Abstract
Genotype‐based algorithms that include VKORC1 and CYP2C9 genotypes are less predictive of warfarin dose variability in Africans as opposed to Europeans. Polymorphisms in GGCX, FPGS, or STX1B are associated with warfarin dose requirements in African‐Americans. We sought to determine if they influenced warfarin dose in European‐Americans, and another African population, specifically Egyptians. We genotyped 529 adults (n = 325 European‐Americans, 204 Egyptians) on a stable warfarin dose for GGCX rs12714145 and rs10654848, FPGS rs7856096, and STX1B rs4889606. Rs12714145, rs10654848, and rs7856096 were not associated with warfarin dose, whereas STX1B rs4889606 was a significant determinant in univariate analysis (P < 0.0001) in both cohorts. However, STX1B rs4889606 was in high linkage disequilibrium with VKORC1‐1639 G>A, and was no longer significant after including VKORC1‐1639 G>A in the regression model. Based on these data, the polymorphisms do not appear to influence, in a clinically important way, warfarin dose requirements in European‐Americans and Egyptians.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THIS TOPIC?
✓ The percent of interindividual variability in the warfarin maintenance dose explained by current genetic‐based algorithms varies by ethnicity, emphasizing the need to examine genetic associations across different ethnic groups.
WHAT QUESTION DID THE STUDY ADDRESS?
✓ Previous studies have shown that GGCX, STX1B, or FPGS influence warfarin dose in African‐Americans. In this study we sought to interrogate these genes for their association with warfarin dose requirements in European‐Americans and Egyptians, a population from the African continent.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓ Results from univariate analysis indicate that only the single nucleotide polymorphism (SNP) in STX1B (rs4889606) was significantly associated with warfarin dose. However, after accounting for VKORC1 rs9923231 SNP, the effect of STX1B rs4889606 was no longer significant. This finding can be explained by high linkage disequilibrium between two SNPs in both populations.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS
✓ Based on these data, there is no reason to consider inclusion of GGCX, FPGS, or STX1B genotypes into warfarin pharmacogenetic dosing algorithms for European‐Americans and Egyptians.
Despite the advent of new oral anticoagulants with a more predictable dose–response profile, fewer drug–drug interactions, and no requirement for frequent monitoring, warfarin remains the mainstay of anticoagulation therapy for the treatment and prevention of thromboembolism. Since its approval in 1954, warfarin dosing has presented significant challenges clinically. Optimal warfarin dosing mandates that an international normalized ratio (INR) in the range of 2 to 3 be achieved for the majority of indications for anticoagulation. Accordingly, regular and vigilant monitoring of the INR is warranted, particularly in the early phases of warfarin initiation since values outside of the target range may have detrimental health consequences, i.e., an INR less than 2 is associated with an increased risk of thrombosis,1, 2 whereas an INR above 3 carries a heightened risk for bleeding complications including intracranial hemorrhage.3, 4 Of note, there is considerable interpatient variability in the warfarin dose that produces therapeutic anticoagulation. As an illustration, the stable warfarin dose could be as low as 0.5 mg per day for some individuals, whereas for others the dose needed for therapeutic anticoagulation could exceed 10 mg/day.5 This has spurred the formulation of several pharmacogenetic‐based algorithms6, 7, 8, 9 that set the framework for a personalized rather than an empiric approach to dosing warfarin. These algorithms incorporate single nucleotide polymorphisms (SNPs) in VKORC1 and CYP2C9 genes, which have been shown to regulate the pharmacodynamics and pharmacokinetics of warfarin, respectively. VKORC1 codes for the target protein of warfarin,10, 11, 12, 13 vitamin K epoxide reductase complex 1, and CYP2C9 encodes the principal cytochrome P450 (CYP2C9), responsible for metabolism of the more potent S‐warfarin enantiomer.14, 15, 16 Together with clinical factors (e.g., age, body surface area, smoking, and amiodarone use), the VKORC1 rs9923231 (‐1639 G>A) and CYP2C9*2 (rs1799853) and CYP2C9*3 (rs1057910) variants account for ∼50% of the variation in the warfarin daily dose among patients of European ancestry.17, 18 However, these variants explain less of the dose variability in African‐Americans and Egyptians,19 also a population residing on the African continent. Conversely, other variants demonstrate a significant association with warfarin dose requirements in African‐Americans, namely, the rs7856096 SNP in the gene coding for folate polyglutamate synthase (FPGS) and rs10654848 in GGCX, which codes for the vitamin K‐dependent enzyme, gamma glutamyl carboxylase. These variants have not been sufficiently interrogated for their association with warfarin in other racial groups. On the other hand, there are genetic markers that have been found to be highly associated with reduced therapeutic warfarin doses in African‐Americans17, 20, 21, 22, 23 only, such as the African‐specific CYP2C9 variants (CYP2C9*5, CYP2C9*6, CYP2C9*8, and CYP2C9*11), and rs127777283 in CYP2C9, which was identified in a recent genome‐wide association study (GWAS).24, 25, 26 Furthermore, STX1B rs4889606, which is 90 kb downstream of the VKORC1 gene, has been associated with VKORC1expression,27, 28 and recent data suggest that the effect of this SNP on warfarin dose requirements in African‐Americans is independent of rs9923231 in the VKORC1 gene.29 Uncovering genetic factors that provide contributions to warfarin response beyond the CYP2C9 and VKORC1 genotypes could potentially improve the accuracy of pharmacogenomics dosing algorithms in predicting warfarin maintenance dose.
We sought to determine the association between the GGCX, FPGS, or STX1B genotypes and warfarin dose requirements in European‐Americans and Egyptians. While these genes have been previously associated with warfarin dose in other populations, their inclusion in dosing algorithms is hampered by the paucity of data across different racial groups, thereby precluding the generalizability of results.
METHODS
Patient selection and intervention
The study design and patient selection are described elsewhere.30, 31 In brief, our patient cohort included a total of 529 patients (325 European‐Americans and 204 Egyptians) who were taking a stable warfarin maintenance dose for the prevention of recurrent venous thromboembolism (VTE) or stroke due to atrial fibrillation. Per protocol, a stable maintenance dose was defined as the dose (not varying by more than 10% between visits) that produced an INR within the target therapeutic range (±0.2) for each patient at three consecutive visits. The study protocol was approved by the University of Florida Review Board (for the European‐American cohort) and the Research Ethics Committee at the Faculty of Medicine, Ain Shams University in Cairo (for the Egyptian cohort). Each patient provided written informed consent for use of genetic material and clinical information for evaluating the genetic determinants of warfarin dose variability.
DNA isolation and genotyping
Genomic DNA was isolated either from buccal cells obtained from mouth wash samples (European cohort) or leukocytes in peripheral blood samples (Egyptian cohort) using the manufacturers’ guidelines.32
Genotyping for GGCX C>T, rs12714145; STX1B A>G, rs4889606; FPGS A>G, rs7856096; VKORC1 ‐1639 G>A (rs9923231); CYP2C9*2 430 C>T (rs1799853); and CYP2C9*3 1075 A>C (rs1057910) was performed by polymerase chain reaction (PCR) and pyrosequencing33 according to the manufacturer's recommendations (Qiagen, Valencia, CA). The PCR and sequencing primers for PCR and pyrosequencing reactions are shown in Supplementary Table S1. The microsatellite (CAA) tandem repeats in intron 6 of GGCX gene (rs10654848) were genotyped by fragment analysis.27
Haplotype analysis
Since the STX1B haplotype block in the Egyptian population had not been identified or characterized in any previous study, the genotyping results obtained for rs9923231 (in VKORC1) and rs4889606 (in STX1B) were uploaded into Haploview software (v. 4.2) to calculate the D’ and r2 linkage disequilibrium values, and also to construct the haplotype block. The Gabriel et al. block method was used to define the confidence interval.34
Statistical analysis
The chi‐square test with one degree of freedom was used to test for deviation from Hardy–Weinburg equilibrium (HWE) for each genotype. The nonparametric Mann–Whitney or Kruskal–Wallis statistical test was used to compare the median weekly maintenance warfarin dose between genotypes. P values less than 0.012, adjusted for multiple comparisons (0.05/4), were considered statistically significant. A stepwise linear regression model was developed to determine whether each of the tested SNPs in the GGCX, FPGS, and STX1B genes remained associated with warfarin dose requirements after accounting for other genotypes and clinical factors (e.g., age, body surface area (BSA), smoking status, SNPs in VKORC1 and CYP2C9). Under the additive model, the STX1B, GGCX, and FPGS genotypes were coded as 0 (homozygous wildtype), 1 (heterozygous), and 2 (homozygous variant). For CYP2C9, a composite score was created, with a score of 0 for those without a variant allele and 1 or 2 for those with 1 or 2 variant alleles (*2 or *3), respectively. All statistical analyses were performed on SAS (v. 9.3, SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of both cohorts (European‐Americans and Egyptians) were previously reported.30, 31 In brief, the mean age of the participants was 69 (±11) years in the European‐American cohort and 47.4 (±14.7) years in the Egyptian cohort; 12.6% of the study participants in the European‐American cohort and 55.1% in the Egyptian cohort were females. The main indications for initiating warfarin in the European‐American and Egyptian cohorts were atrial fibrillation and mitral valve replacement, respectively. The genotypes for the tested SNPs in FPGS, GGCX, and STX1B are shown in Table 1. None of the participants in either cohort was homozygous for the rs7856096 G allele in FPGS. The genotype frequencies for rs7856096, rs12714145, and rs4889606 did not depart from HWE. For the GGCX CAA microsatellite (rs10654848), the 10 CAA tandem repeat was the most prevalent allele; 24.8% of European‐Americans and 28.2% of Egyptians harbored the GGCX 10/10 microsatellite genotype (Table 1). Only one study participant in each cohort had 16 CAA tandem repeats, previously associated with high warfarin dose in African‐Americans.35
Table 1.
Genotype percentages of FPGS rs7856096, GGCX rs12714145, STX1B rs4889606, and GGCX CAA microsatellite in the European‐American and Egyptian cohorts
| Genotype | European‐Americans (n) | Egyptians (n) |
|---|---|---|
| FPGS rs7856096 | ||
| AA | 95.7 (291) | 97 (155) |
| AG | 4.3 (13) | 3 (5) |
| GG | 0 | 0 |
| GGCX rs12714145 | ||
| CC | 32.2 (97) | 42 (55) |
| CT | 47.8 (144) | 42.7 (56) |
| TT | 20 (60) | 15.3 (20) |
| STX1B rs4889606 | ||
| AA | 40 (125) | 22.2 (32) |
| AG | 51 (160) | 56.2 (81) |
| GG | 9 (30) | 21.6 (31) |
| GGCX (CAA) rs10654848 | ||
| 8/10 | 0.3 (1) | 0.4 (1) |
| 10/10 | 24.8 (74) | 28.2 (57) |
| 10–11/11 | 11.4 (34) | 14.3 (29) |
| 10–12/12 | 3.3 (10) | 7.6 (16) |
| 10–13/13 | 34.8 (104) | 26.2 (53) |
| 10–14/14 | 24.1 (72) | 20.6 (42) |
| 10–16/15 | 1.3 (4) | 2.7 (6) |
Analysis of warfarin dose requirement by gene
Only samples with genotype calls for each of the tested SNPs were included in the final analysis. Neither the FPGS rs7856096 nor GGCX rs12714145 genotype was associated with median weekly warfarin dose in either ethnic group (Table 2); however, there was a significant association between the STX1B genotype and warfarin dose on univariate analysis. Each STX1B variant G allele was associated with about a 10 mg reduction in the weekly warfarin dose in the European‐American cohort (Table 2, P < 0.0001), and about a 7 mg reduction in the Egyptian cohort (Table 2, P = 0.0001). However, the association with the STX1B allele was no longer significant in either group when the VKORC1 ‐1639 G>A was added to the regression model (Table 3). Haplotype analysis of the STX1B block (Figure 1) indicated high linkage disequilibrium (LD) between the STX1B and VKORC1 ‐ 1639 G>A SNPs in European‐Americans (D’ = 0.91, r 2 = 0.83) and Egyptians (D’ = 0.88, r 2 = 0.71).
Table 2.
Comparison of median warfarin dose (mg/week) by genotype for GGCX, STX1B and FPGS in the European and Egyptian cohorts
| Median warfarin dose (mg/week) by genotype (IQR) | ||||
|---|---|---|---|---|
| Genotype | European‐Americans | P value | Egyptians | P value |
| GGCX rs12714145 | ||||
| CC | 34.5 (25.5‐40) | 0.46 | 31.5 (24.5‐38.5) | 0.24 |
| CT | 35 (27.5‐45) | 38.5 (28‐49) | ||
| TT | 35 (25‐43.75) | 35 (24.5‐47.2) | ||
| STX1B rs4889606 | ||||
| AA | 42.5 (35‐52.5) | <0.0001 | 42 (29.7‐59.5) | 0.0001 |
| AG | 32.2 (25‐40) | 35 (28‐42) | ||
| GG | 21.5 (17.5‐27.5) | 24.5 (17.5‐35) | ||
| FPGS rs7856096 | ||||
| AA | 35 (25‐42.5) | 0.4 | 33.2 (24.5‐42) | 0.86 |
| AG | 28 (25‐40) | 33.2 (22.7‐45.5) | ||
| GG | ||||
IQR: interquartile range.
Table 3.
Multiple regression stepwise analysis for evaluating effect of STX1B on weekly warfarin dose variability
| European‐Americans | Egyptians | |||||
|---|---|---|---|---|---|---|
| Variable | β | S.E. | P value | β | S.E. | P value |
| STX1B | ‐2.4 | 2.4 | 0.3 | ‐1.4 | 3.3 | 0.6 |
| VKORC1 | ‐9.6 | 2.3 | <0.0001 | ‐ 9.1 | 3.6 | 0.008 |
| CYP2C9 | ‐8.2 | 1.1 | <0.0001 | ‐ 7.4 | 2.1 | 0.0005 |
| Age (years) | ‐0.2 | 0.1 | 0.0003 | ‐ 0.3 | 0.1 | 0.0015 |
| Smoking | 8.0 | 2.0 | <0.0001 | 14.0 | 4.7 | 0.0026 |
| BSA (m2) | 15.6 | 2.6 | <0.0001 | 0.9 | 7.2 | 0.8 |
Figure 1.
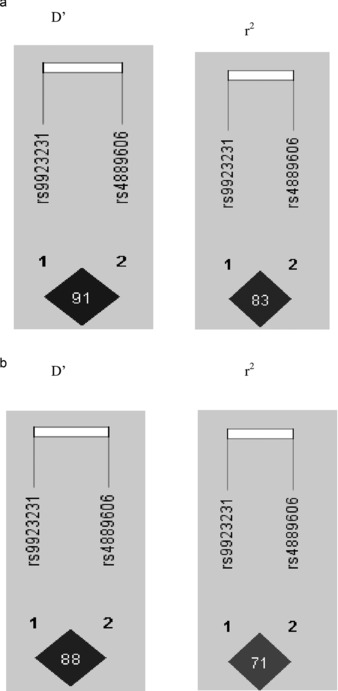
Linkage disequillibrium (LD) plots showing D' and r 2 values for the European‐American population (a) and the Egyptian population (b).
For the GGCX microsatellite (rs1064848), weekly warfarin dose was compared between the following genotype groups: 8–10/10 repeats, 10–11/11 repeats, 10–12/12 repeats, 10–13/13 repeats, 10–14/14 repeats, and 10–16/15 repeats.35 As depicted in Figure 2 a, there was no significant difference in the weekly warfarin dose requirement between these groups in European‐Americans (P = 0.87). On the other hand, the difference in the warfarin dose requirement tended to be higher in Egyptians, with 15 or more repeats compared with those with fewer than 15 repeats, but the difference did not reach statistical significance (Figure 2 b, P = 0.16). Even when the comparison was restricted to the extreme GGCX CAA tandem repeat genotypes, i.e., 8–10/10 vs. 10–16/15, the difference in the weekly warfarin dose was not statistically significant in either population (Figure 2 c,2 d). Only one Egyptian and one European‐American carried the 16 CAA repeat allele; their weekly maintenance warfarin doses were 70 mg and 40 mg, respectively. Both also had the VKORC1 ‐1639 GA genotype associated with intermediate dose requirements.
Figure 2.
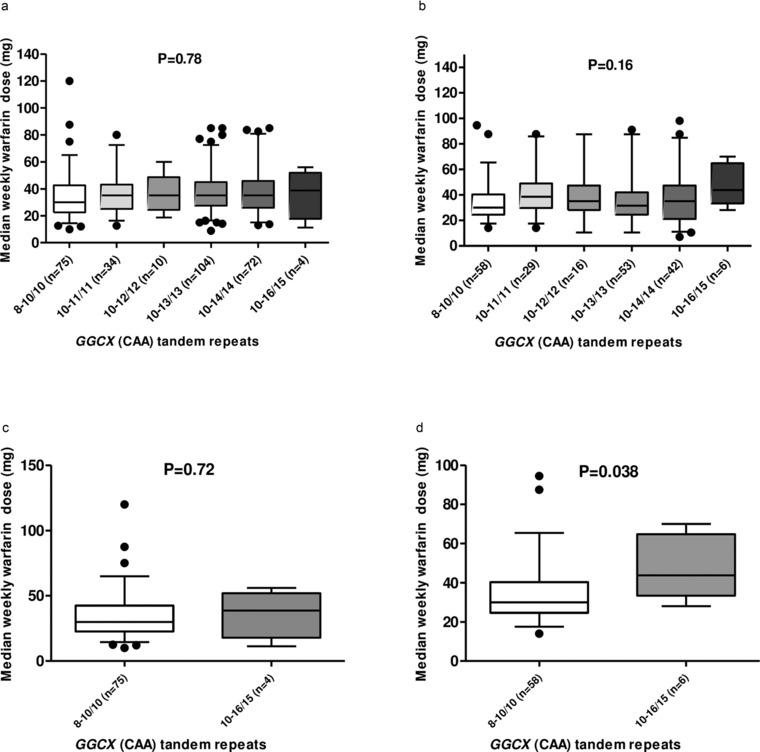
Differences in weekly warfarin maintenance dose based on number of GGCX microsatellites. Comparison of median weekly warfarin dose by number of GGCX CAA tandem repeats in the European‐American cohort (a). The bottom and top of the box represent the 25th and 75th percentiles, respectively, and the band in the middle represents the median (50th percentile). The lower whisker represents 5th percentile of the data and the upper whisker represents 95th percentile of the data. Comparison of median weekly warfarin dose by number of GGCX CAA tandem repeats in the Egyptian cohort (b). The bottom and top of the box represent the 25th and 75th percentiles, respectively, and the band in the middle represents the median (50th percentile). The lower whisker represents 5th percentile of the data and the upper whisker represents 95th percentile of the data. Comparison of median weekly warfarin dose between the extreme CAA tandem repeat genotypes (8–10/10 vs. 10–16/15) in the European‐American cohort (c). The bottom and top of the box represent the 25th and 75th percentiles, respectively, and the band in the middle represents the median (50th percentile). The lower whisker represents 5th percentile of the data and the upper whisker represents 95th percentile of the data. Comparison of median weekly warfarin dose between the extreme CAA tandem repeat genotypes (8–10/10 vs. 10–16/15) in the Egyptian cohort (d). The bottom and top of the box represent the 25th and 75th percentiles, respectively, and the band in the middle represents the median (50th percentile). The lower whisker represents 5th percentile of the data and the upper whisker represents 95th percentile of the data.
DISCUSSION
There are significant differences across race/ancestry groups in the portion of warfarin dose variability explained by known genetic and nongenetic factors, and thus warfarin pharmacogenetic dosing algorithms perform variably across different groups. The most widely cited algorithms6, 7 include only the VKORC1 and CYP2C9*2 and *3 genotypes. It has been shown that inclusion of additional genotypes improves dosing accuracy in African‐Americans.23, 24 However, whether genotypes associated with warfarin dose in African‐Americans, such as FPGS and GGCX, are also important in other populations is not well known.
In this study we evaluated variants in the GGCX, FPGS and STX1B genes to determine whether they contribute to the interindividual variability in the warfarin dose requirement in either the European‐American or Egyptian population. We found no significant association between the GGCX and FPGS variant and warfarin maintenance dose, and the association with the STX1B genotype was no longer observed on regression analysis including VKORC1 ‐1639G>A. A previous study28 reported an association between the STX1B variant and warfarin dose in a small cohort of mostly European‐American patients. Although the authors of that study proposed that the association might be secondary to linkage between the STX1B gene and VKORC1 expression, they did not provide LD values (D’ and r 2) to demonstrate this. While we replicated the results reported by this group in European‐Americans and extended the association to Egyptians, we also ruled out an independent effect of the STX1B genotype on warfarin response in these cohorts. The Egyptian population, by virtue of the geographic location of Egypt, carries a portion of West African ancestry.36 Nevertheless, informative markers were not available to confirm this for our population. In defining the STX1B haplotype structure and LD patterns, we found that the STX1B haplotype block in Egyptians resembled to a great extent the STX1B haplotype block of individuals of European ancestry, indicating that at least in this gene region, Egyptians closely resembled European‐Americans. Similarly, the observed minor allele frequency of FPGS rs7856096 among Egyptians was comparable to that in European‐Americans rather than the frequencies previously reported in Africans. Such findings indicate that the Egyptians are a highly admixed population where the allele frequencies of SNPs vary from being either close to Europeans or close to Africans.31 Hence, results from genetic association studies involving Africans cannot be extrapolated to other populations within the African continent.
The contribution of rs12714145 (C>T) in intron 2 of GGCX to warfarin dose variability was previously assessed in European‐Americans, with contrasting results, where only one37 of three studies demonstrated that homozygous carriers of the T variant required higher warfarin maintenance doses compared to the other genotypes. Specifically, in 201 Caucasian patients, rs12714145 was shown to account for about 3% of the overall variability in dose. Our results in both European‐Americans and Egyptians are consistent with the negative findings by Rieder et al. and King et al.,38, 39 suggesting that GGCX rs12714145 (C>T) is not an important determinant of the warfarin maintenance dose.
As for the microsatellite tandem (CAA) repeats in intron 6 of GGCX (rs10654848), there is some evidence showing an association with warfarin dose requirements. Among Japanese patients, higher warfarin doses were needed to achieve a therapeutic INR in patients with at least 13 CAA repeats compared with those with fewer repeats. In an African‐American population, the presence of at least 16 repeats was found to be higher than previously reported and associated with higher warfarin dose requirements.27 The 16 CAA repeat was observed in only one patient in a previous study in European‐Americans. Similarly, we found that only one European‐American and one Egyptian patient carried 16 CAA repeats, and none carried more than 16 repeats, which translated into an allele frequency of 0.3% and 0.1% in Egyptians and European‐Americans, respectively, compared with 5.6% reported in African‐Americans.35 Interestingly however, both patients with a 16 CAA repeat in our study required doses higher than typically needed to attain therapeutic anticoagulation, despite also having the heterozygous VKORC1 genotype, which typically leads to a requirement for lower than average dose. In the light of this extremely low frequency of the GGCX 16 CAA tandem repeats in both cohorts, we opted to group the 15 and 16 CAA tandem repeat genotypes together to ascertain their impact on warfarin dosing. In both cohorts, our results did not reveal a statistically significant difference in the weekly warfarin maintenance dose between carriers of at least 15 CAA tandem repeats and those with 10 or less. Nonetheless, larger studies are warranted to validate the contribution of 15 and more CAA tandem repeats to the weekly warfarin dose. Unlike previous studies,19, 40 the presence of 13 and 14 CAA tandem repeats in GGCX did not translate into higher warfarin dose requirements in either cohort.
In a recent whole‐exome sequencing study, Daneshjou et al.41 identified a novel association between warfarin dose requirements in an African‐American population and rs7856096 (A>G) in the FPGS gene, which encodes for the mitochondrial enzyme involved in folate homeostasis. They found that the G variant allele was associated with a reduction in warfarin dose by 0.83 mg/day or 5.81 mg/week. Our study was the first study to examine the association between FPGS rs7826096 and warfarin dose in a non African‐American population. In contrast to previous findings in African‐Americans, we did not observe a correlation between FPGS genotype and warfarin maintenance dose in either European‐Americans or Egyptians. However, the prevalence of this risk allele in these cohorts was low (2% in European‐American and 5% in Egyptians) compared with that reported in African‐Americans (23%), and none of the participants in our study was homozygous36 for the variant allele, which could account for our negative association.
Given the multiple gene approach that we undertook in this study, it is important to point out that we did not investigate other polymorphisms such as SNPs in CYP4F2 since they were evaluated elsewhere, and explained a small portion of the warfarin dose variability in European‐Americans,42 whereas in Egyptians the contribution was insignificant.23
In summary, this study shows that GGCX rs12714145, GGCX rs10654848, FPGS rs7856096, and STX1B rs4889606 are not significant determinants of the weekly warfarin dose requirements in either European‐Americans or Egyptians.
Conflicts Of Interest/Disclosure
The authors declared no conflict of interest.
Author Contributions
I.S.H. wrote the article; M.H.S., S.I.K., L.H.C, R.M.C.–D., and J.A.J. designed the research; I.S.H, M.H.S., S.M.L., F.O., and L.W. performed the research; I.S.H., M.H.S., S.M.L., and L.W. analyzed data; T.Y.L. contributed new reagents/analytical tools.
Supporting information
Additional supporting information may/can be found online in the supporting information tab for this article.
Supplementary Table S1
Acknowledgments
This work was supported in part by National Institutes of Health grant GM074492.
References
- 1. Willey, V.J. et al Management patterns and outcomes of patients with venous thromboembolism in the usual community practice setting. Clin. Ther. 26, 1149–1159 (2004). [DOI] [PubMed] [Google Scholar]
- 2. Palareti, G. et al Thrombotic events during oral anticoagulant treatment: results of the inception‐cohort, prospective, collaborative ISCOAT study: ISCOAT study group (Italian Study on Complications of Oral Anticoagulant Therapy). Thromb. Haemost. 78, 1438–1443 (1997). [PubMed] [Google Scholar]
- 3. Optimal oral anticoagulant therapy in patients with nonrheumatic atrial fibrillation and recent cerebral ischemia . The European Atrial Fibrillation Trial Study Group. N. Engl. J. Med. 333, 5–10 (1995). [DOI] [PubMed] [Google Scholar]
- 4. van der Meer, F.J. , Rosendaal, F.R. , Vandenbroucke, J.P. & Briet, E. Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Arch. Intern. Med. 153, 1557–1562 (1993). [DOI] [PubMed] [Google Scholar]
- 5. Wadelius, M. & Pirmohamed, M. Pharmacogenetics of warfarin: current status and future challenges. Pharmacogenomics J. 7, 99–111 (2007). [DOI] [PubMed] [Google Scholar]
- 6. Gage, B.F. et al Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin. Pharmacol. Ther. 84, 326–331 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein, T.E. et al Estimation of the warfarin dose with clinical and pharmacogenetic data. N. Engl. J. Med. 360, 753–764 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wadelius, M. et al The largest prospective warfarin‐treated cohort supports genetic forecasting. Blood 113, 784–792 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finkelman, B.S. , Gage, B.F. , Johnson, J.A. , Brensinger, C.M. & Kimmel, S.E. Genetic warfarin dosing: tables versus algorithms. J. Am. Coll. Cardiol. 57, 612–618 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rost, S. et al Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 427, 537–541 (2004). [DOI] [PubMed] [Google Scholar]
- 11. Li, T. et al Identification of the gene for vitamin K epoxide reductase. Nature 427, 541–544 (2004). [DOI] [PubMed] [Google Scholar]
- 12. Wajih, N. , Hutson, S.M. , Owen, J. & Wallin, R. Increased production of functional recombinant human clotting factor IX by baby hamster kidney cells engineered to overexpress VKORC1, the vitamin K 2,3‐epoxide‐reducing enzyme of the vitamin K cycle. J. Biol. Chem. 280, 31603–31607 (2005). [DOI] [PubMed] [Google Scholar]
- 13. Choonara, I.A. , Haynes, B.P. , Cholerton, S. , Breckenridge, A.M. , Park, B.K. Enantiomers of warfarin and vitamin K1 metabolism. Br. J. Clin. Pharmacol. 22, 729–732 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rettie, A.E. et al Hydroxylation of warfarin by human cDNA‐expressed cytochrome P‐450: a role for P‐4502C9 in the etiology of (S)‐warfarin‐drug interactions. Chem. Res. Toxicol. 5, 54–59 (1992). [DOI] [PubMed] [Google Scholar]
- 15. Yamazaki, H. & Shimada, T. Human liver cytochrome P450 enzymes involved in the 7‐hydroxylation of R‐ and S‐warfarin enantiomers. Biochem. Pharmacol. 54, 1195–1203 (1997). [DOI] [PubMed] [Google Scholar]
- 16. Kaminsky, L.S. & Zhang, Z.Y. Human P450 metabolism of warfarin. Pharmacol. Ther. 73, 67–74 (1997). [DOI] [PubMed] [Google Scholar]
- 17. Limdi, N.A. et al Influence of CYP2C9 and VKORC1 on warfarin dose, anticoagulation attainment and maintenance among European‐Americans and African‐Americans. Pharmacogenomics 9, 511–526 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schelleman, H. et al Dosing algorithms to predict warfarin maintenance dose in Caucasians and African Americans. Clin. Pharmacol. Ther. 84, 332–339 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shikata, E. et al Association of pharmacokinetic (CYP2C9) and pharmacodynamic (factors II, VII, IX, and X; proteins S and C; and gamma‐glutamyl carboxylase) gene variants with warfarin sensitivity. Blood 103, 2630–2635 (2004). [DOI] [PubMed] [Google Scholar]
- 20. Liu, Y. et al Decreased warfarin clearance associated with the CYP2C9 R150H (*8) polymorphism. Clin. Pharmacol. Ther. 91, 660–665 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cavallari, L.H. et al Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin. Pharmacol. Ther. 87, 459–464 (2010). [DOI] [PubMed] [Google Scholar]
- 22. Cha, P.C. et al Genome‐wide association study identifies genetic determinants of warfarin responsiveness for Japanese. Hum. Mol. Genet. 19, 4735–4744 (2010). [DOI] [PubMed] [Google Scholar]
- 23. Perera, M.A. et al Genetic variants associated with warfarin dose in African‐American individuals: a genome‐wide association study. Lancet 382, 790–796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scott, S.A. et al CYP2C9*8 is prevalent among African‐Americans: implications for pharmacogenetic dosing. Pharmacogenomics 10, 1243–1255 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blaisdell, J. et al Discovery of new potentially defective alleles of human CYP2C9. Pharmacogenetics 14, 527–537 (2004). [DOI] [PubMed] [Google Scholar]
- 26. Dickmann, L.J. et al Identification and functional characterization of a new CYP2C9 variant (CYP2C9*5) expressed among African Americans. Mol. Pharmacol. 60, 382–387 (2001). [DOI] [PubMed] [Google Scholar]
- 27. Schadt, E.E. et al Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 6, e107 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang, X. , Li, L. , Ding, X. & Kaminsky, L.S. Identification of cytochrome P450 oxidoreductase gene variants that are significantly associated with the interindividual variations in warfarin maintenance dose. Drug Metab. Dispos. 39, 1433–1439 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hernandez W, G.E. et al Novel regulatory variant for VKORC1 identified: associations with warfarin dose response and gene expression in African Americans. [Under review]. (2015).
- 30. Aquilante, C.L. et al Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin. Pharmacol. Ther. 79, 291–302 (2006). [DOI] [PubMed] [Google Scholar]
- 31. Shahin, M.H. et al Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet Genomics 21, 130–135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andrisin, T.E. , Humma, L.M. & Johnson, J.A. Collection of genomic DNA by the noninvasive mouthwash method for use in pharmacogenetic studies. Pharmacotherapy 22, 954–960 (2002). [DOI] [PubMed] [Google Scholar]
- 33. Langaee, T. & Ronaghi, M. Genetic variation analyses by Pyrosequencing. Mutat. Res. 573, 96–102 (2005). [DOI] [PubMed] [Google Scholar]
- 34. Gabriel, S.B. et al The structure of haplotype blocks in the human genome. Science 296, 2225–2229 (2002). [DOI] [PubMed] [Google Scholar]
- 35. Cavallari, L.H. et al Association of the GGCX (CAA)16/17 repeat polymorphism with higher warfarin dose requirements in African Americans. Pharmacogenet. Genomics 22, 152–158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henn, B.M. et al Genomic ancestry of North Africans supports back‐to‐Africa migrations. PLoS Genet. 8, e1002397 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wadelius, M. et al Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 5, 262–270 (2005). [DOI] [PubMed] [Google Scholar]
- 38. Rieder, M.J. , Reiner, A.P. & Rettie, A.E. Gamma‐glutamyl carboxylase (GGCX) tagSNPs have limited utility for predicting warfarin maintenance dose. J. Thromb. Haemost. 5, 2227–2234 (2007). [DOI] [PubMed] [Google Scholar]
- 39. King, C.R. et al Gamma‐glutamyl carboxylase and its influence on warfarin dose. Thromb. Haemost. 104, 750–754 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen, L.Y. et al Gamma‐glutamyl carboxylase (GGCX) microsatellite and warfarin dosing. Blood 106, 3673–3674 (2005). [DOI] [PubMed] [Google Scholar]
- 41. Daneshjou, R. et al Genetic variant in folate homeostasis is associated with lower warfarin dose in African Americans. Blood 124, 2298–2305 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caldwell, M.D. et al CYP4F2 genetic variant alters required warfarin dose. Blood 111, 4106–4112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may/can be found online in the supporting information tab for this article.
Supplementary Table S1


