromosomes are coinherited, identified loci on chromosomes (Chr) 1 and 7 as being particularly interesting (Fig. S1). The genomic area on Chr1 was proximal to both the Casp8 and cFlip genes, which we then sequenced in B6 and MSM. Although there were no SNPs in MSM Casp8, we identified a 21-bp insertion in the region following the fifth exon of MSM cFLIP involved in alternative splicing of short and long isoforms of cFLIP mRNA (Fig. 4). This region is the 3′ UTR for cFLIPR and the intronic region for cFLIPL. This insertion is not present in B6 mice but is conserved in other wild-derived strains such as MOLF/Ei (Mus musculus molossinus), PWK/Ei (Mus musculus musculus), and SPRETUS/Ei (Mus spretus). Neither of the classical inbred strains from Mus musculus domesticus or the CAST/Ei strain (Mus musculus castaneus) preserved the insertion, suggesting that the polymorphism arose during subspeciation of mouse strains. Furthermore, this insertion is also conserved in R. norvegicus (rat). A striking feature about this insertion is that it contains a putative binding site for the U2 snRNP. In MSM/Ms mice, there is also the presence of a U2AF putative binding site in close proximity to the introduced U2 binding site (Fig. S2)—suggesting that, compared with B6, MSM/Ms may recruit the spliceosome complex more efficiently in this region, either leading to more efficient splicing of the intronic region between exons 5 and 6—producing more cFLIPL transcript—or creating an exon-skipping event of Exon 6 (20) leading to an ineffective transcript, or possibly making it physically difficult for the transcription of cFLIPR, either way decreasing the total amount of cFLIPR transcript that is produced.
Fig. S1.
Linkage analysis identifies Cflar as one of the gene candidates that confer resistance of MSM to Fas-mediated liver failure. (A) QTL-based linkage analysis identifies genomic intervals linked with the trait in an F2 panel plus Jo2. The height of the peak correlates with the extent of linkage. (B) A two- dimensional QTL analysis of the panel from A reveals strong associations between chromosomes 7 and 11 with loci on chromosome 1.
Fig. 4.
Linkage analysis identifies Cflar as one of the gene candidates that confer resistance of MSM to Fas-mediated liver failure. A genetic map of the Cflar locus is shown with a 21-bp insertion in the 3′ UTR of the short variant of cFLIP in MSM/Ms.
Fig. S2.
SNPs identified from the 1000 Genomes Project that may alter putative U2 snRNP binding regions near the splice site that distinguishes cFLIPL from cFLIPR in humans. (A) Table showing data mined from the 1000 Genomes database from exon 5 and the 3′ UTR following exon 5 from the CFLAR gene. The sequence surrounding the SNP is provided where R = A/G, S = C/G, and Y = C/T. The rs557036049 SNP is highlighted in yellow because the underrepresented allele (G) is not preferred by the U2 snRNP and thus may decrease the binding efficacy of this protein, which is crucial for initiating splicing—leading to the production of cFLIPL. We postulate that fewer splicing events may result in greater levels of cFLIPR transcript per protein. (B) Human sequence data from the Ensembl genome browser or sequencing data from MSM mouse genomic DNA showing a portion of the 3′ UTR of exon 5. The highlighted yellow sequence identifies a possible U2 biding site, and the highlighted green sequence identifies a possible U2AF binding sequence proximal to the U2 binding site. In humans, there are polymorphisms in both putative U2 and U2AF sites that may regulate binding of these proteins (and possibly also of the spliceosome). MSM mice introduce a possible U2 binding sequence proximal to a putative U2AF binding site. This insertion is absent in B6 mice. We postulate that this insertion may allow for the recruitment of the spliceosome complex to either enhance splicing or impede the transcription of cFLIPR, either way leading to decreased production of cFLIPR and relatively elevated expression of cFLIPL.
Although the 21-bp insertion is not conserved in humans, human data from the 1000 Genomes Project showed that there are SNPs within the 3′ UTR of the terminal exon for cFLIRR that may alter the binding sequence for U2 as well as for U2AF (Fig. S2). Interestingly, these sequences appear in close proximity to one another—suggesting a possible recruitment locus for the spliceosome–leading to splice modification, depending on the SNPs that are present. SNPs that abrogate the putative binding of U2 or U2AF are present in the minority of the population, suggesting that U2 and U2AF binding may be important in this region (Fig. S3). Intronic sequence polymorphisms have been shown to play a role in human diseases, such as familial dysautonomia (29). In this way, the insertion in MSM mice can serve as a tool for investigating the regulation of alternative splicing of cFLIP.
Fig. S3.
Preliminary model illustrating the effect of the 21-bp insertion in the 3′ UTR of exon 5 of Cflar. This insertion is absent in B6 mice. We postulate that this insertion may allow for the recruitment of the spliceosome complex to either enhance splicing or impede the transcription of cFLIPR, either way leading to decreased production of cFLIPR and relatively elevated expression of cFLIPL.
The 21-bp Insert in Cflar Causes Preferential Splicing to Long cFLIP in MSM Livers.
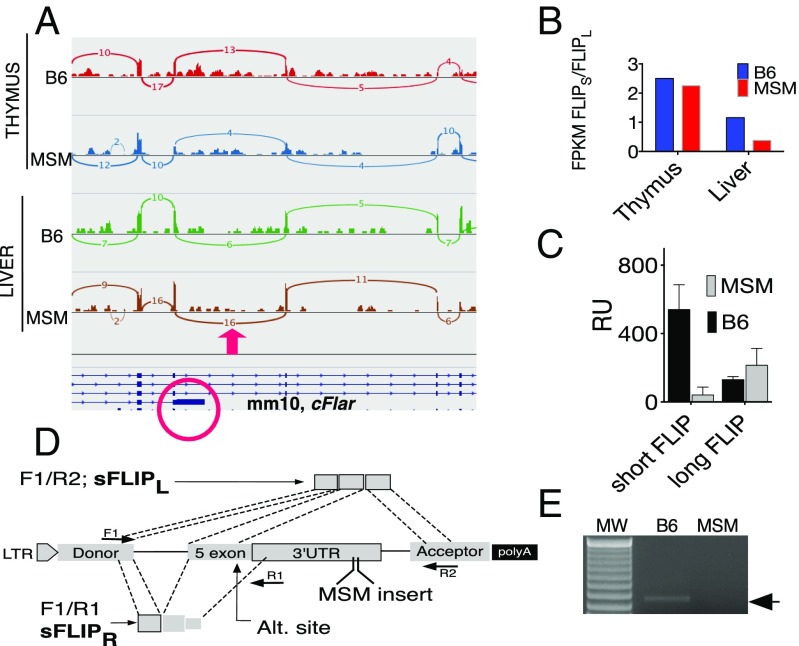
Next generation RNA sequencing (NGS) data allowed for comparison of the ratio of long and short cFLIP in the liver and thymus of B6 and MSM mice at baseline (unstimulated). First, using Sashimi plots and Bam format alignments (30) of gene-expression data, we visualized the RNA-sequencing reads and their splicing for the short and long isoforms of cFLIP. Fig. 5A shows the results of such visualization using the IGV genomic browser for exons 4, 5 (circled in red), and 6–8 of Cflar. Only reads that span two or more exons were included. For example, for B6 liver, relative to 10 reads connecting exons 4 and 5, there were six reads connecting exons 5 and 6 corresponding to the long form of cFLIP. In contrast, all 16 connections between exons 4 and 5 in MSM liver extend to exons 5 and 6—coding for long cFLIP—thus indicating relative skewing of splicing toward the long cFLIP in MSM livers. Furthermore, cFLIP from thymus did not show differential splicing, suggesting that the skewing toward long cFLIP mRNA in MSM could be a liver-specific phenomenon. These data suggested higher levels of transcripts for cFLIPL and lower levels for cFLIPR transcript in MSM than in B6 livers. In further support of the splicing analysis data, we calculated the ratio between long and short cFLIP using the number of reads for a particular transcript normalized per million of mapped reads (FPKM value). The results of this comparison are shown in Fig. 5B and confirm a significant bias toward long cFLIP in MSM livers compared with B6 livers. The ratio between the isoforms becomes equal in the thymus (Fig. 5B). To provide a causative link between the MSM insert and the splicing, we cloned exon 5 of mouse Cflar, together with the flanking intronic regions, into the multiple cloning site of exon-trapping vector pET01 (Fig. 5D) that also contains two additional exons (Donor and Acceptor in Fig. 5D) with 5′ donor and 3′ acceptor sites (31). Next, using high-pressure tail-vein i.v. injection (32), we expressed these constructs in the liver of B6 mice and used complement to exons F1, R1, and R2 primers to compare efficiency of splicing in both B6 and MSM constructs. The semiquantitative PCR (semi-Q-PCR) data in Fig. 5E show that the only PCR product observed is from the B6 construct, which confirms relatively efficient splicing correspondent to the short cFLIP transcript. Furthermore, Q-PCR of the fragment correspondent to the long form of FLIP was more efficient in MSM than B6 constructs (Fig. 5C). Thus, the 21-bp MSM insertion promotes splicing between plasmid exons, resulting in significant attenuation of the short cFLIP transcript. These data confirm that the 21-bp insertion promotes preferential splicing of the long cFLIP. Thus, the insertion likely promotes alternative splicing of cFLIPL in MSM livers, thereby resulting in predominantly cFLIPL mRNA.
Fig. 5.
Liver-specific preferential splicing of Cflar into cFLIPL in MSM. (A) Sashimi plot showing the number of reads between the exons of strain-specific (C57BL6 or MSM) and tissue-specific (liver or thymus) splice variants of long cFLIP. IGV software was used for visualization of the reads. The fifth exon of Cflar is in a red circle; a red arrow on top depicts the splicing of interest; and numbers correspond to the number of spliced reads. (B) Ratios of long/short isoforms quantified from RNA-seq data from unstimulated MSM/Ms and B6. FPKM, number of fragments per kilobase of aligned per million sequenced base pairs. (C) Q-PCR analysis of cDNA correspondent to cFLIPR or cFLIPL amplified with either F1/R1 or F1/R2 primers from the liver of mice injected with plasmids encoding the fifth exon of Cflar. (D) A map of the cloning of exon 5 Cflar into pET01 and the positions of the PCR primers and products correspondent to short (cFLIPR) and long (cFLIPL) isoforms of cFLIP. (E) RT-PCR of the short FLIP from livers of mice after injection with MSM or B6 constructs.
Low Levels of p55 CASP8 and RIP1 Cleavage in MSM Are Linked With Differential Splicing of cFLIP.
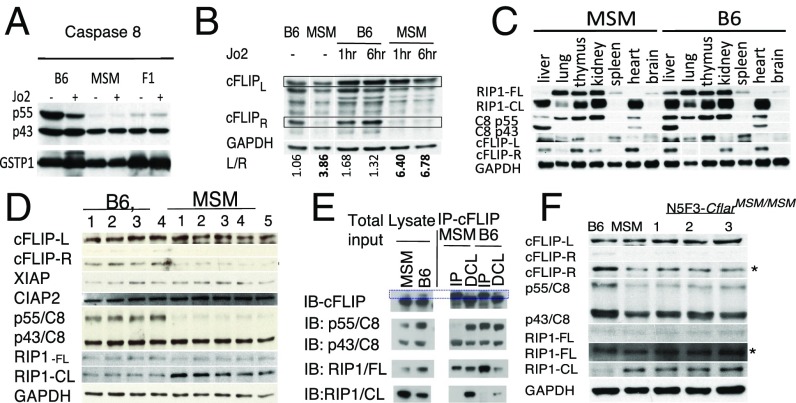
To determine the consequence of high levels of cFLIPL in MSM livers, we analyzed CASP8, FLIP, and RIP1 protein expression. First, the ratio analysis of p55 and p43 isoforms of CASP8 in B6 and MSM livers revealed relatively similar levels of p55 and p43 in B6, with a noticeable shift toward the p43 isoform upon injection of mice with Jo2 (Fig. 6A). In contrast, CASP8 in MSM livers consisted almost exclusively of the p43 isoform, and Jo2 did not significantly shift this ratio toward full-length CASP8 (Fig. 6A). F1 animals also predominantly expressed CASP8 p43. Such a bias toward CASP8 p43 in MSM livers might be the result of self-cleavage events (33) upon constitutive TNF receptor 1 activation, which occur when CASP8 forms a heterodimeric complex with cFLIPL. One of the factors affecting CASP8 processing could be the expression level of the two cFLIP isoforms: If present at high levels, cFLIPR binds to procaspase 8 (p55 CASP8) via homotypic interactions, keeping it intact until receptor-mediated oligomerization of CASP8 is induced. The resultant heterodimers are even more stable than p55 homodimers but cannot form proapoptotic tetramers p10/p18 until Fas-receptor ligation. On the contrary, cFLIPL associates with p55 CASP8 through homotypic interactions to produce enzymatically active heterodimers that potentially could cleave CASP8 (8) p55 to p43 via transcleavage events in the proximity of constitutive “leaky” death receptor-mediated signaling, such as TNFR-mediated oligomerization (34). The RNA sequencing and Q-PCR data show similar levels of TNF mRNA in MSM and B6 livers, thus dismissing differential TNF activation as the reason for p55 CASP8 processing in MSM. cFLIPL itself is not involved in the cleavage events but rather serves to stabilize caspase 8 as p43 caspase8 through the formation of the heterodimer. Accordingly, the relatively low levels of cFLIPR found in MSM livers compared with B6 (Fig. 6B), in agreement with the cFLIP mRNA analysis (Fig. 5), confirms that the long cFLIP is the dominant isoform in the liver of MSM mice. This relative abundance of cFLIPL could also explain our detection of the p43 CASP8 isoform in MSM livers because CASP8/cFLIPL forms an enzymatically active heterodimer capable of cleaving itself as well as other components of the necrotopic pathway, notably RIP1 (13). Given our results suggesting that the resistance was liver-specific (Fig. 1), we decided to look at CASP8, RIP1, and cFLIP (Fig. 6C) in several organs of unstimulated MSM and B6 mice. RIP1 was used as an indicator of p43caspase 8/cFLIPL heterodimer proteolytic activity because this complex has been implicated in cleaving RIP1 in unstimulated cells to maintain cellular homeostasis (35). The protein expression patterns and mouse strain differences observed in the liver were unmatched in other organs, and, as a result, we decided to focus our efforts on the liver. cFLIPR and p55 CASP8 levels were reproducibly lower in a small panel of MSM mice compared with B6 mice (Fig. 6D). Levels of cleaved RIP1 (RIP1-CL) were also higher in MSM mice compared with B6. Interestingly neither XIAP nor CIAP2 protein levels were different between the strains (Fig. 6D).
Fig. 6.
The ratio of CASP8 cleavage products and cFlip isoforms may determine resistance to liver failure in MSM mice. (A) Western blot analysis of caspase 8 from livers of C57BL/6 (B6), MSM/Ms (MSM), or F1 (B6 x MSM) mice. (B) Time course of ratio (L/R beneath) of cFLIP isoforms from livers of Jo2-primed B6 or MSM mice. (C) Tissue-specific RIP1 and CASP8 cleavage and cFLIP isoforms. CL, cleaved RIP; FL, full-length RIP. (D) Differential levels of several apoptotic components in the livers of age-matched unstimulated (four mice) B6 or (five mice) MSM mice. (E) Immunoprecipitation of cFLIP followed by Western blot analysis of CASP8 isoforms and RIP1 in MSM and B6 mouse livers. DCL, depleted cell lysate; IB, immunoblot; IP, immunoprecipitated. (F) Western blot analysis of cFLIP, CASP8, and RIP1 from unstimulated livers from (three mice) genetically distinct N5F3-CflarMSM/MSM mice.
To directly examine possible interaction of cFLIP with CASP8, we immunoprecipitated FLIP from unstimulated MSM and B6 livers and then analyzed the amounts of bound RIP1 and CASP8. Whereas p43 CASP8 almost exclusively bound FLIP in MSM livers, B6 FLIP was bound to both full-length CASP8 and p43 CASP8 (Fig. 6E). This result suggests that both cFLIPR and cFLIPL form complexes with caspase 8 in B6 mouse livers whereas cFLIPL is the predominant isoform that complexes with caspase 8 in MSM mouse livers. Furthermore, higher levels of cleaved RIP1 were detected in MSM livers compared with B6 (Fig. 6E), again suggesting the preferential formation of the p43 CASP8/cFLIPL heterodimer in MSM mouse livers.
To address strain-specific effects on apoptotic protein processing and to determine whether the 21-bp insertion in MSM mice plays a role in creating a prosurvival environment, we generated N5F3.CflarMSM/MSM mice. To do that, we crossed F1 (MSM × B6) mice five times back to B6, selecting mice with heterozygosity for MSM Cflar. Next, we intercrossed N5 progeny to produce mice with ∼96% of the B6 genome and homozygosity for the 21-bp insertion in the 3′ UTR of Exon5 in Cflar (Fig. 4). Compared with unstimulated B6 and MSM livers, protein expression patterns of cFLIP and CASP8 in three individual and genetically distinct N5F3.CflarMSM/MSM mice were more similar to MSM than to B6 (Fig. 6F). Specifically, the congenic mice exhibited low cFLIPR, low p55 CASP8, and a noticeably high level of cFLIPL than B6 mice. Furthermore, we also observed enhanced cleavage of RIP1 in the N5F3.CflarMSM/MSM mice—similar to what we observed in the MSM parental strain (Fig. 6F). This result strongly suggests that all of the differences in cFLIP, CASP8, and RIP1 that we observed in parental strains are due to the 21-bp insertion that alters cFLIP isoform levels in MSM mice.
Discussion
The data presented herein provide previously unidentified insights into the regulation of the death receptor pathway and the role of cFLIP in this process (13). Specifically, we show that resistance to CD95-mediated lethality in MSM correlates with relatively high levels of FLIPL and low levels of FLIPR in the livers of the resistant MSM mice. We identify a 21-bp insertion in the fifth exon of the Cflar in MSM, which corresponds to the 3′ UTR of the short FLIP mRNA and may prevent transcription of short FLIP, leading to preferential alternative splicing and thus increased transcription and translation of long FLIP. In the absence of the short FLIP isoform, which is capable of titrating away and blocking CASP8, long FLIP stabilizes CASP8 and forms an enzymatically active heterodimer that cleaves p55 CASP8 into p43 CASP8 through transcleavage events. This heterodimer of p43 CASP8/cFLIPL is also able to constitutively cleave RIP1, creating an environment of both apoptotic and necroptotic inhibition. Almost complete cleavage of RIP1 and processing of CASP8 into p43 in MSM suggest that components of the DISK complex are preassembled, even in the absence of activation. One of the conditions for CASP8 processing to p43CASP8 might be constitutive activation of the TNF receptor upon leaky TNF-induced signaling. However, neither Q-PCR nor RNA sequencing revealed substantial differences in TNF mRNA between the strains, which would explain preferential processing of CASP8 in MSM.
The identification of the 21-bp insertion in short FLIP mRNA of MSM was made possible by an unbiased genetic analysis of in vivo resistance to death receptor-mediated lethality. Using lethality as a readout, we identified several loci linked to resistance, with a Chr1 locus showing the highest linkage. We complemented this genetic mapping with a genomics approach that used genome-wide RNA sequencing and pathway analysis. Based on NGS analysis of FLIP transcripts and FLIP proteins in various mouse tissues of the MSM mouse, we discerned that the 21-bp insert is a 100% match with the branching splicing motif that might be interacting with the splicing machinery protein in a tissue-specific manner: namely, it affects splicing only in MSM liver tissue, providing an explanation as to why the resistance to apoptosis is liver-specific in MSM mice whereas thymocytes remain susceptible. The fact that the insert does not result in similar splicing in all MSM tissues could be explained by other tissue-specific factors.
The apoptosis resistance model in MSM mice relates to one of the dogmas in the field: i.e., weak versus strong signaling correlates with cell death in type I versus type II cells, respectively (36). Specifically, one of the components of the “weak signaling” cascade might be a lack or low abundance of the DISK components, such as full-length CASP8. In MSM mice, CASP8 predominantly exists in the form of enzymatically active heterodimers as CASP8/cFLIPL because of high levels of long cFLIP and a higher affinity of CASP8 to FLIPL than to itself. CASP8 in the CASP8/cFLIPL heterodimer might potentially cleave itself to p43, but further cleavage to p10/18 CASP8, which is the classically active CASP8 able to cleave CASP3 and drive apoptosis, is prevented.
In conclusion, this previously uncharacterized model of in vivo resistance to Fas-mediated lethality is, to our knowledge, the first that is able to distinguish between two different ligands. We anticipate that other wild-derived strains retaining identical insertions in Cflar, such as MOLF and PWK, will likewise share this resistance phenotype, particularly because N5F3.CflarMSM/MSM congenic mice recapitulate the biochemistry of apoptotic components observed in the parental MSM strain. In fact, wild-derived SPRETUS mice were reported to be resistant to TNF-induced lethality although their resistance was mapped to different loci than in MSM (19, 21).
Experimental Procedures
Phenotype.
A panel of F2 (MSM/Ms × B6) mice was generated from brother–sister mating of F1 mice. When 8 wk old, the mice were injected intraperitoneally with 10 µg of Jo2 or 2 µg of MegaFasL and monitored for 24 h. Kaplan–Meier curves were used to compare the differences in the survival of B6 with MSM/Ms, using the Logrank test to determine significance.
RNA-Sequencing.
Seventy-five–base pair pair-end reads from cDNA libraries were generated on MiSeq (Illumina) and aligned using TopHat2 and Cufflinks software. The data are available at the National Center for Biotechnology Information Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72454).
Tufts University Medical School Animal Care and Use Committees approved all animal work. Please see SI Experimental Procedures for more information on the methods.
SI Experimental Procedures
Cells.
Intraperitoneal macrophages were harvested in a peritoneal lavage with ice-cold PBS from mice 3 d after 1 mL i.p. injection with Thioglycollate (Remel). The macrophages were cultured in complete DMEM [DMEM plus 10% (vol/vol) FBS plus 2% (vol/vol) PenStrep]. Thymocytes were obtained from freshly isolated thymi and cultured in complete DMEM. MEFs were isolated from embryos of pregnant females at d11–d13. After isolation, all cells were allowed to rest for 3 h before in vitro experiments.
Immunoblotting.
Tissue homogenates from thymi, livers, kidneys, spleens, lungs, and brains were harvested from MSM/Ms and B6 mice after perfusion with 6 mL of ice-cold PBS, via the hepatic portal vein. The livers were minced with surgical scissors, and the minced liver was passed through a 70-μm cell strainer. For Western blots, the tissue was then homogenized in cell lysis buffer (BD caspase 3 buffer) supplemented with protease inhibitor (Sigma-Aldrich) on ice for 1 h. Nuclei were pelleted by spinning the lysate at 500 × g for 7 min at 4 °C, and the supernatant was used for Western blotting. Then, 60 μg of protein was run on 12% (vol/vol) bis-acrylamide gels until the desired separation was achieved. The proteins were then transferred onto nitrocellulose membranes (Bio-Rad). The membranes were incubated with 5% (wt/vol) BSA in tris-buffered saline with 0.1% Tween (TBS-T) for 30 min at room temperature. The membrane was then washed in TBS-T for 10 min, followed by overnight incubation with primary antibody diluted in 5% (wt/vol) BSA in TBS-T (1:1,000). The primary antibodies we used were from the following sources: caspase 3 (9662), caspase 8 (4927), caspase 9 (9504), and GAPDH (2118) from Cell Signaling; cFLIP (G11) from Santa Cruz; and cFLIP (AAP440) and caspase 8 (1G12) from Alexis Biochemicals.
Immunoprecipitations.
For immunoprecipitations, the tissue was homogenized in a dounce homogenizer with ice-cold PBS (1×) supplemented with protease inhibitor (Sigma-Aldrich), using 30 strokes. Protein lysate samples were precleared on prewashed Protein G agarose beads for 2 h at 4 °C, and 500 μg of precleared protein lysates supplemented with protease inhibitor (Sigma) were added to 1 μg of antibody (cFLIP G-11 mouse mAb; Santa Cruz) previously coupled to 10 μL of protein G agarose beads at 4 °C. The protein-Ab mix was allowed to rotate at 4 °C overnight. The samples were then pelleted for 1 min at 100 × g, and the supernatants were removed and processed as the “depleted cell lysates” by adding appropriate amounts of 4× Laemmli Sample Buffer (BP-110R; Boston BioProducts). The immunoprecipitate pellet was washed three times, each using ice-cold lysis buffer followed by a 1-min spin at 100 × g. After the final wash, 30 μL of 2× Laemmli Sample buffer was added to the pellet. After adding Laemmli Sample buffer, the samples were incubated at 90 °C for 10 min, and then 10 μL of the sample was used per Western blot run.
Viability Assay.
After isolation from either MSM/Ms or B6 mice as detailed above, 1 × 105 Thymocytes, 50 × 105 Peritoneal, or 5 × 104 MEFs were plated in triplicate in each well of a 96-well plate (Corning). The cells were then treated with various agonists and at the appropriate times (4.5 h for thymocytes and 18 h for macrophages and MEFs). We then carried out a viability assay looking at ATP levels poststimulation using CellTiterGlo using the 96-well protocol as per the manufacturer’s instructions (CellTiterGlo).
Histology.
Livers were isolated from either uninjected mice or mice after Jo2, MegaFasL, or TNFα/DGalN i.p. injections and were fixed using 10% (vol/vol) Formalin. The livers were then sent to the Tufts Medical Histology Core Facility where they were paraffin-embedded and processed for H&E staining.
Mapping.
A panel of 125 F2 mice were phenotyped for Jo2 resistance. In our phenotypic screen, we injected the mice intraperitoneally with either 10 μg of Jo2 resuspended in 500 μL of PBS (1×) and monitored them for 100 h, or until they were moribund, at which point they were euthanized. Those mice that survived until 100 h were considered survivors, and those that did not were susceptible. We used time-to-death measurements as well as binary assignments (0 = death, 1 = survival) as the output data.
These mice were subsequently genome-wide genotyped using microsatellite markers at about 20-cM intervals. We then used the R-based programs J/QTL (Jackson Labs) and R/QTL to identify associations between the phenotypic data and the genotype at each microsatellite marker. Associations were given LOD scores, where the higher the score, the stronger the association at any given locus. The analysis was carried out as outlined in Broman and coworkers (28).
ALT.
ALT levels were detected at various time points by harvesting the sera from mice that were either uninjected or injected with Jo2 or MegaFasL as described in the manufacturer’s protocol [Pointe Liquid ALT(SGPT) reagent set] and after diluting the sera 1:50 in PBS (1×).
Acknowledgments
We thank Bridget Larkin and Brigitte Huber for reading the manuscript. This work was supported by NIH Grants AI056234 and AI090419, Russian Federation Government Grant 220(11.G34.31.0052), RFFI Grant NK13-04-40267-H/15, and Russian Science Fund Project 15-15-00100 (RNA-sequencing, Figs. 3 and 5) (to A.P.). We are grateful for the generous support of the Eshe Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE72454).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517562113/-/DCSupplemental.
References
- 1.Jost PJ, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460(7258):1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenschwender M, Wajant H. The role of FasL and Fas in health and disease. Adv Exp Med Biol. 2009;647:64–93. doi: 10.1007/978-0-387-89520-8_5. [DOI] [PubMed] [Google Scholar]
- 3.Ogasawara J, Suda T, Nagata S. Selective apoptosis of CD4+CD8+ thymocytes by the anti-Fas antibody. J Exp Med. 1995;181(2):485–491. doi: 10.1084/jem.181.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: Secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21(4):461–465. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Schütze S, Tchikov V, Schneider-Brachert W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol. 2008;9(8):655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- 6.Feoktistova M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43(3):449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oztürk S, Schleich K, Lavrik IN. Cellular FLICE-like inhibitory proteins (c-FLIPs): Fine-tuners of life and death decisions. Exp Cell Res. 2012;318(11):1324–1331. doi: 10.1016/j.yexcr.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Pop C, et al. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem J. 2011;433(3):447–457. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and -10 by FLIP(L) Biochem J. 2004;382(Pt 2):651–657. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fricker N, et al. Model-based dissection of CD95 signaling dynamics reveals both a pro- and antiapoptotic role of c-FLIPL. J Cell Biol. 2010;190(3):377–389. doi: 10.1083/jcb.201002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueffing N, et al. Mutational analyses of c-FLIPR, the only murine short FLIP isoform, reveal requirements for DISC recruitment. Cell Death Differ. 2008;15(4):773–782. doi: 10.1038/sj.cdd.4402314. [DOI] [PubMed] [Google Scholar]
- 12.Weinlich R, et al. Protective roles for caspase-8 and cFLIP in adult homeostasis. Cell Reports. 2013;5(2):340–348. doi: 10.1016/j.celrep.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon CP, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Reports. 2012;1(5):401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinlich R, Green DR. The two faces of receptor interacting protein kinase-1. Mol Cell. 2014;56(4):469–480. doi: 10.1016/j.molcel.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter D, et al. Switch from type II to I Fas/CD95 death signaling on in vitro culturing of primary hepatocytes. Hepatology. 2008;48(6):1942–1953. doi: 10.1002/hep.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schüngel S, et al. The strength of the Fas ligand signal determines whether hepatocytes act as type 1 or type 2 cells in murine livers. Hepatology. 2009;50(5):1558–1566. doi: 10.1002/hep.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogasawara J, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura Y, et al. In vivo analysis of Fas antigen-mediated apoptosis: Effects of agonistic anti-mouse Fas mAb on thymus, spleen and liver. Int Immunol. 1997;9(2):307–316. doi: 10.1093/intimm/9.2.307. [DOI] [PubMed] [Google Scholar]
- 19.Staelens J, et al. Hyporesponsiveness of SPRET/Ei mice to lethal shock induced by tumor necrosis factor and implications for a TNF-based antitumor therapy. Proc Natl Acad Sci USA. 2002;99(14):9340–9345. doi: 10.1073/pnas.142293699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao C, et al. Mechanisms for U2AF to define 3′ splice sites and regulate alternative splicing in the human genome. Nat Struct Mol Biol. 2014;21(11):997–1005. doi: 10.1038/nsmb.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahieu T, et al. The wild-derived inbred mouse strain SPRET/Ei is resistant to LPS and defective in IFN-beta production. Proc Natl Acad Sci USA. 2006;103(7):2292–2297. doi: 10.1073/pnas.0510874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beutler B, Krochin N, Milsark IW, Luedke C, Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- 23.Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15(25):3086–3098. doi: 10.3748/wjg.15.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7(7):532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 25.Jodo S, et al. CD95 (Fas) ligand-expressing vesicles display antibody-mediated, FcR-dependent enhancement of cytotoxicity. J Immunol. 2000;165(10):5487–5494. doi: 10.4049/jimmunol.165.10.5487. [DOI] [PubMed] [Google Scholar]
- 26.Neumann L, et al. Dynamics within the CD95 death-inducing signaling complex decide life and death of cells. Mol Syst Biol. 2010;6:352. doi: 10.1038/msb.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong J, Adamson LK, Hatam R, Greenhalgh DG, Cho K. Alterations in the expression and modification of histones in the liver after injury. Exp Mol Pathol. 2003;75(3):256–264. doi: 10.1016/s0014-4800(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 28.Manichaikul A, Moon JY, Sen S, Yandell BS, Broman KW. A model selection approach for the identification of quantitative trait loci in experimental crosses, allowing epistasis. Genetics. 2009;181(3):1077–1086. doi: 10.1534/genetics.108.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmel I, Tal S, Vig I, Ast G. Comparative analysis detects dependencies among the 5′ splice-site positions. RNA. 2004;10(5):828–840. doi: 10.1261/rna.5196404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu E, Nance T, Montgomery SB. SplicePlot: A utility for visualizing splicing quantitative trait loci. Bioinformatics. 2014;30(7):1025–1026. doi: 10.1093/bioinformatics/btt733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogaev EI, Grigorenko AP, Faskhutdinova G, Kittler EL, Moliaka YK. Genotype analysis identifies the cause of the “royal disease”. Science. 2009;326(5954):817. doi: 10.1126/science.1180660. [DOI] [PubMed] [Google Scholar]
- 32.Tward AD, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci USA. 2007;104(37):14771–14776. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallenberger SM, et al. Intra- and interdimeric caspase-8 self-cleavage controls strength and timing of CD95-induced apoptosis. Sci Signal. 2014;7(316):ra23. doi: 10.1126/scisignal.2004738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283(5401):543–546. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- 35.Micheau O, et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277(47):45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 2012;19(1):42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]