Abstract
Background
The immune response in Alzheimer’s disease (AD) involves activation of microglia which may remove β-amyloid. However, overproduction of inflammatory compounds may exacerbate neural damage in Alzheimer’s disease. AD pathology accumulates years before diagnosis, yet the extent to which neuroinflammation is involved in the earliest disease stages is unknown.
Objective
To determine whether neuroinflammation exacerbates neural damage in preclinical AD.
Methods
We utilized cerebrospinal fluid (CSF) and magnetic resonance imaging collected in 192 asymptomatic late-middle-aged adults (mean age=60.98 years). Neuroinflammatory markers chitinase-3-like protein 1 (YKL-40) and monocyte chemoattractant protein-1 (MCP-1) in CSF were utilized as markers of neuroinflammation. Neural cell damage was assessed using CSF neurofilament light chain protein (NFL), CSF total tau (T-Tau), and neural microstructure assessed with diffusion tensor imaging (DTI). With regard to AD pathology, CSF Aβ42 and tau phosphorylated at threonine 181 (P-Tau181) were used as markers of amyloid and tau pathology, respectively. We hypothesized that higher YKL-40 and MCP-1 in the presence of AD pathology would be associated with higher NFL, T-Tau, and altered microstructure on DTI.
Results
Neuroinflammation was associated with markers of neural damage. Higher CSF YKL-40 was associated with both higher CSF NFL and T-Tau. Inflammation interacted with AD pathology, such that greater MCP-1 and lower Aβ42 was associated with altered microstructure in bilateral frontal and right temporal lobe and that greater MCP-1 and greater P-Tau181 was associated with altered microstructure in precuneus.
Conclusion
Inflammation may play a role in neural damage in preclinical AD.
Keywords: Alzheimer’s disease, Inflammation, Diffusion Tensor Imaging, Cerebrospinal Fluid Proteins
1. INTRODUCTION
Inflammation is a well-established feature of Alzheimer’s disease (AD) [1,2]. Several pro-inflammatory cytokines, chemokines, and complement proteins are elevated in the AD brain and both human and animal studies suggest inflammation may occur early in the AD process [3–7]. The underlying cause and temporal sequence of inflammation in relation to AD pathology is not entirely known, although it may occur in response to accumulation of amyloid pathology [8–10]. The inflammatory response in AD is largely mediated by microglia, which are activated to their proinflammatory state via a cytokine-signaling cascade [11]. While the immune response in AD may be necessary to clear amyloid, inflammatory mediators released by chronically active microglia may also contribute to neuronal damage by causing injury and toxicity to neurons [8,12]. In cell culture, microglia activated by β-amyloid have been shown to release reactive oxygen species, TNF-α, and several other molecules that are known to induce apoptosis [13,14]. While this phenomena has been well studied in vitro, the extent to which neuroinflammation may affect neural tissue in vivo is presently unclear, particularly in early disease stages. Understanding the effect of inflammation in preclinical AD could inform the timing of anti-inflammatory therapies, which could play a role in disease prevention.
The primary aim of the current study was to determine the relationship between microglial mediated neuroinflammation and neural damage in asymptomatic adults enriched for AD risk factors including parental family history of AD and apolipoprotein E ε4 (APOE ε4) genotype. Two cerebrospinal fluid (CSF) markers were utilized as proxies of neuroinflammation, chitinase-3-like protein 1 (YKL-40) and monocyte chemoattractant protein-1 (MCP-1). These two proteins are elevated in pre-clinical AD, mild cognitive impairment (MCI), and early AD [4,15–18]. MCP-1 is a potent chemokine, which in the brain serves primarily to attract microglial and peripheral immune cells to sites of inflammation and may also stimulate microglia to change from resting to activated morphology [19]. In turn, YKL-40 is produced by activated microglia, and has been previously associated with reduced cortical thickness and increased CSF total tau (T-Tau) protein in preclinical AD [20].
In order to assess neural damage, outcome markers were derived from CSF and magnetic resonance imaging (MRI). CSF markers included neurofilament light chain protein (NFL) and T-Tau. Both NFL and T-Tau are markers of structural proteins of neurons predominantly localized in large caliber myelinated and thin unmyelinated axons respectively. Because these proteins are released into the CSF from damaged or dying neurons, NFL and T-Tau have been used extensively as neural injury markers in a variety of neurodegenerative diseases [21–24]. MRI based outcomes included hippocampal volume derived from T1-weighted imaging, and whole-brain maps of tissue microstructure derived from diffusion tensor imaging (DTI). Medial temporal lobes are affected early in AD, and hippocampal volume is reduced in AD, MCI, and prodromal stages of the disease [25,26]. In turn, DTI is sensitive to diffusion of water molecules and has been used to assess neural microstructure in upwards of 10,000 human and animal studies. Of relevance to AD, DTI studies have shown neural damage across the spectrum of AD, MCI, and preclinical disease [27–38]. The two summary DTI measures used in this study were fractional anisotropy (FA), a measure of directional water diffusion that is highly sensitive to microstructural features including axonal density, diameter, and myelination, and mean diffusivity (MD), a measure of isotropic diffusion that is sensitive to cellular structure, necrosis, and edema [39,40]. In order to assess the relationship between neuroinflammation and neural microstructure across the brain, we performed a whole brain voxel wise analysis of the DTI maps. We hypothesized that greater neuroinflammation would be associated with higher CSF NFL and T-Tau, in addition to lower hippocampal volume, as well as lower FA and higher MD as shown on DTI.
Given that neuroinflammation may exacerbate neural injury in the presence of existing AD pathology, we also tested for interactions between neuroinflammation and AD pathology—specifically interactions with amyloid and neurofibrillary tangle pathology as measured in CSF. CSF Aβ42 and P-Tau181 are markers of amyloid plaque and neurofibrillary tangle pathology respectively, with lower Aβ42 indicating greater amyloid deposition and higher P-Tau181 indicating greater tangle pathology. High P-Tau181 and low Aβ42 in CSF have been associated with atrophy, cortical thinning, and altered white matter microstructure in preclinical AD [41–45], but the extent to which neuroinflammation may moderate these effects is unknown. Given the potential negative effects of inflammation on neural health, we hypothesized that the negative effects of β-amyloid and neurofibrillary tangle pathology on tissue microstructure would be greater among individuals with higher inflammation.
2. MATERIALS AND METHODS
Study procedures were approved by the University of Wisconsin Health Sciences Institutional Review Board and were in accordance with U.S. federal regulations. All participants provided written informed consent.
2.1 Participants
Participants were asymptomatic late-middle-aged adults (N = 192, mean age = 60.98 years, SD = 7.55, range = 46-85 years) from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) study and the Wisconsin Alzheimer’s Disease Research Center (ADRC) clinical core who underwent brain imaging and lumbar puncture as part of studies on memory, aging, and preclinical AD. Participants were selected from these larger studies based on available DTI and CSF data, which only a subset of participants have available. Both the WRAP and Wisconsin ADRC cohorts comprise well-characterized and longitudinally followed participants who are either positive or negative for parental history of AD. Positive parental family history of AD classification was defined as having one or both parents with AD as determined by a validated interview [46] or autopsy-confirmed or probable AD as outlined by research criteria [47,48], and reviewed by a multidisciplinary diagnostic consensus panel. Detailed medical history and phone interviews were conducted to confirm family history negative participants. Absence of family history of AD required that the participant’s father survive to at least age 70 years and the mother to age 75 years without diagnosis of dementia or cognitive deterioration. Family history was classified as a binary variable. APOE ε4 genotype was performed on a non-fasting blood sample collected at baseline, using standard PCR and DNA sequencing techniques. DNA extracted from whole blood was genotyped with use of a homogeneous Fluorescent Resonance Energy Transfer technology coupled to competitive allele specific PCR (LGC Genomics; Beverly, MA). Genotyping also was performed by NCRAD. There was 100% concordance for APOE genotype between these analyses. Participants were categorized using a binary variable as an APOE ε4 carrier or non-carrier.
General inclusion criteria consisted of: 1) normal cognitive function determined by comprehensive neuropsychological evaluation and consensus review, 2) negative history of psychiatric or neurological disease or untreated depression, and 3) no history of head trauma. While individuals with major medical illness (such as current malignancy and major organ failure) were not included, 12 of the participants had a diagnosis of Type 2 Diabetes. Given that there was no significant difference in levels of neuroinflammation between these participants and participants without diabetes, they remained in the analysis. Participants were also required to have previously undergone MRI and lumbar puncture for CSF assays.
Participants were excluded from the analysis based on one or more of the following: a) missing CSF data due to inadequate CSF collection for assays (n=1), b) unsatisfactory DTI data quality after processing, such as major susceptibility artifact (n=13), c) an incomplete or failed DTI acquisition or transfer (n=11), d) or abnormal radiological report on study MRI (n=31). While not all abnormalities noted on the radiology report required clinical follow-up, we took a conservative approach and removed participants with abnormalities that would affect data processing or interpretation of DTI findings in cerebral white matter (for example, noted presence of small vessel disease or possible history of infection). Of 248 participants identified for possible inclusion in the study, 192 participants met criteria for inclusion. Overall the sample was largely female, Caucasian, well educated, had a parental family history of AD or were APOE ε4 carriers (see Table 1).
Table 1.
Participant Demographics
| Age in years[mean, (SD)] | 60.98 (7.55) |
| Sex [n (%)] | |
| Male | 55 (28.9) |
| Female | 137 (71.3) |
| Ethnicity [n (%)] | |
| Caucasian | 187 (97.4) |
| African American/Black | 2 (1.0) |
| Hispanic/Latino | 0 |
| Other/unknown | 3 (1.5) |
| APOE ε4 status [n (%)] | |
| Carriers | 78 (41) |
| Non-Carriers | 114 (59) |
| Family history [n (%)] | |
| Positive | 145 (75.5) |
| Negative | 47 (24.5) |
| Unknown | 0 |
| Education in years [mean, (SD)] | 16.26 (2.51) |
| RAVLT total | 50.43 (8.65) |
| RAVLT delay | 10.05 (3.07) |
| MMSE | 29.34 (0.886) |
| Category Fluency (animals) | 22.81 (5.39) |
| Trail Making Test A (seconds) | 25.23 (8.56) |
| Trail Making Test B (seconds) | 59.79 (22.23) |
| YKL-40 (ng/mL) | 142760.8 (52093.7) |
| MCP-1 (ng/mL) | 542.8 (135.5) |
| Aβ42 (ng/mL) | 737.7 (212.4) |
| T-Tau (ng/mL) | 307.3 (126.9) |
| P-Tau181 (ng/mL) | 42.2 (15.1) |
| NFL (ng/mL) | 637.2 (316.4) |
| WMHr | 0.014(.0015) |
APOE ε4: Apolipoprotein E ε4; SD: standard deviation; RAVLT: Rey Auditory Verbal Learning Test; MMSE: Mini Mental Status Exam; MCP-1: monocyte chemoattractant protein; Aβ42: 42 residue amyloid beta peptide; T-Tau: Total tau; P-Tau: phosphorylated tau; NFL: neurofilament light chain protein; WMHr: white matter hyperintensity ratio.
2.2 CSF collection and analysis
CSF was collected with a Sprotte 25-or 24-gauge spinal needle at the L3/4 or L4/5 using gentle extraction into polypropylene syringes. Samples were collected in the morning after a 12h fast. Approximately 22mL of CSF were inverted to avoid gradient, gently mixed and centrifuged at 2000g for 10 minutes. Supernatants were frozen in 0.5mL aliquots in polypropylene tubes and stored at −80°C. Samples were analyzed for P-Tau181, T-Tau and Aβ42 using commercially available enzyme-linked immunosorbent assay (ELISA) methods (INNOTEST assays, Fujiurebio, Ghent Belgium) as described previously in detail [49]. MCP-1 levels in CSF were measured using the Meso Scale Discovery technique (MSD Human MCP-1; Meso Scale Discovery, Gaithersburg, MD, USA), and YKL-40 was determined using a sandwich enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, Minn., USA). Likewise, CSF NFL was measured with a sandwich ELISA method (NF-light ELISA kit, UmanDiagnostics AB, Umeå, Sweden). Board-certified laboratory technicians who were blinded to clinical diagnosis performed all analyses on one occasion. All samples were analyzed according to protocols approved by the Swedish Board of Accreditation and Conformity Assessment (SWEDAC) using one batch of reagents (intra-assay coefficients of variation <10%). Two participants had P-Tau181 or T-Tau levels below the detectible threshold of our assays. They were included in the analysis but were assigned the minimum detectible value for the particular marker (90.8ng/mL for T-Tau, 15.6ng/mL for P-Tau181).
2.3 Magnetic Resonance Imaging
The average delay between MRI and lumbar puncture was 6.57 days (SD 19.68). Participants underwent scanning on one of two identical General Electric 3.0 Tesla Discovery MR750 MRI systems with 8 channel head coils and parallel imaging (ASSET). DTI was acquired using a diffusion-weighted, spin-echo, single-shot, echo planar imaging (EPI) pulse sequence in 40 encoding directions with b = 1300 s/mm2, and eight non-diffusion weighted (b = 0) reference images. The cerebrum was covered using contiguous 2.5 mm thick axial slices, FOV = 24 cm, TR = 8000 ms, TE = 67.8 ms, matrix = 96 × 96, resulting in isotropic 2.5 mm3 voxels. High order shimming was performed prior to the DTI acquisition to optimize the homogeneity of the magnetic field across the brain and to minimize EPI distortions. A T1-weighted volume was acquired in the axial plane with a 3D fast spoiled gradient-echo sequence using the following parameters: inversion time (TI) = 450 ms; repetition time (TR) = 8.1 ms; echo time (TE) = 3.2 ms; flip angle = 12°; acquisition matrix = 256 × 256 mm, field of view (FOV) = 256 mm; slice thickness = 1.0 mm. 3D T2-weighted fluid attenuated inversion recovery (FLAIR) scans were acquired in the sagittal plane using the following parameters: TI = 1868 ms; TR = 6000 ms; TE = 123 ms; flip angle = 90°; acquisition matrix = 256 × 256mm, FOV = 256 mm; slice thickness = 2.0 mm, no gap, yielding a voxel resolution of 1 mm × 1 mm × 2 mm.
2.4 MRI Processing
DTI was processed using a customized pipeline, as described in detail in Adluru et al. [50]. Briefly, images underwent eddy current correction, field inhomogeneity correction, and skull stripping using tools from the FMRIB Software Library (FSL) (http://www.fmrib.ox.ac.uk/fsl/). Tensor fitting was performed using the University College London, Camino Diffusion MRI Toolkit (http://cmic.cs.ucl.ac.uk/camino/). Tensor-based registration was implemented utilizing Diffusion Toolkit for DTI analysis (DTI-TK, http://dti-tk.sourceforge.net/pmwiki/pmwiki.php), whereby images were registered to a population specific template created with DTI-TK. FA and MD maps were calculated using DTI-TK. Given that anisotropic water diffusion is less interpretable in gray matter compared to white matter, FA analyses were limited to white matter. A white matter mask was constructed by thresholding the FA template to values > 0.15. MD analyses were conducted in both gray and white matter, using a brain mask constructed by thresholding the population based MD template image to 0.10. Both masks were visually inspected to ensure inclusion of tissue of interest. The MD template was solely used for constructing the brain mask and as an underlay for results (i.e. not used for normalization). In order to control for cerebrovascular disease and ischemic lesion burden, total white matter hyperintensity (WMH) volume for each participant was determined using Lesion Segmentation Tool (version 1.2.2) implemented in SPM8 [51]. The T1-weighted and T2FLAIR images were processed according to the method detailed in Birdsill et al. [52]. WMH was adjusted for variability in head size by dividing total WMH by intracranial volume to yield a WMH ratio (WMHr). Intracranial volume was calculated using the “reverse brain masking” method detailed in Keihaninejad et al. [53].
Hippocampal volume was calculated using FSL’s (FMRIB Software Library) (http://www.fmrib.ox.ac.uk/fsl/) FIRST tool [54]. The shape/appearance models used in FIRST are constructed from manually segmented images provided by the Center for Morphometric Analysis (CMA), MGH, Boston. Hippocampal volumes were corrected for intracranial volume and expressed as a ratio of hippocampal volume to total intracranial volume.
2.5 Statistical Analysis
2.5.1 Effect of Neuroinflammation on CSF markers of neural injury
To test the effect of neuroinflammation and AD pathology on axonal damage, several linear regression models were implemented in SPSS (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp), where YKL-40, MCP-1, P-Tau181, and Aβ42 were the predictor variables, and CSF T-Tau and NFL were the dependent variables, controlling for age, sex, family history of AD, APOE ε4, and WMHr. We also tested for interactions between AD pathology (P-Tau181, and Aβ42) and neuroinflammation (YKL-40 and MCP-1) on CSF T-Tau and NFL. NFL was log transformed to correct for a slight right skew. All other variables were approximately normally distributed. To account for type 1 error caused by multiple comparisons, a Bonferroni correction was implemented (the α level of .05 was divided by the number of contrasts performed, i.e. 16), yielding a corrected alpha of α=0.0031.
2.5.2 Effect of Neuroinflammation on Hippocampal volume
To test the effect of neuroinflammation and AD pathology on hippocampal volume, linear regression models were implemented in SPSS where YKL-40, MCP-1, P-Tau181, and Aβ42 were the predictor variables and left and right hippocampal volume were the dependent variables, covarying for age, sex, family history, APOE ε4 status, and WMHr. Additionally we tested for interactions of pathology (Aβ42 and P-Tau181) and neuroinflammation (YKL-40 and MCP-1) on left and right hippocampal volume. To account for type 1 error caused by multiple comparisons, a Bonferroni correction was implemented (the α level of .05 was divided by the number of contrasts performed, i.e 16), yielding a corrected alpha of α=0.0031.
2.5.3 Effect of Neuroinflammation on microstructure: Voxel-wise DTI analysis
To test the effect of neuroinflammation and AD pathology on tissue microstructure, voxel-wise analyses of FA and MD maps were implemented using statistical parametric mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm/). Multiple regression models tested the main effects of YKL-40 and MCP-1 on FA and MD across the whole brain, in addition to testing for interactions between YKL-40 and MCP-1 and CSF Aβ42 and P-Tau181. Models used age, sex, family history, APOE ε4 status, and WMHr as covariates. Results were determined using a cluster extent based thresholding approach, with a primary voxel level threshold of p < 0.005. This method offers increased sensitivity to spatially extended signals with moderate effect size [55]. To account for spatial non-stationarity of the DTI maps, we used the random field theory based approach documented in Worsley et al. [56]. The results were further corrected using the Bonferroni method (.05/35), yielding a corrected α of 0.0014. Results that survive Bonferroni correction are indicated with asterisks in Table 2.
Table 2.
DTI results
| A: Main effects | Anatomical location | k | Pea k T |
Cluster level P value |
|---|---|---|---|---|
| P-Tau181 | ||||
| Fractional Anisotropy | thalamus (L,R) | 567 | 5.07 | 0.015 |
| Mean diffusivity | inferior frontal gyrus (L) | 211 | 4.00 | 0.031 |
| NFL | ||||
| Mean Diffusivity | cuneus (L) | 689 5 |
5.51 | <0.001* |
| superior parietal lobule (R) | 608 5 |
4.80 | <0.001* | |
| middle frontal gyrus (R) | 252 1 |
4.17 | <0.001* | |
| pre-central gyrus (L) | 989 | 3.96 | 0.025 | |
| hippocampus (R) | 987 | 4.76 | 0.029 | |
| T-Tau | ||||
| Fractional Anisotropy | thalamus (L,R) | 433 | 4.89 | 0.021 |
| Mean Diffusivity | precuneus (L,R) | 144 94 |
5.54 | <0.001* |
| cuneus (L) | 798 | 4.34 | 0.008 | |
| fusiform (R) | 437 | 4.31 | 0.018 | |
| Inferior orbitofrontal (L) | 222 | 4.43 | 0.027 |
| B: Inflammation × Alzheimer's marker interactions |
Anatomical location | k | Pea k T |
Cluster level P value |
|---|---|---|---|---|
| Aβ42x MCP1 | ||||
| Mean Diffusivity | inferior frontal gyrus (R) | 772 | 4.52 | 0.002 |
| inferior temporal gyrus (R) | 329 | 3.46 | 0.027 | |
| fusiform (L) | 357 | 4.20 | 0.029 | |
| Inferior frontal gyrus (L) | 139 | 3.71 | 0.041 | |
| P-Tau181 × YKL-40 | ||||
| Mean Diffusivity | precuneus (L) | 617 | 3.46 | 0.022 |
| P-Tau181 × MCP-1 | ||||
| Fractional Anisotropy | post central gyrus wm (R) | 671 | 4.94 | 0.0018 |
| anterior corona radiata (R) | 395 | 4.37 | 0.013 | |
| anterior internal capsule (L) | 557 | 3.61 | 0.037 | |
| Mean Diffusivity | precuneus (L) | 520 | 3.92 | 0.034 |
| C: Interactions with neural injury |
Anatomical location | k | Pea k T |
Cluster level Pvalue |
|---|---|---|---|---|
| P-Tau181 × NFL | ||||
| Mean Diffusivity | precuneus (R) | 837 | 3.93 | 0.005 |
| YKL-40 × NFL | ||||
| Fractional Anisotropy | internal/external capsule (R) | 777 | 3.59 | 0.028 |
| Mean Diffusivity | angular gyrus (R) | 1353 | 6.77 | <0.001* |
| YKL-40 × T-Tau | ||||
| Fractional Anisotropy | internal/external capsule*, cerebral peduncle (L) |
103 5 |
4.16 | 0.008 |
| cerebral peduncle*, internal/external capsule (R) |
688 | 3.79 | 0.025 | |
| Mean Diffusivity | cerebellum (L) | 256 7 |
4.06 | 0.009 |
| superior frontal gyrus (L) | 113 3 |
3.60 | 0.022 | |
| P-Tau181 × T-Tau | ||||
| Mean Diffusivity | cuneus (L,R) | 757 | 4.23 | 0.012 |
R: right; L: left; MCP-1: monocyte chemoattractant protein; Aβ42: 42 residue amyloid beta peptide; T-Tau: Total tau; P-Tau: phosphorylated tau; NFL: neurofilament light chain protein; WM: white matter;
cluster peak location,
survives Bonferroni correction (α=0.05/36 contrasts, corrected α=0.0014)
Relationship between CSF markers of neural injury and regional microstructure: Voxel-wise DTI analysis
While primary analyses centered on the effects of neuroinflammation and AD pathology, it was of secondary interest to test whether the CSF and imaging outcome markers of neural injury were associated with each other. Voxel-wise regression models were utilized to assess whether NFL and T-Tau were associated with FA and MD. Additionally, interactions of NFL and T-Tau with YKL-40, MCP-1, Aβ42, and P-Tau181 were tested. Age, sex, family history, APOE ε4 status, and WMHr were included as covariates. NFL was log10 transformed to correct for slight right skew. As in the preceding analysis, significant results were determined using a cluster extent based thresholding approach, with a primary voxel level threshold of p < 0.005., further corrected using the Bonferroni method (α =0.0014). Results that survive Bonferroni correction are indicated with asterisks in Table 2.
3. RESULTS
Effect of Neuroinflammation on CSF markers of neural injury
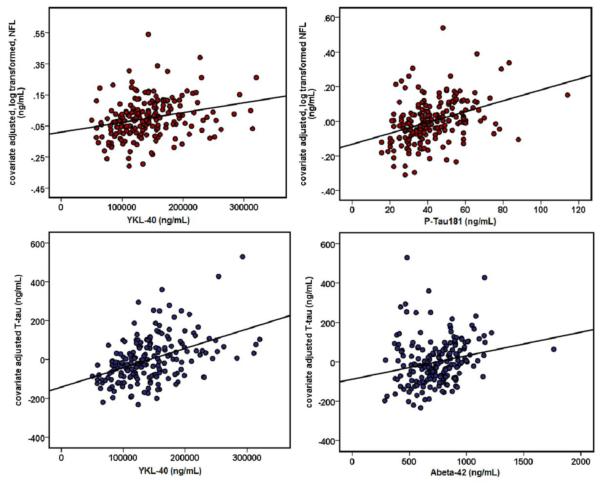
Neuroinflammation was associated with neural injury. The linear regression analysis implemented in SPSS revealed a significant effect of YKL-40 on CSF NFL and T-Tau concentration. As predicted, higher YKL-40 was associated with higher CSF NFL (β=0.254, p<0.001) as well as higher CSF T-Tau (β=0.553, p<0.001)(Figure 1), suggesting degeneration of both large caliber and thin unmyelinated axons. MCP-1 was not associated with CSF NFL or T-Tau levels. Markers of AD pathology were also associated with neural injury. Linear regression analyses in SPSS revealed that higher Aβ42 was associated with higher T-Tau (β=0.244, p=0.001)(Figure 1), although analysis with NFL did not survive Bonferroni correction (β=0.127, p=0.032). Additionally, higher P-Tau181 was associated with greater concentration of CSF NFL (β=0.256, p<0.001) (Figure 1) and T-Tau (β=.869, p<0.001). Neuroinflammation did not interact with AD pathology to have an effect on neural injury, i.e. CSF NFL or T-Tau.
Figure 1. Relationship between neuroinflammation, Alzheimer’s pathology, and CSF markers of neural injury.
Higher YKL-40 and higher P-Tau181 were associated with higher CSF NFL (β=0.254, p<0.001**, and β=0.256, p<0.001**, respectively). Higher YKL-40 and Aβ42 were associated with higher CSF T-Tau (β=0.553, p<0.001** and β=0.244, p=.001**, respectively). NFL was log10 transformed to correct for slight right skew. In the scatterplots, transformed NFL and T-Tau were adjusted for all covariates (age, sex, WMHr, APOE genotype status, and family history status) and plotted against predictor variables.
Effect of Neuroinflammation on Hippocampal volume
No significant effects of MCP-1, YKL-40, Aβ42 or P-Tau181 on hippocampal volume were found. No significant interactions of neuroinflammation and Alzheimer’s pathology on hippocampal volume were found.
Effect of Neuroinflammation on microstructure: Voxel-wise DTI analysis
Cluster level results of the voxel-wise DTI analysis are presented in Table 2, and are organized into sub-tables by category (main effects, interactions with AD pathology, and interactions with neural injury markers), and are further divided into rows based on predictor variables and outcome measures.
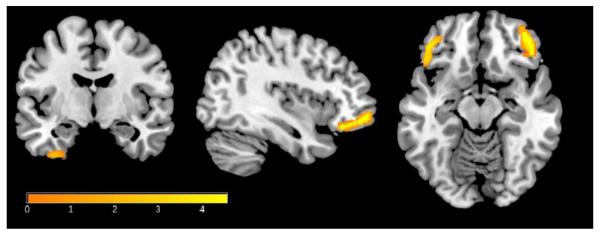
There were no main effects of neuroinflammation on tissue microstructure as indexed by FA and MD (Table 2A); however, neuroinflammation interacted with AD pathology to significantly impact tissue microstructure. Interactions were observed between MCP-1 and Aβ42 on MD in the bilateral inferior frontal lobe, right inferior temporal lobe, and left fusiform gyrus (Figure 2, Table 2B). The relationship was such that among participants with lower CSF Aβ42 (suggesting cerebral amyloid deposition), higher MCP-1 was associated with higher MD, suggestive of tissue damage. The interaction between YKL-40 and Aβ42 did not reach significance.
Figure 2. MCP-1 × Aβ42 interaction on MD.
Higher CSF MCP-1 and lower Aβ42 were associated with increased mean diffusivity in several brain regions including the inferior frontal lobe, right fusiform gyrus, and inferior temporal gyri. Results were corrected for non-stationarity using a random field theory based approach with a voxel-level threshold of p<.005 and a cluster level threshold of p<.05. Variations in the color map correspond to the size of the t-statistic. MD results are overlaid on a T1-weighted image for anatomical detail. Right=Left.
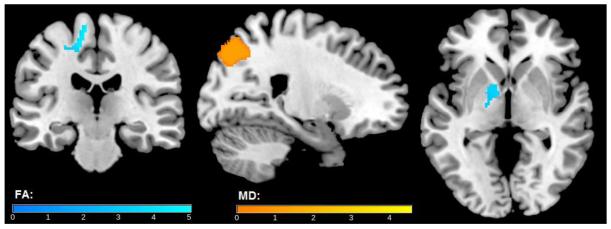
A voxel-wise analysis of FA also revealed an interaction between MCP-1 and CSF P-Tau181 (Table 2B). Specifically, among participants with higher CSF P-Tau181, higher MCP-1 was associated with higher FA in the right post-central gyrus white matter, right corona radiata, and left anterior internal capsule. In the corresponding MD analysis, this interaction was noted in the left precuneus. In both of these interactions, greater neuroinflammation (high MCP-1) in the presence of greater tau pathology (high CSF P-Tau181) was associated with both higher FA and MD. An interaction was also observed between YKL-40 and P-Tau181 in the left precuneus (Figure 3, Table 2B), where higher YKL-40 and higher P-Tau181 were associated with greater MD. Brain regions where main effects of NFL, T-Tau and P-Tau181 were observed differed from the regions where interactions were noted. For example, as is detailed under main effects in Table 2A, higher CSF P-Tau181 was associated with higher FA in bilateral thalamus, and higher MD in the right orbitofrontal gyrus and bilateral post-central gyrus.
Figure 3. MCP-2 × P-Tau181 interaction on FA (cool) and MD (warm).
Higher CSF MCP-1 and Higher P-Tau181 were associated with higher FA in right post central gyrus white matter, right anterior corona radiata, left internal capsule and with higher MD in the left precuneus. Results were corrected for non-stationarity using a random field theory based approach with a voxel level threshold of p<0.005 and a cluster level threshold of p<0.05. Blue clusters indicate FA results, while orange clusters indicate MD results. Variations in color map correspond to the size of the t-statistic. Results are overlaid on a T1-weighted image for anatomical detail. Right=Left.
Relationship between CSF markers of neural injury and regional microstructure: Voxel-wise DTI analysis
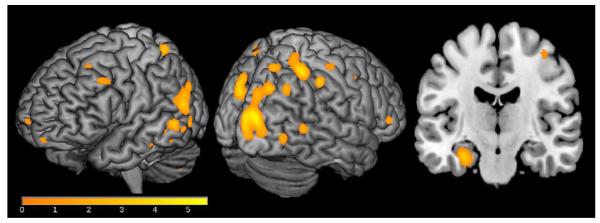
Given that our sample was characterized on NFL and T-Tau, proteins that are primarily localized to axons, we conducted voxel-wise analyses to determine whether CSF NFL and T-Tau levels alone, or in combination with neuroinflammation were associated with regional gray and white matter microstructure. The voxel-wise analyses revealed widespread significant associations between CSF NFL and MD across lateral and medial temporal, parietal, and frontal lobes (Figure 4, Table 2A) and between CSF T-Tau and FA in the bilateral thalamus and MD in bilateral precuneus, left cuneus, right fusiform gyrus, and left orbitofrontal gyrus. As detailed in Table 2C, YKL-40 showed a significant interaction with CSF NFL on FA in right internal and external capsule, in addition to effects on MD, with a peak association in right angular gyrus. Here, higher YKL-40 and higher NFL were associated with greater FA and MD. Finally, YKL-40 interacted with CSF T-Tau on FA in the bilateral internal capsule, and on MD in the left cerebellum and left superior frontal gyrus. In both cases, higher NFL or T-Tau along with greater inflammation was associated with higher FA and MD. CSF NFL also interacted with P-Tau181, where elevated P-Tau181 combined with elevated CSF NFL was associated with higher MD in the right precuneus (Table 2C). Similarly, CSF T-Tau also interacted with P-Tau181 in the cuneus, whereby greater neural damage indicated by higher NFL or T-Tau and higher P-Tau181 were associated with higher MD. In contrast to the YKL40 effects, MCP-1 did not interact with T-Tau or NFL on either DTI measure.
Figure 4. Relationship between CSF NFL and MD.
Higher CSF NFL was associated with greater MD in a variety of brain regions related to AD, including bilateral precuneus, right superior temporal gyrus, right hippocampus, and right superior parietal lobule. Results were corrected for non-stationarity using a random field theory based approach with a voxel level threshold of p<.005 and a cluster level threshold of p<.05. Variations in color map correspond to the size of the t-statistic. MD results are overlaid on a T1-weighted image for anatomical detail. Right=Left.
4. DISCUSSION
Pathological changes associated with AD appear years before the onset of clinically relevant symptoms [57]. Prospective treatments or therapies may be most beneficial in the preclinical stage, before extensive and irreversible neural damage. Microglial activation plays a role in clearance of cellular debris and β-amyloid; however, the associated inflammatory cascade may also contribute to neural damage [12]. In the present study, we assessed for the first time, whether neuroinflammation is associated with preclinical neural damage, particularly in the presence of amyloid and neurofibrillary tangle pathology. As predicted, indicators of a neuroinflammatory response both alone, and in concert with AD pathology, were associated with neural damage. The results of this study suggest that the association between neuroinflammation and neural damage is present in the preclinical stages of AD.
Higher MCP-1 combined with lower Aβ42 was associated with altered tissue microstructure in brain regions known to be affected by amyloid pathology, including bilateral frontal cortex, and lateral temporal lobe. MCP-1 is a chemokine expressed in response to inflammatory signals, and primarily serves to attract peripheral immune cells (monocytes) and microglia to sites of inflammation. Cellular studies show that human monocytes and activated microglia produce MCP-1 in response to Aβ fragments and larger amyloid plaques in order to recruit additional immune cells [58,59]. In humans, MCP-1 is increased in MCI and AD and is predictive of cognitive decline [4,60]. Given that effects of Aβ42 on tissue microstructure were only observed among participants with higher MCP-1, the results suggest that elevated inflammation could underlie the tissue alterations. Indeed, microglial activation along with cell death and degradation of synaptic connections are a distinguishing feature of the post mortem AD brain, compared to cognitively intact controls with comparable levels of plaque and tangle pathology [6].
In addition to relationships with amyloid pathology, we also observed interactions between inflammation and neurofibrillary tangle pathology—specifically a significant interaction between MCP-1 and P-Tau181. The combination of high CSF P-Tau181 and high CSF MCP-1 was associated with both higher FA in right post-central gyrus white matter and bilateral corona radiate, and higher MD in the left precuneus, suggesting cell loss and potentially selective degeneration of axons in these regions. Lower FA in white matter fiber tracts typically suggests microstructural damage, although axonopathy may also be associated with higher FA due to selective degeneration of axons, particularly in areas of crossing fibers [61]. No studies to date have specifically linked MCP-1 expression to tau reactivity on a cellular level; however, histological studies have found that activated microglia are regionally correlated with extracellular neurofibrillary tangles (NFTs) and dystrophic tangle-bearing neurons even in the early stages of tangle formation [62,63].
In addition to interacting with AD pathology, neuroinflammation alone was also associated with markers of neural damage. Elevated neuroinflammation as shown by higher CSF YKL-40, was associated with higher CSF levels of NFL, a structural protein primarily localized to axons, suggesting axonal damage. YKL-40, a 40kDa glycoprotein, is expressed by activated microglia and is elevated with increasing age [64] and at all stages of AD [15,17,18]. Axonal degeneration has been hypothesized to be an early feature of the AD process [65], although the specific effect of neuroinflammation on axonal degeneration is incompletely characterized [66]. Microglia that have been primed by an inflammatory trigger such as Aβ, neural injury, or aging may respond by releasing a variety of neurotoxic factors, which further damage surrounding cells [12]. Given that the effects of YKL-40 were observed regardless of Aβ42 or T-Tau levels, the association of YKL-40 and neural damage in this study could reflect microglial activation in response to age-associated brain changes [64] , or other CNS disorders.
Compared to volumetric imaging, DTI may provide unique insights into early pathological changes. In this study none of the CSF measures were significantly associated with hippocampal volume; however several, especially T-Tau and NFL were associated with the DTI-derived measures of microstructure. Amyloid plaques and neurofibrillary tangle pathology are associated with disruption of cytoskeletal equilibrium, synaptic dysfunction, and axonal degeneration [67–69] while total tau and NFL protein measured in CSF indicate damage to thin unmyelinated and large caliber axons, as damaged cells “leak” tau or NFL protein [70,71]. In this study, higher CSF T-Tau was associated with higher FA and MD in brain regions that are known to undergo pathological changes in early stages of AD including the thalamus, precuneus, cuneus and cingulum. Further, we found a robust relationship between CSF levels of NFL, and MD, involving several brain regions affected in AD, including hippocampus. While higher FA is generally thought of as indicating more intact white matter, several studies suggest that the interpretation of FA may be more complicated. Ryan et al. (2003) reported higher FA among asymptomatic carriers of the PSEN1 mutation that causes autosomal dominant AD, likely due to early and selective loss of axonal fibers [72]. Our group has also found higher FA in select white matter tracts among individuals with parental family history of AD, in addition to higher FA among individuals with greater amyloid deposition [50,73]. Associations between higher T-Tau and higher FA in this study may suggest selective fiber loss. Another study involving 7T DTI imaging found increased FA in the thalamus, left hippocampus, corpus callosum, anterior commissure, and internal capsule in aged APP/presenilin 1 transgenic mice. These increases in FA were associated with presence of injured neurons, swollen neuronal processes, amyloid plaques, and dystrophic axons [74], features that may explain findings of higher FA in our sample. Overall, these findings suggest axonal loss is an early and measurable feature of preclinical AD.
Amyloid in combination with neuroinflammation was associated with neural injury; however, CSF Aβ42 alone did not show a significant association with DTI measures. While amyloid deposition is known to be an early feature of AD, it is thought that the toxic effects on neurons only appear after significant levels of amyloid burden are reached [75]. Another possibility for the lack of direct effect of Aβ42 may be due to a dynamic fluctuation in CSF Aβ42 that has been observed in preclinical AD. While CSF Aβ42 generally declines during the progression of AD, studies in individuals with PSEN1 mutation show elevated CSF Aβ42 levels compared to non-carriers in the asymptomatic stage of the disease [76]. The CSF analyses in this study showed that higher CSF Aβ42 was associated with higher CSF T-Tau, suggesting greater axonal injury in individuals with higher CSF Aβ42. Interestingly, in a recent study by Maia et al. [77] researchers noted that in several APP transgenic mice models CSF Aβ42 and Aβ40 levels showed a slight rise just before the onset of Aβ deposition. CSF Aβ42 levels later dropped in the mice as plaques formed. The association of higher Aβ42 with higher T-Tau in our study may reflect this early stage of plaque formation and neural injury. Main effects of CSF Aβ42 on DTI may have been below detection threshold in the current study. This notion is supported by findings from our group showing that preclinical amyloid deposition as measured with [C11]PiB-PET was associated with altered microstructure on DTI [73].
There are a few limitations that should be noted. Generalizability of the results may be limited, as the sample was largely Caucasian, female, and highly educated with access to quality healthcare. It is also important to note that the study is cross-sectional. While the results of the current study suggest that neuroinflammatory processes are associated with neural damage, it is important to bear in mind that in a cross sectional study such as this, no determination of causality can be made. While we speculate that neural damage may be incurred by the inflammatory process, it is possible that levels of activated microglia reflect a response to tissue injury itself. Given that amyloid levels alone were not predictive of altered microstructure, this seems a less likely scenario, but longitudinal and animal studies are still needed to further investigate this relationship. Furthermore, while several studies support our postulation that neuroinflammation occurs in response to amyloid and tau pathology, it is important to consider that other studies have suggested that it may also drive pathology by causing accumulation of β-amyloid and tau [8,78]. More research is needed to understand the temporal relationship between inflammation and the development of AD pathology. It should also be noted that the specific biochemical and cellular roles of MCP-1 and YKL-40 are still being investigated [79] and more research is needed to elucidate the specific actions of MCP-1 and YKL-40 in the healthy CNS and in preclinical AD.
Summary
In conclusion, this study suggests that neuroinflammation is associated with neural damage—including elevated levels of structural proteins in CSF and altered regional microstructure as shown on brain imaging—among asymptomatic individuals. The results of this study provide evidence that examining markers of AD pathology together with markers of neuroinflammation may provide greater insight into the pathological processes occurring in preclinical AD. Additional studies are needed to further understand the timing of neuroinflammation in the AD process, and to determine at what disease stage neuroinflammation may be beneficial or deleterious.
Acknowledgements
This project was supported by the National Institute on Aging (R01 AG037639, R01 AG027161, ADRC P50 AG033514, R01 AG021155), the University of Wisconsin Institute for Clinical and Translational Research, funded through a National Center for Research Resources/National Institutes of Health Clinical and Translational Science Award, 1UL1RR025011, a program of the National Center for Research Resources, United States National Institutes of Health; and Waisman Center Core Grant P30 HD003352-45 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The project was also facilitated by the facilities and resources at the Geriatric Research, Education, and Clinical Center (GRECC) of the William S. Middleton Memorial Veterans Hospital, Madison, WI. The authors gratefully acknowledge Nancy Davenport, LeAnn DeRungs, Amy Hawley, Sandra Harding, Caitlin Cleary, Jay Fruehling, Paul Cary, Jennifer Oh, and Chuck Illingworth. Above all, we wish to thank our dedicated volunteers for their participation in this research.
REFERENCES
- [1].Akiyama H. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McGeer EG, McGeer PL. Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–9. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- [3].DeKosky ST, Ikonomovic MD, Wang X, Farlow M, Wisniewski S, Lopez OL, Becker JT, Saxton J, Klunk WE, Sweet R, Kaufer DI, Kamboh MI. Plasma and cerebrospinal fluid α1-antichymotrypsin levels in Alzheimer’s disease: Correlation with cognitive impairment. Ann Neurol. 2003;53:81–90. doi: 10.1002/ana.10414. [DOI] [PubMed] [Google Scholar]
- [4].Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corrà B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol Aging. 2006;27:1763–8. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- [5].Tarkowski E. Intrathecal inflammation precedes development of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:1200–5. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Perez-Nievas BG, Stein TD, Tai H-C, Dols-Icardo O, Scotton TC, Barroeta-Espar I, Carballo LF, de Munain EL, Perez J, Marquie M, Pozo AS, Frosch MP, Lowe V, Parisi JE, Peterson RC, Ikonomovic MD, López OL, Klunk W, Hyman BT, Isla TG. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain. 2013;136:2510–26. doi: 10.1093/brain/awt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van Exel E, Eikelenboom P, Comijs H, Frölich M, Smit JH, Stek ML, Scheltens P, Eefsting JE, Westendorp RGJ. Vascular factors and markers of inflammation in offspring with a parental history of late-onset Alzheimer disease. Arch Gen Psychiatry. 2009;66:1263–70. doi: 10.1001/archgenpsychiatry.2009.146. [DOI] [PubMed] [Google Scholar]
- [8].Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–15. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- [9].Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms Underlying Inflammation in Neurodegeneration. Cell. 2010;140:918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kitazawa M. Microglia as a Potential Bridge between the Amyloid -Peptide and Tau. Ann N Y Acad Sci. 2004;1035:85–103. doi: 10.1196/annals.1332.006. [DOI] [PubMed] [Google Scholar]
- [11].Mrak RE, Sheng JG, Griffin WST. Glial cytokines in Alzheimer’s disease: Review and pathogenic implications. Hum Pathol. 1995;26:816–23. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Block ML, Hong J-S. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [13].Ii M, Sunamoto M, Ohnishi K, Ichimori Y. β-Amyloid protein-dependent nitric oxide production from microglial cells and neurotoxicity. Brain Res. 1996;720:93–100. doi: 10.1016/0006-8993(96)00156-4. [DOI] [PubMed] [Google Scholar]
- [14].Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–88. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Antonell A, Mansilla A, Rami L, Lladó A, Iranzo A, Olives J, Balasa M, Sánchez-Valle R, Molinuevo JL. Cerebrospinal Fluid Level of YKL-40 Protein in Preclinical and Prodromal Alzheimer's Disease. J Alzheimer's Dis. 2014;42:901–8. doi: 10.3233/JAD-140624. [DOI] [PubMed] [Google Scholar]
- [16].Olsson B, Hertze J, Lautner R, Zetterberg H, Nägga K, Höglund K, Basun H, Annas P, Lannfelt L, Andreasen N, Minthon L, Blennow K, Hansson O. Microglial Markers are Elevated in the Prodromal Phase of Alzheimer's Disease and Vascular Dementia. J Alzheimer's Dis. 2013;33:45–53. doi: 10.3233/JAD-2012-120787. [DOI] [PubMed] [Google Scholar]
- [17].Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D'Angelo G, Malone JP, Townsend RR, Morris JC, Fagan AM, Holtzman DM. YKL-40: A Novel Prognostic Fluid Biomarker for Preclinical Alzheimer’s Disease. Biol Psychiatry. 2010;68:903–12. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rosén C, Andersson C-H, Andreasson U, Molinuevo JL, Bjerke M, Rami L, Lladó A, Blennow K, Zetterberg H. Increased Levels of Chitotriosidase and YKL-40 in Cerebrospinal Fluid from Patients with Alzheimer’s Disease. Dement Geriatr Cogn Disord extra. 2014;4:297–304. doi: 10.1159/000362164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang G, Meng Y, Li W, Yong Y, Fan Z, Ding H, Wei Y, Luo J, Ke ZJ. Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain Pathol. 2011;21:279–97. doi: 10.1111/j.1750-3639.2010.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alcolea D, Vilaplana E, Pegueroles J, Montal V, Sánchez-Juan P, González-Suárez A, Pozueta A, Rodríguez-Rodríguez E, Bartés-Faz D, Vidal-Pineiro D, González-Ortiz S, Medrano S, Carmona-Iragui María, Sámchez-Saudinós MB, Sala I, Anton-Aguirre S, Sampedro F, Morenas-Rodríguez, Clarimón J, Blesa R, Lleó A, Fortea J. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer’s disease. Neurobiol Aging. 2015;36:2018–23. doi: 10.1016/j.neurobiolaging.2015.03.001. [DOI] [PubMed] [Google Scholar]
- [21].Rosengren LE, Karlsson J-E, Karlsson J-O, Persson LI, Wikkelsø C. Patients with Amyotrophic Lateral Sclerosis and Other Neurodegenerative Diseases Have Increased Levels of Neurofilament Protein in CSF. J Neurochem. 2002;67:2013–8. doi: 10.1046/j.1471-4159.1996.67052013.x. [DOI] [PubMed] [Google Scholar]
- [22].Tullberg M, Rosengren L, Blomsterwall E, Karlsson JE, Wikkelsö C. CSF neurofilament and glial fibrillary acidic protein in normal pressure hydrocephalus. Neurology. 1998;50:1122–7. doi: 10.1212/wnl.50.4.1122. [DOI] [PubMed] [Google Scholar]
- [23].Zemlan FP, Rosenberg WS, Luebbe PA, Campbell TA, Dean GE, Weiner NE, Cohen JA, Rudick RA, Woo D. Quantification of axonal damage in traumatic brain injury: affinity purification and characterization of cerebrospinal fluid tau proteins. J Neurochem. 1999;72:741–50. doi: 10.1046/j.1471-4159.1999.0720741.x. [DOI] [PubMed] [Google Scholar]
- [24].Verbeek MM, de Jong D, Kremer HPH. Brain-specific proteins in cerebrospinal fluid for the diagnosis of neurodegenerative diseases. Ann Clin Biochem. 2003;40:25–40. doi: 10.1258/000456303321016141. [DOI] [PubMed] [Google Scholar]
- [25].Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;71:441–7. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1397. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Comi G, Filippi M. White matter damage in Alzheimer’s disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2002;72:742–6. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Takahashi S, Yonezawa H, Takahashi J, Kudo M, Inoue T, Tohgi H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurosci Lett. 2002;332:45–8. doi: 10.1016/s0304-3940(02)00914-x. [DOI] [PubMed] [Google Scholar]
- [29].Nierenberg J, Pomara N, Hoptman MJ, Sidtis JJ, Ardekani BA, Lim KO. Abnormal white matter integrity in healthy apolipoprotein E epsilon4 carriers. Neuroreport. 2005;16:1369–72. doi: 10.1097/01.wnr.0000174058.49521.16. [DOI] [PubMed] [Google Scholar]
- [30].Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JDE, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging. 2006;27:663–72. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- [31].Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO. White matter damage in Alzheimer disease and mild cognitive impairment: assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology. 2007;243:483–92. doi: 10.1148/radiol.2432051714. [DOI] [PubMed] [Google Scholar]
- [32].Fellgiebel A, Schermuly I, Gerhard A, Keller I, Albrecht J, Weibrich C, Müller MJ, Stoeter P. Functional relevant loss of long association fibre tracts integrity in early Alzheimer’s disease. Neuropsychologia. 2008;46:1698–706. doi: 10.1016/j.neuropsychologia.2007.12.010. [DOI] [PubMed] [Google Scholar]
- [33].Rose SE, Janke AL, Chalk JB. Gray and white matter changes in Alzheimer’s disease: a diffusion tensor imaging study. J Magn Reson Imaging. 2008;27:20–6. doi: 10.1002/jmri.21231. [DOI] [PubMed] [Google Scholar]
- [34].Canu E, McLaren DG, Fitzgerald ME, Bendlin BB, Zoccatelli G, Alessandrini F, Pizzini FB, Ricciardi GK, Beltramello A, Johnson SC, Frisoni GB. Microstructural diffusion changes are independent of macrostructural volume loss in moderate to severe Alzheimer’s disease. J Alzheimers Dis. 2010;19:963–76. doi: 10.3233/JAD-2010-1295. doi:10.3233/JAD-2010-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Honea RA, Vidoni E, Harsha A, Burns JM. Impact of APOE on the healthy aging brain: a voxel-based MRI and DTI study. J Alzheimers Dis. 2009;18:553–64. doi: 10.3233/JAD-2009-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bendlin BB, Ries ML, Canu E, Sodhi A, Lazar M, Alexander AL, Carlsson CM, Sager MA, Asthana S, Johnson SC. White matter is altered with parental family history of Alzheimer’s disease. Alzheimers Dement. 2010;6:394–403. doi: 10.1016/j.jalz.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xiong C, Roe CM, Buckles V, Fagan A, Holtzman D, Balota D, Duchek J, Storandt M, Mintun M, Grant E, Snyder AZ, Head D, Benzinger TL, Mettenburg J, Csernansky J, Morris JC. Role of Family History for Alzheimer Biomarker Abnormalities in the Adult Children Study. Arch Neurol. 2011;68:1313. doi: 10.1001/archneurol.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Westlye LT, Reinvang I, Rootwelt H, Espeseth T. Effects of APOE on brain white matter microstructure in healthy adults. Neurology. 2012;79:1961–9. doi: 10.1212/WNL.0b013e3182735c9c. [DOI] [PubMed] [Google Scholar]
- [39].Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotheraputics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- [41].Bendlin BB, Carlsson CM, Johnson SC, Zetterberg H, Blennow K, Willette AA, Okonkwo OC, Sodhi A, Reis ML, Birdsill AC, Alexander AL, Rowley HA, Puglielli L, Asthana S, Sager MA. CSF T-Tau/Aβ42 Predicts White Matter Microstructure in Healthy Adults at Risk for Alzheimer’s Disease. PLoS ONE. 2012;7:e37720. doi: 10.1371/journal.pone.0037720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Blennow K, Brewer JB, Dale AM. Brain Atrophy in Healthy Aging Is Related to CSF Levels of Aβ1-42. Cereb Cortex. 2010;20:2069–79. doi: 10.1093/cercor/bhp279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fortea J, Vilaplana E, Alcolea D, Carmona-Iragui M, Sánchez-Saudinos M-B, Sala I, Antón-Aguirre S, González S, Medrano S, Pegueroles J, Morenas E, Clarimón J, Blesa R, Lleó A. Cerebrospinal fluid β-amyloid and phospho-tau biomarker interactions affecting brain structure in preclinical Alzheimer disease: CSF β-Amyloid and p-Tau in AD. Ann Neurol. 2014;76:223–230. doi: 10.1002/ana.24186. [DOI] [PubMed] [Google Scholar]
- [44].Mattsson N, Insel P, Nosheny R, Trojanowski JQ, Shaw LM, Jack CR, Tosun D, Weiner M. Effects of cerebrospinal fluid proteins on brain atrophy rates in cognitively healthy older adults. Neurobiol Aging. 2014;35:614–22. doi: 10.1016/j.neurobiolaging.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Aβ 42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–83. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A Validation Study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–6. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- [47].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [48].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, Owenius R, Hägerström D, Wollmer P, Minthon L, Hanssson O. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid β-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282–9. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- [50].Adluru N, Destiche DJ, Lu SY-F, Doran ST, Birdsill AC, Melah KE, Okonkwo OC, Alexander AL, Dowling NM, Johnson SC, Sager MA, Bendlin BB. White matter microstructure in late middle-age: Effects of apolipoprotein E4 and parental family history of Alzheimer’s disease. NeuroImage Clin. 2014;4:730–42. doi: 10.1016/j.nicl.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, Hoshi M, IIg R, Schmid VJ, Zimmer C, Hemmer B, Mühlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. NeuroImage. 2012;59:3774–83. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- [52].Birdsill AC, Koscik RL, Jonaitis EM, Johnson SC, Okonkwo OC, Hermann BP, LaRue A, Sager MA, Bendlin BB. Regional white matter hyperintensities: aging, Alzheimer’s disease risk, and cognitive function. Neurobiol Aging. 2014;35:769–76. doi: 10.1016/j.neurobiolaging.2013.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Keihaninejad S, Heckemann RA, Fagiolo G, Symms MR, Hajnal JV, Hammers A. A robust method to estimate the intracranial volume across MRI field strengths (1.5T and 3T) NeuroImage. 2010;50:1427–37. doi: 10.1016/j.neuroimage.2010.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. NeuroImage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- [56].Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC. Detecting changes in nonisotropic images. Hum Brain Mapp. 1999;8:98–101. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<98::AID-HBM5>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Seimers E, Stern Y, Yaffe K, Carrillo MC, Theis B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ishizuka K, Kimura T, Igata-Yi R, Katsuragi S, Takamatsu J, Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer’s disease. Psychiatry Clin Neurosci. 1997;51:135–8. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- [59].Meda L, Bernasconi S, Bonaiuto C, Sozzani S, Zhou D, Otvos L, Mantovani A, Rossi F, Cassatella MA. Beta-amyloid (25-35) peptide and IFN-gamma synergistically induce the production of the chemotactic cytokine MCP-1/JE in monocytes and microglial cells. J Immunol. 1996;157:1213–8. [PubMed] [Google Scholar]
- [60].Westin K, Buchhave P, Nielsen H, Minthon L, Janciauskiene S, Hansson O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PloS One. 2012;7:e30525. doi: 10.1371/journal.pone.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Douaud G, Jbabdi S, Behrens TEJ, Menke RA, Gass A, Monsch AU, Rao A, Whitcher B, Kindlmann G, Matthews PM, Smith S. DTI measures in crossing-fibre areas: Increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. NeuroImage. 2011;55:880–90. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62].Filipcik P, Zilka N, Bugos O, Kucerak J, Koson P, Novak P. First transgenic rat model developing progressive cortical neurofibrillary tangles. Neurobiol Aging. 2012;33:1448–56. doi: 10.1016/j.neurobiolaging.2010.10.015. [DOI] [PubMed] [Google Scholar]
- [63].Sheffield LG, Marquis JG, Berman NE. Regional distribution of cortical microglia parallels that of neurofibrillary tangles in Alzheimer’s disease. Neurosci Lett. 2000;285:165–8. doi: 10.1016/s0304-3940(00)01037-5. [DOI] [PubMed] [Google Scholar]
- [64].Sutphen CL, Jasielec MS, Shah AR, Macy EM, Xiong C, Vlassenko AG, Benzinger TLS, Stoops EEJ, Vanderstichele HMJ, Brix B, Darby HD, Vandijck M, Ladenson JH, Morris JC, Holtzman DM, Fagan AM. Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age. JAMA Neurol. 2015 doi: 10.1001/jamaneurol.2015.1285. doi:10.1001/jamaneurol.2015.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Terry RD. The cytoskeleton in Alzheimer disease. J Neural Transm Suppl. 1998;53:141–5. doi: 10.1007/978-3-7091-6467-9_12. [DOI] [PubMed] [Google Scholar]
- [66].Aktas O, Ullrich O, Infante-Duarte C, Nitsch R, Zipp F. Neuronal damage in brain inflammation. Arch Neurol. 2007;64:185–9. doi: 10.1001/archneur.64.2.185. [DOI] [PubMed] [Google Scholar]
- [67].Cárdenas AM, Ardiles AO, Barraza N, Baéz-Matus X, Caviedes P. Role of Tau Protein in Neuronal Damage in Alzheimer’s Disease and Down Syndrome. Arch Med Res. 2012;43:645–54. doi: 10.1016/j.arcmed.2012.10.012. [DOI] [PubMed] [Google Scholar]
- [68].Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24:1079–85. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- [69].LaFerla FM, Oddo S. Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol Med. 2005;11:170–6. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- [70].Blennow K, Vanmechelen E, Hampel H. CSF total tau, Abeta42 and phosphorylated tau protein as biomarkers for Alzheimer’s disease. Mol Neurobiol. 2001;24:87–97. doi: 10.1385/MN:24:1-3:087. [DOI] [PubMed] [Google Scholar]
- [71].Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, Blennow K. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–90. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- [72].Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB, Thornton JS, Mancini L, Hyare H, Yousry T, Ridgway GR, Zhang H, Modat M, Alexander DC, Rossor MN, Ourselin S, Fox NC. Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer’s disease. Brain. 2013;136:1399–414. doi: 10.1093/brain/awt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Racine AM, Adluru N, Alexander AL, Christian BT, Okonkwo OC, Oh J, Cleary CA, Birdsill A, Hillmer AT, Murali D, Barnhart TE, Gallagher CL, Carlsson CM, Rowley HA, Dowling NM, Asthana S, Sager MA, Bendlin BB, Johnson SC. Associations between white matter microstructure and amyloid burden in preclinical Alzheimer’s disease: A multimodal imaging investigation. NeuroImage Clin. 2014;4:604–14. doi: 10.1016/j.nicl.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Shu X, Qin Y-Y, Zhang S, Jiang J-J, Zhang Y, Zhao L-Y, Shan D, Zhu W-Z. Voxel-based diffusion tensor imaging of an APP/PS1 mouse model of Alzheimer’s disease. Mol Neurobiol. 2013;48:78–83. doi: 10.1007/s12035-013-8418-6. [DOI] [PubMed] [Google Scholar]
- [75].Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A, Giraldo M, Acosta-Baena N, Tirado V, Munoz C, Reiman RA, Huentelman MJ. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11:1048–56. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Maia LF, Kaeser SA, Reichwald J, Lambert M, Obermuller U, Schelle J, Odenthal J, Martus P, Staufenbiel M, Jucker M. Increased CSF A during the very early phase of cerebral A deposition in mouse models. EMBO Mol Med 2015. 2015 doi: 10.15252/emmm.201505026. doi:10.15252/emmm.201505026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, Van Leuven F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22. doi: 10.1186/1742-2094-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JLM. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation. 2011;8:77. doi: 10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]