Abstract
Rats selectively bred for high (HiS) or low (LoS) saccharin intake are a well-established model of drug abuse vulnerability, with HiS rats being more likely to consume sweets and cocaine (Carroll et al. 2002) than LoS rats. Still, the nature of these differences is poorly understood.
This study examined whether the motivational consequences of cocaine exposure are differentially expressed in HiS and LoS rats by measuring intracranial self-stimulation (ICSS) thresholds following acute injections of cocaine (10 mg/kg).
Reductions in ICSS thresholds following cocaine injection were greater in HiS rats than in LoS rats, suggesting that the reward enhancing effects of cocaine are greater in the drug-vulnerable HiS than LoS rats.
Higher cocaine-induced reward, indicated by lower ICSS thresholds, may explain the higher rates of drug consumption in sweet-preferring animal models, providing a clue to the etiology of cocaine addiction in vulnerable populations.
Keywords: cocaine, ICSS, rat, motivation, sweet preference, selective breeding, rat
1. Introduction
Humans with a greater preference for sweet substances are also more likely to abuse drugs (Janowsky et al. 2003; Kampov-Polevoy et al. 1997, 2001; Pomerleau et al. 1991; Weiss 1982), and rats selectively bred for high (HiS) or low (LoS) saccharin intake (Carroll et al. 2008, 2012, Carroll and Holtz 2014) have consequently been used as a model of drug-abuse vulnerability. HiS rats consume a variety of sweeteners more avidly (Dess 2000; Dess and Minor 1996; Dess et al. 2005) and respond at higher rates than LoS rats in an operant conditioning paradigm for a sucrose reward (Gosnell et al. 2010). HiS and LoS rats also display differences in measures of drug withdrawal (Radke et al. 2013; 2015; Holtz et al. 2015). On measures of cocaine-seeking, HiS rats are more vulnerable to acquisition, escalation, and reinstatement of drug seeking and maintain a higher level of drug intake (Carroll et al. 2002; Perry et al. 2006; Holtz and Carroll 2013) than their LoS counterparts. While we know much about the drug-taking behavior of these animals, the underlying cause of these differences has yet to be determined.
In this study the acute reward-enhancing effects of cocaine were assessed with intracranial self-stimulation (ICSS) thresholds (Markou and Koob 1992), which represent the lowest level of electrical self-stimulation that will maintain operant responding in the rat (Stoker and Markou 2011). ICSS behavior is mediated by the dopaminergic fibers of the medial forebrain bundle (Corbett and Wise 1980; Young and Michael 1993) and acute injections of cocaine (Galankin et al. 2010; Kenny et al. 2003a; Markou and Koob 1992) and other psychostimulants (Lin et al. 2000; Wise and Munn 1995) are thought to decrease thresholds by increasing sensitivity of this brain reward system. This method was used to determine whether HiS and LoS rats are differentially sensitive to the reward-enhancing effects of cocaine, suggesting that their genetic propensity for sweet substances may extend to drug-rewarded behavior.
Methods
Subjects
HiS and LoS rats were selectively bred at the University of Minnesota (Carroll et al. 2002) from Occidental HiS and LoS lines (Occidental College, Los Angeles, CA). Thirteen age-matched, adult, male rats from 7 litters were used in this study (8 HiS rats and 5 LoS). One HiS rat was excluded due to a strong aversive reaction to cocaine injection across multiple days. All procedures conformed to the eighth edition of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Academies Press 2011) and were approved by the University of Minnesota Institutional Animal Care and Use Committee under protocol number 1008A87755. All laboratory facilities were approved by the Association for Assessment and Accreditation of Laboratory Animal Care.
Surgery
Rats were anesthetized with a combination of ketamine (60 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and administered doxapram (5 mg/kg, i.p.) and atropine (0.4 mg/mL, 0.15 ml, s.c.) to facilitate respiration. With the incisor bar of a Kopf stereotaxic instrument set at +5.0 mm, bipolar stainless steel electrodes (#MS303-2-B-SPC, Plastics One, Roanoke, VA) were lowered into the medial forebrain bundle at the lever of the lateral hypothalamus (AP: −0.5 mm, ML: ±1.0 mm, DV: −8.3 mm from Bregma). Jeweler screws were anchored to the skull, and the entire assembly was cemented into place.
Effects of acute cocaine on ICSS thresholds
Rats were housed individually in hanging, wire mesh cages and trained and tested in custom-made operant conditioning chambers according to modified version of the Kornetsky and Esposito (1979) discrete-trial current threshold procedure (Harris et al. 2010; Markou and Koob 1992) previously employed in our laboratory (Holtz et al. 2015). Brain stimulation was administered with constant current stimulators (model #PHM-152, Med Associates, St. Albans, VT). Rats were connected to the stimulation circuit through bipolar leads attached to gold-contact swivel commutators (Plastics One, Roanoke, VA).
Briefly, for threshold testing, each trial was initiated with presentation of a non-contingent stimulus, followed by a 7.5 s window during which a positive response on the wheel produced a second contingent stimulation identical to the first. Lack of responding in the 7.5 s window was considered a negative response. Stimulus intensities were presented in four alternating descending and ascending series (step size, 5 μA), with five trials presented at each current intensity step. The current threshold for each series was defined as the midpoint between two consecutive intensity steps that yielded three or more positive responses and two consecutive intensity steps that yielded three or more negative responses. The overall ICSS threshold for the session was defined as the mean of the current thresholds from the four alternating series. To assess performance effects (e.g., motor disruption), response latencies (time between onset of the non-contingent stimulus and a positive response) were averaged across all trials in which a positive response was made. ICSS sessions were conducted once a day, 7 days a week during training and testing.
Following acquisition of the threshold procedure (defined as less than 10% variation in thresholds over three sessions), rats were given a baseline of five sessions of saline (=baseline) followed by five sessions of cocaine (10 mg/kg, i.p.) injections 10 min before the threshold session. This dose and timing regimen have previously been shown to optimally lower ICSS thresholds without impairing the operant response (Kenny et al. 2003a; Markou and Koob 1992). This dose was also used in our previous work examining cocaine-induced reinstatement of drug-seeking behavior (Perry et al. 2006, Holtz and Carroll 2011) and cocaine-induced locomotor sensitization (Carroll et al. 2007) in the HiS and LoS lines.
Saccharin preference testing
Following standard procedure in our laboratory, two weeks after the final cocaine exposure a phenotype score was derived from a 24 h two-bottle test (see Badia-Elder et al. 1996 for details) in which consumption of 0.1% saccharin solution vs. water was assessed relative to previously attained 24 h water intake, divided by body weight [saccharin score = (saccharin mL − water baseline ml)/body weight × 100].
Drugs
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC), dissolved in 0.9% sterile saline, and injected i.p.
Data Analysis
Throughout the text and figures all data are expressed as mean ± SEM. Thresholds were measured in μA. To minimize variability in thresholds across rats, data were expressed as percent of baseline by averaging each rat’s thresholds from the five sessions of saline injection. Thresholds from each session of saline or cocaine injection were then divided by this average (percent score = cocaine/saline baseline). Data were analyzed using factorial ANOVA, with repeated measures on within-subject factors, or t-tests where appropriate. Follow-up analyses were done with Tukey’s test for multiple comparisons. All statistical analyses were conducted using SPSS (version 17.0) with a Type I error rate of α = 0.05. Outliers were detected with Grubb’s test (GraphPad QuickCalcs).
2. Results
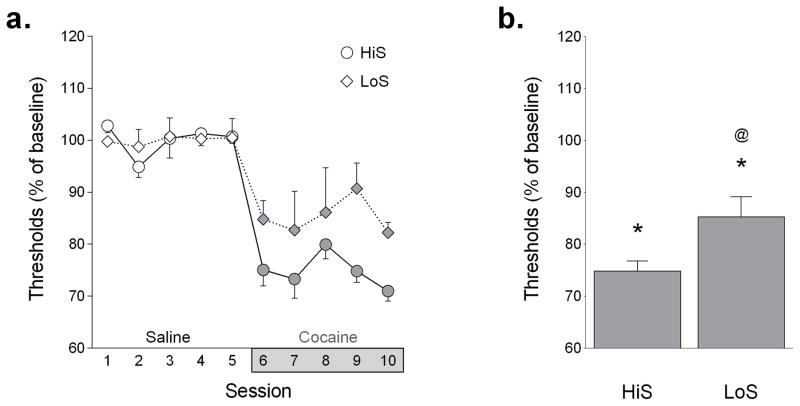
No differences in baseline ICSS thresholds were observed between HiS and LoS rats (Table 1). An initial repeated measures ANOVA (session × line) on the 5 days of cocaine injection revealed a significant main effect of line (F1,10 = 6.841, p < 0.05) but not session (Fig 1A).
Table 1.
Experimental group information and results
| Line | N | Saccharin Phenotype Score* | Saline thresholds (μA) | Cocaine thresholds (μA) | Difference (Saline - Cocaine) (μA)* | Percentage (Cocaine/Saline) (%)* |
|---|---|---|---|---|---|---|
| HiS | 7 | 17.57 ± 3.01 | 130.93 ± 16.48 | 99.32 ± 14.79 | 31.61 ± 2.85 | 74.82 ± 1.97 |
| LoS | 5 | 10.23 ± 3.87 | 99.28 ± 18.67 | 83.23 ± 13.26 | 16.05 ± 6.28 | 85.31 ± 3.91 |
Significant difference between lines, p < 0.05
Fig 1. ICSS thresholds after cocaine injection.
a) ICSS thresholds were measured in HiS (circles, n = 7) and LoS (diamonds, n = 5) rats 10 min after injection with saline or 10 mg/kg cocaine and expressed as a percentage of the average threshold during saline treatment (=baseline). b) Average ICSS thresholds collapsed across sessions. *p < 0.001 compared to saline; @ p < 0.01 compared to HiS rats during cocaine treatment.
When data were collapsed across sessions, a two-way ANOVA (line × treatment) revealed significant main effects of line (F1, 20 = 6.85, p < 0.05) and treatment (F1, 20 = 98.91, p < 0.001) as well as a significant line x treatment interaction (F1, 20 = 6.85, p < 0.05) (Fig 1B). When data were expressed as a percentage of saline baseline, thresholds following cocaine injection were significantly lower than the preceding baseline in both HiS (p < 0.001) and LoS (p < 0.001) rats and thresholds following cocaine injection were also significantly lower in HiS rats than in LoS rats (p < 0.01). Response latencies at during saline treatment did not differ between HiS and LoS rats (HiS: 2.92 ± 0.18 s, LoS: 3.04 ± 0.48 s) and were unaffected by cocaine treatment (HiS: 96.29 ± 9.90% of baseline, LoS: 99.16 ± 2.81%).
After completion of the ICSS experiment, a t-test of saccharin phenotype scores revealed a significant difference in saccharin phenotype scores between HiS and LoS rats (Table 1) (t9 = 2.67; p < 0.05) after one outlier in the LoS group (> 2 standard deviations of the mean) was removed from the data analysis. Removal of this animal from the ICSS analysis did not change any of the results reported above and saccharin preference scores are in line with values typically observed in HiS and LoS rats (Carroll et al., 2008). Water intake did not differ between the lines.
Discussion
Multiple studies have confirmed that HiS rats self-administer drugs at higher rates than LoS rats (Carroll et al. 2002; Perry et al. 2006; Holtz and Carroll 2013), but less is known about the factors that contribute to this difference. The results of this study indicate that HiS rats exhibit lower ICSS thresholds than LoS rats in response to an acute cocaine injection. These results suggest that the reward-enhancing effects of cocaine are greater in the HiS line compared with LoS, indicating that increased reward sensitivity in the HiS vs LoS rats may contribute to increased drug seeking in the HiS line. Indeed, links between ICSS thresholds and cocaine seeking have been demonstrated. For example, cocaine self-administration acutely lowers ICSS thresholds (Kenny et al. 2003b), and this positive affective state is necessary for the acquisition and maintenance of self-administration behavior. Furthermore, pharmacological treatments that attenuate drug-induced lowering of ICSS thresholds (e.g., glutamate receptor blockers) can also reduce drug consumption (Jin et al. 2010; Kenny et al. 2005; Kenny et al. 2009; Paterson et al. 2008).
While enhanced reward sensitivity in the drug-preferring HiS line may seem predictable, such a link between the selectively-bred lines and a measure of drug reward has not previously been explicitly demonstrated. Furthermore, because decreased reward sensitivity has also been associated with increased self-administration rates (Ahmed et al. 2002), it is necessary to define the response of the HiS and LoS lines. The current study makes an important contribution to understanding the neurobiological factors driving cocaine-seeking in HiS and LoS rats.
Overall, greater reward enhancement in sweet-preferring animals may explain their higher rates of drug consumption. This conclusion may provide a clue to the etiology of cocaine addiction in vulnerable human populations, as similar enhancements in reward sensitivity could promote higher rates of drug consumption.
Acknowledgments
We thank Dr. Andrew Harris for advice on intracranial self-stimulation and Seth Johnson for superb technical assistance. This research was supported by NIH grant R01 DA003240-28 (MEC).
Footnotes
Conflict of interest: None declared
References
- Badia-Elder N, Kiefer SW, Dess NK. Taste reactivity in rats selectively bred for high vs. low saccharin consumption. Physiol Behav. 1996;59:749–755. doi: 10.1016/0031-9384(95)02131-0. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Holtz NA. The relationship between feeding and drug-seeking behaviors. In: Brewerton TD, Dennis AB, editors. Eating Disorders, Addictions and Substance Use Disorders: Research, Clinical and Treatment Aspects. Springer-Verlag; New York: 2014. pp. 23–45.pp. 681 [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Higher locomotor response to cocaine in female (vs. male) rats selectively bred for high (HiS) and low (LoS) saccharin intake. Pharmacol Biochem Behav. 2007;88:94–104. doi: 10.1016/j.pbb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Holtz NA, Zlebnik NE. Saccharin preference in rats: Relation to impulsivity and comorbid drug intake, in Animal Models of Eating Disorders. In: Avena NM, editor. Neuromethods. Vol. 74. Springer-Verlag; New York: 2012. pp. 201–234.pp. 380 [Google Scholar]
- Corbett D, Wise RA. Intracranial self-stimulation in relation to the ascending dopaminergic systems of the midbrain: A moveable electrode mapping study. Brain Res. 1980;185:1–15. doi: 10.1016/0006-8993(80)90666-6. [DOI] [PubMed] [Google Scholar]
- Dess NK. Responses to basic taste qualities in rats selectively bred for high versus low saccharin intake. Physiol Behav. 2000;69:247–257. doi: 10.1016/s0031-9384(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Dess NK, Minor TR. Taste and emotionality in rats selectively bred for high versus low saccharin intake. Anim Learn Behav. 1996;24:105–115. [Google Scholar]
- Dess NK, O’Neill P, Chapman CD. Ethanol withdrawal and proclivity are inversely related in rats selectively bred for differential saccharin intake. Alcohol. 2005;37:9–22. doi: 10.1016/j.alcohol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Galankin T, Shekunova E, Zvartau E. Estradiol lowers intracranial self-stimulation thresholds and enhances cocaine facilitation of intracranial self-stimulation in rats. Horm Behav. 2010;58:827–834. doi: 10.1016/j.yhbeh.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Mitra A, Avant RA, Anker JJ, Carroll ME, Levine AS. Operant responding for sucrose by rats bred for high or low saccharin consumption. Physiol Behav. 2010;99:529–533. doi: 10.1016/j.physbeh.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Mattson C, LeSage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacol Biochem Behav. 2010;96:217–227. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. Baclofen has opposite effects on escalation of cocaine self-administration: increased intake in rats selectively bred for high (HiS) saccharin intake and decreased intake in those selected for low (LoS) saccharin intake. Pharmacol Biochem Behav. 2011;100:275–283. doi: 10.1016/j.pbb.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. Escalation of i.v. cocaine intake in peri-adolescent vs. adult rats selectively bred for high (HiS) vs. low (LoS) saccharin intake. Psychopharmacology. 2013;227:243–250. doi: 10.1007/s00213-012-2958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Radke AK, Zlebnik NE, Harris AC, Carroll ME. Intracranial self-stimulation reward thresholds during morphine withdrawal in rats bred for high (HiS) and low (LoS) saccharin intake. Brain Res. 2015;1602:119–126. doi: 10.1016/j.brainres.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, Pucilowski O, Buyinza M. Preference for higher sucrose concentrations in cocaine abusing-dependent patients. J Psychiat Res. 2003;37:35–41. doi: 10.1016/s0022-3956(02)00063-8. [DOI] [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, Conn PJ, Cosford ND, Markou A. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35:2021–2036. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiat. 1997;154:269–270. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy A, Tsoi M, Zvartau E, Neznanov N, Khalitov E. Sweet liking and family history of alcoholism in hospitalized alcoholic and non-alcoholic patients. Alcohol Alcoholism. 2001;36:165–170. doi: 10.1093/alcalc/36.2.165. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Koob GF, Markou A. Conditioned facilitation of brain reward function after repeated cocaine administration. Behav Neurosci. 2003a;117:1103. doi: 10.1037/0735-7044.117.5.1103. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Polis I, Koob GF, Markou A. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur J Neurosci. 2003b;17:191–195. doi: 10.1046/j.1460-9568.2003.02443.x. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology. 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Federation proceedings. 1979:2473. [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Time-dependent alterations in ICSS thresholds associated with repeated amphetamine administrations. Pharmacol Biochem and Behav. 2000;65:407–417. doi: 10.1016/s0091-3057(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A. Positive modulation of GABAB receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J Pharmacol Exp Ther. 2008;326:306–314. doi: 10.1124/jpet.108.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of iv cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology. 2006;186:235–245. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Garcia AW, Drewnowski A, Pomerleau OF. Sweet taste preference in women smokers: comparison with nonsmokers and effects of menstrual phase and nicotine abstinence. Pharmacol Biochem Behav. 1991;40:995–999. doi: 10.1016/0091-3057(91)90118-l. [DOI] [PubMed] [Google Scholar]
- Radke AK, Holtz NA, Gewirtz JC, Carroll ME. Reduced emotional signs of opiate withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake. Psychopharmacology. 2013;227:117–126. doi: 10.1007/s00213-012-2945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Zlebnik NE, Carroll ME. Cocaine withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake. Pharmacol Biochem Behav. 2015;129:51–55. doi: 10.1016/j.pbb.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Markou A. The intracranial self-stimulation procedure provides quantitative measures of brain reward function mood and anxiety related phenotypes in mice. Springer; 2011. pp. 307–331. [Google Scholar]
- Weiss G. Food fantasies of incarcerated drug users. Subst Use Misuse. 1982;17:905–912. doi: 10.3109/10826088209056337. [DOI] [PubMed] [Google Scholar]
- Wise R, Munn E. Withdrawal from chronic amphetamine elevates baseline intracranial self-stimulation thresholds. Psychopharmacology. 1995;117:130–136. doi: 10.1007/BF02245178. [DOI] [PubMed] [Google Scholar]
- Young SD, Michael AC. Voltammetry of extracellular dopamine in rat striatum during ICSS-like electrical stimulation of the medial forebrain bundle. Brain Res. 1993;600:305–307. doi: 10.1016/0006-8993(93)91387-8. [DOI] [PubMed] [Google Scholar]