Summary
Precise patterning of dendritic arbors is critical for the wiring and function of neural circuits. Dendrite-extracellular matrix (ECM) adhesion ensures that the dendrites of Drosophila dendritic arborization (da) sensory neurons are properly restricted in a 2D space, and thereby facilitates contact-mediated dendritic self-avoidance and tiling. However, the mechanisms regulating dendrite-ECM adhesion in vivo are poorly understood. Here, we show that mutations in the semaphorin ligand sema-2b lead to a dramatic increase in self-crossing of dendrites due to defects in dendrite-ECM adhesion, resulting in a failure to confine dendrites to a 2D plane. Furthermore, we find that Sema-2b is secreted from the epidermis and signals through the Plexin B receptor in neighboring neurons. Importantly, we find that Sema-2b/PlexB genetically and physically interacts with TORC2 complex, Tricornered (Trc) kinase and integrins. These results reveal a novel role for semaphorins in dendrite patterning and illustrate how epidermal-derived cues regulate neural circuit assembly.
eTOC Blurb
Meltzer et al. demonstrate that semaphorin ligand derived from epidermis is required to restrict dendrites into a 2D space, thereby facilitating self-avoidance. They further show that Trc/Fry and integrin signaling pathways are components of Sema-2b/PlexB signaling.
Introduction
Precise patterning of dendritic arbors is crucial for the development and function of the nervous system, but the mechanisms that instruct dendrite patterning are only beginning to be elucidated (Jan and Jan, 2010; Dong et al., 2014; Puram and Bonni, 2013). In humans, defects in dendritic morphology are linked to neurodevelopmental disorders including autism, Down syndrome, and Fragile X syndrome (Kaufmann and Moser, 2000; Kulkarni and Firestein, 2012). To refine the location and strength of synaptic or sensory inputs, neurons use various strategies to organize their dendritic arbors, including self-avoidance and tiling (Jan and Jan, 2010; Zipursky and Sanes, 2010; Zipursky and Grueber, 2013).
Self-avoidance, the phenomenon in which dendritic branches of the same neuron avoid overlapping with one another, ensures the non-redundant coverage of sensory or synaptic inputs. Tiling is the phenomenon in which the dendrites of neighboring neurons of the same type avoid overlapping with each other. In both vertebrates and invertebrates, contact-mediated self-repulsion is a well-studied mechanism underlying self-avoidance (Jan and Jan, 2010; Zipursky and Sanes, 2010; Zipursky and Grueber, 2013). In Drosophila, Down syndrome cell adhesion molecule (Dscam) mediates contact-dependent dendritic repulsion through homophilic interactions between identical isoforms of the protein on dendrites of the same neuron (Matthews et al., 2007; Soba et al., 2007; Hughes et al., 2007). In mammalian neurons, protocadherins (Pcdhs) mediate self-avoidance through isoform-specific homophilic interaction (Lefebvre et al., 2012; Chen and Maniatis, 2013).
Importantly, self-avoidance and tiling require not only contact-mediated interactions between dendrites, but also dendrite-ECM adhesion to restrict the dendrites onto a 2D X-Y plane so that dendrites can not stray along the Z-axis and escape contact-mediated repulsion (Han et al., 2012; Kim et al., 2012). Similar spatial restriction of dendrites has been found in Drosophila sensory neurons, fish somatosensory neurons, and within the mammalian retina (Jan and Jan, 2010; Sagasti et al., 2005; Perry and Linden, 1982; Zipursky and Sanes, 2010; Zipursky and Grueber, 2013). In the mammalian retina, laminar stratification of the neurites of retinal neurons is regulated by semaphorins (Sun et al., 2013; Matsuoka et al., 2011a; Matsuoka et al., 2011b). However, how dendrite-ECM adhesion is regulated in vivo remains largely unknown.
Drosophila dendrite arborization (da) sensory neurons are a powerful model system to study the molecular mechanisms underlying dendrite-ECM adhesion. Drosophila da neurons can be divided into four classes (classes I–IV), based on dendritic morphology and central axon projections (Grueber et al., 2002; Grueber et al., 2007). During development, dendrites of da neurons extend mainly in a 2D plane. Defects in dendrite-ECM adhesion lead to a detachment from the ECM and therefore to an increase in the number of non-contacting crossings (Kim et al., 2012; Han et al., 2012). Loss-of-function mutations in integrin subunits cause dendrites to detach from the ECM and become enclosed by the epidermal cell membrane, resulting in excessive non-contacting self-crossings between dendrites (Kim et al., 2012; Han et al., 2012). Additionally, target of rapamycin complex 2 (TORC2) and the evolutionarily conserved protein kinase Tricornered (Trc) as well as its adaptor protein Furry (Fry) were found to be important for self-avoidance and tiling (Emoto et al., 2004), and were shown to function by regulating dendrite-ECM adhesion (Han et al., 2012). To gain insight into how dendrite-ECM adhesion is regulated, we conducted a genetic screen in Drosophila class IV da neurons to examine the contribution of cell surface proteins.
In this study, we show that mutations in the secreted semaphorin ligand sema-2b cause detachment of dendrites from the ECM, leading to an increase in non-contacting dendritic crossings in class IV da neurons. Sema-2b protein is expressed and secreted from epidermal cells, and signals locally through the PlexB receptor in neurons. Moreover, we show that the TORC2 complex and the Trc/Fry signaling pathway act downstream of the Sema-2b/PlexB signaling pathway, and that the PlexB receptor associates with Mys, a β subunit of integrins in vitro and in vivo. Our results reveal an important role for semaphorins in the precise arrangement of dendrites during development and show how epidermis-derived cues shape neural circuit assembly.
Results
Sema-2b Prevents Dendritic Crossings in Class IV da Neurons
In a screen designed to identify cell surface proteins important for the development of sensory neuron dendrites in vivo, we discovered that mutations in sema-2b, which encodes a secreted semaphorin ligand, led to a fully penetrant phenotype in which dendrites of class IV da neurons overlap with each other excessively (Figure 1B–D and 1H), whereas they normally avoid each other in wild-type larvae (Figure 1A and 1H). We examined the phenotypes of two mutant alleles of sema-2b: a P-element insertion called sema-2bf02042 (Figure 1B; Thibault et al., 2004), and a FRT-derived genomic deletion called sema-2bC4 (Figure 1C; Wu et al., 2011), which showed a stronger dendritic self-crossing phenotype than sema-2bf02042 (Figure 1H). We also examined a heteroallelic combination of sema-2bf02042/C4 mutants, which had an intermediate severity of the self-crossing phenotype in between that of sema-2bf02042 and sema-2bC4 mutants (Figure 1D and 1H). The Drosophila genome encodes two secreted semaphorin ligands, Sema-2a and Sema-2b, both of which often act in the same system (Wu et al., 2011; Joo et al, 2013). To determine if Sema-2a is required for preventing dendritic crossings, we examined sema-2a loss-of-function mutants and found that the loss of sema-2a did not lead to any increase in dendritic crossings (Figure 1E and 1H). Moreover, a double mutant of sema-2a and sema-2b did not show a more severe phenotype than sema-2b mutants alone (Figure 1F and 1H), suggesting that Sema-2b, but not Sema-2a, plays a major role in preventing dendritic self-crossings in class IV da neurons.
Figure 1. Sema-2b, but not Sema-2a loss of function leads to an increase in dendritic self-crossings.
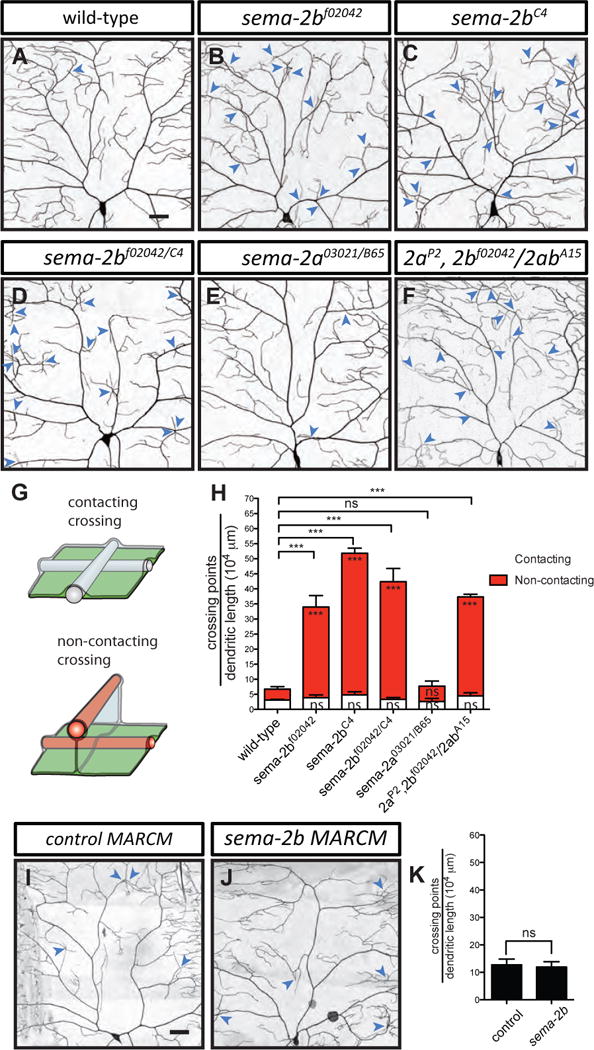
(A–F) Dendritic patterns of wild-type (A), sema-2bf02042 (B), sema-2bC4 (C), sema-2bf02042/C4 (D), sema-2a03021/B65 (E), and sema-2aP2, 2bf02042/2abA15 (F) class IV da neurons. Dendritic crossings are indicated by blue arrowheads. Scale bars represent 30 μm. Wild-type animals are w1118 carrying one copy of ppk-CD4-tdTomato.
(G) Schematic of a contacting crossing (top) and a non-contacting crossing (bottom). In the case of contacting crossings, both dendrites (white bars) are in the same X–Y plane. In the case of non-contacting crossings, one or both dendrites (red bars) detach from ECM (green sheet) and become enclosed by the basal surface of epidermal cells (grey sheet).
(H) Quantification of crossing points normalized to total dendritic length in wild-type (n=6), sema-2bf02042 (n=4), sema-2bC4 (n=4), sema-2bf02042/C4 (n=7), sema-2a03021/B65 (n=4), and sema-2aP2, 2bf02042/2abA15 (n=4) mutant neurons. White bars represent the quantifications of contacting crossings and red bars represent the quantification of non-contacting crossings. Data are plotted as average ± SEM. ns, not significant, and ***p < 0.001 as assessed by one-way analysis of variance and Dunnett’s test. The comparisons of the total number of crossings are labeled on top of the bars. The comparisons of the contacting crossings and non-contacting crossings are labeled in the white bars and red bars, respectively.
(I–J) Dendritic patterns of control (I) and sema-2bC4 (J) class IV neurons generated with MARCM. Scale bars represent 30 μm.
(K) Quantification of total crossing points normalized to total dendritic length in control (n=3) and sema-2bC4 (n=3) class IV da neurons. Data are plotted as average ± SEM. ns, not significant as assessed by a Student’s t test.
Dendrites of class IV da neurons are tightly confined between the basal surface of epidermal cells and the ECM, and branch out in a 2D plane, facilitating contact-mediated dendritic self-avoidance (Han et al, 2012; Kim et al., 2012). Loss of attachment to the ECM leads to enclosure of dendrites into epidermal cells and thus to an increase in the number of non-contacting dendritic crossings (Han et al, 2012; Kim et al., 2012). To understand the nature of the dendritic crossings we observed in the sema-2b mutants, we tested whether these crossings involve direct contact between dendrites using in vivo high-resolution confocal imaging (Figure 1G; Figure S1A). In all mutants examined, we observed no significant increase in contacting crossings compared to wild-type controls (Figure 1H). However, the number of non-contacting crossings increased in sema-2b mutants and sema-2a, sema-2b double mutants (Figure 1H). In addition, we also found an increase in the number of non-contacting crossings in the class I da neurons in sema-2b mutants (Figures S1B–S1D). By contrast, we found no detectable defects in the overall axonal projections of class IV da neurons in the ventral nerve cord of the sema-2b mutants (Figure S2). These observations suggest that the loss of Sema-2b leads to non-contacting dendritic crossings, possibly by an impairment of dendrite-ECM adhesion.
To determine whether Sema-2b is required in class IV da neurons to prevent dendritic crossings, we generated single neuron clones homozygous for a sema-2b mutation in an otherwise heterozygous background using the mosaic analysis with a repressible cell marker (MARCM) technique (Lee and Luo, 1999). Surprisingly, reducing Sema-2b function in class IV da neurons had no significant effect on dendritic self-crossings (Figures 1I–1K). Therefore, Sema-2b is not required in class IV da neurons for preventing self-crossing of dendrites (also see Figures 3J–3L).
Figure 3. Sema-2b is derived from epidermal cells and acts at short range to regulate dendrite adhesion.
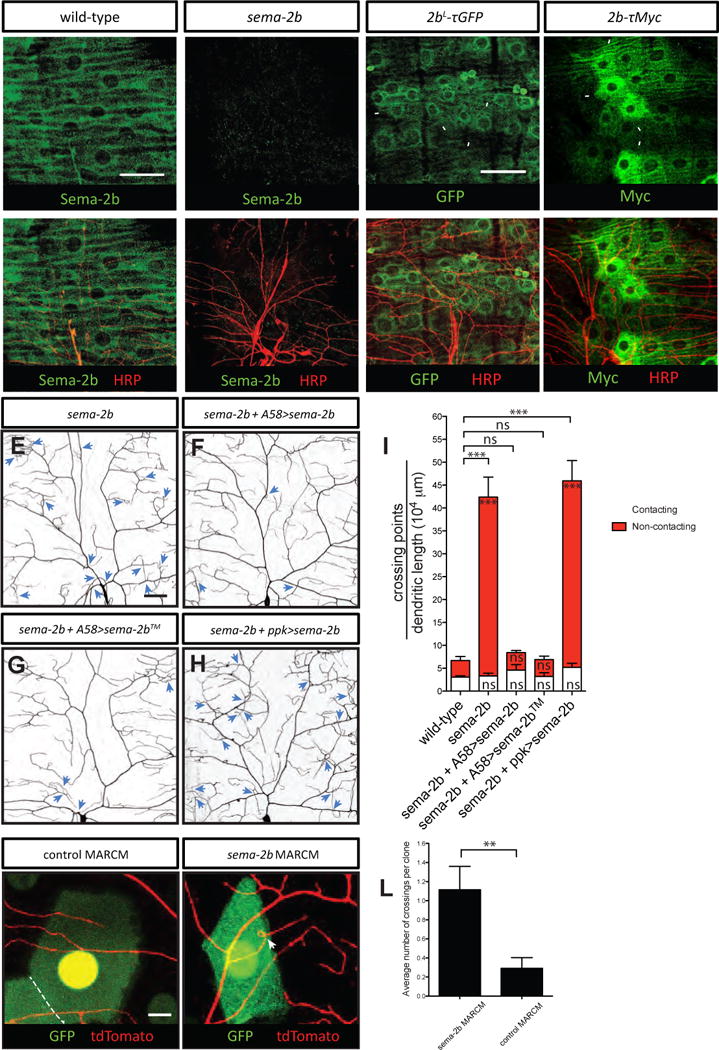
(A–A′) Anti-Sema-2b immunostaining of a w1118 wild-type third instar fillet reveals strong expression in epidermal cells. Anti-HRP immunoreactivity labels neurons in the peripheral nervous system.
(B–B′) No detectable Sema-2b staining in sema-2bC4 mutants. Scale bar represents 50 μm.
(C–C′) The 2bL-τGFP reporter labels epidermal cells that express Sema-2b. 2bL-τGFP animals were immunostained for τGFP and anti-HRP, which labels neurons in the body wall. Some GFP positive epidermal cells are indicated by white arrowheads.
(D–D′) The 2b-τMyc reporter labels a subset of Sema-2b-expressing epidermal cells. 2b-τMyc animals were immunostained for τMyc and anti-HRP, which labels neurons in the body wall. Some τMyc positive epidermal cells are indicated by white arrowheads. All the staining images are positioned with the anterior side on the left and dorsal side on the top.
(E–I) Dendritic patterns and quantifications of class IV da neurons in sema-2bf02042/C4 (E, n=7), sema-2b f02042/C4 mutant with epidermis-expressing full length Sema-2b (F, n=5), epidermis-expressing membrane tethered Sema-2b (G, n=5), and neuronal-expressing full length Sema-2b (H, n=6). Dendritic crossings are indicated by blue arrowheads. Scale bars represent 30 μm. Wild-type animals are w1118 carrying one copy of ppk-CD4-tdTomato. White bars represent the quantifications of contacting crossings and red bars represent the quantification of non-contacting crossings. Data are plotted as average ± SEM. ns, not significant and ***p<0.001 as assessed by one-way analysis of variance and Bonferroni test. The comparisons of the total number of crossings are labeled on top of the bars. The comparisons of the contacting crossings and non-contacting crossings are labeled in the white bars and red bars, respectively.
(J–K) Dendritic patterns of control (J) and sema-2bC4 (K) epidermal clones (labeled by GFP) generated with MARCM. Class IV da neurons are genetically labeled by one copy of ppk-CD4-td-Tomato. Scale bars represent 10 μm. The boundary between two adjacent epidermal cells is marked by a white dashed line.
(L) Quantification of total crossing points among dendrites that are covered by control (n=24) and sema-2bC4 (n=26) class IV da neurons. Data are plotted as average ± SEM. ns, not significant as assessed by a Student’s t test.
Loss of Sema-2b Function Leads to a Progressive Increase in Detachment of Terminal Dendrites from the ECM
To investigate the spatial distribution of dendrites in relation to the ECM, we visualized class IV da neuron dendrites using a class IV-specific membrane marker ppk-CD4-tdTom (Han et al., 2011) and the ECM using viking-GFP (vkg-GFP) (Han et al., 2012) in live third instar larvae. Using a combination of high-resolution confocal imaging and 3D deconvolution, we reconstructed the positions of each dendrite relative to the ECM. At 120 hr after egg laying (AEL), wild-type dendrites of class IV da neurons at the dorsal midline were tightly attached to the ECM (Figure 2C and 2E). In sema-2b mutants, however, the percentage of enclosed dendritic length greatly increased (Figure 2D and 2E), suggesting that defects in dendrite-ECM adhesion contribute to increased non-contacting dendritic crossings.
Figure 2. Enclosure of terminal dendrites by epidermal cells in sema-2b mutants.
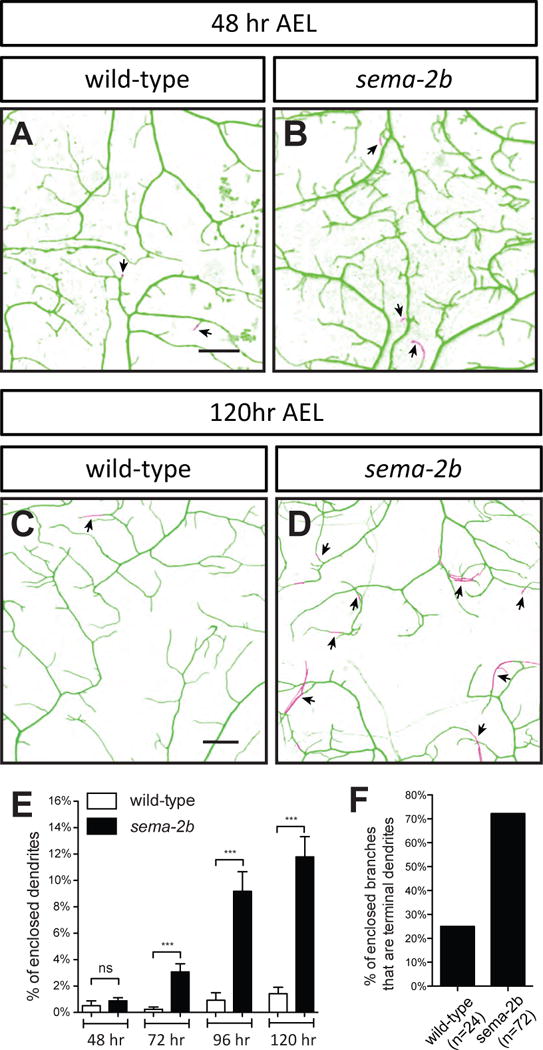
(A–B) Wild-type (A) and sema-2bf02042/C4 (B) dendritic field imaged at 48 hr AEL.
(C–D) Wild-type (C) and sema-2bf02042/C4 (D) dendritic field imaged at 120 hr AEL. Wild-type animals are w1118 carrying one copy of ppk-CD4-tdTomato. Dendrites attached to the ECM are labeled in green and enclosed dendrites are labeled in magenta. The arrowheads point to terminal dendrites that are enclosed by epidermal cells. Scale bars represent 30 μm.
(E) Quantification of percentages of enclosed dendrites at 48 hr, 72 hr, 96 hr and 120 hr AEL in wildtype and sema-2bf02042/C4 neurons. Data are plotted as average ± SEM. ***p <0.001 as assessed by a Student’s t test.
(F) Percentages of detached dendrites that are terminal dendrites in class IV da neurons in wild-type and sema-2b mutants.
Enclosure of growing dendrites by epidermal cells is a dynamic process that takes place during development (Han et al., 2012). To determine the stage during which Sema-2b is required for dendrite-ECM attachment, we characterized the dendrite enclosure in wild-type and sema-2b mutant larvae at different time points throughout development. In wild-type larvae, class IV da neuron dendrites establish their receptive fields by tiling the body wall completely and non-redundantly at 48 hr AEL (Parrish et al., 2007). We found that wild-type dendrites were tightly attached to the ECM from 48 hr AEL to 120 hr AEL (Figure 2A and 2E). Interestingly, in the sema-2b mutants, dendrites initially exhibit normal dendrite-ECM attachment at 48 hr AEL (Figure 2B and 2E). Defects in dendrite-ECM attachment of sema-2b mutants became evident at 72 hr AEL and became worse at 96 hr AEL, revealing that sema-2b mutants exhibit progressive loss of dendrite-ECM attachment after neurons have established their receptive fields (Figure 2E).
Since it has been shown that terminal dendrite branching increases between 72 hr and 96 hr AEL (Parrish et al., 2007), we reasoned that loss of terminal dendrite attachment to the ECM could be the main contributor to the observed increase in dendrite enclosure in the epidermis in sema-2b mutants. Indeed, we found that 72.2% of the enclosed dendrites (n=72) were terminal dendrites in sema-2b mutants, whereas only 25.0% of the enclosed dendrites (n=24) were terminal dendrites in wild-type animals (Figure 2F). In addition, we found that the overall morphology and total number of epidermal cells in the sema-2b mutants were the same as wild-type animals, suggesting that Sema-2b is not required for the development of epidermis (Figure S3).
Collectively, these data reveal that Sema-2b is required for the maintenance of dendrite attachment to the ECM in order to reduce non-contacting crossings (Figure 1G) and ensure non-overlapping coverage of dendritic fields.
Sema-2b is Secreted From Epidermal Cells to Regulate Dendrite-ECM Adhesion
Next, we examined the localization of Sema-2b proteins in the Drosophila larval body wall using a polyclonal antibody specific for Sema-2b (Sweeney et al. 2011). We found that Sema-2b was evenly distributed throughout the wild-type larval body wall (Figure 3A), and was completely absent in sema-2bC4 null mutants (Figure 3B).
To pinpoint the source of Sema-2b, we employed a Sema-2b reporter, 2bL-τGFP, with the τGFP expression driven by the sema-2b promoter (Wu et al. 2011). The 2bL-τGFP reporter resided in epidermal cells (Figure 3C) and colocalized with an epidermal-cell specific marker (Figure S4). We further confirmed the expression pattern using a more selective Sema-2b reporter, 2b-τMyc (Wu et al. 2011). This reporter uses a shorter fragment of the sema-2b promoter to drive the expression of τMyc and labels a subset of cells in the developing Drosophila embryo that normally express a high level of Sema-2b (Wu et al. 2011). We found that 2b-τMyc labeled a subset of epidermal cells in the midline of each abdominal hemisegment, suggesting that these cells express a high level of Sema-2b (Figures 3D and S4). These results suggest that that Sema-2b is expressed in epidermal cells, with a high level of expression in the midline of each segment.
We next tested if restoring Sema-2b in epidermal cells would be sufficient to rescue the dendrite crossing phenotype in the sema-2b mutants. To do this, we used the Gal4/UAS system in Drosophila to specifically express secreted Sema-2b in the epidermal cells using the epidermis-specific driver Gal4A58 in the sema-2b mutant background (Galko and Krasnow, 2004). Indeed, expression of Sema-2b specifically in the epidermis, but not in class IV da neurons, rescued the crossing defects observed in the sema-2b mutants (Figures 3E–3G). To test if the release of Sema-2b protein into the extracellular space is required for dendrite self-avoidance, we expressed a membrane-tethered Sema-2b™ in all the epidermal cells. We found that Sema-2b™ also rescued dendritic crossing defects in sema-2b mutants (Figure 3H–3I), suggesting that secretion of Sema-2b is not required for mediating dendrite self-avoidance. To further examine if locally produced Sema-2b is required to prevent dendrite crossing, we performed MARCM analysis to remove Sema-2b from epidermal cells and we found that dendrites innervating mutant sema-2b clones showed an increase in dendritic crossings (1.1 ± 0.2, N=26), compared to control clones (0.3 ± 0.1, N=26; Figures 3J–3L). Together, these results strongly suggest that Sema-2b is derived from epidermal cells and functions locally to regulate dendrite-ECM adhesion. Further, the fact that a clone of a single sema-2b mutant epidermal cell can produce the dendritic crossing phenotype in adjacent class IV da neuron dendrites strongly suggests that Sema-2b can not diffuse substantially for a distance longer than the diameter of an epidermal cell.
PlexB functions in the Sensory Neurons to Regulate Dendrite-ECM Adhesion
We next explored the mechanisms by which Sema-2b regulates dendrite-ECM adhesion in class IV da neurons. PlexB and Sema-1a have been shown to be receptors for Sema-2a and Sema-2b (Ayoob et al., 2006; Wu et al., 2011; Sweeney et al., 2011; Joo et al., 2013). Sema-1a loss-of-function mutants had normal dendrite morphology with no defects in dendritic crossing (Figure S5B). However, plexB loss-of-function mutants showed a large increase in the number of dendritic crossings (Figures 4A, 4B and 4E), suggesting that reducing PlexB function leads to a defect in dendrite-ECM adhesion.
Figure 4. Plexin B is the Sema-2b Receptor that regulates dendrite-ECM adhesion.
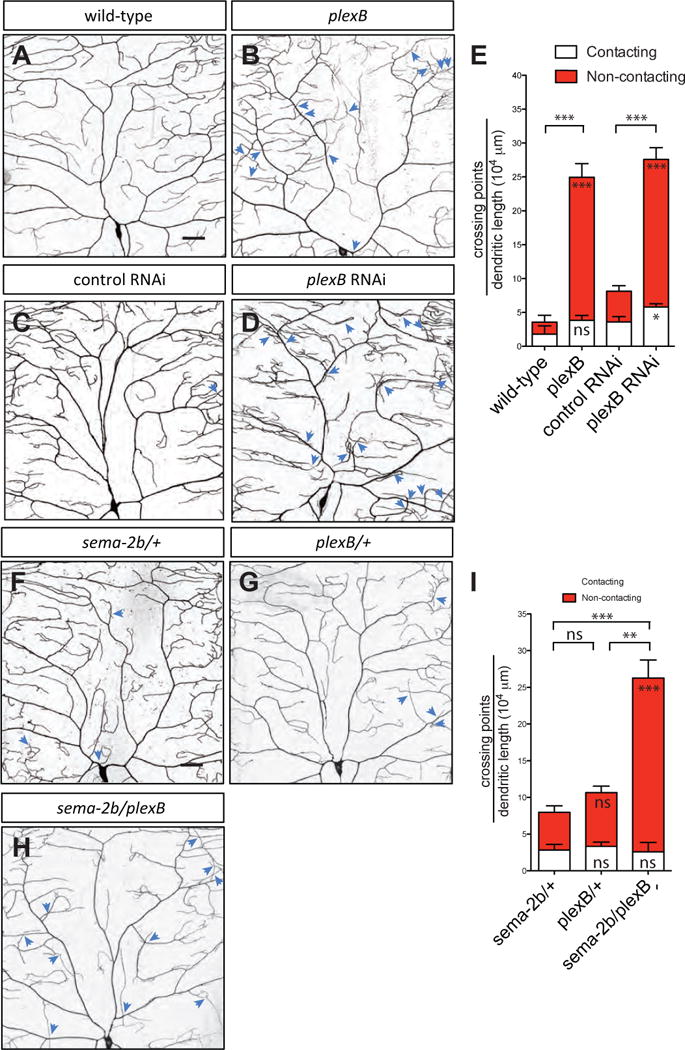
(A–D) Dendritic patterns of wild-type (A), plexBKG00878 (B), control RNAi (C) and plexB RNAi (D) class IV da neurons. Dendritic crossings are indicated by blue arrowheads. Scale bars represent 30 μm. Wild-type animals are w1118 carrying one copy of ppk-CD4-tdTomato.
(E) Quantification of total crossing points normalized to total dendritic length in wild-type (n=4), plexBKG00878 (n=6), RNAi control (n=6), and plexB RNAi (n=6) neurons. Data are plotted as average ± SEM. ns, not significant, *p<0.05, ***p < 0.001 as assessed by a Student’s t test. The comparisons of the total number of crossings are labeled on top of the bars. The comparisons of the contacting crossings and non-contacting crossings are labeled in the white bars and red bars, respectively.
(F–H) Dendritic patterns of sema-2bC4/+ (F), plexBKG00878/+ (G), and sema-2bC4/plexBKG00878 (H) class IV da neurons. Dendritic crossings are indicated by blue arrowheads. Scale bars represent 30 μm.
(I) Quantification of total crossing points normalized to total dendritic length in sema-2bC4/+ (F), plexBKG00878/+ (G), and sema-2bC4/plexBKG00878 (H) class IV da neurons. White bars represent the quantifications of contacting crossings and red bars represent the quantification of non-contacting crossings. Data are plotted as average ± SEM. ns, not significant and **p < 0.01, ***p<0.001 assessed by one-way analysis of variance and Bonferroni test for all pairs of columns. The comparisons of the total number of crossings in each genotype to sema-2bC4/+ are labeled on top of the bars. The comparisons of the contacting crossings and non-contacting crossings each genotype to sema-2bC4/+ are labeled in the white bars and red bars, respectively.
PlexB is expressed in class IV da neurons during the embryonic stages of development (Zlatic et al., 2009). Therefore, we investigated if the PlexB receptor is required cell-autonomously in class IV da neurons to mediate dendrite-ECM adhesion. We used Gal4/UAS-based RNA interference (RNAi) to knock down PlexB in class IV da neurons using Gal421-7 (Song et al., 2007). Using two different RNAi lines, we observed a strong increase in the number of dendritic crossings (Figures 4C–4E, and Figures S5C–S5F), demonstrating that plexB acts cell-autonomously in class IV da neurons to regulate dendrite-ECM adhesion. Additionally, we found an increase in the number of non-contacting crossings when we knocked down plexB in class I da neurons (Figures S5G–S5I).
To determine if sema-2b and plexB function in the same genetic pathway, we examined sema-2b/+, plexB/+, and sema-2b/plexB animals and found an increase in non-contacting crossings but not in contacting crossings in the sema-2b/plexB animals compared to sema-2b/+ and plexB/+ animals (Figures 4F–4I). In addition, we did not find genetic interaction between sema-2b and a null allele of a transmembrane protein Off-track (Otk), which mediates downstream signaling of Sema-1a and PlexinA (Winberg et al., 2001), suggesting that otk is not involved in regulating dendrite-ECM adhesion (Figures S6E–S6G).
Sema-2b Genetically Interacts with TORC2 Complex and Trc/Fry
To uncover the components of Sema-2b/PlexB signaling involved in dendrite-ECM adhesion, we assayed for genetic interactions between sema-2b and other known regulators of dendrite-ECM adhesion, including TORC2 complex and the Trc/Fry signaling pathway (Han et al. 2012). Trc kinases are a subclass of the protein kinase A (PKA)/protein kinase G (PKG)/protein kinase C (PKC) (AGC) group of serine/threonine kinases (Hergovich et al., 2006). In Drosophila, components of the TORC2 complex function in the same pathway by phosphorylating and activating Trc (Koike-Kumagai et al., 2009). In class IV da neurons, loss of components in the TORC2 complex, or the loss of Trc or Fry leads to an increase in dendritic self-crossings and defects in dendrite-ECM adhesion (Emoto et al., 2004; Han et al. 2012; Koike-Kumagai et al., 2009). We tested if Target of Rapamycin (tor), SAPK-interacting protein 1 (sin1), or rapamycin-insensitive companion of Tor (rictor), three components of TORC2 complex, genetically interact with sema-2b. We found strong genetic interactions between sema-2b and all three loss-of-function alleles (Figures 5A–5F and 5K). We further found that sema-2b also interacts genetically with trc and fry (Figures 5G–5K). These genetic interaction results suggest that the Sema-2b/PlexB signaling pathway likely functions together with TORC2 complex and Trc/Fry pathway to regulate dendrite-ECM adhesion.
Figure 5. Sema-2b genetically interacts with TORC2 complex and Trc kinase.
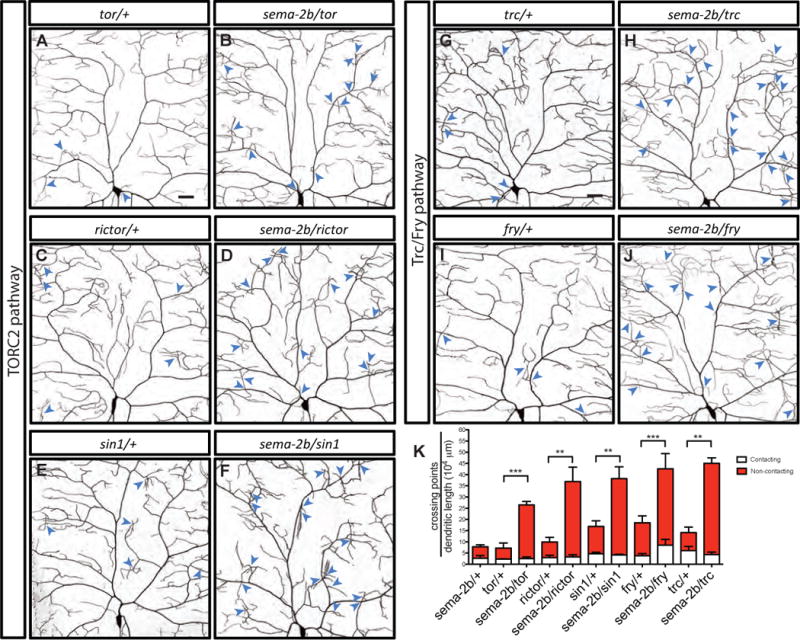
(A–J) Dendritic patterns of torΔP/+ (A), sema-2bC4/torΔP (B), rictorΔ2/+ (C), sema-2bC4/rictorΔ2 (D), sin1e03756/+ (E), sema-2bC4/sin1e03756 (F), trc1/+ (G), sema-2bC4/trc1(H), fry1/+ (I), and sema-2bC4/fry1 (J) class IV da neurons. Dendritic crossings are indicated by blue arrowheads. Scale bars represent 30 μm.
(K) Number of total crossing points normalized to total dendritic length in sema-2bC4/+ (n=5), torΔP/+ (n=4), sema-2bC4/torΔP (n=5), rictorΔ2/+ (n=5), sema-2bC4/rictorΔ2 (n=5), sin1e03756/+ (n=5), sema-2bC4/sin1e03756 (n=7), trc1/+ (n=4), sema-2bC4/trc1 (n=4), fry1/+ (n=5), and sema-2bC4/fry1 (n=5) neurons. White bars represent the quantifications of contacting crossings and red bars represent the quantification of contacting crossings. Data are plotted as average ± SEM. **p < 0.01, and ***p < 0.001 as assessed by one-way analysis of variance and Bonferroni test.
Sema-2b/PlexB Signaling Regulates Dendrite-ECM Adhesion through Tricornered Kinase Activation
Next, we investigated whether Sema-2b/PlexB signaling functions upstream or downstream of the Trc/Fry signaling pathway. The function of the Trc/Fry signaling pathway is evolutionarily conserved, controlling dendrite growth and morphology in worms (Gallegos and Bargmann, 2004), flies (Emoto et al., 2004), and mammals (Ultanir et al., 2012; Rehberg et al., 2014). It has been previously shown that mammalian substrates of Trc/NDR1/2 kinase all contain the consensus sequence HXRXXS/T (Ultanir et al., 2012), which is absent in the Drosophila PlexB receptor. Therefore, we reasoned that PlexB is unlikely to be a direct substrate of Trc kinase.
To assess whether the Sema-2b/PlexB signaling pathway influences Trc kinase activity in vivo, we used a previously generated antibody that specifically detects phosphorylation on threonine residue 449 (T449) of Trc kinase, which is associated with maximal Trc kinase activation (Tamaskovic et al., 2003; Lee at al., 2014). In the larval body wall, Trc P-T449 immunoreactivity was present at high levels in the axons, dendrites and cell bodies of class IV da neurons (Figures 6A and 6D). In the sema-2b and plexB mutants, overall Trc P-T449 levels were greatly reduced (Figures 6B–6D), whereas we did not see any difference in the overall Trc expression levels in class IV da neurons of the sema-2b and plexB mutants (data not shown). Together, these results suggest that Sema-2b/PlexB signaling is required for Trc phosphorylation and activation in class IV da neurons.
Figure 6. Trc kinase acts downstream of Sema-2b/PlexB signaling to promote dendrite adhesion.
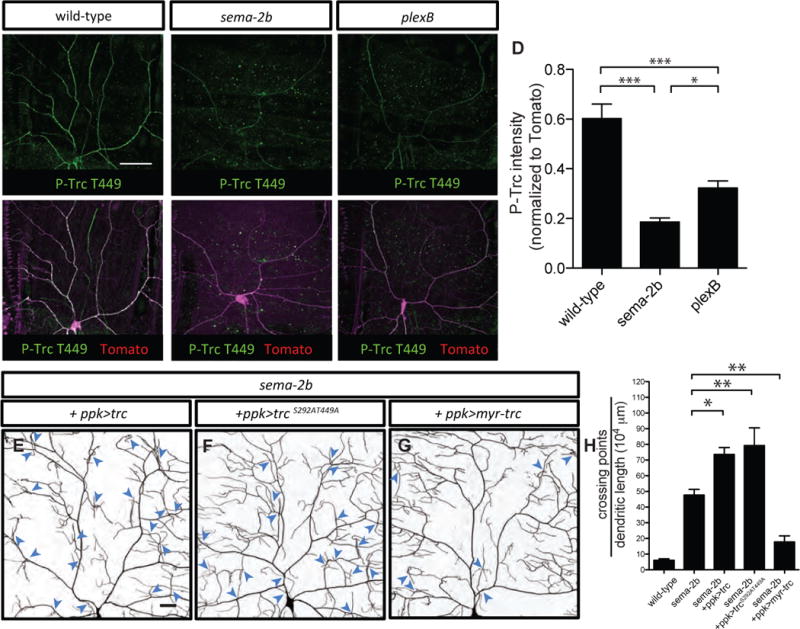
(A–C) Anti-phospho-Trc (P-T449) immunostaining of a wild-type w1118 third instar fillet reveals strong labeling in the wild-type class IV da neuron (A). Anti-phospho-TrcT449 shows weak labeling in sema-2bC4 (B) and plexBKG00878 (C) mutant class IV da neurons. Scale bar represents 50 μm. Class IV da neurons are genetically labeled by ppk-CD4-td-Tomato. All the staining images are positioned with the anterior side on the left and dorsal side on the top.
(D) Quantification of the level of P-T449 normalized to ppk-CD4-td-Tomato in the dendrites of the class IV da neurons in the wild-type, sema-2bC4, and plexBKG00878 animals. ns, not significant, **p < 0.01, and ***p < 0.001 as assessed by one-way analysis of variance and Bonferroni test for all pairs of columns.
(E–G) Dendritic patterns of sema-2bf02042/C4 mutants expressing wild-type Trc (E), phosphorylation-site mutated Trc (S292AT449A) (F), and myristoylated Trc (Myr-Trc) (G) in class IV da neurons. Dendritic crossings are indicated by blue arrowheads. Scale bars represent 30 μm.
(H) Quantification of total crossing points normalized to total dendritic length in sema-2bf02042/C4 mutants expressing wild-type Trc (n=5), TrcS292AT449A (n=5), and Myr-Trc (n=5) in class IV da neurons. Data are plotted as average ± SEM. *p < 0.05 and **p <0.01 as assessed by a one-way analysis of variance and Dunnett’s test.
If Sema-2b/PlexB signaling functions by promoting Trc kinase activation to regulate dendrite-ECM adhesion, we hypothesized that ectopic Trc activation in class IV da neurons of sema-2b mutants would mitigate the dendritic crossing phenotype. First, we tested if overexpressing wild-type Trc could rescue the dendritic crossing phenotype in sema-2b mutants. It has been shown that overexpressing wild-type Trc in a wild-type background is sufficient to activate Trc (Wu et al., 2013). Therefore, if overexpressing wild-type Trc could rescue the sema-2b mutant crossing phenotype, it would suggest that Trc functions downstream of Sema-2b/PlexB signaling, and that its activation is independent of Sema-2b/PlexB signaling. On the other hand, if overexpressing Trc does not rescue the sema-2b mutant crossing phenotype, it would suggest that Sema-2b/PlexB signaling is not only upstream of Trc, but is also required for its activation. Consistent with the second scenario, overexpressing wild-type Trc in sema-2b mutant background led to a more severe dendritic crossing defect (Figures 6E and 6H). Next, we overexpressed dominant negative Trc (S292AT449A), with alanine substitutions of Serine 292 (S292A) and Threonine 449 (T449A) to prevent phosphorylation and activation of Trc (Emoto et al., 2004). Consistent with our hypothesis, overexpressing dominant negative Trc in sema-2b mutants also led to a more severe dendritic crossing defect (Figures 6F and 6H). Finally, overexpressing a constitutively active myristoylated Trc (Myr-Trc), which targets Trc to the membrane (Koike-Kumagai et al., 2009), significantly suppressed the dendritic crossing defects of the sema-2b mutants (Figures 6G and 6H).
Together, these results suggest that Sema-2b/PlexB signaling regulates dendrite-ECM adhesion through the Trc/Fry signaling pathway.
Mys Associates with PlexB and Likely Acts Downstream of the Sema-2b/PlexB Signaling Pathway
Loss of integrins in class IV da neurons causes defects in dendrite-ECM adhesion similar to those found in sema-2b and plexB mutants (Han et al., 2012; Kim et al., 2012). Because Trc promotes integrin-mediated adhesion in class IV da neurons (Han et al., 2012), we hypothesized that defects in integrin-mediated adhesion contribute to the increased dendrite-ECM detachment seen in sema-2b and plexB mutants. If so, we reasoned that increasing the level of integrin expression should rescue the sema-2b mutant dendritic crossing defects, since integrin overexpression is able to suppress dendritic crossing defects of Trc/Fry pathway mutants (Han et al., 2012). To test this prediction, we overexpressed an integrin α subunit encoded by multiple edematous wings (mew), and an integrin β subunit encoded by myospheroid (mys), both of which are required in class IV da neurons for dendrite-ECM adhesion (Han et al., 2012). In fact, overexpressing these two integrin subunits reduced the number of total dendritic crossings in sema-2b mutants to wild-type levels (Figures 7A–7C). It is worth noting that overexpressing integrins greatly reduced the number of non-contacting crossings, suggesting that dendrites become tightly attached to the ECM.
Figure 7. Integrins overexpression suppresses dendrite crossing defects in sema-2b mutant.
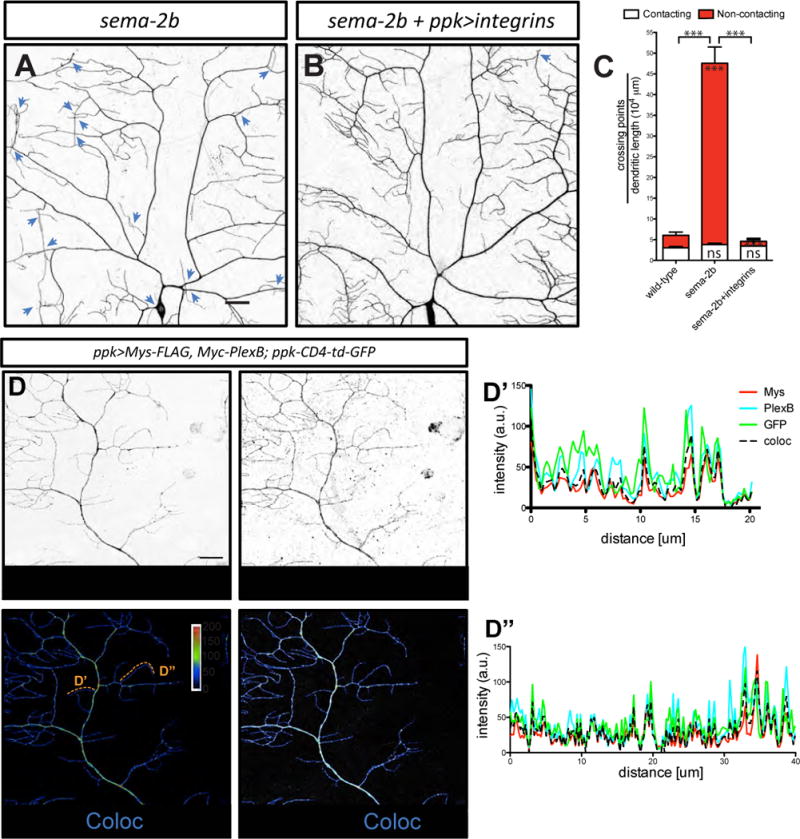
(A–B) Dendritic patterns of class IV da neurons in sema-2bf02042/C4 mutants (A) and sema-2bf02042/C4 mutants with integrins (UAS-mys and UAS-mew) overexpressed in class IV da neurons (B). Dendritic crossings are indicated by blue arrowheads. Scale bars represent 30 μm.
(C) Quantifications of crossing points in wild-type (n=6), sema-2bf02042/C4 (n=5) class IV da neurons and sema-2bf02042/C4 with integrins overexpressed in class IV da neurons (n=4). Data are plotted as average ± SEM. ns, not significant and ***p<0.001 as assessed by one-way analysis of variance and Bonferroni test for all pairs of columns. The comparisons of the total number of crossings in each genotype and wild-type animals are labeled on top of the bars. The comparisons of the contacting crossings and non-contacting crossings are labeled in the white bars and red bars, respectively.
(D) Colocalization of Mys-FLAG and Myc-PlexB in dendrites of class IV da neurons. Mys-FLAG and Myc-PlexB are co-expressed by ppk-Gal4 driver. Class IV da neurons are labeled by ppk-CD4-td-GFP. Scale bar represents 20 m. The line plots of two terminal dendrites are show in D’ and D”.
To test whether Mys and PlexB localize to dendrites, we expressed Mys-FLAG together with Myc-PlexB in class IV da neurons, and found that Mys-FLAG and Myc-PlexB strongly colocalize in the dendrites of class IV da neurons (Figure 7D; Pearson correlation coefficient 0.71 ± 0.02, n=5).
In mammals, activation of integrins signals through focal-adhesion kinase (FAK) family proteins to promote outgrowth of neurites (Ivankovic-Dikic et al., 2000), and semaphorin treatment has been shown to exert changes on Fak phosphorylation (Cho et al., 2012). Therefore, we tested if Fak is also required for dendrite-ECM adhesion in class IV da neurons. We examined the fak56CG1 null allele and found no significant difference in the number of dendritic crossings (Figures S6A and S6D). Moreover, we did not find any significant genetic interaction of fak56 and sema-2b mutations (Figures S6B–S6D). Our results suggest that Fak is not essential for regulating dendrite-ECM adhesion in class IV da neurons.
Plexin receptors have been shown to associate with various other receptors to mediate unique downstream signaling processes (Pasterkamp, 2012; Kolodkin and Pasterkamp, 2013). Whether the Drosophila PlexB receptor associates with other transmembrane receptors is unknown. Therefore, we asked if PlexB could associate with integrins by co-immunoprecipitations. We expressed FLAG-tagged Mys (Mys-FLAG) and Myc-tagged full-length PlexB (Myc-PlexB) in the Drosophila S2 cell line (Schneider, 1972). As a control, we expressed Myc-tagged Dscam, a guidance receptor required for contact-mediated self-avoidance in class IV da neurons (Matthews et al., 2007; Soba et al., 2007; Hughes et al., 2007), to examine if Dscam interacts with Mys. All proteins were expressed robustly and colocalized with membrane tagged GFP (Figures S7A and S7B). We immunoprecipitated Myc-PlexB and Dscam-Myc and found that Mys-FLAG co-immunoprecipitated with Myc-PlexB, but not with Dscam-Myc (Figure 8A). Consistent with these results, immunoprecipitation of Mys-FLAG revealed robust co-immunoprecipitation of both the full-length Myc-PlexB (250kDa) and the cleaved extracellular region of Myc-PlexB (150kDa; Ayoob et al., 2006), but not Dscam-Myc (Figure 8B), demonstrating that the interaction between PlexB and Mys is specific.
Figure 8. Mys, a β subunit of integrin, associates with PlexB.
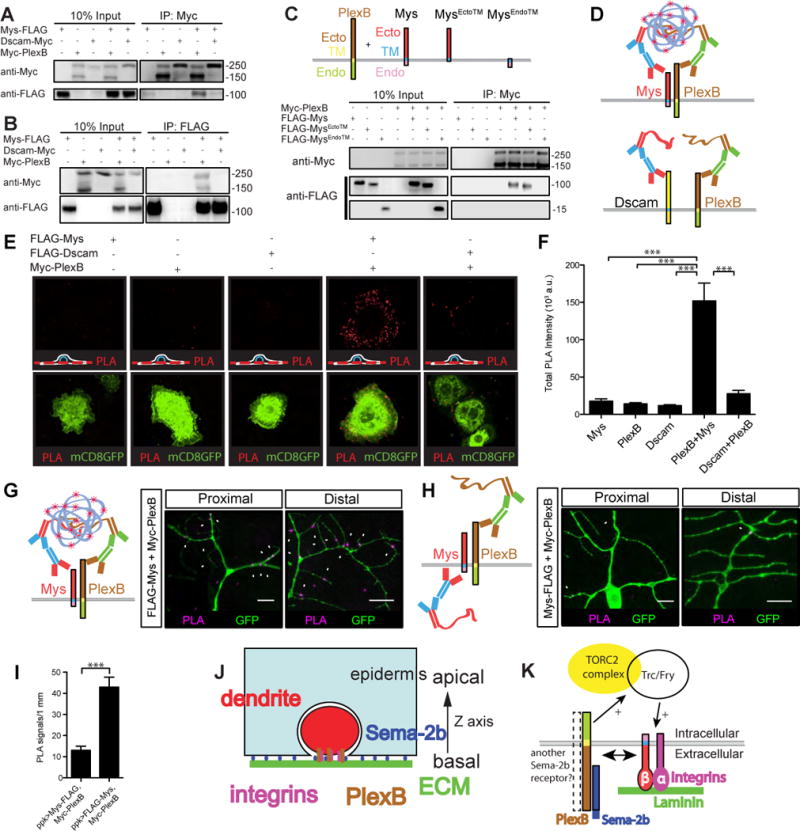
(A–B) Western blot showing co-immunoprecipitation of PlexB and Mys. Lysates from S2 cells expressing Mys-FLAG with Myc-PlexB or Dscam-Myc as depicted were incubated with anti-Myc antibody (A) or FLAG antibody bound beads (B). Immunoprecipitates were probed for the presence of Mys-FLAG (A), Myc-PlexB or Dscam-Myc (B).
(C) Western blot revealing the extracellular region of Mys is required for the association between Mys and PlexB. Lysates from S2 cells expressing either FLAG-Mys, FLAG-MysEctoTM, FLAG-MysEndoTM, and Myc-PlexB as depicted were incubated with Myc antibody. Immunoprecipitates were probed for the presence of FLAG-Mys, FLAG-MysEctoTM and FLAG-MysEndoTM using anti-FLAG antibody.
(D–F) PlexB and Mys interaction detected by in situ PLA on the surface of S2 cells. (D) Diagram showing the principles of in situ PLA: if the two membrane proteins (Mys and PlexB) are within 40nm of each other, the secondary antibodies conjugated with oligonucleotides will be joined together and fluorescently labeled oligonucleotides will then be added to the site by a rolling circle amplification. Both Myc-PlexB and FLAG-Mys are tagged on the extracellular regions. (E) PLA signals in each condition. The interactions were visualized as red fluorescent dots and membrane GFP marked the transfected cells. A cartoon showing the position of the focal plane is shown on the lower left of the panel. (F) Quantification of total PLA intensity per GFP positive cell (n=40 in each condition). Data are plotted as average ± SEM. ***p<0.001 as assessed by one-way analysis of variance and Bonferroni test for all pairs of columns. Scale bars represent 10 μm. A.u. arbitrary unit.
(G–I) PlexB and Mys interaction detected by in situ PLA in dendrites of class IV da neurons. PLA signals in a neuron that co-expresses FLAG-Mys and Myc-PlexB are shown in (G). PLA signals in a neuron that co-expresses Mys-FLAG and Myc-PlexB are shown in (H). (I) Quantification of the number of PLA dots normalized to the dendritic length in each genotype. Data are plotted as average ± SEM. ***p<0.001 as assessed by a Student’s t-test. Staining images are positioned with the anterior side on the left and dorsal side on the top. Scale bars represent 10 μm.
(J) Diagram of a cross section of the ECM (green), epidermis (blue) and dendrites (red). Sema-2b ligands are produced and secreted by epidermal cells and diffuse throughout the whole dendritic field of class IV da neurons. Sema-2b signals through PlexB receptors in the neurons to regulate dendrite-ECM adhesion.
(K) A model of the downstream molecular mechanisms of how Sema-2b/PlexB signaling regulates dendrite-ECM adhesion through the activation of Trc kinase and the binding of integrins to laminin proteins in the ECM.
To define the regions of Mys that interact with PlexB, we generated three FLAG-tagged Mys constructs encoding: the full-length Mys (FLAG-Mys), the Mys extracellular region with transmembrane domain (FLAG-MysEctoTM), and the Mys intracellular region with transmembrane domain (FLAG-MysEndoTM). Surface staining confirmed that these proteins were trafficked to the surface of S2 cells (Figure S7C). We found that immunoprecipitation of Myc-PlexB strongly co-immunoprecipitated FLAG-Mys and FLAG-MysEctoTM, but not FLAG-MysEndoTM (Figure 8C), suggesting that the formation of a complex between Mys and PlexB requires the extracellular region of Mys.
To test if Mys and PlexB interact on the surface of the cells, we performed surface staining and used an in situ proximity ligation assay (PLA), which can visualize specific protein-protein interactions (Söderberg et al., 2006). As a negative control, we generated FLAG-Dscam and confirmed its expression by western blotting and its membrane localization by surface staining (Figures S7D and S7E). We performed surface labeling of FLAG and Myc and found strong PLA signals only in the FLAG-Mys and Myc-PlexB co-expressed condition, and not in FLAG-Dscam and Myc-PlexB co-expressed condition (Figures 8E and 8F), demonstrating that Mys and PlexB interact on the surface of S2 cells.
Next, we examined if Mys and PlexB interact in vivo in dendrites of class IV da neurons. We expressed FLAG-Mys and Myc-PlexB (both tagged at the N-terminus) in class IV da neurons and performed in situ PLA (Figure 8G). As a negative control, coexpressing Mys-FLAG (tagged at the C-terminus) and Myc-PlexB should not give strong signals (Figure 8H). Indeed, even though FLAG-Mys is expressed at a lower level than Mys-FLAG (Figure S7F), we found a significantly higher level of PLA signals in dendrites expressing FLAG-Mys and Myc-PlexB, compared to those expressing Mys-FLAG and Myc-PlexB (Figure 8I). We found PLA signals in both the proximal regions and the distal regions of the dendritic fields, suggesting the interactions of Mys and PlexB occur in these areas.
Discussion
Self-avoidance and tiling are critical mechanisms governing the patterning of dendritic arbors. In Drosophila sensory neurons, these mechanisms depend on the dendrites being restricted onto a 2D plane through dendrite-ECM adhesion. We have uncovered a new role for Sema-2b/PlexB signaling in regulating dendrite-ECM adhesion to promote contact-mediated self-avoidance. We demonstrate that Sema-2b secreted from epidermal cells acts on neuronal PlexB receptors. Furthermore, we identified the TORC2 complex, the Trc/Fry signaling pathway and integrins as components of a Sema-2b/PlexB signaling pathway (Figures 8J and 8K). Our study uncovers previously unknown functions of semaphorin in regulating dendrite-ECM adhesion in neurons to promote dendrite self-avoidance.
Epidermis Secreted Cues Shape Neuron Circuit Formation
Neurons interact with the complex environment that surrounds them at every step of neural development. For example, neuron-glia interaction has been studied in a variety of model organisms from worms to mammals (Corty and Freeman, 2013). Glia-secreted factors play an active role in neuronal migration, axon guidance, and synapse formation during development, and have been implicated in many neurodevelopmental disorders (Sloan and Barres, 2014). In vertebrates and Drosophila, the secretion of TGF-β ligands from glia regulates neuromuscular junction synapse formation and growth (Feng and Ko, 2008; Fuentes-Medel et al., 2012).
Compared to the extensive inquiries into neuron-glia interactions, the importance of neuron-epidermis interactions in neural circuit formation has only begun to be studied. Sensory neurons innervate the epidermis of both invertebrate and vertebrate organisms, and precise innervation must require diverse extrinsic signals. Two recent studies in C.elegans show that the epidermis generates pre-patterned cues to regulate sensory dendrite development (Dong et al., 2013; Salzberg et al., 2013). Our study provides evidence that semaphorin is an epidermis-derived ligand that instructs spatial patterning of sensory dendrites in Drosophila. In mammals, many members of the semaphorin family are expressed in sensory neurons, including those in dorsal root ganglia (Pasterkamp, 2012; O’Malley et al., 2014). It will be of great interest to determine whether epidermis-derived semaphorin also regulates morphogenesis of mammalian sensory neurons.
Role for Semaphorins in Regulating Dendrite-ECM Adhesion to Promote Self-Avoidance
For self-avoidance involving homotypic repulsion, spatial restriction is critical to ensure that contact-mediated interaction between neurites occurs (Zipursky and Grueber, 2013). Similar to class IV da neurons, spatially restricted systems for tiling and self-avoidance also exist in vertebrates, such as fish somatosensory neurons (Sagasti et al., 2005), mammalian cerebellar Purkinje cells (Kaneko et al., 2011), and mammalian retina (Wässle, 2004; Sanes and Zipursky, 2010). Indeed, semaphorin signaling is required for the stratification and symmetric arborization of starburst amacrine cell (SAC) dendrites in the developing mouse retina (Sun et al., 2013). Interestingly, SACs with defects in laminar stratification also show an increase in the number of self-crossings (Sun et al., 2013). In this study, we show that the spatial restriction mediated by dendrite-ECM adhesion is itself modulated by Sema-2b/PlexB signaling, thereby promoting contact-mediated self-avoidance in Drosophila. Our data suggests that Sema-2b/PlexB signaling does not directly promote contact-mediated dendritic repulsion, because the number of contacting crossings did not increase in the plexB mutants (Figure 4B and 4E). Conceptually, the problem of laminar stratification of neurites in vertebrate retina (Sanes and Zipursky, 2010; Kim et al., 2010) and the tethering of dendrites of Drosophila da neurons to the ECM are somewhat similar. In both cases, the growth of the neurites has to be constrained to a 2D space to allow the contact-mediated self-avoidance mechanism to operate. It is therefore very intriguing that semaphorins are involved in both situations (Sun et al., 2013 and this study). In the future, it will be of interest to determine whether the semaphorin regulated dendrite-substrate adhesion observed in our study also occurs in the dendrites of mammalian neurons, and if so whether it also contributes to self-avoidance and tiling in that context.
New Insights into the Semaphorin Signaling Pathway
Semaphorin family proteins play essential roles in refinements of neural circuitry, including neuronal polarization, topographical mapping, dendrite arborization and axon sorting (Pasterkamp, 2012; Koropouli and Kolodkin, 2014). How a small number of proteins can generate such a diverse set of neuronal connections is an intriguing question. One mechanism by which semaphorins may achieve this feat is through activation of divergent downstream signaling pathways at different times during development. In the Drosophila embryonic central nervous system, PlexB receptors in mechanosensory axon terminals mediate both Sema-2b attraction and Sema-2a repulsion (Wu et al., 2011). At the embryonic stage, Sema-2a also acts through PlexB receptor to guide precise axon terminal projections of class IV da neurons (Zlatic et al., 2009). In our study, we found that Sema-2b, but not Sema-2a, acts on PlexB receptor to mediate dendrite adhesion to the ECM. How PlexB binding to Sema-2a and Sema-2b activates distinct downstream pathways is a question that awaits future studies.
Drosophila PlexB receptor has been shown to have opposite effects on cytoskeletal components by simultaneously inhibiting active Rac and enhancing RhoA signaling (Hu et al., 2001). Yet other components downstream of PlexB receptor are less well understood. Our study found that the activation of the Trc/Fry signaling pathway requires Sema-2b/PlexB signaling and provided genetic evidence that TORC2 and Trc activation is downstream of Sema-2b/PlexB signaling (Figure 8K). Our finding is consistent with a previous study that showed TORC2 complex associates with Trc and promotes its activation possibly by recruiting Trc to the membrane in the class IV da neurons (Koike-Kumagai et al., 2009).
The phenotype of the plexB mutants we observed was weaker than the phenotype of sema-2b mutants. It is possible that the plexB allele we used is not a complete null allele (Ayoob et al., 2006), or that there is an unidentified Sema-2b receptor that also mediates Sema-2b/PlexB signaling (Figure 8K). It was recently shown that Ret receptor mediates dendrite-ECM adhesion and that Ret also associates with Mys (Soba et al., 2015), and it would be interesting to determine if Ret mediates part of Sema-2b signaling.
In our model, we proposed that Trc/Fry signaling is upstream of integrins, based on three lines of evidence (Figure 8K). First, overexpressing integrins in trc and fry mutant background rescued their dendrite-ECM adhesion defect (Han et al., 2012). Second, the mammalian homologue of Trc, NDR2 kinase, can induce phosphorylation at the activity and trafficking-relevant site of β1-integrin to stimulate their trafficking to the cell membrane in neurons (Rehberg et al., 2014). Third, our previous study identified AAK1 (AP-2 associated kinase) and Rabin8, a GDP/GTP exchange factor (GEF) of Rab8 GTPase as two direct substrates of NDR1 kinase in mammals, both of which function in intracellular vesicle trafficking (Ultanir et al., 2012). Thus, it is possible that Trc/Fry signaling is important for regulating membrane turnover of integrins in neurons. In the future, it will also be important to determine whether the mammalian NDR kinases mediate semaphorin signaling.
Previous studies show that crosstalk between semaphorin and integrin signaling leads to inhibition of integrin-mediated adhesion in both invertebrates and vertebrates (Tamagnone and Comoglio, 2004; Kruger et al., 2005; Cho et al., 2012). For example, semaphorin-mediated signaling decreases integrin-mediated attachment during vascular morphogenesis (Serini et al., 2003). It has also been observed that semaphorin 4D promotes the formation of focal adhesion complexes through RhoA and Rho kinases (Basile et al., 2007). Here, we found that in Drosophila sensory neurons Sema-2b/PlexB signaling acts in the same pathway as integrins, and showed that PlexB and Mys form a complex (Figure 8). The biological relevance of this association would be of interest for future studies. It is possible that the physical association of PlexB and Mys stabilizes the surface expression of integrins for binding to laminins in the ECM. It is also possible that the interaction induces conformational changes to facilitate signaling pathways downstream of PlexB and/or integrins.
Overall, our results demonstrate that epidermis-secreted semaphorins regulate contact-dependent self-avoidance by promoting dendrite-ECM association and provide insights into the downstream components of semaphorin signaling pathway.
Experimental Procedures
Live Imaging and Image Processing
Animals were reared at 25°C and 29°C for RNAi expe riments in density-controlled vials. Third instar larvae were mounted in glycerol and dendrites of class IV da neurons were imaged using a Leica SP5 laser scanning confocal microscope. For high-resolution imaging in the z axis, images were collected as described in Han et al., 2012. To quantify dendrite enclosure, images were deconvoluted using Autoquant (MediaCybernetics) and analyzed in Imaris (Bitplane) as described in Han et al., 2012.
Immunohistochemistry
Third instar larvae were dissected in PBS, fixed in 4% PFA for 20 min at room temperature, blocked with 5% normal goat serum, and stained with the primary antibodies in a 0.3% Triton X-100 solution overnight at 4°C, and subsequently with secondary antibodies in a 0.3% Triton X-100 solution for 3 hours at room temperature.
Statistical analysis
Two-tailed Student’s t-tests were used to compare two samples. A one-way ANOVA test was used to compare multiple samples.
See Supplemental Experimental Procedures for details on mutant alleles, MARCM analysis, image analysis, immunohistochemistry, immunoprecipitation, in situ proximity ligation assay and western blotting used in this paper.
Supplementary Material
Highlights.
Sema-2b/PlexB signaling confines dendrites in a 2D space.
Sema-2b is secreted from the epidermis and functions through PlexB in neurons.
Sema-2b/PlexB signaling regulates Tricornered kinase activity in vivo.
An integrin β subunit associates with PlexB receptor.
Acknowledgments
We thank Drs. Chun Han, Matthew Klassen, Mu He, and William Joo for technical assistance; Drs. Alex Kolodkin, Liqun Luo, Bingwei Lu, and Tadashi Uemura for reagents; Bloomington Stock Center and VDRC Stock Center for fly stocks; all members of the Jan lab for discussions. S.M. is the recipient of a National Science Foundation Graduate Research Fellowship under grant number 1144247. This work was supported by National Institutes of Health grant number R37NS040929 to Y.N.J. L.Y.J. and Y.N.J. are investigators of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
S.M. designed and performed a genetic screen, confocal imaging, genetic and statistical analysis, co-immunoprecipitations, proximity ligation assays, and wrote the manuscript with help from other authors. S.Y. co-designed and performed co-immunoprecipitations. J.L. co-designed and performed immunohistochemistry with P-Trc antibody. S.H.Y. contributed to generating fly lines. P.S., P.J. and W.Z. contributed to generating several constructs. J.P., L.J. and Y.N.J. supervised the project development.
References
- Ayoob JC, Terman JR, Kolodkin AL. Drosophila Plexin B is a Sema-2a receptor required for axon guidance. Development. 2006;133:2125–2135. doi: 10.1242/dev.02380. [DOI] [PubMed] [Google Scholar]
- Basile J, Gavard J, Gutkind J. Plexin-B1 Utilizes RhoA and Rho Kinase to Promote the Integrin-dependent Activation of Akt and ERK and Endothelial Cell Motility. J Biol Chem. 2007;282:34888–34895. doi: 10.1074/jbc.M705467200. [DOI] [PubMed] [Google Scholar]
- Chen WV, Maniatis T. Clustered protocadherins. Development. 2013;140:3297–3302. doi: 10.1242/dev.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Chak K, Andreone B, Wooley J, Kolodkin A. The extracellular matrix proteoglycan perlecan facilitates transmembrane semaphorin-mediated repulsive guidance. Genes Dev. 2012;26:2222–35. doi: 10.1101/gad.193136.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corty MM, Freeman MR. Cell biology in neuroscience: Architects in neural circuit design: glia control neuron numbers and connesctivity. J Cell Biology. 2013;203:395–405. doi: 10.1083/jcb.201306099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Liu OW, Howell AS, Shen K. An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell. 2013;155:296–307. doi: 10.1016/j.cell.2013.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Shen K, Bülow H. Intrinsic and Extrinsic Mechanisms of Dendritic Morphogenesis. Annu Rev Physiol. 2014;77:1–30. doi: 10.1146/annurev-physiol-021014-071746. [DOI] [PubMed] [Google Scholar]
- Emoto K, He Y, Ye B, Grueber WB, Adler PN, Jan LY, Jan YN. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 2004;119:245–256. doi: 10.1016/j.cell.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Feng Z, Ko CP. Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-beta1. J Neurosci. 2008;28:9599–9609. doi: 10.1523/JNEUROSCI.2589-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Ashley J, Barria R, Maloney R, Freeman M, Budnik V. Integration of a retrograde signal during synapse formation by glia-secreted TGF-β ligand. Curr Biol. 2012;22:1831–1838. doi: 10.1016/j.cub.2012.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galko MJ, Krasnow MA. Cellular and Genetic Analysis of Wound Healing in Drosophila Larvae. PLoS Biol. 2004;2:e239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos ME, Bargmann CI. Mechanosensory neurite termination and tiling depend on SAX-2 and the SAX-1 kinase. Neuron. 2004;44:239–249. doi: 10.1016/j.neuron.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Ye B, Yang CH, Younger S, Borden K, Jan LY, Jan YN. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development. 2007;134:55–64. doi: 10.1242/dev.02666. [DOI] [PubMed] [Google Scholar]
- Han C, Jan LY, Jan YN. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc Natl Acad Sci USA. 2011;108:9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Wang D, Soba P, Zhu S, Lin X, Jan L, Jan YN. Integrins regulate repulsion-mediated dendritic patterning of Drosophila sensory neurons by restricting dendrites in a 2D space. Neuron. 2012;73:64–78. doi: 10.1016/j.neuron.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Stegert MR, Schmitz D, Hemmings BA. NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol. 2006;7:253–264. doi: 10.1038/nrm1891. [DOI] [PubMed] [Google Scholar]
- Hu H, Marton TF, Goodman CS. Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active rac and enhancing rhoa signaling. Neuron. 2001;32:39–51. doi: 10.1016/s0896-6273(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Hughes M, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, Schmucker D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivankovic-Dikic I, Grönroos E, Blaukat A, Barth BU, Dikic I. Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat Cell Biol. 2000;2:574–581. doi: 10.1038/35023515. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo W, Sweeney L, Liang L, Luo L. Linking cell fate, trajectory choice, and target selection: genetic analysis of sema-2b in olfactory axon targeting. Neuron. 2013;78:673–86. doi: 10.1016/j.neuron.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Yamaguchi K, Eiraku M, Sato M, Takata N, Kiyohara Y, Mishina M, Hirase H, Hashikawa T, Kengaku M. Remodeling of monoplanar Purkinje cell dendrites during cerebellar circuit formation. PLoS One. 2011;6:e20108. doi: 10.1371/journal.pone.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Meister M, Sanes J. Laminar Restriction of Retinal Ganglion Cell Dendrites and Axons: Subtype-Specific Developmental Patterns Revealed with Transgenic Markers. J Neurosci. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Shrestha B, Blazeski R, Mason C, Grueber W. Integrins establish dendrite-substrate relationships that promote dendritic self-avoidance and patterning in Drosophila sensory neurons. Neuron. 2012;73:79–91. doi: 10.1016/j.neuron.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin A, Pasterkamp R. SnapShot: Axon guidance II. Cell. 2013;153:722.e1. doi: 10.1016/j.cell.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Koike-Kumagai M, Yasunaga KI, Morikawa R, Kanamori T, Emoto K. The target of rapamycin complex 2 controls dendritic tiling of Drosophila sensory neurons through the Tricornered kinase signalling pathway. EMBO J. 2009;28:3879–3892. doi: 10.1038/emboj.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropouli E, Kolodkin AL. Semaphorins and the dynamic regulation of synapse assembly, refinement, and function. Curr Opin Neurobiol. 2014;27:1–7. doi: 10.1016/j.conb.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- Kulkarni VA, Firestein BL. The dendritic tree and brain disorders. Mol Cell Neurosci. 2012;50:10–20. doi: 10.1016/j.mcn.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lee J, Peng Y, Lin W, Parrish J. Coordinate control of terminal dendrite patterning and dynamics by the membrane protein Raw. Development. 2014;142:162–173. doi: 10.1242/dev.113423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre J, Kostadinov D, Chen W, Maniatis T, Sanes J. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature. 2012;488:517–521. doi: 10.1038/nature11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka R, Chivatakarn O, Badea T, Samuels I, Cahill H, Katayama K, Kumar S, Suto F, Chédotal A, Peachey N, et al. Class 5 Transmembrane Semaphorins Control Selective Mammalian Retinal Lamination and Function. Neuron. 2011a;71:460–73. doi: 10.1016/j.neuron.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chédotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011b;470:259–63. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- O’Malley AM, Shanley DK, Kelly AT, Barry DS. Towards an understanding of semaphorin signalling in the spinal cord. Gene. 2014;553:69–74. doi: 10.1016/j.gene.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Parrish JZ, Emoto K, Jan LY, Jan YN. Polycomb genes interact with the tumor suppressor genes hippo and warts in the maintenance of Drosophila sensory neuron dendrites. Genes Dev. 2007;21:956–972. doi: 10.1101/gad.1514507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ. Getting neural circuits into shape with semaphorins. Nat Rev Neurosci. 2012;13:605–18. doi: 10.1038/nrn3302. [DOI] [PubMed] [Google Scholar]
- Perry VH, Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982;297:683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- Puram S, Bonni A. Cell-intrinsic drivers of dendrite morphogenesis. Development. 2013;140:4657–71. doi: 10.1242/dev.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg K, Kliche S, Madencioglu DA, Thiere M, Müller B, Meineke BM, Freund C, Budinger E, Stork O. The serine/threonine kinase Ndr2 controls integrin trafficking and integrin-dependent neurite growth. J Neurosci. 2014;34:5342–54. doi: 10.1523/JNEUROSCI.2728-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagasti A, Guido MR, Raible DW, Schier AF. Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr Biol. 2005;15:804–814. doi: 10.1016/j.cub.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Salzberg Y, Díaz-Balzac CA, Ramirez-Suarez NJ, Attreed M, Tecle E, Desbois M, Kaprielian Z, Bülow HE. Skin-derived cues control arborization of sensory dendrites in Caenorhabditis elegans. Cell. 2013;155:308–20. doi: 10.1016/j.cell.2013.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J, Zipursky S. Design Principles of Insect and Vertebrate Visual Systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972;27:353–365. [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- Sloan S, Barres B. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr Opin Neurobiol. 2014;27:75–81. doi: 10.1016/j.conb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soba P, Zhu S, Emoto K, Younger S, Yang SJ, Yu HH, Lee T, Jan LY, Jan YN. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–416. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Meth. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- Soba P, Han C, Zheng Y, Perea D, Miguel-Aliaga I, Jan LY, Jan YN. The Ret receptor regulates sensory neuron dendrite growth and integrin mediated adhesion. Elife. 2015;4:e05491. doi: 10.7554/eLife.05491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Onishi M, Jan LY, Jan YN. Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc Natl Acad Sci USA. 2007;104:5199–5204. doi: 10.1073/pnas.0700895104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LO, Jiang Z, Rivlin-Etzion M, Hand R, Brady CM, Matsuoka RL, Yau KW, Feller MB, Kolodkin AL. On and Off retinal circuit assembly by divergent molecular mechanisms. Science. 2013;342:1241947. doi: 10.1126/science.1241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney LB, Chou YH, Wu Z, Joo W, Komiyama T, Potter CJ, Kolodkin AL, Garcia KC, Luo L. Secreted Semaphorins from Degenerating Larval ORN Axons Direct Adult Projection Neuron Dendrite Targeting. Neuron. 2011;72:734–47. doi: 10.1016/j.neuron.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio P. To move or not to move? EMBO Rep. 2004;5:356–361. doi: 10.1038/sj.embor.7400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaskovic R, Bichsel S, Rogniaux H, Stegert M, Hemmings B. Mechanism of Ca2+-mediated Regulation of NDR Protein Kinase through Autophosphorylation and Phosphorylation by an Upstream Kinase. J Biol Chem. 2003;278:6710–6718. doi: 10.1074/jbc.M210590200. [DOI] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Ultanir SK, Hertz NT, Li G, Ge WP, Burlingame AL, Pleasure SJ, Shokat KM, Jan LY, Jan YN. Chemical Genetic Identification of NDR1/2 Kinase Substrates AAK1 and Rabin8 Uncovers Their Roles in Dendrite Arborization and Spine Development. Neuron. 2012;73:1127–1142. doi: 10.1016/j.neuron.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Tamagnone L, Bai J, Comoglio PM, Montell D, Goodman CS. The transmembrane protein Off-track associates with Plexins and functions downstream of Semaphorin signaling during axon guidance. Neuron. 2001;32:53–62. doi: 10.1016/s0896-6273(01)00446-9. [DOI] [PubMed] [Google Scholar]
- Wu Z, Sweeney LB, Ayoob JC, Chak K, Andreone BJ, Ohyama T, Kerr R, Luo L, Zlatic M, Kolodkin AL. A combinatorial semaphorin code instructs the initial steps of sensory circuit assembly in the Drosophila CNS. Neuron. 2011;70:281–298. doi: 10.1016/j.neuron.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Sawada T, Shiba K, Liu S, Kanao T, Takahashi R, Hattori N, Imai Y, Lu B. Tricornered/NDR kinase signaling mediates PINK1-directed mitochondrial quality control and tissue maintenance. Genes Dev. 2013;27:157–62. doi: 10.1101/gad.203406.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky S, Sanes J. Chemoaffinity Revisited: Dscams, Protocadherins, and Neural Circuit Assembly. Cell. 2010;143:343–53. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Zipursky S, Grueber W. The Molecular Basis of Self-Avoidance. Annu Rev Neurosci. 2013;36:547–568. doi: 10.1146/annurev-neuro-062111-150414. [DOI] [PubMed] [Google Scholar]
- Zlatic M, Li F, Strigini M, Grueber W, Bate M. Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis. PLoS Biol. 2009;7:e1000135. doi: 10.1371/journal.pbio.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


