Abstract
Objective
Smoking prevalence is high among substance abusers, making it important to understand when nicotine abstinence will aid, impair, or not affect abstinence from other substances. This study tested novel hypotheses about the coupling of nicotine and stimulant craving over time during stimulant dependence treatment.
Method
Adults (N=538) with cocaine and/or methamphetamine dependence completed a 10-week randomized controlled trial of substance use treatment with or without smoking cessation treatment. Participants reported nicotine and stimulant craving weekly and use twice per week.
Results
Latent Change Score modeling tested the association between weekly increases in nicotine craving and subsequent weekly changes in stimulant craving. Interestingly, results revealed a “substitution” effect: increases in nicotine craving predicted subsequent decreases in stimulant craving (γ=−.37, p=.001). Additionally, increases in nicotine craving predicted subsequent increases in nicotine use (γ=1.26, p=.04) and decreases in stimulant use (γ=−.07, p=.03). As expected, the substitution effect between nicotine and stimulant craving was stronger when stimulants were administered through the same route as nicotine (i.e., smoking; γ=−.56, p=.005) versus other routes (γ=−.32, p=.06). Finally, smoking cessation treatment eliminated the coupling between nicotine craving and stimulant craving (γ=−.07, p=.39).
Conclusions
Contrary to concerns about nicotine abstinence during substance dependence treatment, increases in nicotine craving may be associated with later reductions in stimulant craving and use, and unrelated when smoking cessation treatment is introduced. Weekly changes in nicotine craving convey information that can help clinicians to predict and understand shifts in stimulant craving and use during substance use disorder treatment.
Keywords: substance dependence, smoking cessation, craving, latent change score modeling
Smoking prevalence is high among substance abusers (Lai, Lai, Page, & McCoy, 2000; Stark & Campbell, 1993), making it important to determine how smoking and smoking cessation impact attempted abstinence from other substances. Among treatment providers, there has been significant debate about whether (and how) smoking cessation should be integrated into substance use disorder treatment, with many believing that craving and withdrawal symptoms stemming from nicotine abstinence would be too overwhelming for patients already attempting to quit other substances (Lemon, Friedmann, & Stein, 2003). However, most evidence suggests that smoking cessation does not harm outcomes of drug abuse treatment (Lemon et al., 2003), and sometimes even improves drug abstinence (Baca & Yahne, 2009). To explain these findings, it is important to consider novel mechanisms that can aid the prediction of when abstinence from nicotine will aid, impair, or not affect abstinence from other substances.
Among proposed mechanisms, craving is the most commonly reported and salient symptom of abstinence (Gritz, Carr, & Marcus, 1991; Shiffman & Jarvik, 1976) and is assumed to share many characteristics across substances (Koob & Volkow, 2010). Perhaps because craving is an inconsistent predictor of relapse (Tiffany & Wray, 2009), few researchers have investigated the nature of craving across substances. However, recent conceptual advances have attempted to explain the heterogeneity of the craving - relapse link, leading to new hypotheses about polysubstance craving over time (Kavanagh, Andrade, & May, 2005). These frameworks are helpful for understanding the interrelationships of abstinence from multiple substances over the course of treatment, and have suggested two main competing hypotheses for how increased nicotine craving could impact craving for other substances.
First, it is possible that there is a “carryover” effect across substances, in which increased craving for nicotine triggers subsequently increased craving for a second substance. This idea stems from the cues for craving that are shared across substances (Florsheim et al., 2008), including distressing emotions, physiological states, and associations in memory (Carter & Tiffany, 1999). Similarly, the repeated use of nicotine and other substances in close temporal proximity or via the same administration route (i.e., smoking) could strengthen the associations between cues for two substances via conditioning. Several previous studies using ecological momentary assessment have found that people who craved one substance were more likely to crave the other within the same brief time frame (Epstein, Marrone, Heishman, Schmittner, & Preston, 2010; Preston et al., 2009), such as nicotine craving being associated with craving for cocaine and heroin at single time points (Epstein, Marrone, Heishman, Schmittner, & Preston, 2010). Notably, this pattern has not been tested over longer time frames that align with the timing of clinical practice, such as shifts in craving between weekly sessions.
A second, competing hypothesis is that over time, increased nicotine craving may lessen the craving for another substance, termed a “substitution” effect. This hypothesis is based on evidence that cravings are elaborated in a substance-specific way (Kavanagh et al., 2005; Nosen & Woody, 2007, 2014), allowing the possibility that this elaboration intensifies craving for one substance at the expense of craving for another substance. For instance, when there are multiple possible sources of withdrawal symptoms and the cause of these symptoms is ambiguous, individuals may rely on attributional processes to ascribe those symptoms to a particular substance or to another explanation such as hunger (Kavanagh et al., 2005). This explanation fits with findings that while polysubstance craving is related at individual time points, substance use only follows increases in craving about that particular substance (Preston et al., 2009).
This line of reasoning leads to interesting hypotheses about the experience of polysubstance craving over time. For example, when nicotine users abstain from cocaine, they may initially attribute physiological discomfort to abstinence from cocaine and elaborate a cocaine craving, but over time increasingly attribute the same discomfort to nicotine withdrawal between cigarettes (elaborating a nicotine craving and decreasing cocaine craving). Further, attributions and interpretations are not the only types of elaboration that could lead to a substitution effect, as a similar pattern could result due to the imagery associated with craving. Several studies have demonstrated that craving imagery for a specific substance can be disrupted by presenting alternative, non-substance-related imagery (May, Andrade, Panabokke, & Kavanagh, 2010). In the context of polysubstance craving, these findings suggest that increased nicotine craving may limit elaboration upon craving for a second substance through its imagery, in addition to through attributions or other types of nicotine-related elaboration. More broadly, a substitution effect with polysubstance craving could account for why nicotine abstinence sometimes aids drug abstinence in trials (Baca & Yahne, 2009), as increased nicotine craving during a quit attempt could help to suppress the craving (and use) of other drugs.
In the current study, our primary goal was to evaluate the ties between increases in nicotine craving and subsequent changes in stimulant craving, a relationship that is termed ‘coupling.’ We also examined the coupling between increases in nicotine craving and subsequent changes in nicotine and stimulant use. Based on the literature reviewed above, we expected that nicotine craving would be positively associated with stimulant craving at a single time point. We also expected that their overall trajectories (i.e., slopes) would be associated across treatment, demonstrating a relationship between the two overall change processes during treatment. Given the lack of data about coupling, we evaluated two competing hypotheses about how nicotine craving would relate to stimulant craving over time. According to a carryover effect, one would expect that increases in nicotine craving would predict subsequent increases in stimulant craving. Alternatively, according to a substitution effect, one would expect that increases in nicotine craving would be followed by decreases in stimulant craving.
We also expected that carryover or substitution effects might vary according to several potential moderators. We hypothesized that both possible coupling patterns would be stronger among stimulant users using a smoking administration route versus other methods, due to the enhancement of the mechanisms described above (e.g., more shared cues or an increase in the similarity and ambiguity of physiological withdrawal symptoms). We also explored whether coupling patterns would differ according to the type of stimulant used and/or the presence of smoking cessation treatment. Together, these tests are informative for predicting shifts over time in craving and use among patients who smoke and are completing stimulant dependence treatment. Further, this is the first study in our knowledge to examine weekly shifts in these coupling relationships, a timing that is matched to many clinicians’ typical contacts with patients.
Methods
Participants
This study analyzed data from 538 adults participating in a randomized controlled trial comparing substance use treatment as usual (TAU; N=271), with substance use treatment as usual plus smoking cessation treatment (TAU+SCT; N=267; for more information see Winhusen et al., 2014; Winhusen et al., 2012). Participants met criteria for cocaine dependence (N=295), methamphetamine dependence (N=205), or both (N=38) and were recruited at 12 outpatient treatment sites across the United States. Recruitment occurred via the National Institute on Drug Abuse (NIDA) National Drug Abuse Treatment Clinical Trials Network (CTN) between February 2010 and July 2012, with the protocol being reviewed and approved by each site’s local Institutional Review Board.
Measures
Nicotine craving
Once per week, we assessed nicotine craving over the past 24 hours using an item drawn from the Minnesota Nicotine Withdrawal Scale (Hughes, Hatsukami, & Pickens, 1984). Participants rated their “desire to smoke” on a 5-point scale ranging from 0=“None” to 4=“Severe.”
Stimulant craving
Once per week, four items from the Brief Substance Craving Scale (Mezinskis, Honos-Webb, Kropp, & Somoza, 2001; Somoza, Dyrenforth, Goldsmith, Mezinskis, & Cohen, 1995) assessed patient’s stimulant craving for their self-reported primary drug of choice (cocaine or methamphetamine) over the past 24 hours. Three items were rated on a 5-point scale and queried the intensity of craving (0=“None at all” to 4=“Extreme”), the frequency of craving (0=“Never” to 4=“Almost Constantly”), and the length of time spent craving (0=“None at all” to 4=“Very Long”). For a fourth item, participants were asked to estimate the number of times they had craving for the drug over the past 24 hours. To convert the four items to a common metric, z-scores were computed by comparing each item to that item’s mean and standard deviation for all participants across all six weeks, then rescaling back to a 5-point scale ranging from 0 to 4 to match the scaling for nicotine craving. The average score of the four items within a week was then used as the weekly stimulant craving index for each participant. The average Cronbach’s alpha was .89 across the six weeks.
Nicotine and Stimulant Use
The Timeline Followback (TLFB; Sobell and Sobell, 1996, 1992; Sobell et al., 1979) assessed participants’ daily nicotine use and stimulant use since the last assessment. For nicotine use, participants rated the number of cigarettes they smoked during each day since the last assessment. The numbers were then averaged to compute an average cigarettes smoked per day score for each week. For stimulant use (cocaine, amphetamine, methamphetamine), we computed the total number of days out of the last seven that participants used stimulants.
Administration Route
Participants reported at baseline whether they usually administer stimulants via smoking (N=331) versus nasal, IV or other routes (N=194).
Procedure
During the 10-week treatment phase, participants were scheduled to attend two research visits per week for efficacy and safety assessments. As part of these visits, participants completed the measures of use at each visit and of nicotine and stimulant craving once per week. Participants in the TAU+SCT condition set a uniform target quit date for smoking during week 3. They received one ten-minute counseling session per week and extended-release buproprion (300 mg/day) from weeks 1–10, and contingency management from weeks 4–10. Participants in the TAU condition did not set a uniform quit date for smoking, but were permitted to quit as desired during the treatment phase. For the current analyses, we used data from weeks 4–9, as measures of nicotine craving were only collected following the quit date (week 3) of the TAU+SCT group, and nicotine use was not collected for all days of week 10. Table 1 shows the descriptive statistics for nicotine and stimulant craving and use.
Table 1.
Means and Standard Deviations for Nicotine and Stimulant Craving and Use.
| Week | Nicotine Craving (past 24 hours) | Stimulant Craving (past 24 hours) | Nicotine Use (Cig/day in past week) | Stimulant Use (Use days in past week) |
|---|---|---|---|---|
| 4 | 2.30 (1.13) | 0.31 (0.50) | 7.95 (7.53) | 0.31 (0.89) |
| 5 | 2.08 (1.15) | 0.25 (0.44) | 7.89 (7.20) | 0.28 (0.82) |
| 6 | 2.00 (1.15) | 0.29 (0.49) | 7.80 (7.34) | 0.32 (0.93) |
| 7 | 1.84 (1.20) | 0.26 (0.45) | 7.61 (7.23) | 0.34 (0.94) |
| 8 | 1.78 (1.16) | 0.27 (0.49) | 7.50 (7.69) | 0.34 (0.94) |
| 9 | 1.70 (1.21) | 0.25 (0.46) | 7.26 (7.31) | 0.31 (0.85) |
Note. Nicotine craving refers to the “desire to smoke” over the past 24 hours on a 5-point scale ranging from 0 = “None” to 4 = “Severe.” Stimulant craving over the past 24 hours refers an index scaled to range from 0 to 4. Nicotine use refers to the average number of cigarettes smoked per day over the past week. Stimulant use refers to the number of days in the past week that a stimulant was used.
Results
Analytic Plan
To test our hypotheses, we used Latent Change Score (LCS) models (Ferrer & McArdle, 2010; McArdle, 2009), which are well-suited to the research questions in several important ways. First, LCS models provide information about whether change in one variable precedes change in another variable, while controlling for the general trajectories of each variable. This capability goes beyond alternative tests that only demonstrate a correlation between changes over time in two variables. Second, studying change can be difficult because estimates of change are often highly correlated with initial measurements, a major weakness of methods such as correlating raw change scores (Cronbach & Furby, 1970). LCS models explicitly separate out the portion of change that is correlated with a variable’s previous measurement, removing a source of unreliability that is a problem for measuring and predicting change. Third, LCS models use latent variables to represent change scores, allowing tests of relationships directly at the latent level while excluding the measurement error present in manifest variables.
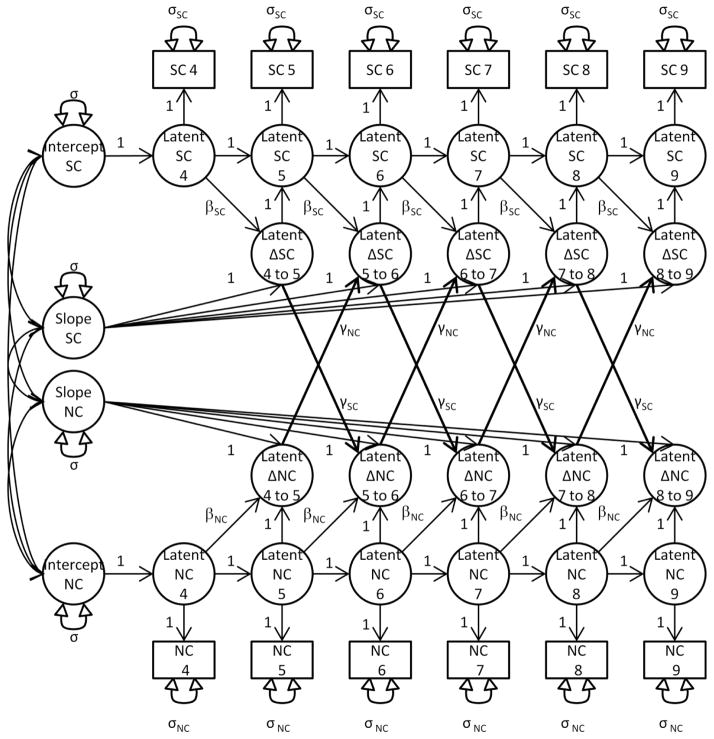
Figure 1 shows an example bivariate LCS model testing the coupling between changes in nicotine craving and stimulant craving. As seen in the model, latent change score variables (e.g., “Latent ΔNC 5 to 6”) representing weekly change are separated into three parameters that correspond to the features described above: 1) α, the portion of change that is associated with the overall linear trajectory for an individual across treatment, represented by regression paths (set to 1) from the slope variable to the latent change score variables; 2) β (the autoproportionality parameter), the portion of change that is associated with the previous level of the same variable; and 3) γ (the coupling parameter), the portion of the change in a variable that is associated with the preceding change in the other variable. This third parameter offers the key test of whether increases in nicotine craving precede changes in stimulant craving (e.g., the coupling parameter γNC denoting the path leading from “Latent ΔNC 5 to 6” to “Latent ΔSC 6 to 7”). Following common practice (McArdle, 2009), we set the intercepts of all manifest variables to zero to identify the model, and constrained each change parameter (i.e., α, β, & γ) to be constant across time. We also constrained the covariances between the manifest variables that were measured at the same time point (e.g., “NC 4 and SC 4”) to be constant across time, as this reduced the number of estimated parameters while not significantly altering model fit or the pattern of results.1 All models were fit using AMOS 21, and full information maximum likelihood methods were used to treat incomplete data as missing at random (Little & Rubin, 1987) unless otherwise noted.
Figure 1.
Path Diagram of a Bivariate Latent Change Score Model for Nicotine Craving and Stimulant Craving. Latent variables are represented by circles, manifest variables by squares, regression paths by single-headed arrows, and variances and covariances by double-headed arrows. Covariances were included between the manifest variables that were measured at the same time point (e.g., “SC 4 and NC 4”) and constrained to be constant across time, but are not represented in Figure 1.
Coupling between Nicotine and Stimulant Craving
Table 2 shows the full results and fit indices for each model. We first examined the model relating nicotine and stimulant craving (Model 1). The correlation between the intercepts for nicotine and stimulant craving did not reach significance (r=.11, p=.08), indicating that levels of the two were only weakly, non-significantly related when measured at a single time point (i.e., week 4). The slopes for nicotine and stimulant craving were positively correlated (r=.43, p<.001), indicating that their overall linear change across weeks 4–9 of treatment was associated (independent of previous measurement levels and any coupling association with the other variable). Finally, we examined our primary question of whether nicotine craving would demonstrate a carryover effect, substitution effect, or neither in its coupling relationship with stimulant craving. Results showed that increases in nicotine craving predicted subsequent decreases in stimulant craving (γ=−.37, p=.001) over the next week, supporting the substitution effect hypothesis. For the coupling parameter representing stimulant craving as the leading indicator, increases in stimulant craving were not related to subsequent increases in nicotine craving (γ=−.71, p=.14). Note that the coupling of nicotine and stimulant craving exists in conjunction with the association of the overall slopes reported above. So, while both types of cravings changed linearly in conjunction across weeks 4–9 of treatment, increases in nicotine craving that deviated above the overall trajectory for nicotine craving predicted subsequent decreases below the overall trajectory for stimulant craving.
Table 2.
Bivariate Latent Change Score Models Examining Polysubstance Craving and Use
| Model | rintercepts | rslopes | Lead variable | γ (SE) | β (SE) | CFI | RMSEA | χ2/df |
|---|---|---|---|---|---|---|---|---|
| 1 | .11+ | .43* | Nic. Craving | −.37* (.12) | −.28* (.04) | .98 | .045 | 2.11 |
| Stim. Craving | −.71 (.48) | −.23 (.14) | ||||||
| 2 | .58* | .42* | Nic. Craving | 1.26* (.60) | −.31* (.06) | .97 | .069 | 3.53 |
| Nic. Use | .20* (.06) | −.30* (.05) | ||||||
| 3 | −.05 | .25+ | Nic. Craving | −.07* (.03) | −.31* (.06) | .94 | .073 | 3.85 |
| Stim. Use | −2.43 (3.00) | .19 (.20) |
Note.
indicates p<.05 or below,
indicates p<.10 or below.
rintercepts and rslopes represent the correlations among the intercepts and slopes of the two model variables. γ represents the amount of change the lead indicator variable predicts in the lag variable. β represents the change in a variable predicted by the preceding measurement of the same variable. CFI, RMSEA, and χ2/df are indices reflecting the fit of the model to the data. Numbers closer to 1 indicate better fit for CFI, and lower numbers indicate better fit for RMSEA and χ2/df.
Coupling between Nicotine Craving and Nicotine and Stimulant Use
Next, we examined the two models relating nicotine craving to the two types of use. Note that when interpreting coefficients, one unit of nicotine use corresponds to one cigarette per day, whereas one unit of stimulant use corresponds to one day of stimulant use in the past week. For the model relating nicotine craving and nicotine use (Model 2), there were positive correlations between the level of nicotine craving and nicotine use at week 4 (r=.58, p<.001), as well as their overall change across treatment (r=.42, p<.001). The coupling parameters showed a bidirectional relationship: increases in nicotine craving predicted subsequent increases in the next week for nicotine use (γ=1.26, p=.04), and increases in nicotine use predicted subsequent increases in the next week for nicotine craving (γ=.20, p<.001).
For the model relating nicotine craving and stimulant use (Model 3), there was no significant relationship between the intercepts (r=−.05, p=.45) and the relationship between the slopes only neared significance (r=.25, p<.06). In terms of coupling, increases in nicotine craving predicted subsequent decreases the next week in stimulant use (γ=−.07, p=.03). There was no relationship between increased stimulant use and subsequent changes in nicotine craving (γ=−2.43, p=.42).
Together, these two models indicated that increases in nicotine craving are associated with different subsequent use patterns depending on the substance. Increases in nicotine craving predict subsequent increases in nicotine use over the next week, but predict decreased stimulant use over the same time frame.
Administration Route, Stimulant Type, and Smoking Cessation Treatment as Moderators of Coupling Relationships
Finally, we examined whether the significant coupling relationships would vary according to three potential moderators: administration route (smoking vs. other methods), the presence of smoking cessation treatment (TAU vs. TAU+SCT), and the type of stimulant dependence (cocaine vs. methamphetamine). For each moderator, we reran the three models, this time estimating the parameters within each subgroup separately.
For administration route, we tested our hypothesis that participants who administered stimulants via smoking (smoking-route stimulant users) would show stronger coupling between their nicotine and stimulant cravings than participants who used other administration routes (other-route stimulant users). As expected, the substitution effect was relatively greater in magnitude and only significant among smoking-route stimulant users. Specifically, increases in nicotine craving predicted subsequent decreases in stimulant craving for smoking-route stimulant users (γ=−.56, p=.005) but only neared significance for other-route stimulant users (γ=−.32, p=.06). Next, we explored differences between administration routes in the other two models. In Model 2, increases in nicotine craving predicted subsequent increases in nicotine use for smoking-route stimulant users (γ=1.49, p<.05), but not for other-route stimulant users (γ=.68, p=.39). In contrast, increases in nicotine use predicted increases in nicotine craving for both smoking-route stimulant users (γ=.20, p=.009) and other-route stimulant users (γ=.33, p=.02). Finally, for Model 3, increases in nicotine craving did not predict subsequent changes in stimulant use among smoking-route stimulant users (γ=−.08, p=.06) but did predict decreases in stimulant use for other-route stimulant users (γ=−.22, p<.001). Across the three models, these results indirectly supported the mechanisms proposed to underlie the substitution effect for polysubstance craving, but identified more complex moderation possibilities in the other models.
Next, we examined differences according to the presence of smoking cessation treatment. For Model 1, increases in nicotine craving predicted subsequent decreases in stimulant craving for the TAU (γ=−.52, p=.001)2 but not the TAU + SCT group (γ=−.07, p=.39). In Model 2, again only the TAU group demonstrated significant relationships: increases in nicotine craving predicted increases in nicotine use for the TAU (γ=1.58, p=.03) but not the TAU + SCT group (γ=−.41, p=.61). Additionally, increases in nicotine use predicted increases in nicotine craving for the TAU (γ=.26, p<.001) but not the TAU + SCT group (γ=.02, p=.77). Finally, for Model 3, convergence problems prevented the model from being estimated for the TAU group. For the TAU + SCT group, there was no relationship between increases in nicotine craving and subsequent changes in stimulant use (γ=−.05, p=.29). Thus, coupling relationships were generally stronger in the TAU group, although convergence issues prevented more conclusive comparisons.
Lastly, we evaluated stimulant type as a moderator of the coupling effects. In Model 1, increases in nicotine craving predicted subsequent decreases in stimulant craving for cocaine (γ=−.30, p=.01) but not for methamphetamine users (γ=−.07, p=.45). In Model 2, increases in nicotine craving did not significantly predict changes in nicotine use for either cocaine (γ=.78, p=.30) or methamphetamine users (γ=1.19, p=.06). In contrast, increases in nicotine use predicted increases in nicotine craving for both cocaine (γ=.16, p=.01) and methamphetamine users (γ=.36, p<.001). For Model 3, increases in nicotine craving did not significantly predict changes in stimulant use for cocaine users (γ=.03, p=.07), but predicted decreases in stimulant use for methamphetamine users (γ=−.14, p=.02). Thus, while there was no single pattern across the three models, the main substitution effect for polysubstance craving appeared to be stronger among cocaine users.
Discussion
This study examined the week to week coupling of nicotine and stimulant craving during stimulant treatment, as well as the coupling of nicotine craving with nicotine and stimulant use. Consistent with theoretical models that emphasize the elaboration of substance-specific cravings, there was a substitution effect for polysubstance craving, as increases in nicotine craving predicted subsequent decreases in stimulant craving (and use). Within nicotine, there was a bidirectional coupling relationship such that increases in both nicotine craving and use predicted subsequent increases in the other. As expected, the substitution effect for polysubstance craving was larger in magnitude and significant only for participants who administered stimulants through smoking, providing additional indirect support for the proposed mechanisms. Finally, the results indicated that the type of stimulant and the presence of smoking cessation treatment are potential moderators of the strength of coupling effects that should be evaluated more extensively. These results demonstrate that, over time, nicotine craving conveys important information about weekly shifts in polysubstance craving and use that have important implications for clinicians.
The finding of a substitution effect for polysubstance craving supported the predictions of theories that emphasize the elaboration of cravings in a substance-specific manner. These results support further inquiry into the possibility that imagery, attributions, or other types of nicotine-related elaboration may limit the elaboration of craving for a second substance, potentially explaining why nicotine abstinence sometimes aids drug abstinence in trials (Baca & Yahne, 2009). As a next step, it would be useful to directly manipulate the to-be-elaborated substance and type of elaboration (e.g., imagery vs. attributions) among polysubstance users to evaluate the resulting effects upon craving for other substances. Similarly, this evidence provides clinicians with preliminary support for assessing elaborative processes such as attributions, interpretations, and imagery that could contribute to patients’ craving for a one substance while inhibiting craving for another.
The timing of treatment activities studied may be important for understanding why nicotine craving was a significant leading indicator, but stimulant craving was not. While all participants began attempts to quit stimulants during week 1, many participants began attempts to quit smoking during week 3, immediately preceding weeks 4–9 that were included in our models. As a result, it is possible that ambiguous physiological symptoms were readily attributed to nicotine craving during the period studied due to the greater salience of attempts to quit that substance, leading to a subsequent decrease in reported stimulant craving. If we had assessed weeks 1–3, stimulant cravings instead may have been the stronger lead indicator, although the general pattern would still be a substitution one. At the same time, we are reticent to over-interpret the possibility of stimulant craving being a leading indicator under different circumstances, given that the relationship did not near significance (p=.14).
When interpreting weekly shifts in patients’ cravings, it is critical to understand that these results reflect craving processes that operate over a weekly time frame, rather than ones that occur over the course of minutes, hours, years, or other informative time frames. The current choice of a weekly time frame has several advantages when translating these results to the information tracked by clinicians. First, the identified coupling process aligns with the weekly timing of sessions in many treatments, allowing a direct mapping of the time sequence. Second, there is an intuitive fit between the meaning of coupling parameters and the way many clinicians monitor progress. With coupling, the focus is on predicted deviations above or below an individual’s overall treatment trajectory. Similarly, clinicians attend not only to whether patients are improving, stable, or worsening (i.e., the overall treatment trajectory), but also to whether patients are experiencing sudden deviations from their trajectories (e.g., “I’m concerned about this patient; she has been improving gradually, but the last two sessions has really taken a step backwards”). These results suggest that sudden increases in nicotine craving should not necessarily alarm clinicians as being signs of looming problems with stimulant craving and use. Instead, knowing the possibility of a substitution effect could help clinicians to prepare for and build upon a higher likelihood of decreased stimulant craving and use the next week. This rationale follows recommendations to advance conceptualizations of addiction treatment in part by better understanding sudden shifts in constructs like craving (Morgenstern & McKay, 2007).
Further indirect support for the proposed mechanisms came from our finding of stronger coupling among participants who used smoking to administer stimulants. Future research could test whether these stronger relationships stem from stronger shared cues across substances, more ambiguous physiological symptoms, or other factors. Given the finding of a substitution effect for craving, another productive route of inquiry may be testing how imagery can be used to disrupt polysubstance craving. While several studies have used alternative imagery to beneficially impact craving for one substance (May et al., 2010; Versland & Rosenberg, 2007), imagery interventions may have particular applicability to polysubstance craving, in which imagery could disrupt craving for multiple substances, or whichever substance is most salient at a particular time. However, this promising direction is speculative pending more direct assessment and manipulation of the current proposed mechanisms.
In terms of the other potential moderators, there were hints of subgroups in which the size of the substitution effects may vary. Notably, substitution effects tended to be present among participants who received treatment as usual, but absent among participants who received added smoking cessation treatment. An intriguing explanation for this finding could be that the smoking cessation treatment group perceived less of an opportunity to use nicotine, which is associated with lower levels of craving (Wertz & Sayette, 2001). In the context of stimulant treatment, smoking cessation treatment could have eliminated a perceived opportunity to use nicotine to cope with stimulant craving, thus lessening not only nicotine craving but also its link to stimulant craving. Alternatively, generally low levels of stimulant and nicotine craving in the smoking cessation treatment group due to a low perceived opportunity to use both substances could have made the link between physiological discomfort from abstinence and a particular substance clearer. By reducing ambiguity about the perceived cause of the discomfort, this would diminish the potential for influence by elaborative processes. Similarly, receiving pharmacological support (buproprion) that often suppresses nicotine craving (Durcan et al., 2002) could have broken or substantially reduce the coupling of craving across substances. In addition to treatment group effects, the tests of substance type as a moderator revealed that cocaine users experienced stronger substitution effects than methamphetamine users, which should be replicated with more direct comparisons.
Limitations and Conclusion
While the current study demonstrated the usefulness of LCS models for understanding week to week changes in craving, multiple measurement methods and designs are needed to tease apart complex constructs such as craving and link them to mechanisms underlying polysubstance relationships. LCS models, while helpful for representing a particular temporal order of changes within a process, do not establish causal relationships between the variables. Additionally, the levels of stimulant craving and use reported by participants were quite low. Although we were able to detect coupling relationships despite this potential floor effect, examinations across more variable levels of stimulant craving and use are important for generalizing these effects. Finally, our examination of smoking cessation treatment as a potential moderator was limited by convergence issues in two of the models, and all of the moderator models were estimated using a smaller sample size than the full sample models.
Overall, nicotine and stimulant craving are linked during stimulant treatment in a way that is not evident when simply examining single time points or overall slopes. This research improves the understanding of how the ebb and flow of craving relates across substances and holds promise for treatments that harness these interrelationships to improve abstinence from co-occurring substance use problems.
Public Health Significance.
This study demonstrates that people completing stimulant dependence treatment who experience increases in nicotine craving over one week are more likely to experience decreases in stimulant craving during the next week. Increases in nicotine craving during stimulant treatment do not appear to put people at risk for triggering increases in their stimulant craving and use.
Acknowledgments
This research was supported by the following grants from the National Institute on Drug Abuse:
U10-DA013732 to University of Cincinnati (Dr. Winhusen);
U10-DA020036 to University of Pittsburgh (Dr. Daley);
U10-DA013720 to University of Miami School of Medicine (Drs. Szapocznik and Metsch);
U10-DA013045 to University of California Los Angeles (Dr. Ling);
U10-DA013727 to Medical University of South Carolina (Dr. Brady);
U10-DA020024 to University of Texas Southwestern Medical Center (Dr. Trivedi);
U10-DA015815 to Universtiy of California San Fransicso (Drs. Sorensen and McCarty).
Footnotes
We initially entered time-invariant covariates (age, gender, and race) for all models, and time-varying covariates for the cross-substance models to tease apart the variance accounted for by craving versus use of a substance. For instance, for the nicotine and stimulant craving model, nicotine use and stimulant use were entered as time-varying covariates on nicotine craving and stimulant craving, respectively. However, neither type of covariate altered the pattern of results, and their inclusion significantly worsened the fit of the models, suggesting that the more parsimonious models were preferable.
Model 1 for the TAU group had convergence issues, but was able to be estimated when removing missing data. To make the TAU + SCT group results more comparable, we estimated Model 1 for the TAU + SCT group using the same missing data procedure, although the pattern of results for TAU + SCT was the same using either method.
Contributor Information
Joshua C. Magee, Email: joshuacmagee@gmail.com.
Theresa Winhusen, Email: winhust@ucmail.uc.edu.
References
- Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. Journal of Substance Abuse Treatment. 2009;36(2):205–19. doi: 10.1016/j.jsat.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Carter B, Tiffany S. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. Retrieved from http://onlinelibrary.wiley.com/doi/10.1046/j.1360-0443.1999.9433273.x/full. [PubMed] [Google Scholar]
- Cronbach LJ, Furby L. How we should measure “change” - or should we? Psychological Bulletin. 1970;74(1):68–80. doi: 10.1037/h0029382. [DOI] [Google Scholar]
- Durcan M, Deener G, White J, Johnston J, Gonzales D, Niaura R, Sachs D. The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clinical Therapeutics. 2002;24:5401–51. doi: 10.1016/S0149-2918(02)85130-X. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: Craving and use during daily life. Addictive Behaviors. 2010;35(4):318–24. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, McArdle JJ. Longitudinal modeling of developmental changes in psychological research. Current Directions in Psychological Science. 2010;19(3):149–154. doi: 10.1177/0963721410370300. [DOI] [Google Scholar]
- Florsheim P, Shiozaki T, Hiraoka R, Tiffany S, Heavin S, Hall S, Clegg C. Craving among polysubstance using adolescents. Journal of Child Adolescent Substance Abuse. 2008;17:101–24. doi: 10.1300/J029v17n02. [DOI] [Google Scholar]
- Gritz ER, Carr CA, Marcus AC. The tobacco withdrawl syndrome in unaided quitters. British Journal of Addiction. 1991;86:57–69. doi: 10.1111/j.1360-0443.1991.tb02629.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Pickens RW, Krahn D, Malin S, Luknic A. Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacology. 1984;83:82–87. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: The elaborated intrusion theory of desire. Psychological Review. 2005;112(2):446–67. doi: 10.1037/0033-295X.112.2.446. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Lai H, Page JB, McCoy CB. The association between cigarette smoking and drug abuse in the United States. Journal of Addictive Diseases. 2000;19(4):11–24. doi: 10.1300/J069v19n04_02. [DOI] [PubMed] [Google Scholar]
- Lemon SC, Friedmann PD, Stein MD. The impact of smoking cessation on drug abuse treatment outcome. Addictive Behaviors. 2003;28(7):1323–1331. doi: 10.1016/S0306-4603(02)00259-9. [DOI] [PubMed] [Google Scholar]
- May J, Andrade J, Panabokke N, Kavanagh D. Visuospatial tasks suppress craving for cigarettes. Behaviour Research and Therapy. 2010;48(6):476–85. doi: 10.1016/j.brat.2010.02.001. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- Mezinskis JP, Honos-Webb L, Kropp F, Somoza E. The measurement of craving. Journal of Addictive Diseases. 2001;20(3):67–85. doi: 10.1300/J069v20n03_07. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, McKay JR. Rethinking the paradigms that inform behavioral treatment research for substance use disorders. Addiction. 2007;102(9):1377–89. doi: 10.1111/j.1360-0443.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- Nosen E, Woody SR. Applying lessons learned from obsessions: Metacognitive processes in smoking cessation. Cognitive Therapy and Research. 2007;33(2):241–54. doi: 10.1007/s10608-007-9180-8. [DOI] [Google Scholar]
- Nosen E, Woody SR. Acceptance of cravings: How smoking cessation experiences affect craving beliefs. Behaviour Research and Therapy. 2014;59:71–81. doi: 10.1016/j.brat.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology. 2009;207(2):291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology. 1976;50(1):35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption: Psychosocial and biological Methods. New Jersey: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback (TLFB) users’ manual. Toronto: Addiction Research Foundation; 1996. [Google Scholar]
- Somoza E, Dyrenforth S, Goldsmith J, Mezinskis J, Cohen M. In search of a universal drug craving scale. Paper presented at the annual meeting of the American Psychiatric Association; Miami, Florida. 1995. [Google Scholar]
- Stark MJ, Campbell BK. Drug use and cigarette smoking in applicants for drug abuse treatment. Journal of Substance Abuse. 1993;5:175–81. doi: 10.1016/0899-3289(93)90060-O. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Wray J. The continuing conundrum of craving. Addiction. 2009;104:1618–19. doi: 10.1111/j.1360-0443.2009.02588.x. [DOI] [PubMed] [Google Scholar]
- Versland A, Rosenberg H. Effect of brief imagery interventions on craving in college student smokers. Addiction Research & Theory. 2007;15(2):177–187. doi: 10.1080/16066350701200582. [DOI] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity on self-reported urge. Experimental and Clinical Psychopharmacology. 2001;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Brigham GS, Kropp F, Lindblad R, Gardin JG, II, Penn P, Ghitza U. A randomized trial of concurrent smoking-cessation and substance use disorder treatment in stimulant-dependent smokers. Journal of Clinical Psychiatry. 2014;75:336–43. doi: 10.4088/JCP.13m08449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Stitzer M, Woody G, Brigham G, Kropp F, Ghitza U, Somoza E. Design considerations for a study to evaluate the impact of smoking cessation treatment on stimulant use outcomes in stimulant-dependent individuals. Contemporary Clinical Trials. 2012;33(1):197–205. doi: 10.1016/j.cct.2011.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]