Abstract
Methylation of the SKA2 gene has recently been identified as a promising biomarker of suicide risk. Based on this finding, we examined associations between SKA2 methylation, cortical thickness, and psychiatric phenotypes linked to suicide in trauma-exposed veterans. 200 trauma-exposed white non-Hispanic veterans of the recent conflicts in Iraq and Afghanistan (91% male) underwent clinical assessment and had blood drawn for genotyping and methylation analysis. 145 participants also had neuroimaging data available. Based on previous research, we examined DNA methylation at the CpG locus cg13989295 as well as DNA methylation adjusted for genotype at the methylation-associated SNP (rs7208505) in relationship to whole-brain cortical thickness, posttraumatic stress disorder symptoms (PTSD), and depression symptoms. Whole-brain vertex-wise analyses identified three clusters in prefrontal cortex that were associated with genotype-adjusted SKA2 DNA methylation (methylationadj). Specifically, DNA methylationadj was associated with bilateral reductions of cortical thickness in frontal pole and superior frontal gyrus, and similar effects were found in the right orbitofrontal cortex and right inferior frontal gyrus. PTSD symptom severity was positively correlated with SKA2 DNA methylationadj and negatively correlated with cortical thickness in these regions. Mediation analyses showed a significant indirect effect of PTSD on cortical thickness via SKA2 methylation status. Results suggest that DNA methylationadj of SKA2 in blood indexes stress-related psychiatric phenotypes and neurobiology, pointing to its potential value as a biomarker of stress exposure and susceptibility.
Keywords: SKA2, cortical thickness, methylation, trauma, posttraumatic stress
The suicide rate among U.S. service members is alarmingly high, with a recent study of 1.3 million veterans reporting that the suicide risk is 41–61% higher for veterans than civilians (1). Studies suggest that warzone trauma, PTSD, and other post-deployment mental health problems put veterans at heightened risk for suicide relative to the general population (2). Although the biological mechanisms involved in this association are not yet well understood, converging lines of evidence implicate stress-related molecular alterations of the hypothalamic-pituitary-adrenal (HPA) axis as well as changes in brain morphology and neural function in the etiology of suicide (3). Furthermore, early adversity and exposure to traumatic stress have been linked to changes in the methylation status and transcriptional activity of genes that regulate HPA-axis function (4, 5). These changes are associated with lower glucocorticoid receptor expression, decreased glucocorticoid negative feedback responses to stress, and hypercortisolism (6, 7). Brain regions densely populated with glucocorticoid receptors, such as the prefrontal cortex and hippocampus, are especially vulnerable to neuronal damage and cell death as a result of these effects (8–11).
One recently identified gene that may play a critical role in this process is SKA2 (spindle and kinetochore associated complex subunit 2). The SKA2 protein is implicated in chaperoning the glucocorticoid receptor from the cytoplasm into the nucleus and, by doing so, it serves as a moderator of negative feedback inhibition of the HPA axis and a neuroprotective function by enhancing activation of glucocorticoid receptors (12). The clinical relevance of the SKA2 locus was recently demonstrated by an epigenome-wide analysis of post-mortem brain tissue conducted to identify genes associated with suicide. Guintivano et al. (13) showed that methylation of probe cg13989295 in the 3′ untranslated region (UTR) of SKA2 in prefrontal neurons was associated with suicide, with suicide decedents showing greater DNA methylation and less SKA2 expression in surrounding tissue than controls. They found that SKA2 methylation in blood predicted suicidal ideation and reduced suppression of waking cortisol in clinical samples after controlling for genotype (which is correlated with cg13989295 methylation), concluding that methylation is a more proximal predictor than genotype. These findings provide preliminary evidence that DNA methylation of SKA2 is an indicator of glucocorticoid signaling dysregulation and a potentially useful biomarker of suicide vulnerability. A recent independent study found decreased expression of SKA2 in blood from violent suicide completers compared to non-suicidal controls (14), replicating the findings of Guintivano et al. An important next step is to determine whether epigenetic variability at SKA2 is also associated with identifiable alterations in neural integrity in psychiatrically relevant regions of the brain and/or stress-related psychiatric disorders linked to suicide.
Thus, building on previous research on SKA2, the primary aims of this study were to examine whether SKA2 methylation is associated with alterations in cortical thickness and suicide-related psychiatric symptoms, specifically PTSD and depression. We focused on cortical rather than subcortical structures (e.g., hippocampus), based on evidence of reduced expression of SKA2 in prefrontal tissue of suicide patients. Based on evidence that the SKA2 protein may be involved in mitigating the neurotoxic effects of stress, we hypothesized that greater DNA methylation of the CpG site identified by Guintivano et al. would be associated with decreased cortical thickness in prefrontal brain regions. We also expected DNA methylation to be associated with greater PTSD and depression symptoms given their associations with suicide risk.
Methods
Sample
The sample consisted of 200 white non-Hispanic service members of Operations Enduring Freedom, Iraqi Freedom, and New Dawn (OEF/OIF/OND) (see Table 1 for sample characteristics).1 Participants were consecutively enrolled in the Translational Research Center for TBI and Stress Disorders, a VA RR&D Traumatic Brain Injury Center of Excellence at VA Boston Healthcare System. DNA methylation data were available for all 200 participants, and neuroimaging data were available for 152 of those participants. The final sample for neuroimaging analyses was 145 after excluding individuals with a history of moderate or severe TBI. Approval for the study was obtained from all relevant Institutional Review Boards and regulatory committees. After complete description of the study to the subjects, written informed consent was obtained.
Table 1.
Characteristics of OEF/OIF/OND Trauma-Exposed Veteran Study Sample
| Mean (SD) | N (%) | |
|---|---|---|
| Age (Years) | 31.8 (8.4) | |
| Sex (Male) | - | 182 (91%) |
| Military Deployment Duration (Months) | 12.9 (8.7) | - |
| Psychiatric Medication Usea | ||
| No | 108 (54%) | |
| Yes | 88 (44%) | |
| Current PTSDb | - | |
| No | 83 (41%) | |
| Yes | 116 (58%) | |
| Current MDDb | - | |
| No | 150 (75%) | |
| Yes | 49 (25%) | |
| Current SUDb | - | |
| No | 171 (86%) | |
| Yes | 28 (14%) | |
Note. PTSD = posttraumatic stress disorder. MDD = major depressive disorder. SUD = substance use disorder.
four missing cases.
one missing case.
Procedures
DNA Genotyping and Methylation
DNA was extracted from peripheral blood samples. Whole-genome genotyping data was obtained by hybridizing DNA samples to Illumina HumanOmni2.5–8 microarrays and scanning with an Illumina iScan System (Illumina, San Diego, CA). SNP imputation was performed using Impute2 (15) and 1000 genomes reference data (The 1000 Genomes Project Consortium). DNA methylation data was obtained by hybridizing bisulfite-modified DNA to Illumina HumanMethylation450K microarrays and scanning with an Illumina iScan System (Illumina, San Diego, CA). Details on genotyping and methylation methods are available in the Supplementary Materials.
Methylation analyses focused on the cytosine-guanine (CpG) dinucleotide implicated by Guintivano et al. (13; Illumina probe cg13989295) and the intervening SNP rs7208505. Because the cytosine (C) at this position of the CpG site measured by cg13989295 is one allele of the (C-T) SNP rs7208505, cg13989295 methylation is highly correlated with rs7208505 genotype. Given that the cytosine measured by cg13989295 is one allele of the SNP rs7208505, the number of alleles that can be methylated depends upon an individual’s genotype (e.g., for the C/T genotype, methylation is possible at one allele). Thus, before evaluating whether methylation associates with the phenotypes of interest, it was prudent to take genotype into account (i.e., adjust for genotype), because the range of possible methylation levels (0–100%, typically) was correlated with genotype and thus restricted in some participants. See supplementary materials for associations between SNP and methylation (Figure S1). The estimated proportion of methylation (Beta-value) of cg13989295 was logit transformed prior to analysis.
Morphometric Acquisition and Processing
Structural imaging data were acquired on a 3T Siemens TIM TRIO whole-body MRI scanner. Two T1-weighted anatomical scans (voxel size = 1mm3, TR = 2530ms, TE = 3.32ms, FOV = 256x256, # of slices = 176) were acquired and averaged to create a single high contrast-to-noise image. The standard Freesurfer v5.1 morphometric pipeline was computed, including reconstruction of the cortical mantle and spatial smoothing of 20mm FWHM. Cortical surface models were manually checked slice-by-slice and edited for accuracy.
Psychiatric Symptoms
Posttraumatic Stress Disorder
Current PTSD symptom severity and PTSD diagnosis were assessed by doctoral level psychologists using the Clinician Administered PTSD Scale (16). The frequency and intensity of each DSM-IV PTSD criterion were assessed for the month preceding the assessment. Severity scores were calculated by summing the frequency and intensity scores for all 17 symptoms. All participants endorsed a DSM-IV PTSD Criterion A event. One participant was missing PTSD data.
Depression
Current depression symptom severity was assessed via the 14-item total depression subscale of the self-report Depression Anxiety Stress Scale (DASS; 17). Current Major Depressive Disorder (MDD) diagnosis was assessed by doctoral level psychologists using the Structured Clinical Interview for DSM-IV Axis I Disorders (18). Twelve participants were missing the DASS, and one participant was missing MDD diagnosis information.
Data Analysis
The data analytic approach was based on Guintivano et al. (13). For each phenotype, we examined associations with rs7208505 genotype, cg13989295 methylation, and methylation adjusted for genotype. Methylation adjusted for genotype (methylationadj) was calculated by taking the residuals of a linear model of SKA2 3′ UTR DNA methylation as a function of rs7208505 genotype. All statistical models were adjusted for age, sex, and population substructure (as indexed by the first three ancestry principal components). Associations between methylation level (unadjusted) and each psychiatric/brain phenotype are presented separately by genotype in the supplemental materials for reference (Table S1 and Figure S2). Cell counts were estimated from the methylation data, and analyses using these cell counts were performed to rule out cell composition as a potential confound. Unless otherwise specified, reported methylationadj results remained significant when cell composition estimates were included in the model. All tests were two-tailed.
We computed vertex-wise analyses across the entire cortex to test the hypothesis that SKA2 methylation would be associated with reduced cortical integrity in prefrontal brain regions. General linear model analyses were run using FreeSurfer’s Qdec. Vertex-wise significance threshold was set at p < .01. Monte Carlo simulations (10,000 iterations) were used to correct for multiple comparisons, resulting in a whole brain corrected threshold of p < .05. Only regions surviving correction for multiple comparisons are reported.
SKA2 methylation associations with suicide-related psychiatric phenotypes were assessed using hierarchical linear regression models in SPSS v22 (SPSS, Chicago, IL). Mediation analyses were conducted in Mplus 7.11 (19) in the subsample with complete data (n = 144) using the maximum likelihood estimator with bootstrapped standard errors and confidence intervals. All brain region phenotypes were examined simultaneously in the model such that each was regressed on methylation, psychiatric phenotype, and rs7208505 genotype. Methylation was also regressed on genotype and psychiatric phenotype. The indirect effect of psychiatric phenotype on the brain phenotypes via methylation was assessed using the ‘model indirect’ procedure.
Results
SKA2 Associations with Cortical Thickness
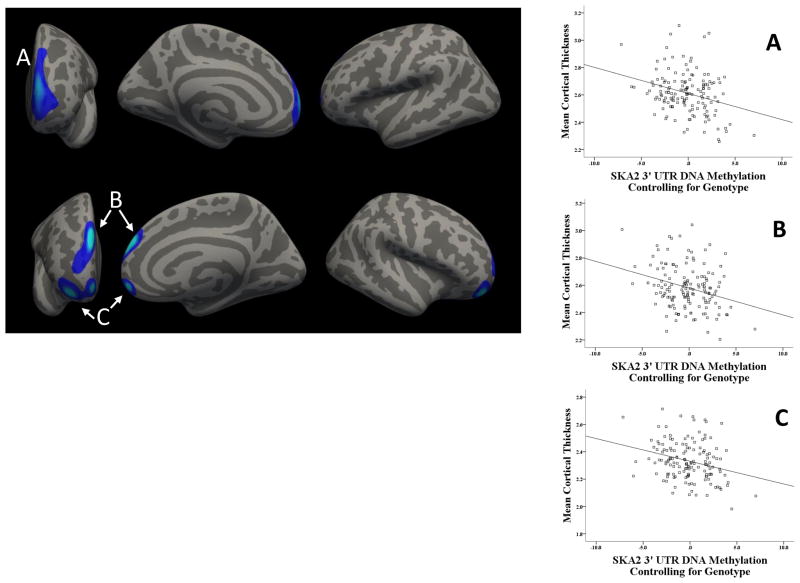
The genotype at rs7208505 did not relate to cortical thickness in the whole brain analyses nor did methylation. As hypothesized, however, SKA2 methylationadj was associated with reduced cortical thickness in prefrontal cortex. Three clusters survived correction for multiple comparisons (Table 2, Figure 1). A left hemisphere cluster spanned frontal pole and the anterior aspects of superior frontal gyrus (SFG) and rostral middle frontal gyrus (MFG) (BA 9/10). Two clusters also emerged in right PFC: one spanning right frontal pole and anterior SFG (BA 9/10) and one in (lateral and medial) orbitofrontal cortex (OFC) and the anterior aspects of inferior frontal gyrus pars orbitalis and rostral MFG (BA 10/11). Greater SKA2 methylationadj was associated with decreased cortical thickness in all clusters (rs < −.28, ps <.001). These results were consistent with those of a similar analysis in rs7208505 genotype groups (Figure S2 and Table S1).
Table 2.
Reductions in Cortical Thickness in Frontal Cortex Associated with SKA2 3′ UTR DNA Methylation Adjusting for Genotype
| Peak F-Value | Peak (x,y,z) | No. of Vertices | Cluster Size (mm2) | |
|---|---|---|---|---|
| L Frontal Pole/SFG/Rostral MFG | −2.87 | −8, 61, 12 | 121563 | 1832 |
| R Frontal Pole/SFG | −3.49 | 7, 55, 25 | 64286 | 1347 |
| R OFC/IFG Pars Orbitalis/Rostral MFG | −3.17 | 27, 49, −12 | 64226 | 1411 |
Note: N = 145. SFG = superior frontal gyrus. MFG = middle frontal gyrus. OFC = orbitofrontal cortex. IFG = inferior frontal gyrus. All clusters survived Monte Carlo Simulation correction for multiple comparisons. R = right hemisphere. L = left hemisphere.
Figure 1. SKA2 Methylation Adjusting for Genotype Relates to Reduced Cortical Thickness in Frontal Cortex.
(A) Frontal pole, superior frontal gyrus, and rostral middle frontal gyrus. (B) Frontal pole and superior frontal gyrus. (C) Orbitofrontal cortex, inferior frontal gyrus, and rostral middle frontal gyrus.
To examine the clinical significance of the alterations in brain morphology associated with SKA2 epigenetic variation, we correlated mean cortical thickness for each cluster with measures of PTSD and depression. PTSD symptom severity was negatively correlated with cortical thickness in both right prefrontal clusters (rs < −.20, ps <.018) and showed a trend for the left prefrontal cluster (r = −.16, p = .06). In contrast, current depression symptom severity was unrelated to cortical thickness in any of the clusters. Analyses performed using dichotomous indices of PTSD and MDD diagnosis produced a similar pattern of results.2
To rule out potential confounds, we tested whether findings were due to the influence of comorbid substance use disorders, psychiatric medication use, or mild TBI (individuals with moderate/severe TBI were already excluded from analyses). We extracted each brain cluster and ran a hierarchical linear regression analysis with these variables added to the statistical models. The associations between DNA methylationadj and cortical thickness for each of the clusters remained significant when these potential confounds were included in the models (ps < .002).
SKA2 Associations with Psychiatric Symptoms
We next examined whether genetic and epigenetic variation at SKA2 associated with psychiatric symptoms that increase risk of suicide – specifically PTSD and depression. Linear regression models revealed that rs7208505 genotype was associated with current PTSD symptom severity (β = −.16, p = .028), such that individuals homozygous for the C minor allele had lower PTSD symptom severity than heterozygotes (MDifference = −16.5, p = .013) and T/T carriers (MDifference = −15.8, p = .024). Methylation was not associated with PTSD, but methylationadj was associated with PTSD symptom severity over and above the effect of genotype (β = .37, p = .021), with methylationadj correlating positively with current PTSD symptoms (see Table 3). Genetic and epigenetic variations at SKA2 were not associated with depression symptoms. Analyses performed using PTSD and MDD diagnostic status yielded a similar pattern of results.3
Table 3.
Hierarchical Linear Regression Analysis of Genetic and Epigenetic Variation at SKA2 and Current PTSD Symptom Severity
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
|
| |||
| β (SE) | β (SE) | β (SE) | |
| Step 1 | |||
| Age | −.10 (0.3) | −.10 (0.3) | −.10 (0.3) |
| Sex | .04 (7.3) | .04 (7.3) | .04 (7.3) |
| Ancestry PC1 | .02 (29.9) | .02 (29.9) | .02 (29.9) |
| Ancestry PC2 | .08 (30.1) | .08 (30.1) | .08 (30.1) |
| Ancestry PC3 | −.16 (30.1)* | −.16 (30.1)* | −.16 (30.1)* |
| Step 2 | |||
| rs7208505 Genotype | −.16 (2.9)* | -------- | −.16 (2.9)* |
| cg13989295 Methylation | -------- | −.07 (0.4) | -------- |
| Step 3 | |||
| cg13989295 Methylation (methylationadj) | -------- | -------- | .37 (0.9)* |
Note. N = 199. PC = principal component.
Model 1. Step1: R2= .043, Step2: Δ R2= .024*.
Model 2. Step1: R2= .043, Step2: Δ R2= .01.
Model 3. Step1: R2= .043, Step2: Δ R2= .024*, Step3: Δ R2= .026*.
p <.05.
SKA2 Epigenetic Variation as a Mediator of Brain-Behavior Relationships
Based on the findings above, we tested whether SKA2 methylationadj mediated the observed association between PTSD symptom severity and reduced cortical thickness in the prefrontal clusters. All phenotypes were examined simultaneously in one model. The indirect effect of PTSD on the right OFC/rostral MFG cluster through DNA methylationadj was significant (standardized β = −.067, p = .039, 95% CI = −.131 to −.003), as was the corresponding effect on the left prefrontal cluster (standardized β = −.063, p = .043, 95% CI = −.125 to −.002). The effect was not statistically significant for the right frontal pole/SFG cluster, although the direction of association was consistent (standardized β = −.061, p = .056, 95% CI = −.124 to .002). In total, the model explained 82% of the variance in methylation, and 16% of the variance in each of the three brain clusters.
Discussion
This study extended the link between SKA2 DNA methylation and suicide by examining associations between this novel epigenetic risk locus, cortical thickness, and suicide-related psychiatric symptoms. We hypothesized that greater DNA methylation at SKA2 would be associated with decreased cortical thickness in prefrontal brain regions in trauma-exposed veterans. Whole-brain vertex-wise analyses revealed that SKA2 methylationadj at this locus was associated with diminished cortical thickness in three regions of prefrontal cortex, and reduced thickness in these regions corresponded to more severe current PTSD symptoms. Although causality cannot be inferred from this cross-sectional dataset, path analysis showed that SKA2 methylationadj significantly mediated the relationship between PTSD and neural integrity in prefrontal cortex. These findings extend previous work by linking SKA2 methylationadj to alterations in cortical thickness - a possible brain endophenotype of stress-related suicide risk - and broaden its potential clinical utility by pointing to its value as a possible PTSD biomarker.
The association between SKA2 methylationadj and reductions in cortical thickness provides novel insight into how alterations in stress-sensitive molecular pathways impact neural integrity and function. In both hemispheres, greater methylationadj was associated with reduced cortical thickness in frontal pole and the anterior aspects of SFG and rostral MFG, areas of the brain crucial for several high-level cognitive-control processes. Specifically, meta-analytic evidence (20) indicates that the observed clusters (i.e., BA 9/10) are involved in multitask control (e.g., holding one task online while performing a separate task), which is necessary for evaluating multiple choices at once, complex reasoning, and cognitive flexibility. Burgess and colleagues (21) have proposed a ‘gateway’ hypothesis, in which these regions coordinate behavior by biasing attention toward internal mental stimuli (thoughts, emotions) or external sensory information. Additionally, frontal pole in particular has been implicated in prospective thinking and introspection (22, 23). Disruption of these higher-order control processes may lead to impulsive decision-making, difficulty imagining future prospects, and emotional dysregulation, which are all risk factors for suicidal behavior (3, 24, 25). An additional cluster emerged in regions of right OFC and IFG pars orbitalis (Figure 1) thought to be crucial for maintaining, updating, and adjusting ongoing behavior based on reward and punishment contingencies (26). Reduced integrity in these regions has been linked to emotional dysregulation and impulsivity (27–29). Given the cognitive functions subserved, reduced cortical integrity in the identified prefrontal clusters may represent a significant neurobiological vulnerability for suicidal behavior in the context of stress-related psychiatric illnesses, an important question that can be addressed in future research that explicitly examines suicidal behavior.
Given that the SKA2 protein has not been studied extensively, the exact mechanisms by which SKA2 may impact cortical integrity are unknown. However, studies suggest that it interacts with the glucocorticoid receptor by chaperoning it from the cytoplasm to the nucleus and is a moderator of negative feedback inhibition of cortisol by the glucocorticoid receptor (12). In prefrontal cortex, where glucocorticoid receptor density is enriched (9), sustained elevations of cortisol can cause neuronal damage and cell death either directly (30–32) or indirectly through mechanisms such as oxidative stress (33). Thus, SKA2 expression may serve a neuroprotective function by enhancing activation of the glucocorticoid receptor. This, in turn, up-regulates the expression of anti-inflammatory proteins in neuronal nuclei, represses the expression of pro-inflammatory proteins in the cytosol, and mitigates oxidative stress. These functions are similar to that of the better-known FK506 binding protein 5 (FKBP5), a co-chaperone of the glucocorticoid receptor, which has been linked in genetic association studies to a variety of relevant phenotypes including PTSD (34), alterations of brain morphology (35, 36) and suicide (37, 38). Epigenetic variation at SKA2, therefore, may represent an important additional locus that confers individual differences in risk and resilience to stress.
Although there are compelling data that SKA2 plays a role in suicide (13, 14), the present findings suggest it is also relevant for understanding the pathophysiology of stress-related disorders more broadly. By associating methylation at this locus with reduced thickness in prefrontal cortex, this study implicates a molecular and neurobiological pathway by which prolonged or extreme stress exposure impacts susceptibility for psychiatric disorders. More precisely, SKA2 may index a transdiagnostic susceptibility to neural deterioration from stress exposure, with suicide and PTSD being two clinical outcomes in which stress exposure is a potent etiological factor, and thus highly relevant. Although novel to SKA2, these hypotheses are consistent with research on the neurotoxic effects of stress exposure (9, 39), including in relation to suicide and PTSD (33, 40).
Moving forward, an important next step will be to focus on clarifying how alterations in stress-sensitive molecular and neurobiological pathways interact with other biological, psychological, and social risk processes to promote suicidal behavior. Not all individuals with stress-related disorders ultimately die by suicide, and thus, examination of more comprehensive etiological models will be needed to individualize risk assessment measures and intervene effectively. For example, present findings suggest that epigenetic modifications from stress exposure may compromise the neural circuits that typically support adaptive decision-making and self-regulation. Expansion of this work to clarify how these molecular and neural mechanisms interact with putative endophenotypes for suicide risk, including HPA-axis dysregulation, serotonin dysfunction, and aberrant cognitive-affective processes (e.g., impulsive-aggressive traits, disadvantageous decision-making), as well as stressful life events and functional genetic/epigenetic influences (41, 42) could elucidate heterogeneity in pathways to suicidal behavior and improve the precision of risk models.
The findings and conclusions need to be considered in the context of the study limitations. First, our results indicate that adjusting methylation levels by the methylation-associated SNP revealed associations that were not apparent for unadjusted methylation or the local SNP. Indeed, no significant relationships were observed for unadjusted methylation at the cg13989295 probe, and no new results emerged for rs7208505 that were not also present for adjusted methylation (i.e., a main effect of SNP was only observed for PTSD). Although we did not observe effects for unadjusted methylation, Guintivano et al. (13) reported associations between DNA methylation and SKA2 expression in brain tissue both before and after controlling for genotype. These discrepant findings for unadjusted methylation may be due to differences in measuring DNA methylation in peripheral blood samples versus brain tissue, which is beyond the scope of the present study but an important avenue of future inquiry. Further, given that it is not clear what adjusting methylation for genotype implies on a biological level and research on SKA2 is in its infancy, further investigation is needed to clarify the functional significance and biological implications of this analytic method. In particular, whether adjusted methylation in blood reflects a specific causal mechanism underlying alterations in neural structure/function or rather serves as a useful indicator of biological processes related to stress exposure and susceptibility more broadly has yet to be determined.
Second, the cross-sectional nature of our data prohibits strong conclusions regarding the direction of the proposed effects, and other mediational models are certainly possibly. We tested SKA2 methylation as a mediator of the association between PTSD (as a measure of ongoing stress) on neural integrity (effects of stress on the brain), but it is also possible that brain phenotypes may mediate the association between methylation and PTSD or that alterations in cortical thickness cause HPA-axis dysregulation and thus alter SKA2 methylation. Thus, prospective research is needed to ascertain the mechanism(s) by which traumatic stress impacts epigenetic variation and neural integrity. Third, the absence of an association between depression symptoms and SKA2 variation in this sample should be interpreted in the context of both the modest size and the makeup of our sample. Given that SKA2 purportedly measures a stress-specific molecular pathway, the high levels of trauma exposure in this sample may have strengthened our ability to detect PTSD effects. However, it is also possible that methylation at SKA2 is less predictive of depression than PTSD phenotypes, and this hypothesis requires testing in larger and more diverse samples. Fourth, we do not have information about the stability of the methylation level of this gene, and prospective studies are needed to ascertain how methylation levels vary over time. Finally, it is important to note that a measure of suicidal behavior was not included in this study, and thus, the findings can only speak to the implications for understanding suicide-related risk processes indirectly, through connections from previous research on SKA2 (13, 14). Despite these limitations, this study also has several strengths, most notably the clinically-relevant sample of veterans with trauma exposure and the innovative research design that integrates neuroimaging and genetic methods to study the biology of stress-related psychiatric phenotypes.
In summary, findings advance our understanding of stress susceptibility for psychiatric disorders by identifying SKA2 as a potential biomarker of the effects of stress exposure on cortical thickness in psychiatrically relevant brain regions. As a putative molecular measure of cumulative dysregulation in stress response systems, SKA2 epigenetic variation may be a useful blood biomarker for screening military personnel prior to deployment to identify individuals with a high lifetime burden of stress who are at risk for developing PTSD and suicide following exposure to warzone stress.
Supplementary Material
Acknowledgments
This research was supported in part by NIMH grant R21MH102834 “Neuroimaging Genetics of PTSD” and the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence (B9254-C), and the Cooperative Studies Program, Department of Veterans Affairs. This research is the result of work supported with resources and the use of facilities at the Pharmacogenomics Analysis Laboratory, Research and Development Service, Central Arkansas Veterans Healthcare System, Little Rock, Arkansas. This work was also supported by a Career Development Award to Erika J. Wolf from the United States (U.S.) Department of Veterans Affairs, Clinical Sciences Research and Development Program. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Footnotes
Three individuals with methylation levels greater than 3 SDs from the sample mean for each genotype were excluded from the study, resulting in a final N of 200. Supplemental analyses indicated that including these cases did not change the study findings.
Current PTSD diagnosis was associated with decreased cortical thickness in the right frontal pole/SFG cluster (F1, 143 = 4.75, p = .031) and right OFC/IFG/rostral MFG cluster (F1, 143 = 6.25, p = .014), with a trend emerging for the left frontal cluster (F1, 143 = 3.39, p = .068). Cortical thickness in these clusters did not differ by MDD diagnostic status (F1, 143s < 1.42, ps > .23).
DNA methylationadj was associated with a greater likelihood of current PTSD diagnosis (Wald χ2 = 4.35, OR = 1.2, p = .037), but not current MDD diagnosis. However, the relationship between DNA methylationadj and PTSD diagnosis were reduced to a trend-level when cell counts were entered in the model (Wald χ2 = 2.75, OR = 1.1, p = .097).
Disclosures
Authors Sadeh, Spielberg, Wolf, Logue, Lusk, Hayes, Sperbeck, Milberg, McGlinchey, Salat, Carter, Stone, Schichman, Humphries, and Miller reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Kang HK, Bullman TA, Smolenski DJ, Skopp NA, Gahm GA, Reger MA. Suicide risk among 1.3 million veterans who were on active duty during the Iraq and Afghanistan wars. Ann Epidemiol. 2014;25:96–100. doi: 10.1016/j.annepidem.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Nock MK, Deming CA, Fullerton CS, Gilman SE, Goldenberg M, Kessler RC, McCarroll JE, McLaughlin KA, Peterson C, Schoenbaum M, Stanley B, Ursano RJ. Suicide among soldiers: A review of psychosocial risk and protective factors. Psychiatry. 2013;7:97–125. doi: 10.1521/psyc.2013.76.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann JJ, Arango VA, Avenevoli S, Brent DA, Champagne FA, Clayton P, Currier D, Dougherty DM, Haghighi F, Hodge SE, Kleinman J, Lehner T, McMahon F, Moşcicki EK, Oquendo MA, Pandey GN, Pearson J, Stanley B, Terwilliger J, Wenzel A. Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psychiatry. 2009;65:556–563. doi: 10.1016/j.biopsych.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 6.Perroud N, Paoloni-Giacobino A, Prada P, Olie E, Salzmann A, Nicastro R, Guillaume S, Mouthon D, Stouder C, Dieben K, Huguelet P, Courtet P, Malafosse A. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: A link with the severity and type of trauma. Transl Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logue MW, Smith AK, Baldwin C, Wolf EJ, Guffanti G, Ratanatharathorn A, Miller MW. An analysis of gene expression in PTSD implicates genes involved in the glucocorticoid receptor pathway and neural responses to stress. Psychoneuroendocrinology. 2015;57:1–13. doi: 10.1016/j.psyneuen.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Kloet ER. Hormones, brain, and stress. Endocr Regul. 2003;37:51–68. [PubMed] [Google Scholar]
- 9.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato H, Takahashi T, Sumitani K, Takatsu H, Urano S. Glucocorticoid generates ROS to induce oxidative injury in the hippocampus, leading to impairment of cognitive function of rats. J Clin Biochem Nutr. 2010;47:224–232. doi: 10.3164/jcbn.10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapolsky RM. Glucocorticoid and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 12.Rice L, Waters CE, Eccles J, Garside H, Sommer P, Kay P, Blackhall FH, Zeef L, Telfer B, Stratford I, Clarke R, Singh D, Stevens A, White A, Ray DW. Identification and functional analysis of SKA2 interaction with the glucocorticoid receptor. J Endocrinol. 2008;198:499–509. doi: 10.1677/JOE-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guintivano J, Brown T, Newcomer A, Jones M, Cox O, Maher BS, Eaton WW, Payne JL, Wilcox HC, Kaminsky ZA. Identification and replication of a combined epigenetic and genetic biomarker predicting suicide and suicidal behaviors. Am J Psychiatry. 2014;17:1287–1296. doi: 10.1176/appi.ajp.2014.14010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niculescu AB, Levey D, Le-Niculescu H, Niculescu E, Kurian SM, Salomon D. Psychiatric blood biomarkers: avoiding jumping to premature negative or positive conclusions. Mol Psychiatry. 2015;20:286–288. doi: 10.1038/mp.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nat. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blake DD, Weathers FW, Nagy LM. A clinical rating scale for assessing current and lifetime PTSD: The CAPS-1. Behav Ther. 1993;18:187–188. [Google Scholar]
- 17.Lovibond SH, Lovibond PF. Manual for the depression anxiety stress scales. 2. Sydney: Psychology Foundation; [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders Research Version (SCID-I) New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- 19.Muthén LK, Muthén BO. Mplus. Statistical analyses with latent variables. User’s guide. 1998;3 [Google Scholar]
- 20.Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional Specialization within Rostral Prefrontal Cortex (Area 10): A Meta-analysis. J Cog Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 21.Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. In: Duncan J, Phillips L, McLeod P, editors. Measuring the mind: speed, control, and age. Oxford University Press; 2005. [Google Scholar]
- 22.Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A. Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- 23.Tsujimoto S, Genovesio A, Wise SP. Frontal pole cortex: encoding ends at the end of the endbrain. Trends Cogn Sci. 2011;15:169–176. doi: 10.1016/j.tics.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30:1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, Castelnau D, Malafosse A, Courtet P. Impaired decision making in suicide attempters. American Journal of Psychiatry. 2005;162:304–310. doi: 10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- 26.Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. Am J Psych. 2001;158:735–741. doi: 10.1176/appi.ajp.158.5.735. [DOI] [PubMed] [Google Scholar]
- 27.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30:1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook SC, Willman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 31.Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 33.Miller MW, Sadeh N. Traumatic stress, oxidative stress and posttraumatic stress disorder: Neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry. 2014;19:1156–1162. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–4110. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fani N, King TZ, Binder EB, Jovanovic T, Bradley B, Ressler KJ. FKBP5 genotype and structural integrity of the posterior cingulum. Neuropsychopharmacology. 2014;39:1206–1213. doi: 10.1038/npp.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii T, Ota M, Hori H, Hattori K, Teraishi T, Sasayama D, Higuchi T, Kunugi H. Association between the common functional FKBP5 variant (rs1360780) and brain structure in a non-clinical population. J Psychiatr Res. 2014;58:96–101. doi: 10.1016/j.jpsychires.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Brent D, Melhem N, Ferrell R, Emslie G, Wagner KD, Ryan N, Vitello B, Birmaher B, Mayes T, Zelazny J, Onorato M, Devlin B, Clarke G, DeBar L, Keller M. Association of FKBP5 Polymorphisms With Suicidal Events in the Treatment of Resistant Depression in Adolescents (TORDIA) Study. Am J Psychiatry. 2010;167(2):190–197. doi: 10.1176/appi.ajp.2009.09040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez-Ortiz JM, García-Gutiérrez MS, Navarrete F, Giner S, Manzanares J. Gene and protein alterations of FKBP5 and glucocorticoid receptor in the amygdala of suicide victims. Psychoneuroendocrinology. 2013;38:1251–8. doi: 10.1016/j.psyneuen.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jollant F, Lawrence NL, Olié E, Guillaume S, Courtet P. The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J Biol Psychiatry. 2011;12(5):319–339. doi: 10.3109/15622975.2011.556200. [DOI] [PubMed] [Google Scholar]
- 41.Courtet P, Gottesman II, Jollant F, Gould TD. The neuroscience of suicidal behaviors: What can we expect from endophenotype strategies? Transl Psychiatry. 2011;1(5):e7. doi: 10.1038/tp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oquendo MA, Perez-Rodriguez MM, Poh E, Sullivan G, Burke AK, Sublette ME, Galfalvy H. Life events: a complex role in the timing of suicidal behavior among depressed patients. Mol Psychiatry. 2014;19(8):902–909. doi: 10.1038/mp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.