Abstract
The development of direct-acting antiviral (DAA) agents has reinvigorated the treatment of hepatitis C virus infection. The availability of multiple DAA agents and drug combinations has enabled the transition to interferon-free therapy that is applicable to a broad range of patients. However, these DAA combinations are not without drug–drug interactions (DDIs). As every possible DDI permutation cannot be evaluated in a clinical study, guidance is needed for healthcare providers to avoid or minimize drug interaction risk. In this review, we evaluated the DDI potential of the novel three-DAA combination of ombitasvir, paritaprevir, ritonavir, and dasabuvir (the 3D regimen) with more than 200 drugs representing 19 therapeutic drug classes. Outcomes of these DDI studies were compared with the metabolism and elimination routes of prospective concomitant medications to develop mechanism-based and drug-specific guidance on interaction potential. This analysis revealed that the 3D regimen is compatible with many of the drugs that are commonly prescribed to patients with hepatitis C virus infection. Where interaction is possible, risk can be mitigated by paying careful attention to concomitant medications, adjusting drug dosage as needed, and monitoring patient response and/or clinical parameters.
Key Points
| The three direct-acting antiviral combination of ombitasvir, paritaprevir, ritonavir, and dasabuvir (3D regimen) is a combination therapy that was recently approved for the treatment of genotype-1 chronic hepatitis C virus infection. |
| Potential drug–drug interactions with the 3D regimen were identified by applying pharmacokinetic study data to known routes of metabolism and disposition of more than 200 prescription and over-the-counter drugs. |
| The majority of concomitant medications assessed are compatible with 3D therapy. Where interaction is possible, guidance on dose adjustment and/or clinical monitoring are provided. |
Introduction
The development of direct-acting antiviral agents (DAAs) has revolutionized the treatment of chronic hepatitis C virus (HCV) infection. In head-to-head comparisons, combination therapy with DAAs has proven to be more effective and better tolerated than interferon-based therapies in both treatment-naïve and treatment-experienced patients [1–4]. One such investigational combination includes ombitasvir, paritaprevir (identified as a lead compound by AbbVie, Inc., North Chicago, IL, USA, and Enanta Pharmaceuticals, Inc., Watertown, MA, USA), ritonavir, and dasabuvir, together known as the 3D regimen. Ombitasvir, paritaprevir, and dasabuvir combine unique antiviral mechanisms of action (nonstructural protein 5A inhibition, nonstructural protein 3/4A protease inhibition, and non-nucleoside nonstructural protein 5B polymerase inhibition, respectively). This potent three-class combination approach has achieved high rates of sustained virologic response in a broad range of patients, including those with cirrhosis or those who have undergone liver transplant [5, 6]. The antiviral activity of paritaprevir is boosted by its co-formulation with a low dose of ritonavir (i.e., 100 mg), facilitating the use of a lower dose of paritaprevir and once-daily dosing. Ritonavir is a strong inhibitor of cytochrome P450 (CYP) 3A4, a major enzyme involved in the metabolism of paritaprevir [7].
In pivotal clinical trials, the 3D regimen with ribavirin achieved sustained virologic response rates at 12 weeks (SVR12) of 94–100 % in treatment-naïve and treatment-experienced non-cirrhotic patients with genotype-1 HCV and 93–100 % after 24 weeks of treatment in patients with genotype-1 HCV and cirrhosis, including prior null responders [5, 8–11]. Additionally, in liver transplant recipients with recurrent HCV genotype-1 infection and no cirrhosis (Metavir ≤F2) at least 12 months after transplantation, 33 of 34 patients (97 %; 95 % confidence interval [CI] 85–100 %) who were treated with the 3D regimen plus ribavirin for 24 weeks achieved SVR12 [6]. No graft rejection events occurred during the study. The 3D regimen was well tolerated when administered with or without ribavirin; treatment discontinuation rates were low and adverse events (AEs) were generally mild [5, 6, 8–12]. In subjects receiving 3D with ribavirin, the most commonly reported AEs (occurring in >10 % of subjects) were fatigue, nausea, pruritus, other skin reactions, insomnia, and asthenia. In subjects receiving 3D regimen without ribavirin, the most commonly reported AEs (occurring in ≥5 % of subjects) were nausea, pruritus, and insomnia. The safety profile of the 3D regimen was similar in patients with cirrhosis [5] or who were post-transplant [6] to that of the overall population and no significant associations were found between ombitasvir, dasabuvir, and ritonavir exposures and AEs or laboratory abnormalities [13]. Exposure-safety analyses showed that increases in paritaprevir exposure of up to 2-fold are not predicted to meaningfully increase AEs or laboratory abnormalities of Grade 3 or greater [13].
Comparisons of 3D pharmacokinetics in subjects with hepatic impairment vs normal hepatic function demonstrated that DAA exposures were not significantly affected (<35 % change) in subjects with mild hepatic impairment (Child-Pugh A) and, hence, no dosage adjustment of 3D therapy is required for such patients [12]. In patients with moderate hepatic impairment (Child-Pugh B), ombitasvir, ritonavir, and dasabuvir area under the plasma concentration-time curves (AUCs) decreased by 30 % or less, whereas paritaprevir AUC increased by 62 % [12]. Because of these exposure changes, 3D therapy is not recommended in patients with moderate hepatic impairment. A more substantial effect on DAA exposures (54 % decrease in ombitasvir and 325 and 945 % increase in dasabuvir and paritaprevir, respectively) was observed in patients with severe hepatic impairment (Child-Pugh C) [12]. Therefore, 3D therapy is contraindicated in patients with severe hepatic impairment.
Pharmacokinetic study data indicate that the presence of mild, moderate, or severe renal impairment does not increase DAA exposures to a clinically significant degree (<50 % change); therefore, no dose adjustment is indicated for 3D therapy when given to patients with renal impairment [14].
Food has been shown to significantly increase exposures for 3D components, by as much as 82, 211, 49, and 30 % for ombitasvir, paritaprevir, ritonavir, and dasabuvir, respectively [12]. Fat and/or calorie content do not appear to influence the magnitude of interaction. In all phase I–III studies, 3D regimen components were administered with food. Given the magnitude of the effect of food, 3D therapy should be administered with food to maximize absorption.
As new therapeutic options for treating HCV become available, it will be important to assess their abilities to interact with established medications, particularly those drugs or drug classes that are commonplace among patients with HCV infection. Adverse drug reactions resulting from concomitantly administered medications are an ongoing concern for patients undergoing HCV treatment because of the heavy burden of polypharmacy that coincides with a high prevalence of comorbidities in this patient population [15–17]. The interplay of therapies influences not only the safety profile (via the potential for increasing drug exposures) but also may reduce therapeutic efficacy in cases where metabolic enzyme induction decreases circulating drug concentrations. Mechanistic data have shown a propensity for all DAAs to act as substrates, inhibitors and/or inducers of metabolic enzymes, and transporters, which is the foundation for the observed drug–drug interactions (DDIs) [18, 19].
In clinical practice, non-HCV medications that have the potential for interactions with HCV treatments are frequently prescribed to patients with chronic HCV infection [20, 21]. In a recent study, more than half of the medications that were commonly prescribed to a large representative sample of patients with chronic HCV infection (N = 53,461) were found to have interaction potential with telapravir and boceprevir.[21] However, later-generation DAAs represent an improvement in DDI propensity compared with the first-generation protease inhibitors.
Pharmacokinetic studies are the ideal method by which to evaluate the DDI potential of the 3D regimen; however, such an undertaking is not feasible given the number of prescription and over-the-counter medications available and their various permutations. In recognition of these limitations, regulatory agencies have created recommendations regarding which drugs should be studied to interrogate potential enzymatic and transporter pathways that may lead to significant DDIs [22, 23]. With results from key DDI studies, data can then be extrapolated to other medications based on what is known about drug metabolism.
To provide clear guidance regarding the clinical use of the 3D regimen and facilitate prescription decisions, in this report, we have gathered information on the metabolism and disposition of more than 200 commonly used drugs. These data, together with DDI studies involving the 3D regimen, were used to develop recommendations for DDI evaluation and management. In addition, these recommendations were successfully implemented for co-medication management in the pivotal phase III clinical trials of the 3D regimen and are also being implemented in the ongoing phase IIIb studies.
Metabolic Characteristics of 3D Regimen Components
In vitro preclinical and human pharmacokinetic studies have demonstrated that several enzymes and transporters are involved in the disposition of 3D regimen components. All DAAs show minimal renal elimination. The presence of ombitasvir, paritaprevir, and dasabuvir in feces suggests possible involvement of biliary excretion for these drugs. Within the family of CYP isoenzymes, paritaprevir and ritonavir are chiefly metabolized by CYP3A, whereas dasabuvir metabolism occurs primarily through CYP2C8 with minor contribution by CYP3A [12, 24, 25]. Ombitasvir is mainly metabolized by amide hydrolysis. The DAAs are not expected to inhibit/induce CYP enzymes, although ritonavir is a known strong inhibitor of CYP3A [12], and the 3D regimen has been shown to modestly induce CYP1A2 and CYP2C19 in vivo [25, 26], which is attributed to the effects of ritonavir.
In general, marketed and in-pipeline DAAs attain sufficient hepatocellular concentrations to inhibit HCV replication because they are substrates of hepatic transporters. This property also explains why these HCV therapeutic agents may interact with multiple drugs.
Components of the 3D regimen act as both substrates and inhibitors of transport proteins. The results of in vitro studies indicate involvement of one or more 3D agents as substrates and/or inhibitors of p-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and organic anion transporting polypeptides 1B1 and 1B3 (OATP1B1/B3) (Table 1). However, none of the 3D components are substrates or inhibitors of renal transport proteins, including organic anion transporters (OAT1, OAT3), organic cation transporters (OCT1, OCT2), or multi-drug and toxin extrusion proteins (MATE1 and MATE2K), at clinically relevant concentrations [12, 24, 25].
Table 1.
3D regimen components as substrates or inhibitors of transport proteinsa
| Transporter | Substrate | Inhibitor |
|---|---|---|
| Ombitasvir | P-gp | |
| Paritaprevir | P-gp, BCRP, OATP1B1/B3 | P-gp, BCRP, OATP1B1/B3 |
| Dasabuvir | P-gp, BCRP | P-gp, BCRP |
| Ritonavir | P-gp | P-gp, BCRP |
BCRP breast cancer resistance protein, OATP1B1 organic anion transporting polypeptide 1B1, OATP1B3 organic anion transporting polypeptide 1B3, P-gp p-glycoprotein, 3D direct-acting antiviral agent combination of ombitasvir, paritaprevir, ritonavir, and dasabuvir
aFindings are based on in vitro data [24]
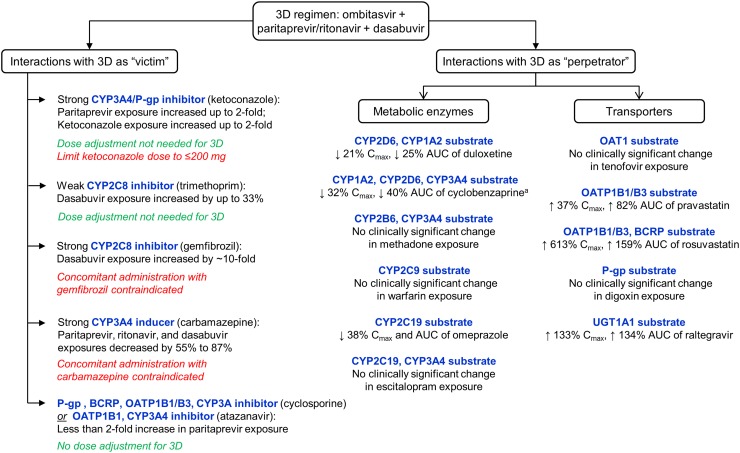
Ombitasvir, paritaprevir, and dasabuvir inhibit uridine diphosphate glucuronosyltransferase (UGT) 1A1 [12, 24, 25]; however, the 3D regimen is not expected to inhibit UGT1A4, UGT1A6, UGT1A9, and UGT2B7 at clinically relevant concentrations. Historically, in vivo data have shown that ritonavir causes UGT1A1 induction [27, 28]; however, based on drug interaction data with raltegravir (a UGT1A1 substrate), the net effect of the 3D regimen is UGT1A1 inhibition (Fig. 1) [29].
Fig. 1.
Formal interaction studies performed with the direct-acting antiviral agent combination of ombitasvir, paritaprevir, ritonavir, and dasabuvir (3D regimen) and their implications on dose recommendations for other drugs. A change in exposure (maximum concentration [C max] or area under the plasma concentration-time curve [AUC]) of ≤20 % is considered to be not clinically significant. a No significant change was observed in exposures for norcyclobenzaprine, the major metabolite of cyclobenzaprine. BCRP breast cancer resistance protein, CYP cytochrome P450, OAT organic anion transporter, OATP organic anion transporting polypeptide, P-gp p-glycoprotein, UGT uridine 5′-diphospho-glucuronosyltransferase. Data sources: [25, 26, 29, 69]
DDI Studies with the 3D Regimen
Eighteen formal interaction studies in healthy volunteers have been performed to evaluate the DDI potential of the 3D regimen with selected drugs, also known as probes, based on their disposition via a metabolic enzyme and/or transporter pathway [25]. In these scenarios, the 3D regimen functioned either as (i) the ‘victim’, wherein the pharmacokinetics of one or more 3D agents were influenced by the presence of another drug, or (ii) the ‘perpetrator’, in which the 3D regimen affected the pharmacokinetics of the probe. Selection of probes was derived from general industry guidance issued by the US Food and Drug Administration and European Medicines Agency [22, 23]. Results from these mechanistic studies form the foundation for recommendations in this paper on dose adjustments for a wide range of drugs in various classes based on similar pharmacokinetic pathways. A summary of the results of these evaluations is presented in Fig. 1.
DDI Potential of Concomitantly Administered Medications by Therapeutic Class
Using the results of the mechanistic studies described above, the potential for interactions of the 3D regimen with other commonly administered medications were determined based on the agents’ pharmacokinetic profiles. Route of elimination information and other relevant influences on metabolism were identified from the published literature and/or prescribing information for more than 200 drugs. The following section describes the results of this analysis parsed by drug class. The ensuing dosing recommendations are summarized in Table 2.
Table 2.
Drug–drug interaction (DDI) potential of commonly administered medications
| Class (use in pivotal trials)a | No expected DDI | Require possible dose adjustment | Contra-indicated |
|---|---|---|---|
| Analgesics/anti-inflammatory agents (30 and 11 % of patients received non-opioid and opioid analgesics, respectively) |
Acetaminophenb
Aspirin Buprenorphineb Celecoxib Codeine Diclofenac Etodolac Fenoprofen Flurbiprofen Ibuprofen Indomethacin Ketoprofen Ketorolac Methadoneb Morphine Naloxoneb Naproxen Piroxicam Sulindac Suprofen |
Fentanyl (↓) Hydrocodoneb (↓) Hydromorphone (↓) Meloxicam (↓) Meperidine (pethidine) (↓) Oxycodone (↓) Tramadol (↓) |
None |
| Diuretics (26 % of patients)c | Amiloride Chlorthalidone Hydrochloro-thiazide Torsemide Triamterene |
Furosemide (↓ ≤50 %)b,d
Xipamide (↓)c |
None |
| Antihypertensive agents (26 % of patients)c | Atenolol Azilsartan Betaxolol Bisoprolol Captopril Carteolol Fosinopril Lisinopril Metoprolol Nadolol Nebivolol Perindopril Propranolol Quinopril Ramipril Sotalol Spironolactone Timolol Zofenopril |
Amlodipine (↓50 %)b
Candesartan (↓) Enalapril (↓) Eprosartan (↓) Irbesartan (↓) Losartan (↓) Olmesartan (↓) Telmisartan (↓) Valsartan (↓) Start lowest dose: Diltiazem Nicardipine Nifedipine Nitrendipine Verapamil Avoid use: Nisoldipine Felodipine |
None |
| Antidiabetic agents (5 % of patients) | Acarbose Glimepiride Glipizide Metforminb Pioglitazone Rosiglitazone Sitagliptine Tolbutamide Vildagliptin |
Repaglinide (↓) Glibenclamide (glyburide) (↓) Saxagliptin (limit dose to 2.5 mg once daily) |
None |
| α1-Adrenergic blockers (26 % of patients)c | None | Use with caution: Doxazosin (↓) Avoid use: Tamsulosin |
Alfuzosin |
| Hypnotic/sedative agentsd (2 % of patients) | Oxazepam Temazepam Zolpidemb |
Alprazolam (↓)b
Clonazepam (↓) Diazepamb (↑)d Estazolam (↓) Eszopiclone (↓) Flunitrazepam (↓) Lorazepam (↓) Midazolam (parenteral)d Prazepam (↓) Quazepam (↓) Zaleplon (↓) Zopiclone (↓) |
Midazolam (oral) Triazolam |
| Antibiotics and antifungals (anti-infectives) (7 % of patients) | Amoxicillin Azithromycin Cephalexin Ciprofloxacin Clarithromycine Sulfamethoxazole/trimethoprimb Caspofungin Micafungin Anidulafungin |
Ketoconazole (limit dose to 200 mg)b
Itraconazole (limit dose to 200 mg) Use with caution: Posaconazole (↓) Voriconazolef |
Fusidic acid Rifampin (rifampicin) |
| Phosphodiesterase type 5 inhibitors (<1 % of patients) | None | Sildenafil (↓ for erectile dysfunction)d,g
Tadalafil (↓)d,h Vardenafil (↓)d,i Avoid use: Avanafil |
Sildenafil (for PAH) |
| Antidepressants (22 % of patients) | Bupropiond
Citalopram Duloxetineb Escitalopramb Fluoxetine Milnacipran Paroxetine |
Fluvoxamined
Mirtazapine (↓) Reboxetine (↓) Sertraline (↓) Trazodone (↓) Venlafaxine (↓) |
St. John’s Wort |
| Antacids/proton pump inhibitors (16 % of patients) | Almagate Aluminum hydroxide Calcium carbonate Cimetidine Famotidine Magaldrate Magnesium hydroxide Ranitidine |
Dexlansoprazole (↑)d
Esomeprazole (↑)d Lansoprazole (↑)d Omeprazole (↑)b,d Pantoprazole (↑)d Rabeprazole (↑)d |
None |
| Antiplatelet agents/anticoagulants (26 % of patients)c | Acenocoumarold
Aspirin Enoxaparin Fluindione Fondaparinux Phenprocoumand Prasugreld Warfarina,b |
Apixaban (restrict dose to 2.5 mg twice daily) Dabigatran (↓)d Avoid use: Clopidogrel Rivaroxaban |
None |
| Antiarrhythmic agents (26 % of patients)c | Digoxinb,d
Flecainided Mexiletined |
Use with caution: Amiodarone (↓)d Disopyramide (↓)d Dronedarone (↓)d Propafenone (↓)d Quinidine (↓)d |
None |
| Lipid-modifying agents (2 % of patients) | Choline fenofibrated
Colesevelemj Colestipolj Fenofibrated Mipomersen Niacin Omega-3 fatty acids |
Pravastatin (↓50 %)b
Rosuvastatin (limit maximum daily dose to 5–10 mg)b Start lowest dose: Cerivastatin Ezetimibe Fluvastatin Pitavastatin Avoid use: Atorvastatin |
Gemfibrozilb
Lovastatin Simvastatin |
| β-Adrenergic agonists (14 % of patients received β-adrenergic agonists/anti-allergics/respiratory agents) | Albuterol (salbutamol) | Use with caution: Formoterol (↓)d Not recommended: Salmeterol |
None |
| Antiepileptic agents (3 % of patients) | Felbamate Gabapentin Levetiracetam Sodium valproated Topiramate Vigabatrin |
Lamotrigine (↑)d
Use with caution: Tiagabine (↓) Zonisamide (↓) |
Carbama-zepine Phenytoin Phenobarbital |
| Steroidsc (8 % of patients) | Beclomethasone Ciclesonide |
Use with caution: Budesonide (↓)f Dexamethasone (↓) Fluticasone (↓)f Methyl-prednisolone (↓) Mometasone (↓) Prednisone (↓) Triamcinolone (↓) |
None |
| Contraceptives (<1 % of patients received hormonal contraceptives) | Progestin-only OCPsb
Non-hormonal contraceptives |
None | Ethinyl estradiol-containing OCPsb |
| Thyroid replacement therapy (7 % of patients) | None | Levothyroxined | None |
OCPs oral contraceptive pills, PAH pulmonary arterial hypertension, 3D direct-acting antiviral agent combination of ombitasvir, paritaprevir, ritonavir, and dasabuvir
↑ dose increase recommended, ↓ dose decrease recommended
aPercentage of patients who received the class of drugs concomitantly for at least 6 weeks during treatment with the 3D regimen in pivotal clinical trials [227]
bFor these drugs, formal DDI studies have been performed with the 3D regimen [25, 26]
cThe percentage represents patients who received antihypertensive agents, antiarrhythmic agents, antiplatelet agents/anticoagulants, diuretics or α1-adrenergic blockers for at least 6 weeks during treatment with 3D regimen in pivotal clinical trials [227]
dSuggest monitoring drug levels or clinical response or side effects during 3D treatment to determine dose adjustment requirements
eNo dose adjustment needed for individuals with normal renal function
fBudesonide, fluticasone, and voriconazole should only be used with the 3D regimen if the potential benefits outweigh the risks of treatment
gLimit dose to 25 mg every 48 h for the treatment of erectile dysfunction
hLimit dose to 10 mg every 72 h for erectile dysfunction; initiate 20 mg once daily for PAH after at least 1 week of 3D treatment. The dose may be increased to 40 mg once daily if tolerated and clinically indicated
iLimit dose to 2.5 mg every 72 h for erectile dysfunction; initiate 20 mg once daily for PAH after at least 1 week of 3D treatment. The dose may be increased to 40 mg once daily if tolerated and clinically indicated
jAdminister 4 h after 3D administration
With the vast size of the pharmaceutical armamentarium, a review of all of the possible drug interactions within the confines of this report is not possible. Therefore, in an effort to simplify the identification and management of drugs that may influence or be influenced by the 3D regimen, we have created the algorithm for screening and dose adjustment shown in Fig. 2.
Fig. 2.
Proposed algorithm for screening, dose adjustment, and monitoring of potential drug interactions with the direct-acting antiviral agent combination of ombitasvir, paritaprevir, ritonavir, and dasabuvir (3D regimen). CYP cytochrome P450, OAT organic anion transporter, OATP organic anion transporting polypeptide, P-gp p-glycoprotein, UGT uridine 5′-diphospho-glucuronosyltransferase
Analgesics/Anti-inflammatory Agents
Within the analgesic/anti-inflammatory class, distinctions can generally be made between the interactions of nonsteroidal anti-inflammatory drugs (NSAIDs)/COX-2-selective inhibitors and opioids. Metabolism of many of the NSAIDs/COX-2 inhibitors predominantly involves CYP2C9 and/or members of the UGT family [30–42]. The lack of interaction between the model CYP2C9 substrate, warfarin, and the 3D regimen indicates that no dose adjustment would be required for drugs predominantly metabolized via CYP2C9 (Fig. 1) [25]. Moreover, the only interaction potential for a UGT species was that of UGT1A1, as demonstrated by the DDI study with raltegravir as the model substrate [24]. The involvement of multiple UGTs in NSAID glucuronidation likely minimizes any effect that the 3D regimen may have on UGT1A1-based catalysis by providing alternate routes of metabolism [37]. Notable exceptions to the general metabolism/elimination pattern for NSAIDs include acetaminophen, for which a 3D DDI study demonstrated minimal pharmacokinetic interaction (<20 % change in exposure) during concomitant therapy [26]; aspirin, which undergoes deacetylation and subsequent glucuronidation [43]; ketorolac, which has a large renal excretion component [44]; and meloxicam, for which CYP3A4 plays a role [45]. The 3D regimen-mediated inhibition of CYP3A4 may increase meloxicam exposure; therefore, a dose reduction for meloxicam should be considered when it is co-administered with the 3D regimen. Table 2 provides a list of analgesics and anti-inflammatory agents that can be used with the 3D regimen without any dose adjustment.
Contribution of CYP3A4 to the metabolism of most opioids (e.g., fentanyl, hydrocodone, hydromorphone, meperidine/pethidine, oxycodone, and tramadol) [46–54] indicates the potential for an interaction with the 3D regimen that would lead to increased drug exposure of the opioids. Hydrocodone AUC increased by 90 % when given with the 3D regimen in a DDI study [26]. A 50 % reduction in hydrocodone dose is, therefore, recommended when administered with the 3D regimen. The other listed opioids should be used with caution and dose reduction is recommended when given concomitantly with the 3D regimen. Buprenorphine, another opioid, is a CYP3A substrate but no dose adjustment is recommended based on a modest interaction with the 3D regimen and the lack of effect on pharmacodynamic parameters [25]. In a DDI study with the 3D regimen, no interaction was observed with naloxone [25]; consequently, no dose adjustment is needed during co-administration. Codeine, morphine, and methadone are unlikely to require dose adjustment when given with 3D therapy because CYP3A4 is not the only or the primary metabolic enzyme for these drugs [55–57]. Indeed, a DDI study with methadone and the 3D regimen revealed no increase in methadone exposure with concomitant administration (Fig. 1) [25].
Antidepressants
Drug–drug interactions with the 3D regimen are not expected for most antidepressants. The 3D regimen showed no significant interaction with the antidepressants duloxetine (a CYP2D6/CYP1A2 substrate) or escitalopram (a CYP2C19/CYP3A4 substrate) in DDI studies (Fig. 1) [25]. Although the 3D regimen would be anticipated to decrease escitalopram exposure through CYP2C19 induction, the effects are likely offset by CYP3A4 inhibition. These results can be extrapolated to citalopram, which is a racemic mixture of escitalopram and (R)-citalopram. In addition, the 100-mg dose of ritonavir that is part of the 3D regimen is not expected to interact with substrates of CYP2D6 based on data with ritonavir 200 mg and the HIV protease inhibitor lopinavir [58]. Thus, fluoxetine and paroxetine are unlikely to exhibit pharmacokinetic interactions with the 3D regimen, as they are also substrates of CYP2D6 [59, 60]. No DDIs are expected for milnacipran because it is primarily excreted renally and shows minimal CYP450-related metabolism [61]. Standard dosing for these antidepressants are warranted when given with the 3D regimen.
Historically, in vivo studies suggest an interaction between bupropion (CYP2B6 substrate) and ritonavir, with long-term administration of ritonavir 200 mg daily reducing bupropion exposure by as much as 22 % [62]. However, no clinically significant interaction between the 3D regimen and bupropion, a CYP2B6 substrate, is expected based on studies of methadone, another CYP2B6 substrate, and the low dose (100 mg) of ritonavir that is co-formulated with the 3D regimen. Hence, initial dose modification is not needed for bupropion when administered with the 3D regimen. Clinical monitoring is recommended, with dose adjustment based on clinical symptoms.
The metabolism of fluvoxamine has not been fully elucidated, but is believed to be predominantly mediated by CYP1A2, with minor involvement of CYP3A4 and CYP2C19 [63]. Fluvoxamine also functions as a moderate inhibitor of CYP3A4 and CYP2C19. As a potential substrate or inhibitor of CYPs, alterations in pharmacokinetics are possible with co-administration of fluvoxamine and the 3D regimen. Caution should be exercised in prescribing fluvoxamine for patients receiving 3D therapy, and dose adjustment of fluvoxamine may be considered, depending on the clinical response.
Dose reduction is recommended for mirtazapine [64], reboxetine [65], sertraline [66], trazodone [67], and venlafaxine [68] when administered with the 3D regimen because of the potential for an increase in drug exposure resulting from the inhibitory effects of the 3D regimen on CYP3A4.
Because of the potential for decreased ombitasvir, paritaprevir, ritonavir, and dasabuvir exposures and commensurate loss of therapeutic efficacy, the herbal antidepressant St. John’s Wort is contraindicated for use with the 3D regimen [12].
Antacids/Proton Pump Inhibitors
The influence of acid reduction on 3D regimen pharmacokinetics was evaluated in a DDI study with omeprazole. The maximum concentration (Cmax) and AUC values for ombitasvir, paritaprevir, ritonavir, and dasabuvir were only minimally effected (<20 % change in exposure); however, a 38 % reduction in omeprazole exposure was observed (Fig. 1) [25, 69]. The results of this study indicate that (i) acid reduction, per se, does not significantly alter the pharmacokinetics of the 3D regimen, and (ii) a dose increase should be considered for substrates of CYP2C19, if a decrease in clinical response is noted. Other proton pump inhibitors (listed in Table 2), follow a similar metabolic pathway [70–74] and dose increases are recommended if clinically indicated. However, dose adjustment is not expected for histamine H2-receptor antagonists (cimetidine, famotidine, ranitidine), which primarily undergo renal elimination [75–77]. In addition, in vitro chelation experiments with the 3D regimen indicate that calcium, magnesium, and aluminum-based antacids will not interact with the 3D regimen.
Diuretics
Dose adjustment is not warranted for the majority of diuretics when given with the 3D regimen. Amiloride, chlorthalidone, and hydrochlorothiazide undergo minimal hepatic metabolism and, hence, are not expected to demonstrate a metabolic interaction with the 3D regimen [78–80]. Triamterene primarily undergoes sulfonation and there is no evidence of overlapping metabolic or transporter pathways with that of the 3D regimen [81]. The metabolism of torsemide predominantly involves CYP2C9, with a minor contribution from CYP2C8 [82, 83]. As demonstrated by the warfarin/3D DDI study, the CYP2C9 substrate does not show significant interaction with the 3D regimen (Fig. 1) [25].
Dose adjustment may be necessary for furosemide and xipamide. In a DDI study, concomitant administration of furosemide and the 3D regimen increased the Cmax of furosemide by 42 %, although minimal change (8 %) in AUC was observed [25]. The exact mechanistic reason for the greater increase in furosemide Cmax vs AUC is unknown but UGTs, including UGT1A1, may be a contributing factor. Clinical monitoring of patients receiving furosemide is recommended, with a possible dose reduction by as much as 50 %. Because the involvement of UGT1A1 cannot be ruled out, clinical monitoring is also suggested for patients who receive concomitant xipamide, with dose adjustment if clinically indicated.
Antiplatelet Agents/Anticoagulants
Antiplatelet agents and anticoagulants are highly diverse in disposition, although none of the pathways are anticipated to lead to clinically relevant DDIs with the 3D regimen. Mechanisms including deacetylation and glucuronidation by various UGT species (aspirin) [43], desulfation and/or depolymerization (enoxaparin) [84], renal excretion (fondaparinux) [85], and CYP2C9/CYP1A2 metabolism (acenocoumerol, fluindione, warfarin) [86–88] are not anticipated to be influenced by or to affect the pharmacokinetics of the 3D regimen. Indeed, no significant pharmacokinetic interaction was observed in a DDI study wherein warfarin was given concomitantly with the 3D regimen (Fig. 1) [25]. Of note, although dose adjustment is not indicated for the vitamin K antagonists warfarin and acenocoumerol when given with the 3D regimen, appropriate international normalized ratio monitoring is advisable according to routine clinical practice.
No dose adjustment is necessary for phenprocoumon, a vitamin K antagonist, although careful international normalized ratio monitoring is recommended when given with the 3D regimen based on its pharmacokinetic profile and drug interaction data with other relevant medications [89]. It is suspected that the impact of CYP3A4, which is involved in the metabolism of phenprocoumon, is mitigated by the role of CYP2C9 and the notable portion of the administered drug that is excreted unchanged [90].
CYP3A4 and/or P-gp inhibition with the 3D regimen has implications for the use of apixaban, rivaroxaban, and dabigatran. Apixaban labeling recommends that the dose be reduced to 2.5 mg twice daily when given in combination with strong inhibitors of CYP3A4 and P-gp, such as ritonavir [91]. Rivaroxaban is contraindicated for use with strong dual inhibitors of CYP3A and P-gp such as ketoconazole and ritonavir [92] and, hence, should not be used with the 3D regimen. As a substrate of P-gp, dabigatran exposure is influenced by P-gp inhibition [93]. Although levels of the P-gp substrate digoxin were not significantly affected by the 3D regimen in a DDI study [25], exposures for dabigatran may increase as a result of intestinal P-gp inhibition by paritaprevir and ritonavir. Dabigatran should be used with caution with 3D therapy and appropriate international normalized ratio monitoring is advisable according to routine clinical practice.
An interaction with the purinergic receptor P2Y, G protein-coupled, 12 (P2Y12) antagonist clopidogrel is possible based on the involvement of CYP2C19 and CYP3A4/5 in its metabolism and bioactivation [94, 95]. While the requirement for dose adjustment of clopidogrel in patients who initiate 3D therapy can be based on clinical judgment, therapeutic drug level monitoring for clopidogrel does not appear to be routine clinical practice. Hence, if clopidogrel cannot be avoided or substituted, it should be used with caution, or consideration can be given to use of an alternative DAA regimen that does not have interaction potential with this medication.
Conversion of prasugrel, a newer generation P2Y12 receptor antagonist, to its active metabolite is primarily dependent on CYP3A4 and CYP2B6, with some contribution by CYP2C9 and CYP2C19 [96]. Treatment with 100 mg ritonavir has been shown to decrease the Cmax and AUC of the active metabolite of prasugrel by 45 and 38 %, respectively [97]. This effect was attributed to inhibition of CYP3A4 by ritonavir. Nevertheless, concomitant administration of ketoconazole, a potent inhibitor of CYP3A4 and CYP3A5, with prasugrel did not impact inhibition of platelet aggregation by prasugrel despite a 34–46 % decrease in the Cmax of the active metabolite of prasugrel [96]. A similar magnitude of interaction to that observed with ritonavir or ketoconazole is expected with the 3D regimen. Dose alteration is, therefore, not needed for prasugrel when initially administered with the 3D regimen; however, monitoring is recommended and dose adjustment should be enacted, if clinically indicated.
Antihypertensive Agents
Beta-blockers can generally be divided into two categories: those that do not undergo extensive metabolism by multiple enzymes in the liver (e.g., atenolol, bisoprolol, nadolol, and sotalol) [98–101] and those with a metabolism that depends primarily on CYP2D6 (betaxolol, carteolol, metoprolol, nebivolol, propranolol, and timolol) [102–107]. An exception to this classification is acebutolol, which undergoes a high degree of first-pass metabolism in the liver [108], but which has not been found to be a substrate of CYP3A4, CYP2D6, CYP2C9, CYP1A2, or CYP2C19 [109]. In all cases, no interaction between members of the beta-blocker class and the 3D regimen is expected.
The metabolic processes and pathways involved in the disposition of angiotensin-converting enzyme inhibitors (e.g., hydrolysis, glucuronidation, dimerization, cyclization, and renal elimination) [110–117] are generally not anticipated to be affected by concomitant administration of the 3D regimen. Thus, no dose adjustment is indicated for captopril, fosinopril, lisinopril, perindopril, quinopril, ramipril, or zofenopril. One exception to this overarching guidance is enalapril, which is an OATP1B1 substrate [118]. The 3D regimen has been shown to increase exposures of OATP1B1/B3 substrates [25] and, hence, a reduction in enalapril dose is advised for patients who are being treated with the 3D regimen.
The importance of CYP3A to the metabolism of calcium channel blockers and the inhibitory effects of certain calcium channel blockers on CYP3A are potential sources of interaction when combined with the 3D regimen [119–126]. With this in mind, a DDI study was conducted between the 3D regimen and the calcium channel blocker, amlodipine. Concomitant administration of the 3D regimen increased amlodipine exposure by up to 157 % [25]. Consequently, a 50 % dose reduction is recommended for amlodipine when given with the 3D regimen. Based on the prescribing information regarding use with CYP3A4 inhibitors and the potential for interaction [120, 122–125], the lowest possible starting dose should be used for diltiazem, nicardipine, nifedipine, nitrendipine, and verapamil when prescribed to patients receiving the 3D regimen. Both felodipine and nisoldipine have demonstrated a substantial interaction with CYP3A inhibitors (e.g., grapefruit juice, ketoconazole), which increases drug exposure by several fold [121, 126]. Therefore, neither felodipine nor nisoldipine should be given in conjunction with the 3D regimen.
Involvement of OATP transporters has been implicated in the disposition of many angiotensin II receptor blocker family members [127–130]. Because of the influence of the 3D regimen on OATP1B1/B3 substrates (Fig. 1) [25], dose reduction is suggested for OATP-associated angiotensin II receptor blockers, including candesartan, eprosartan, irbesartan, losartan, olmesartan, telmisartan, and valsartan. Azilsartan disposition, in contrast, does not appear to involve OATP transporters and no other overlapping metabolic pathways with the 3D regimen have been identified [131]. Dose adjustment is, therefore, not necessary when azilsartan is administered with the 3D regimen.
No overlapping metabolic or transporter pathways have been identified between the aldosterone antagonist spironolactone and the 3D regimen [132, 133]. Hence, no dose adjustment would be required.
Antiarrhythmic Agents
The pharmacokinetics of digoxin were only minimally affected when given as a single dose (0.5 mg) to patients who had achieved steady state on the 3D regimen, suggesting that no dose adjustment is necessary for this combination therapy [25]. Based on the known metabolic pathways, drug–drug interaction is also not expected for other antiarrhythmic drugs whose metabolism involves CYP2D6 (flecainide) [134] or CYP2D6/CYP1A2 (mexiletine) [135]. However, these antiarrhythmic agents have a narrow therapeutic index; therefore, caution and therapeutic drug monitoring is suggested as part of routine clinical practice when these drugs are administered with the 3D regimen.
The involvement of CYP3A4 in the metabolism of amiodarone [136], disopyramide [137], dronedarone [138, 139], propafenone [140], and quinidine [141] signals a possible increase in exposure when given with the 3D regimen owing to inhibitory effects on CYP3A4. If these agents cannot be avoided or substituted, they should be used cautiously, with careful monitoring and consideration for dose reduction, or use of an alternative DAA regimen that does not have interaction potential with these medications.
Antidiabetic Agents
Drug interactions are unlikely with the 3D regimen and most antidiabetic agents. Processes involved in the disposition of most approved antidiabetic agents, including urinary excretion (acarbose, metformin, sitagliptin) [142–144], hydrolysis (vildagliptin) [145], and metabolism via CYP1A1 (pioglitazone) [146], CYP2C8 (pioglitazone, rosiglitazone, sitagliptin) [144, 146, 147], and/or CYP2C9 (glimepiride, glipizide, rosiglitazone, tolbutamide) [147–152], are unlikely to affect the pharmacokinetics of the 3D regimen, nor is the 3D regimen likely to influence exposures to these drugs. In a DDI study of the 3D regimen with metformin, changes in metformin drug exposure during concomitant administration were not clinically significant (≤23 % decrease) [26].
The magnitude of CYP3A4 involvement in the metabolism of other antidiabetic agents influences the degree of interaction potential with the 3D regimen. Labeled guidance suggests a dose limitation to 2.5 mg once daily for saxagliptin, a substrate of CYP3A4, when given in conjunction with a strong CYP3A4 inhibitor, such as the 3D regimen [153]. Dose reduction is recommended for repaglinide, a substrate of CYP2C8, CYP3A4, and OATP1B1 [154], when used with the 3D regimen. Mechanistically, interaction is possible through both inhibition of CYP3A4 and OATP1B1 by the 3D regimen [24]; DDI studies of the 3D regimen with OATP1B1/B3 substrates [25] and repaglinide with OATP1B1 inhibitors support a potential interaction [155, 156]. Inhibition of OATP by the 3D regimen may also increase exposure to glibenclamide (glyburide); hence, dose reduction is recommended for glibenclamide when administered with the 3D regimen [25, 157]. In contrast, because CYP3A4 only plays a minor role in the metabolism of sitagliptin and the majority of the drug (79 %) is excreted unchanged in urine [144], the effect of 3D-mediated CYP3A4 inhibition on sitagliptin pharmacokinetics is expected to be minimal in patients with normal renal function. However, in patients with severe renal impairment or end-stage renal disease, sitagliptin exposure may increase when co-administered with the 3D regimen.
Lipid-Modifying Therapies
As described above, studies of the 3D regimen with pravastatin or rosuvastatin revealed increases in exposure for both of these drugs [25]. To accommodate these changes, it is recommended that pravastatin dose be reduced by half and that the rosuvastatin dose is limited to 5 or 10 mg when prescribed to patients receiving the 3D regimen. As substrates of OATP1B1 and/or OATP1B3 [127], the pharmacokinetics of atorvastatin, cerivastatin, ezetimibe, fluvastatin, and pitavastatin may also be influenced by the 3D regimen. Although CYP3A4 does play a role in the metabolism of the aforementioned statins [127, 158], atorvastatin is the only one for which it is the dominant CYP species [159]. Atorvastatin is, hence, not recommended with the 3D regimen. If a statin is required, clinicians should use the lowest available dose of cerivastatin, ezetimibe, fluvastatin, and pitavastatin, or switch to a reduced recommended doses of pravastatin/rosuvastatin when given concomitantly with the 3D regimen.
No overlap in metabolic or transporter pathways with the 3D regimen was identified for niacin, omega-3 fatty acids, and mipomersen; these agents can be administered with the 3D regimen without dose adjustment [160–162]. Fenofibric acid, the pharmacologically active moiety of fenofibrate and choline fenofibrate, undergoes glucuronidation by several UGT species, including UGT1A1, UGT1A3, UGT1A9, and UGT2B7 [163]. The contribution of UGT1A1 to this process is comparatively modest. Therefore, dose adjustment is not indicated, although monitoring is suggested for fenofibric acid.
As anion exchange resins, the bile acid sequestrants colestipol and colesevelam may bind to molecules other than their intended targets [164, 165]. This may lead to delayed or incomplete absorption of concomitantly administered oral medications. Consistent with prescribing guidance for bile acid sequestrants and the absorption window of the 3D regimen, it is recommended that the administration of bile acid sequestrants should be separated from the 3D regimen administration by at least 4 h.
Several lipid-modifying agents should not be given in conjunction with the 3D regimen, including gemfibrozil, lovastatin, and simvastatin. Gemfibrozil was shown to mediate a substantial increase (approximately ten-fold) in dasabuvir exposure when administered with paritaprevir/r and dasabuvir (Fig. 1) [25]. Recommendations for using lovastatin and simvastatin are based on the labeled contraindication for combination with CYP3A inhibitors because of an increased risk for myopathy, including rhabdomyolysis [58].
α1-Adrenergic Blockers
The use of α1-adrenergic blockers have several restrictions based on their CYP3A4-dependent metabolism [166–168]. Neither tamsulosin or alfuzosin should be given with the 3D regimen because of restrictions on their use with CYP3A inhibitors in their respective labels [166, 168]. However, doxazosin can be used with caution and dose reduction [167].
β-Adrenergic Agonists
There are no overlapping metabolic/transporter pathways between albuterol (salbutamol) and 3D regimen components [169]. Hence, a drug interaction is not expected between albuterol and the 3D regimen and no dose adjustment is necessary when administered together.
The metabolism of formoterol involves an array of enzymes, some of which (UGT1A1, CYP2C19) are known to be affected by the 3D regimen [170]. Given the potential for altering drug pharmacokinetics, formoterol should be used with caution and dose reduction should be implemented if clinically indicated. However, concomitant use of salmeterol, a substrate of CYP3A4, is not recommended with the 3D regimen because of the potential for QTc prolongation, palpitation, and sinus tachycardia [171].
Hypnotic/Sedative Agents
Recommendations for co-administration of hypnotic/sedative agents differ by individual drugs. CYP3A4 plays a dominant role in the metabolism of many hypnotic/sedative agents, including that of alprazolam, clonazepam, eszopiclone, estazolam, flunitrazepam, prazepam, quazepam, and zaleplon and zopiclone [172–180]. Because of the inhibitory effect of the 3D regimen on this enzyme, dose reduction is generally recommended when these agents are administered to patients receiving 3D treatment. The recommended dose adjustment is supported by a DDI study with alprazolam in which the drug’s AUC increased by 34 % when administered with the 3D regimen [25].
Notable exceptions to the dose reduction guidance include diazepam, zolpidem, triazolam, and midazolam. Changes in exposures for diazepam, a substrate of both CYP3A4 and CYP2C19 [181], were modest (22 % decrease in AUC and 18 % increase in Cmax) when co-administered with the 3D regimen in a DDI study [26]. However, the Cmax of nordiazepam, the major metabolite of diazepam, increased by 10 % and the AUC for a dosing interval and AUC from 0 to infinity (AUCinf) decreased by 3 and 44 %, respectively. Because of its long half‐life (137 h), AUCinf was not reliably estimated when diazepam was dosed alone. Given these results, diazepam dose can be increased, if clinically indicated, although pre-emptive dose adjustments are not required. When given concomitantly with the 3D regimen in a DDI study, zolpidem pharmacokinetics were not appreciably affected; therefore, dose reduction is not indicated [25]. Triazolam and oral midazolam, in contrast, are contraindicated for use with medications that impair CYP3A function [58, 182], and should, therefore, not be given with the 3D regimen. Although the effect of the 3D regimen on midazolam exposure is expected to be significantly less with parenteral administration, close monitoring for respiratory depression and/or prolonged sedation are recommended and dose adjustment may be considered [58].
Another potential influence on DDI risk with hypnotics/sedatives is glucuronidation involving different members of the UGT family. Delayed plasma clearance of lorazepam resulting from inhibition of glucuronidation has been observed in clinical studies [183, 184]. Given the influence of the 3D regimen on UGT1A1 substrates (Fig. 1) [25], lorazepam dose reduction is suggested. Dose reduction, however, is not required for oxazepam and temazepam owing to the minimal involvement of UGT1A1 in their glucuronidation [185]. In general, clinical monitoring should be considered for all hypnotic/sedative agents, irrespective of the dose adjustment recommendation.
Antiepileptic Agents
Methods of disposition for many antiepileptic drugs, including renal elimination (felbamate, gabapentin, topiramate, vigabatrin) [186–189] and hydrolysis (levetiracetam) [190], are generally not anticipated to be influenced by the 3D regimen. Dose alteration is also not anticipated for sodium valproate, whose metabolism involves multiple UGT and CYP species [191]; however, therapeutic drug monitoring is recommended when initiating co-administration.
Exposure to drugs that are metabolized by CYP3A4, including tiagabine [192] and zonisamide [193], may increase with concomitant 3D therapy. These drugs should be used with caution and dose reduction should be considered. Dose alteration and patient monitoring may also be required for lamotrigine owing to UGT1A1 induction by ritonavir [28].
Carbamazepine, phenobarbital, and phenytoin are inducers of CYP3A4 [194]. In a DDI study with the 3D regimen, carbamazepine appreciably decreased DAA exposure (Fig. 1) [25]. Because of the potential for loss of therapeutic efficacy, carbamazepine, phenobarbital, and phenytoin are contraindicated for use with the 3D regimen.
Steroids
Co-administration of ritonavir with corticosteroids that are metabolized by CYP3A4 (e.g., budesonide, dexamethasone, fluticasone, methylprednisolone, mometasone, prednisone, and triamcinolone) increases steroid concentrations and can lead to iatrogenic Cushing syndrome and adrenal suppression [58, 195–200]. Caution should be exercised and lowest doses should be used when considering use of these agents with the 3D regimen. In particular, budesonide and fluticasone, especially if prescribed for long-term use, should only be initiated if the potential benefit of treatment outweighs the risk of systemic corticosteroid effects.
Beclomethasone dipropionate appears to be less prone to interactions with ritonavir. Direct combination of ritonavir with beclomethasone increased exposures of its metabolite, beclomethasone-17-monopropionate (17-BMP), but not to a level that was considered to be clinically significant [201]. Interestingly, 17-BMP exposure was not appreciably increased when darunavir and ritonavir were both administered with beclomethasone. Beclomethasone is an acceptable alternative inhaled/intranasal corticosteroid option for patients receiving the 3D regimen. The pharmacokinetic characteristics of ciclesonide generate lower systemic exposure than other inhaled/intranasal corticosteroids [202]. Ciclesonide may be given without dose adjustment to patients treated with the 3D regimen, with the caveat that monitoring for steroid-related side effects should be ongoing.
The use of topical corticosteroid creams or lotions with the 3D regimen is permissible with attention to factors that can increase systemic absorption such as use over large body surface areas or prolonged use, as described in prescribing information.
Antibiotics
Beta-lactams and fluroquinolones can be given with the 3D regimen without dose adjustment because they are predominantly excreted unchanged in urine or bile [203–207] and there are no apparent overlapping metabolic/transporter pathways between these antibiotics and the 3D regimen [203, 205, 206]. The possible involvement of P-gp in azithromycin disposition [208] is not believed to have an appreciable impact on drug pharmacokinetics, given the lack of significant interaction detected with the 3D regimen and digoxin, a model P-gp substrate (Fig. 1) [25]. Clarithromycin is a strong inhibitor of CYP3A4 [207], through which an interaction with the 3D regimen is possible. Nonetheless, a pharmacokinetic evaluation of concomitant ritonavir and clarithromycin determined that no dose adjustment was required for either agent when co-administered to patients with normal renal function [209]. Clarithromycin dose reduction is, however, recommended for patients with moderate or severe renal impairment [207].
Clinically significant interactions were not observed between sulfamethoxazole/trimethoprim and the 3D regimen in a DDI study. The moderate increase (33 %) in dasabuvir exposure is attributable to the weak CYP2C8 inhibitory effects of trimethoprim [26]. Exposures to sulfamethoxazole and trimethoprim modestly increased (<25 %). The magnitude of these changes does not indicate that dose adjustment is needed for either the 3D regimen or sulfamethoxazole/trimethoprim during co-administration.
Metabolic pathways for fusidic acid are not currently known, but interaction with drugs metabolized by CYP3A4 is suspected [210]. It is recommended that fusidic acid not be given to patients who are receiving a drug or drugs that are metabolized by CYP3A4. As such, fusidic acid is contraindicated for use with the 3D regimen.
Antifungals
A potential for interaction with the 3D regimen exists for several antifungal agents, including ketoconazole, itraconazole, posaconazole, and voriconazole, by virtue of their metabolism by or effects on CYP3A4. In a DDI study, the AUC of ketoconazole (a CYP3A4/P-gp inhibitor and CYP3A4 substrate) increased by 117 % when a single dose of the 3D regimen was added to steady-state ketoconazole 400 mg once daily [25]. Although ombitasvir, paritaprevir, ritonavir, and dasabuvir exposures also increased, none of the changes were considered clinically significant. Similar increases are possible with itraconazole, another CYP3A4/P-gp inhibitor and CYP3A4 substrate [211]. As a strong inhibitor of CYP3A4, caution is advised when posaconazole is administered with drugs metabolized by CYP3A4, including ritonavir [212]. Alterations in voriconazole exposure are also possible owing to its metabolism by CYP2C19 and CYP3A4 [213]. Unlike the azoles, echinocandins such as capsofungin, micafungin, and anidulafungin do not have primary interactions with CYP enzymes or P-gp [214–216]; therefore, a drug interaction is not expected between these drugs and the 3D regimen.
In summary, in patients being treated with the 3D regimen, daily doses of greater than 200 mg are not recommended for either ketoconazole or itraconazole, and use of a lower posaconazole dose is advised. Co-administration of voriconazole with the 3D regimen should be avoided unless an assessment of the benefit-to-risk balance justifies its use. Echinocandins can be administered with the 3D regimen without any dose modification.
Oral Contraceptives
Interactions with the 3D regimen have been evaluated for progestin-only contraceptives and combined oral contraceptives. Minimal effects on drug exposures were observed when norethindrone 0.35 mg (a progestin-only contraceptive) was administered with the 3D regimen. However, use of 3D therapy with norgestimate (250 µg) plus ethinyl estradiol (35 µg) significantly increased the levels of norgestimate metabolites and appreciably decreased paritaprevir, ritonavir, and dasabuvir exposures. Moreover, asymptomatic alanine transaminase elevations were observed when the 3D regimen was combined with norgestimate (250 µg) plus ethinyl estradiol (35 µg) or norethindrone (0.40 mg) plus ethinyl estradiol (35 µg) [12, 25]. Given the potential for alanine transaminase elevations in women who use ethinyl estradiol-containing medications such as combined oral contraceptives, contraceptive patches, or contraceptive vaginal rings, these medications must be discontinued at least 2 weeks prior to starting therapy with the 3D regimen. Alternative methods of contraception (e.g., progestin-only contraception or non-hormonal methods) are recommended during 3D therapy. Ethinyl estradiol-containing medications can be restarted approximately 2 weeks after completion of 3D treatment [12, 25]. If ethinyl estradiol-containing oral contraceptives cannot be avoided or substituted, consideration can be given to use of an alternative DAA regimen that does not have interaction potential with these medications.
Phosphodiesterase Type 5 Inhibitors
The predominant route of metabolism for phosphodiesterase type 5 (PDE5) inhibitors is through CYP3A family members [217–220]. As such, PDE5 inhibitor clearance is expected to be reduced in the presence of CYP3A inhibitors, including the 3D regimen. Guidance for use of PDE5 inhibitors with CYP3A inhibitors is dependent on the therapeutic indication. In the absence of data for the 3D regimen, limitations and contraindications suggested for low-dose ritonavir can be applied.
PDE5 inhibitors are used both in the treatment of erectile dysfunction and pulmonary arterial hypertension, at different doses. For the treatment of erectile dysfunction in patients receiving low-dose ritonavir, doses of sildenafil, tadalafil, and vardenafil should be limited to 25 mg every 48 h, 10 mg every 72 h, or 2.5 mg every 72 h, respectively [58]. Avanafil should not be prescribed for patients receiving 3D, as no safe and effective dose has been determined when used with low-dose ritonavir therapy. In patients with pulmonary arterial hypertension, combining sildenafil with low-dose ritonavir is contraindicated because of a lack of an established safe and effective dose. For patients with pulmonary arterial hypertension, consideration can be given to use of an alternative DAA regimen that does not have interaction potential with sildenafil. Tadalafil and vardenafil 20 mg once daily may be initiated after at least 1 week of low-dose ritonavir treatment. The dose may be increased to 40 mg once daily if tolerated and clinically indicated. Increased monitoring for AEs is recommended during concomitant PDE5 inhibitor/3D therapy.
Thyroid Replacement Therapy
Many of the drugs that are commonly used by patients with HCV are covered within the classes discussed above. Based on a recent evaluation of prescription patterns in patients with chronic HCV [21], one additional medication that is often prescribed to patients with HCV is worthy of note. Levothyroxine sodium is a frequent concomitant medication among patients with chronic HCV owing to the elevated prevalence of hypothyroidism in this population [221]. Levothyroxine is a sensitive substrate that is thought to be metabolized by UGT1A1 [222] without the confounding influence of other UGTs or CYPs. Increases in thyroxine concentration have been observed with UGT1A1 inhibitors such as indinavir [223]. Based on results of the 3D/UGT1A1 substrate DDI study (Fig. 1), concomitant use of levothyroxine with the 3D regimen may increase concentrations of levothyroxine. Hence, thyroid-stimulating hormone levels should be carefully monitored in patients receiving levothyroxine and 3D and dose adjustment implemented based on the clinical response.
Two classes of agents that are absent from this discussion are medications used in the treatment of HIV and immunosuppressants used to prevent transplant rejection (e.g., cyclosporine and tacrolimus). The results of ongoing and completed studies [224] with these agents will be presented in a separate review.
Clinical Experience with the 3D Regimen
The 3D regimen has been evaluated with or without ribavirin in six phase III clinical trials that enrolled more than 2300 patients infected with genotype-1 HCV [5, 8–11]. Patients were allowed to continue most of their co-medications while receiving study drug treatment, although dose adjustment was permissible and recommended. More than 1200 co-medications belonging to 15 drug classes and 19 enzyme/transporter inhibitor/inducer categories were given concomitantly with the 3D regimen in these studies [225–227]. Percentage-of-use metrics based on drug class are listed in Table 2. Polypharmacy was commonplace, with approximately 1500 patients (65 %) receiving two or more concomitant medications from multiple drug classes/categories [227]. Patient monitoring and dose adjustment, as necessary, were sufficient to manage any potential DDIs. The 3D regimen with or without ribavirin was well tolerated in treatment-naïve and treatment-experienced HCV-infected patients with and without cirrhosis [12]; the rate of study drug discontinuation due to AEs was low (<1 %) and few serious AEs were reported.
Conclusions
Our analysis suggests that the 3D regimen is compatible with many of the drugs that are commonly used by patients with HCV infection. Where an interaction is possible, risk can be mitigated by careful attention to concomitant medications, adjusting drug dosage as needed, and monitoring patient response and/or clinical parameters. As HCV 3D therapy is indicated for only 12–24 weeks, clinicians may choose to either suspend existing or new interacting medications or clinically manage the potential DDIs over the fixed, short-term, 3D regimen treatment duration. By using these strategies with the drug-specific guidance provided herein, the likelihood for adverse drug reactions can be lessened, thereby maximizing the opportunity for successful treatment of HCV infection. While these recommendations provide general guidelines based on known mechanism of disposition for the 3D regimen and various interaction medications, clinical judgment based on patient response should prevail during co-administration.
Acknowledgments
The authors thank Amit Khatri and Jiuhong Zha for their expert advice on designing/analyses of the drug interaction studies. The authors also thank Crystal Murcia, PhD, of The JB Ashtin Group, Inc., for assistance in preparing this manuscript for publication.
Author contributions
PSB performed literature searches, assessed potential drug interactions/dosing recommendations, and, together with JRK, contributed to the writing of the manuscript. PSB, JRK, ARP, RMM, and SD were involved in designing/analyzing formal drug interaction studies. ARP performed the analysis of concomitant medications in the 3D pivotal clinical trials. RMM, SD, and BHM evaluated the accuracy of the data and dosing recommendations. All authors critically reviewed and approved manuscript content.
Compliance and Ethical Standards
This study was funded by AbbVie, Inc. (North Chicago, IL, USA). AbbVie, Inc., was involved in the study design, collection, and interpretation of data, and writing, reviewing, and approving the manuscript. PSB, JRK, ARP, BHM, SD, and RMM are current or former employees of AbbVie, Inc., and hold AbbVie, Inc., stock and/or stock options.
References
- 1.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 3.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 4.Zeuzem S, Berg T, Gane E, Ferenci P, Foster GR, Fried MW, et al. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology. 2014;146(430–41):e6. doi: 10.1053/j.gastro.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 5.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 6.Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R, Jr, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371:2375–2382. doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]
- 7.Menon RM, Klein CE, Lawal AA, Chiu Y-L, Awni WM, Podsadecki TJ, et al. Pharmacokinetics and tolerability of the HCV protease inhibitor ABT-450 following single ascending doses in healthy adult volunteers with and without ritonavir. HepDART 2009. Kohala Coast; 2009.
- 8.Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97 % and 100 % sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147(359–65):e1. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 9.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 10.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 11.Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourliere M, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604–1614. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 12.Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets; dasabuvir tablets), co-packaged for oral use. North Chicago: AbbVie Inc.; 2014.
- 13.Lin C-W, Menon R, Liu W, Mensing S, Podsadecki T, Shulman N, et al. Exposure-safety response relationship for paritaprevir/ritonavir, ombitasvir, dasabuvir and ribavirin in hepatitis C genotype 1 virus-infected subjects in phase III studies. In: American Society for Clinical Pharmacology and Therapeutics 2015 Annual Meeting. New Orleans; 2015.
- 14.Khatri A, Dutta S, Marbury T, Preston RA, Rodrigues L, Jr., Wang H, et al. The pharmacokinetics and safety of the direct acting antiviral regimen of ABT-450/r. ombitasvir with/without dasabuvir in subjects with mild, moderate and severe renal impairment compared to subjects with normal renal function. In: AASLD/EASL Special Conference on Hepatitis C. New York; 2014.
- 15.Burger D, Back D, Buggisch P, Buti M, Craxi A, Foster G, et al. Clinical management of drug-drug interactions in HCV therapy: challenges and solutions. J Hepatol. 2013;58:792–800. doi: 10.1016/j.jhep.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Ravi S, Nasiri-Toosi M, Karimzadeh I, Khalili H, Ahadi-Barzoki M, Dashti-Khavidaki S. Pattern and associated factors of anti-hepatitis C virus treatment-induced adverse reactions. Expert Opin Drug Saf. 2014;13:277–286. doi: 10.1517/14740338.2014.866091. [DOI] [PubMed] [Google Scholar]
- 17.Louie KS, St Laurent S, Forssen UM, Mundy LM, Pimenta JM. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infect Dis. 2012;12:86. doi: 10.1186/1471-2334-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiser JJ, Burton JR, Jr, Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10:596–606. doi: 10.1038/nrgastro.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furihata T, Matsumoto S, Fu Z, Tsubota A, Sun Y, Matsumoto S, et al. Different interaction profiles of direct-acting anti-hepatitis C virus agents with human organic anion transporting polypeptides. Antimicrob Agents Chemother. 2014;58:4555–4564. doi: 10.1128/AAC.02724-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maasoumy B, Port K, Calle Serrano B, Markova AA, Sollik L, Manns MP, et al. The clinical significance of drug-drug interactions in the era of direct-acting anti-viral agents against chronic hepatitis C. Aliment Pharmacol Ther. 2013;38:1365–1372. doi: 10.1111/apt.12523. [DOI] [PubMed] [Google Scholar]
- 21.Lauffenburger JC, Mayer CL, Hawke RL, Brouwer KL, Fried MW, Farley JF. Medication use and medical comorbidity in patients with chronic hepatitis C from a US commercial claims database: high utilization of drugs with interaction potential. Eur J Gastroenterol Hepatol. 2014;26:1073–1082. doi: 10.1097/MEG.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Food and Drug Administration. Guidance for industry: drug interaction studies—study design, data analysis, implications for dosing, and labeling recommendations. 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM292362.pdf. 26 Aug 2014.
- 23.European Medicines Agency. Guideline on the Investigation of Drug Interactions. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf. 5 Nov 2014.
- 24.Bow DAJ, Liu J, Kavetskaia O, Menon R, de Morais SM, Nijsen M, et al. A mechanistic non-clinical assessment of drug-drug interactions (metabolism and transporters) with the hepatitis C Virus (HCV) regimen: ABT-450/r, ombitasvir and dasabuvir. In: AASLD/EASL Special Conference on Hepatitis C. New York; 2014.
- 25.Menon RM, Badri PS, Wang T, Polepally AR, Zha J, Khatri A, et al. Drug-drug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir, and dasabuvir. J Hepatol. 2015;63(1):20–9. doi: 10.1016/j.jhep.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Polepally AR, King JR, Ding B, Shuster DL, Dumas EO, Khatri A, et al. Drug-drug interactions of commonly used medications with direct acting antiviral HCV combination therapy of paritaprevir/r, ombitasvir and dasabuvir. In: 16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. Washington, DC; 2015.
- 27.van der Lee MJ, Dawood L, ter Hofstede HJ, de Graaff-Teulen MJ, van Ewijk-Beneken Kolmer EW, Caliskan-Yassen N, et al. Lopinavir/ritonavir reduces lamotrigine plasma concentrations in healthy subjects. Clin Pharmacol Ther. 2006;80:159–168. doi: 10.1016/j.clpt.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Burger DM, Huisman A, Van Ewijk N, Neisingh H, Van Uden P, Rongen GA, et al. The effect of atazanavir and atazanavir/ritonavir on UDP-glucuronosyltransferase using lamotrigine as a phenotypic probe. Clin Pharmacol Ther. 2008;84:698–703. doi: 10.1038/clpt.2008.106. [DOI] [PubMed] [Google Scholar]
- 29.Khatri A, Wang T, Wang H, Podsadecki TJ, Trinh RN, Awni WM, et al. Drug-drug interactions of the direct acting antiviral regimen of ABT-450/r, ombitasvir and dasabuvir with emtricitabine + tenofovir, raltegravir, rilpivirine and efavirenz. In: 54th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC; 2014.
- 30.Celebrex (celecoxib) capsules [prescribing information]. New York: GD Searle LLC; 2011.
- 31.King C, Tang W, Ngui J, Tephly T, Braun M. Characterization of rat and human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of diclofenac. Toxicol Sci. 2001;61:49–53. doi: 10.1093/toxsci/61.1.49. [DOI] [PubMed] [Google Scholar]
- 32.Kaji H, Kume T. Identification of human UDP-glucuronosyltransferase isoform(s) responsible for the glucuronidation of 2-(4-chlorophenyl)-5-(2-furyl)-4-oxazoleacetic acid (TA-1801A) Drug Metab Pharmacokinet. 2005;20:212–218. doi: 10.2133/dmpk.20.212. [DOI] [PubMed] [Google Scholar]
- 33.Crespi CL, Chang TK, Waxman DJ. Determination of CYP2C9-catalyzed diclofenac 4′-hydroxylation by high-performance liquid chromatography. Methods Mol Biol. 2006;320:109–113. doi: 10.1385/1-59259-998-2:109. [DOI] [PubMed] [Google Scholar]
- 34.Tougou K, Gotou H, Ohno Y, Nakamura A. Stereoselective glucuronidation and hydroxylation of etodolac by UGT1A9 and CYP2C9 in man. Xenobiotica. 2004;34:449–461. doi: 10.1080/00498250410001691280. [DOI] [PubMed] [Google Scholar]
- 35.Patel M, Tang BK, Kalow W. (S)oxazepam glucuronidation is inhibited by ketoprofen and other substrates of UGT2B7. Pharmacogenetics. 1995;5:43–49. doi: 10.1097/00008571-199502000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Ansaid (flurbiprofen) tablets [prescribing information]. New York: Pharmacia & Upjohn Company; 2010.
- 37.Kuehl GE, Lampe JW, Potter JD, Bigler J. Glucuronidation of nonsteroidal anti-inflammatory drugs: identifying the enzymes responsible in human liver microsomes. Drug Metab Dispos. 2005;33:1027–1035. doi: 10.1124/dmd.104.002527. [DOI] [PubMed] [Google Scholar]
- 38.Hynninen VV, Olkkola KT, Leino K, Lundgren S, Neuvonen PJ, Rane A, et al. Effects of the antifungals voriconazole and fluconazole on the pharmacokinetics of s-(+)- and R-(−)-Ibuprofen. Antimicrob Agents Chemother. 2006;50:1967–1972. doi: 10.1128/AAC.01483-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakajima M, Inoue T, Shimada N, Tokudome S, Yamamoto T, Kuroiwa Y. Cytochrome P450 2C9 catalyzes indomethacin O-demethylation in human liver microsomes. Drug Metab Dispos. 1998;26:261–266. [PubMed] [Google Scholar]
- 40.Sabolovic N, Magdalou J, Netter P, Abid A. Nonsteroidal anti-inflammatory drugs and phenols glucuronidation in Caco-2 cells: identification of the UDP-glucuronosyltransferases UGT1A6, 1A3 and 2B7. Life Sci. 2000;67:185–196. doi: 10.1016/S0024-3205(00)00616-0. [DOI] [PubMed] [Google Scholar]
- 41.Tracy TS, Marra C, Wrighton SA, Gonzalez FJ, Korzekwa KR. Involvement of multiple cytochrome P450 isoforms in naproxen O-demethylation. Eur J Clin Pharmacol. 1997;52:293–298. doi: 10.1007/s002280050293. [DOI] [PubMed] [Google Scholar]
- 42.Feldene (piroxicam) capsules [prescribing information]. New York: Pfizer Labs; 2010.
- 43.Kuehl GE, Bigler J, Potter JD, Lampe JW. Glucuronidation of the aspirin metabolite salicylic acid by expressed UDP-glucuronosyltransferases and human liver microsomes. Drug Metab Dispos. 2006;34:199–202. doi: 10.1124/dmd.105.005652. [DOI] [PubMed] [Google Scholar]
- 44.Allegaert K, van Calsteren K, Hendrickx S, Kelchtermans J, Smits A, Kulo A, et al. Paracetamol and ketorolac pharmacokinetics and metabolism at delivery and during postpartum. Acta Anaesthesiol Belg. 2012;63:121–125. [PubMed] [Google Scholar]
- 45.Gates BJ, Nguyen TT, Setter SM, Davies NM. Meloxicam: a reappraisal of pharmacokinetics, efficacy and safety. Expert Opin Pharmacother. 2005;6:2117–2140. doi: 10.1517/14656566.6.12.2117. [DOI] [PubMed] [Google Scholar]
- 46.Labroo RB, Paine MF, Thummel KE, Kharasch ED. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab Dispos. 1997;25:1072–1080. [PubMed] [Google Scholar]
- 47.Jin M, Gock SB, Jannetto PJ, Jentzen JM, Wong SH. Pharmacogenomics as molecular autopsy for forensic toxicology: genotyping cytochrome P450 3A4*1B and 3A5*3 for 25 fentanyl cases. J Anal Toxicol. 2005;29:590–598. doi: 10.1093/jat/29.7.590. [DOI] [PubMed] [Google Scholar]
- 48.Zohydro ER (hydrocodone bitartrate) extended-release capsules [prescribing information]. San Diego: Zogenix, Inc.; 2014.
- 49.Hutchinson MR, Menelaou A, Foster DJ, Coller JK, Somogyi AA. CYP2D6 and CYP3A4 involvement in the primary oxidative metabolism of hydrocodone by human liver microsomes. Br J Clin Pharmacol. 2004;57:287–297. doi: 10.1046/j.1365-2125.2003.02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramirez J, Innocenti F, Schuetz EG, Flockhart DA, Relling MV, Santucci R, et al. CYP2B6, CYP3A4, and CYP2C19 are responsible for the in vitro N-demethylation of meperidine in human liver microsomes. Drug Metab Dispos. 2004;32:930–936. [PubMed] [Google Scholar]
- 51.Gronlund J, Saari TI, Hagelberg NM, Neuvonen PJ, Olkkola KT, Laine K. Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol. 2010;70:78–87. doi: 10.1111/j.1365-2125.2010.03653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gronlund J, Saari TI, Hagelberg NM, Neuvonen PJ, Laine K, Olkkola KT. Effect of inhibition of cytochrome P450 enzymes 2D6 and 3A4 on the pharmacokinetics of intravenous oxycodone: a randomized, three-phase, crossover, placebo-controlled study. Clin Drug Investig. 2011;31:143–153. doi: 10.2165/11539950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 53.Kummer O, Hammann F, Moser C, Schaller O, Drewe J, Krahenbuhl S. Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Eur J Clin Pharmacol. 2011;67:63–71. doi: 10.1007/s00228-010-0893-3. [DOI] [PubMed] [Google Scholar]
- 54.Subrahmanyam V, Renwick AB, Walters DG, Young PJ, Price RJ, Tonelli AP, et al. Identification of cytochrome P-450 isoforms responsible for cis-tramadol metabolism in human liver microsomes. Drug Metab Dispos. 2001;29:1146–1155. [PubMed] [Google Scholar]
- 55.Codeine sulfate tablets [prescribing information]. Columbus: Roxane Laboratories, Inc.; 2013.
- 56.Pal D, Kwatra D, Minocha M, Paturi DK, Budda B, Mitra AK. Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals. Life Sci. 2011;88:959–971. doi: 10.1016/j.lfs.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108:363–374. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- 58.Kaletra (lopinavir/ritonavir) capsules [prescribing information]. North Chicago: AbbVie Inc.; 2013.
- 59.Prozac (fluoxetine hydrochloride) pulvules for oral use [prescribing information]. Indianapolis: Lilly USA, LLC; 2014.
- 60.Paxil (paroxetine hydrochloride) tablets and oral suspension [prescribing information]. Research Triangle Park: GlaxoSmithKline; 2014.
- 61.Savella (milnacipran HCl) tablets [prescribing information]. St. Louis: Forest Pharmaceuticals, Inc.; 2013.
- 62.Park J, Vousden M, Brittain C, McConn DJ, Iavarone L, Ascher J, et al. Dose-related reduction in bupropion plasma concentrations by ritonavir. J Clin Pharmacol. 2010;50:1180–1187. doi: 10.1177/0091270009359524. [DOI] [PubMed] [Google Scholar]
- 63.Luvox (fluvoxamine maleate) [prescribing information]. New South Wales: Abbot Australasia Pty Ltd; 2011.
- 64.Remeron (mirtazapine) tablets [prescribing information]. Salt Lake City: Cephalon, Inc.; 2014.
- 65.Wienkers LC, Allievi C, Hauer MJ, Wynalda MA. Cytochrome P-450-mediated metabolism of the individual enantiomers of the antidepressant agent reboxetine in human liver microsomes. Drug Metab Dispos. 1999;27:1334–1340. [PubMed] [Google Scholar]
- 66.Ueda N, Yoshimura R, Umene-Nakano W, Ikenouchi-Sugita A, Hori H, Hayashi K, et al. Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry. 2009;10:832–835. doi: 10.1080/15622970802688069. [DOI] [PubMed] [Google Scholar]
- 67.Oleptro (trazodone hydrochloride) extended-release tablets [prescribing information]. Gaithersburg: Angelini Pharma Inc.; 2014.
- 68.Effexor (venlafaxine hydrochloride) tablets [prescribing information]. Philadelphia: Wyeth Pharmaceuticals, Inc.; 2012.
- 69.Polepally AR, Dutta S, Baykal T, Hu B, Podsadecki TJ, Awni WM, et al. Drug-drug interactions of omeprazole with the HCV direct acting antiviral (DAA) combination of ABT-450/r, ombitasvir and dasabuvir. Clin Pharm Ther. 2015;97(Suppl 1):S77–S78. [Google Scholar]
- 70.Dexilant (dexlansoprazole) delayed-release capsules for oral use [prescribing information]. Deerfield: Takeda Pharmaceuticals America, Inc.; 2013.
- 71.Nexium (esomeprazole magnesium) delayed-release capsules, for oral use [prescribing information]. Wilmington: AstraZeneca Pharmaceuticals LP; 2014.
- 72.Prevacid (lansoprazole) delayed-release capsules [prescribing information]. Deerfield: Takeda Pharmaceuticals America, Inc.; 2012.
- 73.Protonix (pantoprazole sodium) delayed-release tablets [prescribing information]. Philadelphia: Wyeth Pharmaceuticals, Inc.; 2013.
- 74.Aciphex (rabeprazole sodium) delayed-release tablets, for oral use [prescribing information]. Eisai, Inc.; 2013.
- 75.Tagamet (cimetidine) [product information]. Victoria: GlaxoSmithKline Australia Pty Ltd; 2008.
- 76.Pepcid (famotidine) tablets [prescribing information]. Northbrook: Marathon Pharmaceuticals, LLC; 2014.
- 77.Zantac (ranitidine hydrochloride) tablets [prescribing information]. Research Triangle Park: GlaxoSmithKline; 2009.
- 78.Midamor (amiloride HCl) tablets [prescribing information]. Whitehouse Station: Merck & Co, Inc.; 2002.
- 79.Thalitone (chlorthalidone) tablets [prescribing information]. Bristol: King Pharmaceticals, Inc.; 2004.
- 80.Microzide (hydrochlorothiazide) capsules [prescribing information]. Morristown: Watson Pharma, Inc.; 2011.
- 81.Dyrenium (triamterene USP) capsules [prescribing information]. Oakville: WellSpring Pharmaceuticals Canada Corp; 2009.
- 82.Miners JO, Rees DL, Valente L, Veronese ME, Birkett DJ. Human hepatic cytochrome P450 2C9 catalyzes the rate-limiting pathway of torsemide metabolism. J Pharmacol Exp Ther. 1995;272:1076–1081. [PubMed] [Google Scholar]
- 83.Ong CE, Coulter S, Birkett DJ, Bhasker CR, Miners JO. The xenobiotic inhibitor profile of cytochrome P4502C8. Br J Clin Pharmacol. 2000;50:573–580. doi: 10.1046/j.1365-2125.2000.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lovenox (enoxaparin sodium injection) for subcutaneous and intravenous use [prescribing information]. Bridgewater: Sanofi-Aventis U.S. LLC; 2013.
- 85.Arixtra (fondaparinux sodium) solution for subcutaneous injection [prescribing information]. Notre Dame de Bondeville: Aspen NDB; 2014.
- 86.Verstuyft C, Delavenne X, Rousseau A, Robert A, Tod M, Diquet B, et al. A pharmacokinetic-pharmacodynamic model for predicting the impact of CYP2C9 and VKORC1 polymorphisms on fluindione and acenocoumarol during induction therapy. Clin Pharmacokinet. 2012;51:41–53. doi: 10.2165/11595560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 87.Coumadin (warfain sodium) tablets, for oral use [prescribing information]. Princeton: Bristol-Myers Squibb Company; 2011.
- 88.Thijssen HH, Flinois JP, Beaune PH. Cytochrome P4502C9 is the principal catalyst of racemic acenocoumarol hydroxylation reactions in human liver microsomes. Drug Metab Dispos. 2000;28:1284–1290. [PubMed] [Google Scholar]
- 89.Jobski K, Behr S, Garbe E. Drug interactions with phenprocoumon and the risk of serious haemorrhage: a nested case-control study in a large population-based German database. Eur J Clin Pharmacol. 2011;67:941–951. doi: 10.1007/s00228-011-1031-6. [DOI] [PubMed] [Google Scholar]
- 90.Toon S, Heimark LD, Trager WF, O’Reilly RA. Metabolic fate of phenprocoumon in humans. J Pharm Sci. 1985;74:1037–1040. doi: 10.1002/jps.2600741003. [DOI] [PubMed] [Google Scholar]
- 91.Eliquis (apixaban) tablets for oral use [prescribing information]. Princeton and New York: Bristol-Myers Sqibb Company and Pfizer, Inc.; 2014.
- 92.Xarelto (rivaroxaban) tablets, for oral use [prescribing information]. Titusville: Janssen Pharmaceuticals, Inc.; 2014.
- 93.Pradaxa (dabigatran etexilate mesylate) capsules [prescribing information]. Ridgefield: Boehringer Ingelheim Pharmaceuticals, Inc.; 2015.
- 94.Plavix (clopidogrel bisulfate) tablets [prescribing information]. Bridgewater: Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; 2013.
- 95.Zhu Y, Zhou J. Identification of the significant involvement and mechanistic role of CYP3A4/5 in clopidogrel bioactivation. ACS Med Chem Lett. 2012;3:844–849. doi: 10.1021/ml3002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Effient (prasugrel) tablets [prescribing information]. Indianapolis: Daiichi Sankyo, Inc. and Lilly USA, LLC; 2013.
- 97.Ancrenaz V, Deglon J, Samer C, Staub C, Dayer P, Daali Y, et al. Pharmacokinetic interaction between prasugrel and ritonavir in healthy volunteers. Basic Clin Pharmacol Toxicol. 2013;112:132–137. doi: 10.1111/j.1742-7843.2012.00932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tenormin (atenolol) tablets [prescribing information]. Wilmington: AstraZeneca Pharmaceuticals LP; 2012.
- 99.Zebeta (bisoprolol fumurate) tablets [prescribing information]. Pamona: Duramed Pharmaceuticals, Inc.; 2011.
- 100.Corgard (nadalol) tablets [prescribing information]. New York: Pfizer, Inc.; 2013.
- 101.Betapace (sotalol HCl) [prescribing information]. Wayne: Bayer HealthCare Pharmaceuticals, Inc.; 2011.
- 102.Zateyshchikov DA, Minushkina LO, Brovkin AN, Savel’eva EG, Zateyshchikova AA, Manchaeva BB, et al. Association of CYP2D6 and ADRB1 genes with hypotensive and antichronotropic action of betaxolol in patients with arterial hypertension. Fundam Clin Pharmacol. 2007;21:437–443. doi: 10.1111/j.1472-8206.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 103.Kudo S, Uchida M, Odomi M. Metabolism of carteolol by cDNA-expressed human cytochrome P450. Eur J Clin Pharmacol. 1997;52:479–485. doi: 10.1007/s002280050322. [DOI] [PubMed] [Google Scholar]
- 104.Lopressor (metoprolol tartrate) injection [prescribing information]. East Hanover: Novartis Pharmaceuticals Corporation; 2013.
- 105.Bystolic (nebivolol) tablets [prescribing information]. St. Louis: Forest Pharmaceuticals, Inc.; 2011.
- 106.Volotinen M, Turpeinen M, Tolonen A, Uusitalo J, Maenpaa J, Pelkonen O. Timolol metabolism in human liver microsomes is mediated principally by CYP2D6. Drug Metab Dispos. 2007;35:1135–1141. doi: 10.1124/dmd.106.012906. [DOI] [PubMed] [Google Scholar]
- 107.Inderal (propranolol hydrochloride) tablets [prescribing information]. Philadelphia: Wyeth Pharmaceuticals, Inc.; 2011.
- 108.Sectral (acebutolol hydrochloride) capsules [presribing information]. Bridgewater: Promius Pharma, LLC; 2011.
- 109.Stringer RA, Strain-Damerell C, Nicklin P, Houston JB. Evaluation of recombinant cytochrome P450 enzymes as an in vitro system for metabolic clearance predictions. Drug Metab Dispos. 2009;37:1025–1034. doi: 10.1124/dmd.108.024810. [DOI] [PubMed] [Google Scholar]
- 110.Capoten (captopril) tablets [prescribing information]. Spring Valley: Par Pharmaceutical Companies, Inc.; 2012.
- 111.Vasotec (enalapril) tablets [prescribing information]. Bridgewater: Valeant Pharmaceuticals North America LLC; 2012.
- 112.Singhvi SM, Duchin KL, Morrison RA, Willard DA, Everett DW, Frantz M. Disposition of fosinopril sodium in healthy subjects. Br J Clin Pharmacol. 1988;25:9–15. doi: 10.1111/j.1365-2125.1988.tb03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zestril (lisinopril) tablets, for oral use [prescribing information]. Wilmington,: AstraZeneca Pharmaceuticals LP; 2014.
- 114.Aceon (perindopril erbumine) tablets [prescribing information]. Berkeley: XOMA (US) LLC; 2013.
- 115.Accupril (quinapril hydrochloride) tablets [prescribing information]. New York: Parke-Davis; 2014.
- 116.Altace (ramipril) capsules, oral [prescribing information]. New York: Pfizer, Inc.; 2014.
- 117.Singhvi SM, Foley JE, Willard DA, Morrison RA. Disposition of zofenopril calcium in healthy subjects. J Pharm Sci. 1990;79:970–973. doi: 10.1002/jps.2600791105. [DOI] [PubMed] [Google Scholar]
- 118.Tian L, Liu H, Xie S, Jiang J, Han L, Huang Y, et al. Effect of organic anion-transporting polypeptide 1B1 (OATP1B1) polymorphism on the single- and multiple-dose pharmacokinetics of enalapril in healthy Chinese adult men. Clin Ther. 2011;33:655–663. doi: 10.1016/j.clinthera.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 119.Norvasc (amlodipine besylate) tablets for oral administration [prescribing information]. New York: Pfizer Labs; 2013.
- 120.Procardia (nifedipine) capsules [prescribing information]. New York: Pfizer Labs; 2013.
- 121.Nisoldipine extended-release tablets [prescribing information]. Mason: Prasco Laboratories; 2013.
- 122.Soons PA, Vogels BA, Roosemalen MC, Schoemaker HC, Uchida E, Edgar B, et al. Grapefruit juice and cimetidine inhibit stereoselective metabolism of nitrendipine in humans. Clin Pharmacol Ther. 1991;50:394–403. doi: 10.1038/clpt.1991.156. [DOI] [PubMed] [Google Scholar]
- 123.Verelan (verapamil hydrochloride) capsules [prescribing information]. Smyrna: Schwarz Pharma, LLC; 2011.
- 124.Cardizem (diltiazem hydrochloride) direct compression tablets [prescribing information]. Bridgewater: BTA Pharmaceuticals, Inc.; 2010.
- 125.Ma B, Prueksaritanont T, Lin JH. Drug interactions with calcium channel blockers: possible involvement of metabolite-intermediate complexation with CYP3A. Drug Metab Dispos. 2000;28:125–130. [PubMed] [Google Scholar]
- 126.Plendil (felodipine) extended-release tablets [prescribing information]. Wilmington: AstraZeneca LP; 2012.
- 127.Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Flynn CA, Hagenbuch BA, Reed GA. Losartan is a substrate of organic anion transporting polypeptide 2B1. FASEB J. 2010;24(758):2. [Google Scholar]
- 129.Karlgren M, Ahlin G, Bergstrom CA, Svensson R, Palm J, Artursson P. In vitro and in silico strategies to identify OATP1B1 inhibitors and predict clinical drug-drug interactions. Pharm Res. 2012;29:411–426. doi: 10.1007/s11095-011-0564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun P, Wang C, Liu Q, Meng Q, Zhang A, Huo X, et al. OATP and MRP2-mediated hepatic uptake and biliary excretion of eprosartan in rat and human. Pharmacol Rep. 2014;66:311–319. doi: 10.1016/j.pharep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 131.European Medicines Agency. Assessment report: Edarbi (azilsartan medoxomil). 2011.
- 132.Karim A. Spironolactone: disposition, metabolism, pharmacodynamics, and bioavailability. Drug Metab Rev. 1978;8:151–188. doi: 10.3109/03602537808993782. [DOI] [PubMed] [Google Scholar]
- 133.Aldactone (spironolactone) tablets [prescribing information]. New York: G.D. Searle; 2013.
- 134.Flecainide acetate [prescribing information]. Glasgow: Amneal Pharmaceuticals; 2013.
- 135.Mexiletine hydrochloride [prescribing information]. Pulaski: AvKARE, Inc.; 2013.
- 136.Cordarone (amiodarone HCl) tablets [prescribing information]. Philadelphia: Wyeth Pharmaceuticals, Inc.; 2014.
- 137.Norpace (disopyramide phosphate) capsules [prescribing information]. New York: G. D. Searle LLC; 2006.
- 138.Multaq (dronedarone) tablets [prescribing information]. Bridgewater: Sanofi-Aventis U.S. LLC; 2014.
- 139.Dorian P. Clinical pharmacology of dronedarone: implications for the therapy of atrial fibrillation. J Cardiovasc Pharmacol Ther. 2010;15:15S–18S. doi: 10.1177/1074248410367792. [DOI] [PubMed] [Google Scholar]
- 140.Rythmol (propafenone hydrochloride) tablets for oral use [prescribing information]. Whippany: Halo Pharmaceutical, Inc.; 2013.
- 141.Quinidine sulfate [prescribing information]. Corona: Watson Pharma, Inc.; 2012.
- 142.Precose (acarbose) tablets [prescribing information]. Wayne: Bayer HealthCare Pharmaceuticals, Inc.; 2012.
- 143.Riomet (metformin hydrochloride) oral solution [prescribing information]. Jacksonville: Ranbaxy Laboratories, Inc.; 2010.
- 144.Januvia (sitagliptin) tablets [prescribing information]. Whitehouse Station: Merck & Co., Inc.; 2013.
- 145.Galvus (vildagliptin) [precsribing information]. New South Wales: Novartis Pharmaceuticals Australia Pty Ltd; 2014.
- 146.Actos (pioglitazone) tablets for oral use [prescribing information]. Deerfield: Takeda Pharmaceuticals America, Inc.; 2013.
- 147.Avandia (rosiglitazone maleate) tablets [prescribing information]. Research Triangle Park: GlaxoSmithKline; 2014.
- 148.Daonil (glibenclamide) tablets [prescribing information]. Auckland: Sanofi-Aventis New Zealand Limited; 2014.
- 149.Amaryl (glimepiride) tablets [prescribing information]. Bridgewater: Sanofi-Aventis U.S. LLC; 2013.
- 150.Tirkkonen T, Heikkila P, Huupponen R, Laine K. Potential CYP2C9-mediated drug-drug interactions in hospitalized type 2 diabetes mellitus patients treated with the sulphonylureas glibenclamide, glimepiride or glipizide. J Intern Med. 2010;268:359–366. doi: 10.1111/j.1365-2796.2010.02257.x. [DOI] [PubMed] [Google Scholar]
- 151.Kirchheiner J, Brockmoller J, Meineke I, Bauer S, Rohde W, Meisel C, et al. Impact of CYP2C9 amino acid polymorphisms on glyburide kinetics and on the insulin and glucose response in healthy volunteers. Clin Pharmacol Ther. 2002;71:286–296. doi: 10.1067/mcp.2002.122476. [DOI] [PubMed] [Google Scholar]
- 152.Lee CR, Pieper JA, Frye RF, Hinderliter AL, Blaisdell JA, Goldstein JA. Tolbutamide, flurbiprofen, and losartan as probes of CYP2C9 activity in humans. J Clin Pharmacol. 2003;43:84–91. doi: 10.1177/0091270002239710. [DOI] [PubMed] [Google Scholar]
- 153.Onglyza (saxagliptin) tablets for oral use [prescribing information]. Princeton and Wilmington: Bristol-Myers Squibb Company and AstraZeneca Pharmaceuticals LP; 2013.
- 154.Prandin (repaglinide) tablets [prescribing information]. Princeton: Novo Nordisk, Inc.; 2012.
- 155.Kalliokoski A, Backman JT, Kurkinen KJ, Neuvonen PJ, Niemi M. Effects of gemfibrozil and atorvastatin on the pharmacokinetics of repaglinide in relation to SLCO1B1 polymorphism. Clin Pharmacol Ther. 2008;84:488–496. doi: 10.1038/clpt.2008.74. [DOI] [PubMed] [Google Scholar]
- 156.Kajosaari LI, Niemi M, Neuvonen M, Laitila J, Neuvonen PJ, Backman JT. Cyclosporine markedly raises the plasma concentrations of repaglinide. Clin Pharmacol Ther. 2005;78:388–399. doi: 10.1016/j.clpt.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 157.Zheng HX, Huang Y, Frassetto LA, Benet LZ. Elucidating rifampin’s inducing and inhibiting effects on glyburide pharmacokinetics and blood glucose in healthy volunteers: unmasking the differential effects of enzyme induction and transporter inhibition for a drug and its primary metabolite. Clin Pharmacol Ther. 2009;85:78–85. doi: 10.1038/clpt.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lipitor (atorvastatin calcium) tablets [prescribing information]. New York: Parke-Davis; 2014.
- 159.Baycol (cerivastatin sodium) tablets [prescribing information]. West Haven: Bayer Corporation Pharmaceutical Division; 2001.
- 160.Niaspan (niacin extended-release) tablets [prescribing information]. North Chicago: Abbott Laboratories; 2013.
- 161.Lovaza (omega-3-acid ethyl esters) capsules [prescribing information]. Research Triangle Park: GlaxoSmithKline; 2014.
- 162.Kynamro (mipomersen sodium) injection [prescribing information]. Cambridge: Genzyme Corporation; 2013.
- 163.Prueksaritanont T, Tang C, Qiu Y, Mu L, Subramanian R, Lin JH. Effects of fibrates on metabolism of statins in human hepatocytes. Drug Metab Dispos. 2002;30:1280–1287. doi: 10.1124/dmd.30.11.1280. [DOI] [PubMed] [Google Scholar]
- 164.Colestid (colestipol hydrochloride) oral suspension [prescribing information]. New York: Pharmacia & Upjohn Company; 2014.
- 165.Welchol (colesevelam hydrochloride) [prescribing information]. Parsippany: Daiichi Sankyo, Inc.; 2014.
- 166.Flomax (tamsulosin hydrochloride) capsules [prescribing information]. Ridgefield: Boehringer Ingelheim Pharmaceuticals, Inc.; 2014.
- 167.Cardura XL (doxazosin mesylate extended-release tablets) [prescribing information]. New York: Roerig; 2011.
- 168.Uroxatral (alfuzosin HCl) extended-release tablets [prescribing information]. Cary: Covis Pharmaceuticals, Inc.; 2014.
- 169.ProAir HFA (albuterol sulfate) inhalation aerosol [prescribing information]. Horsham: Teva Respiratory, LLC; 2012.
- 170.Foradil Certihaler (formoterol fumarate) inhalation powder [prescribing information]. East Hanover: Novartis Pharmaceuticals Corp.; 2010.
- 171.Serevent Diskus (salmeterol xinofoate) inhalation powder [prescribing information]. Research Triangle Park: GlaxoSmithKline; 2014.
- 172.Xanax (alprazolam) tablets [prescribing information]. New York: Pharmacia & Upjohn Co; 2011.
- 173.Klonopin (clonazepam) tablets [prescribing information]. South San Francisco: Genentech USA, Inc.; 2013.
- 174.Lunesta (eszopiclone) tablets for oral use [prescribing information]. Marlborough: Sunovion Pharmaceuticals, Inc.; 2014.
- 175.Miura M, Otani K, Ohkubo T. Identification of human cytochrome P450 enzymes involved in the formation of 4-hydroxyestazolam from estazolam. Xenobiotica. 2005;35:455–465. doi: 10.1080/00498250500111612. [DOI] [PubMed] [Google Scholar]
- 176.Mizuno K, Katoh M, Okumura H, Nakagawa N, Negishi T, Hashizume T, et al. Metabolic activation of benzodiazepines by CYP3A4. Drug Metab Dispos. 2009;37:345–351. doi: 10.1124/dmd.108.024521. [DOI] [PubMed] [Google Scholar]
- 177.Centrax tablets: Summary of product characteristics. Dublin: Pfizer Healthcare Ireland; 2013.
- 178.Miura M, Ohkubo T. In vitro metabolism of quazepam in human liver and intestine and assessment of drug interactions. Xenobiotica. 2004;34:1001–1011. doi: 10.1080/02772240400015214. [DOI] [PubMed] [Google Scholar]
- 179.Sonata (zaleplon) capsules [prescribing information]. Bristol: King Pharmaceuticals, Inc.; 2013.
- 180.Imovane (zopiclone) tablets [prescribing information]. New South Wales: Sanofi-Aventis Australia Pty Ltd; 2011.
- 181.Valium (diazepam) tablets [prescribing information]. South San Francisco: Genentech USA, Inc.; 2013.
- 182.Halcion (triazolam) tablets [prescribing information]. New York: Pharmacia & Upjohn Company; 2008.
- 183.Depacon (valproate sodium) for intravenous injection [prescribing information]. North Chicago: AbbVie, Inc.; 2014.
- 184.Ethell BT, Anderson GD, Burchell B. The effect of valproic acid on drug and steroid glucuronidation by expressed human UDP-glucuronosyltransferases. Biochem Pharmacol. 2003;65:1441–1449. doi: 10.1016/S0006-2952(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 185.Kiang TK, Ensom MH, Chang TK. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther. 2005;106:97–132. doi: 10.1016/j.pharmthera.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 186.Felbatol (felbamate) [prescribing information]. Somerset: Meda Pahrmaceuticals, Inc.; 2012.
- 187.Neurontin (gabapentin) capsules for oral use [prescribing information]. New York: Parke-Davis; 2014.
- 188.Topamax (topiramate) tablets for oral use [prescribing information]. Titusville: Janssen Pharmaceuticals, Inc.; 2014.
- 189.Sabril (vigabatrin) tablets for oral use [prescribing information]. Deerfield: Lundbeck; 2014.
- 190.Keppra (levetiracetam) tablets for oral use [prescribing information]. Smyrna: UCB, Inc.; 2014.
- 191.Argikar UA, Remmel RP. Effect of aging on glucuronidation of valproic acid in human liver microsomes and the role of UDP-glucuronosyltransferase UGT1A4, UGT1A8, and UGT1A10. Drug Metab Dispos. 2009;37:229–236. doi: 10.1124/dmd.108.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gabitril (tiagabine hydrochloride) tablets [prescribing information]. Frazer: Cephalon, Inc.; 2010.
- 193.Zonegran (zonisamide) capsules [prescribing information]. Woodcliff Lake: Eisai, Inc.; 2012.
- 194.Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246–255. doi: 10.1111/j.1365-2125.2005.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Moore CD, Roberts JK, Orton CR, Murai T, Fidler TP, Reilly CA, et al. Metabolic pathways of inhaled glucocorticoids by the CYP3A enzymes. Drug Metab Dispos. 2013;41:379–389. doi: 10.1124/dmd.112.046318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Solu-Medrol (methylprednisolon sodium succinate) for injection [prescribing information]. New York: Pharmacia & Upjohn Co.; 2014.
- 197.Asmanex HFA (mometasone furoate) inhalation aerosol [prescribing information]. Whitehouse Station: Merck & Co., Inc.; 2014.
- 198.Registry GCS. Study No. FNM10004. 2008 [cited 2014 November 20]; Available from: http://www.gsk-clinicalstudyregister.com/study/FNM10004#rs.
- 199.Schwarze-Zander C, Klingmuller D, Klumper J, Strassburg CP, Rockstroh JK. Triamcinolone and ritonavir leading to drug-induced Cushing syndrome and adrenal suppression: description of a new case and review of the literature. Infection. 2013;41:1183–1187. doi: 10.1007/s15010-013-0506-z. [DOI] [PubMed] [Google Scholar]
- 200.Norvir (ritonavir) tablets for oral use [prescribing information]. North Chicago: AbbVie, Inc.; 2013.
- 201.Boyd SD, Hadigan C, McManus M, Chairez C, Nieman LK, Pau AK, et al. Influence of low-dose ritonavir with and without darunavir on the pharmacokinetics and pharmacodynamics of inhaled beclomethasone. J Acquir Immune Defic Syndr. 2013;63:355–361. doi: 10.1097/QAI.0b013e31829260d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006;28:1042–1050. doi: 10.1183/09031936.00074905. [DOI] [PubMed] [Google Scholar]
- 203.Amoxil (amoxicillin) [prescribing information]. Bridgewater: Dr. Reddy’s Laboratories; 2011.
- 204.Zithromax (azithromycin) tablets [prescribing information]. New York: Pfizer Labs; 2007.
- 205.Keflex (cephalexin) capsules [prescribing information]. Germantown: Advancis Pharmaceutical Corporation; 2006.
- 206.Cipro (ciprofloxacin hydrochloride) tablets [prescribing information]. Wayne: Bayer HealthCare Pharmaceuticals, Inc.; 2013.
- 207.Biaxin Filmtab (clarithromycin) tablets [prescribing information]. North Chicago: AbbVie, Inc.; 2014.
- 208.Amsden GW, Nafziger AN, Foulds G, Cabelus LJ. A study of the pharmacokinetics of azithromycin and nelfinavir when coadministered in healthy volunteers. J Clin Pharmacol. 2000;40:1522–1527. [PubMed] [Google Scholar]
- 209.Ouellet D, Hsu A, Granneman GR, Carlson G, Cavanaugh J, Guenther H, et al. Pharmacokinetic interaction between ritonavir and clarithromycin. Clin Pharmacol Ther. 1998;64:355–362. doi: 10.1016/S0009-9236(98)90065-0. [DOI] [PubMed] [Google Scholar]
- 210.Fucidin (fusidic acid) suspension [product information]. Victoria: CSL Biotherapies; 2012.
- 211.Itraconazole (prescribing information). Research Triangle Park: Stiefel Laboratories, Inc.; 2010.
- 212.Noxafil (posaconazole) oral suspension [prescribing information]. Whitehouse Station: Schering Corporation; 2012.
- 213.Vfend (voriconazole) tablets [prescribing information]. New York: Pfizer, Inc.; 2003.
- 214.Cancidas (caspofungin acetate) for injection [prescribing information]. Whitehouse Station: Merck Sharp & Dohme Corp.; 2014.
- 215.Mycamine (micafungin sodium) for injection [prescribing information]. Northbrook: Astellas Pharma US, Inc.; 2013.
- 216.Eraxis (anidulafungin) for injection [prescribing information]. New York: Roerig; 2012.
- 217.Revatio (sildenafil) tablets [prescribing information]. New York: Pfizer Labs; 2014.
- 218.Cialis (tadalafil) tablets [prescribing information]. Indianapolis: Lilly USA, LLC; 2014.
- 219.Levitra (vardenafil hydrochloride) tablets [prescribing information]. Research Triangle Park: GlaxoSmithKline; 2014.
- 220.Stendra (avanafil) tablets [prescribing information]. Mountain View: Vivus, Inc.; 2014.
- 221.Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, et al. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117:10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 222.Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, et al. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004;32:1201–1208. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- 223.Lanzafame M, Trevenzoli M, Faggian F, Marcati P, Gatti F, Carolo G, et al. Interaction between levothyroxine and indinavir in a patient with HIV infection. Infection. 2002;30:54–55. doi: 10.1007/s15010-002-2092-3. [DOI] [PubMed] [Google Scholar]
- 224.Badri P, Dutta S, Coakley E, Cohen D, Ding B, Podsadecki T, et al. Pharmacokinetics and dose recommendations for cyclosporine and tacrolimus when coadministered with ABT-450, ombitasvir, and dasabuvir. Am J Transplant. 2015;15(5):1313–22. doi: 10.1111/ajt.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Menon R, Badri P, Das U, Wang T, Polepally A, Khatri A, et al. Drug-drug interactions with direct acting antiviral combination therapy of ABT-450/r, ombitasvir and dasabuvir. In: AASLD/EASL Special Conference on Hepatitis C. New York; 2014.
- 226.Mensing S, Sharma S, Eckert D, Polepally A, Khatri A, Podsadecki T, et al. Pharmacokinetics of paritaprevir, ombitasvir, dasabuvir, ritonavir and ribavirin in subjects with HCV genotype 1 infection in phase 3 studies. J Hepatol. 2015;62:S644. doi: 10.1016/S0168-8278(15)31023-0. [DOI] [Google Scholar]
- 227.Polepally AR, Badri P, Coakley EP, Parikh A, Rodrigues L, Jr., DaSilva-Tillmann BA, et al. Effect of comedications on paritaprevir, ritonavir, ombitasvir, dasabuvir and ribavirin pharmacokinetics. In: 16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. Washington, DC; 2015.