Abstract
Background
Tranexamic acid (TXA) has been shown to reduce mortality from severe hemorrhage. Although recent data suggest that TXA has anti-inflammatory properties, few analyses have investigated the impact of TXA on infectious complications in trauma patients. We examined the association between TXA administration and infection risk among injured military personnel.
Methods
Patients administered TXA were matched by injury severity score to patients who did not receive TXA. Conditional logistic regression was used to examine risk factors associated with infections within 30 days. A Cox proportional analysis evaluated risk factors in a time-to-first infection model.
Results
A total of 335 TXA recipients were matched to 626 patients not administered TXA. A greater proportion of TXA recipients had an infection compared to the comparative group (P <0.001). The univariate analysis estimated an unadjusted odds ratio (OR) of 2.5 (95 per cent confidence interval [CI]: 1.8–3.4) for the association of TXA with infection risk; however, upon multivariable analysis, TXA administration was not significant (OR: 1.3; CI: 0.8–1.9). Blast injuries, intensive care unit (ICU) admission, and receipt of ≥10 units of blood within 24 hours post-injury were independently associated with infection risk. The Cox proportional model confirmed association with ICU admission and blood transfusions. Moreover, traumatic amputations were also significantly associated with a reduced time-to-first infection.
Conclusion
In life-threatening military injuries matched for injury severity, TXA recipients did not have a higher risk for infections nor was time to developed infections shorter than in non-recipients. Extent of blood loss, blast injuries, extremity amputations, and intensive care stay were associated with infections.
Introduction
A large proportion of mortality among trauma patients is due to hemorrhage and hemorrhagic shock (30 to 40 per cent). In particular, 33–56 per cent of trauma-related deaths that occur prior to the initial hospitalization are the result of exsanguination1. Tranexamic acid (TXA; Cyklokapron®) is an anti-fibrinolytic drug shown to be effective at mitigating severe hemorrhage among surgical patients, including orthopaedic2, cardiac3, and gynecological surgery4. Therefore, the benefit of administering TXA as part of the immediate care of trauma patients has been investigated. Specifically, a randomized placebo-controlled trial (CRASH-2) examined data from 274 hospitals spanning 40 countries and found that mortality in trauma patients due to hemorrhage was significantly reduced with the early administration of TXA5.
The United States Joint Trauma System has encouraged the use of TXA within three hours of injury in combat casualties with “life-threatening hemorrhagic injury and high potential for development of coagulopathy or outright presence of coagulopathy,” for damage control resuscitation6. An analysis of the effect of TXA in combat casualties found that inpatient mortality was reduced by 6.5 per cent 7, and in patients receiving massive (≥ 10 units) blood product transfusions, mortality was significantly decreased by 13.7 per cent. A further reduction in mortality was also seen in combat casualties who were administered both TXA and cryoprecipitate8.
While the anti-fibrinolytic effects of TXA have been described and are being leveraged in the setting of trauma, the clinical effects on inflammation are less clear. In particular, the coagulation cascade is intricately associated with inflammatory pathways9, and the anti-inflammatory effects of TXA are poorly understood in the context of traumatic injury. Although it is presently uncertain through which mechanism TXA imparts a survival benefit in cases of trauma, it is known that TXA affects the fibrinolytic cascade as well as inflammatory pathways, namely through reduction in pro-inflammatory plasmin production10. As innate immunity and inflammation are critical to the host response to infection11, modulation of this response by TXA may influence the development of infectious complications following traumatic injury.
Infections subsequent to traumatic injury and severe hemorrhage are a significant cause of morbidity and mortality in combat wounded. Thus, the objective of our study was to examine the potential association between TXA administration and post-traumatic infection risk among wounded military personnel.
Patients and Methods
This analysis was performed in accordance with the STROBE Statement for observational studies (www.strobe-statement.org).
Study Population and Design
Data for this retrospective cohort analysis were collected through the Trauma Infectious Disease Outcomes Study (TIDOS), which is an ongoing observational cohort study of short- and long-term infectious complications among military personnel injured during deployment in support of recent conflicts in Iraq and Afghanistan12. Subjects were eligible for inclusion in the study population if they sustained injuries during deployment and were medically evacuated to Landstuhl Regional Medical Center (Germany) between 1 June 2009 and 31 December 2013, followed by a subsequent transition to one of three participating US military hospitals: Walter Reed Army Medical Center and National Naval Medical Center in the National Capital Region (Walter Reed National Military Medical Center after September 2011) and Brooke Army Medical Center in San Antonio, Texas (San Antonio Military Medical Center after September 2011).
Inclusion criteria and matched patients
For the analysis, the TXA recipients and non-TXA comparative patients were restricted to those who received blood products (i.e., packed red blood cells and/or whole blood) within 24 hours of injury and were matched using a composite injury severity score13 as a categorical variable (0–9, 10–15, 16–24, and ≥25). The composite injury severity score was calculated for each patient based on the top three maximum Abbreviated Injury Scale anatomical region values across all clinical facilities. Information on trauma history, injury patterns, TXA administration, and clinical characteristics on hospital admission (facilities in the combat zone, Landstuhl Regional Medical Center, and participating U.S. military hospitals) was obtained from the DoD Trauma Registry14. The TIDOS infectious disease module was utilized to gather data on infectious disease events and outcomes.
Ethics approval
The study was approved by the Infectious Disease Institutional Review Board of the Uniformed Services University of the Health Sciences in Bethesda, Maryland.
Outcomes
The outcomes were the rate of any infection occurring within 30 days after injury and time to development of infection associated with TXA.
Statistical Analysis
Statistical analysis was conducted using Stata® version 13 (StataCorp LP, College Station, TX). Comparisons of categorical variables were performed with the Chi-square and Fisher’s exact test, while Kruskal-Wallis test was used to test the difference between the continuous variables. Conditional logistic regression was used to analyze the association between potential risk factors and the development of any infection within 30 days of injury. Effect modification by TXA administration was assessed via stratification and interaction. Potential risk factors were assessed in a univariate analysis for association with risk of developing any infection within 30 days of injury Factors in the unadjusted univariate analysis that were significantly associated with increased risk of infection were selected in a stepwise fashion, corrected for, and used in a multivariable analysis to determine independent risk factors for infection. As a secondary analysis, a Cox proportional model was used to analyze the association between potential risk factors and the time from injury to first infection. The analysis was censored on the date of discharge from the U.S. military hospitals. In cases where the patient was transitioned through multiple U.S. military hospitals, the last date of discharge was used. Results are presented as hazard/odds ratios (HR/OR) with 95 per cent confidence intervals (CI). P-values of <0.050 are considered significant.
Results
Study Population
From 1 June 2009 to 31 December 2013, 2593 patients were admitted to Landstuhl Regional Medical Center and subsequently transferred to a participating military hospital in the U.S., of which 1124 received blood products within 24 hours of injury. Among this population, 335 patients (29.9 per cent of patients with blood product transfusion data) received TXA and were matched to 626 comparative patients who were not administered TXA. The majority of TXA recipients (236, 70.4 per cent) received TXA after the publication of the guidance document in August 2011. Patient demographics are presented in Table 1.
Table 1.
Demographic and injury characteristics of injured patients
| Characteristics | TXA Recipients (N=335) | non-TXA Recipients (N=626) | P value |
|---|---|---|---|
| Demographics, No. (per cent) | |||
| Age, median (IQR) | 24.2 (21.7, 27.2) | 24.2 (21.8, 28.7) | 0.029 |
| Male | 334 (99.7) | 616 (98.4) | 0.071 |
| Enlisteda | 304 (90.7) | 570 (91.1) | 0.567 |
| Injury Circumstance, No. (per cent) | |||
| Combat-related | 333 (99.4) | 620 (99.0) | 0.557 |
| Blast Injury | 294 (87.8) | 481 (76.8) | <0.001 |
| Composite ISS, median (IQR) | 33 (27, 45) | 33 (26, 43) | 0.135 |
| Composite ISS category, No. (percent) | 0.966 | ||
| 0–9 | 2 (0.6) | 4 (0.6) | |
| 10–15 | 11 (3.3) | 22 (3.5) | |
| 16–24 | 55 (16.4) | 110 (17.6) | |
| ≥25 | 267 (79.7) | 490 (78.3) | |
| Traumatic Amputations, No. (per cent) | 225 (67.2) | 223 (35.6) | <0.001 |
| Both lower and upper extremities | 36 (10.7) | 22 (3.5) | |
| Lower extremities only | 185 (55.2) | 193 (30.8) | |
| Upper extremities only | 4 (1.2) | 8 (1.3) | |
| Fractures, No. (per cent) | 0.002 | ||
| Both open and closed | 174 (51.9) | 252 (40.3) | |
| Closed only | 24 (7.2) | 46 (7.4) | |
| Open only | 77 (23.0) | 207 (33.1) | |
| Shock Index, median (IQR) | 1.1 (0.8, 1.4) | 0.9 (0.7, 1.2) | <0.001 |
| PRBCs transfused within 24 hours of injury, median units (IQR) | 17 (10, 26) | 7 (3, 15) | <0.001 |
| ICU Admission, No. (per cent)b | <0.001 | ||
| LRMC only | 53 (15.8) | 105 (16.8) | |
| U.S. MTFs ± LRMC | 260 (77.6) | 399 (63.7) | |
| Non-ICU | 22 (6.6) | 122 (19.5) | |
ICU – intensive care unit; ISS – injury severity score; IQR – interquartile range; LRMC – Landstuhl Regional Medical Center; MTFs – military treatment facilities; PRBC – packed red blood cells plus whole blood; TXA – tranexamic acid
Records with missing rank were omitted from the categorical analyses, accounting for a 14 records.
Admission to ICU during the first week of care at the respective sites
Infectious Complications
Among patients who received TXA, 255 (76 per cent) developed an infection compared to 366 (59 per cent) of the non-TXA comparative patients (P <0.001; Table 2). The duration following injury to first infection was also shorter for patients who received TXA compared to patients who did not (median of 5 versus 7 days, respectively; P=0.002). Furthermore, TXA patients had a greater number of infections per patient (upper interquartile range of 4 versus 3; P =0.002). For both groups, skin and soft-tissue infections contributed the highest proportion followed by miscellaneous (e.g., urinary tract infections and pneumonia) and bloodstream infections. Lastly, there was no significant difference in mortality between the two groups (10 [3.0 per cent] and 11 [1.8 per cent] for the TXA recipients and non-TXA comparative patients, respectively; P=0.215).
Table 2.
Rate of infections, infection types and associated outcomes for injured patients
| TXA Recipients (N=335) | non-TXA Recipients (N=626) | P value | |
|---|---|---|---|
| Time from injury to 1st infection, median days (IQR) | 5 (3, 9) | 7 (4, 11) | 0.002 |
| Patients with any infection during inpatient hospitalization, No. (per cent) | 255 (76.1) | 366 (58.5) | <0.001 |
| Infections per patient within 30 days of injury, median (IQR) | 2 (1, 4) | 2 (1, 3) | 0.002 |
| Types of Infections, No. (per cent)a | 0.001 | ||
| Bloodstream | 109 (13.0) | 137 (15.0) | |
| Central nervous system | 9 (1.1) | 21 (2.3) | |
| Sepsis | |||
| Sepsis | 22 (2.6) | 26 (2.8) | |
| Septic shock | 21 (2.5) | 9 (1.0) | |
| Skin and soft-tissue (SSTI) | 429 (51.2) | 394 (43.1) | |
| Osteomyelitis ± SSTI | 40 (4.8) | 73 (8.0) | |
| Miscellaneousb | 187 (22.3) | 219 (23.9) | |
| Undifferentiated | 20 (2.4) | 36 (3.9) | |
| Death, No. (per cent) | 10 (3.0) | 11 (1.8) | 0.215 |
IQR – interquartile range; TXA – tranexamic acid
Patients may have more than one type of infection. Percentages are calculated based upon total number of infections (TXA recipients: N=837; non-TXA comparative patients: N=914).
Examples of miscellaneous infections includes urinary tract infections, pneumonia, and Clostridium difficle infections
Risk for any infection within 30 days after injury
Blast injury mechanism, traumatic amputations, and fractures were associated with an increased risk of infection (Table 3). Other indicators of injury severity (e.g., admission to the intensive care unit, shock index, and ≥10 units of blood products transfused within 24 hours of injury) were also associated with infection risk. Furthermore, TXA use was associated with an increased risk of infection by an OR of 2.5.
Table 3.
Univariate analysis of factors associated with risk of infection
| Potential Risk Factor | Univariate Odds Ratio (95 per cent CI) | P value |
|---|---|---|
| Combat-related injury | 2.3 (0.2 – 24.4) | 0.489 |
| Age at injury (continuous) | 1.0 (1.0 – 1.0) | 0.259 |
| Blast injuries | 3.6 (2.2 – 5.7) | <0.001 |
| Any traumatic amputation | 2.9 (2.0 – 4.1) | <0.001 |
| Any fracture | 1.7 (1.0 – 2.7) | 0.033 |
| Admission to the ICUa | ||
| LRMC only | 2.1 (1.2 – 3.7) | 0.015 |
| U.S. MTF ± LRMC | 5.1 (3.0 – 8.6) | <0.001 |
| PRBC transfusions within 24 hours of injuryb | ||
| ≥10 units | 4.7 (3.2 – 7.0) | <0.001 |
| Shock indexc | ||
| 0.57–0.69 | 0.8 (0.4 – 1.7) | 0.512 |
| 0.69–0.82 | 1.7 (0.9 – 3.4) | 0.124 |
| >0.82 | 2.3 (1.3 – 4.1) | 0.004 |
| Tranexamic acid use | 2.5 (1.8 – 3.4) | <0.001 |
CI – confidence interval; ICU – intensive care unit; LRMC – Landstuhl Regional Medical Center; MTFs – military treatment facilities; PRBC – packed red blood cells plus whole blood
The reference value was non-ICU.
The reference value was <10 units.
The reference value was shock index of <0.57.
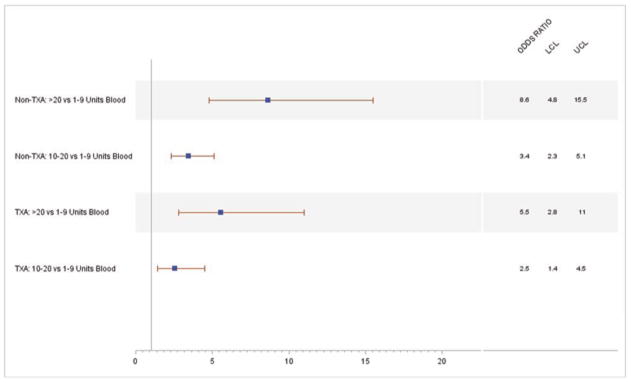
In a separate assessment, the univariate relationship of blood transfusion and the risk of infection within 30 days from injury was further stratified by TXA status. For non-TXA recipients, blood transfusion ≥10 units revealed an increased risk of infection with an OR of 4.7 (CI: 3.3–6.8) compared to <10 units of blood. Patients who received both TXA and ≥10 units of blood products transfused within 24 hours of injury had a lower, although still elevated, increased risk of infection within 30 days post injury (OR: 3.5; CI: 2.0–6.0). A continued downward trend in risk estimates was observed when stratifying categorical blood transfusion levels by TXA status (Figure 1). Among the non-TXA recipients, patients who received 10–20 units of blood products had a risk estimate of OR: 3.4 (CI: 2.3–5.1) when compared to those who received 1–9 units. In contrast, TXA recipients who received 10–20 units of blood products compared to 1–9 units had a risk estimate of OR: 2.5 (CI: 1.4–4.5). Similarly, non-TXA recipients who received >20 units of blood products compared to those who received 1–9 units had a nearly 9 fold increased risk (OR: 8.6; CI: 4.8–15.5) for infection within 30 days. In the presence of TXA, this comparison revealed a lower estimated risk of infection at 5.5 (CI: 2.8–11.0). When effect modification by TXA administration was assessed via stratification, and as an interaction term with blood volume in a multivariable model, the results were not statistically significantly different.
Figure 1.
Multivariable risk model for 30-day infectious complication
When adjusting for other factors, the independent risk factors for infection included blast injury mechanism, admission to the intensive care unit in the U.S., and receipt of ≥10 units of blood products within 24 hours of injury (Table 4). The adjusted association of TXA administration with the occurrence of any infection within 30 days post-injury was not statistically significant (OR: 1.3; CI: 0.8–1.9).
Table 4.
Multivariable analysis of factors associated with the risk of infection
| Risk Factor | Multivariable Odds Ratio (95 per cent CI) | P value |
|---|---|---|
| Blast injury | 2.6 (1.4 – 4.8) | 0.001 |
| Any traumatic amputation | 1.5 (0.9 – 2.4) | 0.124 |
| Any fracture | 1.6 (0.9 – 3.0) | 0.094 |
| Admission to the ICUa | ||
| LRMC only | 1.4 (0.7 – 2.7) | 0.320 |
| U.S. MTF ± LRMC | 3.3 (1.8 – 6.2) | <0.001 |
| PRBC transfusions within 24 hours of injuryb | ||
| ≥ 10 units | 2.4 (1.5 – 4.0) | <0.001 |
| Shock indexc | ||
| 0.57–0.69 | 0.5 (0.3 – 1.1) | 0.079 |
| 0.69–0.82 | – | – |
| >0.82 | – | – |
| Tranexamic acid use | 1.3 (0.8 – 1.9) | 0.243 |
CI – confidence interval; ICU – intensive care unit; LRMC – Landstuhl Regional Medical Center; MTFs – military treatment facilities; PRBC – packed red blood cells plus whole blood
The reference value was non-ICU.
The reference was transfusion of <10 units.
The reference value was shock index of <0.57.
Stratification of the multivariable model by TXA status revealed similar results from the univariate blood transfusion model. Specifically, holding other risk factors constant, non-TXA recipients had a statistically significantly increased risk of infection for blood transfusions ≥10 units compared to <10 units. The risk of infection for ≥10 units of blood transfusion, compared to <10 units, was also elevated for TXA recipients, but was no longer statistically significant.
Although the results in the stratified multivariable model were similar, this model was unable to be conditioned on a matched set due to the limitations of sample size and the lack of variation among the risk factors and outcome. Results for the stratified multivariable model were achieved using multivariable logistic regression with injury severity included as a risk factor in the model.
Multivariable analysis for time-to-infection
As a secondary analysis, risk factors were examined in a Cox proportional analysis with the endpoint of time following injury to first infection. Overall, the results were similar. Independent risk factors for infection included sustaining a traumatic amputation, admission to the Landstuhl Regional Medical Center or U.S. intensive care unit, and receipt of ≥10 units of blood products within 24 hours of injury (Table 5). As with the logistic analysis, administration of TXA was not a significant factor.
Table 5.
Multivariable Analysis of Factors Associated with the Time from Injury to First Infection
| Risk Factor | Multivariable Hazard Ratio (95 per cent CI) | P value |
|---|---|---|
| Any traumatic amputation | 1.64 (1.39 – 1.96) | <0.001 |
| Admission to the ICUa | ||
| LRMC only | 1.60 (1.12 – 2.30) | 0.011 |
| U.S. MTF ± LRMC | 2.73 (1.99 – 3.74) | <0.001 |
| PRBC transfusions within 24 hours of injuryb | ||
| ≥ 10 units | 1.64 (1.35 – 1.98) | <0.001 |
| Tranexamic acid use | 1.16 (0.98 – 1.38) | 0.078 |
CI – confidence interval; ICU – intensive care unit; LRMC – Landstuhl Regional Medical Center; MTFs – military treatment facilities; PRBC – packed red blood cells plus whole blood
The reference value was non-ICU.
The reference was transfusion of <10 units.
Discussion
The current study does not support the hypothesis of an increased risk for infectious complications associated with TXA when adjusted for other factors. Despite the fact that the unadjusted OR indicated that TXA use was a possible risk factor for infections following traumatic injury in our matched analysis, the adjusted analyses did not reveal an independent association with increased risk of infection.
Clinical trials have examined infections as a secondary outcome to TXA administration among surgical patients; however, less data from trauma patients are available2,15. One analysis of civilian trauma examined the use of TXA, but did not find a significant association with the occurrence of any infection within 30 days of injury16, which corresponds to the result of our analysis. Nonetheless, the population utilized in the civilian study was not comparable to our military cohort. Specifically, the majority of injuries sustained in the civilian study were blunt force trauma (93 per cent), which is different than the majority of military injuries, namely blast (70–74 per cent) and gunshot wounds (18–19 per cent)17,18.
The risk of infection are likely surrogates for severe injuries, including blast injury mechanism, large-volume blood product transfusions within 24 hours of injury, traumatic amputations, fractures, admission to intensive care unit, a high shock index, and use of TXA that follows with associated life threatening injuries. These observations are confirmed by previous work 19,20, which demonstrated increased risk of infection in combat casualties with blast injuries and high injury severity scores. Thus, it is not surprising that our data revealed similar associations between infection risk and injury severity, as well as an association with administration of TXA.
Although one may suspect that TXA’s attenuation of the immune and inflammatory response would increase susceptibility to infection, it may in fact result in the opposite. Several species of bacteria secrete substances that activate plasminogen. For instance, Group A Streptococci emit streptokinase, Staphylococcus aureus secretes staphylokinase, and Yersina pestis produces Pla protein21. Fibrin aggregation and thrombus formation may be a host’s local barrier to infection. Bacteria, through increasing plasmin production, attempt to break down that local barrier to gain access to the systemic vasculature21. On the other hand, TXA inhibits plasminogen, preventing plasmin formation and dissolution of local clots; thus, increasing local clot and host defenses. This may explain why TXA was not an independent risk factor of infections in wounded personnel.
The use of TXA in relation to traumatic injuries is aimed at correcting coagulopathy, decreasing bleeding, and ultimately reducing the need for allogenic blood product transfusions. In general, blood transfusions modulate the immune response to trauma22, and perioperative blood transfusions have been associated with increased infection rates in combat casualties23. While TXA usage was not independently associated with an increased risk of infection in our study, patients given TXA did receive a significantly larger volume of blood products compared to the non-TXA comparative group (median of 17 versus 7 units, respectively). As blood transfusions have been associated with wound healing disturbances24, it is not unexpected that receipt of large-volume of blood products (≥ 10 units) was significant for an increased risk of infection and with a shorter duration to first infection. The anti-inflammatory properties of TXA may add to impaired wound healing, which may explain the non-significant finding of reduced time-to-first infection.
Timing of TXA administration in traumatic injury may also provide a hypothesis for the absence of TXA influence in post-traumatic infection. Combat related infections predominantly occur between 3 and 15 days after injury12. According to the Joint Trauma System CPG, TXA is administered within 3 hours of injury6; however, information on the administration of TXA with regards to hours is not available through the Department of Defense Trauma Registry. In addition, the half-life of TXA ranges from 2 to 11 hours depending on formulation25,26 and the TXA-plasminogen complex is reversible26, so presumably after TXA is eliminated, its impact on formation of plasmin will cease. This may provide evidence as to why our data did not reveal an independent association of increased risk for infection with TXA as the occurrence of an infection may be more of an indirect consequence.
Our study has limitations as a retrospective observational cohort. First, there was the possibility of selection and/or misclassification bias within the study population. The possible selection bias stemmed from the clinician decision on whether to administer TXA as it is evident that not everyone who had blood transfusions received it. In addition, due to the often chaotic combat theater environment, it is possible that TXA use was not always properly documented and, thus, patients may have been misclassified as non-recipients when they did receive TXA. Quality assurance checks within the trauma registry were undertaken to best mitigate errors. In addition, although our dataset did not capture specific information on antibiotic prophylaxis, this has been examined previously and revealed approximately 75% adherence to clinical practice guideline recommendations regarding antibiotic prophylaxis to prevent combat-related infections27. Patients that received TXA in our study were more likely to have blast injuries, traumatic amputations, and overall high injury severity. All patients included in the analysis received blood following traumatic injuries; however, we chose to not match on blood volume. Although this could be considered a limitation, we wanted to evaluate the impact of blood product transfusions on the risk of infection. These devastating injury burdens make this population unique; therefore, our data may not be applicable to civilian trauma scenarios.
Acknowledgments
Funding Statement: This work (IDCRP-024) was supported by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health [Inter-Agency Agreement Y1-AI-5072], and the Department of the Navy under the Wounded, Ill, and Injured Program.
We are indebted to the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project. Drs. Ross and Lewis had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A portion of this manuscript has been accepted for presentation at the American College of Surgeons Clinical Congress meeting (October 4–8, 2015), Chicago, Illinois.
Disclaimer: The views expressed are those of the authors and do not necessarily reflect the official views or policies of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., National Institutes of Health or the Department of Health and Human Services, Brooke Army Medical Center, Walter Reed National Military Medical Center, the U.S. Army Medical Department, U.S. Army Office of the Surgeon General, the Department of Defense or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organization does not imply endorsement by the U.S. Government. A number of the co-authors are military service members (or employees of the U.S. Government). This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Sa-Ngasoongsong P, Channoom T, Kawinwonggowit V, Woratanarat P, Chanplakorn P, Wibulpolprasert B, et al. Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-hour clamp drain: a prospective triple-blinded randomized controlled trial. Orthop Rev (Pavia) 2011;3:e12. doi: 10.4081/or.2011.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Later AF, Bruggemans EF, Romijn FP, van Pelt J, Klautz RJ. A comparative study of the immune modulating properties of antifibrinolytics in cardiac surgery. Cytokine. 2013;61:438–444. doi: 10.1016/j.cyto.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Senturk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: a double-blind, placebo-controlled, randomized clinical trial. Arch Gynecol Obstet. 2013;287:641–645. doi: 10.1007/s00404-012-2624-8. [DOI] [PubMed] [Google Scholar]
- 5.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 6.Joint Theater Trauma System. [accessed 7 May 2015];Clinical Practice Guideline: Damage control resuscitation at Level IIb/III treatment facilities. http://www.usaisr.amedd.army.mil/cpgs/Damage%20Control%20Resuscitation%20-%201%20Feb%202013.pdf.
- 7.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg. 2012;147:113–119. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 8.Morrison JJ, Ross JD, Dubose JJ, Jansen JO, Midwinter MJ, Rasmussen TE. Association of cryoprecipitate and tranexamic acid with improved survival following wartime injury: findings from the MATTERs II Study. JAMA Surg. 2013;148:218–225. doi: 10.1001/jamasurg.2013.764. [DOI] [PubMed] [Google Scholar]
- 9.Petaja J. Inflammation and coagulation. An overview Thromb Res. 2011;127(Suppl 2):S34–S37. doi: 10.1016/S0049-3848(10)70153-5. [DOI] [PubMed] [Google Scholar]
- 10.Syrovets T, Lunov O, Simmet T. Plasmin as a proinflammatory cell activator. J Leukoc Biol. 2012;92:509–519. doi: 10.1189/jlb.0212056. [DOI] [PubMed] [Google Scholar]
- 11.Abbas A, Lichtman A. Basic Immunology: Functions and Disorders of the Immune System. 2. Saunders; Philadelphia: 2004. Introduction to the immune system; pp. 1–20. [Google Scholar]
- 12.Tribble DR, Conger NG, Fraser S, Gleeson TD, Wilkins K, Antonille T, et al. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. J Trauma. 2011;71(Suppl 1):S33–S42. doi: 10.1097/TA.0b013e318221162e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linn S. the injury severity score - Important and uses. Ann Epidemiol. 1995;5:440–446. doi: 10.1016/1047-2797(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 14.Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB. Trauma system development in a theater of war: experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2006;61:1366–1372. doi: 10.1097/01.ta.0000245894.78941.90. discussion 1372–1373. [DOI] [PubMed] [Google Scholar]
- 15.Oertli D, Laffer U, Haberthuer F, Kreuter U, Harder F. Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. Br J Surg. 1994;81:856–859. doi: 10.1002/bjs.1800810621. [DOI] [PubMed] [Google Scholar]
- 16.Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes. Ann Surg. 2015;261:390–394. doi: 10.1097/SLA.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 17.Schoenfeld AJ, Dunn JC, Bader JO, Belmond PJ., Jr The nature and extent of war injuries sustained by combat speciality personnel killed and wounded in Afghanistan and Iraq, 2003–2011. J Trauma. 2013;75:287–291. doi: 10.1097/TA.0b013e31829a0970. [DOI] [PubMed] [Google Scholar]
- 18.Belmont PJ, Jr, McCriskin BJ, Sieg RN, Burks R, Schoenfeld AJ. Combat wounds in Iraq and Afghanistan from 2005 to 2009. J Trauma. 2012;73:3–12. doi: 10.1097/TA.0b013e318250bfb4. [DOI] [PubMed] [Google Scholar]
- 19.Murray CK, Wilkins K, Molter NC, Li F, Yu L, Spott MA, et al. Infections complicating the care of combat casualties during Operations Iraqi Freedom and Enduring Freedom. J Trauma. 2011;71(Suppl 1):S62–S73. doi: 10.1097/TA.0b013e3182218c99. [DOI] [PubMed] [Google Scholar]
- 20.Murray CK, Wilkins K, Molter NC, Yun HC, Dubick MA, Spott MA, et al. Infections in combat casualties during Operations Iraqi and Enduring Freedom. J Trauma. 2009;66(Suppl 4):S138–S144. doi: 10.1097/TA.0b013e31819d894c. [DOI] [PubMed] [Google Scholar]
- 21.Chang WC, Shi GY, Chow YH, Chang LC, Hau JS, Lin MT, et al. Human plasmin induces a receptor-mediated arachidonate release coupled with G proteins in endothelial cells. Am J Physiol. 1993;264(2 Part 1):C271–C281. doi: 10.1152/ajpcell.1993.264.2.C271. [DOI] [PubMed] [Google Scholar]
- 22.Jackman RP, Utter GH, Muench MO, Heitman JW, Munz MM, Jackman RW, et al. Distinct roles of trauma and transfusion in induction of immune modulation after injury. Transfusion. 2012;52:2533–2550. doi: 10.1111/j.1537-2995.2012.03618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunne JR, Riddle MS, Danko J, Hayden R, Petersen K. Blood transfusion is associated with infection and increased resource utilization in combat casualties. Am Surg. 2006;72:619–625. discussion 625–626. [PubMed] [Google Scholar]
- 24.Weber EW, Slappendel R, Prins MH, van der Schaaf DB, Durieux ME, Strumper D. Perioperative blood transfusions and delayed wound healing after hip replacement surgery: effects on duration of hospitalization. Anesth Analg. 2005;100:1416–1421. doi: 10.1213/01.ANE.0000150610.44631.9D. [DOI] [PubMed] [Google Scholar]
- 25.Pilbrant A, Schannong M, Vessman J. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol. 1981;20:65–72. doi: 10.1007/BF00554669. [DOI] [PubMed] [Google Scholar]
- 26.Collen D, Tytgat G, Claeys H, Verstraete M, Wallen P. Metabolism of plasminogen in healthy subjects: effect of tranexamic acid. J Clin Invest. 1972;51:1310–1318. doi: 10.1172/JCI106927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd BA, Weintrob AC, Hinkle MK, Fortuna GR, Murray CK, Bradley W, et al. Adherence to published antimicrobial prophylaxis guidelines for wounded service members in the ongoing conflicts in Southwest Asia. Mil Med. 2014;179:324–328. doi: 10.7205/MILMED-D-13-00424. [DOI] [PMC free article] [PubMed] [Google Scholar]