Abstract
Aspirin and clopidogrel are the mainstay oral antiplatelet regimens, yet a substantial number of major adverse cardiac events (MACE) still occur. Herein, we investigated genetic and nongenetic factors associated with clopidogrel response in Egyptians. In all, 190 Egyptians with acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI), treated with clopidogrel (75 mg/day) for at least a month, were genotyped for CYP2C19 *2, *3, *6, *8, *10, and *17, CES1 G143E and ABCB1*6 and *8. These variants along with nongenetic factors were tested for association with the risk of having MACE in clopidogrel‐treated patients. CYP2C19 loss‐of‐function (LOF) alleles carriers had increased risk of MACE vs. noncarriers (odds ratio 2.52; 95% confidence interval 1.23–5.15, P = 0.011). In a logistic regression, CYP2C19 LOF variants (P = 0.011), age (P = 0.032), and body mass index (BMI, P = 0.039) were significantly associated with the incidence of MACE in patients taking clopidogrel. CYP2C19 genetic variants, age, and BMI are potential predictors associated with variability to clopidogrel response in Egyptians.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ Genetic polymorphisms, with different levels of evidence, have been associated with variability in clopdogrel response. CYP2C19 loss‐of‐function (LOF) alleles have been associated in numerous studies with increased risks for adverse cardiovascular events among clopidogrel‐treated patients with acute coronary syndrome undergoing PCI. However, there are no data on Egyptians.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ This study addresses the genetic and nongenetic factors associated with increased risk of having major adverse cardiac events (MACE) in Egyptians treated with clopidogrel.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓ The results of this study emphasize the influence of CYP2C19 LOF alleles on adverse cardiovascular outcomes in clopidogrel‐treated patients within Mediterranean populations, like Egyptians. Additionally, it suggests that age and body mass index are also important factors affecting response to clopidogrel in Egyptians.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS?
✓ These results further support the importance of CYP2C19 genotype on clopidogrel efficacy, regardless of race or geographical locale, and suggest CYP2C19 genotyping to guide antiplatelet therapy may be of value globally.
Aspirin and clopidogrel are the standard of care for patients experiencing acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI).1 Despite the unambiguous clinical benefit of aspirin plus clopidogrel, a number patients continue to experience major adverse cardiac events (MACE).2 Studies have shown that platelet response to clopidogrel is heterogeneous,3 and that polymorphisms in the genes responsible for the metabolism (and perhaps transport) of clopidogrel (e.g., CYP2C19, and potentially ABCB1 and CES1) contribute to interindividual variability in clopidogrel response.4 Metabolic activation of the prodrug clopidogrel to its active metabolite by CYP2C19 has emerged as a crucial determinant of clopidogrel pharmacodynamic response and clinical efficacy.5, 6 CYP2C19 is a highly polymorphic gene with more than 25 known variants.7 CYP2C9*2 and *3 are the most commonly studied CYP2C19 loss‐of‐function (LOF) alleles, and they have differences in minor allele frequencies (MAFs) based on continental ancestry, ranging from ∼13–33%, and zero to 5%, respectively. CYP2C19 *4‐*8 are less commonly studied LOF variants, with very low MAFs among most racial groups.4 In contrast, the CYP2C19*17 allele (–806C>T) is associated with increased enzyme activity and its allele frequency ranges from 3% in Asians to 21% in Caucasians.4, 8 Besides CYP2C19, carboxyl esterase 1 (CES1) is also an important enzyme that is involved in clopidogrel metabolic pathway, responsible for transforming clopidogrel into inactive metabolites.9 A missense single nucleotide polymorphism (SNP) (rs71647871) in CES1 was reported to change the amino acid glycine (G) to glutamic acid (E) at position 143 (G143E) in the catalytic site, resulting in greatly reduced CES1 catalytic function.10 As such, it has been reported that the G143E variant is associated with higher clopidogrel active metabolite levels and increased clopidogrel efficacy.11
Moreover, genetic variants in ABCB1 gene have been associated with reduced clopidogrel absorption and adverse clinical response in some but not all studies.12, 13, 14 The relative importance of ABCB1 polymorphisms compared with other well‐described genetic variants continues to be a focus of evaluation.15, 16, 17
In March 2010, the US Food and Drug Administration (FDA) issued a boxed warning on the clopidogrel label, where they stated that CYP2C19 poor metabolizers (homozygous loss of function variant carriers) are at risk of cardiovascular events because of the suboptimal response to clopidogrel in those patients. This FDA warning was based on several studies from populations of European ancestry.18, 19 Nothing is known about the genetic, clinical, and demographic factors affecting clopidogrel response in Egyptians, a highly admixed Mediterranean population. Accordingly, this study aimed to determine the influence of genetic and nongenetic variables on the response of clopidogrel therapy in Egyptians.
METHODS
Study population
A retrospective case–control design was used to evaluate the contribution of genetic and nongenetic factors underlying the risk of cardiovascular events in Egyptian patients with established coronary artery disease who were treated with clopidogrel. Between January 2012 and June 2013, the data were obtained from: (i) the pharmacy dispensary unit of the outpatient clinic of the National Heart Institute in relation to the dispensing of clopidogrel (75 mg), and (ii) the computer management system of the cardiovascular intensive care unit admissions at the Ain Shams Specialized Hospital and El‐Demerdash Hospital. Case history was available to decide whether it was a first ischemic event or a recurrent event.
The study protocol was approved by the Research Ethics Committee at Faculty of Pharmacy, Ain Shams University, Cairo, Egypt. All patients provided written informed consent. Clinical data were collected from patients' records at each site. Eligible patients were interviewed and the following information was documented: age, gender, body weight, height, smoking, medical history, and coadministered drugs. A blood sample for DNA extraction and genotyping was obtained from each patient.
Patients recruited in this study had an established coronary artery disease defined by an episode of ACS (ST‐elevation or non‐ST‐elevation myocardial infarction) or revascularization (any type of PCI or coronary‐artery bypass grafting, CABG), with a consequent exposure to clopidogrel therapy (75 mg per day). Patients taking clopidogrel for an indication other than cardiovascular indications, such as stroke prophylaxis, advanced hepatic renal diseases, and significant valvular disease or active pathological bleeding (e.g., gastrointestinal (GI) bleeding), were excluded.
Control subjects were selected as those prescribed clopidogrel (75 mg per day) for the duration maximally of 12 months and not less than 3 months, as a consequence of ACS and/or PCI and without experiencing any MACE during the period of treatment, as confirmed from patient records obtained from the pharmacy dispensary unit of the outpatient clinic of the National Heart Institute, in their follow‐up visits.
Case subjects were chosen from patients who experienced a recurrence of cardiovascular events while on clopidogrel, for at least 3 months and up to 12 months, which was defined by hospital admission. The case subjects were made up of patients with hospitalization during therapy with clopidogrel for the following: recurrent ACS, ischemic stroke, coronary arterial occlusion, and stent‐related revascularization, as documented in their medical records in the cardiovascular intensive care unit. Data were collected on all patients prior to determination of their genotype; thus, case definition and clinical data collection were blinded to genotype.
Genotyping procedure
Genomic DNA was extracted from peripheral blood leukocytes using an automated QIAcube device (Qiagen, Cairo, Egypt) according to the manufacturer's guidelines. Genotyping for CYP2C19 polymorphisms: CYP2C19*2 (G681A; rs4244285), CYP2C19*3 (G636A; rs4986893), CYP2C19*6 (G395A; rs72552267), CYP2C19*8 (T358C; rs41291556), CYP2C19*10 (G227C; rs6413438), and CYP2C19*17 (–806C>T; rs12248560) and CES1 variant (G143E; rs71647871) were performed by polymerase chain reaction (PCR) followed by pyrosequencing.20 The pyrosequencing genotyping assay for CYP2C19*2,*3, *6, *8, *10, and *17 and CES1 G143E were designed by Pyrosequencing assay design software (Qiagen, Valencia, CA). The ABCB1 polymorphisms: ABCB1*6 (C3435T; rs1045642) and ABCB1*8 (C1236T; rs1128503) were genotyped by TaqMan assay as recommended by the manufacturer (Life Technologies, Grand Island, NY). The standard PCR reaction mixture used for the amplification of the target sequence consisted of 12.5 μL including 6.5 μL of ABI PCR master mix with Taq DNA polymerase for all the SNPs except CES1 G143E, for which HotStarTaq Master Mix Kit (Cat. No. 203445) was used, 1 μL of each primer (Invitrogen, Carlsbad, CA), 2 μL of water for DNA, and 2 μL of genomic DNA.
Statistical analysis
Chi‐square and Fisher's exact tests were used to compare the allele and genotype frequencies as appropriate and descriptive analysis was used to compare allele frequencies between the Egyptian population and published data of other populations. Numerical variables were represented as mean ± SD. Categorical variables are presented as percentages. Hardy–Weinberg equilibrium was tested by allele counting and χ2 analyses with one degree of freedom. Univariate analysis was used to compare the quantitative dependent factors between the MACE (cases) and no‐MACE (controls) groups. Because all of the genes being tested have been previously reported to be significantly associated with a risk of MACE in post‐ACS or post‐PCI patients, we considered a two‐sided P‐value of less than 0.05 to indicate statistical significance. Age, gender, and BMI along with covariates associated with clinical outcome of P < 0.2 were further analyzed by a logistic regression model and described as odd ratios (ORs) with 95% confidence intervals (CI). Because CES1 and ABCB1 showed no association with outcomes in univariate analysis, but CYP2C19 did, only CYP2C19 was included in the multivariate logistic regression analysis. In the logistic regression model, the CYP2C19 genetic variants were coded as: (i) 0 for absence of CYP2C19 LOF alleles (*1*1, *1*17, *17*17), and (ii) 1 for one or two LOF allele carriers (*1*2, *1*3, *2*3, *2*17). All statistical analyses were carried out using SPSS software (v. 17.0 for Windows; Chicago, IL).
RESULTS
We identified 84 patients who experienced MACE during the study period (cases) and 106 patients who did not experience any MACE while on clopidogrel (controls). Hereafter, the cases are referred to as the MACE group and controls as the no‐MACE group. All patients had experienced ACS and/or PCI as the reason for a clopidogrel prescription. The mean age was 57.1 ± 9.1 years for MACE patients and 54.8 ± 9.5 years for the no‐MACE group. The MACE group included 60 males (71.4%), while the no‐MACE group included 70 (66%) males. Patients' demographics, medical history, and concomitant medications are shown in Table 1.
Table 1.
Baseline demographics of study participants
| MACE group | No‐MACE group | P‐value | |
|---|---|---|---|
| (n = 84) | (n = 106) | ||
| Mean age, y (SD) | 57.1 (9.12) | 54.78 (9.45) | 0.74 |
| Male gender (%) | 60 (71.4%) | 70 (66%) | 0.427 |
| Current smoker (%) | 28 (33.3%) | 25(23.6%) | 0.329 |
| Weight, kg (SD) | 84.66 (10.7) | 81.92 (11.5) | 0.14 |
| Height, cm (SD) | 167.05 (6.8) | 166.6 (5.4) | 0.34 |
| BMI by class, kg/m² (SD) | 30.02 (5) | 29.5 (3.7) | 0.265 |
| Medical historya | |||
| ACS | 63 (75%) | 92 (86.7%) | 0.058 |
| PCI | 56 (66.6%) | 74 (69.8%) | 0.76 |
| Diabetes (%) | 31 (36.9%) | 26 (24.5%) | 0.064 |
| Hypertension (%) | 57 (67.9%) | 54 (51 %) | 0.028 |
| Drug therapy during follow upb | |||
| Statin | 60 (71.4%) | 69 (65%) | 0.353 |
| Beta blocker | 60 (71.4%) | 63(59.4%) | 0.086 |
| Aspirin | 84 (100%) | 106 (100%) | — |
PCI, percutaneous coronary intervention; CABG, coronary‐artery bypass grafting; ACE, angiotensin‐converting enzyme; ARBs, angiotensin‐receptor blockers; ACS, acute coronary syndrome; DM, diabetes mellitus.
Patients might have more than one concomitant disease.
Patients might have more than one concomitant drug.
The MAFs of the studied SNPs in CYP2C19, CES1, and ABCB1 are illustrated in Table 2, and compared with published MAFs data of other populations. These data confirm our previous observation that the polymorphism frequencies in Egyptians cannot be presumed to match those of Africans, European Caucasians, or any other continental population.21
Table 2.
Comparison of minor allele frequencies of studied polymorphisms in Egyptians compared with previously reported frequencies from different ethnic populations
| Gene variant | Genotype | Egyptian | Africana | Asiana | Europeana | Americana |
|---|---|---|---|---|---|---|
| CYP2C19*2 | G>A | 0.126 | 0.169 | 0.334 | 0.148 | 0.115 |
| CYP2C19*3 | G>A | 0.0025 | 0.004 | 0.051 | 0.000 | 0.000 |
| CYP2C19*6 | G>A | 0.000 | — | — | — | — |
| CYP2C19*8 | C<T | 0.000 | 0.004 | — | 0.003 | — |
| CYP2C19*10 | C>T | 0.000 | — | — | — | — |
| CYP2C19*17 | C>T | 0.17 | 0.215 | 0.019 | 0.228 | 0.205 |
| ABCB1*6 | C>T | 0.387 | 0.146 | 0.397 | 0.529 | 0.189 |
| ABCB1*8 | C>T | 0.392 | 0.132 | 0.326 | 0.404 | 0.133 |
| CES1 (G143E) | G>A | 0.01 | 0.040 | 0.000 | 0.024 | 0.016 |
Information from 1,000 Genomes (http://browser.1000genomes.org/Homo_sapiens/Info/Index).
Table 3 depicts genotype frequencies in the MACE and no‐MACE patients and reveals that only CYP2C19*2 was more common in MACE patients. CYP2C19*3 occurred in only one person and *6, *8, and *10 were nonvariant in this population. All the genotypes were in Hardy–Weinberg equilibrium.
Table 3.
Genotype frequencies in MACE and no‐MACE patients
| Observed frequency | |||||
|---|---|---|---|---|---|
| Gene variant | Genotype | MACE (n = 84) | NO‐MACE (n = 106) | Overall (n = 190) | P * |
| CYP2C19*2, G681A | CC | 58 | 87 | 145 | 0.03 |
| 69% | 82.1% | 76.3% | |||
| CT | 25 | 17 | 42 | ||
| 29.8% | 16% | 22.1% | |||
| TT | 1 | 2 | 3 | ||
| 1.2% | 1.9% | 1.6% | |||
| T allele | 27 | 21 | 48 | ||
| 16.1% | 9.9% | 12.65% | |||
| CYP2C19*17, ‐806C>T | CC | 63 | 73 | 136 | |
| 75% | 68.9% | 71.6% | 0.274 | ||
| CT | 16 | 29 | 45 | ||
| 19% | 27.4% | 23.7% | |||
| TT | 5 | 4 | 9 | ||
| 6.0% | 3.8% | 4.7% | |||
| T allele | 26 | 37 | 63 | ||
| 16.5% | 17.4% | 16.55% | |||
| ABC1*6, C3435T | CC | 33 | 40 | 73 | |
| 39.3% | 37.7% | 38.4% | 0.661 | ||
| CT | 40 | 47 | 87 | ||
| 47.6% | 44.3% | 45.8% | |||
| TT | 11 | 19 | 30 | ||
| 13.1% | 17.9% | 15.8% | |||
| T allele | 62 | 85 | 147 | ||
| 36.9% | 40% | 38.7% | |||
| ABC1*8, C1236T | CC | 32 | 41 | 73 | |
| 38.1% | 38.7% | 38.4% | 0.934 | ||
| CT | 37 | 48 | 85 | ||
| 44% | 45.3% | 44.7% | |||
| TT | 15 | 17 | 32 | ||
| 17.9% | 16% | 16.8% | |||
| T allele | 67 | 82 | 149 | ||
| 39.9% | 38.65% | 39.15% | |||
| CES1, G143E | GG | 82 | 104 | 186 | 1.000 |
| 97.6% | 98.1% | 97.9% | |||
| GA | 2 | 2 | 4 | ||
| 2.4% | 1.9% | 2.1% | |||
| A allele | 2 | 2 | 4 | ||
| 1.2% | 0.94% | 1.05% | |||
*P < 0.05 was considered significant between the two studied groups. Only the variant alleles that are carried by at least three individuals in the studied population are included.
Results of the multiple logistic regression analysis are presented in Figure 1. In the multiple regression analysis, carriers of one and two LOF alleles were combined, as only three individuals were homozygous for an LOF allele. Our analysis suggests that the overall estimated OR of having one or more LOF alleles is ∼2.5 times that of noncarriers (OR 2.52; 95% CI 1.23–5.15 at P = 0.011). Along with CYP2C19*2, getting older and increasing BMI significantly contribute to the variability of clopidogrel response in Egyptians, being a year older increases the odds of MACE by 3% (OR 1.03; 95% CI 1.003–1.07, P = 0.032), and every one unit increase in BMI increases the odds of MACE by 8% (OR 1.08; 95% CI 1.004–1.181, P = 0.039).
Figure 1.
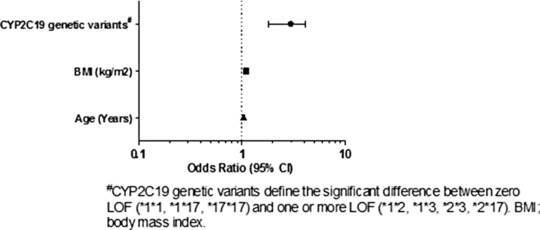
Multiple logistic regression association between MACE and significant genetic and nongenetic factors (P < 0.05) in clopidogrel‐treated Egyptians.
DISCUSSION
Populations in the Mediterranean were one of the first populations to migrate out of Africa, which makes genetic research in these populations of great interest. The Egyptian population is a highly admixed population, which makes it an ideal population for genetic studies.22 This study demonstrated the prevalence and frequencies of nine genetic polymorphisms of enzymes and transporters, postulated to be associated with clopidogrel efficacy, and assessed their influence, as well as other nongenetic factors, on the variability in clopidogrel response.
Globally, clopidogrel use, in conjunction with aspirin, is recommended to prevent acute coronary events in patients with recent ACS or stent placement.1, 2, 23, 24 Despite the importance of clopidogrel, studies have shown that about 30% of clopidogrel‐treated patients do not respond effectively.25 Several mechanisms of clopidogrel resistance are possible and can be categorized to genetic factors including polymorphisms in the genes involved in the processing of clopidogrel in body, e.g., CYP2C19, CES1, and ABCB1, and nongenetic factors as age, gender, BMI, smoking status, concomitant treatment, and diseases.
To our knowledge, our study is the first to address the effect of CYP2C19 genotype on the clinical outcome of clopidogrel in Egyptians and the magnitude of risk is consistent with numerous other studies and a widely cited meta‐analysis of post‐PCI patients.12 In the current study, it was revealed that the CYP2C19 LOF polymorphism significantly influences the clinical outcome in patients with ACS and/or PCI on extended clopidogrel treatment. The carriers of one or two LOF alleles of CYP2C19 (*1*2, *1*3, *2*3, *2*17) had worse clinical outcomes than noncarriers of CYP2C19 loss of function alleles, with an estimated OR ∼2.5 times that of noncarriers (OR 2.52; 95% CI 1.23–5.15 at P = 0.011). Several clinical studies have recently demonstrated the relationship between variants in the CYP2C19 gene and the risk for adverse cardiovascular outcome in clopidogrel‐treated patients.26 Studies have shown up to 3.6‐fold increased risk of adverse events among patients carrying two CYP2C19 LOF alleles undergoing PCI compared with noncarriers.27 Meta‐analysis of clopidogrel outcome after treatment of ACS showed a significant association between the CYP2C19 polymorphism and an increased risk of thrombosis‐related events among patients with one or more variant alleles, documenting that the risk of the CYP2C19 polymorphism is associated with its interference of the normal metabolism of clopidogrel, and confirmed a reduction in platelet inhibition among carriers of CYP2C19 LOF alleles who received standard dose clopidogrel.12, 28, 29
We found no significant association between ABCB1 polymorphisms and the variability of clopidogrel response, in accordance with previous reports from other populations.30, 31, 32, 33, 34 As for the CES1 variant, only four of our study participants carried the variant allele, which made us underpowered to test this association.
A genome‐wide association study has pointed out that the polymorphic hepatic CYP2C19 enzyme, which is the main predictor of altered response to clopidogrel, accounts for only 12% of the variation in response to the thienopyridine agent clopidogrel.25, 26, 35 Other genetic and environmental factors may play a role.26 Studies have reported that some nongenetic factors might influence the inhibitory effect of clopidogrel treatment on platelet function.13, 36, 37, 38 Our data suggest that age and BMI had a significant influence on the risk of MACE in Egyptian patients on clopidogrel. Getting a year older and being more obese by one BMI unit increase the risk of MACE exposure by 3.7% and 8.9%, respectively. As reported by Cuisset et al.,39 age and BMI were significantly associated with nonresponse to clopidogrel. Additionally, we observed a trend for smokers to be more susceptible to MACE compared with nonsmokers, with a marginally significant association (P = 0.057). As reported by many studies, smoking has a negative effect on the cardiovascular system, and it increases the ability of platelets to aggregate.40 However, surprisingly, a recent study suggested that nonsmokers had a reduced response to clopidogrel compared with smokers.41
There are several important limitations to our study. First is the retrospective nature of the enrollment of our participants. The demographics of the MACE and no‐MACE groups were largely similar, differing minimally for those risk factors that would be expected to differ in the two groups. However, there could be unmeasured demographic or clinical differences in the two groups that we have not accounted for that contributed to the observed differences in clinical outcomes, independent of the CYP2C19 genotype. We also had a small sample size, which may have precluded noting an effect for ABCB1 and CES1. However, despite the small sample size, our finding of an ∼2.50‐fold increased risk of MACE in LOF carriers is completely consistent with previous literature in European populations.
In conclusion, our findings in Egyptians support studies in other populations suggesting reduced clopidogrel efficacy, and thus increased risk of MACE during clopidogrel treatment, in those who carry a CYP2C19 LOF allele. Clinical Pharmacogenetics Implementation Guidelines suggest alternative treatment in CYP2C19 LOF carriers who have ACS and/or PCI,4 and our data suggest these guidelines could be applied to Egyptians.
Conflict of Interest/Disclosure
The authors declare no conflicts of interest.
Author Contributions
J.A.J., B.M.K., M.H.S., and M.H.M.S. wrote the article; J.A.J., B.M.K., T.L., M.F.S., L.N.H., H.O.A‐M., N.M.H., and W.A.E‐H. designed the research; J.A.J., B.M.K., M.H.S., and M.H.M.S. performed the research; J.A.J., B.M.K., M.H.S., and Y.G. analyzed the data; T.L. contributed new reagents/analytical tools.
Acknowledgments
This work was partially supported by a grant from Misr International University, Cairo, Egypt and the University of Florida, Center of Pharmacogenomics and NIH grant U01 GM074492 and IGNITE Network grant U01 HG007269. M.H. Shahin is supported by an American Heart Association predoctoral fellowship grant no. 14PRE20460115. M.H.M. Solayman is funded by a grant from the Embassy of the Arab Republic of Egypt. We thank Nihal Abdel Khalek at National Heart Institute, Cairo, Egypt, for help in subject recruitment and Lynda Stauffer at the University of Florida, Center for Pharmacogenomics, for laboratory assistance.
References
- 1. Bhatt, D.L. et al Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J. Invasive Cardiol. 13, 767–771 (2001). [PubMed] [Google Scholar]
- 2. Han, Y.L. et al A high maintenance dose of clopidogrel improves short‐term clinical outcomes in patients with acute coronary syndrome undergoing drug‐eluting stent implantation. Chin. Med. J. (Engl). 122, 793–797 (2009). [PubMed] [Google Scholar]
- 3. Serebruany, V.L. , Steinhubl, S.R. , Berger, P.B. , Malinin, A.I. , Bhatt, D.L. & Topol, E.J. Variability in platelet responsiveness to clopidogrel among 544 individuals. J. Am. Coll. Cardiol. 45, 246–251 (2005). [DOI] [PubMed] [Google Scholar]
- 4. Scott, S.A. et al Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 94, 317–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angiolillo, D.J. et al Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J. Am. Coll. Cardiol. 49, 1505–1516 (2007). [DOI] [PubMed] [Google Scholar]
- 6. Bennett, D. & Yan, B. Suboptimal response to clopidogrel: a genetic risk factor for recurrent ischaemic stroke. J. Clin. Neurosci. 20, 767–770 (2013). [DOI] [PubMed] [Google Scholar]
- 7. Scott, S.A. et al PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet. Genomics. 22, 159–165 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frere, C. , Cuisset, T. , Gaborit, B. , Alessi, M.C. & Hulot, J.S. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non‐ST acute coronary syndrome. J. Thromb. Haemost. 7, 1409–1411 (2009). [DOI] [PubMed] [Google Scholar]
- 9. Tang, M. et al Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. J. Pharmacol. Exp. Ther. 319, 1467–1476 (2006). [DOI] [PubMed] [Google Scholar]
- 10. Zhu, H.J. et al Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am. J. Hum. Genet. 82, 1241–1248 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis, J.P. et al The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet. Genomics. 23, 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mega, J.L. et al Reduced‐function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta‐analysis. JAMA. 304, 1821–1830 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mega, J.L. et al Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON‐TIMI 38 trial: a pharmacogenetic analysis. Lancet. 376, 1312–1319 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fung, K.L. & Gottesman, M.M.A. Synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim. Biophys. Acta. 1794, 860–871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taubert, D. et al Impact of P‐glycoprotein on clopidogrel absorption. Clin. Pharmacol. Ther. 80, 486–501 (2006). [DOI] [PubMed] [Google Scholar]
- 16. Mega, J.L. et al Cytochrome p‐450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 360, 354–362 (2009). [DOI] [PubMed] [Google Scholar]
- 17. Wessler, J.D. , Grip, L.T. , Mendell, J. & Giugliano, R.P. The P‐glycoprotein transport system and cardiovascular drugs. J. Am. Coll. Cardiol. 61, 2495–2502 (2013). [DOI] [PubMed] [Google Scholar]
- 18. Berger, P.B. et al Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation. 121, 2575–2583 (2010). [DOI] [PubMed] [Google Scholar]
- 19. Pride, Y.B. et al Angiographic and clinical outcomes among patients with acute coronary syndromes presenting with isolated anterior ST‐segment depression: a TRITON‐TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel‐Thrombolysis In Myocardial Infarction 38) substudy. JACC Cardiovasc. Interv. 3, 806–811 (2010). [DOI] [PubMed] [Google Scholar]
- 20. Langaee, T. & Ronaghi, M. Genetic variation analyses by Pyrosequencing. Mutat. Res. 573, 96–102 (2005). [DOI] [PubMed] [Google Scholar]
- 21. Shahin, M.H. et al Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet. Genomics. 21, 130–135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ozcelik, T. et al Collaborative genomics for human health and cooperation in the Mediterranean region. Nat. Genet. 42, 641–645 (2010). [DOI] [PubMed] [Google Scholar]
- 23. Kushner, F.G. et al 2009 focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 54, 2205–2241 (2009). [DOI] [PubMed] [Google Scholar]
- 24. Sofi, F. , Marcucci, R. , Gori, A.M. , Giusti, B. , Abbate, R. & Gensini, G.F. Clopidogrel non‐responsiveness and risk of cardiovascular morbidity. An updated meta‐analysis. Thromb. Haemost. 103, 841–848 (2010). [DOI] [PubMed] [Google Scholar]
- 25. Shuldiner, A.R. et al Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 302, 849–857 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hulot, J.S. et al Cardiovascular risk in clopidogrel‐treated patients according to cytochrome P450 2C19*2 loss‐of‐function allele or proton pump inhibitor coadministration: a systematic meta‐analysis. J. Am. Coll. Cardiol. 56, 134–143 (2010). [DOI] [PubMed] [Google Scholar]
- 27. Simon, T. et al Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 360, 363–375 (2009). [DOI] [PubMed] [Google Scholar]
- 28. Collet, J.P. et al Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 373, 309–317 (2009). [DOI] [PubMed] [Google Scholar]
- 29. Holmes, M.V. , Perel, P. , Shah, T. , Hingorani, A.D. & Casas, J.P. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta‐analysis. JAMA. 306, 2704–2714 (2011). [DOI] [PubMed] [Google Scholar]
- 30. Rideg, O. et al Impact of genetic variants on post‐clopidogrel platelet reactivity in patients after elective percutaneous coronary intervention. Pharmacogenomics. 12, 1269–1280 (2011). [DOI] [PubMed] [Google Scholar]
- 31. Leschziner, G.D. , Andrew, T. , Pirmohamed, M. & Johnson, M.R. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J. 7, 154–179 (2007). [DOI] [PubMed] [Google Scholar]
- 32. Tang, X.F. et al Effect of the CYP2C19 2 and 3 genotypes, ABCB1 C3435T and PON1 Q192R alleles on the pharmacodynamics and adverse clinical events of clopidogrel in Chinese people after percutaneous coronary intervention. Eur. J. Clin. Pharmacol. 69, 1103–1112 (2013). [DOI] [PubMed] [Google Scholar]
- 33. Wallentin, L. et al Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 376, 1320–1328 (2010). [DOI] [PubMed] [Google Scholar]
- 34. Jaitner, J. et al No association of ABCB1 C3435T genotype with clopidogrel response or risk of stent thrombosis in patients undergoing coronary stenting. Circ. Cardiovasc. Interv. 5, 82–88, S81‐82 (2012). [DOI] [PubMed] [Google Scholar]
- 35. Damani, S.B. & Topol, E.J. The case for routine genotyping in dual‐antiplatelet therapy. J. Am. Coll. Cardiol. 56, 109–111 (2010). [DOI] [PubMed] [Google Scholar]
- 36. Silvain, J. et al High on‐thienopyridine platelet reactivity in elderly coronary patients: the SENIOR‐PLATELET study. Eur. Heart J. 33, 1241–1249 (2012). [DOI] [PubMed] [Google Scholar]
- 37. Berger, J.S. et al Smoking, clopidogrel, and mortality in patients with established cardiovascular disease. Circulation. 120, 2337–2344 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park, K.W. et al Enhanced clopidogrel response in smokers is reversed after discontinuation as assessed by VerifyNow assay: additional evidence for the concept of ‘smokers’ paradox'. Heart. 98, 1000–1006 (2012). [DOI] [PubMed] [Google Scholar]
- 39. Cuisset, T. et al Relationship between aspirin and clopidogrel responses in acute coronary syndrome and clinical predictors of non response. Thromb. Res. 123, 597–603 (2009). [DOI] [PubMed] [Google Scholar]
- 40. Colivicchi, F. , Mocini, D. , Tubaro, M. , Aiello, A. , Clavario, P. & Santini, M. Effect of smoking relapse on outcome after acute coronary syndromes. Am. J. Cardiol. 108, 804–808 (2011). [DOI] [PubMed] [Google Scholar]
- 41. Gurbel, P.A. et al The influence of smoking status on the pharmacokinetics and pharmacodynamics of clopidogrel and prasugrel: the PARADOX study. J. Am. Coll. Cardiol. 62, 505–512 (2013). [DOI] [PubMed] [Google Scholar]


