Abstract
Susceptibility to the human autoimmune disease insulin-dependent diabetes mellitus is strongly associated with particular haplotypes of the major histocompatibility complex (MHC). Similarly, in a spontaneous animal model of this disease, the nonobese diabetic (NOD) mouse, the genes of the MHC play an important role in the development of diabetes. We have produced transgenic NOD mice that express the class II MHC molecule I-Ad in addition to the endogenous I-Ag7 molecules in order to study the role of these molecules in the disease process. Although the inflammatory lesions within the islets of Langerhans in the pancreas appear similar in transgenic and nontransgenic animals, transgenic mice develop diabetes with greatly diminished frequency compared to their nontransgenic littermates (10% of transgenic females by 30 weeks of age compared to 45% of nontransgenic females). Furthermore, adoptive transfer experiments show that T cells present in the transgenic mice are able to interfere with the diabetogenic process caused by T cells from nontransgenic mice. Thus, the mechanism by which I-Ad molecules protect mice from diabetes includes selecting in the thymus and/or inducing in the periphery T cells capable of inhibiting diabetes development.
Full text
PDF

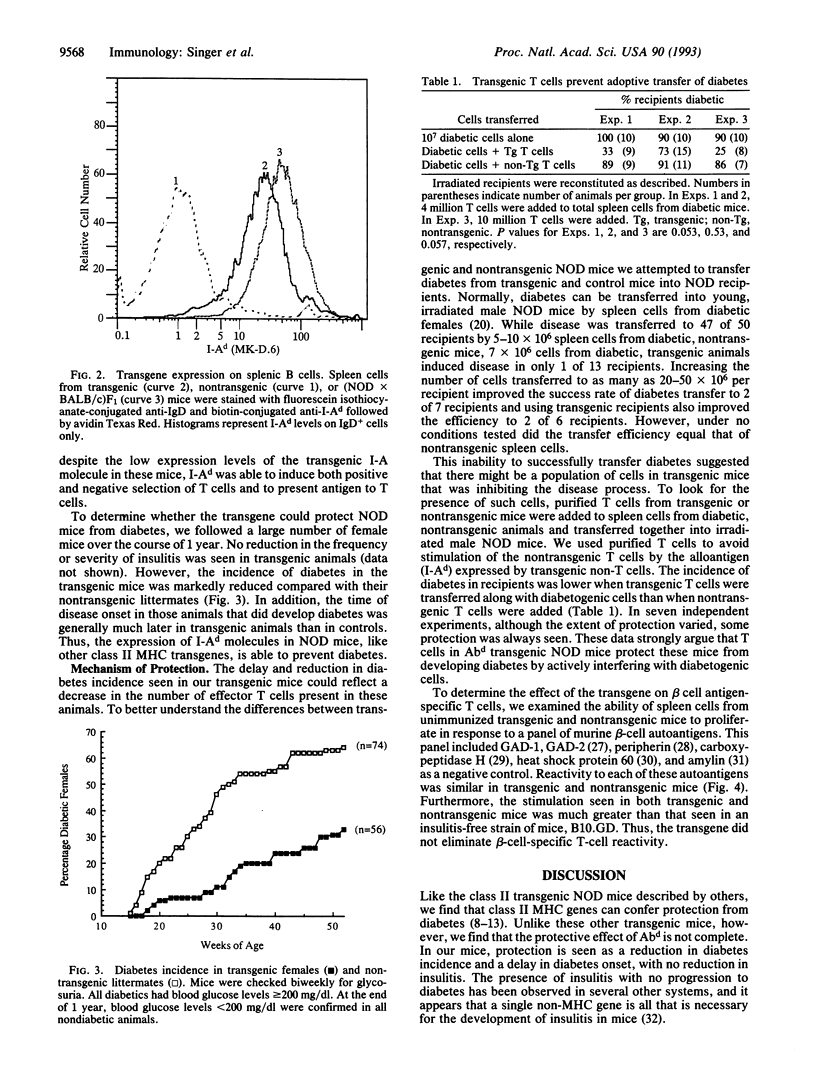
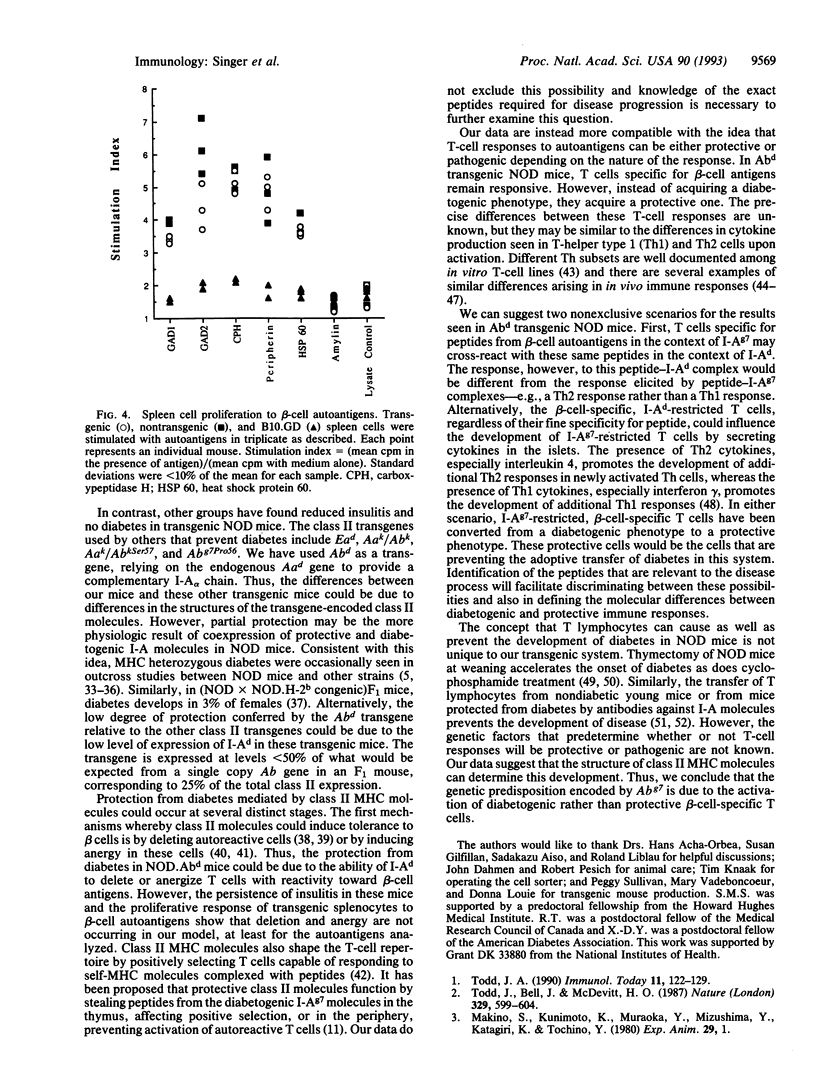

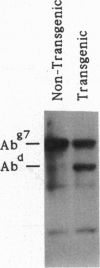
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., McDevitt H. O. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2435–2439. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Bedossa P., Bendelac A., Bach J. F., Carnaud C. Syngeneic T cell transfer of diabetes into NOD newborn mice: in situ studies of the autoimmune steps leading to insulin-producing cell destruction. Eur J Immunol. 1989 Oct;19(10):1947–1951. doi: 10.1002/eji.1830191028. [DOI] [PubMed] [Google Scholar]
- Boitard C., Bendelac A., Richard M. F., Carnaud C., Bach J. F. Prevention of diabetes in nonobese diabetic mice by anti-I-A monoclonal antibodies: transfer of protection by splenic T cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9719–9723. doi: 10.1073/pnas.85.24.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C., Villa M. C., Becourt C., Gia H. P., Huc C., Sempe P., Portier M. M., Bach J. F. Peripherin: an islet antigen that is cross-reactive with nonobese diabetic mouse class II gene products. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):172–176. doi: 10.1073/pnas.89.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C., Yasunami R., Dardenne M., Bach J. F. T cell-mediated inhibition of the transfer of autoimmune diabetes in NOD mice. J Exp Med. 1989 May 1;169(5):1669–1680. doi: 10.1084/jem.169.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme J., Schuhbaur B., Kanagawa O., Benoist C., Mathis D. MHC-linked protection from diabetes dissociated from clonal deletion of T cells. Science. 1990 Jul 20;249(4966):293–295. doi: 10.1126/science.2115690. [DOI] [PubMed] [Google Scholar]
- Castaño L., Russo E., Zhou L., Lipes M. A., Eisenbarth G. S. Identification and cloning of a granule autoantigen (carboxypeptidase-H) associated with type I diabetes. J Clin Endocrinol Metab. 1991 Dec;73(6):1197–1201. doi: 10.1210/jcem-73-6-1197. [DOI] [PubMed] [Google Scholar]
- Charlton B., Bacelj A., Mandel T. E. Administration of silica particles or anti-Lyt2 antibody prevents beta-cell destruction in NOD mice given cyclophosphamide. Diabetes. 1988 Jul;37(7):930–935. doi: 10.2337/diab.37.7.930. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Lepault F., Bendelac A., Bach J. F. Acceleration of the onset of diabetes in NOD mice by thymectomy at weaning. Eur J Immunol. 1989 May;19(5):889–895. doi: 10.1002/eji.1830190516. [DOI] [PubMed] [Google Scholar]
- Elias D., Markovits D., Reshef T., van der Zee R., Cohen I. R. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M., Makino S. Promotion of spontaneous diabetes in non-obese diabetes-prone mice by cyclophosphamide. Diabetologia. 1984 Dec;27(6):604–606. doi: 10.1007/BF00276978. [DOI] [PubMed] [Google Scholar]
- Hattori M., Buse J. B., Jackson R. A., Glimcher L., Dorf M. E., Minami M., Makino S., Moriwaki K., Kuzuya H., Imura H. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986 Feb 14;231(4739):733–735. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- Hattori M., Fukuda M., Ichikawa T., Baumgartl H. J., Katoh H., Makino S. A single recessive non-MHC diabetogenic gene determines the development of insulitis in the presence of an MHC-linked diabetogenic gene in NOD mice. J Autoimmun. 1990 Feb;3(1):1–10. doi: 10.1016/0896-8411(90)90002-a. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Shreiber M. Neonatal injection of CD3 antibody into nonobese diabetic mice reduces the incidence of insulitis and diabetes. J Immunol. 1989 Sep 1;143(5):1555–1559. [PubMed] [Google Scholar]
- Hutchings P., Rosen H., O'Reilly L., Simpson E., Gordon S., Cooke A. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature. 1990 Dec 13;348(6302):639–642. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Marrack P. The role of H-2 linked genes in helper T-cell function. IV. Importance of T-cell genotype and host environment in I-region and Ir gene expression. J Exp Med. 1978 Dec 1;148(6):1510–1522. doi: 10.1084/jem.148.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Serreze D. V. Antigen presenting cells and the immunogenetics of autoimmune diabetes in NOD mice. Reg Immunol. 1992 Sep-Oct;4(5):263–273. [PubMed] [Google Scholar]
- Livingstone A., Edwards C. T., Shizuru J. A., Fathman C. G. Genetic analysis of diabetes in the nonobese diabetic mouse. I. MHC and T cell receptor beta gene expression. J Immunol. 1991 Jan 15;146(2):529–534. [PubMed] [Google Scholar]
- Locksley R. M., Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991 Mar;12(3):A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Lund T., O'Reilly L., Hutchings P., Kanagawa O., Simpson E., Gravely R., Chandler P., Dyson J., Picard J. K., Edwards A. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-A beta-chain or normal I-E alpha-chain. Nature. 1990 Jun 21;345(6277):727–729. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Uno M., Uehira M., Kikutani H., Kishimoto T., Kimoto M., Nishimoto H., Miyazaki J., Yamamura K. Direct evidence for the contribution of the unique I-ANOD to the development of insulitis in non-obese diabetic mice. Nature. 1990 Jun 21;345(6277):722–724. doi: 10.1038/345722a0. [DOI] [PubMed] [Google Scholar]
- Nishi M., Chan S. J., Nagamatsu S., Bell G. I., Steiner D. F. Conservation of the sequence of islet amyloid polypeptide in five mammals is consistent with its putative role as an islet hormone. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5738–5742. doi: 10.1073/pnas.86.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto H., Kikutani H., Yamamura K., Kishimoto T. Prevention of autoimmune insulitis by expression of I-E molecules in NOD mice. 1987 Jul 30-Aug 5Nature. 328(6129):432–434. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Prochazka M., Leiter E. H., Serreze D. V., Coleman D. L. Three recessive loci required for insulin-dependent diabetes in nonobese diabetic mice. Science. 1987 Jul 17;237(4812):286–289. doi: 10.1126/science.2885918. [DOI] [PubMed] [Google Scholar]
- Prochazka M., Serreze D. V., Worthen S. M., Leiter E. H. Genetic control of diabetogenesis in NOD/Lt mice. Development and analysis of congenic stocks. Diabetes. 1989 Nov;38(11):1446–1455. doi: 10.2337/diab.38.11.1446. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Fowlkes B. J. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science. 1990 Jun 15;248(4961):1342–1348. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- Reich E. P., Sherwin R. S., Kanagawa O., Janeway C. A., Jr An explanation for the protective effect of the MHC class II I-E molecule in murine diabetes. Nature. 1989 Sep 28;341(6240):326–328. doi: 10.1038/341326a0. [DOI] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H. Development of diabetogenic T cells from NOD/Lt marrow is blocked when an allo-H-2 haplotype is expressed on cells of hemopoietic origin, but not on thymic epithelium. J Immunol. 1991 Aug 15;147(4):1222–1229. [PubMed] [Google Scholar]
- Sher A., Gazzinelli R. T., Oswald I. P., Clerici M., Kullberg M., Pearce E. J., Berzofsky J. A., Mosmann T. R., James S. L., Morse H. C., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992 Jun;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Shizuru J. A., Taylor-Edwards C., Banks B. A., Gregory A. K., Fathman C. G. Immunotherapy of the nonobese diabetic mouse: treatment with an antibody to T-helper lymphocytes. Science. 1988 Apr 29;240(4852):659–662. doi: 10.1126/science.2966437. [DOI] [PubMed] [Google Scholar]
- Slattery R. M., Kjer-Nielsen L., Allison J., Charlton B., Mandel T. E., Miller J. F. Prevention of diabetes in non-obese diabetic I-Ak transgenic mice. Nature. 1990 Jun 21;345(6277):724–726. doi: 10.1038/345724a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Swain S. L. Helper T cells: polarized patterns of cytokine secretion. Curr Biol. 1993 Feb;3(2):115–117. doi: 10.1016/0960-9822(93)90169-o. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Todd J. A. Genetic control of autoimmunity in type 1 diabetes. Immunol Today. 1990 Apr;11(4):122–129. doi: 10.1016/0167-5699(90)90049-f. [DOI] [PubMed] [Google Scholar]
- Uehira M., Uno M., Kürner T., Kikutani H., Mori K., Inomoto T., Uede T., Miyazaki J., Nishimoto H., Kishimoto T. Development of autoimmune insulitis is prevented in E alpha d but not in A beta k NOD transgenic mice. Int Immunol. 1989;1(2):209–213. doi: 10.1093/intimm/1.2.209. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Appel M. C., Dotta F., Pressey A., Miller B. J., DeLarato N. H., Fischer P. A., Boltz R. C., Jr, Peterson L. B. Autoimmune syndromes in major histocompatibility complex (MHC) congenic strains of nonobese diabetic (NOD) mice. The NOD MHC is dominant for insulitis and cyclophosphamide-induced diabetes. J Exp Med. 1992 Jul 1;176(1):67–77. doi: 10.1084/jem.176.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Coker L. Z., McNally S. E., Scott S., Mullen Y., Appel M. C. Genetic control of diabetes and insulitis in the nonobese diabetic (NOD) mouse. J Exp Med. 1987 Jun 1;165(6):1639–1654. doi: 10.1084/jem.165.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Fischer P. A., Pressey A., Peterson L. B. Genetic control of diabetes and insulitis in the nonobese diabetic mouse. Pedigree analysis of a diabetic H-2nod/b heterozygote. J Immunol. 1989 Feb 1;142(3):781–784. [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Mullen Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes. 1986 Aug;35(8):855–860. doi: 10.2337/diab.35.8.855. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Kisielow P. Self-nonself discrimination by T cells. Science. 1990 Jun 15;248(4961):1369–1373. doi: 10.1126/science.1972594. [DOI] [PubMed] [Google Scholar]