Abstract
Purpose
An impaired metaboreflex is associated with abnormal ventilatory and peripheral vascular function in heart failure (HF), whereas its influence on cardiac function or pulmonary vascular pressure remain unclear. We aimed to assess whether metabolite-sensitive neural feedback (metaboreflex) from locomotor muscles via post-exercise regional circulatory occlusion (RCO) attenuates pulmonary vascular capacitance (GXCAP) and/or circulatory power (CircP) in HF patients.
Methods
Eleven HF patients (NYHA class: I/II; ages, 51±15; ejection fraction: 32±9%) and 11 age and gender matched controls (ages, 43±9) completed three cycling sessions (four-minutes, 60% peak oxygen uptake [VO2]). Session one: control trial=normal recovery (NR). Sessions two or three: bilateral upper-thigh pressure tourniquets inflated suprasystolic at end-exercise (RCO) for 2-minute recovery with or without inspired CO2 (RCO+CO2) (randomized). Mean arterial pressure (MAP), heart rate, and VO2 were continuously measured. Estimates of central hemodynamics; CircP=(VO2×MAP)/weight, oxygen pulse index (O2pulseI=(VO2/heart rate)/body surface area), and GXCAP=O2pulseI×end-tidal partial pressure CO2 were calculated.
Results
At rest and end-exercise, CircP and GXCAP were lower in HF versus controls (P<0.05), with no differences between transients (P>0.05). At 2-minute recovery, GXCAP was lower during RCO versus NR in both groups (72±23 versus 98±20 and 73±34 versus 114±35 mL·beat−1·mm Hg·m−2, respectively; P<0.05); whereas, CircP did not differ between transients (P>0.05). Differences (% and Δ) between baseline and 2-minute recovery amongst transients suggest the metaboreflex attenuates GXCAP in HF. Differences (% and Δ) between baseline and 2-min recovery amongst transients suggest the metaboreflex may attenuate CircP in controls.
Conclusion
The present observations suggest locomotor muscle metaboreflex activation may influence CircP in controls but not in HF. However, metaboreflex activation may evoke decreases in GXCAP (increased pulmonary vascular pressures) in HF and controls.
Keywords: cardiac power, ergoreceptor, metaboreceptor, mechanoreceptor, circulatory power
Introduction
Secondary pulmonary hypertension (PH) in patients with chronic heart failure (HF) is a major complicating factor that increases mortality risk and decreases exercise capacity in this population (1, 3, 4). Although the pathophysiology of secondary PH in HF remains incompletely understood, it is likely that augmented pulmonary vascular pressures first occur because of passive downstream increases in left heart pressures due to ventricular dysfunction (3, 4, 23), which later transgresses to a mixed form of PH related to vascular remodeling resulting in persistently elevated pulmonary vascular resistance (3, 4, 23). Exercise further exacerbates elevations in pulmonary vascular pressures in HF patients with secondary PH (4, 36). Therefore, because an ample body of evidence suggests that exercise measurements of both cardiac and pulmonary system function are clear markers of syndrome severity and prognosis in HF (4, 10, 18-20), it is necessary to study potential pathophysiological mechanisms contributing to changes in pulmonary pressures in HF during a paradigm that includes rest and exercise testing.
Growing evidence in support of the “muscle hypothesis” suggests that feedback from group III/IV skeletal muscle nerve fibers contributes to altered ventilatory and peripheral arterial pressure control during exercise in HF (16, 26-28). Post-exercise regional circulatory occlusion (RCO) is a valid non-invasive technique used to selectively study the influence of group IV neural feedback from skeletal muscle (i.e., metaboreflex) on cardiovascular responses in humans (32). Activation of the metaboreflex influences sympathetically mediated vasoconstriction and augmented ventilation that leads to ventilatory inefficiency in HF (16, 27). In contrast, the metaboreflex has a favorable influence on peripheral vascular conductance and both ventilatory and cardiac function during exercise in healthy individuals (2, 6-9). Similarly, others also demonstrate that the metaboreflex may influence increased pulmonary arterial pressure during and following exercise in healthy individuals (21, 38), however this has not been examined in HF patients.
Consistent with peripheral pressor responses to metaboreflex activation in healthy individuals (2, 6-9), the metaboreflex evokes increases in cardiac function (e.g., heart rate [HR], stroke volume [SV], and cardiac output [Q]) during and immediately following submaximal exercise in healthy canines (12, 30, 33). In this context, experimental testing in healthy canine models suggest that a substantial contribution to metaboreflex-mediated increases in arterial pressure are largely driven by increases in cardiac hemodynamics as opposed to marked vasomotor adjustments (12, 30, 33). In contrast, because of low cardiac reserve in HF, the metaboreflex may influence increases in arterial pressure as a result of robust increases in peripheral vasomotor tone (11, 13, 24, 29), whereby exacerbating afterload in these patients.
The pressure flow product, cardiac power, is a strong direct index of cardiac function and is related to prognosis in HF (19, 35). Circulatory power (CircP) is a robust non-invasive surrogate for cardiac power that is also indicative of prognosis in HF (5, 39). We have also recently shown that oxygen pulse (O2pulse) during exercise strongly correlates with invasive measurements of SV in HF patients with or without secondary PH (36). Equally important, end-tidal partial pressure of CO2 (PETCO2) is related to changes in pulmonary vascular pressures during exercise in HF (36). Therefore, it is noteworthy that the product of O2pulse and PETCO2 (i.e., GXCAP) directly relates with invasive determination of pulmonary vascular capacitance [PVCAP=stroke volume/mean pulmonary arterial pressure (mPAP) (36)] in HF patients, which shows strong prognostic power in PH patients (22).
As such, because of the clinical implications of secondary PH in HF, the afterload sensitivity of systolic HF patients, and the associations between metaboreflex activation and increased peripheral arterial pressure in this population (16); the aim of this study was to examine the influence of the locomotor muscle metaboreflex, via post-exercise RCO, on CircP and GXCAP in HF. We hypothesized that isolation of the locomotor muscle metaboreflex during post-exercise recovery would contribute to the attenuation of both CircP and GXCAP in HF patients.
Methods
Subjects
Eleven HF patients were recruited through the Mayo Clinic Heart Failure Service and the Cardiovascular Health Clinic. Eleven healthy control participants were recruited through advertisement in the surrounding community with attempts to match the HF group for age and gender (demographics, Table 1). Inclusion criteria for HF included diagnosis of ischemic or dilated cardiomyopathy with duration of HF symptoms >one-year; stable HF symptoms (>three-months); left ventricular (LV) ejection fraction percentage ≤35% (from clinical records within three months); body mass index (BMI) <35.0 kg/m2 (at enrolment); and current non-smokers with a past smoking history <15 pack-years (at enrolment). All HF patients were on standard pharmacological therapy at the time of the study. Control participants had normal cardiac function without evidence of exercise-induced ischemia and were without history of hypertension, lung disease, or coronary artery disease. The Mayo Clinic Institutional Review Board approved all experimental procedures. Prior to study, all participants provided written informed consent, and all aspects of the study were performed in accordance with the ethical standards of the Declaration of Helsinki.
Table 1.
Participant Characteristics
| Healthy Control | Heart Failure | P | |
|---|---|---|---|
|
|
|||
| Demographics | |||
| Age, years | 43 ± 9 | 51 ± 15 | 0.21 |
| Gender, male/female | 7/4 | 7/4 | 1.00 |
| Height, m | 1.76 ± 0.06 | 1.73 ± 0.08 | 0.35 |
| Weight, kg | 78.3 ± 10.9 | 87.4 ± 18.5 | 0.18 |
| BMI, kg · m−2 | 25.2 ± 3.6 | 29.1 ± 6.1 | 0.10 |
| BSA, m2 | 2.0 ± 0.1 | 2.0 ± 0.2 | 0.33 |
| VO2peak, mL · kg−1 · min−1 | 36.3 ± 9.2 | 17.5 ± 4.8 | <0.001 |
| Medications | |||
| ACE inhibitor | 6 (55) | ||
| Angiotensin II receptor blockers | 4 (36) | ||
| β-blocker | 10 (91) | ||
| Digitalis | 4 (36) | ||
| Aspirin | 7 (64) | ||
| Diuretics | 7 (64) | ||
Data are mean ± SD, n, or percentages that are contained within parentheses. BMI, body mass index; BSA, body surface area; ACE, angiotensin converting enzyme.
Protocol Overview
This study protocol has been published previously by our group for the separate study of an a priori determined hypothesis (16). Therefore, in brief, this study consisted of two separate days of exercise testing procedures, in an environmentally controlled physiological laboratory, separated by ≥48-hours. For all study visits, participants were asked to avoid strenuous physical activity for 24-hours and refrain from eating or consuming caffeine for >3 hours prior to arrival at the physiological laboratory for testing. Day one of testing consisted of a peak exercise test to volitional fatigue (peak oxygen consumption [VO2peak]). Day two consisted of three separate and randomized submaximal exercise sessions at 60% of the previously determined VO2peak.
For each testing day, upon arrival, participants were fitted with a 12-lead electrocardiogram (Marquette Electronics, Milwaukee, WI) to monitor HR and rhythm. Participants were seated on a recumbent cycle ergometer and fitted with a nose clip and standard mouthpiece attached to a PreVent Pneumotach (Medical Graphic, St Paul, MN) connected to a metabolic measurement system (MedGraphics CPX/D; Medical Graphics) which was calibrated for volume (3.0 liter [L] syringe) and gases immediately prior to each test (27). Resting simultaneous measures of gas-exchange and ventilation included: VO2, carbon dioxide production (VCO2), respiratory exchange ratio (RER), respiratory rate (RR), tidal volume (VT), minute ventilation (VE), ventilatory equivalent for carbon dioxide production (VE/VCO2), and PETCO2 were performed. Blood pressure was measured via manual sphygmomanometer at rest, near the end of each stage during peak exercise testing, and each minute during constant-load submaximal exercise sessions. For all exercise testing, measures of gas-exchange and flow analysis as well as HR and oxygen saturation were continuously monitored and averaged every three-seconds at rest and throughout exercise sessions. For analysis and data reporting, at rest we averaged the entire three min period for all measures, for exercise we averaged the final 30-seconds of each exercise stage. During recovery, data were averaged in 10-second intervals. Additional calculations included the SV estimate O2pulse (36) adjusted for body surface area (BSA) (VO2/HR)/BSA) since effect sizes for height and weight between groups were 0.42 and 0.60 (i.e., medium effect of HF), respectively, the non-invasive surrogate for PVCAP (GXCAP=O2pulseI×PETCO2) (36), and cardiac power estimated by CircP (VO2/weight)×MAP) (5, 39). We calculated O2pulseI, GXCAP, and CircP at rest, end-exercise, and at 2 minutes (min) post-exercise.
During day two of testing, participants performed three separate randomized bouts of constant-load submaximal locomotor exercise at 60% of VO2peak (measured on day one of testing). Each of the three exercise sessions were identical in procedure and consisted of three-min of resting data collection, followed by four-min of constant-load cycle ergometry, and five-min of passive recovery that were randomized between cuffing conditions. Session one was the control trial that included a normal recovery at end-exercise (NR). Sessions two and three were randomized and included, immediately at cessation of exercise, RCO via inflation of bilateral upper-thigh pressure tourniquets to ≈20 mm Hg above peak exercise arm systolic blood pressure (SBP) measured during the VO2peak test on day one. Session two or three also included addition of CO2 (RCO+CO2) to inspired air to clamp end-exercise PETCO2 to account for potential reduced venous return of CO2 due to RCO and potential influence on central chemoreceptor activity leading to abnormal adjustments in cardiovascular function as suggested in Olson et al. (25). The RCO protocol is valid in HF and not associated with causing significant pain or discomfort that could potentially bias measures of cardiovascular function (27, 32).
Statistical Analyses
This study is the first to test CircP and GXCAP in the context of this study design. However, in setting a two-tailed alpha at 0.05 and power=0.80, we determined that group sample sizes were adequate to detect effect sizes=0.80 for testing our experimental conditions. No dropouts or test failures occurred during the collection of study data and all data were included in the analyses. Where appropriate, data are presented as means ± standard deviation (SD). The data was distributed normally. Homogeneity of variance of the data was tested using Levene’s test. Unpaired two-tailed Student’s t-tests were used for specific comparisons between groups. Multiple comparisons for within group differences for treatment condition were tested using the repeated measures one-way analysis of variance test. When the F-test statistic was significant from the analysis of variance test, Bonferroni post-hoc analysis was used to correct for multiple comparisons and to identify between which comparisons significance occurred. Relationship testing between variables were assessed using univariate linear regression with coefficient of determination (R2) and 95% confidence limits (CL). Two-tailed statistical significance was determined using an alpha level set at 0.05. All computations were made using SAS statistical software, v.9.4 (SAS Institute, Cary, NC).
Results
Participants
Participant characteristics are presented in Table 1, and have been reported previously during testing of a separate hypothesis in HF (16). Heart failure patients were NYHA class I (n=4) or II (n=7). Mean ejection fraction percentage was 32.1±9.2% in HF patients. Four HF patients had an ischemic etiology, whereas seven were idiopathic.
End-exercise intensity and symptoms
The mean absolute workload performed was 115±16 vs 36±7 watts in control and HF (P<0.05), respectively. However, RPE (Borg, 6-20) did not differ between control and HF, or within groups at cessation of exercise (NR=11.4±1.2 vs 10.7±1.8; RCO=11.4±1.4 vs 11.3±1.8; 11.2±1.7 vs 11.6±2.0, respectively, all P>0.05).
Heart rate
Baseline measures of HR did not differ between or within groups (NR=64±13 vs 76±14; RCO=68±11 vs 77±14; RCO+CO2=68±11 vs 77±14 beats/min in control vs HF, respectively, all P>0.05). At end-exercise, no between or within group differences at rest persisted for HR (NR=116±10 vs 105±16; RCO=115±11 vs 104±15; RCO+CO2=117±13 vs 106±16 beats/min in control vs HF, respectively, all P>0.05). This HR trend continued at 2 min post-exercise (NR=75±14 vs 80±16; RCO=77±11 vs 87±18; RCO+CO2=76±11 vs 85±17 beats/min in control vs HF, respectively, all P>0.05) (16).
Mean arterial pressure
Although reported previously (16), MAP was used in the calculation of CircP in the present study. Therefore, in brief, baseline MAP did not differ between or within group (NR=90±12 vs 91±14; RCO=93±12 vs 91±15; RCO+CO2=92±10 vs 92±14 mm Hg in control vs HF, respectively, all P>0.05). Similar to HR, no differences between and within group at baseline persisted at end-exercise for MAP (NR=111±14 vs 99±14; RCO=112±14 vs 100±13; RCO+CO2=111±14 vs 101±16 mm Hg in control vs HF, respectively, all P>0.05). This trend continued at 2 min post-exercise between and within group (NR=98±12 vs 96±15; RCO=97±34 vs 107±13; RCO+CO2=108±13 vs 108±16 mm Hg in control vs HF, respectively, P>0.05) (16).
Oxygen uptake
Also reported previously (16), we report VO2 because it is used in the calculation of both CircP and O2pulse (36, 39). Except for between group in RCO+CO2, absolute measures of VO2 did not differ significantly between or within group at baseline (NR=0.4±0.1 vs 0.4±0.1; RCO=0.4±0.2 vs 0.4±0.1; RCO+CO2=0.5±0.1 vs 0.4±0.1 L/min in control vs HF, respectively). At end-exercise, controls had significantly higher VO2 compared to HF for all three transients in the absence of significant within group differences in both controls and HF (NR=1.7±0.4 vs 1.0±0.3; RCO=1.8±0.5 vs 1.0±0.3; RCO+CO2=1.8±0.5 vs 1.1±0.3 L/min for control vs HF, respectively, between group all P<0.05). Although VO2 measures at 2 min post-exercise were similar to baseline levels, no between and within group differences were present (NR=0.4±0.1 vs 0.5±0.1; RCO=0.3±0.1 vs 0.4±0.1; RCO+CO2=0.4±0.1 vs 0.4±0.1 L/min for control vs HF, respectively, all P>0.05) (16).
End-tidal partial pressure CO2
End-tidal partial pressure CO2 is a factor that is necessary in the calculation of GXCAP (36), but has been reported previously (16). Similar to HR and MAP, PETCO2 did not differ significantly between or within group at baseline (NR=36.6±4.3 vs 34.1±3.3; RCO=35.7 ± 3.8 vs 33.7 ± 4.0; RCO+CO2=36.0±3.6 vs 33.5±3.7 mm Hg for control vs HF, respectively, all P>0.05). However, consistent with VO2 at end-exercise, PETCO2 was higher in control compared to HF for all three transients in the absence of significant within group differences (NR=41.4±5.2 vs 35.4±4.7; RCO=40.6±4.3 vs 35.3±4.7; RCO+CO2=40.3±3.8 vs 34.7±4.8 mm Hg for control vs HF, respectively, between group all P<0.05). At 2 min post-exercise, there was a variable influence of experimental transient between and within group on PETCO2. For NR, PETCO2 was less in HF vs control (33.8±4.5 vs 36.4±4.0 mm Hg, p<0.05). For RCO, PETCO2 was less in HF vs control (30.2±3.4 vs 33.2±4.9, P>0.05). For RCO+CO2, PETCO2 was less in HF vs control (34.8±4.0 vs 38.4±3.9, P<0.05). However, in both control and HF, PETCO2 during RCO was significantly lower than NR; but, RCO+CO2 was significantly higher than RCO (16).
At 2 min post-exercise, relationships between PETCO2 and VE were low in strength. Linear regressions (R2 and 95% CL) in HF were: NR=0.34 (0.00, 0.63), RCO=0.25 (0.00, 0.57), and RCO+CO2=0.01 (0.00, 0.29). Controls had R2 and 95% CL of 0.19 (0.00, 0.53), 0.04 (0.00, 0.37), and 0.00 (0.00, 0.24) for NR, RCO, and RCO+CO2, respectively. These relationships suggested that metaboreflex mediated adjustments in PETCO2 were not directly influenced by the contributions of the metaboreflex on changes in VE.
Surrogates of central hemodynamics at baseline, end-exercise, and 2 minutes post-exercise
Absolute mean values at rest, end-exercise, and 2 min post-exercise for CircP, O2pulseI, and GXCAP are presented in Table 2. Interestingly, at baseline, lower O2pulseI for NR and RCO+CO2 sessions in HF contributed to significantly lower GXCAP compared to controls.
Table 2.
Estimates of central hemodynamics
| NR | RCO | RCO+CO2 | |
|---|---|---|---|
|
|
|||
| Baseline | |||
| O2pulseI, mL/beat/m2 | |||
| CTL | 3.3±0.8 | 3.4±1.6 | 3.6±1.1 |
| HF | 2.5±0.5* | 2.6±0.8 | 2.4±0.5* |
| CircP, mL/kg/min · mm Hg | |||
| CTL | 465±102 | 510±199 | 542±105 |
| HF | 391±76 | 418±152 | 397±87* |
| GXCAP, mL/beat · mm Hg/m2 | |||
| CTL | 122±38 | 122±59 | 128±43 |
| HF | 84±16* | 88±30 | 80±15* |
| End-exercise | |||
| O2pulseI, mL/beat/m2 | |||
| CTL | 7.6±1.6 | 7.9±1.7 | 7.8±1.7 |
| HF | 4.9±1.1* | 4.8±1.0* | 5.0±1.2* |
| CircP, mL/kg/min · mm Hg | |||
| CTL | 2451±572 | 2579±750 | 2527±673 |
| HF | 1217±367* | 1187±323* | 1241±301* |
| GXCAP, mL/beat · mm Hg/m2 | |||
| CTL | 318±86 | 323±86 | 315±79 |
| HF | 175±51* | 170±43* | 172±50* |
| Post-exercise (2 min) | |||
| O2pulseI, mL/beat/m2 | |||
| CTL | 3.1±0.9 | 2.2±0.8‡ | 2.6±1.0‡ |
| HF | 2.9±0.7 | 2.4±0.7 | 2.5±0.7 |
| CircP, mL/kg/min · mm Hg | |||
| CTL | 557±132 | 453±233 | 510±189 |
| HF | 505±83‡ | 492±138 | 520±105‡ |
| GXCAP, mL/beat · mm Hg/m2 | |||
| CTL | 114±35 | 73±34†‡ | 100±47 |
| HF | 98±20 | 72±23† | 86±22 |
| Slope | |||
| O2pulseI, mL/beat/m2 | |||
| CTL | 16.1±3.1 | 21.8±7.8 | 19.3±6.3 |
| HF | 12.5±6.6 | 15.8±4.6* | 16.1±5.4 |
| CircP, mL/kg/min · mm Hg | |||
| CTL | −947±263 | −1084±339 | −1009±289 |
| HF | −356±187* | −348±161* | −361±134* |
| GXCAP, mL/beat · mm Hg/m2 | |||
| CTL | −102±28 | −125±34 | −108±25 |
| HF | −39±24* | −49±24* | −43±19* |
Data are mean±SD. CTL, control; CircP, circulatory power; GXCAP, pulmonary vascular capacitance; HF, heart failure; NR, normal recovery; O2pulseI, oxygen pulse indexed to body surface area; RCO, regional circulatory occlusion; RCO+CO2, added carbon dioxide during RCO; Slope, rate of change from end-exercise to 2-min post-exercise;
p<0.05, compared to CTL after Bonferroni correction;
p<0.05, RCO compared to NR;
p<0.05, compared to baseline.
For all transients at end-exercise, lower O2pulseI and PETCO2 in HF compared to control contributed to significantly lower GXCAP in HF versus controls. However, at end-exercise there were no within group differences present for O2pulseI, CircP, or GXCAP in HF or controls (P>0.05). Consistent with lower VO2 (P<0.05) and MAP (P>0.05) at end-exercise for all transients, CircP was significantly lower in HF compared to controls.
At 2 min post-exercise, because of significantly lower PETCO2 in RCO versus NR in both HF and controls, GXCAP was lower in both groups for RCO versus NR by magnitudes of −26.1±2.5 and −41.1±0.7 mL/beat · mm Hg/m2, respectively; P<0.05. Whereas, consistent with no between or within group differences for VO2 and MAP at 2 min post-exercise (P>0.05), CircP did not differ between or within groups at 2 min post-exercise (P>0.05).
Lastly, although we were unable to assess arterial-venous oxygen content difference, which is assumed to be invariable in the modeling of cardiac output using the direct Fick equation, we note that although not equivalent to the arterial-venous oxygen content difference measurement, peripheral oxygen saturation did not differ between experimental transients at 2 min post-exercise in HF or control participants. In HF, oxygen saturation was 99.4±1.0, 98.8±1.4, and 98.7±1.3 % in NR, RCO, and RCO+CO2, respectively; P>0.05. In controls, oxygen saturation was 99.6±0.9, 99.4±1.2, and 99.3±1.4 % in NR, RCO, and RCO+CO2, respectively; P>0.05. No between or within group differences were detected (P>0.05).
Changes in central hemodynamics
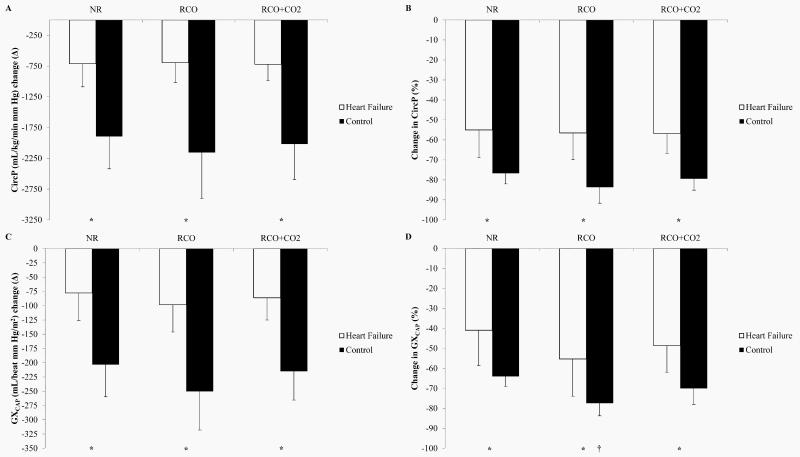
Absolute changes (Δ) in both CircP and GXCAP from end-exercise to 2 min post-exercise are shown in Figure 1 (A and C), respectively. Figure 1 (B and D) shows both CircP and GXCAP at 2 min post-exercise as percentages (%) of CircP or GXCAP at end-exercise, respectively.
Figure 1.
Differences in circulatory power (CircP) or pulmonary vascular capacitance (GXCAP) between end-exercise and 2 min post-exercise. Data presented as means±SD. (A) CircP, absolute change (Δ). (B) CircP, percentage (%) change. (C) GXCAP, absolute Δ. (D) GXCAP, % change. †NR vs. RCO or RCO+CO2 in controls (P<0.05). *Heart failure vs. control (P<0.05).
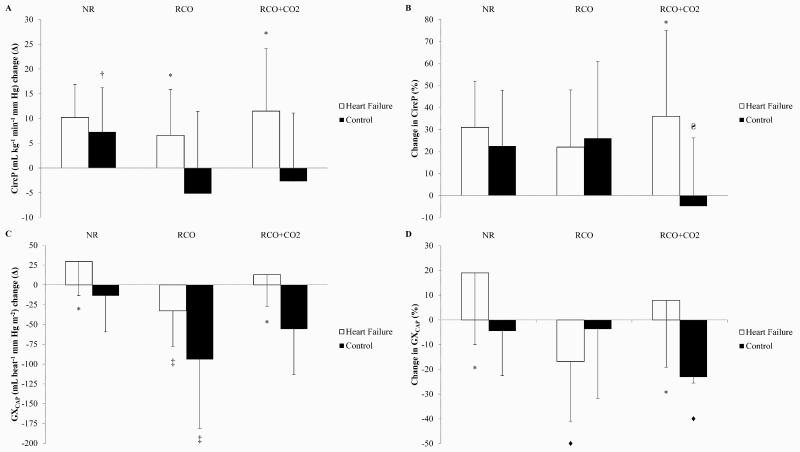
Absolute Δ in both CircP and GXCAP 2 min post-exercise minus baseline are shown in Figure 2 (A and C), respectively. Figure 2 (B and D) shows both CircP and GXCAP at 2 min post-exercise as % of CircP or GXCAP at baseline, respectively.
Figure 2.
Differences in circulatory power (CircP) or pulmonary vascular capacitance (GXCAP) between baseline and 2 min post-exercise. Data presented as means±SD. (A) CircP, absolute change (Δ). (B) CircP, percentage (%) change. (C) GXCAP, absolute Δ. (D) GXCAP, % change. †NR vs RCO in controls, (P<0.05); ΔDifferent between RCO and RCO+CO2 in controls, (P<0.05); ‡Different between NR and RCO in heart failure and controls, (P<0.05); ♦Different between NR and RCO in heart failure, and RCO+CO2 vs NR or RCO in controls, (P<0.05); *Heart failure vs. controls (P<0.05).
Overall, in the complete absence of an influence from central command and/or mechanoreceptor activation (Figure 2), CircP in HF patients did not appear to respond to locomotor metaboreflex activation. Whereas, locomotor metaboreflex activation appeared to have an influence on lowering CircP in controls. In contrast to CircP, however, locomotor metaboreflex activation appeared to have a lowering effect on GXCAP in both HF and controls.
Relationships between changes in VE and changes in CircP or GXCAP
Because there is the potential for aberrant changes in VE to influence changes in CircP or GXCAP since both contain VO2 in their calculation, we present in Table 3 univariate linear regressions between differences (Δ or %) in VE and differences (Δ or %) in CircP or GXCAP (differences between 2 min post-exercise and baseline). Linear regressions in HF strongly suggest that variance in CircP or GXCAP associated with our experimental paradigm were not explainable by variance in VE.
Table 3.
Linear regressions between differences in VE and differences in CircP or GXCAP.
| R2 (95% CL) | Intercept | Slope | Std. slope | P-value | |
|---|---|---|---|---|---|
|
|
|||||
| ΔVE and ΔCircP (2 min post-exercise minus baseline) | |||||
| Normal recovery | |||||
| CTL | 0.43 (0.00, 0.68) | −0.14 | 0.99 | 0.65 | 0.03 |
| HF | 0.03 (0.00, 0.35) | 7.97 | 0.44 | 0.17 | 0.61 |
| Regional circulatory occlusion | |||||
| CTL | 0.79 (0.37, 0.89) | −10.16 | 3.68 | 0.90 | <0.01 |
| HF | 0.08 (0.00, 0.43) | 3.81 | 0.56 | 0.29 | 0.39 |
| RCO+CO2 | |||||
| CTL | 0.01 (0.00, 0.27) | −4.34 | 0.23 | 0.08 | 0.81 |
| HF | 0.00 (0.00, 0.00) | 11.47 | 0.00 | 0.00 | 0.99 |
| %VE and %CircP (2 min post-exercise as percentage of baseline) | |||||
| Normal recovery | |||||
| CTL | 0.26 (0.00, 0.57) | 5.86 | 0.26 | 0.51 | 0.11 |
| HF | 0.13 (0.00, 0.48) | 17.28 | 0.34 | 0.37 | 0.27 |
| RCO | |||||
| CTL | 0.63 (0.11, 0.79) | −39.9 | 3.00 | 0.81 | <0.01 |
| HF | 0.19 (0.00, 0.53) | 12.18 | 0.24 | 0.44 | 0.17 |
| RCO+CO2 | |||||
| CTL | 0.36 (0.00, 0.64) | −36.81 | 0.61 | 0.60 | 0.05 |
| HF | 0.00 (0.00, 0.00) | 40.00 | −0.05 | −0.06 | 0.87 |
| ΔVE and ΔGXCAP (2 min post-exercise minus baseline) | |||||
| Normal recovery | |||||
| CTL | 0.00 (0.00, 0.00) | −12.94 | −0.10 | −0.01 | 0.97 |
| HF | 0.03 (0.00, 0.35) | 15.01 | 2.84 | 0.17 | 0.62 |
| Regional circulatory occlusion | |||||
| CTL | 0.49 (0.02, 0.72) | −118.55 | 15.46 | 0.70 | 0.02 |
| HF | 0.06 (0.00, 0.40) | −44.31 | 2.29 | 0.24 | 0.47 |
| RCO+CO2 | |||||
| CTL | 0.03 (0.00, 0.35) | −41.87 | −2.03 | −0.17 | 0.62 |
| HF | 0.04 (0.00, 0.37) | −2.87 | 1.60 | 0.20 | 0.56 |
| %VE and %GXCAP (2 min post-exercise as percentage of baseline) | |||||
| Normal recovery | |||||
| CTL | 0.02 (0.00, 0.32) | −7.50 | 0.05 | 0.13 | 0.70 |
| HF | 0.06 (0.00, 0.41) | 5.82 | 0.33 | 0.25 | 0.45 |
| Regional circulatory occlusion | |||||
| CTL | 0.55 (0.05, 0.75) | −65.56 | 2.73 | 0.74 | <0.01 |
| HF | 0.03 (0.00, 0.36) | −13.2 | −0.09 | −0.17 | 0.61 |
| RCO+CO2 | |||||
| CTL | 0.29 (0.00, 0.60) | −39.90 | 0.32 | 0.54 | 0.09 |
| HF | 0.00 (0.00, 0.00) | 4.78 | 0.04 | 0.07 | 0.85 |
Univariate linear regressions between differences (Δ or %) in minute ventilation (VE) and differences (Δ or %) in circulatory power (CircP) or pulmonary vascular capacitance (GXCAP). VE was always the independent variable. R2=coefficient of determination, and upper and lower 95% confidence limits. Std.slope=standardized slope, which is the change in the dependent variable expressed as a fraction of the standard deviation, per standard deviation of the change in the independent variable.
Discussion
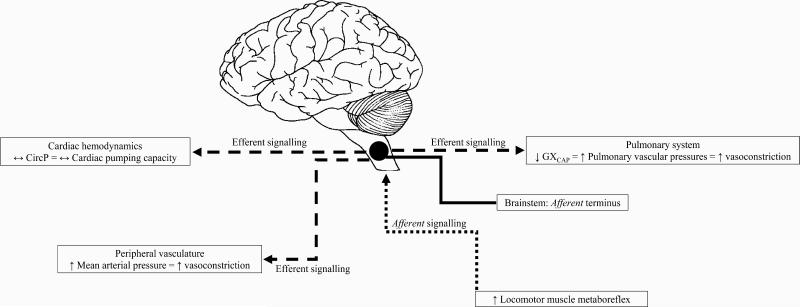
Our study demonstrates that HF patients have different CircP and GXCAP responses to locomotor metaboreflex activation compared to older healthy individuals. The general effect of the locomotor metaboreflex pathway on CircP and GXCAP in HF can be seen schematically in Figure 3. Specifically, we show that sustained metaboreflex activation following locomotor exercise does not contribute to adjustments in CircP and O2pulseI (estimates of cardiac function) in HF. In contrast, HF patients demonstrate reduced GXCAP suggesting an augmented pulmonary vascular pressure response in these patients (36, 39). Further, CircP and O2pulseI responses in our controls are in direct contrast to observations suggesting SV increases as a result of RCO following exercise (7, 8). Whereas, we demonstrate that changes (Δ and %) in CircP and GXCAP closely mirror the findings of others, which show systolic pulmonary arterial pressure may increase in the absence of adjustments in Q during metaboreflex activation following handgrip or plantar-flexor exercise in healthy individuals (21, 38). These latter findings in healthy individuals are consistent with the present CircP and GXCAP observations in HF, suggesting pulmonary and perhaps also peripheral vascular pressures may increase in the absence of marked increases in Q.
Figure 3.
Schematic representation of the linked pathways between locomotor muscle metaboreflex afferent signaling and adjustments in circulatory power (CircP) or pulmonary vascular capacitance (GXCAP) in heart failure patients. Heightened afferent signaling associated with metaboreflex activation of locomotor muscles contributes to efferent signaling toward the pulmonary system causing decreases in GXCAP and, hence, pulmonary vasoconstriction and elevations in pulmonary pressures. In contrast, efferent signaling toward the myocardium contributes little to increases in CircP (cardiac pumping capacity), and that increases in peripheral pressures are not likely contributed to by increasing cardiac hemodynamics, but more likely due to robust peripheral vasoconstriction in heart failure patients.
Prior to locomotor metaboreflex isolation using the RCO technique, we show that exercise results in anticipated responses in CircP and GXCAP in both HF and controls. CircP and GXCAP responses consisted of uniformly higher values at end-exercise compared to rest, suggesting a metabolic dose-dependent response of these indices. However, we also show that CircP and O2pulseI, but not GXCAP, demonstrate consistent and appropriate metabolic-dependent declines during isolated metaboreflex activation (RCO) compared to NR in HF patients. These data suggest that locomotor metaboreflex initiated sympathetically-mediated vasoconstriction of the pulmonary vasculature may contribute to aberrant GXCAP responses in the absence of changes in O2pulseI or CircP, and hence null adjustments in cardiac function.
Stimulation of the metaboreflex using RCO or comparable muscle ischemia techniques are valid for promoting neural feedback from skeletal muscle group IV sensory fibers during or immediately following exercise in HF and healthy models (6, 28, 29, 32, 33). The RCO technique during post-exercise is particularly useful for examining metabolite-sensitive neural feedback from skeletal muscle and its influence on inotropy and SV since central command and mechanical deformation from muscle are not present at this time; and, also because increases in HR (i.e., chronotropy) are not present during post-exercise RCO likely due to a robust return of parasympathetic activity related to the baroreflex influence in response to pronounced metaboreflex sensitization (6, 13, 30).
Recent observations suggest that although activation of the metaboreflex during or immediately following exercise in HF or controls contributes to increases in arterial pressure (6, 12, 30, 33), mechanisms underlying adjustments in peripheral vascular hemodynamics may not be similar in HF patients compared to healthy individuals. In this context, Ichinose et al. and Spranger et al. demonstrate that the metaboreflex may evoke profound lowering of systemic vascular conductance and increased vascular resistance to maintain adequate arterial pressure in the absence of metaboreflex-mediated increases in myocardial contractility (12, 33). As such, because HF patients are known to have severely depressed cardiac reserve related to impaired inotropy (and chronotropy when on rate modulating pharmacotherapy), observations in this study suggest that activation of the locomotor metaboreflex likely influences decreases in GXCAP via increases in pulmonary vascular tone with little contribution from O2pulseI and recruitment of myocardial contractile reserve in HF patients (5, 16, 19, 24, 29).
Although we and others show hyperpnea that is accompanied by attenuated gas-exchange occur during exercise in HF compared to healthy individuals (25, 27, 28, 40), we demonstrate in this study that impaired ventilation during exercise across all HF transients compared to controls does not persist during experimental post-exercise recovery. As noted in our present and previous observations (27), we show marked differences in PETCO2 in the absence of differences in ventilation (i.e., VE, RR, VT, and VO2) in RCO (no CO2) versus NR in HF at 2 min post-exercise; whereas, during exercise these measures appear similar between RCO and NR. In this context, we further demonstrate that variance in GXCAP during metaboreflex activation and/or NR cannot be explained by the variance in VE as indicated by linear regression models (Δ and %) in HF patients. Consistent with this finding, VE does not relate to PETCO2 during locomotor metaboreflex activation (RCO or RCO+CO2) in HF patients. Therefore, we suggest post-exercise adjustments in GXCAP during locomotor metaboreflex activation are not likely due to adjustments in ventilation alone. Indeed, because we have previously shown a direct negative association between PETCO2 and mPAP during exercise in HF (36), we suggest that GXCAP measures during RCO in HF are the consequences of augmented mPAP related to locomotor metaboreflex activation. The present observations extend findings of others by examining and demonstrating that locomotor metaboreflex influence on GXCAP may be independent of adjustments in the SV index estimate O2pulseI in both controls and HF (21, 36, 38).
Lastly, adjustments in GXCAP or CircP in the comparison of NR to RCO or RCO+CO2 in participants, suggest there may be a potential synergistic interaction between feedback from central chemoreceptors with feedback from ergoreceptors. This may reaffirm the critical role of the regions within the dorsal medulla (e.g., caudal nucleus tractus solitarius) in receiving, organizing, and integrating neural feedback from central and peripheral origins.
Clinical Implications
While cardiac and pulmonary system abnormalities are most evident during physical activity in HF, to date, there is an incomplete understanding of the pathophysiology of exertional symptoms and functional limitations of these patients. However, the present findings are hypotheses generating in suggesting that neural feedback from metabolite-sensitive skeletal muscle afferents may facilitate chronic increases in pulmonary vascular pressures, contributing to impairment in pulmonary vascular reactivity, permeability, and vascular resistance in HF. Understanding mechanisms of pulmonary vascular function are important because common complications of HF includes ventilatory dysfunction, impaired gas exchange, dyspnea, and exercise intolerance, which may be related to tonic increases in pulmonary vascular resistance (4, 23, 36). More importantly, augmented pulmonary vascular pressures are directly related to increased mortality in HF patients (3, 23).
Limitations
Patients with systolic HF are disproportionately represented by males in the population and in this study (34), and therefore the present findings may not be representative of females with systolic HF. Also, because HF patients were on optimum pharmacological therapy, it remains unclear what influence medications such as β-blockers, ACE inhibitors, and/or angiotensin II receptor blockers may have on the interaction between the metaboreflex and CircP, O2pulseI, and GXCAP. The potential influence of ACE inhibitors and angiotensin II receptor blockers on increasing inotropy and cardiac reserve in HF suggest that these pharmacologically treated patients may provide the ideal setting for assessing CircP, O2pulseI, and GXCAP responsiveness to metaboreflex activation (15, 31). However, we suggest that the unique responsiveness of CircP, O2pulseI, and GXCAP to metaboreflex activation in HF, despite being on such rate-limiting and/or inotropic promoting therapy, further underscores our hypotheses that the metaboreflex does little to contribute to increased cardiac hemodynamics, while facilitating pulmonary and peripheral increases in vasomotor tone in these patients.
Although post-exercise RCO is a validated technique (32), we did not directly measure intramuscular metabolite concentration and do not know what specific metabolites are contributing to metaboreflex activation. Further, we did not directly measure Q or mPAP and do not know whether there was unanticipated bias that accompanied our surrogate estimates. Use of ventilation-based central hemodynamic surrogates may misrepresent respective invasive counterparts in circumstances where there may be profound pulmonary congestion resulting in ventilation and perfusion mismatch, or peripheral oxygen extraction dysfunction. In circumstances such as these, use of the validated Doppler echocardiography technique, despite its own limitations (e.g., high user dependence, pulmonary and tricuspid valve regurgitation not always visible/present, aliasing, or low resolution), may serve as a reliable method for assessing cardiac and pulmonary hemodynamics in HF (17, 37). Nevertheless, HF patients in this study were without diagnosis of secondary pulmonary disease and did not demonstrate signs or symptoms consistent with pulmonary congestion on exertion (e.g., feeling of suffocating, gasping for breath, chest pain, rapid irregular heartbeat). Moreover, it is suggested that arterial-venous oxygen content difference may be normal at rest and during exercise in HF (14).
CircP, O2pulseI, and GXCAP are valid surrogates for each respective invasive counterpart during exercise in HF (5, 20, 36, 39), while also maintaining these relationships at rest (unpublished data from our lab). Therefore, use of indirect estimates of central hemodynamics are adequate as the intent of this study design was to assess changes in central hemodynamics in response to metaboreflex augmentation, not absolute measurements.
Summary
Administrating RCO immediately at cessation of submaximal constant-load locomotor exercise in HF patients and healthy individuals facilitates stimulation of metabolite-sensitive neural feedback from skeletal muscle. This neural feedback pathway contributes to decreases in GXCAP (i.e., increased pulmonary vascular pressures) in low- to- moderate severity HF patients, and perhaps to a lesser extent in older healthy individuals. Moreover, although the metaboreflex pathway may favorably contribute to increased cardiac function in healthy individuals, because of null changes in CircP and O2pulseI, we suggest that it may be unlikely that this pathway contributes to adjustments in cardiac function in HF patients.
Acknowledgments
We thank the participants who took part in this research. This work was supported by: American Heart Association (AHA) grant 12GRNT1160027 (Thomas P. Olson), National Center for Advancing Translational Science (NCATS) grant KL2TR000136 (Thomas P. Olson). The results of the present study do not constitute endorsement by ACSM.
Footnotes
Conflict of Interest
None.
References
- 1.Abramson SV, Burke JF, Kelly JJ, et al. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Annals of internal medicine. 1992;116(11):888–95. doi: 10.7326/0003-4819-116-11-888. [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. The Journal of physiology. 2011;589:5299–309. doi: 10.1113/jphysiol.2011.213769. Pt 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson D, Eitan A, Dragu R, Burger AJ. Relationship between reactive pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circulation. Heart failure. 2011;4(5):644–50. doi: 10.1161/CIRCHEARTFAILURE.110.960864. [DOI] [PubMed] [Google Scholar]
- 4.Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. Journal of the American College of Cardiology. 1999;34(6):1802–6. doi: 10.1016/s0735-1097(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 5.Cohen-Solal A, Tabet JY, Logeart D, Bourgoin P, Tokmakova M, Dahan M. A non-invasively determined surrogate of cardiac power ('circulatory power') at peak exercise is a powerful prognostic factor in chronic heart failure. Eur Heart J. 2002;23(10):806–14. doi: 10.1053/euhj.2001.2966. [DOI] [PubMed] [Google Scholar]
- 6.Crisafulli A, Piras F, Filippi M, et al. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. The Journal of Physiological Sciences. 2011;61(5):385–94. doi: 10.1007/s12576-011-0163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crisafulli A, Salis E, Pittau G, et al. Modulation of cardiac contractility by muscle metaboreflex following efforts of different intensities in humans. American Journal of Physiology-Heart and Circulatory Physiology. 2006;291(6):H3035–H42. doi: 10.1152/ajpheart.00221.2006. [DOI] [PubMed] [Google Scholar]
- 8.Crisafulli A, Scott AC, Wensel R, et al. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc. 2003;35(2):221–8. doi: 10.1249/01.MSS.0000048639.02548.24. discussion 9. [DOI] [PubMed] [Google Scholar]
- 9.Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH, Fadel PJ. Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. The Journal of physiology. 2010;588:1117–27. doi: 10.1113/jphysiol.2009.185470. Pt 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guazzi M, Reina G, Tumminello G, Guazzi MD. Exercise ventilation inefficiency and cardiovascular mortality in heart failure: the critical independent prognostic value of the arterial CO2 partial pressure. Eur Heart J. 2005;26(5):472–80. doi: 10.1093/eurheartj/ehi060. [DOI] [PubMed] [Google Scholar]
- 11.Ichinose M, Sala-Mercado JA, O'Leary DS, et al. Spontaneous baroreflex control of cardiac output during dynamic exercise, muscle metaboreflex activation, and heart failure. Am J Physiol Heart Circ Physiol. 2008;294(3):H1310–6. doi: 10.1152/ajpheart.01187.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ichinose MJ, Sala-Mercado JA, Coutsos M, et al. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol. 2010;298(1):H245–50. doi: 10.1152/ajpheart.00909.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iellamo F, Sala-Mercado JA, Ichinose M, et al. Spontaneous baroreflex control of heart rate during exercise and muscle metaboreflex activation in heart failure. Am J Physiol Heart Circ Physiol. 2007;293(3):H1929–36. doi: 10.1152/ajpheart.00564.2007. [DOI] [PubMed] [Google Scholar]
- 14.Katz SD, Maskin C, Jondeau G, Cocke T, Berkowitz R, LeJemtel T. Near-maximal fractional oxygen extraction by active skeletal muscle in patients with chronic heart failure. J Appl Physiol (1985) 2000;88(6):2138–42. doi: 10.1152/jappl.2000.88.6.2138. [DOI] [PubMed] [Google Scholar]
- 15.Kawai H, Fan T-HM, Dong E, et al. ACE inhibition improves cardiac NE uptake and attenuates sympathetic nerve terminal abnormalities in heart failure. American Journal of Physiology-Heart and Circulatory Physiology. 1999;277(4):H1609–H17. doi: 10.1152/ajpheart.1999.277.4.H1609. [DOI] [PubMed] [Google Scholar]
- 16.Keller ML, Johnson BD, Joyner M, Olson TP. Influence of the metaboreflex on arterial blood pressure in heart failure patients. Am Heart J. 2014;167:521–8. doi: 10.1016/j.ahj.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim A, Deo SH, Vianna LC, et al. Sex differences in carotid baroreflex control of arterial blood pressure in humans: relative contribution of cardiac output and total vascular conductance. Am J Physiol Heart Circ Physiol. 2011;301(6):H2454–65. doi: 10.1152/ajpheart.00772.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleber FX, Vietzke G, Wernecke KD, et al. Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation. 2000;101(24):2803–9. doi: 10.1161/01.cir.101.24.2803. [DOI] [PubMed] [Google Scholar]
- 19.Lang CC, Karlin P, Haythe J, Lim TK, Mancini DM. Peak cardiac power output, measured noninvasively, is a powerful predictor of outcome in chronic heart failure. Circulation. Heart failure. 2009;2(1):33–8. doi: 10.1161/CIRCHEARTFAILURE.108.798611. [DOI] [PubMed] [Google Scholar]
- 20.Lavie CJ, Milani RV, Mehra MR. Peak exercise oxygen pulse and prognosis in chronic heart failure. The American journal of cardiology. 2004;93(5):588–93. doi: 10.1016/j.amjcard.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Lykidis CK, White MJ, Balanos GM. The pulmonary vascular response to the sustained activation of the muscle metaboreflex in man. Experimental physiology. 2008;93(2):247–53. doi: 10.1113/expphysiol.2007.09487. [DOI] [PubMed] [Google Scholar]
- 22.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. Journal of the American College of Cardiology. 2006;47(4):799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 23.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation. 2000;102(14):1718–23. doi: 10.1161/01.cir.102.14.1718. [DOI] [PubMed] [Google Scholar]
- 24.O'Leary DS, Sala-Mercado JA, Augustyniak RA, Hammond RL, Rossi NF, Ansorge EJ. Impaired muscle metaboreflex-induced increases in ventricular function in heart failure. Am J Physiol Heart Circ Physiol. 2004;287(6):H2612–8. doi: 10.1152/ajpheart.00604.2004. [DOI] [PubMed] [Google Scholar]
- 25.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. The Journal of physiology. 2010;588:2487–501. doi: 10.1113/jphysiol.2009.186056. Pt 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson TP, Joyner MJ, Eisenach JH, Curry TB, Johnson BD. Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Experimental physiology. 2014;99(2):414–26. doi: 10.1113/expphysiol.2013.075937. [DOI] [PubMed] [Google Scholar]
- 27.Olson TP, Joyner MJ, Johnson BD. Influence of locomotor muscle metaboreceptor stimulation on the ventilatory response to exercise in heart failure. Circulation. Heart failure. 2010;3(2):212–9. doi: 10.1161/CIRCHEARTFAILURE.109.879684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piepoli M, Ponikowski P, Clarka AL, Banasiak W, Capucci A, Coats AJ. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J. 1999;137(6):1050–6. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- 29.Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O'Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol. 2007;292(5):H2159–66. doi: 10.1152/ajpheart.01240.2006. [DOI] [PubMed] [Google Scholar]
- 30.Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O'Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol. 2006;290(2):H751–7. doi: 10.1152/ajpheart.00869.2005. [DOI] [PubMed] [Google Scholar]
- 31.Sato H, Yaoita H, Maehara K, Maruyama Y. Attenuation of heart failure due to coronary stenosis by ACE inhibitor and angiotensin receptor blocker. American Journal of Physiology-Heart and Circulatory Physiology. 2003;285(1):H359–H68. doi: 10.1152/ajpheart.00615.2002. [DOI] [PubMed] [Google Scholar]
- 32.Scott AC, Francis DP, Coats AJ, Piepoli MF. Reproducibility of the measurement of the muscle ergoreflex activity in chronic heart failure. Eur J Heart Fail. 2003;5(4):453–61. doi: 10.1016/s1388-9842(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 33.Spranger MD, Sala-Mercado JA, Coutsos M, et al. Role of cardiac output versus peripheral vasoconstriction in mediating muscle metaboreflex pressor responses: dynamic exercise versus postexercise muscle ischemia. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;304(8):R657–63. doi: 10.1152/ajpregu.00601.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stromberg A, Martensson J. Gender differences in patients with heart failure. Eur J Cardiovasc Nurs. 2003;2(1):7–18. doi: 10.1016/S1474-5151(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 35.Tan LB. Cardiac pumping capability and prognosis in heart failure. Lancet. 1986;2(8520):1360–3. doi: 10.1016/s0140-6736(86)92006-4. [DOI] [PubMed] [Google Scholar]
- 36.Taylor BJ, Olson TP, Chul Ho K, Maccarter D, Johnson BD. Use of noninvasive gas exchange to track pulmonary vascular responses to exercise in heart failure. Clinical medicine insights. Circulatory, respiratory and pulmonary medicine. 2013;7:53–60. doi: 10.4137/CCRPM.S12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Temporelli PL, Scapellato F, Eleuteri E, Imparato A, Giannuzzi P. Doppler Echocardiography in Advanced Systolic Heart Failure A Noninvasive Alternative to Swan-Ganz Catheter. Circulation: Heart Failure. 2010;3(3):387–94. doi: 10.1161/CIRCHEARTFAILURE.108.809590. [DOI] [PubMed] [Google Scholar]
- 38.White MJ, Lykidis CK, Balanos GM. The pulmonary vascular response to combined activation of the muscle metaboreflex and mechanoreflex. Experimental physiology. 2013;98(3):758–67. doi: 10.1113/expphysiol.2012.068528. [DOI] [PubMed] [Google Scholar]
- 39.Williams SG, Tzeng BH, Barker D, Tan LB. Comparison and relation of indirect and direct dynamic indexes of cardiac pumping capacity in chronic heart failure. The American journal of cardiology. 2005;96(8):1149–50. doi: 10.1016/j.amjcard.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 40.Witte KK, Notarius CF, Ivanov J, Floras JS. Muscle sympathetic nerve activity and ventilation during exercise in subjects with and without chronic heart failure. Canadian Journal of Cardiology. 2008;24(4):275–8. doi: 10.1016/s0828-282x(08)70176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]