Abstract
Successful implementation of the 2013 American College of Cardiology/American Heart Association cholesterol guidelines hinges on a clear understanding of the clinician-patient risk discussion (CPRD). This is a dialogue between the clinician and patient about potential for atherosclerotic cardiovascular disease risk reduction benefits, adverse effects, drug-drug interactions, and patient preferences. Designed especially for primary prevention patients, this process of shared decision making establishes the appropriateness of a statin for a specific patient. CPRD respects the autonomy of an individual striving to make an informed choice aligned with personal values and preferences. Dedicating sufficient time to high-quality CPRD offers an opportunity to strengthen clinician-patient relationships, patient engagement, and medication adherence. We review the guideline-recommended CPRD, the general concept of shared decision making and decision aids, the American College of Cardiology/American Heart Association Risk Estimator application as an implementation tool, and address potential barriers to implementation.
Keywords: cerebrovascular disease, coronary heart disease, lipid-lowering therapy, myocardial infarction, risk estimation, shared decision making
Tell me, I may listen.
Teach me, I may remember.
Involve me, I will do it.
—Chinese proverb
The 2013 American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guidelines provide clinical recommendations based on high-quality randomized controlled trial evidence for reducing atherosclerotic cardiovascular disease (ASCVD) risk (1–6). The ultimate number of ASCVD events that will be prevented rests in the hands of clinicians and patients who are striving to optimally interpret and apply the recommendations in clinical practice. Successful guideline implementation, especially in primary prevention, hinges on clear understanding and attention to the clinician-patient risk discussion (CPRD). This is a dialogue between the clinician and patient about potential for ASCVD risk reduction benefits, adverse effects, drug-drug interactions, and patient preferences. In this review, we expand on the guideline-recommended CPRD, examine supporting evidence on shared decision making (SDM) and decision aids, highlight the ACC/AHA Risk Estimator application (app) as an implementation tool (Figure 1), and address potential barriers to implementation. We aim to synthesize evidence with our clinical experience to maximize clinical relevance.
FIGURE 1. Smartphone Screenshot of the 2013 ACC/AHA Guidelines ASCVD Risk Estimator App.
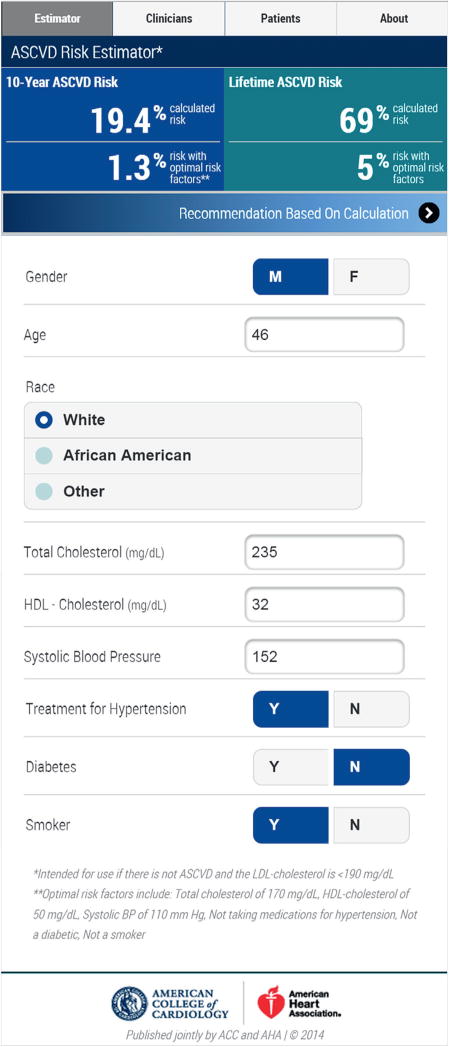
Additional screenshots are provided in the Online Appendix 1. The app can also be downloaded from iTunes (16) or Google Play (17), or the web version can be launched from a computer (18). ACC = American College of Cardiology; AHA = American Heart Association; ASCVD = atherosclerotic cardiovascular disease.
WHAT THE GUIDELINES SAY
In primary prevention, the 2013 ACC/AHA cholesterol guidelines recommend estimating the 10-year ASCVD risk with pooled cohort equations every 4 to 6 years in adults 40 to 75 years of age without clinical ASCVD or diabetes who have a low-density lipoprotein cholesterol (LDL-C) level of 70 to 189 mg/dl. However, the pooled cohort equations should not be used to re-estimate risk in statin-treated patients or after a short course of lifestyle change. The guidelines advise that a statin may be indicated when a patient has either 1) an estimated 10-year ASCVD (heart attack/stroke) risk of ≥7.5% (Class I, Level of Evidence: A) or 2) estimated risk of 5% to 7.4% (Class IIa, Level of Evidence: B).
The guideline panel compared the estimated number needed-to-treat (NNT) to avoid an ASCVD event with the estimated number needed-to-harm (NNH) (with respect to diabetes; not considered equivalent to an ASCVD event) to identify evidence-based 10-year ASCVD risk thresholds for initiation of moderate-intensity or high-intensity statin therapy. For a moderate-intensity statin, the estimated NNT vs. NNH was 36 to 44 vs. 100 for the ≥7.5% risk threshold and 57 to 67 vs 100 for patients with 5% to 7.4% risk. For a high-intensity statin, respective estimates were 30 vs. 33 for ≥7.5% risk and 44 vs. 33 for 5% to 7.4% risk. Therefore, these estimates supported net clinical benefit with moderate-intensity statin therapy in each risk group and also for high-intensity statin therapy in those with ≥7.5% risk.
Starting a statin, which is commonly a lifelong therapy, is not a trivial decision and the guidelines recommend holding a CPRD (1,6). Elements of a comprehensive CPRD are shown in Table 1. Importantly, the appropriateness of statin therapy can only be established through this process of SDM (7–9). Per the guidelines, the estimation of 10-year risk per se should not be used in isolation to prescribe a statin.
TABLE 1.
Checklist for Clinician-Patient Risk Discussion
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The 2013 ACC/AHA guidelines are reflective of the idea that: “Guidelines must not replace clinicians’ compassionate and mindful engagement of the patient in making decisions together. This is the optimal practice of evidence-based medicine” (10). Accordingly, the 10-year ASCVD risk estimate serves as a starting point for a patient-clinician dialogue. While discussion about the expected benefits and risks of medication should generally occur with all prescriptions, such discussions too often occur haphazardly or not at all (7–9). By bringing the CPRD to the forefront, the 2013 ACC/AHA cholesterol guidelines reinforce the need for a more consistent and reliable dialogue in connection with writing a prescription. Emphasis on the CPRD acknowledges that each case is unique and that patient preferences matter, providing a flexible framework that values the art of medicine.
IMPORTANCE OF SDM
The clinical role of SDM was recently reviewed (9,11). By definition, the decision is shared, but not necessarily equally. Depending on the situation, either the patient or clinician may take the lead in the process (11). Attention to SDM has increased in the United States, in particular because of changes in health policy, the availability of greater numbers of decision aids, and new funding opportunities through the Patient-Centered Outcomes Research Institute (9). It is not a matter of if—but rather of when—SDM will be more widely implemented.
This is supported by numerous studies. A survey of patients undergoing angiograms indicated that patients generally want to take an active role in SDM, although they appreciate their physician taking a lead on problem-solving tasks (12). In a survey of 1,340 patients older than 40 years of age, approximately 70% reported a preference for SDM (13). This is consistent with a survey of 6,636 participants in contemporary acute myocardial infarction registries, where 68% preferred being actively involved in decision making (14).
DECISION AIDS AND THE ACC/AHA RISK ESTIMATOR APP
Participation in SDM requires an informed patient. In the randomized Statin Choice Trial of 150 patients with diabetes, a decision aid improved risk communication, beliefs, and decisional conflict (15). Whereas the focus is on the patient, decision aids can be used by both patients and clinicians, either together during the clinical encounter, or separately between visits. To increase relevant health knowledge and promote patient-clinician dialogue, decision aids may be produced digitally or on paper.
The ACC/AHA Risk Estimator app was developed to help clinicians and patients implement SDM related to the 2013 ACC/AHA cholesterol guidelines. The app is widely available and easily accessed via computers, tablets, or smartphones (Figure 1, Online Appendix 1) (16–18). Easy availability compared with previous risk scores was a critical step in facilitating use by patients and care providers alike. It was also designed with the hope that it could be integrated into the electronic medical record for automatic calculation and display.
The app not only facilitates estimation of 10-year ASCVD risk for those 40 to 79 years old, but also allows for lifetime risk estimation for those 20 to 59 years old. This latter risk is based on the grouping of risk factor levels into 5 strata, related to whether all risk factors are optimal, ≥1 risk factor is not optimal, ≥1 risk factor is elevated, 1 major risk factor is present, or ≥2 major risk factors are present (19). Using the app will facilitate linkage to lifestyle (20) and obesity/over-weight (21) guideline recommendations, as these are highlighted in the app. For example, the app’s patient-oriented weight management section advises that “losing just 3–5% of body weight can improve blood pressure and cholesterol levels and reduce the risk for cardiovascular disease and diabetes.”
COMMUNICATING ASCVD RISK AND POTENTIAL FOR STATIN BENEFIT
A key aspect of CPRD is communicating that an individual’s risk estimate is a group average from a representative sample. One straightforward and reasonable way to communicate an estimated 10-year ASCVD risk of 19% is to say, “of 100 patients like you, 19 would be expected to have a stroke or heart attack in the next 10 years.” Whereas for some patients this description of risk may be sufficient, others may require additional cues. An option is a visual aid showing absolute risk and incremental benefits/risks associated with drug therapy. This is currently available in a web-based tool (22) and is being considered for inclusion in future versions of the ACC/AHA Risk Estimator app.
A recent systematic review on risk communication did not establish the superiority of any single method for conveying risk, but it suggests that using visual aids (e.g., icon arrays, risk pictographs) can improve patient understanding and satisfaction (23). Whereas some tools have focused exclusively on longevity (24), nonfatal ASCVD outcomes are costly and nontrivial to the patients and their families (25,26). The NNT data appeals to clinicians, but it appears to decrease patient understanding (23). Previous studies have generally been small and heterogeneous in methodology, thus further high-quality, patient-oriented research comparing different presentation styles is needed to better understand how to best enhance patient-clinician interactions (9,23,27,28). In the meantime, a group of experts has provided a comprehensive set of “best practices” in presenting quantitative risk information through decision aids (29).
In our experience, we have found it useful to compare an individual’s risk estimate with someone of the same age, sex, and race who has optimal risk factors, defined as a total cholesterol of 170 mg/dl, high-density lipoprotein cholesterol of 50 mg/dl, untreated systolic blood pressure of 110 mm Hg, no diabetes history, and absence of current smoking. Personalizing the presentation may lead to more effective SDM.
Once patients understand their ASCVD risk, the next step is a discussion about risk reducing interventions, including lifestyle changes and initiation of a statin. Patients at higher risk generally understand that they are more likely to benefit from more intensive therapy. For those in a statin benefit group, high-intensity statin regimens (≥50% anticipated LDL-C lowering) are recommended to those at highest risk. As noted previously (4), meta-analyses have shown a ~20% relative risk reduction per 39 mg/dl lowering of LDL-C. Moderate-intensity regimens (30% to <50% LDL-C lowering) are reserved for those at lower risk who still may benefit from statin therapy. The ACC/AHA Risk Estimator app helps providers make appropriate treatment choices by listing the various statins and respective doses for the different intensities. In general, patients and clinicians should aim for the maximum tolerated regimen, however for those predisposed to adverse effects or for whom drug-drug interactions require a lower dose and favor specific statins, a moderate-intensity statin is preferred.
WHEN THE RISK-BASED TREATMENT DECISION IS UNCERTAIN
As indicated in the ACC/AHA Risk Estimator app, if the risk-based treatment decision is uncertain after 10-year risk is estimated, which may be common in cases where risk is not greatly elevated, then the patient and clinician should take into consideration additional factors that modify the risk estimate. These include a family history of premature ASCVD, elevated lifetime risk, LDL-C ≥160 mg/dl, an elevated coronary artery calcium score or ≥75th percentile based on age, sex, and ethnicity, a high-sensitivity C-reactive protein level ≥2.0 mg/l, or an ankle-brachial index <0.9. Importantly, lifetime risk is primarily intended to enhance discussion and promotion of an optimal lifestyle; it is not intended per se to dictate statin prescriptions (19).
DISCUSSING RISK OF STATIN ADVERSE EFFECTS
In the discussion of potential adverse effects, an ideal CPRD weighs expected benefits and risks, conveying the concept of net benefit. When addressing statin adverse effects with a patient, one may consider the 5 Ms: metabolism, muscle, medication interactions, major organ effects, and memory (30). The 2013 ACC/AHA cholesterol guideline emphasizes the safety of statins when used in patients similar to those enrolled in clinical trials. The guideline indicates that adverse effects may only begin to outweigh benefits in lower-risk groups and that different margins of safety may occur in patients excluded from clinical trials (e.g., elderly, serious comorbidities).
We see value in being attuned to concerns popularized in the mainstream media, as patients will often be aware of these reports and factor them into decisions. To this end, statin-related diabetes risk is a topic worth prioritizing. When diabetes risk factors are present (metabolic syndrome, hemoglobin A1c ≥6%, fasting glucose ≥100 mg/dl, body mass index ≥30 kg/m2), statins are associated with a higher likelihood of developing diabetes or acceleration in its diagnosis by ~5 weeks due to a small hyperglycemic effect (31). Similar risk factors predict a new diagnosis of diabetes in those with pre-existing ASCVD (32). Comparable drug-induced increases in glucose from other medications, such as thiazides, have not increased ASCVD risk. Regarding microvascular disease risk, statin use preceding diabetes diagnosis was not associated with a higher risk over a median follow-up of 2.7 years (33).
Therefore, statins generally seem to unmask an underlying propensity to develop diabetes, but this may not have major adverse macrovascular or microvascular risk implications. It could be an opportunity to underscore the benefit of lifestyle changes at an earlier stage in the natural history of the disorder. Lifestyle changes, including avoidance of weight gain and promotion of weight loss (34,35), are useful ways to prevent diabetes. Moreover, crossing the threshold to diabetes does not reduce the expected benefits of statins and reinforces the need for effective ASCVD risk reduction (1). Generally, an estimated 5 to 9 ASCVD events may be prevented per case of diabetes that might develop with statin therapy (36,37).
For patients concerned about statin-induced cognitive impairment, reassurance can be provided that this is exceedingly rare, if it exists at all (1,38–40). Patients should also be informed that severe muscle damage is extremely rare and muscle complaints are usually no more common in statin-treated patients than in those given placebo in clinical trials, even in ones without a drug run-in period (1). Nevertheless, myalgias and arthralgias are common and important issues in real-world settings, and their significance—whether causally related or not to statin therapy—should not be minimized. It may be reassuring that most patients who develop myalgias can tolerate a statin when rechallenged (41,42), especially when it is given in a lower dose or less frequently during the week. A personal or family history of muscle problems should be determined and addressed, however, before statin therapy is initiated.
If new symptoms develop, the clinician should be alerted so that they can be evaluated appropriately. Moderate to severe symptoms that are progressive or concerning should prompt cessation of statin therapy until a clinical evaluation can occur. This, in part, is why the guidelines recommend post-treatment lipid and safety evaluations 3 to 12 weeks after initiation of statin therapy and then at intervals thereafter deemed appropriate through the SDM period.
WHEN TO USE THE APP
The ACC/AHA Risk Estimator app was designed as a resource that could be used during and between clinical encounters. Some practices have encouraged patients to download the app, review information, and enter data to prepare for a CPRD in advance of the office visit. Beyond the in-office uses discussed earlier, patient-centered information contained in the app can serve as an ongoing resource after the visit. This section includes information on understanding cardiovascular risk, diet and physical activity recommendations, weight management recommendations, blood cholesterol management recommendations, statin benefit groups, and common cardiovascular terms.
For some patients, the SDM process may span multiple encounters. After the initial visit, further testing to refine risk assessment may be pursued or the patient may desire more time to review information before making a decision. As opposed to decisions in the acute care setting, decisions regarding chronic therapy (e.g., a statin) are, by definition, less pressured, and patients should be given adequate time to learn about their risk status and treatment options. Goals of this process include alignment with the patients’ values and preferences, along with engagement of the patients in their care. We provide 2 narrative case examples in Online Appendix 2.
POTENTIAL BARRIERS TO IMPLEMENTATION: ADDRESSING COMMON CONCERNS
MY PATIENT ISN’T 40 TO 75 YEARS OLD
Patients falling outside the age boundaries of the ASCVD risk estimator present an added opportunity to exercise clinical judgment and weigh patient preference because there is less evidence to guide clinical decision making. For patients <40 years of age, we recommend prioritizing discussion about lifetime risk and other selected risk factors (particularly family history and LDL-C ≥160 mg/dl). For patients ≥75 years of age, we recommend paying particular attention to the potential for adverse effects (e.g., from drug-drug interactions).
I DON’T AGREE WITH THE CONTENT
Clinicians who disagree with the guidelines’ core content and associated decision aids may find that their beliefs prohibit implementation. For example, some clinicians have raised concerns that women may not tolerate statins as well as men do and the associated benefit may be less. Such is not the case in secondary prevention trials, where strong evidence exists that women derive the same magnitude of benefit as men in terms of the reduction in nonfatal ASCVD events (1). Whereas the guidelines acknowledge that less data are available in primary prevention, results from multiple trials (43,44) support the selective use of statins in women who are at a sufficient level of estimated risk. The new guidelines’ use of separate risk equations by sex and race should decrease the number of white women who would have been given statins based only on a mildly elevated LDL-C, instead focusing treatment on those most likely to benefit. Treatment decisions ultimately reside with the patient and it is the responsibility of the clinician to share information that is scientifically accurate and bias-free with their patients (45).
I ALREADY DO THIS
Many clinicians already carry out CPRDs. Nonetheless, a systematic review suggested that most could improve (46). A study of well-educated, affluent patients from the West Coast of the United States who participated in focus groups conveyed a reluctance to disagree or assert their preference for fear of being perceived as difficult and compromising their quality of care (47). Moreover, a study formally asking clinicians what their patients believe found that physicians tend to misdiagnose patients’ health beliefs and preferences (48). On this basis, it should come as no surprise that long-term adherence to evidence-based medical therapies, including statins, is only about 50% (49).
Concentrating efforts on high-quality CPRD offers an opportunity to strengthen clinician-patient relationships, patient engagement, and medication adherence (9). In our own practices, we find that patients appreciate the opportunity to discuss the meaning of their estimated risk and lipid results, their other nonlipid risk factors and what to do about them, components of a healthy lifestyle and how they may be incorporated, and the potential role of statin therapy. Our patients appreciate that evidence-based guidelines exist to inform these discussions, but judgment is required, and they appreciate that guidelines with thresholds of estimated risk do not mandate medication prescription.
I DON’T HAVE THE TIME
We have heard colleagues say that the CPRD is difficult, too involved and time-consuming, and may not be practically implemented in an era of appointments lasting <15 min. However, in a nation where almost 1 in 3 people will die of a myocardial infarction or stroke and almost 6 of 10 will have an event before they die, it seems prudent to endorse a CPRD. For straightforward cases, a CPRD may take <5 min. For more complicated cases, it may take a full visit or multiple visits to ensure that all elements have been discussed, the patient has had enough time to consider the options, and the patient’s questions are fully answered. Working with patients on the issues of greatest consequence to their health—is this not precisely what we are supposed to do?
Some physicians, such as those in busier practices, may work with other members of the care team on the CPRD. For example, a nurse practitioner or physician assistant may initiate the discussion, present evidence, and elicit patient preferences, to be later joined by the physician in the SDM process. Additionally, portions of the CPRD may then be reinforced and extended by other care team members, such as a dietitian who builds on the initial diet discussion. We not only need dedicated and collaborative clinicians, but also reimbursement models that value these team-based efforts.
Pay for process could be a reasonable option that does not discriminate against clinicians who treat those with the toughest problems (e.g., statin intolerance) and those with the fewest resources. For example, a reasonable process-based goal in primary prevention could be documentation that each component of the CPRD checklist was addressed (Table 1). This could be easily accomplished collaboratively in one’s electronic health record with a dot or smart phrase, or via the ACC/AHA Risk Estimator app if integrated into the electronic health record. Such an approach is compatible with recommendations from implementation science leaders to use checklists to enhance guideline implementation (50).
CONCLUSIONS
Conceptually, the CPRD has been summarized as an intersection between evidence, clinical judgment, and patient preference (Central Illustration). It is dynamic in nature, as clinicians and patients will focus on a variety of issues over time, including review of medication types and doses. The CPRD respects the autonomy of patients as the persons who are balancing the expected risks and available therapeutic options to reach decisions that best fits with their preferences. Whereas the patient makes the decision, the physician provides essential guidance and may adjust the content and strength of their guidance based on the patient’s clinical profile, learning style, and comfort in making the decision. Shining the spotlight on CPRD serves as a reminder that we are not treating only numbers, but rather we are addressing the cardiovascular health concerns of individual patients.
CENTRAL ILLUSTRATION. Conceptual Framework for CPRD.
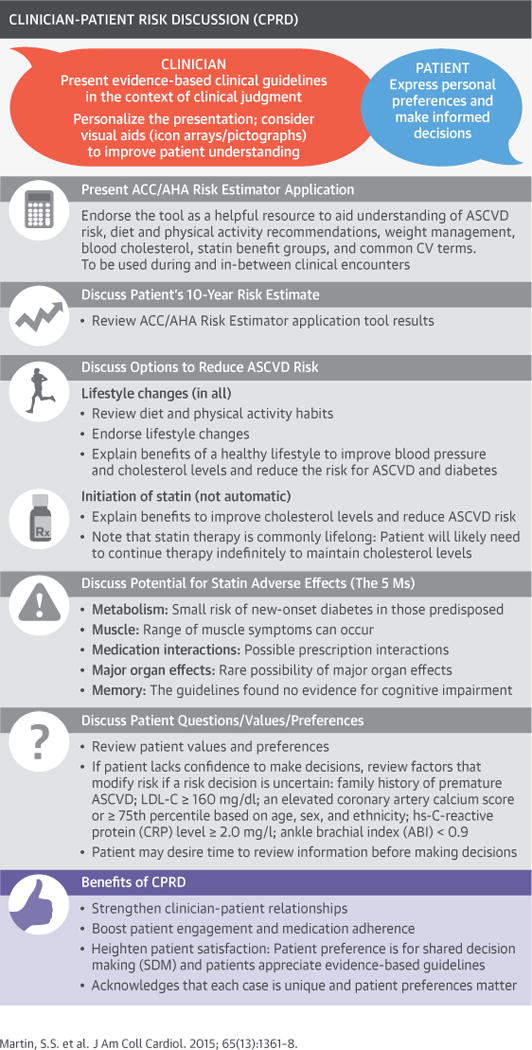
The clinician-patient risk discussion (CPRD) is an intersection of evidence, clinical judgment, and patient preference. ACC = American College of Cardiology; AHA = American Heart Association; ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; CRP = C-reactive protein; CV = cardiovascular; LDL-C = low-density lipoprotein cholesterol; SDM = shared decision making.
Supplementary Material
Acknowledgments
The authors would like to recognize Dr. Sanjay Kaul for putting forth the concept of the risk discussion as an intersection between evidence, clinical judgment, and patient preference.
Dr. Martin is supported by the Pollin Cardiovascular Prevention Fellowship, Marie-Josée and Henry R. Kravis endowed fellowship, and a National Institutes of Health training grant (T32HL07024). Dr. Blaha has served on the Advisory Board of Pfizer. Dr. Gluckman has served as an expert witness for Takeda Pharmaceuticals. Dr. Blumenthal is supported by the Kenneth Jay Pollin Professorship in Cardiology.
ABBREVIATIONS AND ACRONYMS
- ACC
American College of Cardiology
- AHA
American Heart Association
- app
application
- ASCVD
atherosclerotic cardiovascular disease
- CPRD
clinician-patient risk discussion
- LDL-C
low-density lipoprotein cholesterol
- NNH
number needed-to-harm
- NNT
number needed-to-treat
- SDM
shared decision making
APPENDIX
For supplemental materials, please see the online version of this article.
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. for the 2013 ACC/AHA Cholesterol Guideline Panel Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med. 2014;160:339–43. doi: 10.7326/M14-0126. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein A. The new cholesterol treatment guidelines. N Engl J Med. 2014;370:1957. doi: 10.1056/NEJMc1403438. [DOI] [PubMed] [Google Scholar]
- 4.Martin SS, Abd TT, Jones SR, Michos ED, Blumenthal RS, Blaha MJ. 2013 ACC/AHA cholesterol treatment guideline: what was done well and what could be done better. J Am Coll Cardiol. 2014;63:2674–8. doi: 10.1016/j.jacc.2014.02.578. [DOI] [PubMed] [Google Scholar]
- 5.Martin SS, Blumenthal RS. Concepts and controversies: the 2013 American College of Cardiology/American Heart Association risk assessment and cholesterol treatment guidelines. Ann Intern Med. 2014;160:356–8. doi: 10.7326/M13-2805. [DOI] [PubMed] [Google Scholar]
- 6.Martin SS, Stone NJ, Blumenthal RS. The Risk Discussion: A Key Virtue of the 2013 ACC/AHA Cholesterol Treatment Guidelines. Cardiology Today. 2014 Jan 27; [serial online] Available at: http://www.healio.com/cardiology/guidelines/news/online/%7Bb672ff07-1288-4fee-9407-82cb3821a8c9%7D/the-risk-discussion-a-key-virtue-of-the-2013-accaha-cholesterol-treatment-guidelines. Accessed January, 27, 2015.
- 7.Barry MJ, Edgman-Levitan S. Shared decision making–pinnacle of patient-centered care. N Engl J Med. 2012;366:780–1. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 8.Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368:6–8. doi: 10.1056/NEJMp1209500. [DOI] [PubMed] [Google Scholar]
- 9.Hess EP, Coylewright M, Frosch DL, Shah ND. Implementation of shared decision making in cardiovascular care: past, present, and future. Circ Cardiovasc Qual Outcomes. 2014;7:797–803. doi: 10.1161/CIRCOUTCOMES.113.000351. [DOI] [PubMed] [Google Scholar]
- 10.Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2503–4. doi: 10.1001/jama.2013.281422. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann TC, Légaré F, Simmons MB, et al. Shared decision making: what do clinicians need to know and why should they bother? Med J Aust. 2014;201:35–9. doi: 10.5694/mja14.00002. [DOI] [PubMed] [Google Scholar]
- 12.Deber RB, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Arch Int Med. 1996;156:1414–20. [PubMed] [Google Scholar]
- 13.Adams JR, Elwyn G, Légaré F, Frosch DL. Communicating with physicians about medical decisions: a reluctance to disagree. Arch Intern Med. 2012;172:1184–6. doi: 10.1001/archinternmed.2012.2360. [DOI] [PubMed] [Google Scholar]
- 14.Krumholz HM, Barreto-Filho JA, Jones PG, Li Y, Spertus JA. Decision-making preferences among patients with an acute myocardial infarction. JAMA Intern Med. 2013;173:1252–7. doi: 10.1001/jamainternmed.2013.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann DM, Ponieman D, Montori VM, Arciniega J, McGinn T. The Statin Choice decision aid in primary care: a randomized trial. Patient Educ Couns. 2010;80:138–40. doi: 10.1016/j.pec.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 16.American College of Cardiology. ASCVD Risk Estimator. iTunes. 2014 Feb 22; [mobile application] Available at: https://itunes.apple.com/us/app/ascvd-risk-estimator/id808875968?mt=8. Accessed January 27, 2015.
- 17.American College of Cardiology Foundation. ASCVD Risk Estimator. Google Play. 2014 Feb 3; [mobile application] Available at: https://play.google.com/store/apps/details?id=org.acc.cvrisk&hl=en. Accessed January 27, 2015.
- 18.American College of Cardiology, American Heart Association. ASCVD Risk Estimator. 2014 [online tool] Available at: http://tools.cardiosource.org/ASCVD-Risk-Estimator/. Accessed January 27, 2015.
- 19.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960–84. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayo Clinic. Statin/Aspirin Choice Decision Aid. 2013 Jul 13; [online tool] Available at: http://statindecisionaid.mayoclinic.org/. Accessed January 27, 2015.
- 23.Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270–80. doi: 10.7326/M14-0295. [DOI] [PubMed] [Google Scholar]
- 24.Fontana M, Asaria P, Moraldo M, et al. Patient-accessible tool for shared decision making in cardiovascular primary prevention: balancing longevity benefits against medication disutility. Circulation. 2014;129:2539–46. doi: 10.1161/CIRCULATIONAHA.113.007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czarny MJ, Martin SS, Kohli P, Metkus T, Blumenthal RS. Nonfatal outcomes in the primary prevention of atherosclerotic cardiovascular disease: is all-cause mortality really all that matters? Circ Cardiovasc Qual Outcomes. 2014;7:481–5. doi: 10.1161/CIRCOUTCOMES.114.000871. [DOI] [PubMed] [Google Scholar]
- 26.O’Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29:693–704. doi: 10.2165/11584620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 28.Barry MJ. Health decision aids to facilitate shared decision making in office practice. Ann Intern Med. 2002;136:127–35. doi: 10.7326/0003-4819-136-2-200201150-00010. [DOI] [PubMed] [Google Scholar]
- 29.Trevena LJ, Zikmund-Fisher BJ, Edwards A, et al. Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S7. doi: 10.1186/1472-6947-13-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz DH, Intwala SS, Stone NJ. Addressing statin adverse effects in the clinic: the 5 Ms. J Cardiovasc Pharmacol Ther. 2014;19:533–42. doi: 10.1177/1074248414529622. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–71. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters DD, Ho JE, Boekholdt SM, et al. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J Am Coll Cardiol. 2013;61:148–52. doi: 10.1016/j.jacc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol. 2014;2:894–900. doi: 10.1016/S2213-8587(14)70173-1. [DOI] [PubMed] [Google Scholar]
- 34.Knowler WC, Barrett-Connor E, Fowler SE, et al. for the Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong KL, Waters DD, Messig M, DeMicco DA, Rye KA, Barter PJ. Effect of change in body weight on incident diabetes mellitus in patients with stable coronary artery disease treated with atorvastatin (from the Treating to New Targets study) Am J Cardiol. 2014;113:1593–8. doi: 10.1016/j.amjcard.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 37.Cannon CP. Balancing the benefits of statins versus a new risk-diabetes. Lancet. 2010;375:700–1. doi: 10.1016/S0140-6736(10)60234-6. [DOI] [PubMed] [Google Scholar]
- 38.Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc. 2013;88:1213–21. doi: 10.1016/j.mayocp.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688–97. doi: 10.7326/0003-4819-159-10-201311190-00007. [DOI] [PubMed] [Google Scholar]
- 40.Jukema JW, Cannon CP, de Craen AJ, Westendorp RG, Trompet S. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875–81. doi: 10.1016/j.jacc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Mampuya WM, Frid D, Rocco M, et al. Treatment strategies in patients with statin intolerance: the Cleveland Clinic experience. Am Heart J. 2013;166:597–603. doi: 10.1016/j.ahj.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–34. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizuno K, Nakaya N, Ohashi Y, et al. for the MEGA Study Group Usefulness of pravastatin in primary prevention of cardiovascular events in women: analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA study) Circulation. 2008;117:494–502. doi: 10.1161/CIRCULATIONAHA.106.671826. [DOI] [PubMed] [Google Scholar]
- 44.Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation. 2010;121:1069–77. doi: 10.1161/CIRCULATIONAHA.109.906479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henriquez AR. Bias control in shared decision making: still too many loose ends. BMJ. 2012;345:e8291. doi: 10.1136/bmj.e8291. [DOI] [PubMed] [Google Scholar]
- 46.Elwyn G, Scholl I, Tietbohl C, et al. Many miles to go…”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S14. doi: 10.1186/1472-6947-13-S2-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frosch DL, May SG, Rendle KA, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled ’difficult’ among key obstacles to shared decision making. Health Aff (Millwood) 2012;31:1030–8. doi: 10.1377/hlthaff.2011.0576. [DOI] [PubMed] [Google Scholar]
- 48.Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345:e6572. doi: 10.1136/bmj.e6572. [DOI] [PubMed] [Google Scholar]
- 49.Akincigil A, Bowblis JR, Levin C, Jan S, Patel M, Crystal S. Long-term adherence to evidence based secondary prevention therapies after acute myocardial infarction. J Gen Intern Med. 2008;23:115–21. doi: 10.1007/s11606-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pronovost PJ. Enhancing physicians’ use of clinical guidelines. JAMA. 2013;310:2501–2. doi: 10.1001/jama.2013.281334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


