Abstract
AIM
To describe the neurocognitive and adaptive behavior profile of children and adolescents with Niemann–Pick Disease type C1 (NPC1), a rare genetic disease that frequently presents in childhood, with variable onset and symptom complex involving neurodegeneration.
METHOD
Thirty-eight participants (20 males, 18 females; mean age 8y 10mo, SD 4y 8mo, range 1–18y) with NPC1 were evaluated through a natural history protocol.
RESULTS
NPC1 severity was in the mild to moderate range for most participants. Cognitive scores (n=32) ranged from very low to above average; about half of the participants exhibited a clinically significant advantage of Verbal IQ over Non-verbal IQ. Adaptive behavior scores (n=21) were generally in the borderline to impaired range. Longitudinal cognitive data (n=19) suggested a pattern of decreasing scores over time. However, most participants remained at the same general level of functioning throughout the study.
INTERPRETATION
This study begins to systematically describe the neurocognitive phenotype of children and adolescents with NPC1, identifying heterogeneity and decline, aiding in understanding the natural history of the disease to plan treatment studies.
Niemann–Pick disease type C1 (NPC1) (OMIM #257220) is a fatal autosomal recessive neurodegenerative disorder in which lipid storage is altered. The most recent incidence estimate is 1 per 92 000,1 and there is no Food and Drug Administration-approved therapy. Onset of symptoms is variable, with both pediatric and adult presentations.2 The classic childhood phenotype includes infantile hepatosplenomegaly (enlarged liver and spleen), which may be transient, followed by onset of neurologic symptoms, typically around school age. Death in those with the classical pediatric presentation typically occurs by the late teens or early adulthood.3
Neurological and neurocognitive symptoms are ubiquitous but heterogeneous in NPC1, including general cognitive decline, dysarthria, dysphasia, cerebellar ataxia, eye movement abnormalities (including vertical supranuclear gaze palsy), and seizures.4–5 One study of neuropsychological functioning in 10 adults with NPC1 found that performance on specific neuropsychological domains (e.g. fine motor, verbal fluency, memory, and attention) generally corresponded to the ratings of disease progression, though the testing was not consistent across participants, precluding comprehensive conclusions.6 Another report of adolescents and adults found receptive vocabulary to be relatively spared compared to expressive language, non-verbal, executive functioning, and memory skills.7 Standardized measures of cognitive abilities and adaptive behavior have not been reported upon for children with NPC1.
Yanjanin et al.8 proposed a clinical severity scale for NPC1 based on neurological examination and parent/patient-reported history of symptoms. The scale includes several types of cognitive, neurological, and sensory symptoms, as well as specific modifiers that relate to symptoms in other system domains. Yanjanin et al.8 described increasing severity over time since diagnosis, but not age at onset, in 19 historical/retrospective patients and 18 prospective patients (mean age 12y 11mo, range 4–51y). This severity scale has also been utilized by Shin et al.9 to expand the NPC1 patient cohort and description of neurological disease progression. Stampfer et al.10 used the scale along with another to describe cognitive decline as one of the earliest and most frequently documented neurological symptoms of NPC1 disease, further demonstrating the importance of reporting on standardized, descriptive measures of this aspect of disease presentation. Thus, in the current study, we present cross-sectional and longitudinal data on the cognitive and adaptive behavior profiles of children and adolescents with NPC1.
METHOD
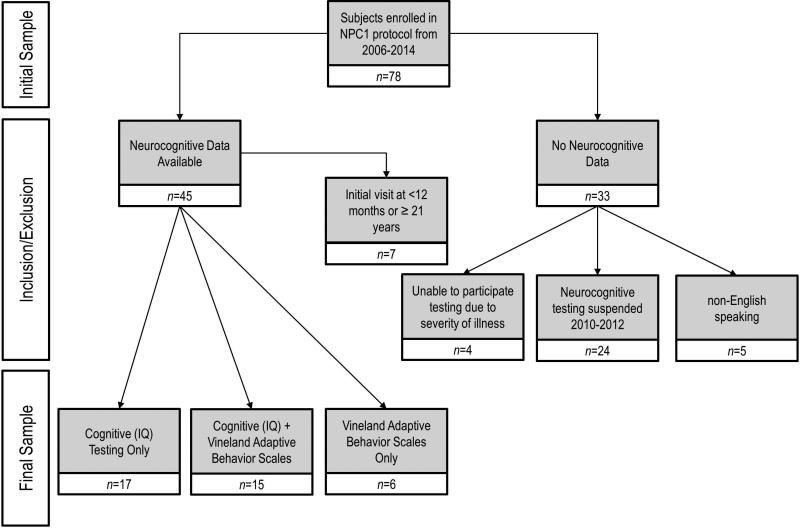
Individuals diagnosed with NPC1 were enrolled in a natural history study performed at the National Institutes of Health (NIH) from 2006 to 2014. The study was approved by the NICHD Institutional Review Board (IRB). Informed consent and assent (where appropriate) was obtained for all participants. Participants underwent neuropsychological evaluations by experienced doctoral-level clinicians. A number of participants were re-evaluated at intervals of at least 5 months (see Fig. 1).
Figure 1.
Patient disposition.
Participants
This study included 38 children and adolescents ranging from ages 1 year 10 months to 18 years (mean 8y 10mo, SD 4y 8mo). Of these participants, 14 were included in Yanjanin et al.,8 a study of general disease severity, and there were five sibling pairs. Twenty participants were male (53%) and 35 were white/non-Hispanic (92%).
Measures
The NPC1 Clinical Severity Scale8 was used to rate disease severity. Items rated on a 0 to 5 scale comprise nine major domain scores: eye movement, speech, fine motor, hearing, seizures, ambulation, swallow, cognition, and memory. Relevant to this report, possible cognition scores were 0 (normal cognition); 1 (mild learning delay: grade appropriate for age); 3 (moderate learning delay: individualized curriculum or modified work setting); 4 (severe delay/plateau: some loss of cognitive function, no longer in school or no longer able to work); and 5 (minimal cognitive function). Speech scores were 0 (normal speech); 1 (mild dysarthria); 2 (severe dysarthria); 3 (non-verbal/functional communication skills for needs); and 5 (minimal communication) – with no score of 4. Severity score determination was independent of neurocognitive evaluation.
The neurocognitive evaluation utilized a multi-tiered approach, as recommended in other studies of CNS dysfunction.11 The appropriate Wechsler scale – including the Wechsler Abbreviated Scales of Intelligence (WASI or WASI-II), Wechsler Preschool and Primary Scale of Intelligence – Third Edition, Wechsler Intelligence Scales for Children – Fourth Edition,12–14 or the Mullen Scales of Early Learning15 – was used to assess cognitive ability. Verbal IQ (VIQ), Non-verbal IQ (NVIQ)/Perceptual Reasoning IQ, and Full Scale IQ were reported. Clinically significant (≥10) discrepancies between VIQ and NVIQ were also tallied.
The Vineland Adaptive Behavior Scales – Second Edition (VABS-II)16 is a semi-structured caregiver interview that assesses adaptive functioning in domains of Communication, Daily Living Skills, and Socialization. Motor Skills are also evaluated but only for children under the age of 7 years. The overall level of adaptive behavior is reflected in the Adaptive Behavior Composite score. Both the VABS and Wechsler scales produce standard scores with a mean of 100 (SD 15). Scores less than 70 are considered impaired.
Finally, medical records were used to determine the highest language level each participant had attained (single words, sentences, or complex sentences) and parent-reported history was used to indicate the age of first reported neurological symptom (e.g. ataxia, dysarthria).
Procedures
Participants were drawn from a pool of 78 participants enrolled in a natural history study between 2006 and 2014. With the exception of a period from 2010 to 2012 when neurocognitive testing was temporarily suspended (n=24 participants), this cohort represents consecutive enrollments into the natural history study. Sixteen participants were excluded if their symptoms were too advanced to allow participation in testing, English was not their first language, or they were outside the targeted age range (12mo–21y). The final participant disposition is shown in Figure 1. Thirty-eight participants had neurocognitive data from either cognitive/IQ testing (n=17), VABS-II (n=6), or both IQ and VABS-II (n=15). The majority of participants with IQ data (n=32) received the WASI (or WASI-II) (n=16, 50%); 10 (31%) participants received the WPPSI-III, four (13%) participants received the Wechsler Intelligence Scales for Children – Fourth Edition, and two (6%) participants received the Mullen Scales of Early Learning. In some cases, when allowed for by the test manual, pro-rated scores were used. In these cases, following test rules, Full Scale IQ were not calculated. Of the 32 participants, 19 (59%) with IQ data had at least one follow-up visit with additional cognitive testing, with minimum intervals of at least 5 months. We present no longitudinal VABS data. The mean time to the final follow-up visit was 3 years 4 months (SD 2y 10mo, range 5mo–8y 3mo).
Statistical Analysis
The majority of this report is descriptive in nature. Pearson's (continuous variables) and Spearman's (ordinal variables) correlations were calculated where appropriate.
RESULTS
Cross-sectional cohort
The average NPC1 severity score across participants at their first visit (n=38) was 11.71 (SD 7.84, median 12), ranging from 1 to 33 on a scale of increasing severity (Table I). Thus, the mean level of severity in this sample was mild to moderate (mild, 0–10; moderate, 11–25; and severe, ≥26). Cognition and speech scores are individually reported in Table I, and are again indicative of the generally mild to moderate scores of this cohort at first study visit. Eight (21%) participants had no self- or parent-reported neurological symptoms at the first evaluation (years since onset was set to zero for these participants). Twenty-one (55%) participants experienced neurological onset during early childhood (<5y) and 9 (24%) had onset during late childhood (5–13y). The mean time between onset of neurological symptoms and evaluation was 4.10 years (SD 4.01, range 0–14).
Table I.
Results of initial evaluation
| VABS-II | Cognitive | NPC1 severity | Other | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant | Age, years | Sex | Mig-lustat | Communication | Daily Living | Socialization | ABC | FSIQ | NVIQ | VIQ | test | Overall | Speech | Cognition | Language level | Years Since Onset |
| 2a | 7.24 | M | Y | 98 | 97 | 108 | 99 | 89 | 79 | 104 | 2 | 5 | 1 | 1 | 3 | 2.24 |
| 3 | 13.14 | F | Y | 31 | 33 | 38 | 31 | 33 | 3 | 3 | 2 | 11.14 | ||||
| 4a | 5.02 | M | N | 65 | 73 | 66 | 61 | 59 | 3 | 11 | 1 | 1 | 2 | 2.02 | ||
| 5a | 10.04 | M | N | 69 | 62 | 69 | 65 | 68 | 54 | 83 | 2 | 14 | 1 | 1 | 3 | 7.04 |
| 6a | 16.39 | M | N | 58 | 57 | 66 | 2 | 18 | 1 | 3 | 3 | 13.39 | ||||
| 7a | 11.8 | F | N | 69 | 58 | 68 | 64 | 62 | 61 | 69 | 2 | 22 | 1 | 3 | 3 | 10.3 |
| 8 | 3.45 | F | Y | 44 | 60 | 51 | 50 | 23 | 3 | 5 | 1 | 1.45 | ||||
| 9a | 7.7 | M | Y | 102 | 83 | 104 | 94 | 83 | 81 | 87 | 2 | 7 | 1 | 1 | 3 | 5.7 |
| 10a | 11.9 | M | Y | 55 | 60 | 58 | 2 | 18 | 1 | 3 | 3 | 5.9 | ||||
| 11a | 4.48 | M | Y | 79 | 87 | 94 | 77 | 63 | 49 | 81 | 3 | 5 | 1 | 0 | 3 | 2.48 |
| 12a | 5.73 | F | Y | 89 | 81 | 77 | 74 | 57 | 81 | 3 | 12 | 2 | 1 | 2 | 4.73 | |
| 14 | 3.1 | M | N | 97 | 105 | 101 | 101 | 88 | 3 | 3 | 0 | 0 | 3 | 0 | ||
| 16 | 5.69 | M | Y | 97 | 96 | 100 | 3 | 3 | 1 | 0 | 3 | 0 | ||||
| 17a | 6.16 | M | N | 65 | 70 | 66 | 2 | 7 | 1 | 1 | 3 | 4.16 | ||||
| 19 | 3.79 | F | N | 74 | 3 | 3 | 0 | 1 | 2 | 1.79 | ||||||
| 20 | 4.52 | F | N | 47 | 58 | 57 | 53 | 9 | 1 | 1 | 1 | 2.52 | ||||
| 21 | 1.85 | M | N | 84 | 77 | 82 | 74 | 9 | 0 | 3 | 1 | 0.35 | ||||
| 22 | 10.68 | M | Y | 84 | 77 | 94 | 2 | 12 | 1 | 1 | 3 | 2.68 | ||||
| 23 | 7.72 | F | N | 100 | 96 | 107 | 2 | 1 | 0 | 0 | 3 | 0 | ||||
| 28 | 3.36 | F | N | 110 | 105 | 113 | 3 | 3 | 0 | 0 | 3 | 0.36 | ||||
| 29a | 9.67 | F | Y | 96 | 104 | 89 | 4 | 1 | 0 | 0 | 3 | 0 | ||||
| 30a | 11.84 | F | Y | 115 | 115 | 104 | 4 | 1 | 0 | 0 | 3 | 0 | ||||
| 32 | 2.99 | M | Y | 68 | 74 | 3 | 20 | 1 | 3 | 1 | 1.33 | |||||
| 33 | 12.75 | F | N | 92 | 102 | 4 | 14 | 1 | 1 | 3 | 4.75 | |||||
| 34b | 3.26 | F | Y | 65 | 73 | 70 | 65 | 20 | 1 | 3 | 1 | 1.6 | ||||
| 35 | 4.52 | M | N | 74 | 60 | 81 | 64 | 18 | 1 | 0 | 2 | 3.02 | ||||
| 40a | 4.75 | M | N | 95 | 103 | 116 | 101 | 87.33 | 87.77 | 86.89 | 1 | 3 | 0 | 0 | 3 | 0 |
| 42a | 17.19 | M | Y | 71 | 58 | 86 | 2 | 10 | 1 | 1 | 3 | 5.19 | ||||
| 47 | 6.63 | M | N | 63 | 66 | 74 | 64 | 52 | 49 | 64 | 3 | 13 | 1 | 3 | 2 | 3.13 |
| 49a | 5.57 | M | N | 84 | 82 | 93 | 3 | 1 | 0 | 0 | 3 | 0 | ||||
| 50a | 12.71 | F | Y | 73 | 63 | 86 | 2 | 17 | 2 | 3 | 3 | 4.71 | ||||
| 52 | 15.64 | F | Y | 65 | 57 | 76 | 2 | 12 | 1 | 3 | 3 | 13.97 | ||||
| 61a | 12.92 | F | Y | 79 | 74 | 82 | 76 | 70 | 62 | 79 | 2 | 16 | 1 | 3 | 3 | 9.92 |
| 64b | 13.32 | F | Y | 48 | 47 | 42 | 45 | 17.35 | 16.88 | 17.82 | 1 | 20 | 0 | 4 | 3 | 10.32 |
| 65 | 14.51 | M | N | 83 | 77 | 79 | 77 | 72 | 79 | 83 | 4 | 5 | 0 | 3 | 3 | 0 |
| 67 | 18.02 | M | Y | 68 | 69 | 85 | 72 | 64 | 69 | 64 | 2 | 19 | 2 | 3 | 3 | 9.02 |
| 68b | 13.91 | F | Y | 75 | 79 | 82 | 76 | 70 | 69 | 76 | 2 | 15 | 2 | 3 | 3 | 5.91 |
| 76a,b | 10.68 | F | Y | 69 | 61 | 66 | 64 | 42 | 46 | 47 | 2 | 22 | 2 | 4 | 3 | 4.68 |
Denotes participant who had longitudinal data (n=19).
Participants received medication for seizures (n=4).
IQ test scores: 1=Mullen Scales of Early Learning (these scores are developmental quotients rather than standard scores), 2=Wechsler Abbreviated Scales of Intelligence – First or Second Edition; 3=Wechsler Preschool and Primary Scale of Intelligence – Third Edition; and 4=Wechsler Intelligence Scales for Children – Fourth Edition. Where necessary, and allowed for by the test manual, some pro-rated scores were used; when either NVIQ or VIQ was pro-rated, FSIQ was not calculated. NPC1 severity scores: speech 0=normal speech, 1=mild dysarthria, and 2=severe dysarthria. Cognition 0=normal cognition, 1=mild learning delay, grade appropriate for age, 3= moderate learning delay: individualized curriculum or modified work setting, 4=severe delay/plateau: some loss of cognitive function, no longer in school or no longer able to work, and 5=minimal cognitive function. Language level scores: 1=single words, 2=sentences, and 3=complex sentences.
M, male; F, female; Y, yes; N, no; Years since onset, number of years since neurological onset (set to zero where there are no self- or parent-reported neurological symptoms); VABS-II, Vineland Adaptive Behavior Scales; ABC, Adaptive Behavior Composite; FSIQ, Full Scale IQ; NVIQ, Non-verbal IQ; VIQ, Verbal IQ.
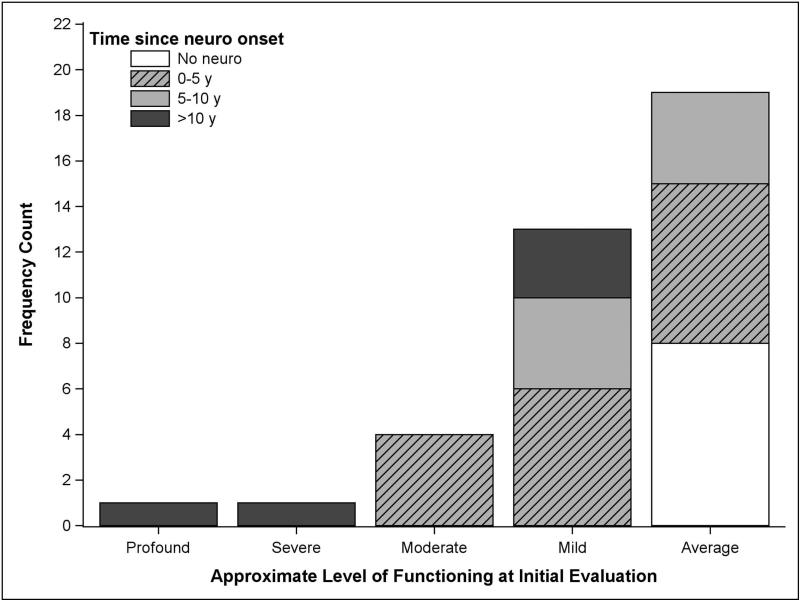
The general level of current functioning was estimated using cognitive data, if available, or the VABS-II Adaptive Behavior Composite. As shown in Figure 2, the majority of participants were classified as borderline to average (scores >70; n=19, 50%) or mildly impaired (scores 55–70; n=13, 34%), using the Diagnostic and Statistical Manual for Mental Disorders – Fourth Edition17 intellectual disability severity classifications. The cognitive profile was explored in the 29 participants whose NVIQ and VIQ scores could both be calculated. Thirteen (45%) had VIQ scores at least 10 points higher than NVIQ scores (mean 20.08, SD 7.33, range 10–32 points difference), and two (7%) had higher NVIQ than VIQ scores (11- and 15-point difference respectively).
Figure 2.
Approximate level of functioning by time since neurological onset at initial evaluation (n=38). Approximate level of functioning was determined using the following hierarchy of scores: Full Scale IQ, Non-verbal IQ or Verbal IQ, or Vineland Adaptive Behavior Composite. Scores were classified as average (>70), mild (55–69), moderate (40–54), severe (20–39), and profound (<20).
Among the 20 participants with full VABS-II administrations, the mean Adaptive Behavior Composite score was 70.60 (SD 18.57, median 68.5, range 31–101). The profile of mean standard scores across domains was even: Communication, 71.62 (SD 18.70, median 69, range 31–102); Daily Living Skills, 71.53 (SD 17.63, median 73, range 33–105); and Socialization, 76.90 (SD 20.62, median 79, range 38–108). Among the participants with both cognitive data and VABS-II scores (n=15), estimates of cognitive and adaptive abilities were consistent, with no pattern suggesting that either IQ or adaptive functioning was stronger.
Standardized language measures were not available for most participants. However, the highest level of speech attained, as determined by parental report during admission assessment, was recorded for each individual (Table I). All participants were verbal; most had achieved complex sentences (n=27, 71%).
Pearson's and Spearman's correlations between age, time since neurological onset, NPC1 severity scores, and neurocognitive scores are shown in Table II. Both age and time since neurological onset were positively and significantly correlated with overall severity, though neither relationship was strong. Time since neurologic onset was negatively and moderately correlated with both IQ and VABS-II scores. All cognitive and adaptive variables were moderately to strongly and negatively correlated with overall disease severity. It is important to note that IQ and adaptive behavior were obtained in slightly different subsamples, so comparison between these correlations should be made with care.
Table II.
Pearson's or Spearman's correlations between NPC1 severity and age, IQ, and adaptive functioning (initial evaluation)
| n | Mean (SD) | Median | Age | Time since neurological onset | NPC1 Overall Severity | NPC1 Speech Severity | NPC1 Cognition Severity | |
|---|---|---|---|---|---|---|---|---|
| Full-Scale IQ | 27 | 73.36 (20.86) | 70 | –0.36 | –0.63a | –0.81a | –0.47a | –0.77a |
| Verbal IQ | 32 | 79.93 (19.31) | 82 | –0.26 | –0.53a | –0.70a | –0.39a | –0.75a |
| Non-verbal IQ | 29 | 70.78 (21.2) | 69 | –0.27 | –0.63a | –0.69a | –0.48a | –0.66a |
| VABS Communication | 21 | 71.62 (18.70) | 69 | –0.18 | –0.40 | –0.77a | –0.49a | –0.51a |
| VABS Daily Living Skills | 21 | 71.52 (17.63) | 73 | –0.36 | –0.61a | –0.84a | –0.40 | –0.51a |
| VABS Socialization | 21 | 76.90 (20.62) | 79 | –0.22 | –0.43a | –0.77a | –0.34 | –0.58a |
| VABS Adaptive Behavior | 20 | 68.50 | ||||||
| Composite | 70.60 (18.57) | –0.22 | –0.47a | –0.82a | –.45a | –0.58a | ||
| Age, years | 38 | 8.81 (4.66) | 7.71 | 1.00 | 0.69a | 0.33a | 0.23 | 0.36a |
| Years since neurologic onset | 38 | 4.10 (4.01) | 2.86 | 1.00 | 0.63a | 0.42a | 0.51a | |
| NPC1 Overall Severity | 38 | 11.71 (7.84) | 12 | 1.00 | 0.73a | 0.76a | ||
| NPC1 Speech Severity | 38 | 0.95 (0.80) | 1 | 1.00 | 0.52a | |||
| NPC1 Cognition Severity | 38 | 1.74 (1.46) | 1 | 1.00 |
p<0.05. Correlation coefficients are Pearson's r for NPC1 total severity (continuous variable) and Spearman's rho for speech and cognition severity (ordinal variables). NPC1, Niemann–Pick Disease type C1; VABS, Vineland Adaptive Behavior Scales.
Longitudinal cohort
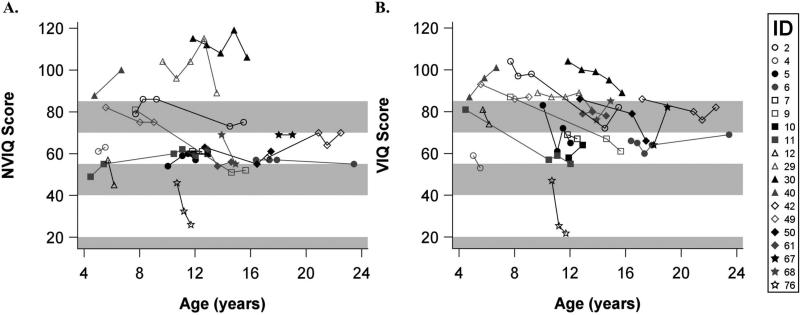
Nineteen participants were seen for a follow-up evaluation where at least part of an IQ measure was obtained (Fig. 3). No longitudinal data were available for the VABS-II. The majority of the longitudinal sample was male (n=11, 58%), and the average age at the first visit was 10 years 5 months (SD 4y 3mo, range 4y 6mo–18y). A general statement about change over time is qualified by the small number of participants with longitudinal data and the varying amount of time between assessments. To crudely summarize the data, we present information on total IQ change and change per year for the 19 participants. On average, the rate of change per year for NVIQ was –3.62 (SD 8.02, median –0.42, IQR –3.65 to 0). The average rate of VIQ change per year was –2.38 (SD 9.32, median –2.05, IQR –4.19 to 0). When the entire study period is considered, 8 (42%) of 19 participants decreased by more than five points in NVIQ (range 6–29 points), while 11 (58%) of 19 decreased by five or more points in VIQ (range 6–26 points). Although the predominant pattern was one of variability and perhaps decline, it was clear that most participants remained in the same general level of functioning across longitudinal visits.
Figure 3.
Non-verbal IQ (NVIQ) and Verbal IQ (VIQ) scores over time in participants with visits at least 5 months apart (n=19). IDs correspond to IDs listed in Table I. Bands represent the DSM levels of intellectual disability (70–85, borderline; 55–69, mild; 40–54, moderate; <20–39, severe). In six instances, the cognitive test differed from that of the previous administration; four of these were transitions into higher-level Wechsler scales (Participant#30 visit 4 to 5, Participant#29 visit 4 to 5, Participant#11 visit 2 to 3, Participant#50 visit 1 to 2), one from Mullen Scales of Early Learning to a Wechsler (Participant#40 visit 1 to 2), and one from Wechsler to Mullen Scales of Early Learning (Participant#76).
DISCUSSION
This is the first report on the cognitive ability and adaptive behavior of pediatric patients with NPC1. Keeping in mind that participants who were too severely impaired to participate in neurocognitive testing were excluded, our findings indicated that IQ scores range from very low to above average, with about half of the testable participants above the range of cognitive impairment (Full Scale IQ>70). About 40% of the patients demonstrated a clinically significant advantage of VIQ over NVIQ. In the participants with available data, adaptive behavior was generally in the borderline to impaired range. There was no consistent difference between IQ and adaptive behavior in participants with both measures.
Although cognitive and adaptive functioning scores below 70 are generally indicative of intellectual disability, in patients with medical conditions known to contribute to cognitive impairment the more appropriate term is neurocognitive disorder.18 In the case of NPC1 and other neurodegenerative disorders, this term refers both to the later onset of cognitive impairment as well as to the progressive cognitive decline (previously referred to as dementia). Heterogeneity in cognitive ability and adaptive behavior among children and adolescents with NPC1 is consistent with findings in adults with NPC1,6 as well as in other genetic disorders with a neurologic component.19–20 The full range of ‘age at onset’ and a wide range of ‘time since diagnosis’ were also observed in this sample, which may have resulted in uneven progression of disease and/or variant exposure to environmental factors. We reported here on major neuropsychological constructs, but future research may reveal more informative patterns through a fine-grained analysis of specific neuropsychological abilities (i.e. memory, executive function, fine motor, language profile) and other aspects of psychological functioning. Further serial evaluations will also be necessary in understanding the unique natural progression NPC1 compared to other neurodegenerative conditions affecting youth (see Freeman et al.20).
Disease severity was generally described as mild to moderate in this study. Even in this potentially restricted sample, we demonstrated that disease severity was correlated with increased cognitive and adaptive impairment in children and adolescents with NPC1. While cognition comprises only 5 of 62 potential points in the severity score, it is not surprising that a strong relationship exists between the total NPC1 severity score and standardized cognitive and adaptive behavior scores. While this correlation helps validate this novel type of severity measure, measures of intellectual functioning and adaptive behavior provide practical indicators of daily functioning and are thus integral to understanding the progression of the disorder. In this case it is noteworthy that the NPC1 severity scores are clinical impressions and are not expected to be fully redundant with standardized scores. This is especially evident in the wide range of functioning within each cognition score level, suggesting that the anchors of the NPC1 cognition severity score may benefit from slight revision.
In the cross-sectional data, a moderate and negative relationship was observed between time since neurological onset and IQ scores, consistent with a progressive neurodegenerative disorder. In general, this was corroborated by a general pattern of decreasing scores within participants who had longitudinal assessments. This observation is tempered by the fact that IQ scores are expected to vary within a range (usually ±5 points, depending on the test). In fact, recent studies of typically developing children and adolescents have demonstrated that the rate of significant change in IQ score may be much larger than most test manuals predict,21–22 although scores tend to increase, rather than decrease over time. Further, variability in individuals with lower IQ scores may be more strongly influenced by external factors, such as inattention and fatigue.23 Testing was scheduled in the morning, whenever possible, and at least 24 hours after travel to the testing site in order to minimize the impact of fatigue; however, many other “background” variables were uncontrolled. Finally, the use of the brief IQ in measures like the WASI may have contributed to variability over time; in participants with uneven cognitive development, as is often seen in the context of cognitive impairment, brief IQ scores may be biased when compared to Full Scale IQ scores. Importantly, these data indicate that an individual's score should be considered within the context of his or her history of scores; true declination will be evidenced by a persistent pattern of lower scores.
While the general trajectory in the sample was downward, few participants experienced clinically meaningful and persistent changes in cognitive ability. Of particular interest, given the demonstrated advantage of VIQ in nearly half of the cross-sectional participants, was the potential for steeper decline in of VIQ compared to NVIQ. Although our results were not conclusive in this respect, it is possible that dysarthria or memory deficits, known progressive symptoms of the disorder, had differential effects on VIQ. In addition, the initial advantage of verbal over non-verbal skills suggests that visual-spatial and visual-motor skills in particular may be affected early in disease progression, compared to verbal skills, which may have had more room to decline. More longitudinal data on a representative sample will help to elucidate the responsible mechanisms for these observations.
The major limitation of this study is selection bias that is inherent to the study of neurocognitive abilities in populations with the potential for severe impairment. Patients who could not tolerate cognitive testing but received administration of the VABS-II (n=6) almost certainly would have received very low IQ scores had appropriate cognitive tests been available. Further, the administration of the VABS-II was variable and may have been preferentially administered in patients where adaptive behavior was considered a potential weakness. This variation in assessment battery is a documented limitation of many studies of neuropsychiatric outcomes in genetic disorders. While a working group recommends the use of separate batteries for three levels of general functioning (very low, low, and average) to appropriately assess changes both within and between individuals,11 standardized cognitive tests that account for confounding impairments such as cerebellar ataxia are lacking. Thus, while these data are unique and contribute significantly to our understanding of the neurocognitive profile of NPC1 on commonly used tests, they should be interpreted with caution.
Another potentially confounding variable was medication use, including the off-label use of miglustat (Zavesca), a glycosphingolipid inhibitor which is approved for treatment of NPC1 in the European Union and several other countries, but not in the US. Access to the drug is primarily limited by its cost. Two-thirds (65%) of the participants were taking off-label miglustat at the time of initial testing, though no difference in neurocognitive ability was apparent. Four participants in the cross-sectional cohort were on antiepileptic medications, but none of the participants in the longitudinal cohort were taking medication for seizures at the time of testing. Still, the natural history element of this study, which included a significant portion not on miglustat, makes it a critical comparison for studies of open-label treatment with miglustat or other drugs.24
While the current study did not address all facets of the neuropsychological profile of children with NPC1 (e.g. executive functioning, speech issues such as dysarthria, and short and long term memory), it provides important information related to the feasibility of neurocognitive testing in both a cross-sectional cohort and in a patient population over time. It also highlights the importance of natural history studies in rare diseases, especially those with phenotypic heterogeneity, in order to approximate the amount of inter- and intra-patient variability that treatment studies may confront. Although this study demonstrates the feasibility of obtaining standardized cognitive data on a group of patients while disease progression is occurring, it also underscores the primary source of difficulty in this type of investigation: consistency in testing can be very difficult, limited by the participant (e.g. fatigue), and the instrumentation (e.g. tests do not span the full range of ability). However, we submit that this limitation is not insurmountable. As has been recently articulated, when researchers carefully plan and implement a protocol with the intent of consistently collecting basic data elements, useful data can be gleaned, even when there is variability in the test used.25
In a heterogeneous, multisystem disease such as NPC1, clinical outcome measures are difficult to establish, either to monitor natural disease progression or to evaluate pharmacologic efficacy in a therapeutic trial. This study begins to quantify the functional neurocognitive impact of disease in individuals with NPC1. While neurocognitive testing may be useful for revealing a slowing or stabilization of disease progression in response to specific treatments, further longitudinal study of the disorder is a necessary precursor in order to better understand the variability in the natural progression of the disease.
What this paper adds
First standardized neurocognitive data from children with NPC1.
Significant impairment was common but not universal, and was associated with time since neurological onset.
A pattern of gradual decline was observed in repeated assessments.
Variability over time was common.
Individual assessments should be interpreted with caution.
ACKNOWLEDGEMENTS
The authors thank the guardians and patients who participated in this study, Riley Kessler for reviewing this manuscript and assisting with data management, and Joseph Iluore for his work on this project. This work was supported by the Intramural Research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health of the National Institutes of Health; Dana's Angels Research Trust; the Ara Parseghian Medical Research Foundation; and the Bench to Bedside Program of the NIH Clinical Center.
ABBREVIATIONS
- NPC1
Niemann–Pick Disease type C1
- NVIQ
Non-verbal IQ
- VABS
Vineland Adaptive Behavior Scales
- VIQ
Verbal IQ
- WASI
Wechsler Abbreviated Scales of Intelligence
Footnotes
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
REFERENCES
- 1.Wassif CA, Cross JL, Iben J, et al. High incidence of unrecognized visceral/neurological late-onset Niemann–Pick disease, type C1, predicted by analysis of massively parallel sequencing data sets. Genet Med. 2015 Mar 12; doi: 10.1038/gim.2015.25. doi: 10.1038/gim.2015.25. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sevin M, Lesca G, Baumann N, et al. The adult form of Niemann–Pick disease type C. Brain. 2007;130:120–33. doi: 10.1093/brain/awl260. [DOI] [PubMed] [Google Scholar]
- 3.Vanier MT, Millat G. Niemann–Pick disease type C. Clin Genet. 2003;64:269–81. doi: 10.1034/j.1399-0004.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 4.Mengel E, Klunemann HH, Lourenco CM, et al. Niemann–Pick disease type C symptomatology: an expert-based clinical description. Orphanet J Rare Dis. 2013;8:166. doi: 10.1186/1750-1172-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijburg FA, Sedel F, Pineda M, et al. Development of a suspicion index to aid diagnosis of Niemann–Pick disease type C. Neurology. 2012;78:1560–7. doi: 10.1212/WNL.0b013e3182563b82. [DOI] [PubMed] [Google Scholar]
- 6.Klarner B, Klunemann HH, Lurding R, Aslanidis C, Rupprecht R. Neuropsychological profile of adult patients with Niemann–Pick C1 (NPC1) mutations. J Inherit Metab Dis. 2007;30:60–7. doi: 10.1007/s10545-006-0417-6. [DOI] [PubMed] [Google Scholar]
- 7.Hinton VJ, Vecchio D, Prady H, Wraith E, Patterson MC. The cognitive phenotype of Niemann–Pick type C (NPC): neuropsychological characteristics of patients at baseline in a clinical trial with oral miglustat.. American Society of Human Genetics Annual Meeting; Salt Lake City, UT. 2005. [Google Scholar]
- 8.Yanjanin NM, Velez JI, Gropman A, et al. Linear clinical progression, independent of age of onset, in Niemann–Pick disease, type C. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:132–40. doi: 10.1002/ajmg.b.30969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin J, Epperson K, Yanjanin NM, et al. Defining natural history: assessment of the ability of college students to aid in characterizing clinical progression of Niemann–Pick disease, type C. PLoS One. 2011;6:e23666. doi: 10.1371/journal.pone.0023666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stampfer M, Theiss S, Amraoui Y, et al. Niemann–Pick disease type C clinical database: cognitive and coordination deficits are early disease indicators. Orphanet J Rare Dis. 2013;8:35. doi: 10.1186/1750-1172-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson PI, Pariser AR, Groft SC, et al. Research challenges in central nervous system manifestations of inborn errors of metabolism. Mol Genet Metab. 2011;102:326–38. doi: 10.1016/j.ymgme.2010.11.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wechsler D. Wechsler Preschool and Primary Scale of Inteligence. Third Edition. Pearson; San Antonio, TX: 2002. [Google Scholar]
- 13.Wechsler D. Wechsler Intelligence Scale for Children. Fourth Edition Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- 14.Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 15.Mullen EM, editor. Mullen Scales of Early Learning. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- 16.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. Second Edition AGS Publishing; Circle Pines, MN: 2005. [Google Scholar]
- 17.American Psychiatric Association., American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed. xxv. American Psychiatric Association; Washington, DC: 1994. Task Force on DSM-IV. p. 886. [Google Scholar]
- 18.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- 19.Drotar D, Aylward G, Beebe D. Introduction to the special section: Psychological outcomes of pediatric conditions that affect the central nervous system. J Pediatr Psychol. 2012;37:707–12. doi: 10.1093/jpepsy/jss079. [DOI] [PubMed] [Google Scholar]
- 20.Freeman K, Gregory A, Turner A, Blasco P, Hogarth P, Hayflick S. Intellectual and adaptive behaviour functioning in pantothenate kinase-associated neurodegeneration. J Intellect Disabil Res. 2007;51:417–26. doi: 10.1111/j.1365-2788.2006.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsden S, Richardson FM, Josse G, et al. Verbal and non-verbal intelligence changes in the teenage brain. Nature. 2011;479:113–6. doi: 10.1038/nature10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waber DP, Forbes PW, Almli CR, Blood EA. Four-year longitudinal performance of a population-based sample of healthy children on a neuropsychological battery: the NIH MRI study of normal brain development. J Int Neuropsychol Soc. 2012;18:179–90. doi: 10.1017/S1355617711001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenni OG, Fintelmann S, Caflisch J, Latal B, Rousson V, Chaouch A. Stability of cognitive performance in children with mild intellectual disability. Dev Med Child Neurol. 2015;57:463–9. doi: 10.1111/dmcn.12620. [DOI] [PubMed] [Google Scholar]
- 24.Fecarotta S, Romano A, Della Casa R, et al. Long term follow-up to evaluate the efficacy of miglustat treatment in Italian patients with Niemann–Pick disease type C. Orphanet J Rare Dis. 2015;10:22. doi: 10.1186/s13023-015-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Summar ML, Endo F, Kolker S. On the Creation, Utility and Sustaining of Rare Diseases Research Networks: Lessons learned from the Urea Cycle Disorders Consortium, the Japanese Urea Cycle Disorders Consortium and the European Registry and Network for Intoxication Type Metabolic Diseases. Mol Genet Metab. 2014;113:105–8. doi: 10.1016/j.ymgme.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]