Abstract
Prematurity is the leading cause of infant mortality worldwide. In developed countries, extremely preterm infants contribute disproportionately to both neonatal and infant mortality. Survival of this high-risk population has incrementally improved in recent years. Despite these improvements, approximately 1 in 4 extremely preterm infants dies during the birth hospitalization. Among those who survive, respiratory and other morbidities are common, although their effect on quality of life is variable. In addition, long-term neurodevelopmental impairment is a large concern for patients, clinicians and families. However, the interplay of multiple factors contribute to neurodevelopmental impairment, with measures that change over time and outcomes that can be difficult to define and predict. Understanding outcomes of extremely preterm infants can help better counsel families regarding antenatal and postnatal care and guide strategies to improve survival without morbidity. This review summarizes recent evidence to provide an overview into the short- and long-term outcomes for extremely preterm infants.
Keywords: prematurity, low birth weight, neurodevelopment, survival, morbidity, periviable
Introduction
Extremely preterm (EPT) birth is a leading cause of infant death and morbidity. The World Health Organization defines EPT infants as those born before 28 weeks (wk) gestational age (GA) and studies from the NICHD Neonatal Research Network (NRN) define EPT infants as those born before 29wk GA. Despite the subtle variability in definitions of EPT, these infants contribute disproportionately to preterm-related morbidity and mortality. Globally, EPT births account for 5.2% of all preterm births < 37wk GA1. In the United States in 2013, 11.4% of infants were born preterm, with 0.7% of infants born before 28wk GA, a proportion that has been relatively constant since 20002. Despite the relatively small number of EPT births, these infants and slightly more mature infants born between 28 and < 32wk GA account for over half of all infant deaths in the US3. This review focuses on providing an overview of outcomes for EPT infants, using data from the US and other developed countries. Given the wide ranging literature on the multitude of neonatal outcomes covered, this review is intended to be an overview, rather than a systematic review, of short- and long-term outcomes for EPT infants. The goal is to provide the reader with an understanding of EPT outcomes in the context of other important review articles in this special theme issue on preterm birth.
Survival
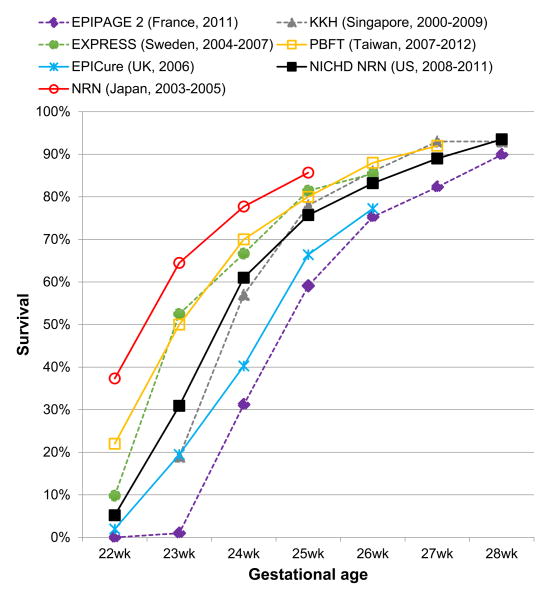
Following decades long trends, survival continues to improve for EPT infants. Recent data from several sources indicate improvements in survival for EPT infants in the US4-6 and other international developed nations7-11. Based on estimates from the NRN, 74% of EPT infants survive the initial birth hospitalization4,5, although each decreasing GA week has substantial effects on mortality, particularly for infants born at 22-25wk GA (Figure 1).
Figure 1. Gestational Age Specific Survival for Extremely Preterm Infants.
Characteristics of the data sources are shown in Table 1.
Gestational age-specific survival
A number of recent cohort studies in the US4,5, France7, Japan10, Taiwan11, UK12, Sweden13, and Singapore14 have provided estimates of GA-specific survival that can be utilized as a starting point for understanding outcomes and international variations in survival for EPT infants (Table 1). However, international comparisons of EPT survival among countries are limited by differences in the data sources, ascertainment of death, selection of denominators, and definitions of live-births15. International collaborative groups, such as the International Network for Evaluating Outcomes (iNEO) have undertaken efforts to provide a better framework for comparisons of health outcomes for preterm infants amongst developed countries16. Such efforts should ensure consistent reporting of outcomes, including systematic measurement of numerators and denominators for comparisons.
Table 1. Characteristics of Select Cohort Studies Reporting Gestational Age Specific Survival for Extremely Preterm Infants.
| Study | NICHD NRN | EPIPAGE-2 | NRN | PBFT | EPICure | EXPRESS | KKH |
|---|---|---|---|---|---|---|---|
| Country | United States | France | Japan | Taiwan | United Kingdom | Sweden | Singapore |
| Year(s) | 2008-2011 | 2011 | 2003-2005 | 2007-2012 | 2006 | 2004-2007 | 2000-2009 |
| Sample size | 7124 | 1911 | 1057 | 1718 | 2034 | 707 | 887 |
| Gestational ages | 220/7-286/7 wk | 220/7-286/7 wk | 22-25 wk | <29 wk | <27 wk | <27 wk | <29 wk |
| Numerator | Survival to discharge | Survival to discharge | Survival to discharge | Survival to discharge | Survival to discharge | Survival to 1 year | Survival to discharge |
| Denominator | Live births (inborn) | Live births | Live births | Live births admitted to centers | Live births | Live births | Live births admitted to center |
| Study cohort | 25 academic medical centers | Population-based cohort | 48 tertiary centers | 5 centers part of PBFT | Population-based cohort | Population-based cohort | Single large tertiary referral center |
| Reference | 5 | 7 | 10 | 11 | 12 | 13 | 14 |
Abbreviations: NICHD, National Institute of Child Health and Human Development; NRN, Neonatal Research Network; EPIPAGE-2, Etude Epidémiologique sur les Petits Ages Gestationnels 2; PBFT, Premature Baby Foundation of Taiwan; EXPRESS, Extremely Preterm Infants in Sweden Study; KKH, KK Women's and Children's Hospital.
Effect of variation in active treatment on survival
Variation in active treatment with resuscitation after birth, particularly for those infants less than 25wk GA, is likely to account for a significant portion of the variation in GA-specific survival between countries (Figure 1). In the US, between-hospital variation in active treatment for infants less than 24wk GA has a large effect on center differences in survival of the most immature infants17. In a study by Rysavy et al., the investigators found that 78% of variation in survival for infants born before 26wk GA among 24 academically-affiliated hospitals was accounted for by variation in the use of active treatment with potentially lifesaving interventions after birth (e.g. intubation, ventilation). For infants at 22wk and 23wk GA, the variation in mean rates of active treatment by hospital ranged from 0% to 100% and 25% to 100%, respectively. Understanding the frequency and spectrum of adverse outcomes for EPT infants is important, as the decision to forgo active lifesaving treatment is often reached after antenatal counselling regarding EPT outcomes. As the authors note, data from infants that did not receive active treatment are often included in population estimates of survival, which may lead to a “self-fulfilling prophecy” and provide potentially misleading estimates when considering the possible outcomes if active treatment (i.e. resuscitation) is pursued for an EPT infant. For example, overall survival to hospital discharge for inborn live births at 22wk and 23wk GA is 5% and 24%, respectively17. However, when only infants receiving active treatment with life-saving interventions are evaluated (i.e. excluding infants receiving only comfort care), survival estimates change to 23% at 22wk GA (n=79) and 33% at 23wk GA (n=542). Understanding these data are important in counselling families and caring for fetuses and neonates at a periviable GA, which is broadly defined as 20 0/7 to 25 6/7 weeks by a recent NICHD workshop18. Even at a periviable GA, long-term neurodevelopmental disability is not universal and prediction can be uncertain as discussed later in this review.
Causes of death
Death among EPT infants is often broadly attributable to preterm birth or short-gestation, particularly in national vital statistics datasets3. Identifying the specific causes of death from underlying complications of preterm birth is important in understanding contributors to mortality in EPT infants, although attribution of singular causes of death can be challenging as EPT infants often have multiple co-morbid complications of prematurity. Among very preterm infants less than 32wk GA in the United Kingdom, complications of preterm birth predominated as the cause of neonatal and infant death, while malformations largely accounted for deaths among more mature preterm infants 32-36wk GA19. Recent data from the NRN reports “immaturity” as the leading cause of death among EPT infants, with most of these infants receiving comfort care in the delivery room without active treatment and dying within 12 hours of birth5. The second most common cause of death is pulmonary, comprised of respiratory distress syndrome and bronchopulmonary dysplasia (BPD). Over half of recent improvements in survival for EPT infants from 2000 to 2011 in the NRN was accounted for by decreases in pulmonary-related deaths. By contrast, data from the NRN5, Sweden20 and the UK21 suggest a greater proportion of EPT infants are dying from necrotizing enterocolitis (NEC) in recent years. NEC becomes a larger proportionate cause of mortality as GA increases, particularly at 26-27wk GA. In addition, data from a prospective study in 46 US neonatal intensive care units (NICU) found NEC to be the second most common cause of death among all infants (term and preterm) born at ≥22wk GA, accounting for 10% of NICU deaths22. Only deaths due to extreme prematurity were more common, accounting for 14% of all NICU deaths.
Short-term morbidities
EPT birth leads to loss of months of fetal development, leaving the infant vulnerable to morbidities, many of which are unique to the preterm population. Among infants who survive, the percent who leave the hospital without severe morbidities range from 0% at 22wk GA to 54% at 28wk GA based on data from the NRN4. Overall, 39% of EPT infants <29wk GA who survive to discharge leave the hospital without severe morbidity. A large component of morbidity among surviving infants is driven by the high frequency of BPD, ranging from 88% of infants at 22wk to 24% of infants at 28wk GA based on NRN data from 2008-2012 4. Data from EPIPAGE-2 in France, by contrast, shows a higher proportion of surviving infants leaving the hospital without serious morbidity (70% for infants 23-28wk GA)7. This may be due to the lower incidence of BPD, likely due to differences in definitions of BPD as well as fewer infants surviving at 22-24wk GA. Survival with 3 severe morbidities at hospital discharge (BPD, serious brain injury and severe ROP) is an important predictor of interval death or disability at 5 years of age23. A summary of common and serious comorbidities among EPT infants is reviewed below.
Respiratory distress syndrome (RDS)
RDS is a leading cause of death among EPT infants, as previously discussed, and many infants with RDS will go on to develop BPD. The understanding of the ubiquitous respiratory disease, also known as hyaline membrane disease, that currently affects almost all EPT infants changed in 1959, when Avery and Mead reported on the relationship between surface tension and RDS24. Follow-up studies identified surfactant as the missing lipid in preterm lungs, and in 1980 Fujiwara et al. first described the successful treatment of RDS with surfactant25. RDS presents soon after birth in the delivery room and affects 86% to 95% of EPT infants, depending on the GA26. The mainstay of treatment is to provide continuous positive airway pressure (CPAP) therapy and surfactant, when necessary27. The use of surfactant to treat RDS increased from 65% in 1993-1997 to 76% in 2008-2012 among EPT infants4. In addition, data supports the early use of surfactant among infants requiring mechanical ventilation, compared to delaying treatment until the severity of RDS worsens28. In the delivery room, studies suggest that initial CPAP, rather than intubation and surfactant, is a potentially more favorable approach to reduce long-term respiratory morbidity in EPT infants with RDS29 and is a recommended approach in EPT infants who are spontaneously breathing in the delivery room30.
Bronchopulmonary dysplasia
First characterized by Northway et al. in 1967 in a description of the sequela of RDS in a cohort of 32 infants31, BPD is the most common serious morbidity affecting EPT infants. Neonates with BPD are at high-risk of long-term pulmonary disease, adverse neurodevelopmental outcomes, and readmission to the hospital in the first year of life32. Older data suggest that the effects of BPD persist into adolescence and early adulthood, with problems such as airway hyperreactivity, decreased lung function and airway obstruction33. However, for many infants with BPD, symptoms improve over time.
Recent epidemiological data indicate an increasing incidence of BPD among EPT infants4, which may be a result of improved survival. Pharmacologic therapies to prevent BPD include caffeine34,35, intramuscular administration of vitamin A36 and corticosteroids37. While both caffeine and vitamin A have been shown to be safe, concerns regarding the adverse central nervous system effects of corticosteroids, particularly dexamethasone, have limited its use38. Inhaled corticosteroids may be a potential alternative to systemic administration, and the effects of early prophylactic use on preventing BPD are promising, although further studies are needed to ensure its safety39. In addition, earlier initiation of caffeine within the first 2 days after birth may have more favorable treatment effects that later initiation on the risk of BPD40-42.
Patent ductus arteriosus (PDA)
A PDA is a common finding in EPT infants, occurring in 32-60% of infants 22-28wk GA26. Observational studies have reported associations with a PDA and adverse neonatal outcomes, including intraventricular hemorrhage (IVH)43,44, BPD45 and death46,47. A recent observational study found that more frequent early screening for a PDA, which leads to more treatment, is associated with a decreased risk of hospital death (OR 0.73; 95% CI 0.54-0.98)48. However, a contrasting study found that surgical ligation of a PDA, after accounting for the propensity to receive such a treatment, is associated with worse outcomes including a higher risk of a composite of death or BPD, severe IVH, NEC, and severe ROP (adjusted OR 2.0; 95% CI 1.57-2.54)49. In the Trial of Indomethacin Prophylaxis in Preterms (TIPP), treatment with indomethacin, compared to placebo, substantially reduced the risk of a PDA (adjusted OR 0.3; 95% CI 0.2-0.4) and severe IVH (aOR 0.6; 95% CI 0.4-0.9) but had no effect on BPD (aOR 1.2; 95% CI 0.9-1.6), NEC (aOR 1.1; 95% CI 0.8-1.7), or death or neurosensory impairment (aOR 1.1; 0.8-1.4)50. These data add more uncertainty as to the benefit of treating a PDA, and highlight the need for randomized trials in which confounding factors associated with a decision to evaluate and treat a PDA can be appropriately accounted for to better understand the risks and benefits of pharmacologic and surgical treatment51.
Infection
Infection is a serious and potentially lethal complication in EPT infants. Common pathogens include coagulase-negative Staphylococcus, Staphylococcus aureus, Eschericha coli and Candida albicans, which in one study comprised 48%, 8%, 5% and 6%, respectively, of late-onset sepsis episodes among very low birth weight (VLBW) infants52. By contrast, early-onset sepsis within the first 72 hours after birth is mostly caused by Eschericha coli and Group B streptococcal infections. However, culture-proven early-onset sepsis is relatively uncommon, affecting 1.1% of VLBW infants53. Although gram-positive infections are more frequent in EPT infants, case-fatality rates are higher among fungal and gram-negative organisms, with rates as high as 44% for Candida albicans and 75% for Pseudomonal sepsis52.
For those infants that survive an episode of sepsis, infection is associated with poor growth and adverse long-term neurodevelopmental outcomes. In a study by Stoll et al. of extremely low birth weight (ELBW) infants from 1993-200154, 65% developed at least 1 infection. ELBW infants with infection had a higher risk neurodevelopmental impairment (ORs 1.3-1.8 depending on the definition of infection) and cerebral palsy (ORs 1.3-1.6). An additional study reported on the association between candida infection and worse adverse neurodevelopmental outcomes in ELBW infants55. Although fluconazole prophylaxis has been shown to substantially reduce the risk of invasive candida infection, its use has not had an effect on either mortality or neurodevelopmental impairment56, raising questions of whether invasive candida infection is a comorbid complication or causal factor in neonates with death or neurodevelopmental impairment. Fortunately, the incidence of late-onset sepsis has decreased over the last 20 years from 1993 to 20124, following similar trends in the incidence of invasive fungal infections57.
Necrotizing enterocolitis
NEC is the most common serious gastrointestinal complication in EPT infants, affecting approximately 1 in 1058. Cause-specific mortality from NEC is high, estimated at 30-40% for ELBW infants59. Infants who survive the disease, particular those who undergo surgical intervention, commonly have long-term complications, including poor growth, short bowel syndrome, and neurodevelopmental impairment60. Often, the interval between initial clinical symptoms and extensive intestinal necrosis is short, limiting the effectiveness of therapeutic interventions and underscoring the importance of prevention61. Although the etiology of NEC is not fully understood, several key factors in addition to prematurity are postulated to be important determinants: abnormal intestinal bacterial colonization, immature gut barrier, impaired intestinal blood flow, and type of enteral feeding62. One of the most important strategies to prevent NEC is breastfeeding63, and the use of an exclusive human milk diet with human milk-based fortifiers has shown promise in decreasing the risk of NEC64. However, the effect of an exclusive human milk diet on growth and long-term neurodevelopment needs further study. Additional strategies to prevent NEC include probiotic therapy, which has been widely studied and has a strong treatment effect in reducing NEC65. However, questions regarding the optimal dose and preparation of probiotic therapy, lack of a Food and Drug Administration (FDA) approved preparation, and risk of probiotic-associated sepsis have limited widespread use. Reducing the use of acid-suppression medications66 and decreasing prolonged empiric antibiotic therapy67,68 may also have benefit in decreasing the risk of NEC.
Retinopathy of prematurity (ROP)
ROP is a leading cause of blindness in EPT infants and is thought to be caused by excessive supplemental oxygen administration that leads to suppression of vascular endothelial growth factor (VEGF) and delayed retinal vascular growth69. Prevention of ROP has focused on limiting supplemental oxygen administration, which has been shown to decrease the risk of ROP. However, based on trials finding an increased risk of mortality with lower oxygen saturation targets (85-89% vs 91-95%)70,71, many centers have abandoned lower oxygen targeting. The effect of these changes in practice may be leading to increases in ROP72. Once an infant develops severe ROP, treatment has historically been limited to cryotherapy or laser photocoagulation. Because these treatments can impact residual vision, particularly peripheral vision, newer treatments such as intravitreal bevacizumab that inhibit VEGF are promising alternatives73. However, further studies are needed to assess the safety of these therapies, given their potential for antiangiogenic effects in the developing EPT infant. A second later phase of ROP is characterized by hypoxia-induced pathological vessel growth. This mechanistic insight was the rationale for a trial of supplemental oxygen to target oxygen saturations of 96-99% to prevent progression of ROP74. Although a negative overall trial, the findings suggested some potential benefit of higher oxygen targeting in infants with pre-threshold ROP without plus disease in a post-hoc subgroup analysis. However, these potential benefits were offset by an increased risk of adverse pulmonary outcomes, including longer duration of need for supplemental oxygen and pneumonia.
Neurodevelopmental outcomes, including effects of short-term neurologic injury
Neurodevelopmental impairment among EPT infants who survive the initial birth hospitalization follows a wide spectrum of outcomes, with some measures demonstrating dynamic improvements over time and others remaining severe and fixed. As both clinicians and families share in the concern for adverse long-term neurologic outcomes among EPT infants, understanding the spectrum of impairment is important to guide conversations with families and provide estimates of outcomes that are as unbiased as possible. Estimates of neurodevelopmental impairment should be considered alongside the competing outcome of death in EPT infants, particularly at early GAs where overall survival is low.
Cognitive impairment and dynamic changes over time
Cognitive impairment, commonly measured using the cognitive scale or mental developmental index (MDI) of the Bayley Scales of Infant and Toddler Development (Bayley), is typically the most common measure of neurodevelopmental impairment among EPT infants. The challenges of assigning a diagnosis of cognitive impairment includes the selection of an appropriate cut-point for a Bayley score as well as the variability of the reference population on which the Bayley is standardized75. While several studies suggest neurodevelopmental outcomes are improving in EPT infants76-78, the changes in the measurement tool from the 2nd to 3rd Bayley edition have made it difficult to determine how much of the changes in neurodevelopmental outcomes are related to changes in measurement. In a study by Vohr et al., the incidence of neurodevelopmental impairment among ELBW infants decreased from 43% in 2006-2007 to 13% in 2008-2011, but the measurement tool also changed from the 2nd edition to 3rd edition of the Bayley between the periods76.
Studies have shown that the MDI component of the Bayley-2nd edition improves over time, with a mean score increase of 20 points between measures of cognition using the Bayley-2nd edition at 18 months and the Wechsler Preschool and Primary Scale of Intelligence III IQ at 5 years35. This may lead to misclassification of infants as impaired at 18 months who fall well within the normal population distribution of IQ scores at 5 years. These findings suggest that the Bayley-2nd edition may measure developmental delay, rather than fixed impairment, and may potentially overestimate adverse neurodevelopmental outcomes. By contrast, the Bayley-3rd edition, using the scale mean of 100 as a reference, may underestimate delay at 2yr of age79. Concerns have also been raised regarding how well the Bayley-3rd edition performed at 2yr predicts 4yr cognitive outcomes80. In addition, a recent study demonstrates that parental socioeconomic status has an important effect in cognitive gains over time81. In this study, infants born to parents with higher education and whose caregivers were employed had greater cognitive gains in neurodevelopmental assessments between 18 months and 5 years of age.
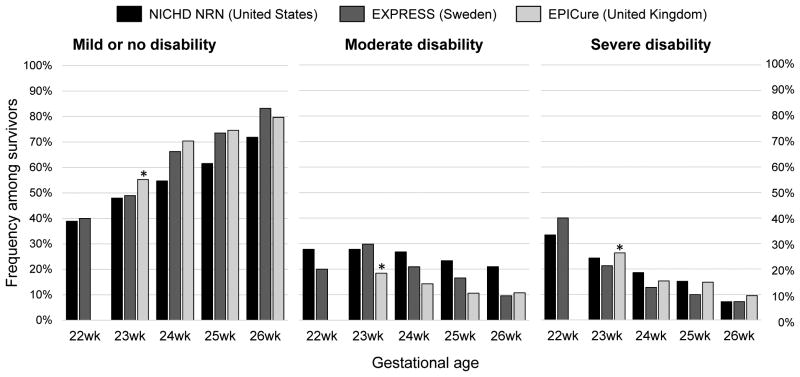
As the cognitive scales of the Bayley accounts for a large portion of commonly used composite measures of neurodevelopmental impairment defined by researchers82, it is important to evaluate the specific measures of neurodevelopmental impairment (e.g. mild or none, moderate, severe) to better understand the spectrum of impairment among survivors (Table 2). Marlow et al., on behalf of the EPICure Study Group, compared the assignment of the severity of disability at 30mo with that at 6yr of age82. At 6yr, approximately 2 out of 5 of infants who were diagnosed with severe disability at 30mo no longer had severe disability. By contrast, 1 in 4 infants without any disability at 30mo were found to have moderate or severe disability at 6yr. These findings highlight the importance of evaluation of school-age outcomes in ascertaining neurodevelopment impairment in EPT infants. Data from 3 cohort studies, two population-based cohorts from Sweden (EXPRESS Group)83 and the United Kingdom (EPICure)78 and a multicenter cohort of US academic centers (NRN)17, provides recent estimates of neurodevelopmental impairment among EPT infants, including the spectrum of gestational age-specific disability (Figure 2).
Table 2. Characteristics of Select Cohort Studies Evaluating Long-term Outcomes for Extremely Preterm Infants.
| Study (Country) | Follow-up | Details | Definition of moderate disability | Definition of severe disability |
|---|---|---|---|---|
| NICHD NRN17 (United States) | 18-22 mo corrected age | Multicenter cohort evaluating 2630 (65%) of 4329 inborn live births receiving active treatment with survival to 18-22 corrected age. | Bayley-III cognitive or motor score 1-2 SD below mean, moderate CP, or a GMFCS level of 2 or 3. | Bayley-III cognitive or motor score >2 SD below mean, severe CP, GMFCS level of 4 or 5, bilateral blindness, or severe hearing impairment not corrected with bilateral amplification. |
| EXPRESS83 (Sweden) | 2.5 yr | Population-based cohort evaluating 491 (69%) of 707 live births surviving to 30mo corrected age. | Bayley-III score 2-3 SD below mean (any scales), moderate CP, moderate visual or hearing impairment. | Bayley-lll composite cognitive, language or motor score <3 SD below mean, severe CP, or bilateral blindness or deafness. |
| EPICure78 (United Kingdom) | 3 yr (range of 27-48 mo) | Population based cohort evaluating 584 (57%) of 1031 live births surviving to 3 yr. Age at assessment was variable, with use of multiple scales. | Ambulant CP (GMFCS 2), functionally impaired vision, hearing loss improved by aids, or a developmental score within 2-3 SD below mean. | Non-ambulant CP (GMFCS 3-5), blindness, profound sensorineural hearing loss not improved by aids, or a developmental quotient <3 SD below mean. |
Abbreviations: Bayley-III, Bayley Scales of Infant and Toddler Development-3rd edition; SD, standard deviation; CP, cerebral palsy; GMFCS, Gross Motor Function Classification System.
Figure 2. The Spectrum of Disability Among Surviving Extremely Preterm Infants.
Characteristics of the data sources are shown in Table 2. *Estimates reported for infants ≤ 23wk gestational age.
Cerebral palsy and motor impairment
Cerebral palsy (CP), a permanent neurologic disorder that impairs movement and muscle coordination, is estimated to occur in approximately 8-9% of infants 22-32 wk GA78,84 and 14% of infants 22-25 wk GA78. The most common type of CP in EPT infants is bilateral spastic cerebral palsy, accounting for over two-thirds of cases. The assessment of motor impairment in EPT infants is performed by physical examination, as well as formal assessments including the Bayley-3rd edition and Gross Motor Function Classification System (GMFCS). Similar to the problems with the Bayley cognitive scales, use of a higher cut-point of a motor composite score of < 85 for a Bayley-3rd edition may overestimate impairment85. However, many ELBW infants have motor coordination difficulties that persist into adulthood86.
Intraventricular hemorrhage and periventricular leukomalacia (PVL)
Intraventricular and periventricular hemorrhage is a common finding in EPT infants, with worse outcomes associated with higher grades of IVH. A study from 2006-2008 by Payne et al. reported IVH in 31% of infants <27wk GA who underwent ultrasound screening87. The study reported severe IVH (Grade 3 or 4) was associated with an increased risk of all adverse neurodevelopmental outcomes, including CP (OR 3.4; 95% CI 2.2-4.3), GMFCS > 2 (OR 2.5; 95% CI 1.4-4.4) and a Bayley 3rd edition MDI <85 (OR 1.8; 95% CI 1.3-2.6), when compared to infants without IVH. Infants with low grade IVH had a similar risk of adverse outcomes as those without IVH. By contrast, a study of infants 23-28 wk GA from 1998-2004, found that even low grade IVH, compared to no IVH, was associated with a higher risk of neurosensory impairment (OR 1.7; 95% CI 1.2-2.5), with an overall IVH incidence of 22%88. Other studies have found that late imaging (cranial ultrasound or magnetic resonance imaging (MRI)) is a better predictor of long-term outcomes at 18-22 months when compared to early cranial ultrasound89. Additional studies have demonstrated a potential benefit to late MRI imaging among high-risk EPT infants90, as MRI is better at identifying diffuse white matter and cerebellar injury than ultrasound. However, the presence of PVL on ultrasound strongly correlates with a high risk of CP91.
Among those infants with severe IVH, the development of post-hemorrhagic hydrocephalus requiring shunt placement confers a worse outcome92. Neurodevelopmental impairment was seen in 92% of ELBW infants with a Grade 4 IVH requiring shunt placement, compared to 55% of ELBW infants with a Grade 3 IVH without need for a shunt and 35% of infants without IVH. Among infants with severe IVH, the rates of hearing impairment ranged from 2-6% and vision impairment from 17-33%, both higher than the respective rates of 1% and 9% for infants without IVH. Despite the challenges that disabilities may pose, many former EPT infants report quality of life that is equivalent to term counterparts and that is less impacted by EPT birth over time93,94.
Counseling at periviable gestation
A recent consensus statement from the American College of Obstetricians and Gynecologists (ACOG) and Society for Maternal-Fetal Medicine (SMFM) recommends clinicians provide accurate, balanced, and unbiased guidance when counseling families regarding care for fetuses and infants at perviable gestational ages18. Information presented in several ways, including providing estimates of both survival and mortality to prevent framing bias95, using visual aids96 and providing estimates for only infants receiving active treatment17 are some strategies to accomplish this goal. The consensus statement also suggests institutions develop consensus guidelines, because individual providers may have variable approaches based on personal beliefs or professional experiences.
Conclusion
Survival continues to incrementally improve for EPT infants. Understanding short- and long-term outcomes may help in caring for EPT infants and informing discussions with families. Ultimately, reducing preterm birth is necessary to substantially reduce the burden of mortality and morbidity for EPT infants.
Acknowledgments
This review was supported, in part, by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers KL2TR000455 and UL1TR000454. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. There are no relevant conflicts of interest. The author would like to acknowledge Ira Adams-Chapman M.D., M.P.H. for her thoughtful review of the manuscript.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJK. Reports NVS, ed. Vol. 64. Hyattsville, MD: National Center for Health Statistics; 2015. Births: Final Data for 2013. [PubMed] [Google Scholar]
- 3.Rothwell CJ, Mathews TJ, MacDorman MF, Thoma ME. Reports NVS, ed. Vol. 64. Hyattsville, MD: National Center for Health Statistics; 2015. Infant mortality statistics from the 2013 period linked birth/infant death data set. [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314(10):1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel RM, Kandefer S, Walsh MC, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–340. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6):1019–1026. doi: 10.1542/peds.2011-3028. [DOI] [PubMed] [Google Scholar]
- 7.Ancel PY, Goffinet F, Group E-W, et al. Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015;169(3):230–238. doi: 10.1001/jamapediatrics.2014.3351. [DOI] [PubMed] [Google Scholar]
- 8.Shah PS, Sankaran K, Aziz K, et al. Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J Perinatol. 2012;32(2):132–138. doi: 10.1038/jp.2011.68. [DOI] [PubMed] [Google Scholar]
- 9.Kusuda S, Fujimura M, Uchiyama A, Totsu S, Matsunami K, Neonatal Research Network J. Trends in morbidity and mortality among very-low-birth-weight infants from 2003 to 2008 in Japan. Pediatr Res. 2012;72(5):531–538. doi: 10.1038/pr.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M, Neonatal Research Network J Outcomes of infants born at 22 and 23 weeks' gestation. Pediatrics. 2013;132(1):62–71. doi: 10.1542/peds.2012-2857. [DOI] [PubMed] [Google Scholar]
- 11.Su BH, Hsieh WS, Hsu CH, et al. Neonatal outcomes of extremely preterm infants from taiwan: comparison with Canada, Japan, and the USA. Pediatr Neonatol. 2015;56(1):46–52. doi: 10.1016/j.pedneo.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies) BMJ. 2012;345:e7976. doi: 10.1136/bmj.e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group E, Fellman V, Hellstrom-Westas L, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301(21):2225–2233. doi: 10.1001/jama.2009.771. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal P, Sriram B, Rajadurai VS. Neonatal outcome of extremely preterm Asian infants 28 weeks over a decade in the new millennium. J Perinatol. 2015;35(4):297–303. doi: 10.1038/jp.2014.205. [DOI] [PubMed] [Google Scholar]
- 15.Guillen U, DeMauro S, Ma L, et al. Survival rates in extremely low birthweight infants depend on the denominator: avoiding potential for bias by specifying denominators. Am J Obstet Gynecol. 2011;205(4):329, e321–327. doi: 10.1016/j.ajog.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Shah PS, Lee SK, Lui K, et al. The International Network for Evaluating Outcomes of very low birth weight, very preterm neonates (iNeo): a protocol for collaborative comparisons of international health services for quality improvement in neonatal care. BMC Pediatr. 2014;14:110. doi: 10.1186/1471-2431-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rysavy MA, Li L, Bell EF, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801–1811. doi: 10.1056/NEJMoa1410689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of O, Gynecologists, the Society for Maternal-Fetal M. #3: Periviable birth. Am J Obstet Gynecol. 2015;213(5):604–614. doi: 10.1016/j.ajog.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Ward Platt M. The MBRRACE-UK perinatal surveillance report. Arch Dis Child Fetal Neonatal Ed. 2015 doi: 10.1136/archdischild-2015-309271. [DOI] [PubMed] [Google Scholar]
- 20.Ahle M, Drott P, Andersson RE. Epidemiology and trends of necrotizing enterocolitis in Sweden: 1987-2009. Pediatrics. 2013;132(2):e443–451. doi: 10.1542/peds.2012-3847. [DOI] [PubMed] [Google Scholar]
- 21.Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND. Deaths in preterm infants: changing pathology over 2 decades. J Pediatr. 2012;160(1):49–53. e41. doi: 10.1016/j.jpeds.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 22.Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics. 2015;135(1):e59–65. doi: 10.1542/peds.2014-2967. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt B, Roberts RS, Davis PG, et al. Prediction of Late Death or Disability at Age 5 Years Using a Count of 3 Neonatal Morbidities in Very Low Birth Weight Infants. J Pediatr. 2015 doi: 10.1016/j.jpeds.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 24.Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959;97(5, Part 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;1(8159):55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 26.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polin RA, Carlo WA, Committee on F, Newborn, American Academy of P Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133(1):156–163. doi: 10.1542/peds.2013-3443. [DOI] [PubMed] [Google Scholar]
- 28.Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2012;11:CD001456. doi: 10.1002/14651858.CD001456.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens TP, Finer NN, Carlo WA, et al. Respiratory outcomes of the surfactant positive pressure and oximetry randomized trial (SUPPORT) J Pediatr. 2014;165(2):240–249. doi: 10.1016/j.jpeds.2014.02.054. e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyllie J, Perlman JM, Kattwinkel J, et al. Part 7: Neonatal resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2015;95:e169–201. doi: 10.1016/j.resuscitation.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 31.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 32.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 33.Northway WH, Jr, Moss RB, Carlisle KB, et al. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;323(26):1793–1799. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt B, Anderson PJ, Doyle LW, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307(3):275–282. doi: 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- 36.Tyson JE, Wright LL, Oh W, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999;340(25):1962–1968. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 37.Doyle LW, Ehrenkranz RA, Halliday HL. Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2014;5:CD001145. doi: 10.1002/14651858.CD001145.pub3. [DOI] [PubMed] [Google Scholar]
- 38.Committee on F, Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109(2):330–338. doi: 10.1542/peds.109.2.330. [DOI] [PubMed] [Google Scholar]
- 39.Bassler D, Plavka R, Shinwell ES, et al. Early Inhaled Budesonide for the Prevention of Bronchopulmonary Dysplasia. N Engl J Med. 2015;373(16):1497–1506. doi: 10.1056/NEJMoa1501917. [DOI] [PubMed] [Google Scholar]
- 40.Patel RM, Leong T, Carlton DP, Vyas-Read S. Early caffeine therapy and clinical outcomes in extremely preterm infants. J Perinatol. 2013;33(2):134–140. doi: 10.1038/jp.2012.52. [DOI] [PubMed] [Google Scholar]
- 41.Dobson NR, Patel RM, Smith PB, et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J Pediatr. 2014;164(5):992–998. e993. doi: 10.1016/j.jpeds.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lodha A, Seshia M, McMillan DD, et al. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr. 2015;169(1):33–38. doi: 10.1001/jamapediatrics.2014.2223. [DOI] [PubMed] [Google Scholar]
- 43.Dykes FD, Lazzara A, Ahmann P, Blumenstein B, Schwartz J, Brann AW. Intraventricular hemorrhage: a prospective evaluation of etiopathogenesis. Pediatrics. 1980;66(1):42–49. [PubMed] [Google Scholar]
- 44.Evans N, Kluckow M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75(3):F183–186. doi: 10.1136/fn.75.3.f183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schena F, Francescato G, Cappelleri A, et al. Association between Hemodynamically Significant Patent Ductus Arteriosus and Bronchopulmonary Dysplasia. J Pediatr. 2015;166(6):1488–1492. doi: 10.1016/j.jpeds.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Dudell GG, Gersony WM. Patent ductus arteriosus in neonates with severe respiratory disease. J Pediatr. 1984;104(6):915–920. doi: 10.1016/s0022-3476(84)80499-0. [DOI] [PubMed] [Google Scholar]
- 47.Noori S, McCoy M, Friedlich P, et al. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123(1):e138–144. doi: 10.1542/peds.2008-2418. [DOI] [PubMed] [Google Scholar]
- 48.Roze JC, Cambonie G, Marchand-Martin L, et al. Association Between Early Screening for Patent Ductus Arteriosus and In-Hospital Mortality Among Extremely Preterm Infants. JAMA. 2015;313(24):2441–2448. doi: 10.1001/jama.2015.6734. [DOI] [PubMed] [Google Scholar]
- 49.Mirea L, Sankaran K, Seshia M, et al. Treatment of patent ductus arteriosus and neonatal mortality/morbidities: adjustment for treatment selection bias. J Pediatr. 2012;161(4):689–694. e681. doi: 10.1016/j.jpeds.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt B, Davis P, Moddemann D, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344(26):1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 51.Bose CL, Laughon MM. Patent ductus arteriosus: lack of evidence for common treatments. Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F498–502. doi: 10.1136/adc.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 53.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 55.Adams-Chapman I, Bann CM, Das A, et al. Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. J Pediatr. 2013;163(4):961–967. e963. doi: 10.1016/j.jpeds.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benjamin DK, Jr, Hudak ML, Duara S, et al. Effect of fluconazole prophylaxis on candidiasis and mortality in premature infants: a randomized clinical trial. JAMA. 2014;311(17):1742–1749. doi: 10.1001/jama.2014.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aliaga S, Clark RH, Laughon M, et al. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics. 2014;133(2):236–242. doi: 10.1542/peds.2013-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitzgibbons SC, Ching Y, Yu D, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44(6):1072–1075. doi: 10.1016/j.jpedsurg.2009.02.013. discussion 1075-1076. [DOI] [PubMed] [Google Scholar]
- 60.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 61.Grave GD, Nelson SA, Walker WA, et al. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62(4):510–514. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 62.Patel RM, Denning PW. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res. 2015;78(3):232–238. doi: 10.1038/pr.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 64.Cristofalo EA, Schanler RJ, Blanco CL, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163(6):1592–1595. e1591. doi: 10.1016/j.jpeds.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 65.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4):CD005496. doi: 10.1002/14651858.CD005496.pub4. [DOI] [PubMed] [Google Scholar]
- 66.Terrin G, Passariello A, De Curtis M, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics. 2012;129(1):e40–45. doi: 10.1542/peds.2011-0796. [DOI] [PubMed] [Google Scholar]
- 67.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–725. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10(2):133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 70.Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. BOOST II United Kingdom Collaborative Group; BOOST II Australia Collaborative Group; BOOST II New Zealand Collaborative Group. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368(22):2094–2104. doi: 10.1056/NEJMoa1302298. [DOI] [PubMed] [Google Scholar]
- 71.Carlo WA, Finer NN, Walsh MC, et al. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362(21):1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manley BJ, Kuschel CA, Elder JE, Doyle LW, Davis PG. Higher Rates of Retinopathy of Prematurity after Increasing Oxygen Saturation Targets for Very Preterm Infants: Experience in a Single Center. J Pediatr. 2015 doi: 10.1016/j.jpeds.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Mintz-Hittner HA, Kennedy KA, Chuang AZ, Group B-RC. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105(2):295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 75.L, Orton J, McGinley JL, Fox LM, Spittle AJ. Challenges of neurodevelopmental follow-up for extremely preterm infants at two years. Early Hum Dev. 2015;91(12):689–694. doi: 10.1016/j.earlhumdev.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 76.Vohr BR, Stephens BE, Higgins RD, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012;161(2):222–228. e223. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schlapbach LJ, Adams M, Proietti E, et al. Outcome at two years of age in a Swiss national cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatr. 2012;12:198. doi: 10.1186/1471-2431-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW, Victorian Infant Collaborative G Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164(4):352–356. doi: 10.1001/archpediatrics.2010.20. [DOI] [PubMed] [Google Scholar]
- 80.Spencer-Smith MM, Spittle AJ, Lee KJ, Doyle LW, Anderson PJ. Bayley-III Cognitive and Language Scales in Preterm Children. Pediatrics. 2015;135(5):e1258–1265. doi: 10.1542/peds.2014-3039. [DOI] [PubMed] [Google Scholar]
- 81.Manley BJ, Roberts RS, Doyle LW, et al. Social variables predict gains in cognitive scores across the preschool years in children with birth weights 500 to 1250 grams. J Pediatr. 2015;166(4):870–876. e871–872. doi: 10.1016/j.jpeds.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 82.Marlow N, Wolke D, Bracewell MA, Samara M, Group EPS. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 83.Serenius F, Kallen K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309(17):1810–1820. doi: 10.1001/jama.2013.3786. [DOI] [PubMed] [Google Scholar]
- 84.Beaino G, Khoshnood B, Kaminski M, et al. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Dev Med Child Neurol. 2010;52(6):e119–125. doi: 10.1111/j.1469-8749.2010.03612.x. [DOI] [PubMed] [Google Scholar]
- 85.Duncan AF, Bann C, Boatman C, et al. Do currently recommended Bayley-III cutoffs overestimate motor impairment in infants born <27 weeks gestation? J Perinatol. 2015;35(7):516–521. doi: 10.1038/jp.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poole KL, Schmidt LA, Missiuna C, Saigal S, Boyle MH, Van Lieshout RJ. Motor Coordination Difficulties in Extremely Low Birth Weight Survivors Across Four Decades. J Dev Behav Pediatr. 2015;36(7):521–528. doi: 10.1097/DBP.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 87.Payne AH, Hintz SR, Hibbs AM, et al. Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr. 2013;167(5):451–459. doi: 10.1001/jamapediatrics.2013.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bolisetty S, Dhawan A, Abdel-Latif M, et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014;133(1):55–62. doi: 10.1542/peds.2013-0372. [DOI] [PubMed] [Google Scholar]
- 89.Hintz SR, Barnes PD, Bulas D, et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135(1):e32–42. doi: 10.1542/peds.2014-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 91.Graham M, Levene MI, Trounce JQ, Rutter N. Prediction of cerebral palsy in very low birthweight infants: prospective ultrasound study. Lancet. 1987;2(8559):593–596. doi: 10.1016/s0140-6736(87)92986-2. [DOI] [PubMed] [Google Scholar]
- 92.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R, Network NR. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121(5):e1167–1177. doi: 10.1542/peds.2007-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saigal S. Quality of life of former premature infants during adolescence and beyond. Early Hum Dev. 2013;89(4):209–213. doi: 10.1016/j.earlhumdev.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 94.Zwicker JG, Harris SR. Quality of life of formerly preterm and very low birth weight infants from preschool age to adulthood: a systematic review. Pediatrics. 2008;121(2):e366–376. doi: 10.1542/peds.2007-0169. [DOI] [PubMed] [Google Scholar]
- 95.Kim UO, Basir MA. Informing and educating parents about the risks and outcomes of prematurity. Clin Perinatol. 2014;41(4):979–991. doi: 10.1016/j.clp.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 96.Guillen U, Suh S, Munson D, et al. Development and pretesting of a decision-aid to use when counseling parents facing imminent extreme premature delivery. J Pediatr. 2012;160(3):382–387. doi: 10.1016/j.jpeds.2011.08.070. [DOI] [PubMed] [Google Scholar]