Significance
Much progress has been made toward the development of drug and cell therapies for the treatment of severely injured skeletal muscle, although reliable clinical therapies still do not exist. In contrast, this work demonstrates that biphasic ferrogels with a capacity for large, fatigue-resistant deformations can be used to mechanically stimulate and regenerate injured muscle tissue without the use of growth factors or cells. These biologic-free scaffolds exhibit a potential immunomodulatory role when stimulated and could potentially translate rapidly to the clinic. The therapeutic use of direct mechanical stimulation of injured tissues via externally actuated biomaterials could establish a new paradigm for regenerative medicine broadly.
Keywords: mechano-therapy, magnetic hydrogel, massage-mimetic, fibrous capsule, immunomodulation
Abstract
Severe skeletal muscle injuries are common and can lead to extensive fibrosis, scarring, and loss of function. Clinically, no therapeutic intervention exists that allows for a full functional restoration. As a result, both drug and cellular therapies are being widely investigated for treatment of muscle injury. Because muscle is known to respond to mechanical loading, we investigated instead whether a material system capable of massage-like compressions could promote regeneration. Magnetic actuation of biphasic ferrogel scaffolds implanted at the site of muscle injury resulted in uniform cyclic compressions that led to reduced fibrous capsule formation around the implant, as well as reduced fibrosis and inflammation in the injured muscle. In contrast, no significant effect of ferrogel actuation on muscle vascularization or perfusion was found. Strikingly, ferrogel-driven mechanical compressions led to enhanced muscle regeneration and a ∼threefold increase in maximum contractile force of the treated muscle at 2 wk compared with no-treatment controls. Although this study focuses on the repair of severely injured skeletal muscle, magnetically stimulated bioagent-free ferrogels may find broad utility in the field of regenerative medicine.
Skeletal muscle comprises a large percentage of the human body mass (40–50%) and plays an essential role in locomotion, postural support, and breathing. In response to minor injuries, skeletal muscle possesses a remarkable capacity for regeneration. Small exercise-induced tears, lacerations, and contusions typically heal without therapeutic intervention (1, 2). However, severe injuries resulting in a muscle mass loss of greater than 20% can lead to extensive fibrosis and loss of muscle function (2). Unfortunately, traumatic injuries resulting from motor vehicle accidents, aggressive tumor ablation, and prolonged denervation are common clinical situations and frequently lead to volumetric muscle loss (2–4). Although surgical reconstruction can lead to improved outcomes, this technique typically does not fully regenerate lost muscle tissue, and often leads to donor site morbidity (5, 6). As a result, the development of therapeutic strategies to treat severe skeletal muscle injuries is an area of active investigation.
Typical current approaches to skeletal muscle repair rely on the delivery of biologics such as growth factors and cells to enhance muscle regeneration. In early clinical trials, the intramuscular injection of cultured myoblasts was proven to be a safe but ineffective cell therapy for human myopathies, likely as a result of rapid death, poor migration, and immune rejection of the donor cells (7, 8). Recently, the identification of several important microenvironmental cues that regulate satellite cell fate has led to the development of cell-instructive biomaterials that improve cell engraftment and muscle regeneration. Biomaterial-based delivery of myogenic [IGF (insulin-like growth factor), FGF-2 (fibroblast growth factor-2), HGF (hepatocyte growth factor)] and angiogenic [VEGF (vascular endothelial growth factor)] factors that appear during the normal regenerative process has proven successful in animal models of severe muscle injury (9–13). Furthermore, the synergistic presentation of cells and growth factors that mimic normal in vivo presentation has led to improved functional muscle regeneration in mice (13, 14). However, although much progress has been made toward the development of cell and growth factor-based approaches for the treatment of severely injured skeletal muscle in rodents, reliable clinical therapies still do not exist. Cell and drug therapies have likely been limited by rapidly depleted local concentrations, inappropriate gradients, and loss of bioactivity of delivered growth factors (13, 15), as well as the loss of regenerative potential associated with myogenic cell isolation and manipulation (16).
Skeletal muscle and satellite cells are sensitive to biophysical microenvironmental cues (16), and mechanical loading in particular is a strong regulator of muscle biology and function (17, 18). Stretch activation has been shown to play a role in modulating satellite cell activation, glucose uptake, and immune cell recruitment (19–21), while mechanical conditioning has been used extensively in cell culture to replicate the in vivo microenvironment and produce more physiologically relevant skeletal muscle constructs. Specifically, cyclic mechanical stretching of cell-seeded scaffolds has been shown to improve myoblast alignment, myofiber diameter, and skeletal muscle construct hypertrophy (22, 23). Importantly, mechanically loaded tissue-engineered skeletal muscle constructs often more closely mimic the tissue elasticity, structural organization, and force generation capabilities of native skeletal muscle (24–26). In addition, there is some clinical evidence that massage therapy or the physical manipulation of injured skeletal muscle and connective tissue may reduce pain and promote recovery by increasing blood flow (27–29). Because of its wide-ranging benefits on myogenesis, mechanical stimulation may offer a simple, yet effective, approach to skeletal muscle regeneration.
This manuscript addresses the hypothesis that direct mechanical stimulation can enhance the regeneration of severely damaged skeletal muscle, obviating the need for exogenous growth factors or cells. Previously, it has been demonstrated that magnetically responsive biomaterials termed ferrogels are capable of rapid, on-demand deformations in response to externally applied magnetic fields (30, 31). In particular, ferrogels containing an iron oxide gradient (biphasic ferrogels) possess a capacity for large, fatigue-resistant deformations even at device sizes appropriate for small animal implantation. Biphasic ferrogel-driven cyclic mechanical compressions, driven by an external magnet, were tested here for their ability to affect skeletal muscle regeneration. Unlike previous reports focusing on the effect of cyclic compression on exercise-induced muscle damage, these studies were carried out using a murine model of severe muscle injury involving both myotoxin-induced direct muscle damage and hind limb ischemia. If left untreated, this model leads to substantial muscle necrosis, fibrosis, and contractile function loss (32), mimicking severe injuries in humans.
Results
Experimental Design and Muscle Stimulation Profiles.
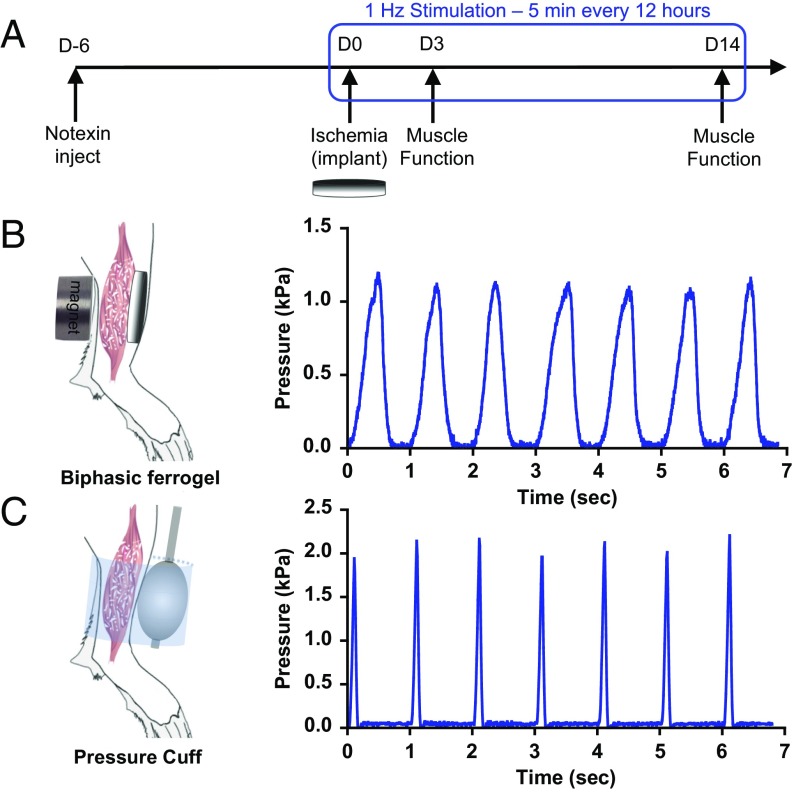
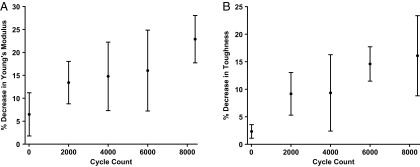
The tibialis anterior muscle of each C57BL6/J mouse was subjected to a severe dual injury involving an intramuscular injection of notexin followed by induction of hind limb ischemia 6 d later, as previously described (32). Complete loss of locomotion of the injured hind limb was observed immediately after induction of ischemia. After ischemic surgery, the injured muscle was treated with a s.c. implanted biphasic ferrogel, subsequently stimulated at 1 Hz for 5 min every 12 h noninvasively, using a permanent magnet (Fig. 1A). Control conditions included a pressure cuff stimulated at 1 Hz for 5 min every 12 h, a biphasic ferrogel without stimulation, magnetic field only, and no treatment. Although the biphasic ferrogel provides stimulation directly to the muscle, the pressure cuff externally compresses the muscle through the skin. Both stimulation of the biphasic ferrogel and the pressure cuff led to uniform cyclic compressions on the injured muscle. Importantly, biphasic ferrogels exhibited fatigue resistance as Young’s modulus and toughness changed minimally throughout the 2-wk study (Fig. S1 A and B). Stimulated biphasic ferrogels were able to exert a peak pressure of 1.2 kPa, whereas the pressure cuff was able to exert a slightly larger peak pressure of 2.0 kPa. The kinetics of the compressions varied between the biphasic ferrogel and the pressure cuff, with the biphasic ferrogels exhibiting more gradual changes in pressure while the pressure cuff demonstrated more rapid changes in pressure (Fig. 1 B and C).
Fig. 1.
Biphasic ferrogels and pressure cuffs generate cyclic mechanical compressions. (A) Experimental design showing injury, implant, and stimulation profile. (B) Schematic of biphasic ferrogel implant in mouse hind limb depicting orientation of ferrogel relative to skin, muscle tissue, and magnet (Left). Pressure profile of biphasic ferrogel undergoing repeated magnetic stimulations (Right). (C) Schematic of pressure cuff on mouse hind limb depicting orientation of balloon and polycarbonate cuff relative to skin and muscle tissue (Left). Pressure profile of balloon cuff undergoing repeated inflations and deflations (Right).
Fig. S1.
Biphasic ferrogels exhibit fatigue resistance. Percentage decrease in biphasic ferrogel (A) Young's modulus and (B) toughness at 50% strain after 8,400 cyclic compressions to 50% strain, compared with the values calculated from the first cycle of compression. Values represent the mean and SD (n = 4).
Host Response to Ferrogel Implant.
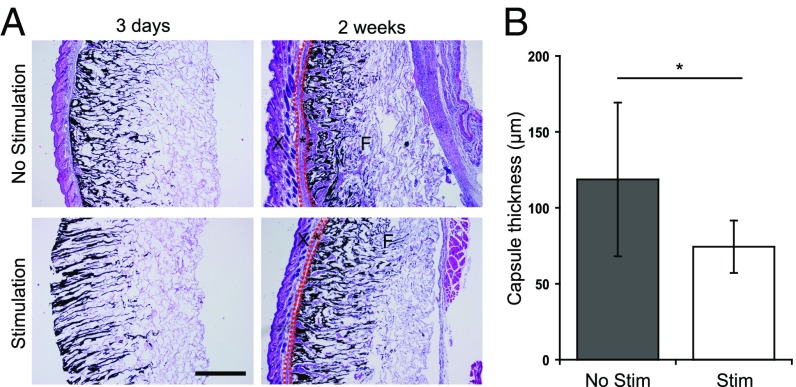
Biphasic ferrogels were histologically examined 3 d and 2 wk after implantation to determine the host response to the implants. On retrieval, all scaffolds were found localized at the initial site of implantation. Initial orientation of the gel relative to the skin and injured muscle tissue remained unchanged throughout the study. In addition, no significant differences in gel thickness were observed between stimulated and nonstimulated ferrogels. At 3 d, both stimulated and nonstimulated biphasic ferrogels remained largely acellular, and no fibrous capsule was seen surrounding the implants. At 2 wk, a fibrous capsule surrounding all biphasic ferrogel implants was observed (Fig. 2A). Nonstimulated biphasic ferrogels were surrounded with a capsule of ∼120 μm thickness, and stimulated biphasic ferrogels were surrounded with a significantly thinner capsule of ∼75 μm thickness (Fig. 2B).
Fig. 2.
Magnetic stimulation of ferrogel implants decreases fibrous capsule thickness. (A) Cross-sections of biphasic ferrogels stained with H&E at 3 d and 2 wk after implantation. Skin (X), fibrous capsule (*), and ferrogels (F) are indicated. It is important to note that significant fibrous capsule formation was not observed at 3 d in either ferrogel condition, and surrounding tissues were often lost during processing. (B) Quantified fibrous capsule thickness of nonstimulated and stimulated biphasic ferrogels after 2 wk of implantation. Fibrous capsule boundaries are marked with red dashed lines in A. (Scale bar, 500 μm.) Data were compared using a two-tailed unpaired Student's test with Welch's correction (n = 9; *P < 0.05). Error bars represent SDs.
Markers of Muscle Regeneration: Centrally Located Nuclei and Muscle Fiber Size.
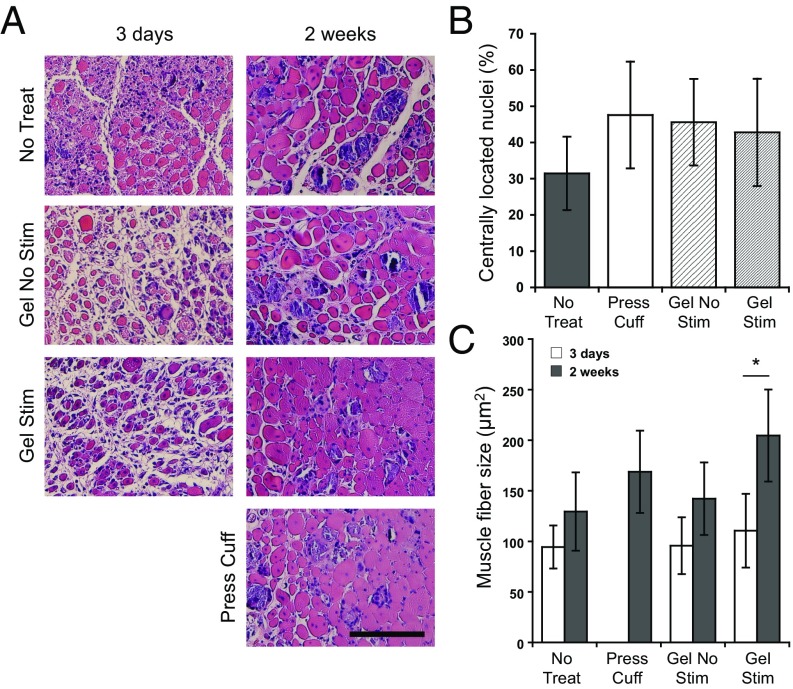
To determine the histological quality of muscle regeneration, several markers of muscle regeneration were examined. Areas of active muscle regeneration were present in all treatment conditions, as indicated by muscle fibers containing centrally located nuclei (33). Although no statistically significant differences were observed at 2 wk, greater than 40% of fibers contained centrally located nuclei in all conditions except the no-treatment control (30%) (Fig. 3 A and B). Mean muscle fiber size, as measured by cross-sectional area, remained fairly constant (∼100 μm2) in all tested conditions 3 d postinjury, as expected. However, at 2 wk, mean muscle fiber size was generally greater in muscles treated with stimulated biphasic ferrogels (205 μm2) compared with no-treatment controls (130 μm2) (Fig. 3 A and C). Interestingly, a significant increase in mean muscle fiber size from 3 d to 2 wk was only seen in muscles treated with stimulated biphasic ferrogels.
Fig. 3.
Ferrogel stimulation leads to improved muscle regeneration. (A) Histological cross-sections of tibialis anterior muscles stained with H&E 3 d and 2 wk after no treatment (No Treat), treatment with a pressure cuff (Press Cuff), treatment with a nonstimulated biphasic ferrogel (Gel No Stim), or treatment with a stimulated biphasic ferrogel (Gel Stim). (Scale bar, 100 μm.) (B) Quantification of myofibers residing in the defect containing centrally located nuclei 2 wk posttreatment. Values are expressed as a percentage of the total number of myofibers in the defect. (C) Quantified mean muscle fiber size in the defect area 3 d and 2 wk posttreatment. Data were compared using ANOVA with Bonferroni's post hoc test (n = 5; *P < 0.05). Error bars represent SDs.
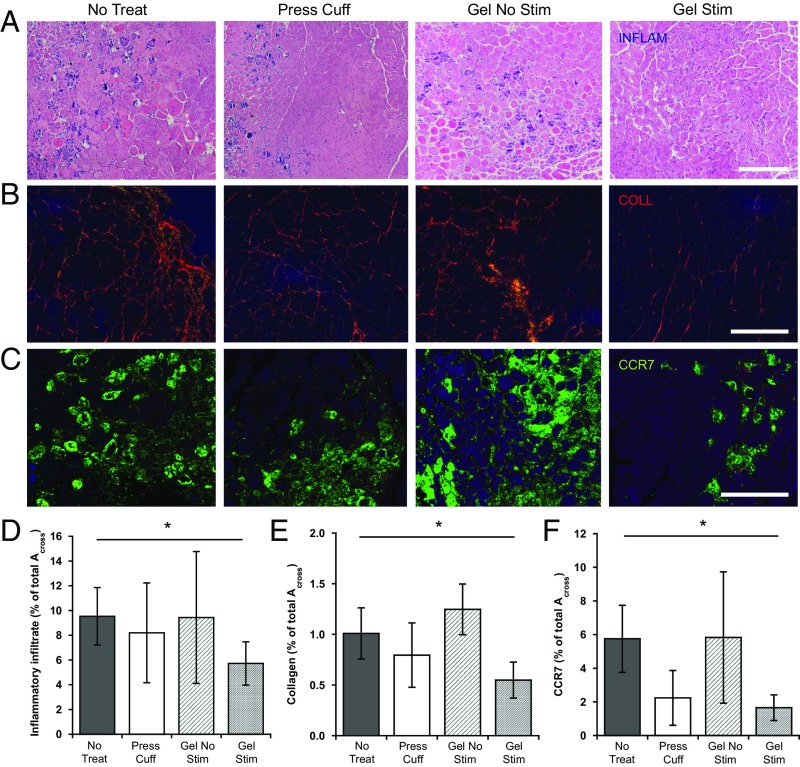
Inflammation: Interstitial Fibrosis, Inflammatory Infiltrate, and Macrophage Presence.
The influence of cyclic mechanical compressions on inflammation and fibrosis was next examined using histologic sections. The extent of muscle fibrosis was assessed by visualizing picrosirius red-stained collagen I and III under polarized light. Interestingly, at 2 wk, stimulated biphasic ferrogels showed significantly less interstitial fibrosis compared with no-treatment controls (Fig. 4 B and E). In contrast, all other treatment conditions remained statistically equivalent at this time. In addition, quantification of the inflammatory infiltrate followed a similar trend. Unlike all other conditions, a significantly lower number of muscle-infiltrating inflammatory cells were observed in stimulated biphasic ferrogel conditions compared with no-treatment controls (Fig. 4 A and D). Finally, stimulation of injured muscles by biphasic ferrogels and pressure cuff controls significantly reduced M1 macrophage infiltration, as measured by CCR7 staining (Fig. 4 C and F).
Fig. 4.
Ferrogel stimulation decreases inflammation and fibrosis. (A and D) Representative images and quantification of the inflammatory infiltrate (INFLAM) in histological cross-sections of tibialis anterior muscles stained with H&E 2 wk posttreatment. (B and E) Representative images and quantification of tissue collagen (COLL) from picrosirius red stained cross-sections 2 wk posttreatment. (C and F) Representative images and quantification of M1 macrophages from CCR7-stained cross-sections 2 wk posttreatment. All values are expressed as a percentage of the total cross-section area (Across) of the tissue section. (Scale bars, 200 μm.) Data were compared using ANOVA with Dunnett's post hoc test (n = 5–10; *P < 0.05). Error bars represent SDs.
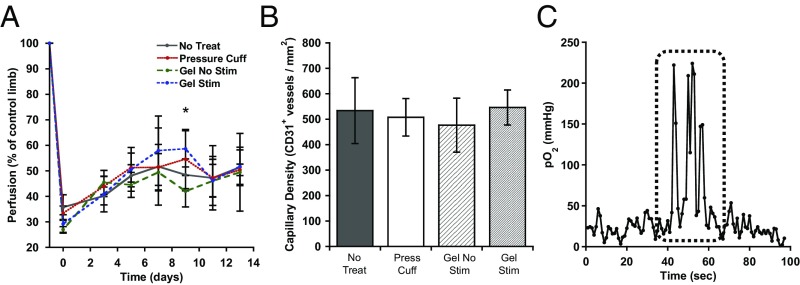
Angiogenesis, Hind Limb Perfusion, and Oxygen.
The ability of cyclic mechanical compressions to promote hind limb reperfusion and angiogenesis was examined next. Induction of hind limb ischemia led to a dramatic decrease in perfusion relative to the contralateral control limb, from 100% to ∼30% immediately after surgery in all treatment groups (Fig. 5A), as expected. At 3 d, perfusion increased to ∼40% in all conditions. A difference between the stimulated and nonstimulated biphasic ferrogel conditions appeared at day 9, but did not persist to day 14. Differences in the average capillary density in muscle sections between the various conditions, as measured by immunostaining for the endothelial cell marker CD31, were not observed (Fig. 5B). The oxygen concentration within the muscle remained at a fairly constant baseline level of ∼20 mm Hg before biphasic ferrogel stimulation (Fig. 5C). Upon stimulation, however, intramuscular oxygen concentration rapidly increased and remained elevated until stimulation ceased. Upon cessation of ferrogel stimulation, the oxygen concentration rapidly returned to the previous baseline levels.
Fig. 5.
Intramuscular oxygen concentration increases during ferrogel stimulation. (A) Quantified perfusion of injured hind limbs normalized to contralateral controls, as measured by Laser Doppler perfusion imaging. *A difference between the stimulated and nonstimulated biphasic ferrogel conditions appeared at day 9. (B) Quantified capillary density in injured muscle, as assessed by CD31+ staining 2 wk posttreatment. (C) Representative oxygen probe trace with stimulation period marked by a dashed line. Data were compared using ANOVA with Bonferroni's post hoc test (n = 5; *P < 0.05). Error bars represent SDs.
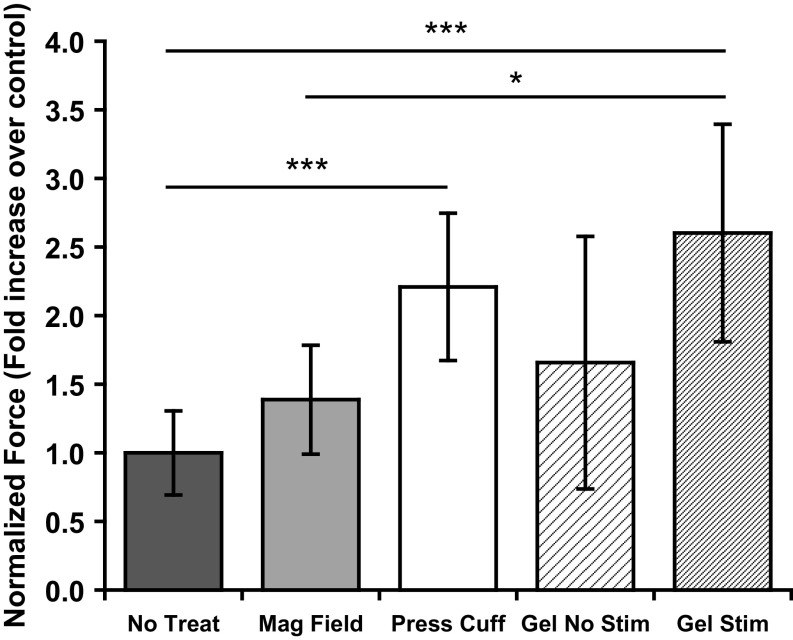
Muscle Function: Contraction Force.
To assess the functional quality of muscle regeneration, the contractile force of each injured muscle was measured. At 2 wk, injured muscles treated with stimulated biphasic ferrogels and pressure cuffs showed significant increases in specific peak tetanic force, 2.6- and 2.2-fold over no-treatment controls, respectively (Fig. 6). Stimulated biphasic ferrogels also showed a significant increase in specific peak tetanic force over the magnetic field-only control (1.9-fold). In contrast, no significant difference in specific peak tetanic force was observed between the no-treatment, magnetic field-only, and nonstimulated biphasic ferrogel conditions.
Fig. 6.
Cyclic mechanical compressions enhance functional muscle regeneration. Maximum contractile force after tetanic stimulation of injured muscles 2 wk after no treatment (No Treat), treatment with a magnetic field only (Mag Field), treatment with a pressure cuff (Press Cuff), treatment with a nonstimulated biphasic ferrogel (Gel No Stim), or treatment with a stimulated biphasic ferrogel (Gel Stim). Force measurements were normalized to muscle wet weight. Data were compared using ANOVA with Bonferroni’s post hoc test (n = 5–10; *P < 0.05; ***P < 0.001).
Discussion
Actuation of biologic-free ferrogels resulted in mechanical compressions that affected the host inflammatory response toward the gel and led to a reduction in fibrous capsule thickness after 2 wk of implantation. Strikingly, mechanical stimulation led to a significant reduction in fibrosis and inflammation of the injured muscle, demonstrating a potential immunomodulatory role for ferrogel-driven cyclic compressions. As assessed histologically, severe muscle injury resulting from myotoxin injection and hind limb ischemia led to subsequent active muscle regeneration that was enhanced by stimulated biphasic ferrogels. In addition, mechanical stimulation led to a temporary increase in oxygen concentration at the site of injury. Biologic-free ferrogel and pressure cuff-driven mechanical compressions led to enhanced muscle regeneration and muscle function compared with no-treatment controls, demonstrating the therapeutic potential of these mechanical interventions.
Actuation of both the biphasic ferrogel and the pressure cuff results in uniform cyclic compressions of the severely injured muscle tissue. The pressure cuff was roughly tuned so that the peak pressure values achieved by each system were comparable. However, the peak pressure value achieved by the pressure cuff remained slightly larger than that achieved by the biphasic ferrogel because of limitations associated with control of the pressure cuff air valves. The kinetics of the pressure profile also varied, as the rate at which the pressure cuff air valves opened and shut was not finely controlled with this system. Nevertheless, both systems were able to apply a similar force (normalized to tibialis anterior wet weight) of ∼2 N/g, a value similar to that used previously for massage-like compressive loading of rabbit tibialis anterior muscles injured with eccentric exercise (34). Although stimulation parameters were chosen to yield mechanical compressions that approximate those achieved with massage, future studies are needed to optimize the frequency, amplitude, and duration of the stimulations.
Biphasic ferrogel stimulation leads to a reduction in fibrous capsule thickness and inflammatory cells present in the surrounding muscle after 2 wk of implantation, and this may relate to expulsion of inflammatory cells as a result of cyclic compression of the ferrogels. A key event leading to fibrous capsule formation is the adhesion of profibrotic macrophages, foreign body giant cells, and fibroblasts to the implant surface, as these cells secrete proteins that modulate fibrosis and increase collagen deposition (35, 36). Previous in vitro experiments have demonstrated significant fibroblast and myoblast cell expulsion from RGD peptide-containing ferrogels in vitro, even with stimulation patterns shorter and less frequent than used here (30, 31). It is possible that invading cells near the scaffold edges were expelled from the ferrogel system upon stimulation, because of fluid convection resulting from large gel deformations, leading to an overall diminished cell presence within the scaffold. The decrease in M1 macrophage presence with ferrogel stimulation further suggests a potent immunomodulatory role for cyclic mechanical compressions. These results are perhaps not surprising, as a beneficial role for massage-like compressive loading after exercise-induced muscle damage has recently been demonstrated (34, 37). In addition, massage and massage mimics have been previously hypothesized to moderate the inflammatory response (27, 34, 37). The beneficial effects of massage-like loading were found to be dose-dependent and more potent when administered immediately after injury (34, 38). In addition, a role for massage therapy as a potent immunomodulator after exercise-induced muscle damage in humans has been reported (27). A similar reduction in fibrosis and inflammation was demonstrated with pressure cuff controls, although the effect was not always significant. Taken together, these studies provide evidence that ferrogel-driven cyclic compressions may be useful to alleviate inflammation in certain muscle injuries. Further, this ability to inhibit fibrous capsule formation with cyclic compressions has potential utility for implantable drug delivery devices and sensors that require unobstructed diffusion around the implant for proper function.
Severe muscle injury resulting from myotoxin injection and hind limb ischemia leads to active muscle regeneration that can be enhanced with stimulated biphasic ferrogels. Although centrally located nuclei were observed in all conditions, significant increases in the mean muscle fiber size of regenerating fibers over time were only observed in muscles treated with stimulated biphasic ferrogels. Past reports suggest increased mean fiber diameter is indicative of tissues that have progressed further in the regenerative process (13, 39). Although pressure cuff-treated muscles were not harvested at 3 d, mean muscle fiber size values remained remarkably consistent among all treatment groups at this time. At 2 wk, pressure cuff controls exhibited a smaller mean fiber size than stimulated ferrogels, suggesting ferrogel-driven cyclic mechanical compressions provide an additional, likely convection-based benefit. Specifically, enhanced fluid transportation around the implant site may accelerate immune cell and/or metabolic waste product removal. Interestingly, ferrogel-driven mechanical stimulation produces a therapeutic effect on muscle fiber size of the same order of magnitude as that achieved by previous approaches that delivered cells and drugs to severely injured muscle (13, 39).
Cyclic mechanical compressions do not lead to an enhancement in hind limb reperfusion and angiogenesis, but may instead lead to temporary increases in convection through the tissue or blood flow to the injured limb. This is perhaps not surprising, as ferrogels in this study did not deliver any proangiogenic growth factors or cells, such as VEGF and endothelial progenitor cells, that specifically target and support new blood vessel growth (12, 13, 32, 39, 40). Although small differences in perfusion were present 9 d postinjury, they did not persist to the end of the study, suggesting that growth factor or cell support may be required to maintain any increases in perfusion that appear as a result of cyclic mechanical compressions alone. However, longer-duration studies would be useful to confirm that no differences in angiogenesis or perfusion result from the ferrogel treatment. Strikingly, although steady-state hind limb perfusion and capillary density were not significantly affected, oxygen probe experiments demonstrated an increase in oxygen concentration during the time in which biphasic ferrogel-driven cyclic mechanical compressions were being generated in the muscle. Massage has previously been shown to temporarily increase intramuscular temperature, which may lead to enhanced nutrient transport throughout the injured tissue (41), and a similar phenomenon could have occurred here. More likely, enhanced intramuscular convection driven by tissue compressions may have led to increased oxygen levels and expedited removal of metabolic byproducts that inhibit regeneration. Alternatively, cyclic compressions may increase oxygen concentration by locally and temporarily increasing blood flow to the injured muscle. A parallel to this situation may occur in massage therapy, as controlled clinical trials aimed at identifying a physiological mechanism for massage have often implicated, although not proven, transient increases in blood flow (29, 42, 43).
Strikingly, biphasic ferrogel and pressure cuff-driven cyclic mechanical compressions of injured muscle led to significant functional muscle regeneration. After 2 wk of treatment, biphasic ferrogel and pressure cuff treatment conditions showed 2.6- and 2.2-fold increases, respectively, in peak tetanic force over no-treatment conditions. Previous studies using mouse models of severe muscle injury report comparable enhancements of muscle contractile force at 2 wk after delivery of proregenerative growth factors and/or myogenic cells to the injured muscle. In these studies, scaffold delivery of VEGF and IGF resulted in a 2.4-fold increase in peak tetanic force over blank scaffolds, whereas scaffold delivery of VEGF, IGF, and myoblasts resulted in 1.5–6.0-fold increases in peak tetanic force over control conditions (13, 32, 39). Although additional biomaterial-based approaches have induced similar improvements in muscle function through the codelivery of myogenic bioagents with endothelial cells to enhance vascularization (5.5-fold increase in active stress over blank scaffold) or neural stem cells to promote innervation of muscle constructs (2.0-fold increase in maximum tetanic force over nerve-deficient controls) (44–47), no bioagents were delivered from the scaffolds in this study. It is possible that the magnetic field directly affected regeneration in the current study, as past studies involving treatment of myoblasts and myotubes with static magnetic fields have demonstrated increased myogenic differentiation and cell alignment (48, 49). However, although a slight increase in peak tetanic force was observed with magnetic field application in the current study, results were not significantly different from the untreated controls, perhaps because of the relatively short duration of magnetic field exposure. Alternatively, cyclic mechanical compressions have been shown to improve muscle regeneration after exercise-induced muscle injury in rabbits and humans (27, 34, 37). Specifically, after 4 d of massage-like compressive loading in rabbits, 3.0–4.1-fold increases in peak torque recovery over untreated controls were observed. However, the current study is the first report, to our knowledge, of functional muscle regeneration resulting from cyclic mechanical compressions in a severe model of muscle damage.
Strikingly, the results of these studies indicate a ferrogel scaffold can be used to mechanically stimulate and regenerate severely injured muscle tissue without the use of growth factors or cells. The demonstration of functional muscle regeneration with a biologic-free material system may offer a simple yet effective alternative to cell-based therapies when treating certain types of muscle injuries. Further, incorporation of cyclic mechanical compressions into existing drug and cell delivery systems could potentially lead to new combinatorial therapies that generate enhanced regenerative outcomes. Although this study focuses on the repair of skeletal muscle, bioagent-free ferrogels and the concept of mechanically driven regeneration are expected to find broad utility and can likely be applied to other tissues and diseases.
Materials and Methods
Materials.
Medical-grade, high-molecular weight (∼250 kDa) sodium alginate with high guluronate content (Protanal LF 20/40) was purchased from FMC Biopolymers. Alginates were used after covalent RGD peptide modification and dialysis purification, as previously described (50). All other chemicals including adipic acid dihydrazide, 1-ethyl-3-(dimethylaminopropyl) carbodiimide, 2-(N-morpholino)ethanesulfonic acid (MES), 1-hydroxybenzotriazole (HOBt), and Iron(II,III) oxide powder (<5 μm, 310069) were purchased from Sigma-Aldrich.
Animals and Surgical Procedures.
All animal work was performed in compliance with NIH and institutional guidelines. For myotoxin injuries, tibialis anterior muscles of 6-wk-old female wild-type C57BL/6J mice were injected with 10 μL of 10 μg/mL Notexin Np myotoxin. Six days after notexin injection, hind limb ischemia was induced by unilateral external iliac and femoral artery and vein ligation, as described in SI Materials and Methods (12, 51).
Ischemia and Perfusion Analysis.
Blood perfusion measurements of the ischemic and normal limb were performed on anesthetized animals, using a Laser Doppler Perfusion Imaging analyzer, as described in SI Materials and Methods. Perfusion was calculated as the ratio of ischemic to nonischemic limb perfusion for each animal.
Ferrogel Scaffold and Pressure Cuff Stimulation.
Biphasic ferrogel scaffolds (7 wt% iron oxide) were fabricated with alginate covalently modified with RGD peptide, as previously described (30). Scaffolds were placed s.c. on the tibialis anterior muscle and stimulated for 5 min at 1 Hz every 12 h by approaching and retracting a permanent magnet with a surface field of 6,510 Gauss. Similarly, the balloon pressure cuff was programmed to inflate and deflate for 5 min at 1 Hz every 12 h, as described in SI Materials and Methods.
Histologic Assessment of Skeletal Muscle.
Mice were euthanized and hind limb muscle tissues were processed for histologic analyses. Tissue sections were stained with H&E, CCR7, CD31, and picrosirius red for quantification of regenerative markers and the inflammatory infiltrate, as described in SI Materials and Methods.
Muscle Function Testing.
Intact tibialis anterior muscles were dissected and mounted vertically midway between two cylindrical parallel steel wire electrodes. Muscles were attached by their tendons to microclips connected to a force transducer and bathed in a physiologic saline solution with continuously bubbled oxygen at 37 °C. Contractions were evoked every 5 min, as described in SI Materials and Methods. Specific peak tetanic force was determined as the difference between the maximum force during contraction and the baseline level normalized to muscle wet weight.
Oxygen Probe.
An OxyLab pO2 instrument and implantable optical probe were used to measure rapid temporal changes in intramuscular dissolved oxygen and temperature during biphasic ferrogel stimulation, as described in SI Materials and Methods. Oxygen and temperature readings were recorded continuously before, during, and after biphasic ferrogel stimulation at 1-s intervals.
Statistical Analyses.
All statistical comparisons were performed using ANOVA with Bonferroni’s or Dunnett’s post hoc test and a two-tailed unpaired Student’s t test with Welch’s correction and analyzed using INSTAT 3.1a (GraphPad Software, Inc.) software. Differences between conditions were considered significant if P < 0.05.
SI Materials and Methods
Animals and Surgical Procedures.
All animal work was performed in compliance with NIH and institutional guidelines. Six-week-old female wild-type C57BL/6J mice (Jackson Laboratories) were anesthetized with an i.p. injection of ketamine (80 mg⋅kg−1) and xylazine (5 mg⋅kg−1). For myotoxin injuries, the tibialis anterior muscles of the right legs of anesthetized mice were injected with 10 μL of 10 μg/mL Notexin Np myotoxin from Notechis Scutatus snake venom (Latoxan), using a 25-μL Hamilton syringe. Six days after notexin injection, hind limb ischemia was induced by unilateral external iliac and femoral artery and vein ligation, as previously described (12, 51). After vessel ligation, a hydrated ferrogel scaffold was placed s.c. on the tibialis anterior muscle in certain conditions, and the incision was surgically closed.
Ischemia and Perfusion Analysis.
Blood perfusion measurements of the ischemic and normal limb were performed on anesthetized animals (n = 10), using a Laser Doppler Perfusion Imaging analyzer (PeriScan PIM II; Perimed Instruments). Entire hind limbs were scanned under basal conditions and then every other day after surgery. Perfusion was calculated as the ratio of ischemic to nonischemic limb perfusion for each animal.
Ferrogel Scaffold Stimulation.
Biphasic ferrogels (7 wt% iron oxide) were fabricated with alginate covalently modified with RGD peptide (DS10, 10 RGD peptides per alginate chain), as previously described (30). Although nondegradable alginate was used to simplify the system, oxidized alginate can be used in the future to create ferrogels with controlled degradation (52). In this case, optimization of iron oxide nanoparticle size, shape, and surface charge may be necessary to ensure particle absorption by the lymphatic capillary system and clearance from the body (53). Before implantation, biphasic ferrogel scaffolds were hydrated with 100 μL PBS. Biphasic ferrogel scaffolds were then placed s.c. on the tibialis anterior muscle and stimulated for 5 min at 1 Hz every 12 h by approaching and retracting a permanent magnet with a surface field of 6,510 Gauss (K&J Magnetics, DX0Z0). The ferrogel implant and magnet were orientated so that magnetic stimulation resulted in compression of the ferrogel against the injured muscle. After retrieval at 3 d and 2 wk, scaffolds were fixed in 10% (vol/vol) neutral-buffered formalin overnight. Ferrogels were then paraffin embedded, sectioned at 7 μm thickness, and stained with H&E at the Harvard Rodent Histopathology Core. For pressure profile generation, an Instron 3342 single column apparatus (10 N load cell) configured for tensile testing was used. A custom adapter that allowed for stimulation of the ferrogel far from the load cell was attached to the top tensile grip, and the ferrogel was cyclically stimulated with a permanent magnet.
Pressure Cuff Stimulation.
For mechanical stimulation using the balloon pressure cuff, a spherical low durometer urethane balloon (10–15 mm diameter 10000000FA, Vention Medical) was attached to flexible silicone tubing (1.78 mm outer diameter, 1.1 mm inner diameter; McMaster Carr) and bonded at each neck with ultraviolet cure adhesive (Loctite 3943; Henkel). The output from two solenoid valves (TE miniature switching solenoid valves, sensor technics) was joined and connected to the balloon. Valve inlets were connected to a pneumatic air pump (pneumatic pump M0019057; Parker Electronics) at the pressure and vacuum outlets. The valves were wired to two-channel relay board with two SRD-05VDC-SL-C relays that were controlled by an Arduino Uno microcontroller developer board. The microcontroller was programmed to sequentially open the valves for 500 ms every second so that the balloon would be pressurized for 500 ms and then evacuated for 500 ms at a 1-Hz frequency. The balloon was placed inside a polycarbonate cylindrical cuff made from a 20-mL syringe body trimmed to length with the plunger removed (20-mm outer diameter, 40-mm length, adjustable wall thickness from 2 to 4 mm, using polycarbonate cylindrical liners). For pressure cuff stimulation, the mouse leg was placed in the cuff and the balloon was fixed directly above the tibialis anterior muscle outside the skin. The balloon was then inflated and deflated for 5 min at 1 Hz every 12 h. For pressure profile generation, an Instron 3342 single column apparatus (10 N load cell) configured for compression testing was used. The balloon was removed from the cylindrical cuff and cyclically inflated and deflated between two stationary compression platens separated by a distance equal to the diameter of the deflated balloon.
Histologic Assessment of Skeletal Muscle.
Mice were euthanized and hind limb muscle tissues (n = 10) were processed for histologic analyses. Tibialis anterior muscles were fixed in 10% (vol/vol) neutral-buffered formalin overnight, paraffin embedded, and sectioned at 7 μm thickness. Sections stained with H&E were used for quantification of mean fiber diameter, inflammatory infiltrate, and fibers with centrally located nuclei. All histologic analyses performed on H&E-stained sections were performed in a blinded fashion. M1 macrophages were identified with immunostaining for mouse CCR7 (Abcam ab32527;Invitrogen A-11035). Vascular endothelial cells were identified by immunostaining for mouse CD31 (Abcam ab28364; Invitrogen A-11035). Interstitial fibrosis was assessed in picrosirius-stained sections imaged with polarized light. Quantification of CCR7 and CD31 immunostaining was performed using ImageJ.
Muscle Function Testing.
Intact tibialis anterior muscles were dissected (n = 5/condition), mounted vertically midway between two cylindrical parallel steel wire electrodes (1.6 mm diameter, 21 mm long) attached by their tendons to microclips connected to a force transducer (FORT 25, WPII), and bathed in a physiologic saline solution in a chamber with continuously bubbled oxygen at 37 °C. Muscle length was adjusted to a physiological relevant length. A wave pulse was initiated using a custom-written LabVIEW program and delivered to the stimulation electrodes via a purpose-built power amplifier (QSC USA 1310). Contractions were evoked every 5 min. Tetani was evoked at 250–300 Hz and 25–30 V, with a constant pulse width of 2 ms and a train duration of 1 s. Peak tetanic force was determined as the difference between the maximum force during contraction and the baseline level. Forces were then normalized to muscle wet weight.
Oxygen Probe.
An OxyLab pO2 instrument and implantable optical probe (Oxford Optronix) were used to measure rapid temporal changes in intramuscular dissolved oxygen and temperature during biphasic ferrogel stimulation. Briefly, the probe was inserted using a 16-g needle catheter, and the tip was carefully placed in the midbelly region of the tibialis anterior muscle. A biphasic ferrogel was then implanted s.c. above the muscle containing the probe, as before (30), and the incision was closed around the probe. Oxygen and temperature readings were recorded continuously before, during, and after biphasic ferrogel stimulation at 1-s intervals.
Acknowledgments
The authors would like to thank Dr. Roderick Bronson of the Harvard Rodent Histopathology Core for his pathological assessment of histology sections. This work was supported by the National Institutes of Health (R01 DE013349), National Science Foundation Graduate Student Fellowship (DMR-0820484 to C.A.C.), and the Materials Research Science and Engineering Center at Harvard University. E.T.R. acknowledges a Fulbright International Science and Technology Award and the Wyss Institute Director’s Challenge Cross-Platform Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517517113/-/DCSupplemental.
References
- 1.Juhas M, Bursac N. Engineering skeletal muscle repair. Curr Opin Biotechnol. 2013;24(5):880–886. doi: 10.1016/j.copbio.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347(3):759–774. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- 3.Järvinen TAHJ, Järvinen TLN, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: Biology and treatment. Am J Sports Med. 2005;33(5):745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 4.Rossi CA, Pozzobon M, De Coppi P. Advances in musculoskeletal tissue engineering: Moving towards therapy. Organogenesis. 2010;6(3):167–172. doi: 10.4161/org.6.3.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma CH, et al. Reconstruction of upper extremity large soft-tissue defects using pedicled latissimus dorsi muscle flaps--technique illustration and clinical outcomes. Injury. 2008;39(Suppl 4):67–74. doi: 10.1016/j.injury.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Tu YK, et al. Soft-tissue injury management and flap reconstruction for mangled lower extremities. Injury. 2008;39(Suppl 4):75–95. doi: 10.1016/j.injury.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Tedesco FS, Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol. 2012;25(5):597–603. doi: 10.1097/WCO.0b013e328357f288. [DOI] [PubMed] [Google Scholar]
- 8.Palmieri B, Tremblay JP, Daniele L. Past, present and future of myoblast transplantation in the treatment of Duchenne muscular dystrophy. Pediatr Transplant. 2010;14(7):813–819. doi: 10.1111/j.1399-3046.2010.01377.x. [DOI] [PubMed] [Google Scholar]
- 9.Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 2010;224(1):7–16. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]
- 10.Shansky J, Creswick B, Lee P, Wang X, Vandenburgh H. Paracrine release of insulin-like growth factor 1 from a bioengineered tissue stimulates skeletal muscle growth in vitro. Tissue Eng. 2006;12(7):1833–1841. doi: 10.1089/ten.2006.12.1833. [DOI] [PubMed] [Google Scholar]
- 11.Pelosi L, et al. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 2007;21(7):1393–1402. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- 12.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5(3):590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 13.Borselli C, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA. 2010;107(8):3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheehan SM, Allen RE. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J Cell Physiol. 1999;181(3):499–506. doi: 10.1002/(SICI)1097-4652(199912)181:3<499::AID-JCP14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Hammers DW, et al. Controlled release of IGF-I from a biodegradable matrix improves functional recovery of skeletal muscle from ischemia/reperfusion. Biotechnol Bioeng. 2012;109(4):1051–1059. doi: 10.1002/bit.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cezar CA, Mooney DJ. Biomaterial-based delivery for skeletal muscle repair. Adv Drug Deliv Rev. 2015;84:188–197. doi: 10.1016/j.addr.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornberger TA, Armstrong DD, Koh TJ, Burkholder TJ, Esser KA. Intracellular signaling specificity in response to uniaxial vs. multiaxial stretch: Implications for mechanotransduction. Am J Physiol Cell Physiol. 2005;288(1):C185–C194. doi: 10.1152/ajpcell.00207.2004. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Chaudhry I, Reid MB, Boriek AM. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J Biol Chem. 2002;277(48):46493–46503. doi: 10.1074/jbc.M203654200. [DOI] [PubMed] [Google Scholar]
- 19.Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res. 2001;267(1):107–114. doi: 10.1006/excr.2001.5252. [DOI] [PubMed] [Google Scholar]
- 20.Peterson JM, Pizza FX. Cytokines derived from cultured skeletal muscle cells after mechanical strain promote neutrophil chemotaxis in vitro. J Appl Physiol (1985) 2009;106(1):130–137. doi: 10.1152/japplphysiol.90584.2008. [DOI] [PubMed] [Google Scholar]
- 21.Chambers MA, Moylan JS, Smith JD, Goodyear LJ, Reid MB. Stretch-stimulated glucose uptake in skeletal muscle is mediated by reactive oxygen species and p38 MAP-kinase. J Physiol. 2009;587(Pt 13):3363–3373. doi: 10.1113/jphysiol.2008.165639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandenburgh H, Kaufman S. In vitro model for stretch-induced hypertrophy of skeletal muscle. Science. 1979;203(4377):265–268. doi: 10.1126/science.569901. [DOI] [PubMed] [Google Scholar]
- 23.Vandenburgh HH, Karlisch P. Longitudinal growth of skeletal myotubes in vitro in a new horizontal mechanical cell stimulator. In Vitro Cell Dev Biol. 1989;25(7):607–616. doi: 10.1007/BF02623630. [DOI] [PubMed] [Google Scholar]
- 24.Moon G, Christ G, Stitzel JD, Atala A, Yoo JJ. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng Part A. 2008;14(4):473–482. doi: 10.1089/tea.2007.0104. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T, et al. Three-dimensional cell and tissue patterning in a strained fibrin gel system. PLoS One. 2007;2(11):e1211. doi: 10.1371/journal.pone.0001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell CA, Smiley BL, Mills J, Vandenburgh HH. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol. 2002;283(5):C1557–C1565. doi: 10.1152/ajpcell.00595.2001. [DOI] [PubMed] [Google Scholar]
- 27.Crane JD, et al. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Transl Med. 2012;4(119):119ra13. doi: 10.1126/scitranslmed.3002882. [DOI] [PubMed] [Google Scholar]
- 28.Cherkin DC, Sherman KJ, Deyo RA, Shekelle PG. A review of the evidence for the effectiveness, safety, and cost of acupuncture, massage therapy, and spinal manipulation for back pain. Ann Intern Med. 2003;138(11):898–906. doi: 10.7326/0003-4819-138-11-200306030-00011. [DOI] [PubMed] [Google Scholar]
- 29.Weerapong P, Hume PA, Kolt GS. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005;35(3):235–256. doi: 10.2165/00007256-200535030-00004. [DOI] [PubMed] [Google Scholar]
- 30.Cezar CA, et al. Biphasic ferrogels for triggered drug and cell delivery. Adv Healthc Mater. 2014;3(11):1869–1876. doi: 10.1002/adhm.201400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, et al. Active scaffolds for on-demand drug and cell delivery. Proc Natl Acad Sci USA. 2011;108(1):67–72. doi: 10.1073/pnas.1007862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32(34):8905–8914. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawke TJ, Garry DJ. Myogenic satellite cells: Physiology to molecular biology. J Appl Physiol (1985) 2001;91(2):534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 34.Haas C, et al. Dose-dependency of massage-like compressive loading on recovery of active muscle properties following eccentric exercise: Rabbit study with clinical relevance. Br J Sports Med. 2013;47(2):83–88. doi: 10.1136/bjsports-2012-091211. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32(28):6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 37.Butterfield TA, Zhao Y, Agarwal S, Haq F, Best TM. Cyclic compressive loading facilitates recovery after eccentric exercise. Med Sci Sports Exerc. 2008;40(7):1289–1296. doi: 10.1249/MSS.0b013e31816c4e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas C, et al. Massage timing affects postexercise muscle recovery and inflammation in a rabbit model. Med Sci Sports Exerc. 2013;45(6):1105–1112. doi: 10.1249/MSS.0b013e31827fdf18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, et al. Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold. Mol Ther. 2014;22(8):1441–1449. doi: 10.1038/mt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shvartsman D, et al. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol Ther. 2014;22(7):1243–1253. doi: 10.1038/mt.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drust B, Atkinson G, Gregson W, French D, Binningsley D. The effects of massage on intra muscular temperature in the vastus lateralis in humans. Int J Sports Med. 2003;24(6):395–399. doi: 10.1055/s-2003-41182. [DOI] [PubMed] [Google Scholar]
- 42.Hovind H, Nielsen SL. Effect of massage on blood flow in skeletal muscle. Scand J Rehabil Med. 1974;6(2):74–77. [PubMed] [Google Scholar]
- 43.Hansen TI, Kristensen JH. Effect of massage, shortwave diathermy and ultrasound upon 133Xe disappearance rate from muscle and subcutaneous tissue in the human calf. Scand J Rehabil Med. 1973;5(4):179–182. [PubMed] [Google Scholar]
- 44.Koffler J, et al. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci USA. 2011;108(36):14789–14794. doi: 10.1073/pnas.1017825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shandalov Y, et al. An engineered muscle flap for reconstruction of large soft tissue defects. Proc Natl Acad Sci USA. 2014;111(16):6010–6015. doi: 10.1073/pnas.1402679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morimoto Y, Kato-Negishi M, Onoe H, Takeuchi S. Three-dimensional neuron-muscle constructs with neuromuscular junctions. Biomaterials. 2013;34(37):9413–9419. doi: 10.1016/j.biomaterials.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 47.Larkin LM, Van der Meulen JH, Dennis RG, Kennedy JB. Functional evaluation of nerve-skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim. 2006;42(3-4):75–82. doi: 10.1290/0509064.1. [DOI] [PubMed] [Google Scholar]
- 48.Coletti D, et al. Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment. Cytometry A. 2007;71(10):846–856. doi: 10.1002/cyto.a.20447. [DOI] [PubMed] [Google Scholar]
- 49.Sakurai T, Hashimoto A, Kiyokawa T, Kikuchi K, Miyakoshi J. Myotube orientation using strong static magnetic fields. Bioelectromagnetics. 2012;33(5):421–427. doi: 10.1002/bem.21701. [DOI] [PubMed] [Google Scholar]
- 50.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 51.Chen RR, et al. Integrated approach to designing growth factor delivery systems. FASEB J. 2007;21(14):3896–3903. doi: 10.1096/fj.06-7873com. [DOI] [PubMed] [Google Scholar]
- 52.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26(15):2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 53.Reddy LH, Arias JL, Nicolas J, Couvreur P. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev. 2012;112(11):5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]