Abstract
The low-cost, primarily phone-based Coordinated-Transitional Care (C-TraC) program reduced 30-day rehospitalizations by 1/3, leading to significant cost savings at one VA hospital. Non-VA hospitals have expressed interest in launching C-TraC, but non-VA hospitals differ in important ways from VA hospitals, particularly in terms of context, culture and resources. The objective of this project was to successfully adapt C-TraC to the specific context of one non-VA setting using a modified Replicating Effective Programs (REP) implementation theory model, and to test the feasibility of this protocolized implementation approach. The modified-REP model 1) uses a mentored phased-based implementation with intensive pre-implementation activities, and 2) harnesses local key stakeholders to adapt processes and goals to local context. Using this protocolized implementation approach, an adapted C-TraC protocol was created and launched at the non-VA hospital in July 2013. In its first 16 months, C-TraC successfully enrolled 1,247 patients with 3.2 full-time nurse case managers, achieving good fidelity for core protocol steps. C-TraC patients experienced a 30-day rehospitalization rate of 10.8%, as compared to 16.6% for a contemporary comparison group of similar patients for whom C-TraC was not available (n=1,307) [p-value < 0.001]. The new C-TraC program continues in operation to this day. In conclusion, use of a modified REP model to guide protocolized adaptation to local context resulted in a C-TraC program that was feasible and sustained in a real-world non-VA setting. A modified-REP implementation framework may be an appropriate foundational step for other clinical programs seeking to harness protocolized adaptation in mentored dissemination activities.
Keywords: transitional care, rehospitalization, implementation science, dissemination, nursing
INTRODUCTION
Overview of the VA Coordinated-Transitional Care (C-TraC) Program
The VA C-TraC program is a low-cost transitional care program that utilizes hospital-based nurse case managers, inpatient team integration and in-depth post-hospital phone contacts to support high-risk patients and their caregivers as they transition from hospital to community.1 The goals of C-TraCinclude empowering patients and their caregivers in medication management, medical follow-up and when and whom to contact if issues arise.1–5 C-TraC was specifically designed to complement evidence-based home-visit transitional care programs2,3,5 by offering a similar, but phone-based, protocolized, transitional care option for patients who refuse home-visits, are not ill enough or live too far away to qualify for home-visit based transitional care, or who cannot access such programs because they are in low-resource health care settings.1 Pilot testing at the VA Hospital in Madison, Wisconsin, demonstrated that C-TraC reduced 30-day rehospitalizations by one-third, leading to a net savings of over $1200 per enrollee. The VA program continues in operation to this day, has enrolled over 2000 veterans (since 2010) with only 8 refusals, and has disseminated to other VA hospitals.
Importance of Adapting to Local Culture and Context to Achieve Sustainability
Non-VA hospitals have expressed interest in launching their own C-TraC programs, but non-VA hospitals differ in important ways from VA hospitals, particularly in terms of context, culture and resources. In order for newly disseminated clinical programs to be valued by their new system and ultimately sustained, they must be sensitive to that new system’s pre-existing context, culture and resources and to local programmatic goals.7–9 Externally defined, nonadaptable programs which are not sensitive to the local context, culture and goals of a new system are less likely to be seen as ‘successful’ by local leadership or sustained after initial dissemination.9 Each new setting may differ markedly,7 so if a program is to disseminate widely this adaptation process should be protocolized and achieve a balance of local-adaptation and core intervention fidelity.7–10
It was not clear whether this balance could be feasibly accomplished in a C-TraC dissemination, but the Replicating Effective Programs (REP) Implementation Theory model held promise as a pathway towards this goal.
The REP Implementation Theory Model
Originally developed by the Centers for Disease Control (CDC) as a strategy for closing the gap between research and community practice, the REP model has been applied widely since its development in 1996.10 The REP model recognizes that operationalization of an intervention’s core features may vary from one setting to another (because each setting varies), but that the intent of each core step must remain the same to achieve a desired impact. The REP model has been used extensively in the dissemination of community-level HIV treatment interventions by the CDC, with its effectiveness proven in a national randomized-controlled trial.11,12 Although REP is well-proven in population-level (macro-level) dissemination efforts, many theorize that its framework would be highly suitable to use in the dissemination of health systems interventions at the micro-level.10
Health systems are remarkably diverse entities. Each health system is unique in terms of general culture and context, but each also contains a diverse array of micro-level contexts (e.g., hospitals→units→teams) which may, themselves, contain diversities in terms of resources, goals and culture. Although it has been successfully applied at the population level,10,13,14 to our knowledge REP has not been used to guide health system intervention adaptation at the hospital or smaller micro-level. We theorized that the REP model could be modified to allow for protocolized program adaptation to local micro-level health system context in a C-TraC dissemination.
Disseminating C-TraC
An opportunity to test this theory arose when our team was approached to launch C-TraC at the University of Wisconsin Hospitals and Clinics (UWHC), a large tertiary-care academic hospital with an expansive geographic referral region. Our goal was to successfully adapt the VA C-TraC program to the specific micro-level context of UWHC by employing a modified-REP model, and to test the feasibility of this protocolized implementation approach. We defined a ‘successful adaptation’ as one which maintained fidelity of the intervention’s core steps, yet allowed for enough programmatic evolution for the implementation to fit local context, meet locally-defined goals and, ultimately, achieve sustainability.
METHODS
Local Context: The Decision to Launch C-TraC at UWHC
The UWHC is a 592 bed academic hospital in Madison, Wisconsin. Over half of UWHC’s inpatients reside beyond the reach of a home visit; some hundreds of miles away. To improve transitional care quality and decrease rehospitalizations, UWHC piloted an evidence-based home-visit transitional care program in 2012 for high-risk medical inpatients. However, the program’s viability was threatened by much lower than expected enrollment due to two main factors: 1.) Many patients lived too far away to be eligible for the home-visit based program; and 2.) About half of patients who lived closer refused to allow clinical staff to enter their homes.
The UWHC Senior Vice President of Patient Care Services – Chief Nursing Officer and the UWHC Director of Transitional Care Programs became aware of the C-TraC program,1 and decided that by using C-TraC these enrollment challenges could potentially be overcome. They approached the VA C-TraC team to mentor a pilot C-TraC launch on inpatient medicine services at UWHC. UWHC financed the full program.
Given the multiple differences in clinical culture and context between the VA and UWHC, including models of inpatient rounding, electronic medical record interconnectivity and typical assignment/association of primary care providers within and outside the systems, intervention adaptation was clearly needed prior to any implementation. A modified-REP model was harnessed to meet this need.
Applying the Modified-REP Model
We created a pilot implementation protocol for C-TraC adaptation based upon the original REP model and on practical modifications derived from our team’s real-world experience with REP’s application in two regional VA C-TraC disseminations (Figure 1). This modified-REP protocol included simple modifications (outlined below) to enable practical application to the hospital micro-level and to our specific issue of transitional care. The protocol harnesses four key phases: 1.) Pre-conditions, 2.) Pre-implementation, 3.) Implementation, and 4.) Maintenance and Evolution.
Figure 1.
Modified-Replicating Effective Programs (REP) Implementation Modela Used for Mentoring in C-TraC. aAdapted from CDC’s Replicating Effective Programs Implementation Theory Model
Pre-Conditions
Pre-conditions in the modified-REP model are those pre-existing characteristics of the targeted health system and micro-level context which are fundamental to a new intervention and merit careful consideration. This phase involves two primary tasks: 1.) identification of a local programmatic champion(s) who is well-connected within the target system and who has the skills/positioning to become the primary local advocate and director for the program; and 2.) a detailed review of existing resources and processes related to the new intervention. Ideally, institutional leaders are engaged at this step as well.
In the original REP model for macro-level dissemination, researchers determine “need” and chose an intervention to implement; however in this micro-level implementation, UWHC leadership had already independently accomplished these tasks. In our experience with microlevel implementation, it is typical for definition of need and choice of specific intervention to lie primarily with system-leadership, not with an external researcher group.
In the specific case of C-TraC, the UWHC Chief Nursing Officer and the Director of Transitional Care Programs were identified as local programmatic champions. Local discharge resources and processes, as well as any existing related programs within the system, were noted. These were initially documented by the C-TraC implementation mentoring team (comprised of MD Director and nurse mentor of VA C-TraC) in partnership with the local champions and later validated (or revised) in discussions with local key stakeholders.
Pre-Implementation
Pre-implementation in the modified-REP model is a preparation phase in which wider local stakeholder buy-in is achieved to facilitate programmatic adaptation to local culture/context while maintaining core step fidelity, ultimately readying for initial local program launch. This phase involves three primary tasks.
1) Key-Stakeholders
The first is convening of a local, multidisciplinary key-stakeholder group. “Key-stakeholders,” in this case, are individuals embedded within the local system and who can advocate on behalf of their particular discipline, employee type or patient group, with all groups potentially impacted by the new program/intervention being represented. The local champion helps identify key-stakeholders. For example, the UWHC C-TraC key-stakeholder group included the UWHC Chief Executive Officer and aforementioned UWHC C-TraC champions, and had representatives from the UWHC executive team, inpatient nurse managers and physicians, outpatient primary care providers and UWHC patients. The implementation mentoring team then guides this group through a series of sessions in which the key-stakeholders validate and/or refine the ‘pre-existing related health system processes’ list created during the pre-condition step, are engaged in a detailed discussion of the core target intervention (e.g., C-TraC) steps and its previous impact in other settings,1 and are coached to clearly and specifically define what outcomes/goals would be indicative of local ‘success’ for a new program. Locally defined goals for the UWHC C-TraC program included mitigating confusion around the discharge plan, identifying and correcting medication discrepancies, and reducing readmissions.
2) Customizing Delivery
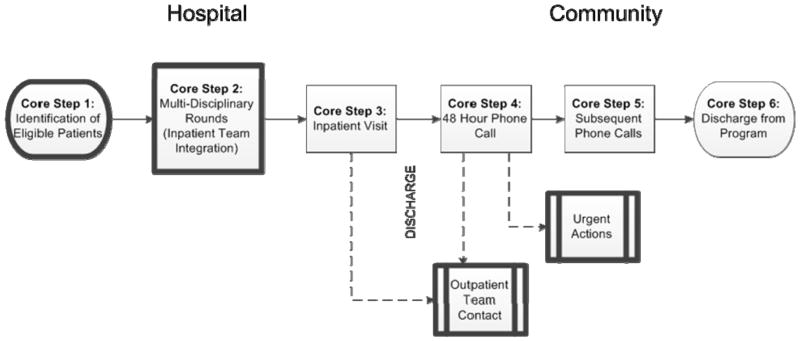
The second task of pre-implementation involves customizing delivery of the intervention protocol to ensure adaptation to local health system and micro-level context. To accomplish this customization, the implementation mentor team guides the local key-stakeholder group in formally adapting each core step of the intervention protocol while ensuring that the intent of each step is maintained. (A full and detailed description of the original C-TraC protocol has been published previously1 and can also be found at http://www.hipxchange.org/C-TraC.) Additionally, the mentoring team works with the key-stakeholders to ensure that the resultant adaptation is fully integrated with, and not duplicative of, pre-existing related health system processes. Some core steps may need more adaptation than others depending upon local health system characteristics or the particular micro-level context being considered. For example, when the C-TraC protocol1 was formally adapted for UWHC (Figure 2), core step #2 required a change in operationalization because multidisciplinary discharge rounds were not present on all hospital services. The intent of this core step was to achieve C-TraC nurse integration with the inpatient team, so the key-stakeholder group identified other ways that this core step’s intent could be realized (e.g., integration with unit rounds, regular check-ins with the team). Additionally, because UWHC inpatient pharmacists review the discharge medication list in detail with the patient on the discharge day, the UWHC C-TraC adaptation omitted this specific task in core step #3 (the inpatient visit). Other tasks included in core step #3 were maintained, including establishing rapport, scheduling a 48 hour post-discharge phone call, and ensuring that the patient/family know how and when to directly contact their C-TraC nurse. A complete medication reconciliation was still conducted in core step #4 (the 48 hour post-discharge phone call). The other core steps involving direct communication with patients and caregivers required no changes. This customization/adaptation process resulted in a full and highly detailed protocol that could be harnessed for initial C-TraC launch at UWHC. This protocol was then used to construct electronic medical record-based templates which directly reflected the new protocol, reinforced fidelity of each core step, and allowed for collection of key process measures/outcomes identified as important by local stakeholders.
Figure 2.
C-TraC Core Steps. aDark boxes are highly sensitive to context and may need system-specific adaptations
The stakeholder group and the UWHC C-TraC leadership decided that eligibility criteria for the UWHC C-TraC program would remain identical to that used in the original VA C-TraC pilot.1 Therefore, patients eligible for UWHC C-TraC had to be hospitalized at UWHC and be discharged to a non-institutional community setting with one or more of the following: 1) cognitive impairment, 2) age 65 years or older and lives alone, or 3) at least one previous hospitalization in the last year. Patients who were receiving intensive, on-going, longitudinal outpatient case management (e.g., transplant patients) were excluded.
3) Hiring, Training, Coaching
In this last task of pre-implementation, clinical program staff are hired and trained, and program leadership is coached in program assessment, reporting and administrative barrier reduction. The implementation mentoring team leads each of these activities, coaching local leadership on characteristics of ideal candidates for C-TraC nurse hire and tailoring training for those new hires as needed.15 Two experienced full time UWHC Registered Nurse case managers (RNs) were initially dedicated to the new C-TraC program, but this was increased to three full time nurses at the nine month point because of strongly positive initial results. Each was trained using a combination of didactics and practice-based learning over an intensive one week apprenticeship and through weekly one-hour mentoring team meetings during the subsequent four months. The UWHC C-TraC program staff continued to meet daily to discuss challenges and operations.
The time needed to accomplish pre-implementation activities with local key-stakeholders will vary based upon how easy or difficult it is to obtain participation and majority consensus, and based upon how widely the target system varies from the original. In the UWHC C-TraC pre-implementation, three months were required to accomplish all key tasks. Pre-implementation for C-TraC launches in systems more similar to the initial VA C-TraC pilot hospital have required less time. However, regardless of the length, this is time well invested as strong pre-implementation allows for a smoother implementation with fewer unexpected barriers which could threaten sustainability.
Implementation
Implementation in the modified-REP model occurs with actual local program launch, maintaining continuous process and fidelity monitoring to guide rapid-cycle iterative protocol refinement during the post-launch period. This key phase involves four primary tasks: 1.) Garner widespread front-line stakeholder engagement and launch the adapted program; 2.) Perform continuous process monitoring to understand core step fidelity and implementation barriers, if any; 3.) Using continuous monitoring data, perform rapid cycle iterative-phased protocol refinement (i.e., plan, do, study, act cycles) to ensure program feasibility and fidelity; and 4.) Perform real-time monitoring of outcomes identified as important by the local key-stakeholders. Any protocol refinements made during this period are immediately and clearly documented in the formal protocol and discussed with the full implementation team to ensure transparency and understanding. Any necessary updates to the electronic medical record templates should also be completed at this time.
In the UWHC C-TraC implementation, each of these four tasks was achieved. Prior to launch, the local C-TraC champion and members of the key-stakeholder group addressed all nursing, physician and other front-line inpatient and outpatient staff to introduce C-TraC, its goals and planned operations, and to ask for feedback. When possible, these sessions were performed in small group or individual settings, and were discipline concordant (e.g., nurses presented to nurses). The UWHC C-TraC program was launched July 2013 on two inpatient medical services, and quickly expanded to cover four total medical services. Only minor protocol alterations were necessary post-launch, and primarily centered on how to best achieve C-TraC integration with the inpatient teams, as multi-disciplinary rounding practice varied between services.
Maintenance/Evolution
The final phase of the modified-REP model is Maintenance/Evolution in which program results are compiled and reported to leadership and other relevant stakeholders, and, if the program is deemed successful, local sustainment is achieved and next steps for dissemination are considered. In this final step, which began approximately 12 months post-launch, the implementation mentoring team worked closely with the UWHC C-TraC team to help design and support results feedback to the local health system leaders and stakeholder groups. This included mentorship in data presentation, messaging and resource sustainability requests, as well as advice on potential next steps for local dissemination. A cornerstone of this phase of mentorship was advice on the production of a locally-targeted financial case for program growth and sustainability. Although the implementation mentoring team assisted in the creation of materials and strategy, UWHC C-TraC leadership served as the primary champions and presenters of this information to local health system leaders. Once sustainability was achieved the new UWHC C-TraC program reached full independence.
Assessment
Program evaluation for the UWHC C-TraC launch focused on two primary areas—core step fidelity and locally-targeted outcomes. Core step fidelity measures, extracted directly from the electronic medical record templates, included indicators for each core step’s occurrence for each enrolled patient, as well as key content items included within the initial post-hospital phone call (core step #4). Outcomes data included medication discrepancies identified and rectified during the 48–72 hour post-discharge phone call and the presence of any acute care rehospitalization within 30 days back to UWHC. Medication discrepancies were collected within the electronic medical record templates. Rehospitalizations were extracted directly from UWHC internal administrative data by the UWHC Business Planning and Analysis Department (BPAD), an analytics branch independent from both the UWHC C-TraC team and the C-TraC implementation mentoring team. These program metrics, as well as enrollment numbers, patient characteristics and staff work-time data were compiled for the first 16 months of UWHC C-TraC operation (July 2013 through October 2014). These data were compiled separately for C-TraC enrolled patients on acute-care status as well as C-TraC enrolled patients on observation status, because UWHC only compiles 30-day rehospitalization data for those on acute-care status. A contemporaneous comparison group (n=1,307) was drawn from UWHC acute-care medical patients who would have met C-TraC criteria but who did not receive C-TraC because the program was not offered on their particular hospital medical service. Rehospitalizations for this comparison group were also extracted administratively by BPAD, using the identical methods to those noted above. Differences in rehospitalization frequencies between the intervention and contemporaneous comparison group were assessed via Chi-squared tests. The UW Institutional Review Board designated this project as exempt from review.
RESULTS
Enrollment
In its first 16 months of operation, UWHC C-TraC successfully enrolled 1247 patients (964 acute-care and 283 observation status) with enrollment capacity increasing monthly due to sequential improvements in operational efficiency as well as the addition of new nursing staff at the 9 month point. In the first month of operation 8.5 patients were enrolled per C-TraC nurse, but this increased to the expected target volume of 35–45 patients per nurse per month by the third month of operation. During the 16 month assessment period, 61 patients (~5%) actively refused to participate when approached during the in-hospital visit. The two most common reason patients gave C-TraC nurses for active refusal were: 1) the patient felt he/she did not need the service; and 2) the patient felt that they had enough resources already in place. Additionally, another 129 patients (~10%) did not engage in the program. These patients either did not answer their post-hospital phone call despite multiple attempts or were rehospitalized within 48 hours of discharge prior to engaging in the post-hospital call. Although these refusal rates were higher than those observed for the VA C-TraC program (<1% refusal rates), they were still much lower than those noted for the prior UWHC home-visit based transitional care program. Characteristics of patients who enrolled in the program are in Table 1.
Table 1.
Characteristics of Patients Within the UW C-TraC Program (N=1247)a
| Characteristic | Enrolled % (N = 1247) |
|---|---|
| Sociodemographics | |
| Average Age (y) | 77 |
| Race, White | 93 |
| Male | 47 |
| Lives Alone | 43 |
| Medicaid | 0 |
| Medicare | 81 |
| Education Level: | |
| Less Than 8 Years | 2 |
| Some High School | 5 |
| High School Graduate/GED | 24 |
| Some College | 12 |
| College Graduate | 27 |
| Previous Hospitalization During Prior 12 Months | 43 |
Values represent percents unless otherwise specified
Core-Step Fidelity
The UWHC C-TraC program operated with a reasonable level of core step fidelity. Program clinical staff identified eligible patients (Figure 2, Core Step #1) and integrated with inpatient teams (Core Step #2) on a daily basis. Eighty-nine percent (1112/1247) of enrolled patients received a protocolized inpatient visit (Core Step #3), >95% had an attempted protocolized post-hospital phone call within 48–72 hours and 65% of patients engaged in that call within 48–72 hours (Core Step #4); the rest of the enrolled patients engaged in calls outside of the 72 hour timeframe (i.e., multiple calls were needed to reach these patients). These calls averaged 16 minutes in length (range 5–120 minutes), and included a full patient/caregiver-led medication reconciliation with all medication discrepancies noted and rectified.1 The C-TraC nurses rectified all medication discrepancies by employing patient education and, when necessary, obtaining new orders from either the patient’s inpatient or outpatient prescribing provider (per the nurse’s judgment). Caregivers were included in C-TraC transitional care contacts for 29% of patients. C-TraC nurses noted that the electronic medical record templates designed for each core step helped reinforce protocol fidelity.
UWHC C-TraC Pilot: Outcomes, Business Case
Outcomes for the UWHC C-TraC pilot met stakeholder pre-defined goals. Twenty-five percent of all patients (n=312) had at least one medication discrepancy identified/rectified during the 48–72 hour post-hospital C-TraC call, with cardiovascular medications being the most commonly discrepant. This was less than the 47% discrepancy rate observed in the original VA C-TraC pilot. The average number of medication discrepancies per patient was 2.4 but ranged from 1 to 29. (Table 2)
Table 2.
Medication Discrepancies Identified and Rectified by C-TraC During the 48–72 Hour Post-Discharge Follow-up Phone Call Performed by the Transitional Care Manager (N = 1247)a
| Medication Discrepancy Characteristic | Number or Percent |
|---|---|
| Prevalence of Medication Discrepancies | |
| Number of Patients with at Least One Medication Discrepancy | 312 (25%) |
| Average Number of Medications per Patient (Range) | 14 (0–44) |
| Total Number of Medication Discrepancies in Sample | 592 |
| Average Number of Medication Discrepancies Identified/Rectified per Patient, For Those With Any Discrepancy (Range) | 2.4 (1 – 29) |
| Most Common Classes of Medications with Discrepancies (% of all Discrepancies) | |
| Cardiovascular | 25 |
| Vitamins/Supplements | 15 |
| Gastroenterology | 14 |
| Analgesics | 13 |
| Endocrine/Metabolic | 7 |
| Pulmonary | 4 |
| Most Common Specific Medications with Discrepancies (% of all Discrepancies) | |
| Aspirin | 4 |
| Acetaminophen | 3 |
| Lisinopril | 3 |
| Multivitamin | 3 |
| Omeprazole | 2 |
129 patients enrolled in the C-TraC program, but met program discharge criteria prior to the first follow-up phone call. The data shown above is for those who did receive the post-hospital phone call.
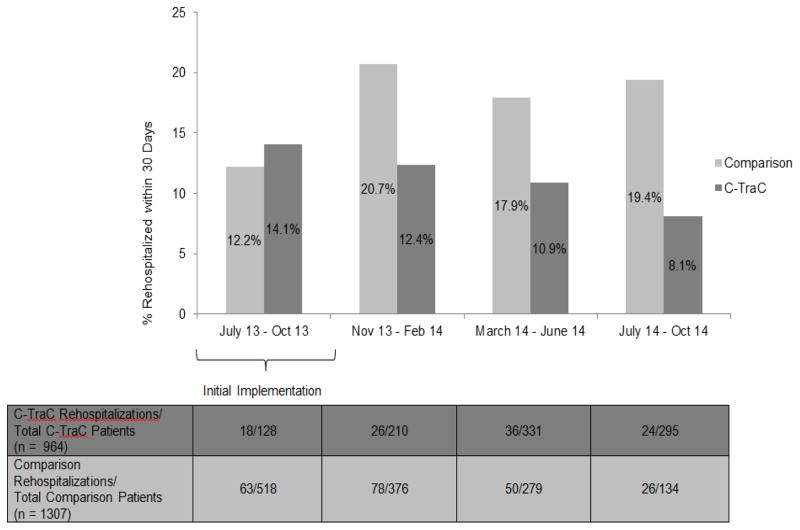
C-TraC intervention acute-care status patients experienced lower rates of 30-day rehospitalization than the contemporaneous comparison group of acute care status patients who did not receive C-TraC. Overall, 10.8% (104/964) of C-TraC patients experienced a rehospitalization as compared to 16.6% (217/1,307) of medical comparison patients (p-value < 0.001). [UWHC’s overall rehospitalization rate for the same time-period for all older adult patients was 12.5% (4,259 readmissions of 34,184 discharges)]. This 5.8% absolute decrease in rehospitalizations represents a relative risk reduction of 35%; a magnitude of reduction similar to that noted in the original VA C-TraC pilot. When examined by 4-month time-periods, rehospitalization reductions were not fully observed until after the initial 4-month implementation period (Figure 3). No rehospitalization data were available for the observation status groups.
Figure 3.
Rehospitalization Frequencies for Coordinated-Transitional Care (C-TraC) Intervention Acute Care Patients as Compared to Usual Care Contemporaneous Comparison Groupa, by 4 Month Intervals. aComparison Group = Contemporaneous study hospital medical patients who would have met C-TraC program criteria (i.e., were 65 years of age or older and who were discharged to home or to an assisted living facility) but who did not receive C-TraC because the program was not offered on their particular hospital medical service
Estimated total up-front investment for this C-TraC pilot was $300 per patient enrolled, which includes all staff, administrative and implementation costs. The average direct cost for an acute medical/surgical bed day of care at UWHC is $3,325/day, and the average rehospitalization length of stay is 5 days. If one were to assume a capitated system, given the observed decrease in rehospitalizations of −5.8% versus the comparison group, it is estimated that the UWHC C-TraC program avoided 361.6 days in acute care over the first 16 months, leading to an estimated gross savings of $1,202,420. After accounting for all programmatic costs, this led to an estimated net savings of $826,337 overall or $663 per patient enrolled over the first 16 months of the program (including the less efficient early implementation months). However, UWHC is not a capitated system; most of these theoretical savings were actually realized by Medicare. Nonetheless, given the observed drop in rehospitalizations, strong reports of patient satisfaction, and decreased risk of receiving Medicare rehospitalization penalties, the UWHC C-TraC program continues in operation to this day, fully sustained by UWHC. Efficiency continues to improve and expansion plans are under-way.
DISCUSSION
To achieve sustainability, newly disseminated clinical programs must be valued by their new health micro-systems. To be valued, these programs need to be sensitive and adaptable to the new system’s pre-existing context, resources and locally-defined goals.8–10 We offer the modified-REP model as a promising option to achieve protocolized micro-system adaptation in clinical program dissemination, balancing local-adaptation with core intervention fidelity. The modified-REP framework has now functioned successfully as an approach for protocolized implementation within this UWHC C-TraC dissemination as well as in regional VA C-TraC disseminations. To our knowledge, this is the first publication of a modified-REP framework being used to guide protocolized adaptation and implementation of a clinical intervention at a hospital or smaller micro-system level. This work is innovative in that it could potentially serve as a foundation for the spread of other patient-level interventions across a large variety of health system sizes, types and cultures.
The modified-REP model is founded upon the CDC’s REP implementation model, which has a strong track-record in national macro-level dissemination efforts.10 This model fits well as a specific, health system micro-level protocol for achieving the context-specific ‘adoption’ and ‘implementation’ steps present within most theoretical models of dissemination, including The Practical, Robust Implementation and Sustainability Model (PRISM) and others.8,9 It incorporates basic tenets of dissemination, including consideration of organizational characteristics, needs and perspectives; staff and patient perspectives, needs; and attention to the implementation process and sustainability infrastructure.8 Nonetheless, there are many other theoretical implementation models available; an approach should be chosen based upon a project’s specific implementation needs.
Use of the modified-REP model should be tempered in light of some limitations arising primarily from the pilot nature of the work presented here. This modified-REP model has been used only in a handful of Midwestern health system C-TraC launches, and it is unclear whether similar results could be realized in other micro-system regions/cultures or with other interventions. Although it is extremely promising and holds strong face-validity, the modified-REP model should be tested in a more formal, rigorous and quantitative implementation trial to better understand its impact and generalizability across a wider array of micro-systems. Additionally, the outcomes collected as part of this clinical C-TraC implementation arise from a prospective quality improvement-level program evaluation, and lack the rigor of more stringent assessment modalities. In particular, we were unable to assess rehospitalizations to outside facilities. This may have been particularly important at the UWHC given the high rate of older patients who were receiving care a long distance from their home. The UWHC C-TraC outcomes are provided here primarily to offer a detailed real-world example of program implementation and the underpinnings of the financial argument made for local sustainment. An NIH-funded C-TraC randomized-controlled trial is underway and will provide a more rigorous assessment of C-TraC’s impact.6
In conclusion, use of a modified-REP implementation model to guide protocolized adaptation to local context resulted in a good fidelity C-TraC program which was feasible and sustained in the non-VA setting. A modified-REP implementation framework may be an appropriate foundational step for other clinical programs seeking to harness protocolized adaptation in mentored micro-system level dissemination activities.
Acknowledgments
Funding Source: This project was supported by the University of Wisconsin Hospitals and Clinics; a National Institute on Aging Beeson Career Development Award (K23AG034551 [PI Kind], National Institute on Aging, The American Federation for Aging Research, The John A. Hartford Foundation, The Atlantic Philanthropies and The Starr Foundation); National Institute on Aging 2P50AG033514-06 and by the Madison VA Geriatrics Research, Education and Clinical Center (GRECC-Manuscript #2015-008). Dr. Kind’s time was also partially supported by the University of Wisconsin School of Medicine and Public Health from the Wisconsin Partnership Program. Additional support was provided by the Community-Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR), grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Footnotes
Related Paper Presentations: This project was presented as a poster during the 2014 American Geriatrics Society Annual Meeting.
Author Contributions
Study concept and design: Amy JH Kind, Maria Brenny-Fitzpatrick, Beth Houlahan
Acquisition of subjects and/or data: Amy JH Kind, Maria Brenny-Fitzpatrick, Kris Leahy-Gross, Jacquelyn Mirr, Elizabeth Chapman, Brooke Frey
Analysis and interpretation of data: Amy JH Kind, Kris Leahy-Gross, Jacquelyn Mirr, Brooke Frey
Preparation of manuscript: Amy JH Kind, Maria Brenny-Fitzpatrick, Kris Leahy-Gross, Jacquelyn Mirr, Elizabeth Chapman, Brooke Frey, Beth Houlahan
Contributors
The authors would like to acknowledge Peggy Troller, Hilary Krieger, Matt Lakosky, and Jennifer Hendricks for program support and Laury Jensen for nurse mentoring.
Conflict of Interest: Dr. Kind has received institutional grant support from the Department of Veterans Affairs, the National Institutes of Health-NIA and the John Hartford Foundation, and serves as a consultant for the State of Maryland. No other co-authors have any conflicts of interest to disclose.
Sponsor’s Role: No funding source or sponsor had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
References
- 1.Kind AJH, Jensen L, Barczi S, et al. Low-cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff. 2012;31:2659–2668. doi: 10.1377/hlthaff.2012.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: A randomized, controlled trial. J Am Geriatr Soc. 2004;52:675–684. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 3.Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: A randomized clinical trial. JAMA. 1999;281:613–620. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 4.Coleman EA. Falling through the cracks: Challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51:549–555. doi: 10.1046/j.1532-5415.2003.51185.x. [DOI] [PubMed] [Google Scholar]
- 5.Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: Results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 6.National Institute of Health. [Accessed November 11th, 2014];Research Portfolio Online Reporting Tools. 2014 http://projectreporter.nih.gov/reporter.cfm.
- 7.Moulding NT, Silagy CA, Weller DP. A framework for effective management of change in clinical practice: Dissemination and implementation of clinical practice guidelines. Qual Health Care. 1999;8:177–183. doi: 10.1136/qshc.8.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34:228–243. doi: 10.1016/s1553-7250(08)34030-6. [DOI] [PubMed] [Google Scholar]
- 9.Glasgow RE, Lichtenstein E, Marcus AC. Why don’t we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health. 2003;93:1261–1267. doi: 10.2105/ajph.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilbourne A, Neumann M, Pincus H, et al. Implementing evidence-based interventions in health care: Application of the replicating effective programs framework. Implementation Sci. 2007;2:42. doi: 10.1186/1748-5908-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson JL, Milam J, McCutchan A, et al. Effect of brief safer-sex counseling by medical providers to HIV-1 seropositive patients: A multi-clinic assessment. AIDS. 2004;18:1179–1186. doi: 10.1097/00002030-200405210-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kelly JA, Somlai AM, DiFranceisco WJ, et al. Bridging the gap between the science and service of HIV prevention: Transferring effective research-based HIV prevention interventions to community AIDS service providers. Am J Public Health. 2000;90:1082–1088. doi: 10.2105/ajph.90.7.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens AB, Lancer K, Smith ER, et al. Engaging communities in evidence-based interventions for dementia caregivers. Fam Community Health. 2009;32(1 Suppl):S83–92. doi: 10.1097/01.FCH.0000342843.28477.72. [DOI] [PubMed] [Google Scholar]
- 14.Goodrich DE, Bowersox NW, Abraham KM, et al. Leading from the middle: Replication of a re-engagement program for veterans with mental disorders lost to follow-up care. Depress Res Treat. 2012;2012:325249. doi: 10.1155/2012/325249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilmore-Bykovskyi A, Jensen L, Kind AJ. Development and Implementation of the Coordinated-Transitional Care (C-TraC) Program. Fed Pract. 2014;31:30–34. [PMC free article] [PubMed] [Google Scholar]