Abstract
Purpose
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a bladder pain disorder associated with voiding symptomatology and other systemic chronic pain disorders. Currently diagnosis of IC/BPS is complicated, as patients present with wide ranges of symptoms, physical examination findings, and clinical test responses. One hypothesis is that IC symptoms arise from increased bladder permeability to urine solutes. This study established the feasibility of using contrast-enhanced magnetic resonance imaging (CE-MRI) to quantify bladder permeability in IC patients.
Materials and Methods
Permeability alterations in bladder urothelium were assessed with intravesical administration of a MRI contrast agent (Gd-DTPA) in a small cohort of patients. MRI signal intensities (SI) in IC patient and control bladders were compared regionally and for entire bladders.
Results
Quantitative assessment of MRI SI indicated a significant increase in SI within anterior bladder regions (p<0.01) compared to posterior regions in IC patients, and significant increases in SI within anterior bladder regions (p<0.001) and kurtosis (descriptor of shape of probability distribution) and skewness (measure of asymmetry of probability distribution) associated with contrast enhancement in total bladders (p<0.05) for IC patients compared to controls. Regarding symptomatology, IC cases differed significantly from controls for the SF-36, PPUF and ICPI questionnaires with no overlap in range of scores for each group, and were significantly different for ICSI but with a slight overlap in range of scores.
Conclusions
The data suggests that CE-MRI provides an objective, quantifiable measurement of bladder permeability that could be used to stratify bladder pain patients and monitor therapy.
Keywords: Bladder, CE-MRI, IC/BPS, permeability, symptoms
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a bladder pain disorder associated with voiding symptomatology and other systemic chronic pain disorders [1]. Originally IC was considered rare and diagnosed on the basis of finding a Hunner’s lesion [2], but now the definition has broadened to include bladder pain, urgency and frequency syndromes [1,3]. Cystoscopy biopsy and hydrodistension to observe petechial bleeding are no longer routinely performed [3], resulting in reduced use of imaging or physiological methods as objective, quantifiable criteria for diagnosis. IC/BPS prevalence is a 10:1 female-to-male ratio [1]. IC/BPS is thought to be either a type of hypersensitivity disorder that affects bladder and other somatic/visceral organs with many overlapping symptoms and pathophysiology, or a continuum of painful vs. non-painful overactive bladder syndrome [1]. Two distinct pain location phenotypes for IC/BPS occur, including pelvic pain only (19%) and pelvic pain beyond (81%), from pain diagnosis criteria [4]. Also 25% of individuals with IC have an emotional component associated with their physical concerns [5].
Basic assessments for IC/BPS includes: medical history and physical examination, symptom questionnaires, assessing pain, urination frequency/volume, and post-void residual, urinalysis, urine culture and cytology [1]. Characteristic clinical features for BPS include bladder filling pain and wall tenderness [6]. For patients with symptoms associated with complicated IC/BPS, further assessment for incontinence, gastrointestinal and/or gynecological signs/symptoms, microscopic/gross evaluation, and other tests (e.g. imaging, cystoscopy and laparoscopy) need to be conducted [1]. IC/BPS is essentially a diagnosis of several exclusion criteria [1]. Associated with IC diagnosis, psychiatric illnesses such as depression, anxiety, addiction, and also a history of child abuse, as well as bowel/gastrointestinal problems are also assessed [7,8].
Currently IC/BPS diagnosis is complicated, as patients present with a range of symptoms, physical examination findings, and clinical test responses [1]. A simple and conclusive diagnostic test to establish if some structural abnormalities associated with IC/BPS, such as bladder urothelial permeability, would provide a means to stratify or phenotype patients with lower abdominal pain and urinary voiding symptoms. Although the etiology of the disorder is not known, a constant observation is that IC patients have increased permeability that allows urinary toxins to penetrate into the urothelium and muscularis. Earlier Parsons [9] showed increased uptake of urea from the bladder of IC patients vs. controls, and Buffington showed abnormal kinetics of excretion of fluorescein due to recycling from the bladder [10]. Parsons also proposed a potassium sensitivity test (PST) where a pain reaction due to instillation of dilute KCl (but not NaCl) was diagnostic of increased bladder permeability [11].
In this report we demonstrate the feasibility of using contrast-enhanced magnetic resonance imaging (CE-MRI) to assess bladder permeability within a small cohort of IC patients. A MRI contrast agent was introduced via an intravesical catheter to assess permeability alterations in bladder urothelium, compared to normal controls. We suggest that CE-MRI could provide an objective assessment of bladder permeability alterations that could be used to stratify bladder pain patients and monitor therapy.
Materials and Methods
Participants
Six IC cases and 4 controls participated in the study. IC cases were recruited by their physicians from the Female Pelvic Medicine and Bladder Health Clinic at the University of Oklahoma Medical Center (Oklahoma City, OK) (2013–2015). None of the IC patients had recently been hydrodistended nor had received any intravesical therapy. Healthy controls were recruited on campus via flyers. MRI measurements were not available for one IC case due to technical difficulties (five IC cases). Signed informed consent was obtained from each participant in accordance with the University of Oklahoma Health Sciences Center Institutional Review Board which approved the study (protocol no. 1684).
Clinical CE-MRI
All scans were performed on a GE Discovery 450 3.0 T 70 cm wide bore clinical MRI system with a 50mT/m and 200 T/m/s gradient system. Pre- and post-contrast (Gd-DTPA (Magnevist); 0.05 mmol/kg body weight; 5 mL in 200 mL total volume, diluted in sterile saline; instilled via an intravesical catheter (16F silicone foley); 2–2.5 cc/s over 5–10 min) two-dimensional T1-weighted spin echo images were obtained in the coronal plane with TR=700 ms; TE=10.0 ms; 320×160 matrix; 36×36 cm2 field-of-view (FOV); and slice thickness=4 mm. Three-dimensional (3D) LAVA (liver acquisition with volume acceleration) sequences were also used to obtain pre- and post-contrast (Gd-DTPA) images taken in the coronal, sagittal and axial planes. 3D LAVA images were obtained in sagittal, axial and coronal planes with the following parameters: TR=5.3 (sagittal), 4.3 (axial) or 4.9 (coronal); TE=1.9 (sagittal), 1.8 (axial) or 2.1 (coronal); TI=5.0 ms; 320×224 (sagittal and axial) or 384×256 matrix (coronal); 12° flip angle; and 4.0 mm slice thicknesses. Images were obtained over 30 min, and taken at 3, 6, 9, 12, 15, 20, 25 and 30 min post-contrast.
Region-of-Interest (ROI) Analysis of MRI SI
Selective regions-of-interest were taken in bladder dome (anterior region), posterior region, and thigh muscle regions, and percent (%) difference in bladder/muscle MRI SI were calculated for IC patients and controls from post-contrast images. Data reported as means ± S.D.
Segmentation Analysis of MRI SI
All image slices were semi-automatically segmented [12], with additional corrections to remove the catheter and improve bladder wall definition. Contrast enhancement at each pixel was reported as a post-contrast (9-min)/pre-contrast ratio, and log10 transformed, for entire bladders for each image slice. Amount of kurtosis and skewness were calculated for each distribution.
Clinical symptoms assessment measures
Anxiety was measured with the Beck Anxiety Index (BAI), a twenty-one-item self-report measure that uses a four-point response scale ranging from “not at all” to “severely” [13]. Levels of depressive symptoms were measured with the Beck Depression Inventory-2nd Edition (BDI-2), a twenty-one-item self-report measure that uses a four-point response scale ranging from “not at all” to “severely” [14]. A Medical Outcomes Study Short Form 36 (SF-36) was used to assess health-related quality of life [15]. A Pelvic Pain, Urgency, and Frequency (PPUF) questionnaire was also given [16]. Interstitial Cystitis Symptom Index (ICSI) and Pain Index (ICPI) are brief (4 questions each) questionnaires used to measure the amount of symptoms and problems, respectively, caused by urinary frequency and urgency, nocturia and bladder pain [17]. The Trauma Symptom Checklist-40 (TSC-40) measures symptomatology in adults related to previous traumatic experiences in childhood or adulthood, which includes a 40-item self-report comprised of 6 subscales: anxiety, depression, dissociation, sexual abuse trauma index, sexual problems, sleep disturbance.
Statistical analysis
Group descriptive statistics were expressed as means ± S.D.. Differences between groups were assessed using Student’s t-tests for individual data comparisons, or two-way analysis of variance (ANOVA) for the kinetics data (GraphPad Prism 6, GraphPad Software, Inc., La Jolla, CA). Multivariate analysis of variance (MANOVA) was used on the global measures of kurtosis and skewness for each histogram (SAS v9.2, Statistical Analysis System (SAS), Carey, NC), followed by a pre-planned comparison (post-hoc) for each kurtosis/skewness measures. Symptomatology data analyses used IBM SPSS Statistics (2011. Version 20.0 for Windows. Armonk, NY: IBM Corp). P-values <0.05 were considered significant.
Results
Nine participants were included in the study (five IC cases, four controls; see Table 1). All were Caucasian women ranging in age from 20–60 years (47±10 yrs) with a BMI of 26±7. 3/5 cases and 2/4 controls had BMI > 25. Cases and controls did not differ significantly in age, height, weight or BMI means (Table 1).
Table 1.
Group comparisons of IC cases to controls for demographic, MRI and questionnaire outcomes (means±SD, [range]).
| IC cases | Controls | p-value | |
|---|---|---|---|
| N | 5 | 4 | |
| Age (yr) | 47 ± 7 [39 – 59] | 47 ± 14 [28 – 60] | 0.90 |
| Height (m) | 1.64 ± 0.06 [1.57 – 1.74] | 1.64 ± 0.06 [1.59 – 1.73] | 0.64 |
| Weight (kg) | 66.0 ± 8.1 [54.4 – 76.2] | 73.3 ± 27.7 [44.5 – 111.1] | 0.64 |
| BMI | 24.5 ± 2.6 [20.0 – 26.6] | 27.6 ± 11.4 [16.8 – 43.4] | 0.62 |
| Bladder void volume (mL) | 448.2 ± 179.4 [237 – 651] | 575.5 ± 269.1 [266 – 920] | 0.39 |
| Diagnosis since (years) | 3.04 ± 1.49 [1.15 – 4.93] | 0.00 ± 0.00 [0.00 – 0.00] | 0.004 |
|
| |||
| MRI Anterior bladder | 162.4 ± 13.0 [151.0 – 182.8] | 117.6 ± 6.8 [109.9 – 125.9] | 0.0005 |
| MRI Posterior bladder | 131.7 ± 15.6 [109.6 – 150.4] | 113.1 ± 12.6 [101.3 – 128.0] | 0.094 |
|
| |||
| BAI | 6.3 ± 8.5 [1 – 23] | 5.3 ± 5.3 [1 – 13] | 0.81 |
| BDI-II | 8.7 ± 7.4 [2 – 23] | 1.0 ± 1.4 [0 – 3] | 0.053 |
| SF-36 Total | 60.6 ± 14.8 [32.8 – 74.3] | 89.5 ± 3.7 [85.6 – 94.0] | <0.0001 |
| SF-36 physical | 64 ± 28 [19 – 95] | 93 ± 15 [70 – 100] | 0.070 |
| SF-36 emotional | 63 ± 12 [43 – 77] | 81 ± 12 [63 – 90] | 0.052 |
| PPU/F Total | 14.2 ± 4.6 [7 – 20] | 1.3 ± 0.5 [1 – 2] | <0.0001 |
| PPU/F symptom | 9.8 ± 3.4 [5 – 15] | 1.3 ± 0.5 [1 – 2] | <0.0001 |
| PPU/F bother | 4.3 ± 1.6 [2 – 6] | 0 ± 0 [0 – 0] | <0.0001 |
| ICSI | 7.2 ± 2.6 [3 – 10] | 1.3 ± 1.9 [0 – 4] | <0.0001 |
| ICPI | 8.0 ± 4.0 [2 – 11] | 0.3 ± 0.5 [0 – 1] | <0.0001 |
| TSC-40 Total | 15. 7 ± 11.5 [5 – 37] | 6.0 ± 7.0 [0 – 15] | 0.14 |
Abbreviations: MRI = magnetic resonance imaging; SI = signal intensity; BAI = Beck anxiety index; BDI-II = Beck depression inventory; SF-36 = medical outcomes study short-form 36; PPUF_symp = pelvic pain, urgency and frequency symptoms; ICSI = interstitial cystitis symptom index; ICPI = interstitial cystitis pain index; TSC-40 = trauma symptom check-list 40 dissociation.
Clinical MRI
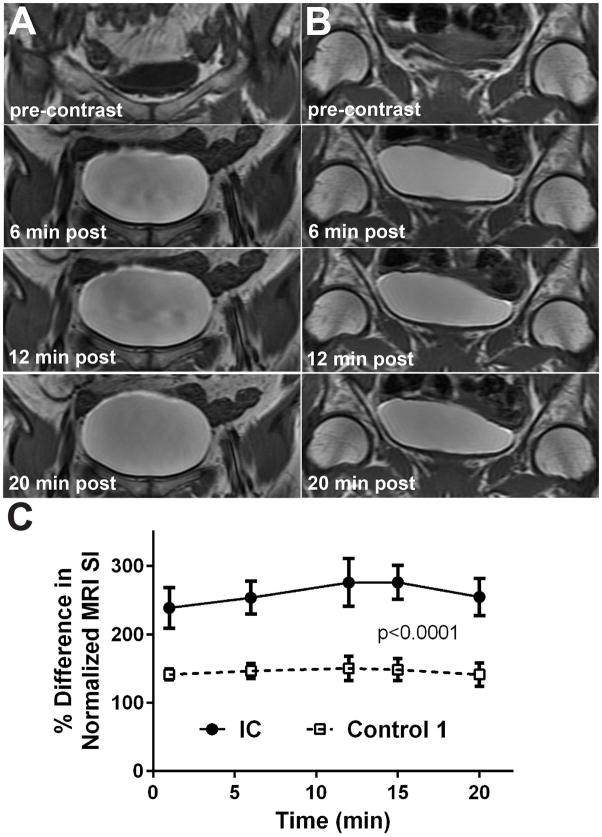
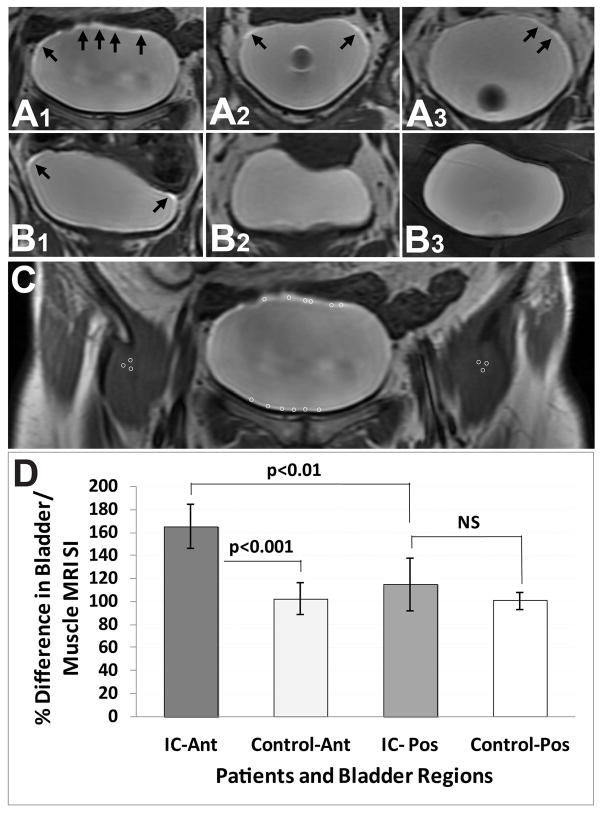
An increased MRI signal intensity (SI) was observed in the dome or anterior bladder regions of IC patients compared to controls, with the use of CE-MRI. Representative CE-MR images from an IC patient with an increased MRI SI in the dome region, compared to a control, are shown in Figure 1. Kinetic uptake of the Gd-DTPA contrast agent, measured as an increase in MRI SI, is shown in Figure 2. There was a sustained significant increase in MRI SI in the bladder dome regions of IC patients (n=5) over the entire 20 min period (p<0.0001), reaching a maximum SI at 12–15 min, compared to controls (n=4). Quantitative assessment of MRI SI in IC patients, compared to controls, indicated a significant increase in SI within the anterior bladder region (p<0.001) compared to controls. There was also a significant increase in SI in the anterior (p<0.01) compared to the posterior bladder region in IC patients (Fig. 3D). Representative CE-MR images from IC patients (Fig. 3A) and controls (Fig. 3B) depict high SI regions (black arrows). Quantitative data of MRI SI in IC patients and controls is also reported in Table 1. With the use of segmentation analysis, the IC patients appear to have significantly greater amounts of skewness and kurtosis in their distributions across their entire bladders (p<0.021; MANOVA) compared to controls (see Fig. 4).
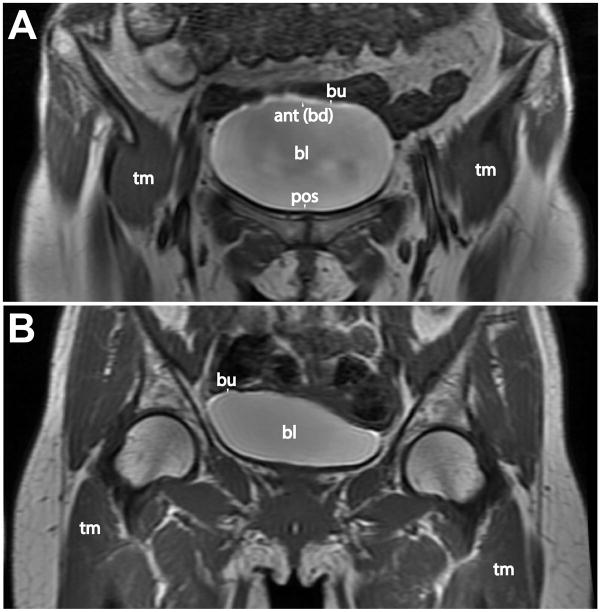
Figure 1.
Representative clinical MR post-contrast (Gd-DTPA) images of the bladder regions of an IC patient (A) and a normal control (B). Note the enhanced signal intensity of the bladder urothelium in the dome region for the IC patient (A). Anatomical labels: bl=bladder; bu=bladder urothelium; tm=thigh muscle; ant(bd)=anterior region or bladder dome; and pos=posterior region.
Figure 2.
Post-contrast MR imaging kinetics in representative IC (A) and normal control (B) subjects. Displayed bladder region images are at pre-contrast and 6, 12 and 20 min post-contrast (Gd-DTPA). (C) Graphic representation of the relative uptake of the Gd-DTPA contrast agent in the bladder urothelium (dome region) over 20 min. post-contrast. There was a significant increase in the uptake of Gd-DTPA in IC patients (n=6) compared to normal controls (n=4) (p<0.0001; ANOVA). Data represented as mean ± S.D.
Figure 3.
Quantitative assessment of the percent difference in MRI signal intensity (SI) relative to muscle tissue in IC patients (A) and normal controls (B) 12 min post-contrast. Arrows in panels A and B depict high MRI SI post-contrast. All IC patients (A1–3) had increased SI in the bladder urothelium (dome region), in comparison to normal controls. There was one example of a normal control (B1) that had a slight increase in SI. (C) Regions-of-interest (ROIs) depicted in bladder urothelium (anterior (Ant) and posterior (Pos) regions) and thigh muscle regions. (D) Quantitative assessment of the percent difference in bladder MRI SI relative to muscle tissues in IC patients and normal controls. There was a significant increase in SI for IC patients compared to normal controls in the anterior region (p<0.0001), and a significant increase in SI in the anterior region compared to the posterior region in IC patients (p<0.01). Data represented as mean ± S.D.
Figure 4.
Global evaluation of bladders in IC patients and controls. Representative CE-MR images with contrast uptake (one representative image slice) in the bladder of an IC patient (A) and a control (B). Representative histograms of 3D voxel analysis due to the amount of contrast enhancement (ratio of enhancement at 9-min. post-contrast divided by pre-contrast values, which were then log10 transformed) in an IC patient (C) compared to a control (D). Note the observed enhancement for the IC patient appears to be more asymmetric in appearance for the kurtosis histogram. The IC patients were tested on degree of kurtosis and skewness in their distributions across their entire bladders (p<0.021; MANOVA with a F(2,6)=7.896) compared to controls. Following the MANOVA, a post-hoc analysis demonstrated a p<0.031 for the kurtosis measure t(5.33)=2.34 using a Satterthwaite approximation. This also produced on the kurtosis measure a mean difference=20.4 with S.D.=8.68 with an upper confidence interval (CI) of 42.348 and a lower CI difference of −1.541, with a confidence interval of 0.95. No significant differences were found on the skewness measure of the distributions between IC patients and controls. Data represented as mean ± S.D.
Symptomatology
Quantitative reporting of symptomatology data is also presented in Table 1. IC cases differed significantly from controls for SF-36, PPU/F and ICPI questionnaires with no overlap in range of scores for each group. They also were significantly different for ICSI but with a slight overlap in range of scores. Differences between IC cases and controls were not significant for BAI, BDI-II and TSC-40 due to the small sample size which may offer little power for these comparisons. This may be the case for BDI-II, which approaches significance (p=0.053) and has a large difference between group means and only a slight overlap in ranges like ICSI. It is less likely for BAI, which shows little difference and extensive overlap of ranges.
Discussion
In this study we established that CE-MRI was able to detect a significantly increased uptake of Gd-DTPA in the bladder urothelia of IC patients, compared to controls who did not report IC symptoms. The increased MRI signal intensity was found to be predominantly in the dome bladder region for IC patients. Globalized measures of perfusion included kurtosis and skewness were used for comparison across all bladders between IC and control patients in addition to the previously mentioned selective regions-of-interest (ROI). The selective ROIs analysis provided a more local evaluation while the globalized measures via histograms were used to assess the entire bladder. The histogram shape (kurtosis) and asymmetry (skewness), through a lack of symmetry would suggest a potential for greater dysregulation in the underlying level of perfusion as compared to the more symmetric distributions of the control patients.
Gd-DTPA is a charged small molecule routinely used to assess neurological disorders, which normally does not cross the blood-brain-barrier unless it is disrupted. The bladder epithelium also forms a tight barrier, but has been found to be more permeable in IC patients (in part due to the loss of the zona occludens protein ZO-1 which is responsible for tight junctions, and an increase in E-cadherin which inhibits proliferation) [18], which may allow increased uptake of the MRI contrast agent Gd-DTPA.
Detrusor smooth muscle differentiation initiates in the bladder dome region and subsequently progresses caudally with the leading edge extending down the right posterior surface of the bladder during murine bladder development [19]. There is also evidence of 2 main interstitial cell populations in the upper and deeper lamina propria in the human bladder dome region, which have distinct ultrastructural and immunohistochemical phenotypes [20]. This differentiation in detrusor muscle development and ultrastructural composition may play a role in the pathophysiology associated with IC. It is known for instance that low bladder wall compliance is associated with IC, and that low bladder wall compliance is attributable to increased detrusor muscle tone during bladder filling or changes in the viscoelastic properties of the bladder wall that impede the ability of the bladder wall to stretch [21]. Our group has previously reported on changes in urinary bladder smooth muscle contractility in response to colonic inflammation [22]. IC patients have a characteristic oak leaf pattern with protrusions of the sarcolemma in the smooth muscle cells, which may be due to degeneration [23]. It is also known that altered detrusor gap junction communication induces storage symptoms in bladder inflammation in a mouse model for IC [24].
Disease-specific questionnaires and voiding diaries were no better than measures of quality of life at distinguishing between women with IC and their unaffected counterparts. This likely reflects the heterogeneity of symptoms reported by IC patients. If MRI proves better at identifying patients with IC associated with permeability, then physicians who treat these patients will be better positioned to identify, individualize and optimize their therapy.
Structural mechanistic abnormalities associated with IC/BPS include the absence of epithelium, mucosal ulceration, mucosal ruptures, abnormal and leaky tight junctions, a widening of spaces between cells, cell vacuolization, and urothelial detachment [25]. IC/BPS functional abnormalities include increased intravesical urea absorption, increased paracellular permeability, and increased sensitivity to intravesical potassium ions, as well as reported abnormal protein expressions [25,26]. Pathophysiological changes associated with IC/BPS include inflammation and mast cell activation, urothelial dysfunction – thought to be associated with glycosaminoglycans (GAGs) layer defects, an increase in nitric oxide related enzymes, a defect in the Tamm-Horsfall protein (THP) – which prevents damage to the urothelium, an increase in antinuclear antibodies – autoimmune response, and a genetic predisposition [26].
Other diagnostic studies for IC patients include bladder biopsy microarray gene expression assessment [27], and metabolomic profiling of urine [3]. Leukocyte transendothelial migration pathway-associated genes were upregulated in low bladder capacity patients, and tight junction pathway-associated genes were down-regulated in normal bladder capacity patients [27]. Metabolomics found that a small glycosylated peptide, anti-proliferative factor (APF), has increased bioactivity in urine samples from IC patients [3]. A novel biomarker, phenylacetylglutamine, is elevated in IC patient urine samples [3].
An earlier study which infused 99mTc (technetium)-DTPA into IC patient and normal subject bladders, found no significant differences in bladder permeability from measured radioactivity in serum [28]. Our method directly measured changes in MRI contrast within the bladder urothelium from Gd-DTPA instillation in the bladder. Recently our group also used CE-MRI to detect alterations in bladder permeability in rat bladders exposed to protamine sulfate, and also detect an increased MRI signal intensity within the colon due to the bladder-colon crosstalk [29].
Future studies should include a larger cohort of patients to establish if CE-MRI can be used as a diagnostic tool for IC patients, and to correlate MRI data with symptomatology. In addition, other painful bladder diseases/disorders (e.g. bladder cancer, bladder stones) should be included as additional control groups. This method could also be useful for following the response of IC patients given various therapies.
Conclusions
In this study CE-MRI was able to differentiate bladder permeability alterations in IC patients, compared to controls. This small study was designed mainly to test the feasibility of using MRI to assess bladder permeability alterations. Our approach assessing the bladder directly in IC patients, in a relatively short time period, may provide an additional diagnostic outcome that could be used to stratify the spectrum of lower pelvic pain and voiding dysfunction patients into those with a defined leak and those without a leak, and also stratify “leakers” according to the degree of leakage. This stratification could prove particularly useful for clinical trials and for monitoring the efficacy of therapy and tissue repair. The feasibility of using contrast-enhanced MR imaging to provide information associated with bladder permeability complications is presented.
Acknowledgments
Funding obtained from National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant no. P20DK097799 (REH). We thank Raquel Zamora and Kristy J. Scott for their nursing assistance, and Alisha Parrett for her clinical management assistance.
References
- 1.Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA Guideline Amendment. J Urol. 2015;193:1545–53. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- 2.Hunner GL. A rare type of bladder ulcer in women: Report of cases. Boston Med Surg J. 1915;172:660. [Google Scholar]
- 3.Evans RJ, Sant GR. Current diagnosis of interstitial cystitis: an evolving paradigm. Urology. 2007;69:64–72. doi: 10.1016/j.urology.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Nickel JC, Tripp DA International Interstitial Cystitis Study Group. Clinical and psychological parameters associated with pain pattern phenotypes in women with interstitial cystitis/bladder pain syndrome. J Urol. 2015;193:138–44. doi: 10.1016/j.juro.2014.07.108. [DOI] [PubMed] [Google Scholar]
- 5.Fazio RL, Wunderlich T, Wilson N, et al. MMPI-2-RF characteristics of individuals with interstitial cystitis. J Psychosom Res. 2014;77:359–62. doi: 10.1016/j.jpsychores.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Tirlapu SA, Priest L, Wojdyla D, et al. Bladder pain syndrome: validation of simple tests for diagnosis in women with chronic pelvic pain: BRaVADO study protocol. Reprod Health. 2013;10:61. doi: 10.1186/1742-4755-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937–940. doi: 10.1097/01.ju.0000169258.31345.5d. [DOI] [PubMed] [Google Scholar]
- 8.Nickel JC, Tripp DA, Pontari M, et al. Psychosocial phenotyping in women with interstitial cystititis/painful bladder syndrome: a case control study. J Urol. 2010;183:167–172. doi: 10.1016/j.juro.2009.08.133. [DOI] [PubMed] [Google Scholar]
- 9.Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis) J Urol. 1991;145:732–5. doi: 10.1016/s0022-5347(17)38437-9. [DOI] [PubMed] [Google Scholar]
- 10.Buffington CA, Woodworth BE. Excretion of fluorescein in the urine of women with interstitial cystitis. J Urol. 1997;158:786–9. doi: 10.1097/00005392-199709000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Parsons CL, Greenberger M, Gabal L, et al. The role of urinary potassium in the pathogenesis and diagnosis of interstitial cystitis. J Urol. 1998;159:1862–6. doi: 10.1016/S0022-5347(01)63178-1. [DOI] [PubMed] [Google Scholar]
- 12.Wu DH, Shaffer AD, Thompson DM, et al. Iterative active deformational methodology for tumor delineation: evaluation across radiation treatment stage and volume. J Magn Reson Imaging. 2008;28:1188–1194. doi: 10.1002/jmri.21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson KA, Chambless DL, de Beurs E. Beck anxiety inventory. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment. 3. Mahwah, NJ: Lawrence, Erlbaum; 1999. pp. 399–421. [Google Scholar]
- 14.Dozios DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychol Assess. 1998;10:83–90. [Google Scholar]
- 15.McHorney CA, Ware JE, Jr, Rogers W, et al. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care. 1992;30:MS253–65. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 16.Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573–8. doi: 10.1016/s0090-4295(02)01829-0. [DOI] [PubMed] [Google Scholar]
- 17.O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. J Urol. 1996;156:2123. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 18.Slobodov G, Feloney M, Gran C, et al. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with insterstitial cystitis. Clin Urol. 2004;171:1554–58. doi: 10.1097/01.ju.0000118938.09119.a5. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter A, Paulus A, Robinson M, et al. 3-Dimensional morphometric analysis of murine bladder development and dysmorphogenesis. Dev Dynamics. 2012;241:522–33. doi: 10.1002/dvdy.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gevaert T, Vanstreels E, Daelemans D, et al. Identification of different phenotypes of interstitial cells in the upper and deep lamina propria of the human bladder dome. J Urol. 2014;192:1555–63. doi: 10.1016/j.juro.2014.05.096. [DOI] [PubMed] [Google Scholar]
- 21.Gray M. Traces: making sense of urodynamics testing – Part 6: evaluation of bladder filling/storage: bladder wall compliance and the detrusor leak point pressure. Urol Nurs. 2011;31:215–21. [PubMed] [Google Scholar]
- 22.Noronha R, Akbarali H, Malykhina A, et al. Changes in urinary bladder smooth muscle function in response to colonic inflammation. Am J Physiol Renal Physiol. 2007;293:F1461–7. doi: 10.1152/ajprenal.00311.2007. [DOI] [PubMed] [Google Scholar]
- 23.Horn T, Holm NR, Hald T. Interstitial cystitis. Ultrastructural observations on detrusor smooth muscle cells. APMIS. 1998;106:909–16. [PubMed] [Google Scholar]
- 24.Okinami T, Imamura M, Nishikawa N, et al. Altered detrusor gap junction communications induce storage symptoms in bladder inflammation: a mouse cyclophosphamide-induced model of cystitis. PLoS One. 2014;9:e104216. doi: 10.1371/journal.pone.0104216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keay SK, Birder LA, Chai TC. Evidence for bladder urothelial pathophysiology in functional bladder disorders. Biomed Res Int. 2014;2014:865463. doi: 10.1155/2014/865463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser PJ, Dozmorov MG, Bane BL, et al. Abnormal expression of differentiation related proteins and proteoglycan core proteins in the urothelium of patients with interstitial cystitis. J Urol. 2008;179:764–9. doi: 10.1016/j.juro.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colaco M, Koslov DS, Keys T, et al. Correlation of gene expression with bladder capacity in interstitial cystitis/bladder pain syndrome. J Urol. 2014;192:1123–9. doi: 10.1016/j.juro.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 28.Chelsky MJ, Rosen SI, Knight LC, et al. Bladder permeability in interstitial cystitis is similar to that of normal volunteers: direct measurement by transvesical absorption of 99mtechnetium-diethylenetriaminepentaacetic acid. J Urol. 1994;151:346–9. doi: 10.1016/s0022-5347(17)34945-5. [DOI] [PubMed] [Google Scholar]
- 29.Towner RA, Smith N, Saunders D, et al. Contrast enhanced magnetic resonance imaging as a diagnostic tool to assess bladder permeability and associated colon crosstalk: preclinical studies in a rat model. J Urology. 2015;193:1394–400. doi: 10.1016/j.juro.2014.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]