Abstract
Taking advantage of the property of high surface-area-to-volume ratio of microfluidic droplets, we developed an innovative interfacial nanosensing strategy based on aptamer-functionalized graphene oxide nanosensors in microfluidic droplets for high-sensitivity one-step detection of 17β-estradiol and other low-solubility molecules, with detection sensitivity improved by about 3 orders of magnitude than conventional systems.
17β-estradiol, a sex hormone, is the most potent and ubiquitous member of the mammalian estrogenic steroids. However, 17β-estradiol is also an endocrine disrupting compound (EDC), and becomes one of the most potential environmental endogenous estrogens, due to its detrimental effects on endocrine function of human and aquatic organisms.1 Estradiol has a critical impact on reproductive and sexual functions,2 and affects the functions of other organs as well.3 Estradiol has been widely used in animal fattening because of its anabolic effects.4 But it is harmful to aquatic organisms and drastic problems can be caused through the food chain to human being.5 Therefore, the rapid and sensitive detection of estradiol is of significance for environmental and food safety monitoring.
However, a broad range of molecules such as 17β-estradiol and a number of drugs, fats, proteins, vitamins and hormones have limited solubility in aqueous solutions,6 which often affect their detection in many widely-used detection systems. Current methods for estradiol detection include high-performance liquid chromatography (HPLC),7 gas chromatography-mass spectrometry (GC-MS),8 and liquid chromatography-mass spectrometry (LC-MS).9 Despite the high sensitivity, these methods rely on sophisticated and expensive instruments, complicated sample preparation procedures, long assay time and intensive labour. Recently, aptamer-based electrochemical biosensors1b, 10 and aptamer-based optical biosensor1a, 11 have been developed for simple estradiol detection. However, because 17β-estradiol is almost insoluble in water,6a water miscible organic solvents are required in these methods to dissolve estradiol, and the distribution ratio of each component needs to be carefully optimized to ensure that estradiol is completely dissolved. Thus, these assays usually require multi-step complicated procedures. These limitations compromise the advantages of detection simplicity from aptamers, and hinder the extensive application of such detection approaches. More importantly, because of insolubility of estradiol, the detection sensitivity of most methods are not high. Most limits of detection (LOD) for estradiol are in the range of nanomolar to subnanomolar (e.g. 2.1 nM1a), making them incapable of detecting trace contaminant of estradiol.
Recently, microfluidic lab-on-a-chip provides a versatile platform for rapid biosensing and environmental monitoring, because of various advantages associated with its integration, miniaturization, portability and automation.12 Along with the advantage of high throughput, droplet microfluidic systems enable rapid mixing of fluids in the droplet microreactors with high reaction efficiency.13 In addition, the high surface-area-to-volume ratio from microfluidic droplets makes it promising in developing high-sensitivity interfacial biosensing between two different phases, thus overcoming the insolubility issues of many organic compounds like 17β-estradiol in various aqueous phase-based detection systems. Therefore, taking advantage of high surface-to-volume property of microfluidic droplets, we have developed a simple interfacial nano-biosensing strategy for high-sensitivity detection of low-solubility compounds like 17β-estradiol.
We previously developed a paper/polymer hybrid microfluidic device for simple one-step pathogen detection, using aptamer-functionalized graphene oxide (GO) nanosensors.14 Because of the quenching property of graphene oxide and simplicity offered by aptamers, this nanosensing system provides a simple method for one-step pathogen detection. However it is not feasible to use this nanosensing system to detect estradiol due to the insolubility issue of estradiol. Combination of droplet microfluidics with aptamer-functionalized GO nanosensors enables a new interfacial nano-biosensing method for detection of low-insolubility organic compounds, with high simplicity and high sensitivity.
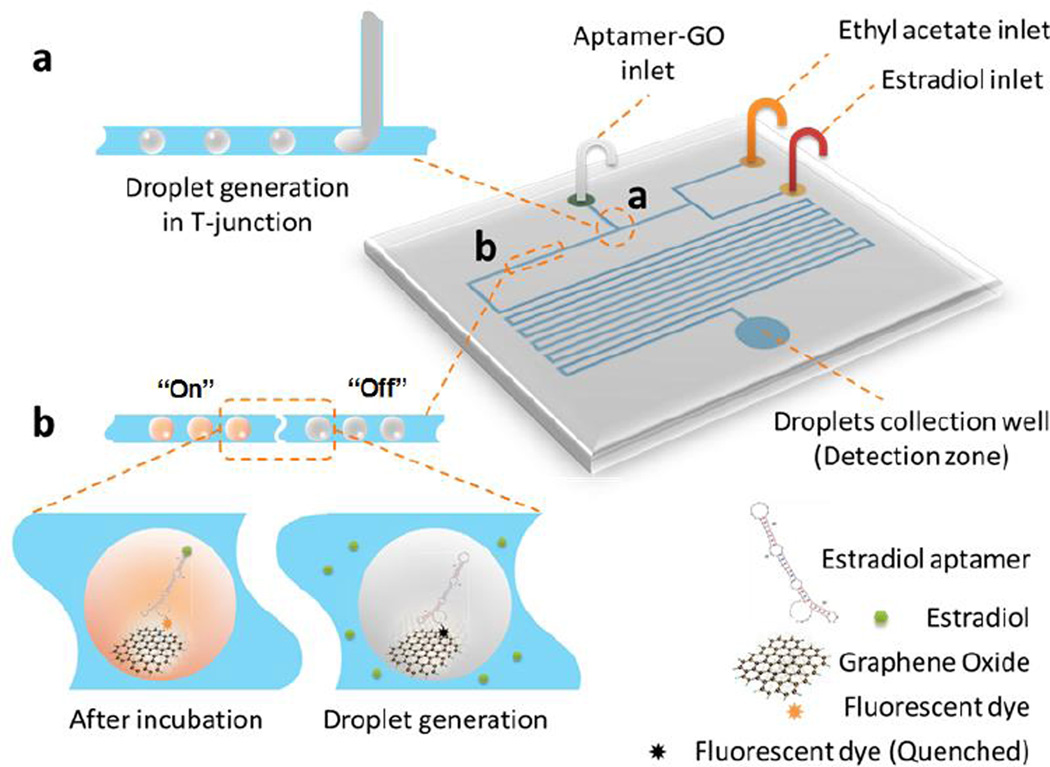
The microfluidic chip design and the detection principle of interfacial nano-biosensing strategy are shown in Figure 1. The chip has two layers. The top layer is a polydimethylsiloxane (PDMS) layer consisting of three inlets for the delivery of estradiol, ethyl acetate and the aptamer-GO complex, microchannels, and an outlet for droplet collection. The outlet region is also used as the detection zone. A glass layer at the bottom is used for structure support. We used the T-junction method for microfluidic droplet generation, as shown in the inset (a) of Figure 1. Estradiol is dissolved in ethyl acetate as the oil phase, whereas an aqueous solution of aptamer-functionalized graphene-oxide nanosensors are used as the aqueous phase. Because of the extraordinary distance-dependent fluorescence quenching property of GO,14–15 fluorescence of the Cy3-labeled aptamer will be pre-quenched in the aptamer-GO aqueous phase (fluorescence ‘off’; see inset (b) in Figure 1). By changing different flow rates of ethyl acetate and estradiol, different concentrations of estradiol solutions can readily detected by this droplet microfluidic system. When the water phase and the oil phase introduced at different flow rates meet at the T-junction, water-in-oil emulsion droplets will be generated due to the shear stress from the continuous oil flow which is set at a higher flow rate (2.4 µL/min) than the water phase (0.6 µL/min). Figure 2 shows a series of images during and after the droplet generation process. After water-in-oil droplet generation, aptamer-functionalized GO nanosensors in aqueous droplets will start to react with the target of estradiol from the oil phase at the droplet interface between these two immiscible phases. The large surface from millions of droplets significantly enhances the interaction possibilities between aptamer-GO nanosensors with the target. In the presence of the target, the aptamer will bind specifically to the corresponding target estradiol. The competitive binding of the aptamer and target estradiol lowers affinity of the adsorption with GO and spontaneously liberates the aptamer from the GO surface, thus resulting in the fluorescence recovery (fluorescence ‘turn-on’; see inset (b) in Figure 1). After 30-min incubation at room temperature, recovered fluorescence will be detected by a fluorescence microscope at the outlet region. No fluorescence restoration is observed in the absence of the target. Hence, the aptamer-functionalized GO nanosensors in droplets enables a simple one-step “turn-on” mechanism for high-sensitivity estradiol detection. To the best of our knowledge, this is the first time to use the large effective area of microfluidic droplets to develop high-sensitivity nano-biosensing system based on enhanced interfacial reactions.
Figure 1.
Schematic of the droplet microfluidic system for one-step estradiol detection using aptamer-functionalized GO nanosensors. The high surface-to-volume ratio from droplet microfluidics enables high-sensitivity interfacial nano-biosensing. Sections (a) and (b) of the chip channel were zoomed in inset (a) and (b) to show the detection principle, respectively.
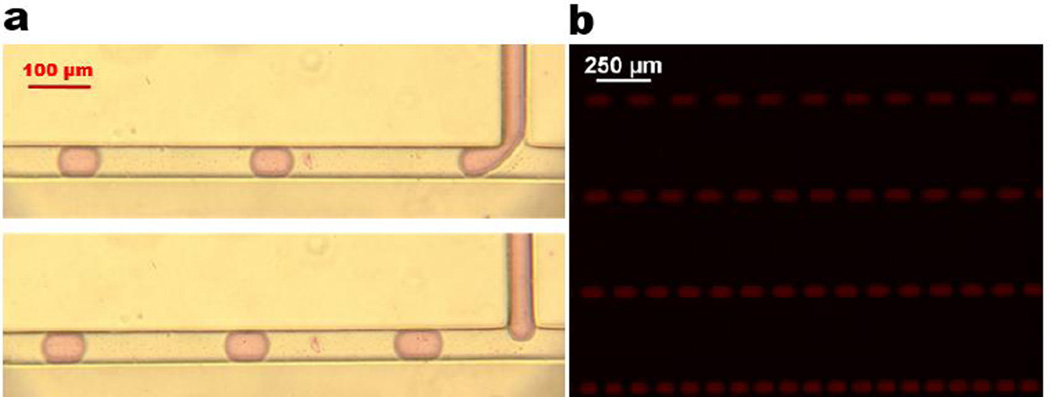
Figure 2.
Droplet generation. (a) Captured images during the droplet generation process by using the T-junction method. Food dye was add to distinguish droplets from the continuous flow. (b) A fluorescence image after droplet generation with Cy3-labeled aptamer in droplets.
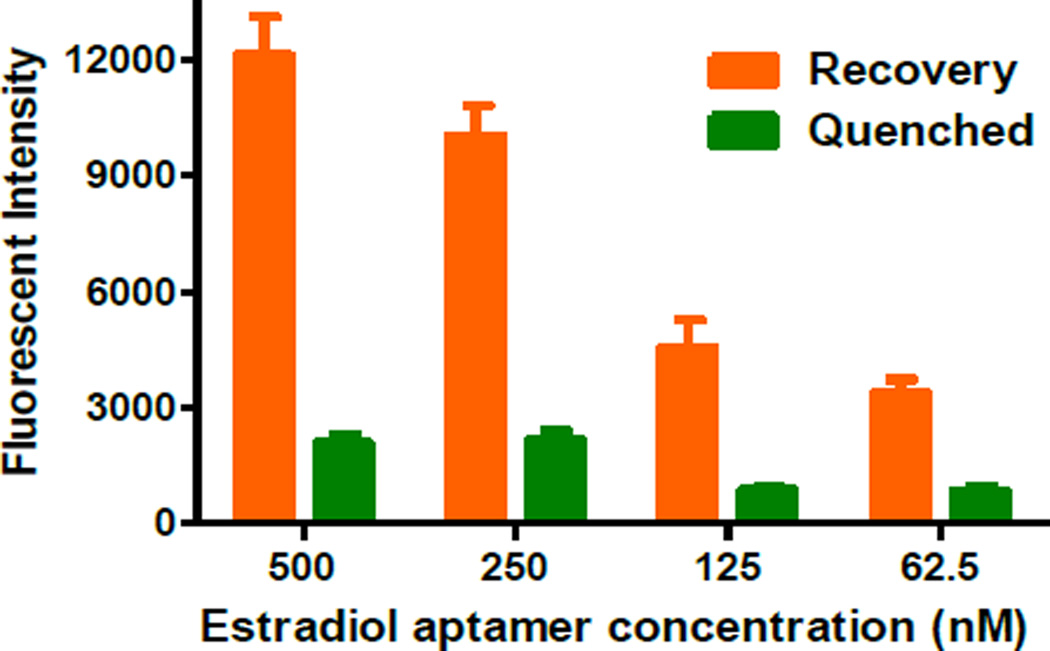
Optimization of the aptamer concentration is critical for high-sensitivity detection of estradiol. Therefore, four different concentrations of the aptamer ranging from 62.5 to 500.0 nM were tested to optimize the aptamer concentration for the droplet microfluidic system by using 1000.0 pM estradiol. The corresponding fluorescent intensities at different aptamer concentrations after quenching and recovery are shown in Figure 3. Two important factors, both the recovered fluorescent intensity and the net fluorescence increase (i.e. the difference between the recovered and quenched fluorescent intensity) need to be considered since they can directly affect the detection sensitivity. From Figure 3 we can see that the fluorescence of the aptamer were significantly quenched by GO for all aptamer concentrations, without significant differences between different concentrations. However, the restored fluorescence varied a lot at different aptamer concentrations, ranging from 3700 to 12300 a.u. corresponding to the aptamer concentrations from 62.5 nM to 500.0 nM. 500.0 nM of the aptamer exhibited the maximal net fluorescence recovery (~7 folds increase). At lower aptamer concentrations, the recovered fluorescent intensities were much lower. Given the highest recovered fluorescence intensity and maximal difference between the recovered and quenched fluorescent intensity, we chose 500.0 nM as the optimal aptamer concentration for the subsequent experiments.
Figure 3.
Estradiol aptamer concentration optimization. Estradiol concentration, 1000.0 pM.
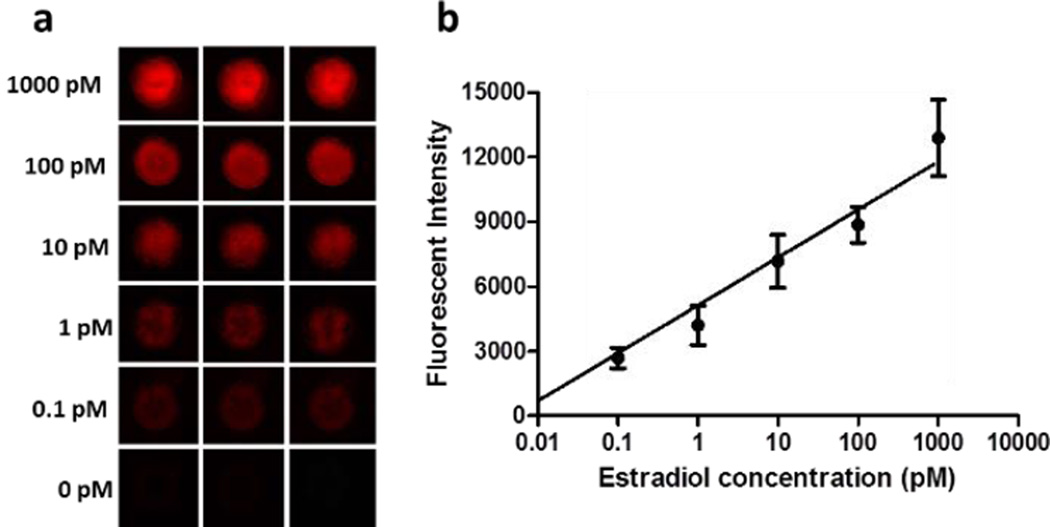
Under the optimized conditions, different concentrations of the standard estradiol were tested with their corresponding recovered fluorescence intensities recorded. Figure 4 shows the recovered fluorescent images and the calibration curve plotted by using recovered fluorescence intensities versus various concentrations of estradiol ranging from 0.1 pM to 1000 pM. Compared to the negative control (0 pM of estradiol), even 0.1 pM of estradiol showed well distinguishable fluorescence signal. With the increase of estradiol concentration, stronger recovered fluorescence intensity was observed. As can be seen in Figure 4b, a linear calibration curve was established between the recovered fluorescence and the estradiol concentration, with the square of the correlation coefficient (i.e. R2) of 0.997. The limit of detection (LOD) of estradiol was calculated to be as low as 0.07 pM on the basis of the 3-fold standard deviations of the negative control signal, whereas the LODs of most aptamer-based biosensors were in the range of nM (e.g. 2.1 nM1a) or above the pM range (e.g. 100 pM10a and 2.0 pM1b). It is noteworthy to mention that the reported LODs from two previous conventional methods using the same aptamer and the same optical detection method of fluorescence are 0.22 nM and 2.1 nM. Hence, our LOD is 2000 and 20000 folds lower than those two methods,1a, 11 respectively, indicating the high sensitivity of our interfacial nano-biosensing method using microfluidic droplets. As far as we know, this LOD from our droplet microfluidics is the lowest one reported one for estradiol detection.
Figure 4.
Fluorescence images (a) and calibration curve (b) of the detection of different concentrations of estradiol by using droplet microfluidic nanosensing system. Estradiol aptamer concentration, 500.0 nM.
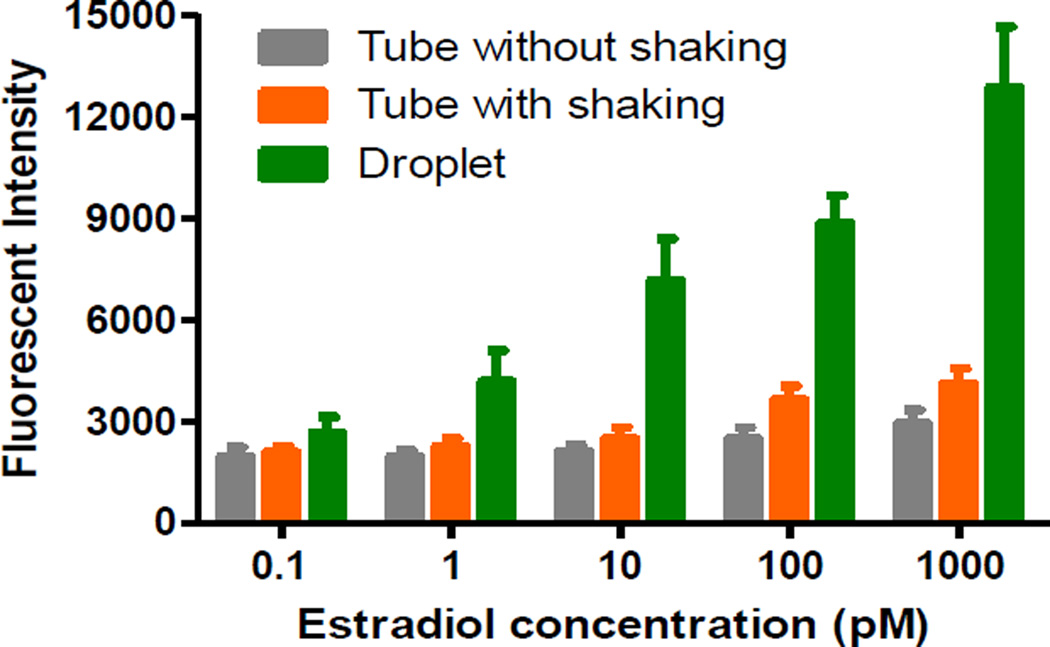
We further compared the sensitivity of our droplet microfluidic nanosensing system with conventional off-chip methods by testing various concentrations of estradiol. During the off-chip detection, the mixed aptamer-GO and estradiol solutions were incubated at room temperature for 30 min in different microtubes with continuous shaking and without shaking, respectively. The fluorescent intensities generated by our droplet microfluidic method and these two conventional off-chip methods were recorded. Their comparison is shown in Figure 5. For the off-chip detection method without shaking, there were no obvious enhanced fluorescent intensities after incubation at all estradiol concentrations; for the off-chip detection method with shaking, slightly enhanced fluorescent intensities were observed at only higher estradiol concentrations (>100 pM), indicating the low performance of the off-chip detection methods. It is estimated that the LODs of estradiol from these two conventional methods with and without shaking are about 150.0 and 20.0 pM, respectively. The LOD of 150.0 pM is consistent with previously published value of 0.22 nM.11 Although shaking can lower the LOD down to 20.0 pM, its LOD is still about 200 folds higher than that of our interfacial nano-biosensing system. In addition, we found the LOD of estradiol using commercial ELISA kits is above 18.3 pM (5.0 pg/mL) with longer assay time (~3 h) (see Supplementary Table S-1), which is also about 200 folds higher than that of our interfacial nano-biosensing system. Compared with the off-chip detection methods, the on-chip detection method showed high performance with much higher sensitivity. This is mainly because the droplet system greatly enhances the reaction kinetics and efficiency between the two phase interfaces due to a tremendous increase in effective contact areas from a number of droplets. In addition, when millions of droplets are uniformly distributed in the analyte solution, which creates millions of bioreactors in various locations in the analyte solution. This miniaturized droplet microfluidic system allows the analyte to quickly diffuse and access to nanosensors in droplets, resulting in faster fluorescence recovery in a given time. Compartmentalization of droplets enhances assay sensitivity by an enormous increase in their reaction rate due to shortened access distances.16
Figure 5.
Comparison of estradiol detection results between on-chip and off-chip detection methods. Estradiol aptamer concentration, 500 nM.
Taking advantage of large effective surface area from microfluidic droplets, we have developed an interfacial nano-biosensing strategy based on aptamer-functionalized GO nanosensors in droplets for high-sensitivity one-step 17β-estradiol detection. The LOD was calculated to be as low as 0.07 pM. This study should have great potential for high-sensitivity food safety and environmental monitoring. This interfacial nano-biosening system can also be used to solve the detection problems of many low-solubility compounds (e.g. a number of hormones, proteins, drugs, vitamins, fats, polymers and other organic compounds) in numerous aqueous solutions-based detection systems. It is estimated 40% of approved drugs are poorly water soluble.
Supplementary Material
Acknowledgments
The authors would like to acknowledge financial support of the UTEP URI Program, the NIH/NIAID under award number R21AI107415, and the NIH/NIGMS under award number SC2GM105584. Financial support from the IDR Program at the University of Texas at El Paso (UTEP) and the NIH RCMI Pilot Grant and some preliminary work by Dr. Peng Zuoare also gratefully acknowledged.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.(a) Yildirim N, Long F, Gao C, He M, Shi H-C, Gu AZ. Environmental science & technology. 2012;46:3288. doi: 10.1021/es203624w. [DOI] [PubMed] [Google Scholar]; (b) Lin Z, Chen L, Zhang G, Liu Q, Qiu B, Cai Z, Chen G. Analyst. 2012;137:819. doi: 10.1039/c1an15856b. [DOI] [PubMed] [Google Scholar]
- 2.(a) Akingbemi BT. Reproductive Biology and Endocrinology. 2005;3:51. doi: 10.1186/1477-7827-3-51. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Fertility and Sterility. 2002;77:42. doi: 10.1016/s0015-0282(02)03001-7. [DOI] [PubMed] [Google Scholar]
- 3.(a) McCARTHY MM. Physiological reviews. 2008;88:91. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Clemons M, Goss P. N engl J med. 2001;344:276. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 4.Noppe H, Le Bizec B, Verheyden K, De Brabander HF. Analytica Chimica Acta. 2008;611:1. doi: 10.1016/j.aca.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 5.(a) Cakmak G, Togan I, Severcan F. Aquatic toxicology. 2006;77:53. doi: 10.1016/j.aquatox.2005.10.015. [DOI] [PubMed] [Google Scholar]; (b) Seki M, Fujishima S, Nozaka T, Maeda M, Kobayashi K. Environmental Toxicology and Chemistry. 2006;25:2742. doi: 10.1897/05-647r.1. [DOI] [PubMed] [Google Scholar]
- 6.(a) Combined estrogen-progestogen contraceptives and combined estrogen-progestogen menopausal therapy. World Health Organization; 2007. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, International Agency for Research on Cancer. [PMC free article] [PubMed] [Google Scholar]; (b) Li X, Chen Y, Li PC. Lab on a chip. 2011;11:1378. doi: 10.1039/c0lc00626b. [DOI] [PubMed] [Google Scholar]; (c) Li X, Ling V, Li PC. Analytical chemistry. 2008;80:4095. doi: 10.1021/ac800231k. [DOI] [PubMed] [Google Scholar]
- 7.(a) Shi Y, Peng D-D, Shi C-H, Zhang X, Xie Y-T, Lu B. Food chemistry. 2011;126:1916. doi: 10.1016/j.foodchem.2010.12.020. [DOI] [PubMed] [Google Scholar]; (b) Mishra A, Joy K. General and comparative endocrinology. 2006;145:84. doi: 10.1016/j.ygcen.2005.07.006. [DOI] [PubMed] [Google Scholar]; (c) Jiang T, Zhao L, Chu B, Feng Q, Yan W, Lin J-M. Talanta. 2009;78:442. doi: 10.1016/j.talanta.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 8.(a) Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S. Steroids. 2007;72:666. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]; (b) Budzinski H, Devier M, Labadie P, Togola A. Analytical and bioanalytical chemistry. 2006;386:1429. doi: 10.1007/s00216-006-0686-9. [DOI] [PubMed] [Google Scholar]
- 9.(a) Hosogi J, Tanaka H, Fujita K, Kuwabara T, Ikegawa S, Kobayashi N, Mano N, Goto J. Journal of Chromatography B. 2010;878:222. doi: 10.1016/j.jchromb.2009.08.010. [DOI] [PubMed] [Google Scholar]; (b) Vega-Morales T, Sosa-Ferrera Z, Santana-Rodríguez J. Journal of Hazardous Materials. 2010;183:701. doi: 10.1016/j.jhazmat.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 10.(a) Kim YS, Jung HS, Matsuura T, Lee HY, Kawai T, Gu MB. Biosensors and Bioelectronics. 2007;22:2525. doi: 10.1016/j.bios.2006.10.004. [DOI] [PubMed] [Google Scholar]; (b) Huang K-J, Liu Y-J, Zhang J-Z, Cao J-T, Liu Y-M. Biosensors and Bioelectronics. 2015;67:184. doi: 10.1016/j.bios.2014.08.010. [DOI] [PubMed] [Google Scholar]; (c) Huang K-J, Liu Y-J, Shi G-W, Yang X-R, Liu Y-M. Sensors and Actuators B: Chemical. 2014;201:579. [Google Scholar]
- 11.Long F, Shi H, Wang H. RSC Advances. 2014;4:2935. [Google Scholar]
- 12.(a) Dou M, Dominguez DC, Li X, Sanchez J, Scott G. Analytical chemistry. 2014;86:7978. doi: 10.1021/ac5021694. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dou M, Sanjay ST, Benhabib M, Xu F, Li X. Talanta. 2015;145:43. doi: 10.1016/j.talanta.2015.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sanjay ST, Fu G, Dou M, Xu F, Liu R, Qi H, Li X. Analyst. 2015;140:7062. doi: 10.1039/c5an00780a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liu P, Li X, Greenspoon SA, Scherer JR, Mathies RA. Lab on a Chip. 2011;11:1041. doi: 10.1039/c0lc00533a. [DOI] [PubMed] [Google Scholar]; (e) Shen F, Li X, Li PC. Biomicrofluidics. 2014;8:014109. doi: 10.1063/1.4866358. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Li X, Valadez AV, Zuo P, Nie Z. Bioanalysis. 2012;4:1509. doi: 10.4155/bio.12.133. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Li XJ, Zhou Y. Microfluidic devices for biomedical applications. Elsevier; 2013. [Google Scholar]
- 13.(a) Nightingale AM, Bannock JH, Krishnadasan SH, O'Mahony FT, Haque SA, Sloan J, Drury C, McIntyre R. Journal of Materials Chemistry A. 2013;1:4067. [Google Scholar]; (b) Song H, Chen DL, Ismagilov RF. Angewandte chemie international edition. 2006;45:7336. doi: 10.1002/anie.200601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuo P, Li X, Dominguez DC, Ye B-C. Lab Chip. 2013;13:3921. doi: 10.1039/c3lc50654a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang H, Tang L, Wang Y, Jiang J, Li J. Analytical Chemistry. 2010;82:2341. doi: 10.1021/ac9025384. [DOI] [PubMed] [Google Scholar]
- 16.Guo MT, Rotem A, Heyman JA, Weitz DA. Lab on a Chip. 2012;12:2146. doi: 10.1039/c2lc21147e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.