Bipolar disorder (BP), also known as manic-depressive illness, is a brain disorder that causes unusual shifts in mood, energy, and activity levels (1). Some of the most prominent and characteristic symptoms of BP include dramatic disturbances in sleep:wake or rest:activity cycles, with restlessness in the manic phase and extremely low levels of activity during the depressive phase (“having difficulty getting out of bed”) (2, 3). Also, rest:activity cycles can profoundly change the course of clinical manifestations; pharmacological agents such as lithium and antidepressants, which are commonly used to treat this illness, significantly alter the cycling of affective episodes over the longitudinal course of the disorder (2, 3). In PNAS, Pagani et al. (4) report, to their knowledge, the first large-scale systematic analysis of rest:activity phenotypes in 26 pedigrees segregating for severe BP. This study involved monitoring of actigraphy for two weeks in 136 euthymic (between episodes of mania or depression) BP subjects and 422 of their non-BP relatives. To identify sleep, activity, and circadian rhythm phenotypes with a significant genetic component, the authors estimated heritability. In simple terms, they compared the degree of familial relationships with the level of similarity in 73 phenotypes collected for all family members. In addition to identifying heritable rest:activity traits, this analysis also identified a subset of phenotypes correlated with the disease status. Systematic delineation of heritable disease-associated traits, as illustrated in the Pagani et al. paper (4), will be critical in ongoing efforts to link genomic lesions—such as those identified by next-generation sequencing—to complex psychiatric disorders.
Genetic investigations of BP in several multigenerational pedigrees from the Central Valley of Costa Rica and Antioquia in Colombia started more than 20 y ago with classical linkage studies aimed at identifying “major genes” linked to the overall syndrome (5–8). The message was loud and clear: Even in families from genetically isolated populations, there was significant variation in the genetic basis for BP in different families with no high-impact risk alleles. An international collaborative team, which included clinicians and scientists from Costa Rica and Colombia, embarked on an ambitious project to measure a wide range of quantitative traits in euthymic BP subjects and their non-BP family members. In addition to clinical assessments, and measurements of sleep, activity, and circadian rhythms (4), they also assessed the familial aggregation of 169 traits from neurocognitive batteries and neuroimaging (9, 10).
Using sophisticated statistical methodology, Fears et al. (9) identified 126 significantly heritable and 53 were associated with severe BP (BP-I). Among phenotypes that are heritable and associated with BP are proneness to delusions, perceptual creativity, and impairments in processing speed, verbal learning, and memory. The Pagani et al. study (4) extends the list of heritable behavioral traits associated with BP-I to sleep and circadian rhythms. Of the 73 activity and sleep-related phenotypes assessed through actigraphy, 12 phenotypes were heritable and significantly associated with BP-I. Consistent with previous smaller-scale studies (11), Pagani et al. report that euthymic BP subjects have lower activity levels compared with their non-BP family members. BP subjects awoke later and slept longer and had more variability in the total minutes scored as sleep, possibly reflecting the robustness of the subject’s circadian system. Euthymic BP-I subjects also display lower amplitude, mainly due to the lower activity levels during their least active hours of a day. The reduction in the amplitude of activity in BP subjects is particularly striking because it may relate to the reduced amplitude in the expression of several core circadian genes in fibroblasts of subjects with BP from another population (12). Pagani et al. (4) also report results from a genome-wide linkage study of 13 disease-associated rest:activity phenotypes. Although the majority of analyzed traits did not give a genome-wide significant linkage signal, the team identified a locus for interdaily stability of activity on chromosome 12 [maximum logarithm of odds (LOD) score 4.73] and a locus for four correlated phenotypes related to the SD of sleep onset and activity on chromosome 1 (LOD score >3.0).
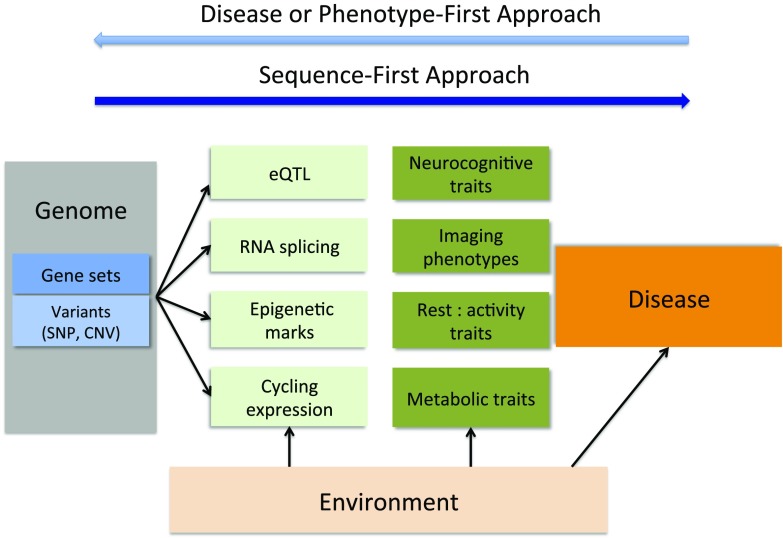
This work is a necessary step in building a better understanding of the pathogenic mechanisms underlying BP. However, it is unclear if a combined analysis of the neurocognitive, sleep and circadian, and neuroanatomical components of this complex clinical phenotype will lead to a more unified picture of its genetic basis (Fig. 1, disease or phenotype-first approach). An alternative approach, obviously already underway, is to obtain whole genome sequence for all of the members of a pedigree, and interpret phenotypic variation within and between pedigrees in the context of almost complete genetic information (Fig. 1, sequence-first approach).
Fig. 1.
Flowchart for the analysis of genome–endophenotype–disease association in psychiatric disorders. Light blue arrow (at top) illustrates a conventional disease or phenotype-first approach for identification of disease risk alleles. The dark blue arrow depicts the sequence-first approach. Exome or whole-genome sequences will identify variants (single-nucleotide polymorphisms or copy number variants) or variants in defined gene sets. These variants are tested for functional impact at a molecular level (eQTL, RNA splicing, epigenetic elements, cycling gene expression, or reporter assays) and on organismal endophenotypes (neuroimagining, neurocognitive traits, rest:activity traits). A subset of these endophenotypes will be disease-associated. Heritability estimates will identify endophenotypes with a strong genetic component and those more influenced by environment.
Major advances in genetic technologies, from high-density genotyping to exome and whole-genome sequence analysis, have transformed the field of psychiatric genetics. Genome-wide association studies (GWAS) on hundreds or thousands of subjects with and without a disease (cases and controls) have led to the identification of significant associations with common genetic polymorphisms (13). In an effort to interpret how common alleles associated with a disease lead to disease pathology, and to explore the role of rarer alleles, geneticists are now using exome or whole-genome sequencing to obtain a more detailed picture of the genetic landscape of these diseases (13, 14). In studies of BP, linking a disease to a specific DNA variant identified through genomic characterization (GWAS and sequencing) has proved difficult. As in many other psychiatric disorders, BP is genetically heterogeneous, with a handful of significant variants identified by GWAS (15). However, the small relative risk attributed to alleles associated with the disease makes the identification of causal mutations challenging. Similarly, early insights from whole-genome sequence analysis of extended families implicated hundreds of genes with variants predicted to have an effect on phenotype (13). Analysis of endophenotypes and their prioritization based on covariance with bipolar illness in extended families, as described by Pagani et al. (4) and Fears et al. (9), may bridge the gap between genomic lesions—single-nucleotide polymorphisms and copy number variants—and clinical phenotypes (Fig. 1). For example, a subset of detected deleterious variants may have a pleiotropic effect on several endophenotypes, whereas others may have a more specific phenotypic manifestation. Also, taking an example from circadian biology, linking specific variants in clock or clock-controlled genes to a heritable behavioral trait that is not necessarily associated with BP will still contribute to a better understanding of the neurobiological basis of behavior.
Further studies of the extended pedigrees from the Central Valley and Antioquia, described by Pagani et al. (4), will likely be directed toward genomic characterization and transcriptome analysis. In addition to obtaining the whole-genome sequence of each family member, the availability of induced pluripotent stem cell lines (iPSC) and expression data for several cell types (blood cells and iPSC-derived cell types) would offer the possibility of
In PNAS, Pagani et al. report, to their knowledge, the first largescale systematic analysis of rest:activity phenotypes in 26 pedigrees segregating for severe BP.
exploring transcript-based endophenotypes or regulatory variation (Fig. 1). Recent estimates suggest that more than 85% of common phenotype-associated genetic variants are non-protein-coding polymorphisms (16). A coupling of whole-genome sequence with RNA sequencing analysis of individual-specific cell types in extended families provides sufficient power to test for expression Quantitative Trait Loci (eQTLs). In addition, the integrated analysis of eQTLs and disease-associated endophenotypes (17, 18) may make it possible to identify the causal mechanisms, genes, or pathways underlying disease risk.
The analysis of sleep, activity, and circadian rhythms performed by Pagani et al. (4) focused on two groups of subjects: those with BP-I, a form of BP with pronounced episodes of mania and depression, and their non–BP-I family members. Future studies should include family members with other BP-related disorders, such as BP type II (a form with episodes of depression and hypomania), major depressive disorder, and other psychiatric manifestations (such as anxiety disorder, postpartum depression, or alcohol and drug use disorders) observed in non-BP family members in these extended pedigrees. Moreover, in addition to the BP-I phenotype, which is the focus of the Pagani et al. (4) and Fears et al. (9) publications, disrupted daily patterns of sleep and wakefulness, as well as variability of these cycles over longer time periods, represent core features of several common BP-related psychiatric, neurodevelopmental, and neurodegenerative disorders (2, 3, 19, 20). Long-term monitoring of rest:activity patterns, using either actigraphy devices developed for research studies or ubiquitously available consumer-oriented electronic activity monitoring devices, will provide insights into disease-specific anomalies in cycles of sleep and wakefulness and, potentially, help define disease subtypes. However, even with these advances, more integrated approaches will be needed to resolve a decades-old question: whether rest:activity disturbances arise from anomalies in core mechanisms underlying disease pathology or are an inability of a diseased brain to sustain consolidated sleep and wakefulness during development (in Autism Spectrum Disorder, for example) or at an advanced age as in neurodegenerative disorders.
After many years of transformative advances in genomic analysis, the combined work of the Fears et al. (9) and Pagani et al. (4) teams help to expand the phenotype half of the genotype−phenotype space (18), and demonstrate the value of family-based studies for the analysis of complex psychiatric disorders.
Acknowledgments
This work was supported by US National Institutes of Health (NIMH) Grants MH103963 and MH093415.
Footnotes
The author declares no conflict of interest.
See companion article on page E754.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th Ed Am Psych Publ; Arlington, VA: 2013. [Google Scholar]
- 2.Rumble ME, White KH, Benca RM. Sleep disturbances in mood disorders. Psychiatr Clin North Am. 2015;38(4):743–759. doi: 10.1016/j.psc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Jagannath A, Peirson SN, Foster RG. Sleep and circadian rhythm disruption in neuropsychiatric illness. Curr Opin Neurobiol. 2013;23(5):888–894. doi: 10.1016/j.conb.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Pagani L, et al. Genetic contributions to circadian activity rhythm and sleep pattern phenotypes in pedigrees segregating for severe bipolar disorder. Proc Natl Acad Sci USA. 2016;113:E754–E761. doi: 10.1073/pnas.1513525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McInnes LA, et al. A complete genome screen for genes predisposing to severe bipolar disorder in two Costa Rican pedigrees. Proc Natl Acad Sci USA. 1996;93(23):13060–13065. doi: 10.1073/pnas.93.23.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzberg I, et al. Convergent linkage evidence from two Latin-American population isolates supports the presence of a susceptibility locus for bipolar disorder in 5q31-34. Hum Mol Genet. 2006;15(21):3146–3153. doi: 10.1093/hmg/ddl254. [DOI] [PubMed] [Google Scholar]
- 7.Service S, et al. 2006. Results of a SNP genome screen in a large Costa Rican pedigree segregating for severe bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 141B(4):367−373, and erratum (2008) 147B(4):540.
- 8.Kremeyer B, et al. Genome-wide linkage scan of bipolar disorder in a Colombian population isolate replicates Loci on chromosomes 7p21-22, 1p31, 16p12 and 21q21-22 and identifies a novel locus on chromosome 12q. Hum Hered. 2010;70(4):255–268. doi: 10.1159/000320914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fears SC, et al. Multisystem component phenotypes of bipolar disorder for genetic investigations of extended pedigrees. JAMA Psychiatry. 2014;71(4):375–387. doi: 10.1001/jamapsychiatry.2013.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fears SC, et al. Brain structure-function associations in multi-generational families genetically enriched for bipolar disorder. Brain. 2015;138(Pt 7):2087–2102. doi: 10.1093/brain/awv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff EA, 3rd, Putnam FW, Post RM. Motor activity and affective illness. The relationship of amplitude and temporal distribution to changes in affective state. Arch Gen Psychiatry. 1985;42(3):288–294. doi: 10.1001/archpsyc.1985.01790260086010. [DOI] [PubMed] [Google Scholar]
- 12.Yang S, Van Dongen HP, Wang K, Berrettini W, Bućan M. Assessment of circadian function in fibroblasts of patients with bipolar disorder. Mol Psychiatry. 2009;14(2):143–155. doi: 10.1038/mp.2008.10. [DOI] [PubMed] [Google Scholar]
- 13.Neale BM, Sklar P. Genetic analysis of schizophrenia and bipolar disorder reveals polygenicity but also suggests new directions for molecular interrogation. Curr Opin Neurobiol. 2015;30:131–138. doi: 10.1016/j.conb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Shinozaki G, Potash JB. New developments in the genetics of bipolar disorder. Curr Psychiatry Rep. 2014;16(11):493. doi: 10.1007/s11920-014-0493-5. [DOI] [PubMed] [Google Scholar]
- 15.Georgi B, et al. Genomic view of bipolar disorder revealed by whole genome sequencing in a genetic isolate. PLoS Genet. 2014;10(3):e1004229. doi: 10.1371/journal.pgen.1004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurano MT, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337(6099):1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glahn DC, et al. Arguments for the sake of endophenotypes: Examining common misconceptions about the use of endophenotypes in psychiatric genetics. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(2):122–130. doi: 10.1002/ajmg.b.32221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almasy L. The role of phenotype in gene discovery in the whole genome sequencing era. Hum Genet. 2012;131(10):1533–1540. doi: 10.1007/s00439-012-1191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds AM, Malow BA. Sleep and autism spectrum disorders. Pediatr Clin North Am. 2011;58(3):685–698. doi: 10.1016/j.pcl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Hastings MH, Goedert M. Circadian clocks and neurodegenerative diseases: Time to aggregate? Curr Opin Neurobiol. 2013;23(5):880–887. doi: 10.1016/j.conb.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]