Summary
In patients presenting with a clinically isolated syndrome (CIS), magnetic resonance imaging (MRI) can support and substitute clinical information for multiple sclerosis (MS) diagnosis demonstrating disease dissemination in space (DIS) and time (DIT) and helping to rule out other conditions that can mimic MS. From their inclusion in the diagnostic work-up for MS in 2001, several modifications of MRI diagnostic criteria have been proposed, in the attempt to simplify lesion-count models for demonstrating DIS, change the timing of MRI scanning for demonstrating DIT, and increase the value of spinal cord imaging.
Since the last update of these criteria, new data regarding the application of MRI for demonstrating DIS and DIT have become available and improvement in MRI technology has occurred. State-of-the-art MRI findings in these patients were discussed in a MAGNIMS workshop, the goal of which was to provide an evidence-based and expert-opinion consensus on diagnostic MRI criteria modifications.
Keywords: Multiple Sclerosis, Magnetic Resonance Imaging, Diagnosis, Criteria
Introduction
Magnetic resonance imaging (MRI) was formally included in the diagnostic work-up of patients presenting with a clinically isolated syndrome (CIS) suggestive of multiple sclerosis (MS) in 2001 by an International Panel of experts.1 MS diagnosis requires demonstration of disease dissemination in space (DIS) and time (DIT) and exclusion of other conditions that can mimic MS by their clinical and laboratory profile. MRI can support and substitute clinical information for MS diagnosis, allowing an earlier and accurate diagnosis and, consequently, earlier treatment.
MRI criteria for MS are based on the presence of focal lesions in the white matter (WM) of the central nervous system (CNS), which are considered typical for this condition in terms of distribution, morphology, evolution, and signal abnormalities on conventional MRI sequences (e.g., T2-weighted, T2-FLAIR, pre- and post-contrast T1-weighted scans).2–4 Several modifications of MRI diagnostic criteria have been proposed over the years. These revisions have simplified the lesion-count models for demonstrating DIS, changed the timing of MRI scanning for demonstrating DIT, and increased the value of spinal cord imaging.5–8 In 2007, the European collaborative research network that studies MRI in MS (MAGNIMS) reviewed the findings of studies that addressed these issues and proposed new MRI criteria to be applied in MS.9 Those MAGNIMS criteria are currently included in the most recent of the MS diagnostic criteria, known as the 2010 McDonald criteria.10 Recent consensus guidelines for clinicians regarding optimization of the planning, performance, and interpretation of brain and spinal cord MRI in the MS diagnostic process have also been published and are complementary to the recommendations in this paper.11
Since 2011, new data regarding the application of MRI for demonstrating DIS and DIT have become available, and these deserve consideration for future revisions of the MS criteria. Additionally, many improvements in MRI technology have occurred, which resulted in the development of innovative acquisition sequences, the identification of novel pathophysiological mechanisms that may help in the differential diagnosis, and new insights into MS disease activity as evidenced by high-field and ultra-high-field scanners. The MAGNIMS members felt the need for timely revision of these recent findings and consideration of how they should modify the MRI criteria for the diagnosis of MS. A summary of the main revisions or clarifications to the MRI component of the 2010 McDonald criteria for MS we propose is presented in the stand alone panel.
Stand alone panel.
Recommended modifications to the criteria
Three or more lesions should be required to define the involvement of the periventricular region for disease dissemination in space (DIS) (expert consensus, 100% agreement);
The presence of a lesion in the optic nerve should be added to the DIS criteria as an additional CNS area, increasing the number of DIS locations from 4 to 5 (expert consensus, 100% agreement);
At present, intracortical, leukocortical and juxtacortical lesions cannot be reliably and consistently distinguished on conventional MRI scans using most available MRI scanners in the clinical setting. These should be combined in a single term (“cortical/juxtacortical lesions”) that indicates the involvement of the white matter next to the cortex and/or the involvement of the cortex, thereby expanding the term “juxtacortical” lesion that was used in the 2010 McDonald DIS criteria. When available, advanced imaging sequences should be applied to visualize CLs (expert consensus, 100% agreement);
No distinction needs to be made between symptomatic and asymptomatic MRI lesions for both DIS and DIT (evidence based);12–15
Imaging of the whole spinal cord is recommended to define DIS (particularly in those patients not fulfilling brain MRI for DIS). There is a limited role of spinal cord imaging for DIT (evidence based);16–19
Use identical DIS criteria for primary progressive (PP) MS and relapse-onset MS (expert consensus, 100% agreement). Consider cerebrospinal fluid (CSF) results for clinically uncertain PPMS cases (evidence based);20
In children ≥ 11 years with non-ADEM-like presentation, use MRI DIS and DIT criteria identical to those applied in adults (evidence based);21–26
Use caution when applying the 2010 criteria solely at baseline in a patient younger than age 11 years, even in those with a non-ADEM presentation. Clinical and MRI serial evaluation to confirm new lesions over time may be particularly important in this age group (evidence based);27
Identical DIS and DIT MRI criteria used in MS should be applied for the evaluation of radiologically isolated syndromes (RIS). When a clinical attack occurs in RIS-DIT positive subjects (who, by definition, have DIS), a diagnosis of MS can be made (expert consensus, 100% agreement);
MRI criteria apply equally well to MS in Asia or Latin America, once alternative neurological conditions (e.g., NMOSD) have been carefully excluded (evidence based).28–31
Additional clarifications and summary statements
The criteria for disease dissemination in time (DIT) can remain unchanged;
The presence of non-enhancing black holes is not useful as a potential alternative criterion for demonstrating DIT in adults. The contribution of non-enhancing black holes appears more robust in distinguishing pediatric patients with MS from children with monophasic demyelination (acute disseminated encephalomyelitis in particular);
In the case of atypical imaging presentation, always consider other acquired and inherited WM diseases in the differential diagnosis;
At present, there is limited evidence of an earlier diagnosis of MS when using high- or ultra-high-field scanners. However, lesion features distinctive of MS could emerge from the use of these scanners and may eventually enhance the differentiation of MS from other diseases.
Methods
In March 2015, an international workshop was held in Milan, Italy, under the auspices of MAGNIMS. The workshop involved clinical and imaging experts in the diagnosis and management of patients with MS, and included neurologists and neuroradiologists. Before the meeting, two co-chairs (MF and FB) identified areas where revision and/or clarification might be necessary in future MS diagnostic criteria. Experts for each topic were invited to provide a summary during the meeting of the main findings related to their argument, based on revision of the literature and on their personal experience. Afterward, they were asked to define whether such a measure would be useful or not in the diagnostic process, and whether it would move the field forward in a promising way, in order to stimulate group discussion. For each measure, a group agreement was reached during the workshop and summarized in a first draft, which was circulated among the meeting participants as well as some additional experts in the field for critical discussion and revision.
MRI criteria for DIS
According to the 2010 McDonald criteria for MS,10 DIS can be demonstrated with at least one T2 lesion in at least 2 of 4 locations characteristic for MS (juxtacortical, periventricular, infratentorial, and spinal cord). We propose to increase the number of lesions necessary to confirm the involvement of the periventricular area from 1 to 3, and to add an additional cardinal CNS location, the optic nerve (Table 1).
Table 1.
Proposed 2015 MAGNIMS DIS criteria
| DIS can be demonstrated by the involvement* of at least 2 out of 5 areas of the CNS as follows: |
| ≥ 3 periventricular lesions |
| ≥ 1 infratentorial lesion |
| ≥ 1 spinal cord lesion |
| ≥ 1 optic nerve lesion |
| ≥ 1 cortical/juxtacortical lesion^ |
If a subject has a brainstem or spinal cord syndrome, or optic neuritis, the symptomatic lesion(s) are not excluded from the criteria and contribute to lesion count.
This combined terminology indicates the involvement of the white matter next to the cortex and/or the involvement of the cortex, thereby expanding the term “juxtacortical” lesion.
Periventricular lesions
A single lesion was deemed not sufficiently specific to define whether the involvement of the periventricular region is due to a demyelinating inflammatory event. Indeed, incidental periventricular lesions can be detected in healthy individuals and patients with other neurological conditions, including up to 30% of patients with migraine.32 Importantly, ≥3 periventricular lesions was the most accurate threshold determined by receiver-operating curve analysis in Barkhof et al.4 and was therefore applied in previous McDonald criteria.1, 8 A formal validation of the value of 1 periventricular lesion for DIS has never been performed. The analysis of a large cohort of 652 CIS patients has shown that in patients not satisfying DIS criteria for MS, the presence of 3 periventricular lesions, combined with age or presence of oligoclonal bands (OB), is helpful in identifying those at risk for MS.33 In a retrospective study in patients with spinal cord CIS, a prediction model, including age ≤40 years, ≥3 periventricular lesions, and intrathecal immunoglobulin synthesis identified, with an accuracy of 78%, patients who would evolve to MS.34 In a multicenter trial of 468 CIS patients, the presence of at least 3 periventricular lesions had a strong prognostic value for conversion to MS over a 3-year period.35 In a study comparing patients with MS and those with primary and secondary CNS vasculitis, the presence of ≥3 periventricular lesions was the only individual component of the Barkhof criteria that was able to distinguish MS from systemic lupus erythematosus and Sjögren’s syndrome.36
In the pediatric arena, however, the presence of a single periventricular lesion (as well as one or more T1 hypointense lesions) powerfully distinguished children with MS from children with monophasic demyelination.37
In summary, expert consensus was that three or more periventricular lesions are required to contribute to the demonstration of DIS.
Optic nerve lesions
Around 20–31% of CIS patients present with acute optic neuritis.38–40 Compared to other clinical presentations, adult patients with optic neuritis are more likely than those with acute demyelination in other CNS locations to have a monophasic illness,38, 41, 42 as also confirmed by a recent study that enrolled 1058 CIS patients.39 Importantly, in this cohort and in other studies, the likelihood of optic neuritis being a monophasic illness is dramatically reduced in the presence of CSF OB and/or clinically silent brain MRI lesions (with a hazard ratio [HR] of 5.1 for patients with one to three lesions and 11.3 for patients with 10 or more lesions). The presence of even one clinically silent T2 hyperintense brain lesion in children with optic neuritis is highly associated with confirmation of a MS diagnosis,43 whereas the absence of brain lesions strongly predicts a monophasic illness.27
Clinical features of optic neuritis (visual impairment, scotoma, red-green desaturation, pain with ocular movement), MRI evidence of optic nerve inflammation (increased T2 signal, gadolinium enhancement, optic nerve swelling), ocular coherence tomography (evidence of retinal nerve fiber layer thinning) and neurophysiological abnormalities (particularly delayed visual evoked potentials) all support inclusion of the optic nerve as an additional CNS area that may be affected at CIS onset. Clinical documentation of optic nerve atrophy or pallor, neurophysiological confirmation of optic nerve dysfunction (slowed conduction), or imaging features of clinically silent optic nerve inflammation (MRI lesions or retinal nerve fiber layer thinning) support DIS, and in patients without concurrent visual symptoms, also support DIT.
In summary, expert consensus was that optic nerve involvement should constitute an additional item to meet DIS criteria.
Cortical lesions
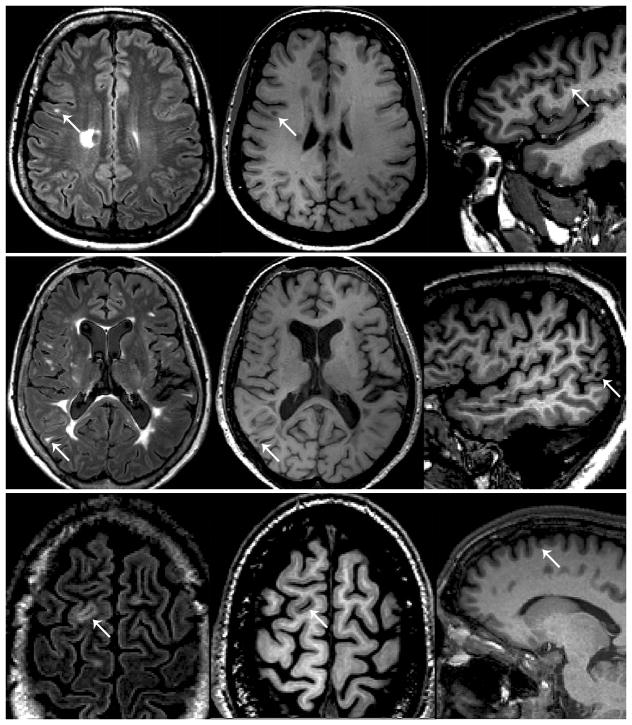
Pathology studies have shown extensive involvement of the gray matter (GM) in MS.44–46 According to their location within the GM, different cortical lesion (CL) locations (subpial, purely intracortical, and leukocortical lesions abutting the GM-WM border) have been identified.45 Imaging CL is challenging, particularly using conventional clinical MRI protocols. Different MRI techniques have been proposed and are currently being compared for their sensitivity to CL detection, including double inversion recovery (DIR),47 phase-sensitive inversion recovery (PSIR)48–50 and magnetization-prepared rapid acquisition with gradient echo51 sequences (Figure 1). Despite this, correlative MRI-pathology studies have shown that many CLs remain invisible on MRI, at least at 1.5 and 3.0 tesla MRI strengths.52, 53
Figure 1.
Examples of lesion classification based on integrated analysis of double inversion recovery (DIR) (left column) and magnetization-prepared rapid acquisition with gradient echo (MPRAGE) (middle and right columns) sequences. Top row: a hyperintense lesion close to the cortex (white arrow) is visible on DIR, but MPRAGE shows that the lesion is located in the white matter. Middle row: a hyperintense lesion close to the cortex (white arrow) is visible on DIR, and MPRAGE shows that the location abuts the cortex (juxtacortical). Bottom row: a hyperintense lesion close to the cortex (white arrow) is visible on DIR, and MPRAGE shows that the lesion is intracortical. Under the proposed system, the lesions in the middle and bottom rows would be classified as “cortical/juxtacortical.”
Using DIR sequences, CLs have been identified in more than 30% of CIS patients.54, 55 In a cohort of 80 CIS patients with 4-year follow-up, the accuracy of MRI diagnostic criteria for MS was increased when considering the presence of at least 1 intracortical (IC) lesion on baseline scans.55 CL assessment may also help in the differential diagnosis between MS and MS-mimicking conditions, since they have not been found in patients with migraine with WM T2 lesions32 or neuromyelitis optica (NMO).56 IC lesions are also rare in healthy controls (1/60 subjects using PSIR sequences).49
Even with these promising results, there remain many unsolved issues related to the inclusion of CL assessment in the diagnostic work-up of CIS. First, the MRI sequences used in the research setting for the identification of these lesions may not be available and easily implementable on most clinical scanners. Second, the acquisition parameters for these sequences still need to be standardized on systems from different manufacturers and of various field strengths. Third, inter-observer agreement in the assessment of these sequences is at best moderate (complete agreement=19% for DIR), and guidelines for their evaluation are evolving.49, 57 Fourth, different criteria and terms are currently being applied by different research groups for the distinction between IC, leukocortical, mixed WM/GM and juxtacortical lesions.47–50, 55 Additionally, subpial demyelination, which can be quite extensive, is usually not scored.46
In summary, expert consensus was that the combined term “cortical/juxtacortical” is recommended to expand the concept of juxtacortical lesion in the DIS criteria, by including all MS CLs types and/or the involvement of the WM next to the cortex. When available, advanced imaging sequences should be applied to visualize CLs.
MRI criteria for DIT
According to the 2010 McDonald criteria,10 DIT can be demonstrated by: (1) at least one new T2 and/or gadolinium-enhancing lesion on follow-up MRI, with reference to a baseline scan, irrespective of the timing of the baseline MRI; or (2) the simultaneous presence of asymptomatic gadolinium-enhancing and non-enhancing lesions at any time.
Non-enhancing T1-hypointense lesions (black holes)
Non-enhancing T1-hypointense lesions (black holes) are chronic lesions characterized by severe axonal damage.58 In relapsing-remitting (RR) MS, brain T1-hypointense lesion volume increases by approximately 11% per year and correlates with long-term disability progression.59, 60 T1-hypointense lesion formation is more common in patients with longer disease durations and progressive disease subtypes. For that reason, their presence in CIS patients is indicative of an already-established MS disease process. The prevalence of non-enhancing T1-hypointense lesions and their added value in identifying adult patients with MS was analyzed in a large multicenter study of 520 CIS patients.61 Non-enhancing black holes were relatively common in adult CIS patients (36%) and were associated with a higher likelihood of MS diagnosis. However, the value of this MR finding for predicting a second clinical attack in these patients was lost when added to the other criteria.61 Of note, T1-hypointese lesion assessment is still rather subjective and highly dependent on the type of T1-weighted sequence and field strength. Nevertheless, in pediatric patients with acute demyelination, the presence of one or more T1-hypointense lesion was highly correlated with subsequent confirmation of MS.37
In summary, the criteria for DIT remain unchanged. The presence of non-enhancing black holes should not be considered as a potential alternative criterion for demonstrating DIT in adult MS patients.
Symptomatic lesions
In CIS patients, symptomatic lesions that align with an acute clinical deficit currently do not contribute to the DIS or DIT component of the MS diagnostic criteria.10 Specifically, in patients with brainstem or spinal cord syndromes, lesions within the symptomatic region cannot be counted for demonstration of DIS. The simultaneous presence of asymptomatic gadolinium-enhancing and non-enhancing lesions at any time is a criterion to define DIT.
In CIS patients presenting with brainstem symptoms, a 2004 study showed that the specificity of MRI criteria for DIS (Barkhof’s criteria at that time) was lower (61%) than that found in other CIS (myelitis and optic neuritis) (73%).62 A recent investigation12 assessed the likelihood of MS confirmation in 35/954 patients (3%) with one single symptomatic lesion in the brainstem or spinal cord with a follow-up of almost 8 years. The HR of MS was higher for patients with a symptomatic lesion (HR=7.2) than for those with a single asymptomatic lesion (HR=5.7), or with no lesions (HR=1), in the same regions. Another retrospective study in 146 CIS patients who fulfilled the 2010 McDonald criteria10 found that the presence of a symptomatic lesion identifies with a high sensitivity those patients with MS.13 In a recent study of 30 CIS patients who were studied for a mean of 7.3 years after onset, the sensitivity/specificity/accuracy of the DIS criteria was 73/73/73% for the 2010 McDonald criteria, 80/73/77% when asymptomatic lesions in the symptomatic region were included, and 87/73/80% when any lesion in the symptomatic region was included.14 These results suggest that including lesions in the symptomatic region in DIS may increase the sensitivity of MRI criteria for diagnosing MS without compromising specificity.
The diagnostic impact of allowing any gadolinium-enhancing and non-enhancing lesions (not only asymptomatic, but also symptomatic) to count for demonstrating DIT has also been recently analyzed.15 Inclusion of symptomatic lesions in the DIT criteria increased the proportion of patients satisfying the MRI diagnostic criteria for MS to 33%, compared to 30% of those diagnosed without including such lesions, with three additional patients meeting the 2010 McDonald criteria. In fact, deciding what is symptomatic or not is often very difficult. It is relatively easy to apply in brainstem and spinal cord presentations but not in other clinical scenarios.
In summary, both symptomatic and asymptomatic lesions act for demonstrating DIS and DIT.
Spinal cord imaging
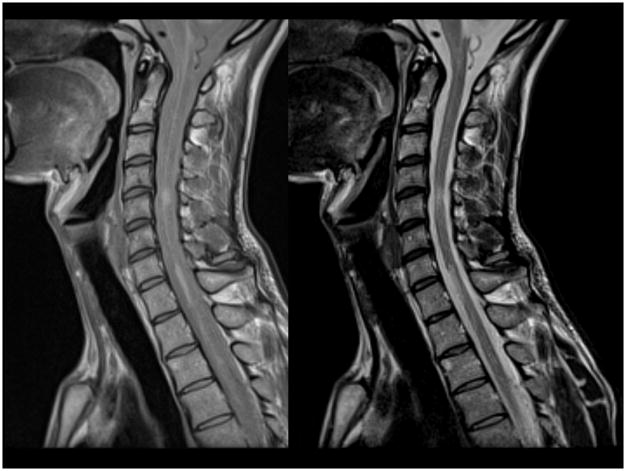
Based on the 2010 McDonald criteria,10 clinically silent spinal cord lesions can contribute to both DIS and DIT. At symptom onset, spinal cord imaging is recommended in patients with clinical features localized to the spinal cord to rule out alternative cord pathology (e.g., compression, spinal cord tumor, NMO, vasculitides) and in those with non-spinal CIS not fulfilling brain MRI for DIS. In this second group, whole cord imaging showed that the presence of one spinal cord lesion identifies patients at higher risk of MS confirmation.16 Imaging of the entire cord, using at least two MR sequences (e.g., T2 and STIR, T2 and DIR, T2 and post-contrast T1) is preferable to increase confidence in lesion identification, in part because approximately 40% of spinal cord lesions are found in the thoracolumbar region (Figure 2).17–19 The value of spinal cord imaging for DIT in patients without accrual of deficits referable to the spine is limited, since new clinically silent cord lesions are not frequent.
Figure 2.
Sagittal intermediate and T2-weighted dual echo fast-spin echo images of the spinal cord in a patient with multiple sclerosis. Note the presence of abnormalities both at the cervical and thoracic level of the cord.
In summary, whole spinal cord MRI is recommended to meet DIS criteria, whereas it has a limited role for DIT.
Primary progressive MS
In the different formulations of the diagnostic criteria, the diagnosis of primary progressive (PP) MS has always been kept separate from that of the more common relapse-onset form of the disease. In 2009, there was a proposal of unification of DIS MRI criteria for PPMS and relapsing-MS,63 which was only partially integrated in the 2010 McDonald criteria.10 Indeed, according to these criteria, DIS in PPMS was defined by the occurrence of two of the following three criteria: (1) DIS in the brain, based on the presence of at least one lesion in at least one area characteristic for MS (periventricular, juxtacortical, or infratentorial); (2) DIS in the spinal cord, based on the presence of at least two lesions in the spinal cord; and (3) positive CSF examination.
The sensitivity of the spinal cord criteria and the utility of CSF examination was retrospectively analyzed in a cohort of 95 PPMS patients.20 That study found that if the requirement for two or more cord lesions was changed to one or more cord lesions (whether symptomatic or not), a higher number of patients would meet the spinal cord criteria for diagnosis, with increasing sensitivity and simplification of the criteria. However, specificity of these simplified criteria still requires testing.
In summary, expert consensus was that identical DIS criteria should be used for PPMS and relapse-onset MS.
MRI criteria in pediatric populations
The 2010 consensus was that the proposed MRI criteria also served for most pediatric MS patients. An alert was specified that the use of the 2010 McDonald criteria for MS at baseline was not applicable for children with encephalopathy and multifocal neurological deficits meeting criteria for ADEM.10 Such children have multiple lesions, some of which may enhance, yet when defined using international consensus criteria for ADEM, 95% of such children have a monophasic illness.27 The diagnosis of MS in pediatric patients manifesting initially with an ADEM-like first attack requires clinical and/or MRI evidence of further non-ADEM attacks and/or accrual of clinically silent MRI lesions.
Several studies have confirmed that the 2010 McDonald criteria perform better or similar to previous proposed pediatric MS criteria in children with non-ADEM presentations and in pediatric patients older than 11 years.21–26 While a study from 52 patients has suggested that inclusion of spinal cord imaging at first attack does not increase the accuracy of the 2010 McDonald criteria,25 a retrospective investigation of 85 patients showed that the addition of spinal cord MRI was helpful in reaching DIS and DIT in 10% of the cases.22
In summary, the use of MRI DIS and DIT criteria identical to those applied in adult is suggested in children ≥ 11 years, with non-ADEM-like presentation.
MRI criteria in non-Caucasian populations
The 2010 McDonald criteria have been developed and mostly tested in typical adult Caucasian European and North American populations, and their current formulation states that they require validation in Asian and Latin American populations.10 Between 2011 and 2015, the performance of MRI diagnostic criteria has been tested in Korean,28 Taiwanese,29 Argentinean (including a sub-analysis applied only to non-European descendants, i.e., mestizos, natives, and zambos),30 and Russian31 CIS patients, after careful exclusion of alternative neurological conditions, such as NMO/NMOSD in Korean patients.28 All these studies provided evidence that the 2010 McDonald criteria apply well irrespective of world region.
In summary, DIS and DIT MRI criteria apply equally well to MS patients from Asia and Latin America.
Radiologically isolated syndromes (RIS)
The availability of MRI evaluation for indications unrelated to MS has led to an increased recognition of individuals with incidental brain lesions consistent with MS. Criteria have been proposed to identify imaging features that may be suggestive of a clinically asymptomatic demyelinating condition, including the fulfillment of at least three of four Barkhof criteria for DIS.64, 65 The 2010 McDonald criteria concluded that “a firm diagnosis of MS based on incidental findings on MRI alone, even with additional supportive findings on evoked potentials or typical CSF findings in the absence of MS-relevant clinical symptoms, is problematic.” We propose that identical DIS and DIT MRI criteria used in MS should be applied for the evaluation of RIS, and that when a clinical attack occurs in RIS-DIT positive subjects (who by definition have DIS), a diagnosis of MS can be made. Thus, it was agreed that persons should not be diagnosed with MS on the basis of MRI findings alone, at least one clinical event consistent with acute demyelination remains a cornerstone for MS diagnosis.
The use of advanced MRI techniques to characterize CNS involvement in RIS subjects has shown extensive axonal damage (measured using MR spectroscopy)66 and a perhaps surprisingly high percentage (40%) of subjects with CLs (which were more frequent in subjects with CSF OB, cervical cord lesions, and DIT on brain MRI).67
Approximately two-thirds of RIS subjects develop new lesions on longitudinal MRI scans and one-third of people with RIS develop neurological symptoms within five years, especially those with gadolinium-enhancing or spinal cord lesions.68 In persons with clinically silent brain lesions consistent with MS, the presence of OBs, younger age, male sex, and abnormal visual evoked potentials identify individuals more likely to experience a sentinel clinical attack. Just focusing on MRI, the presence of gadolinium-enhancing lesions69 and of asymptomatic spinal cord lesions (cervical or thoracic) are predictors of clinical evolution.68, 70
At present, there is the need for a more specific characterization of people with RIS and of prospective long-term studies to estimate the risk for these subjects to become MS. As a consequence, a firm recommendation concerning RIS is not possible. It is clear, however, even at this stage, that individuals bearing several risk factors need to be distinguished from those without these factors, since they are likely to have a prodromal condition, and that specific requirements are needed for a prompt diagnosis when the first symptom of CNS involvement occurs.
In summary, expert consensus was that RIS patients should be evaluated with DIS and DIT criteria identical to those used for MS.
MRI in differential diagnosis (including atypical demyelination and NMO)
The exclusion of alternative diagnoses that can mimic MS is imperative in applying the 2010 McDonald criteria.10 From an imaging perspective, many inherited and acquired disorders may manifest with evidence of DIT, DIS, or both, and these should be included in the differential diagnosis of MS-like lesions. A timely recognition of imaging “red flags” in the work-up of patients suspected of having MS should alert clinicians to reconsider the differential diagnosis more extensively and perform some additional analyses.71 Several reviews have been published on imaging features of the main acquired and inherited conditions that can enter the differential diagnosis of MS.71–73
In the 2010 McDonald criteria, a specific focus was the differential diagnosis between MS and NMO and NMO spectrum disorders (NMOSD). Up to 70% of NMOSD patients at onset have brain MRI lesions. The brain, optic nerve and spinal cord MRI findings of NMOSD patients have been recently reviewed,74 and revised diagnostic criteria for NMOSD have been proposed.75 The International Panel for NMO diagnosis proposed the use of the unifying term NMOSD, which was stratified further by aquaporin-4 immunoglobulin G antibody (AQP4-IgG) testing. According to this revision, for patients with a positive AQP4-IgG test, at least one core clinical characteristic is required for NMOSD diagnosis; these include clinical syndromes or MRI findings related to optic nerve, spinal cord, area postrema, other brainstem, diencephalic, or cerebral presentations. For AQP4-IgG negative patients or patients with unknown AQP4-IgG status, more stringent clinical criteria, with additional neuroimaging findings, are required. In particular, acute optic neuritis requires brain MRI showing (1) normal findings or only nonspecific WM lesions, or (2) optic nerve MRI with a T2-hyperintense or T1-weighted gadolinium-enhancing lesion extending over half the optic nerve length or involving the optic chiasm. Acute myelitis requires an associated intramedullary MRI lesion extending over 3 contiguous segments (longitudinally extensive transverse myelitis, or LETM) or 3 contiguous segments of focal spinal cord atrophy in patients with a history compatible with acute myelitis. The area postrema syndrome requires associated dorsal medulla/area postrema lesions. Finally, an acute brainstem syndrome requires associated peri-ependymal brainstem lesions.
In summary, when atypical imaging presentation occurs other acquired and inherited WM conditions should be considered.
High field and ultra-high field scanners
High-field scanners (3.0 tesla)
Compared to 1.5 tesla, the use of high field-strength scanners (3.0) allows detection of a significantly higher number of lesions in CIS patients,76, 77 with improved recognition of lesions involving the cortical,78 infratentorial, and periventricular regions.76 The comparison of MRI criteria performance at 1.5 vs 3.0 tesla in 40 CIS patients showed that one additional patient was diagnosed with DIS at high field, without improvement for DIT.79
Ultra-high-field scanners (7.0 tesla)
Ultra-high-field MRI allows detection of a significantly higher number of lesions,80 as well as better definition of lesions located in the WM and GM with respect to their morphology and association with the vasculature,81–85 than what was previously shown by using 1.586 or 3.087 tesla scanners. Whether the assessment of lesion number and distribution using ultra-high-field MRI scanners assists in making an earlier diagnosis of MS in CIS patients has not yet been evaluated. Several studies have identified some interesting lesion characteristics, which can aid the differential diagnosis between MS and other neurological conditions. The better definition of the relationship between demyelinating lesions and the intraparenchymal venous system, obtained by using T2*-weighted magnitude and phase imaging, confirms pathological studies demonstrating that many MS plaques form around the microvasculature.81–85, 88, 89 The perivenular lesion location can help to distinguish WM lesions in MS patients from incidental (ischemic) WM lesions.85, 89 This finding has been reinforced by investigation of blood-brain barrier abnormalities in MS at 7.0 (but also 3.0) tesla, which showed that the majority of enhancing lesions are perivenular and that the smallest lesions have a centrifugal pattern of enhancement, suggesting that they grow outward from a central vein.90, 91 The presence of a central small vein and a rim of hypointensity on 7.0 tesla T2*-weighted magnitude or FLAIR*85 could be a distinctive feature of MS WM lesions, which may assist in the differentiation from lesions of patients with NMOSD92 or Susac syndrome.93
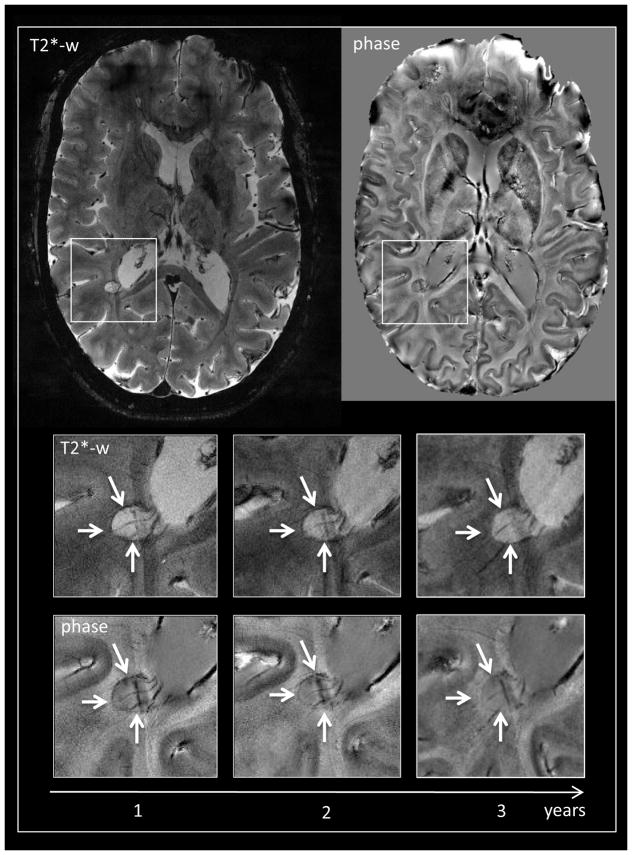
A limited number of studies have tracked the longitudinal evolution of the above-mentioned abnormalities (Figure 3). A longitudinal study of 29 patients with possible but unclear diagnosis has shown that the presence of a central vein in most lesions accurately identifies MS patients.80 Another study has shown that phase-ring lesions remained unchanged over a 2.5 year period in five RRMS patients,94 whereas such a ring can be transient in acute lesions.90, 91
Figure 3.
7T T2*-weighted gradient-echo and phase axial images of a 36-year-old woman with relapsing-remitting MS. A periventricular non-enhancing lesion with a paramagnetic rim (white arrows) is displayed. A prominent central vein is visible on all images. The lesion maintains the same morphological features in a medium-term follow up (magnified boxes).
In summary, the use of high- or ultra-high-field scanners is not likely to result in an earlier diagnosis.
Future perspectives
The Panel also noted that some promising measures deserve further investigation before being moved (or not) to diagnostic criteria in the future.
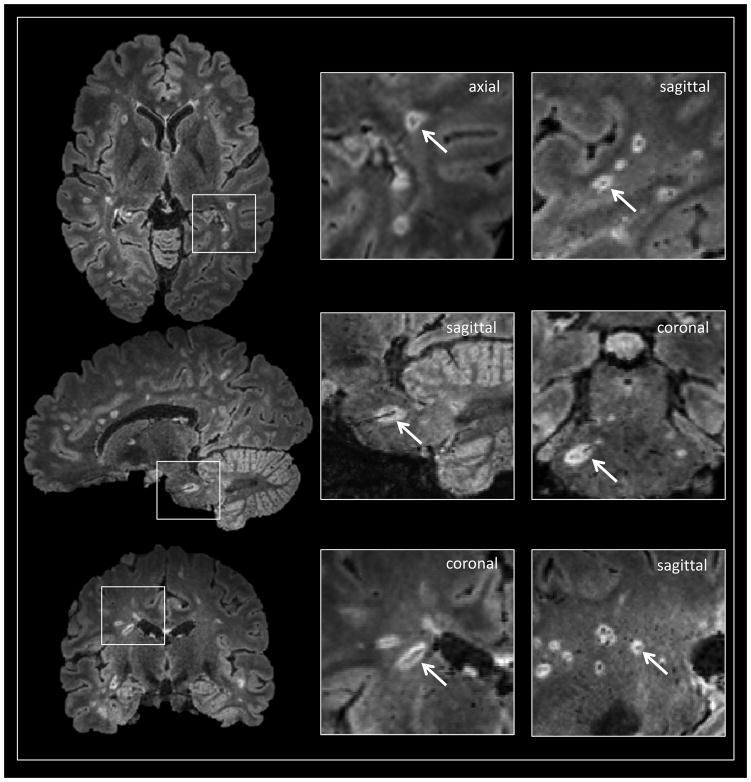
Concerning the identification of the central vein, what remains to be done is the standardization of sequences capable of showing these features on 3.0 and 1.5 tesla scanners, as well as the creation of standardized definitions for identification of central vessels. To date, central vessels have been if they (1) could be visualized in at least 2 perpendicular planes, (2) appeared linear in at least one plane, and (3) were completely surrounded by hyperintense signal in at least one plane (Figure 4).89 Whether central veins are indeed confirmatory for MS lesions requires further study with appropriate disease comparisons.
Figure 4.
Noncontrast 3T FLAIR* images (axial, sagittal and coronal views) in a 33-year-old woman with MS. A conspicuous central vessel is clearly visible in the majority of hyperintense lesions. The definition of “perivenular” lesion requires the visualization of the central vessel in at least two perpendicular views (arrows in magnified boxes).
Concerning the identification of the hypointense lesional rim on T2*-weighted magnitude and/or phase images, what remains to be done is the performance of longitudinal studies at both 3.0 and 7.0 tesla; the clinical implementation and standardization of MR sequences among different vendors at 3.0 tesla; the analysis of the value in predicting conversion to MS and disability progression in CIS patients; the study of different MS clinical phenotypes and other neurological conditions that can mimic MS.
Concerning the identification of cortical pathology, higher field-strength imaging is expected to identify CLs more reliably than conventional MRI but is not likely to be available in clinical practice in the near term. More advanced techniques for CL identification at 3.0 tesla may prove valuable in MS diagnosis. The definition of standardized, up-to-date guidelines for CL classification is also pending.
Closing remarks
Reading of MRI scans should be done in the appropriate clinical context. The premise of these guidelines and criteria is that we assume a basic knowledge of what constitutes a lesion. The largest linear measurement for lesion definition should be ≥3 mm in at least one plane. Therefore, lesion identification should be done by trained, expert personnel. Image quality must be of a high standard. A conservative approach in identifying lesions should be adopted.
In the diagnostic work-up of patients with a suspicion of MS, the use of post-contrast sequences provides important information for the differential diagnosis. However, it should be noted that the Food and Drug Administration (FDA) has recently made a safety communication for the long-term effects of repeated administration of gadolinium-based contrast agents (GBCAs), following the description of deposition of gadolinium in the brains of some patients who underwent multiple contrast-enhanced MRI scans (http://www.fda.gov/downloads/Drugs/DrugSafety/UCM455390.pdf). At the time of this writing, the impact of these safety concerns remains unknown.
MRI remains a valuable tool in identification of children and adults with MS, both at the time of an incident attack and when applied serially to confirm the chronic nature of this disease. More advanced imaging techniques inform on regional CNS involvement with greater sensitivity and may add to diagnostic specificity. Whether MRI features consistent with MS in the absence of clinical involvement can confirm MS diagnosis remains an area of controversy that requires further study and deliberation, particularly given evidence that some such individuals demonstrate focal and global loss of tissue integrity yet are not currently eligible for MS-directed therapies. As higher field-strength imaging and newer sequences better approximate pathology-level interrogation of the CNS, the fundamental question of what defines a disease like MS will need to be answered.
Acknowledgments
We are very grateful to Professor Douglas Arnold (Brain Imaging Center, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada) for his fruitful discussion during the meeting and his subsequent thoughtful comments to the manuscript. We also thank Drs. Martina Absinta (Neuroimaging Research Unit, Institute of Experimental Neurology, Division of Neuroscience, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy) and Pascal Sati (Translational Neuroradiology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland, USA) for providing us Figures 3 and 4.
Sponsor- The panel meeting was supported by an unrestricted educational grant from Novartis. The funding source had no role in the preparation of this article.
Search strategy and selection criteria
References for this Review were identified through searches of PubMed with the search terms “Clinically Isolated Syndrome”, “Multiple Sclerosis”, “McDonald criteria”, “Diagnosis”, “Differential diagnosis”, “Cortical Lesions”, “White matter”, “Lesions”, “Cortical Lesions”, “Brain”, “Spinal Cord”, “MRI”, “Optic Nerve”; “Disease Dissemination in Spce”; “Disease Dissemination in Time”; “Radiologically Isolated Syndromes”; “Pediatric MS”; “T1-hypointense lesions”; “Symptomatic Lesions”, “Primary Progressive Multiple Sclerosis”; “Non-Caucasian Populations”; “Neuromyelitis Optica”; “Neuromyelitis Optica Spectrum Disorders”; “High Field”; and “Utra-high field” from 1979 until 15th November 2015. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review.
Authors’ contribution
MF and FB had the idea of organizing the meeting, chaired it, and framed the structure of this manuscript. AR, FB, JSG, LK, MAR, MT, NDS, NE and OC participated to the meeting, summarized different aspects for the discussion and took part to the discussion. JF, CG, JP and DSR participated in the meeting and the discussion. BB and XM were involved after the meeting for critical discussion and revision. The complete manuscript was commented, revised and approved also by all the authors.
Conflict of interest statement
M. Filippi is Editor-in-Chief of the Journal of Neurology; serves on scientific advisory boards for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Biogen Idec, Excemed, Novartis, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Teva Pharmaceutical Industries, and Novartis.
M.A. Rocca received speakers honoraria from Biogen Idec, Novartis, Genzyme, Sanofi-Aventis and Excemed.
O. Ciccarelli serves as a consultant for GE, Biogen and Novartis and all the payments are made to the institution.
N. De Stefano has received honoraria from Schering, Biogen-Idec, Teva, Novartis, Genzyme, and Merck Serono S.A. for consulting services, speaking and travel support. He serves on advisory boards for, Biogen-Idec, Merck Serono S.A and Novartis.
N. Evangelou has received honoraria from Biogen, Novartis and Genzyme for consulting services, speaking and travel support. He serves on advisory boards for Biogen, Merck, and Novartis.
L. Kappos’ Institution (University Hospital Basel) received in the last 3 years and used exclusively for research support: steering committee, advisory board and consultancy fees (Actelion, Addex, Bayer Health Care, Biogen, Biotica, Genzyme, Lilly, Merck, Mitsubishi, Novartis, Ono Pharma, Pfizer, Receptos, Sanofi-Aventis, Santhera, Siemens, Teva, UCB, Xenoport); speaker fees (Bayer Health Care, Biogen, Merck, Novartis, Sanofi-Aventis, Teva ); support of educational activities (Bayer Health Care, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi, Teva); royalties (Neurostatus Systems GmbH); grants (Bayer Health Care, Biogen, Merck, Novartis, Roche, Swiss MS Society, the Swiss National Research Foundation, the European Union, Roche Research Foundations).
A. Rovira serves on scientific advisory boards for Biogen Idec, Novartis, Genzyme, and OLEA Medical, has received speaker honoraria from Bayer, Genzyme, Bracco, Merck-Serono, Teva Pharmaceutical Industries Ltd, OLEA Medical, Stendhal, Novartis and Biogen Idec, and has research agreements with Siemens AG.
J. Sastre-Garriga has received compensation for serving on scientific advisory boards or in speaker’s bureaus from Biogen, Merck-Serono, Novartis, Teva and Sanofi-Aventis.
M. Tintorè has received compensation for consulting services and speaking from Bayer-Schering, Merck-Serono, Biogen-Idec, Teva, Sanofi-Aventis, and Novartis.
J.L. Frederiksen has served on scientific advisory boards for and received funding of travel for these activities and honoraria from Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi-Aventis, Takeda and Teva.
C. Gasperini has received compensation for consulting from Bayer HealthCare and Biogen and as a speaker for lectures from Biogen, Bayer HealthCare, Genzyme, Merck Serono, Novartis and Teva.
J. Palace reports personal fees from Biogen Idec, personal fees from Teva Pharmaceuticals and an unrestricted research grant, personal fees from Merck Serono, grants from Merck Serono, personal fees from Bayer Schering, grants from Bayer Schering, personal fees from Novartis, grants from Novartis, personal fees from Chugai Pharma, personal fees from Ono Pharmaceuticals Co Ltd, personal fees from CI consulting, grants from MSS UK, grants from Guthy-Jackson Foundation, outside the submitted work.
D. Reich reports grants from Vertex Pharmaceuticals, outside the submitted work; In addition, Dr. Reich has a patent PCT/US2012/067997 pending, and a patent PCT/US2013/033334 pending.
B. Banwell serves as a centralized MRI reviewer for Novartis, and serves as an unpaid advisor regarding pediatric MS clinical trial design for Novartis, Biogen Idec, and Teva Neuroscience.
X. Montalban has received speaking honoraria and travel expenses for scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Actelion, Almirall, Bayer, Biogen, Genzyme, Merck, Novartis, Octapharma, Receptos, Roche, Sanofi-Genzyme, Teva, Trophos.
F. Barkhof serves as a scientific consultant to Bayer-Schering Pharma, Sanofi-Aventis, Biogen-Idec, TEVA, Merck-Serono, Novartis, Roche, Synthon BV, Janssen, Genzyme, and Toshiba Medical systems; has served on speakers’ bureaus for Serono Symposia Foundation and MedScape; and receives research support from Neugrid4you (FP7 European committee) and Dutch Foundation for MS Research—centre grant 2010–2014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 2.Paty DW, Oger JJ, Kastrukoff LF, et al. MRI in the diagnosis of MS: a prospective study with comparison of clinical evaluation, evoked potentials, oligoclonal banding, and CT. Neurology. 1988;38(2):180–5. doi: 10.1212/wnl.38.2.180. [DOI] [PubMed] [Google Scholar]
- 3.Fazekas F, Offenbacher H, Fuchs S, et al. Criteria for an increased specificity of MRI interpretation in elderly subjects with suspected multiple sclerosis. Neurology. 1988;38(12):1822–5. doi: 10.1212/wnl.38.12.1822. [DOI] [PubMed] [Google Scholar]
- 4.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120( Pt 11):2059–69. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- 5.Swanton JK, Fernando K, Dalton CM, et al. Modification of MRI criteria for multiple sclerosis in patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry. 2006;77(7):830–3. doi: 10.1136/jnnp.2005.073247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanton JK, Rovira A, Tintore M, et al. MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: a multicentre retrospective study. Lancet Neurol. 2007;6(8):677–86. doi: 10.1016/S1474-4422(07)70176-X. [DOI] [PubMed] [Google Scholar]
- 7.Rovira A, Swanton J, Tintore M, et al. A single, early magnetic resonance imaging study in the diagnosis of multiple sclerosis. Arch Neurol. 2009;66(5):587–92. doi: 10.1001/archneurol.2009.49. [DOI] [PubMed] [Google Scholar]
- 8.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 9.Montalban X, Tintore M, Swanton J, et al. MRI criteria for MS in patients with clinically isolated syndromes. Neurology. 2010;74(5):427–34. doi: 10.1212/WNL.0b013e3181cec45c. [DOI] [PubMed] [Google Scholar]
- 10.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovira A, Wattjes MP, Tintore M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11(8):471–82. doi: 10.1038/nrneurol.2015.106. [DOI] [PubMed] [Google Scholar]
- 12.Tintoré M, Rovira A, Río J, et al. Factors that determine disease course: the symptomatic lesion matters. 1000 CIS subgroup analysis. ECTRIMS conference. 2014:P759. [Google Scholar]
- 13.Caucheteux N, Maarouf A, Genevray M, et al. Criteria improving multiple sclerosis diagnosis at the first MRI. J Neurol. 2015;262(4):979–87. doi: 10.1007/s00415-015-7668-9. [DOI] [PubMed] [Google Scholar]
- 14.Brownlee WJ, Swanton JK, Miszkiel KA, Miller DH, Ciccarelli O. Should we include lesions in the symptomatic site in dissemination in space in patients with clinically isolated syndromes? Mult Scler. 2015;23(September):65. [Google Scholar]
- 15.Kang H, Metz LM, Traboulsee AL, et al. Application and a proposed modification of the 2010 McDonald criteria for the diagnosis of multiple sclerosis in a Canadian cohort of patients with clinically isolated syndromes. Mult Scler. 2014;20(4):458–63. doi: 10.1177/1352458513501230. [DOI] [PubMed] [Google Scholar]
- 16.Sombekke MH, Wattjes MP, Balk LJ, et al. Spinal cord lesions in patients with clinically isolated syndrome: A powerful tool in diagnosis and prognosis. Neurology. 2013;80(1):69–75. doi: 10.1212/WNL.0b013e31827b1a67. [DOI] [PubMed] [Google Scholar]
- 17.Bot JC, Barkhof F, Polman CH, et al. Spinal cord abnormalities in recently diagnosed MS patients: added value of spinal MRI examination. Neurology. 2004;62(2):226–33. doi: 10.1212/wnl.62.2.226. [DOI] [PubMed] [Google Scholar]
- 18.Weier K, Mazraeh J, Naegelin Y, et al. Biplanar MRI for the assessment of the spinal cord in multiple sclerosis. Mult Scler. 2012;18(11):1560–9. doi: 10.1177/1352458512442754. [DOI] [PubMed] [Google Scholar]
- 19.Nair G, Absinta M, Reich DS. Optimized T1-MPRAGE sequence for better visualization of spinal cord multiple sclerosis lesions at 3T. AJNR Am J Neuroradiol. 2013;34(11):2215–22. doi: 10.3174/ajnr.A3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly SB, Kinsella K, Duggan M, Tubridy N, McGuigan C, Hutchinson M. A proposed modification to the McDonald 2010 criteria for the diagnosis of primary progressive multiple sclerosis. Mult Scler. 2013;19(8):1095–100. doi: 10.1177/1352458512464829. [DOI] [PubMed] [Google Scholar]
- 21.Bigi S, Marrie RA, Verhey L, Yeh EA, Banwell B. 2010 McDonald criteria in a pediatric cohort: is positivity at onset associated with a more aggressive multiple sclerosis course? Mult Scler. 2013;19(10):1359–62. doi: 10.1177/1352458513486519. [DOI] [PubMed] [Google Scholar]
- 22.Hummel HM, Bruck W, Dreha-Kulaczewski S, Gartner J, Wuerfel J. Pediatric onset multiple sclerosis: McDonald criteria 2010 and the contribution of spinal cord MRI. Mult Scler. 2013;19(10):1330–5. doi: 10.1177/1352458513493033. [DOI] [PubMed] [Google Scholar]
- 23.Sadaka Y, Verhey LH, Shroff MM, et al. 2010 McDonald criteria for diagnosing pediatric multiple sclerosis. Ann Neurol. 2012;72(2):211–23. doi: 10.1002/ana.23575. [DOI] [PubMed] [Google Scholar]
- 24.Tantsis EM, Prelog K, Brilot F, Dale RC. Risk of multiple sclerosis after a first demyelinating syndrome in an Australian Paediatric cohort: clinical, radiological features and application of the McDonald 2010 MRI criteria. Mult Scler. 2013;19(13):1749–59. doi: 10.1177/1352458513484377. [DOI] [PubMed] [Google Scholar]
- 25.Kornek B, Schmitl B, Vass K, et al. Evaluation of the 2010 McDonald multiple sclerosis criteria in children with a clinically isolated syndrome. Mult Scler. 2012;18(12):1768–74. doi: 10.1177/1352458512444661. [DOI] [PubMed] [Google Scholar]
- 26.Williams MT, Tapos DO, Juhasz C. Use of the 2010 McDonald criteria can facilitate early diagnosis of pediatric multiple sclerosis in a predominantly black cohort. Pediatr Neurol. 2014;51(6):826–30. doi: 10.1016/j.pediatrneurol.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Banwell B, Bar-Or A, Arnold DL, et al. Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: a prospective national cohort study. Lancet Neurol. 2011;10(5):436–45. doi: 10.1016/S1474-4422(11)70045-X. [DOI] [PubMed] [Google Scholar]
- 28.Huh SY, Kim SH, Kim W, et al. Evaluation of McDonald MRI criteria for dissemination in space in Korean patients with clinically isolated syndromes. Mult Scler. 2014;20(4):492–5. doi: 10.1177/1352458513496881. [DOI] [PubMed] [Google Scholar]
- 29.Hsueh CJ, Kao HW, Chen SY, et al. Comparison of the 2010 and 2005 versions of the McDonald MRI criteria for dissemination-in-time in Taiwanese patients with classic multiple sclerosis. J Neurol Sci. 2013;329(1–2):51–4. doi: 10.1016/j.jns.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Patrucco L, Rojas JI, Miguez JS, Cristiano E. Application of the McDonald 2010 criteria for the diagnosis of multiple sclerosis in an Argentinean cohort of patients with clinically isolated syndromes. Mult Scler. 2013;19(10):1297–301. doi: 10.1177/1352458513475492. [DOI] [PubMed] [Google Scholar]
- 31.Belova AN, Shalenkov IV, Shakurova DN, Boyko AN. Revised McDonald criteria for multiple sclerosis diagnostics in central Russia: sensitivity and specificity. Mult Scler. 2014;20(14):1896–9. doi: 10.1177/1352458514539405. [DOI] [PubMed] [Google Scholar]
- 32.Absinta M, Rocca MA, Colombo B, et al. Patients with migraine do not have MRI-visible cortical lesions. J Neurol. 2012;259:2695–8. doi: 10.1007/s00415-012-6571-x. [DOI] [PubMed] [Google Scholar]
- 33.Ruet A, Arrambide G, Brochet B, et al. Early predictors of multiple sclerosis after a typical clinically isolated syndrome. Mult Scler. 2014;20(13):1721–6. doi: 10.1177/1352458514533397. [DOI] [PubMed] [Google Scholar]
- 34.Ruet A, Deloire MS, Ouallet JC, Molinier S, Brochet B. Predictive factors for multiple sclerosis in patients with clinically isolated spinal cord syndrome. Mult Scler. 2011;17(3):312–8. doi: 10.1177/1352458510386999. [DOI] [PubMed] [Google Scholar]
- 35.Moraal B, Pohl C, Uitdehaag BM, et al. Magnetic resonance imaging predictors of conversion to multiple sclerosis in the BENEFIT study. Arch Neurol. 2009;66(11):1345–52. doi: 10.1001/archneurol.2009.243. [DOI] [PubMed] [Google Scholar]
- 36.Kim SS, Richman DP, Johnson WO, Hald JK, Agius MA. Limited utility of current MRI criteria for distinguishing multiple sclerosis from common mimickers: primary and secondary CNS vasculitis, lupus and Sjogren’s syndrome. Mult Scler. 2014;20(1):57–63. doi: 10.1177/1352458513491329. [DOI] [PubMed] [Google Scholar]
- 37.Verhey LH, Branson HM, Shroff MM, et al. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: a prospective national cohort study. Lancet Neurol. 2011;10(12):1065–73. doi: 10.1016/S1474-4422(11)70250-2. [DOI] [PubMed] [Google Scholar]
- 38.Miller D, Barkhof F, Montalban X, Thompson A, Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol. 2005;4(5):281–8. doi: 10.1016/S1474-4422(05)70071-5. [DOI] [PubMed] [Google Scholar]
- 39.Tintore M, Rovira A, Rio J, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain. 2015;(138):1863–74. doi: 10.1093/brain/awv105. [DOI] [PubMed] [Google Scholar]
- 40.Kuhle J, Disanto G, Dobson R, et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult Scler. 2015;8(21):1013–24. doi: 10.1177/1352458514568827. [DOI] [PubMed] [Google Scholar]
- 41.Sorensen TL, Frederiksen JL, Bronnum-Hansen H, Petersen HC. Optic neuritis as onset manifestation of multiple sclerosis: a nationwide, long-term survey. Neurology. 1999;53(3):473–8. doi: 10.1212/wnl.53.3.473. [DOI] [PubMed] [Google Scholar]
- 42.Tintore M, Rovira A, Rio J, et al. Is optic neuritis more benign than other first attacks in multiple sclerosis? Ann Neurol. 2005;57(2):210–5. doi: 10.1002/ana.20363. [DOI] [PubMed] [Google Scholar]
- 43.Wilejto M, Shroff M, Buncic JR, Kennedy J, Goia C, Banwell B. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006;67(2):258–62. doi: 10.1212/01.wnl.0000224757.69746.fb. [DOI] [PubMed] [Google Scholar]
- 44.Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain. 1999;122( Pt 1):17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- 45.Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50(3):389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 46.Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62(7):723–32. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- 47.Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology. 2005;236(1):254–60. doi: 10.1148/radiol.2361040450. [DOI] [PubMed] [Google Scholar]
- 48.Nelson F, Poonawalla AH, Hou P, Huang F, Wolinsky JS, Narayana PA. Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. AJNR Am J Neuroradiol. 2007;28(9):1645–9. doi: 10.3174/ajnr.A0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sethi V, Yousry TA, Muhlert N, et al. Improved detection of cortical MS lesions with phase-sensitive inversion recovery MRI. J Neurol Neurosurg Psychiatry. 2012;83(9):877–82. doi: 10.1136/jnnp-2012-303023. [DOI] [PubMed] [Google Scholar]
- 50.Favaretto A, Poggiali D, Lazzarotto A, Rolma G, Causin F, Gallo P. The Parallel Analysis of Phase Sensitive Inversion Recovery (PSIR) and Double Inversion Recovery (DIR) Images Significantly Improves the Detection of Cortical Lesions in Multiple Sclerosis (MS) since Clinical Onset. PLoS One. 2015;10(5):e0127805. doi: 10.1371/journal.pone.0127805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson F, Poonawalla A, Hou P, Wolinsky JS, Narayana PA. 3D MPRAGE improves classification of cortical lesions in multiple sclerosis. Mult Scler. 2008;14(9):1214–9. doi: 10.1177/1352458508094644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seewann A, Vrenken H, Kooi EJ, et al. Imaging the tip of the iceberg: visualization of cortical lesions in multiple sclerosis. Mult Scler. 2011;17:1202–10. doi: 10.1177/1352458511406575. [DOI] [PubMed] [Google Scholar]
- 53.Seewann A, Kooi EJ, Roosendaal SD, et al. Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology. 2012;78(5):302–8. doi: 10.1212/WNL.0b013e31824528a0. [DOI] [PubMed] [Google Scholar]
- 54.Calabrese M, De Stefano N, Atzori M, et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol. 2007;64(10):1416–22. doi: 10.1001/archneur.64.10.1416. [DOI] [PubMed] [Google Scholar]
- 55.Filippi M, Rocca MA, Calabrese M, et al. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology. 2010;75(22):1988–94. doi: 10.1212/WNL.0b013e3181ff96f6. [DOI] [PubMed] [Google Scholar]
- 56.Calabrese M, Oh MS, Favaretto A, et al. No MRI evidence of cortical lesions in neuromyelitis optica. Neurology. 2012;79(16):1671–6. doi: 10.1212/WNL.0b013e31826e9a96. [DOI] [PubMed] [Google Scholar]
- 57.Geurts JJ, Roosendaal SD, Calabrese M, et al. Consensus recommendations for MS cortical lesion scoring using double inversion recovery MRI. Neurology. 2011;76(5):418–24. doi: 10.1212/WNL.0b013e31820a0cc4. [DOI] [PubMed] [Google Scholar]
- 58.van Walderveen MA, Kamphorst W, Scheltens P, et al. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology. 1998;50(5):1282–8. doi: 10.1212/wnl.50.5.1282. [DOI] [PubMed] [Google Scholar]
- 59.Giorgio A, Stromillo ML, Bartolozzi ML, et al. Relevance of hypointense brain MRI lesions for long-term worsening of clinical disability in relapsing multiple sclerosis. Mult Scler. 2014;20(2):214–9. doi: 10.1177/1352458513494490. [DOI] [PubMed] [Google Scholar]
- 60.Enzinger C, Fuchs S, Pichler A, et al. Predicting the severity of relapsing-remitting MS: the contribution of cross-sectional and short-term follow-up MRI data. Mult Scler. 2011;17(6):695–701. doi: 10.1177/1352458510394454. [DOI] [PubMed] [Google Scholar]
- 61.Mitjana R, Tintore M, Rocca MA, et al. Diagnostic value of brain chronic black holes on T1-weighted MR images in clinically isolated syndromes. Mult Scler. 2014;20(11):1471–7. doi: 10.1177/1352458514526083. [DOI] [PubMed] [Google Scholar]
- 62.Sastre-Garriga J, Tintore M, Rovira A, et al. Specificity of Barkhof criteria in predicting conversion to multiple sclerosis when applied to clinically isolated brainstem syndromes. Arch Neurol. 2004;61(2):222–4. doi: 10.1001/archneur.61.2.222. [DOI] [PubMed] [Google Scholar]
- 63.Montalban X, Sastre-Garriga J, Filippi M, et al. Primary progressive multiple sclerosis diagnostic criteria: a reappraisal. Mult Scler. 2009;15(12):1459–65. doi: 10.1177/1352458509348422. [DOI] [PubMed] [Google Scholar]
- 64.Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology. 2009;72(9):800–5. doi: 10.1212/01.wnl.0000335764.14513.1a. [DOI] [PubMed] [Google Scholar]
- 65.Okuda DT. Unanticipated demyelinating pathology of the CNS. Nat Rev Neurol. 2009;5(11):591–7. doi: 10.1038/nrneurol.2009.157. [DOI] [PubMed] [Google Scholar]
- 66.Stromillo ML, Giorgio A, Rossi F, et al. Brain metabolic changes suggestive of axonal damage in radiologically isolated syndrome. Neurology. 2013;80(23):2090–4. doi: 10.1212/WNL.0b013e318295d707. [DOI] [PubMed] [Google Scholar]
- 67.Giorgio A, Stromillo ML, Rossi F, et al. Cortical lesions in radiologically isolated syndrome. Neurology. 2011;77:1896–9. doi: 10.1212/WNL.0b013e318238ee9b. [DOI] [PubMed] [Google Scholar]
- 68.Okuda DT, Siva A, Kantarci O, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One. 2014;9(3):e90509. doi: 10.1371/journal.pone.0090509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lebrun C, Bensa C, Debouverie M, et al. Association between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid, and visual evoked potential: follow-up of 70 patients. Arch Neurol. 2009;66(7):841–6. doi: 10.1001/archneurol.2009.119. [DOI] [PubMed] [Google Scholar]
- 70.Okuda DT, Mowry EM, Cree BA, et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology. 2011;76(8):686–92. doi: 10.1212/WNL.0b013e31820d8b1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Charil A, Yousry TA, Rovaris M, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lancet Neurol. 2006;5(10):841–52. doi: 10.1016/S1474-4422(06)70572-5. [DOI] [PubMed] [Google Scholar]
- 72.Miller DH, Weinshenker BG, Filippi M, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler. 2008;14(9):1157–74. doi: 10.1177/1352458508096878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aliaga ES, Barkhof F. MRI mimics of multiple sclerosis. Handbook of clinical neurology. 2014;122:291–316. doi: 10.1016/B978-0-444-52001-2.00012-1. [DOI] [PubMed] [Google Scholar]
- 74.Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84(11):1165–73. doi: 10.1212/WNL.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;14:177–89. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wattjes MP, Lutterbey GG, Harzheim M, et al. Higher sensitivity in the detection of inflammatory brain lesions in patients with clinically isolated syndromes suggestive of multiple sclerosis using high field MRI: an intraindividual comparison of 1.5 T with 3.0 T. Eur Radiol. 2006;16(9):2067–73. doi: 10.1007/s00330-006-0195-4. [DOI] [PubMed] [Google Scholar]
- 77.Nielsen K, Rostrup E, Frederiksen JL, et al. Magnetic resonance imaging at 3.0 tesla detects more lesions in acute optic neuritis than at 1. 5 tesla. Invest Radiol. 2006;41(2):76–82. doi: 10.1097/01.rli.0000188364.76251.28. [DOI] [PubMed] [Google Scholar]
- 78.Simon B, Schmidt S, Lukas C, et al. Improved in vivo detection of cortical lesions in multiple sclerosis using double inversion recovery MR imaging at 3 Tesla. Eur Radiol. 2010;20(7):1675–83. doi: 10.1007/s00330-009-1705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wattjes MP, Harzheim M, Lutterbey GG, et al. Does high field MRI allow an earlier diagnosis of multiple sclerosis? J Neurol. 2008;255(8):1159–63. doi: 10.1007/s00415-008-0861-3. [DOI] [PubMed] [Google Scholar]
- 80.Mistry N, Tallantyre EC, Dixon JE, et al. Focal multiple sclerosis lesions abound in ‘normal appearing white matter’. Mult Scler. 2011;17(11):1313–23. doi: 10.1177/1352458511415305. [DOI] [PubMed] [Google Scholar]
- 81.Ge Y, Zohrabian VM, Grossman RI. Seven-Tesla magnetic resonance imaging: new vision of microvascular abnormalities in multiple sclerosis. Arch Neurol. 2008;65(6):812–6. doi: 10.1001/archneur.65.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hammond KE, Metcalf M, Carvajal L, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol. 2008;64(6):707–13. doi: 10.1002/ana.21582. [DOI] [PubMed] [Google Scholar]
- 83.Tallantyre EC, Brookes MJ, Dixon JE, Morgan PS, Evangelou N, Morris PG. Demonstrating the perivascular distribution of MS lesions in vivo with 7-Tesla MRI. Neurology. 2008;70(22):2076–8. doi: 10.1212/01.wnl.0000313377.49555.2e. [DOI] [PubMed] [Google Scholar]
- 84.Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44(9):491–4. doi: 10.1097/RLI.0b013e3181b4c144. [DOI] [PubMed] [Google Scholar]
- 85.Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261(7):1356–64. doi: 10.1007/s00415-014-7351-6. [DOI] [PubMed] [Google Scholar]
- 86.Tan IL, van Schijndel RA, Pouwels PJ, et al. MR venography of multiple sclerosis. AJNR Am J Neuroradiol. 2000;21(6):1039–42. [PMC free article] [PubMed] [Google Scholar]
- 87.Sati P, George IC, Shea CD, Gaitan MI, Reich DS. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology. 2012;265(3):926–32. doi: 10.1148/radiol.12120208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mainero C, Benner T, Radding A, et al. In vivo imaging of cortical pathology in multiple sclerosis using ultra-high field MRI. Neurology. 2009;73(12):941–8. doi: 10.1212/WNL.0b013e3181b64bf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76(6):534–9. doi: 10.1212/WNL.0b013e31820b7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gaitan MI, Sati P, Inati SJ, Reich DS. Initial investigation of the blood-brain barrier in MS lesions at 7 tesla. Mult Scler. 2013;19:1068–73. doi: 10.1177/1352458512471093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Absinta M, Sati P, Gaitan MI, et al. Seven-tesla phase imaging of acute multiple sclerosis lesions: A new window into the inflammatory process. Ann Neurol. 2013;74(5):669–78. doi: 10.1002/ana.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sinnecker T, Dorr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79(7):708–14. doi: 10.1212/WNL.0b013e3182648bc8. [DOI] [PubMed] [Google Scholar]
- 93.Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18(11):1592–9. doi: 10.1177/1352458512441270. [DOI] [PubMed] [Google Scholar]
- 94.Bian W, Harter K, Hammond-Rosenbluth KE, et al. A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. Mult Scler. 2012;19(1):69–75. doi: 10.1177/1352458512447870. [DOI] [PubMed] [Google Scholar]