Abstract
Background
Iron deficiency is very common in a number of medical conditions. Ferric carboxymaltose is a new stable iron preparation that can be administered in single infusions over short periods of time. The aim of this study was to conduct a systematic review of randomised controlled trials (RCTs) regarding the efficacy and safety of the novel complex compared with other iron formulations. In addition, the feasibility of a network meta-analysis for indirect comparisons was investigated.
Methods
A systematic literature review was performed for published RCTs on the use of ferric carboxymaltose in iron deficiency between July and October 2014. Indirect comparisons were also addressed using terms referring to competing iron formulations. We further supported the qualitative results of the systematic review by a network meta-analysis that allows pooling the evidence around different intervention outcomes in the absence of trials involving a direct comparison.
Results
The initial search yielded 1027 citations, which was decreased to 21 studies eligible for inclusion in the review. Studies were heterogeneous in the number of patients randomised, iron deficiency-related conditions addressed, trial inclusion criteria, time horizon, treatment dosage and outcomes assessed. Six studies with the same time horizon (i.e. 6 weeks) were included in the network meta-analysis. Considering the differences between final and initial outcome values for each iron formulation, the mean difference of these differences (delta) was estimated for each couple of treatments involving ferric carboxymaltose. Significant improvements in serum ferritin (µg/l) were obtained with ferric carboxymaltose compared to oral iron (delta 172.8; 95 % CI 66.7–234.4) and in haemoglobin (g/dl) with respect to ferric gluconate (delta 0.6; 95 % CI 0.2–0.9), oral iron (delta 0.8; 95 % CI 0.6–0.9) and placebo (delta 2.1; 95 % CI 1.2–3.0).
Conclusions
All currently available intravenous iron preparations appear to be safe and effective, but ferric carboxymaltose seems to provide a better and quicker correction of haemoglobin and serum ferritin levels in iron-deficient patients.
Key Points
| Ferric carboxymaltose is a new intravenous iron formulation that allows the administration of high doses of iron over a limited time. Although it was proved to be effective and safe in different clinical studies, extensive use in iron-deficient patients was not supported by strong scientific evidence. |
| The aim of this study was to conduct a systematic review and network meta-analysis of randomised controlled trials to combine the highest quality (direct and indirect) evidence regarding the efficacy and safety of ferric carboxymaltose compared with other available iron formulations. |
| Ferric carboxymaltose seems to provide a better and more rapid correction of haemoglobin and serum ferritin levels in iron-deficient patients compared to other iron formulations. |
Introduction
Iron deficiency (ID) is the most common nutritional disorder in the world affecting most preschool children and pregnant women in developing countries and at least 30–40 % in industrialised countries [1, 2]. This condition also occurs frequently across multiple therapeutic areas and especially in patients with chronic disease (such as inflammatory bowel disease and chronic kidney disease). ID can result from chronic blood loss, decreased dietary intake, reduced intestinal absorption, or impaired use of endogenous iron due to chronic inflammations [3]. Common symptoms that may result from ID are fatigue, susceptibility to stress, lack of concentration and underperformance. ID is also associated with increased risk of infections [3], besides representing the most common cause of anaemia worldwide. In particular, the WHO defines anaemia as haemoglobin (Hb) <12 g/dl in non-pregnant women and <13 g/dl in men [2]. In developed countries, iron deficiency anaemia (IDA) occurs in 2–5 % of adult men and postmenopausal women and represents a common cause of hospitalisation, morbidity and quality-of-life impairment [4].
Treatment for anaemic patients should involve prompt iron replacement plus diagnostic steps directed towards identifying the underlying cause of IDA [5]. Non-anaemic subjects with low serum ferritin concentration with symptoms of fatigue may also benefit from iron therapies [6]. Oral iron supplementation is usually the first treatment choice for iron repletion; however, intravenous iron may be better suited to those patients who are unable to tolerate oral iron intake due to gastrointestinal side effects or whose chronic iron loss exceeds the replacement rate achievable with oral therapy [7]. Parenteral iron formulations are also prescribed when there is a need for rapid delivery of iron as in pregnancy or following traumas [7–9] and in any situations where blood transfusions should be avoided [10, 11].
The first iron intravenous preparations were associated with acute toxicity deriving from the release of free iron. Nowadays, all parenteral therapies are formulated so that each iron particle is surrounded by a carbohydrate molecule which allows a slow release of iron and limits toxicity. Current intravenous iron formulations include high or low molecular weight iron dextran, ferric gluconate, iron sucrose and, very recently, ferric carboxymaltose [12]. They all share the same structure, but differ from each other by the size of the core and the identity and density of the surrounding carbohydrate.
Ferric carboxymaltose is an innovative, intravenous iron preparation surrounded by a non-dextran carbohydrate shell—the stable ferric carboxymaltose complex, which allows the release of iron in a controlled manner. Thanks to this intrinsic property, ferric carboxymaltose can be administered in a short period of time (15 min) and at large doses (up to 750 mg in the USA and 1000 mg in EU) ensuring the amount of iron needed to promptly relieve patients from the debilitating effects of ID [13]. Ferric gluconate was approved in 2002 for use in patients undergoing haemodialysis and rapidly replaced iron dextran due to severe adverse events (AEs; i.e. anaphylaxis reactions) associated with the latter formulation; its recommended dose for adult patients is 125 mg per treatment (from the leaflet [14]). Iron sucrose represents another intravenous alternative to treat haemodialysis-related IDA; iron sucrose can be safely administered as a bolus infusion over 2 min or as a short infusion for doses up to 300 mg. A last option, ferumoxytol, is available to treat adult patients with IDA associated with chronic kidney disease in the USA; however, since it may cause serious hypersensitivity reactions (including death), from 2014 this drug is no longer authorised in Europe.
In synthesis, current treatments with intravenous iron either risk anaphylaxis when using iron dextran or require multiple injections of low doses when using non-dextran-containing agents (i.e. ferric gluconate and iron sucrose). Although ferric carboxymaltose appears as an attractive option in terms of both efficacy and safety, a widespread use of this formulation is not yet supported by a high level of evidence. Moore and colleagues [15] examined the available trials of intravenous ferric carboxymaltose using details from both published and unpublished literature. The study increased the scientific evidence supporting recommendations for intravenous iron treatments—and for ferric carboxymaltose in particular—versus oral iron, but also highlighted the paucity of trial data comparing different parenteral iron preparations [15].
The aim of this study was to conduct a systematic review and perform a network meta-analysis (NMA) of randomised controlled trials (RCTs) to combine the highest quality evidence regarding the efficacy and safety of the novel iron complex (ferric carboxymaltose) compared (directly or indirectly) to other existing (intravenous and oral) formulations.
Methods
The present review was conducted in keeping with the current guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16]. All reviewing activities were conducted by two independent reviewers (CR and MM) with disagreements resolved by consensus.
Literature Search
Literature searches were performed for published RCTs on the use of intravenous ferric carboxymaltose for the treatment of ID in any medical condition. Published studies were identified by searching PubMed, MEDLINE, EMBASE and The Cochrane Library using “ferric carboxymaltose” OR “Ferinject” OR “Injectafer” as keywords in the title, abstract or anywhere in a document. In order to perform (direct or indirect) comparisons of ferric carboxymaltose with other preparations, terms referring to competing iron formulations (i.e. “Ferlixit” OR “ferric gluconate”; “Venofer” OR “iron sucrose”; “oral iron” OR “ferrous sulphate”) were included in the query. Due to current restrictions involving the prescription of ferumoxytol, this intravenous iron formulation was not considered in the analysis. The search was limited to RCTs using highly sensitive filters; electronic searches were conducted originally between July and October 2014. No language or journal restriction was enforced in order to minimise the risk of publication bias.
Studies combining the administration of iron and erythropoiesis-stimulating agents were excluded from the analysis in order to evaluate the effect of iron treatment alone. Moreover, trials addressing anaemia following surgical interventions or studies not reporting the haematopoietic response among the outcomes were not considered.
Data Extraction
The following data were extracted from each study: first author’s last name, publication year, title, study horizon, number of participants (per arm), ID-related medical condition, intervention and comparator, drug dosage, post-intervention efficacy [i.e. Hb, serum ferritin, transferring saturation (TSAT)] and safety (i.e. number and type of AEs) outcome values.
Quality Assessment
The evaluation of potential biases in the selected studies is an essential element of a systematic literature review or meta-analysis. The internal validity of the eligible studies was assessed according to the Cochrane Collaboration’s Risk of Bias tool in Review Manager (RevMan 5—http://tech.cochrane.org/revman). The risk of bias assessment was performed with reference to the following domains: sequence generation; allocation concealment; blinding of participants and personnel and outcome assessors; blinding of outcome assessment; incomplete outcome data; selective outcome reporting.
Outcome Measures
The primary efficacy endpoint to be evaluated in the included studies was the haematopoietic response, usually defined as the improvement in Hb levels (g/dl) achieved by the two (or more) iron formulations compared. Secondary outcomes were the proportion of patients achieving correction (or avoiding a recurrence) of IDA, time to reach the target haematopoietic response (Hb ≥12 g/dl), increase in TSAT (percentage) and serum ferritin (ng/ml), and improvement in symptoms of ID-related diseases. Safety outcomes as the proportion of study participants reporting serious or mild treatment-related AEs were also included.
Categorical variables were described as absolute (and percentage) frequencies and continuous variables as mean (±standard deviation).
Network Meta-analysis
In order to quantitatively support the findings of the systematic review, a network meta-analysis (NMA) was conducted using data extracted from the RCTs identified through the same literature search.
Systematic reviews of RCTs are considered the standard basis in evidence-based medicine to inform clinical treatment guidelines and reimbursement policies. Many systematic reviews use meta-analysis to combine quantitative results of comparable studies and summarise the available evidence [17]. In the absence of trials involving a direct comparison of all the treatments of interest, an indirect comparison can represent an effective alternative to generate enough evidence to select the best treatment option [18]. NMA is a generalization of standard pairwise meta-analysis that allows pooling both the direct and the indirect evidence available for a given intervention. In this way, it is possible to obtain a more precise estimate of the relative effect for each pair of treatments considered [19, 20].
In the past few years, NMAs have been increasingly adopted for comparing healthcare interventions [21–23] in different therapeutic areas and, subsequently, endorsed by several health-technology assessment bodies [e.g. Canadian Agency for Drugs and Technologies in Health, National Institute for Health and Clinical Excellence (NICE; UK)].
A Bayesian NMA implemented by using either fixed or random effects [24] has been carried out in WinBUGS [25]. The choice between the two models has been addressed by comparing the associated information criterion [i.e. the Deviance Information Criterion (DIC)], where the lower the value, the better the fit of the model to the data.
Results
Study Selection
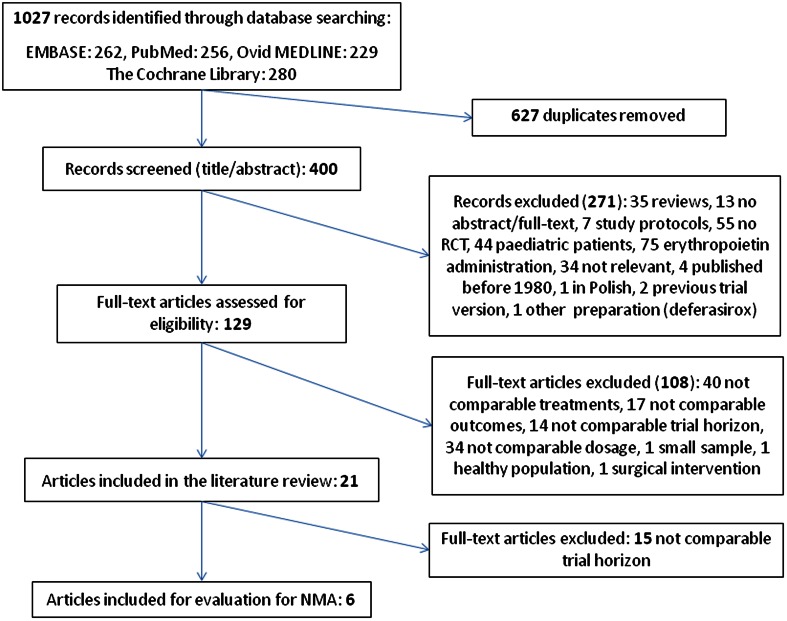
The initial search from multiple databases yielded 1027 citations, decreasing to 400 after removal of duplicates. A subsequent review of the abstracts yielded 129 articles that were evaluated in depth; eventually, 21 studies were eligible for inclusion in our systematic review (Fig. 1).
Fig. 1.
Study selection process
Study Characteristics
The characteristics of the 21 studies included in the systematic review are summarised in Table 1. All were RCTs comparing iron treatments in anaemic (or non-anaemic) patients requiring therapies for ID. Studies were published between 2003 and 2014. The time horizon of each trial spanned between 2 weeks [8, 26] and 9 months [27]. The number of patients enrolled ranged between 18 [26] and 2584 [28], averaging at 472 subjects (±703). The vast majority of studies (n = 16) [8, 9, 12, 28–39] recruited adult patients (≥18 years) with anaemia (i.e. Hb <13 g/dl in men and <12 g/dl in women), although the haematological inclusion criteria varied among studies. Indeed, in some articles the target population included patients with severe IDA (i.e. Hb <10.5 g/dl) only [7, 9, 32, 38], while in others [33] these severe cases were explicitly excluded from the analysis. Two studies enrolled premenopausal non-anaemic women with symptoms of fatigue and low ferritin levels (≤50 ng/ml) [6, 40], while two others [26, 41] addressed a neurological disorder (i.e. the restless legs syndrome) which may be caused or worsened by ID. Finally, one study [42] evaluated IDA recurrence in patients with inflammatory bowel disease whose Hb levels where above IDA upper limits at the time of study entry.
Table 1.
Main features and results of the 21 randomised controlled trials included in the systematic review
| Study (year) | Patients randomised | Medical condition | Clinical inclusion criteria | Intervention drug (dosage) | Comparator drug (dosage) | Time horizon | Efficacy outcome(s) | Safety outcome |
|---|---|---|---|---|---|---|---|---|
| Agarwal et al. (2006) [29]a | 89 | Chronic kidney disease | Hb <12 g/dl; serum ferritin <100 ng/ml and or TSAT <20 % | Intravenous ferric gluconate (250 mg; weekly; 4 weeks) | Oral ferrous sulphate (325 mg; 3 times daily; 6 weeks) | 43 days | Increase in Hb levels (0.4 g/dl in ferric gluconate and 0.2 g/dl in ferrous sulphate) was statistically significant (p <0.01) for intravenous iron only. Compared to ferrous sulphate, ferric gluconate achieved greater improvements in ferritin (232.0 ± 160.8 vs. 55.9 ± 236.2 g/dl) and TSAT (8.3 ± 7.5 vs. 2.9 ± 8.8%, p = 0.007) | Intravenous ferric gluconate: drug-related AEs occurred in 13 out of 44 patients (three of which designated as serious AEs). Hypotension and nausea occurred in six (out of 44) subjects, while vomiting was seen in five and diarrhoea in two. Oral ferrous sulphate: drug-related AEs occurred in nine of 45 patients Constipation was the most frequent effect (four of 45 subjects) followed by dark stools (three patients), nausea (two patients), and diarrhoea (two patients). No serious AEs were seen in this group |
| Bager et al. (2014) [30] | 97 | Non-variceal acute upper gastrointestinal bleeding | Hb <12 g/dl for women and <13 g/dl for men | Intravenous ferric carboxymaltose (1000 mg; single infusion) | Oral ferrous sulphate (100 mg; twice daily; 3 months) or intravenous placebo (saline) | 13 weeks | At week 13, mean Hb (g/dl) in ferric carboxymaltose group was 13.9 (13.4–14.3) vs. 13.5 (12.9–14.1) in oral iron and 11.5 (10.3–12.9) in placebo (p < 0.01) | Re-admission for bleeding was reported in 2.4 % of patients in both the oral and intravenous groups (p = NS). Admission due to cardiovascular symptoms was reported in 4.9, 4.8 and 7.1 % of oral, intravenous and placebo groups, respectively (p = NS) |
| Bailie et al. (2010) [8] | 559 | Iron deficiency anaemia (various conditions) | Hb <12 g/dl; TSAT ≤25 %; serum ferritin <300 ng/ml (CKD and IBD patients) or ≤100 ng/ml (other) | Intravenous ferric carboxymaltose (1000 mg; single infusion) | Intravenous placebo (normal saline, 100–250 ml) | 14 days | Not reported | During the first 24 h of the treatment, at least one TEAE was experienced by 15.0 % of subjects after receiving ferric carboxymaltose and 11.4 % after receiving placebo (p = 0.066). During the 7-day treatment period, at least one TEAE was experienced by 29.3 % of the subjects after receiving ferric carboxymaltose and 19.7 % after receiving placebo (p < 0.001). The most common events in the ferric carboxymaltose group were headache (5.4 %), nausea (3.8 %) and dizziness (1.8 %) |
| Breymann et al. (2008) [9] | 349 | Post-partum anaemia | Hb ≤10.5 g/dl | Intravenous ferric carboxymaltose (1000 mg; up to 3 weekly doses) | Oral ferrous sulphate (100 mg; twice per day; 12 weeks) | 12 weeks | At week 12, Hb levels increased to a mean of 130.4 g/l in ferric carboxymaltose vs. 128.9 g /l in ferrous sulphate (p = NS). Mean ferritin was 161.2 µg/l vs. 43.3 µg/l (p < 0.0001) | Overall, AEs were experienced by 26.0 % in ferric carboxymaltose vs. 22.2 % in ferrous sulphate (p = 0.51). Statistically significant differences (ferric carboxymaltose vs. ferrous sulphate) were seen in gastrointestinal disorders (3.5 vs. 10.3 %; p = 0.015), general disorders and administration site conditions (6.2 vs. 0.0 %; p = 0.003), and musculoskeletal and connective tissue disorders (0.0 vs. 2.6 %; p = 0.039). Constipation was less common in ferric carboxymaltose group (0.4 vs. 6.8 %). Two patients in ferric carboxymaltose group and none in the control group experienced severe AEs (i.e. hypersensitivity). There were no cases of anaphylactic shock/reaction |
| Coyne et al. (2003) [27] | 144 (dextran-sensitive) and 2194 (dextran-tolerant) | Haemodialysis | Not reported | Intravenous ferric gluconate (12 mg; single infusion) | Placebo (bacteriostatic saline, 10 ml) | 9 months | Not reported | Among 143 iron dextran-sensitive patients exposed to ferric gluconate, three (2.1 %) had suspected allergic events, including one serious reaction (0.7 %). One patient (0.7 %) had a suspected allergic reaction after placebo. In contrast, among 2194 iron dextran-tolerant patients, ferric gluconate intolerance occurred in seven patients (0.3 %; p = 0.020), including five (0.2 %) who had suspected allergic events (p = 0.010), but none who had serious events (0.0 %; p = 0.061). Two iron dextran-tolerant patients (0.09 %) had allergic-like reactions following placebo |
| Earley et al. (2009) [26] | 18 | Idiopathic restless legs syndrome | Periodic leg movements of sleep (PLMS) >15/h | Intravenous iron sucrose (500 ml; two infusions) | Intravenous placebo (500 ml; two infusions) | 2 weeks | Iron treatment resulted in a small but significant (p = 0.0001) increase in ferritin and a decrease in disease severity | The most commonly reported treatment-related AEs were oedema in either hands or feet (36 %) and nausea or vomiting (36 %), followed by hypotension (18 %), dizziness (18 %) and abdominal pain (9 %). All the reported AEs occurred during treatment and resolved within minutes to hours of completing the infusion. No AEs were reported at 2-week follow-up after the iron treatment |
| Evstatiev et al. (2013) [42] | 256 | Inflammatory bowel disease | Hb ≥12 g/dl in women and ≥13 g/dl in men | Intravenous ferric carboxymaltose (500 mg; single infusion) | Placebo (saline solution; 500 mg) | 8 months | Anaemia recurred in 26.7 % of patients given ferric carboxymaltose vs. 39.4 % given placebo. Time to anaemia recurrence was longer in the ferric carboxymaltose group (p = 0.049). Ferritin and TSAT increased by 30.3 µg/l and 0.6 %, respectively, in ferric carboxymaltose group; the same parameters decreased by 36.1 µg/l and 4.0 %, respectively, in placebo group | AEs were reported in 59.0 % in ferric carboxymaltose group vs. 50.5 % of placebo group, and serious AEs in 6.7 and 8.1 %, respectively. The most common AEs were ulcerative colitis-specific symptoms and nasopharyngitis (7.8 vs. 7.3 %). Gastrointestinal symptoms and flares of ulcerative colitis were somewhat less frequent with ferric carboxymaltose compared with placebo: 20.0 vs 28.3 % (p = 0.17) and 6.7 vs. 12.1 % (p = 0.18), respectively. No anaphylactic reaction was reported and no death occurred |
| Evstatiev et al. (2011) [31] | 485 | Inflammatory bowel disease | Hb = 7–12 g/dl (women) and 7–13 g/dl (men) | Intravenous ferric carboxymaltose (500 or 1000 mg; up to 3 infusions) | Intravenous iron sucrose (200 mg; up to 11 infusions, twice weekly) | 12 weeks | More patients with ferric carboxymaltose than iron sucrose achieved Hb response (65.8 vs. 53.6 %; p = 0.004) or Hb normalisation (72.8 vs. 61.8 %; p = 0.015) | The frequency of treatment-related AEs was comparable between the two groups (p = 0.413). One treatment-related serious AE (i.e. pulmonary embolism) was reported in ferric carboxymaltose group. Skin and subcutaneous tissue disorders such as rash, dermatitis and pruritus were reported in 3.7 % of ferric carboxymaltose patients and 0.8 % of the iron sucrose group (p = 0.063). No true hypersensitivity reactions were reported |
| Favrat et al. (2014) [40] | 294 | Fatigue in premenopausal, non-anaemic women | Serum ferritin <50 µg/l and TSAT <20 %; Hb ≥115 g/l. Piper Fatigue Scale (PFS) score ≥5 | Intravenous ferric carboxymaltose (1000 mg; single infusion) | Intravenous placebo (saline, 1000 mg) | 56 days | Fatigue was reduced in 65.3 % (ferric carboxymaltose) and 52.7 % (placebo) of patients (OR = 1.68; p = 0.03). All ferric carboxymaltose -treated patients (vs. 86 % in placebo group) had haemoglobin levels ≥120 g/l | TEAEs were experienced by 57.2 % of ferric carboxymaltose and 49.0 % of placebo-treated patients (p = 0.16). Five ferric carboxymaltose -treated patients reported severe AEs (in four, the events were considered drug-related) |
| Grote et al. (2009) [41] | 60 | Restless legs syndrome | Serum ferritin <30 µg/l; International Restless Legs Study Group Rating Scale (IRLS) ≥10 | Intravenous iron sucrose (200 mg; 5 infusions in 3 weeks) | Placebo (saline, 1000 mg) | 11 weeks | Median IRLS score decreased from 24 to 7 in iron sucrose group vs. from 26 to 17 in placebo group (p = 0.123, NS). Serum ferritin increased from 20.1 to 118.4 µg/l and Hb from 129.3 to 134.5 g/l in the iron sucrose group; no changes were observed after placebo | In the iron sucrose group the most common AEs were dysaesthesia (7.1 %), taste perversion (4.8 %), headache (9.5 %). In the placebo group they were: headache (11.9 %), nausea and urticaria (4.8 %) |
| Krayenbuehl et al. (2011) [6]a | 90 | Fatigue in premenopausal, non-anaemic women | Serum ferritin ≤50 ng/ml; Hb ≥120 g/l | Intravenous iron sucrose (200 mg; twice weekly for 2 weeks) | Intravenous placebo (saline, four infusions of 200 ml) | 6 weeks | Improvement in fatigue was reported by 65 % of iron-treated and 40 % of placebo-treated patients (p = 0.02). In iron-treated patients, a significant increase in serum ferritin concentration (98 vs. 1 ng/ml; p < 0.001) and TSAT (9 vs. 2 %; p = 0.006) was observed compared to placebo-treated patients | Drug-associated AEs were observed in 21 % of iron-treated patients and in 7 % of placebo-treated patients (p = 0.05) |
| Kulnigg et al. (2008) [32] | 200 | Inflammatory bowel disease | Hb ≤10 g/dl; TSAT <20 % or serum ferritin <100 µg/l | Intravenous ferric carboxymaltose (maximum 1000 mg repeated weekly until deficit was corrected) | Oral ferrous sulphate (100 mg; twice daily; 12 weeks) | 12 weeks | The median Hb improved from 8.7 to 12.3 g/dl (ferric carboxymaltose) vs. from 9.1 to 12.1 g/dl (ferrous sulphate, p = 0.70). Median ferritin increased from 5.0 to 43.5 µg/l (ferric carboxymaltose) vs. from 6.5 to 28.5 µg/l (ferrous sulphate) | Treatment-related AEs occurred in 28.5 % (ferric carboxymaltose) and 22.2 % (ferrous sulphate) of patients, respectively. Most commonly reported (>2 % of patients overall) treatment-related AEs for ferric carboxymaltose and ferrous sulphate respectively were abdominal pain (2.9 vs. 3.2 %), nausea (2.2 vs. 4.8 %), headache (2.9 vs. 1.6 %) and diarrhoea (0.7 vs. 6.3 %) |
| Kulnigg-Dabsch et al. (2013) [33]a | 25 | IBD-related thrombocytosis | Platelet count >450 g/l; Hb >10.5 g/dl (no severe anaemia); TSAT <20 % or ferritin <100 µg/l | Intravenous ferric carboxymaltose (500, mg; once weekly for 3 weeks) | Intravenous Placebo | 6 weeks | Mean platelet counts dropped on ferric carboxymaltose but not on placebo treatment (p = 0.0024). Hb, TSAT and ferritin levels improved in the ferric carboxymaltose but not in the placebo group | Fourteen AEs were reported in nine patients (34.6 %), 11 in ferric carboxymaltose patients and three in the placebo group. Five effects were rated as serious: bowel obstruction, deterioration of ulcerative colitis, systemic inflammatory response syndrome, sepsis-associated heart failure and hospitalization because of fistula |
| Onken et al. (2014) [28] | 2584 | Non-dialysis-dependent CKD | Hb ≤11.5 g/dl; glomerular filtration rate (GFR) <60 ml/min/1.73 m2 | Intravenous ferric carboxymaltose (750 mg; twice weekly) | Intravenous iron sucrose (200 mg; five infusions in 14 days) | 56 days | The mean Hb increase was 1.13 g/dl (ferric carboxymaltose) vs. 0.92 g/dl (iron sucrose; 95 % CI 0.13–0.28). More patients in the ferric carboxymaltose group achieved Hb increase ≥1.0 g/dl (48.6 vs. 41.0 %; 95 % CI 3.6–11.6) | During the study, at least one drug-related TEAE occurred in 298 of 1276 (23.4 %) subjects in ferric carboxymaltose group and 202 of 1285 (15.7 %) subjects in the iron sucrose group. The most common events were nausea (8.6 % in ferric carboxymaltose group vs. 1.6 % in iron sucrose), hypertension (4.6 vs. 2.0 %), flushing (3.0 vs. 0.1 %), dizziness (2.4 vs. 1.2 %) and dysgeusia (2.4 vs. 1.2 %). The majority of drug-related TEAEs were mild or moderate in severity. At least one serious AE was experienced by 202 of 1276 (15.8 %) participants receiving ferric carboxymaltose and 197 of 1285 (15.3 %) participants receiving iron sucrose (p = 0.74), with congestive heart failure being the most commonly reported (2.4 vs. 2.3 %; p = 0.90) |
| Onken et al. (2014) [13] | 507 (A vs. B) 504 (C vs. D) |
Iron deficiency anaemia (various conditions) | Hb ≤11 g/dl; ferritin <100 ng/ml (or <300 ng/ml when TSAT <30 %) | (A) or (C) intravenous ferric carboxymaltose (750 mg; twice weekly) | (B) Oral ferrous sulphate (325 mg; three times a day; 14 days); (D) intravenous iron standard-of-care (IVSC) | 35 days | Mean (±SD) Hb was significantly higher in group A than in group B: 1.57 (±1.19) vs. 0.80 (±0.80) g/dl (p = 0.001). Group C vs. group D: Hb = 2.90 (±1.64) vs. 2.16 (±1.25) g/dl (p = 0.001) | During the treatment phase, at least one drug-related TEAE was experienced by 22.8 % of participants in Group A, 6.3 % in Group B, 25.3 % in Group C, and 26.5 % in Group D. The most common events were hypophosphatemia (Group C 5.5 %; Group A 3.7 %), nausea (Group A 4.1 %; Group D 3.3 %), protocol-defined hypotension (Group D 3.7 %), and constipation (Group B 3.2 %). Serious events were described in eight (3.3 %) patients in group A, 10 (4.0 %) in group B, 17 (6.7 %) in group C, and 16 (6.5 %) in group D. Hypersensitivity events were reported in eight participants: three (0.8 %) were in group A, two (0.8 %) were in group C and six (2.4 %) in group D |
| Quinibi et al. (2011) [34] | 255 | Non-dialysis-dependent CKD | Hb ≤11 g/dl; TSAT ≤25 %; serum ferritin ≤300 ng/ml; glomerular filtration rates ≤45 ml/min/1.73 m2 | Intravenous ferric carboxymaltose (1000 mg; up to two additional doses at two-week intervals) | Oral ferrous sulphate (325 mg; 3 times daily; 56 days) | 56 days | Mean increase in Hb was 0.95 ± 1.12 (ferric carboxymaltose) vs. 0.50 ± 1.23 g/dl (ferrous sulphate; p = 0.005); mean increase in ferritin was 432 ± 189 vs. 18 ±45 ng/ml (p < 0.001); mean increase in TSAT was 13.6 ± 11.9 % vs. 6.1 ± 8.1 % (p < 0.001) | The proportion of subjects who experienced at least one possibly drug-related AE was significantly lower in ferric carboxymaltose group compared with the oral iron group: 2.7 % in ferric carboxymaltose group and 26.2 % in the oral iron group (p = 0.0001). The most commonly AEs the ferric carboxymaltose group were peripheral oedema (6.1 %), hyperkalaemia (4.1 %), urinary tract infection (3.4 %), hypotension (3.4 %), bronchitis, headache and infusion site reaction (2.0 % each). The most commonly experienced AEs in the oral iron group were constipation (17.5 %), nausea (4.9 %), diarrhoea, upper respiratory tract infection (3.9 % each), discoloured faeces and gastrointestinal haemorrhage (2.9 % each). Serious AEs were recorded in 13 (8.8 %) subjects in ferric carboxymaltose group and ten (9.7 %) in the oral iron group |
| Schatz et al. (2013) [35] | 50 | Apheresis-related anaemia | Serum ferritin <100 µg/l; or ferritin <300 µg/l; TSAT <20 % | Intravenous ferric carboxymaltose (500–1000 mg; single infusion) | Intravenous ferric gluconate (62.5 mg; once weekly until deficit was corrected) | 8 weeks | Serum ferritin (mean) at week 8: 117.9 µg/l in ferric gluconate vs. 140.2 µg/l in ferric carboxymaltose (p = 0.4). TSAT: 22 % (ferric gluconate) vs. 26.4 % (ferric carboxymaltose; p = 0.02) | There were no serious AEs in either group, especially no anaphylactic shocks. Six patients per group showed AEs, mainly gastrointestinal distress and flu-like symptoms. All AEs were mild, transient and self-limiting. There were no injection site reactions in either group |
| Seid et al. (2008) [36]a | 289 | Post-partum anaemia | Hb ≤10 g/dl | Intravenous ferric carboxymaltose (1000 mg; weekly for a maximum of 2500 mg) | Oral ferrous sulphate (325 mg; 3 times daily; 6 weeks) | 6 weeks | Ferric carboxymaltose -treated patients were significantly more likely to achieve Hb >12 g/dl (91 vs. 67 %; p < 0.0001) in a shorter time period (14 vs. 27 days; p < 0.0002) and attain higher TSAT (36 vs. 28 %) and ferritin (647 vs. 12 ng/ml) levels (p < 0.0001) | Patients reporting one or more drug-related AEs were lower in ferric carboxymaltose (10.6 %) than in oral ferrous sulphate (21.8 %) group. Urticaria (2 %) was the only AE occurring in ≥2 % of the ferric carboxymaltose -treated patients. Four (2.8 %) subjects in ferric carboxymaltose group and four (2.7 %) subjects in the oral iron group experienced at least one serious AE, none of which was considered to be related to study medication or led to premature discontinuation |
| Toblli et al. (2007) [37] | 40 | Chronic heart failure and chronic renal failure | Hb ≤12.5 g/dl; TSAT <20 %; ferritin <100 ng/ml; creatinine clearance <90 ml/min; left ventricular ejection fraction ≤35 % | Intravenous iron sucrose (200 mg weekly; 5 weeks) | Placebo (saline solution, 200 mg) | 6 months | The treatment group showed better haematological values: mean (±SD) Hb was 11.8 (±0.7) vs. 9.8 (±0.6) g/dl in placebo; mean ferritin: 240.4 (±55.6) ng/ml vs. 78.9 (±30.1); TSAT (%): 0.25 (±0.04 %) vs. 0.20 (±0.01) | Therapy with iron sucrose was well tolerated in all patients, and there were no side effects reported in the two groups throughout the study |
| Van Wyck et al. (2009) [39]a | 477 | Uterine bleeding | Hb ≤11 g/dl; serum ferritin ≤100 ng/ml; TSAT ≤25 % | Intravenous ferric carboxymaltose (maximum 1000 mg repeated weekly to achieve a total replacement dose) | Oral ferrous sulphate (325 mg; 3 times daily; 6 weeks) | 6 weeks | Compared to ferrous sulphate, more patients assigned to ferric carboxymaltose achieved correction (Hb ≥12 g/dl) of anaemia (73 % vs. 50 %; p < 0.001) and experienced greater improvement in symptoms of fatigue (p < 0.05) | No hypotensive or serious drug-related AEs were reported in either treatment group. There were no deaths. Patients assigned to oral iron therapy were more likely to experience drug-related gastrointestinal complaints, particularly constipation (14.2 vs. 3.0 %), diarrhoea (4.4 vs. 1.7 %), nausea (11.9 vs. 3.5 %), and vomiting (3.1 vs. 0.4 %). Patients assigned to intravenous iron therapy were more likely to report transient fatigue (2.2 vs. 0 %), headache (6.5 vs. 4.4 %), dizziness (2.2 vs. 0.4 %), dysgeusia (2.6 vs. 0.9 %), and rash (2.2 vs. 0 %) |
| Van Wyck et al. (2007) [38]a | 352 | Post-partum anaemia | Hb ≤10 g/dl | Intravenous ferric carboxymaltose (maximum 1000 mg repeated weekly to achieve a total replacement dose) | Oral ferrous sulphate (325 mg; 3 times daily; 6 weeks) | 6 weeks | Patients assigned to intravenous ferric carboxymaltose compared with those assigned to oral iron were more likely to achieve Hb >12 g/dl (90.5 vs. 68.6 %; p < 0.001) | No serious drug-related AEs occurred in either treatment group. Patients assigned to oral iron therapy were more likely to report gastrointestinal complaints, particularly constipation (11.2 vs. 3.4 %; p = 0.07), diarrhoea (3.9 vs. 0 %; p = 0.015) and nausea (7.3 vs. 1.1 %; p = 0.006) in comparison to ferric carboxymaltose. Patients assigned to intravenous ferric carboxymaltose were more likely to experience skin disorders (5.2 vs. 2.2 %; p = 0.164), principally mild pruritus and rash that usually resolved within 15 min from infusion |
AE adverse event, CKD chronic kidney disease, Hb haemoglobin, HS hydroxide sucrose, IBD inflammatory bowel disease, TEAEs treatment-emergent adverse events, TSAT transferrin saturation
aIndicates the studies included in the network meta-analysis
IDA patients across the studies were affected by a variety of conditions including inflammatory bowel disease (and related thrombocytosis), chronic kidney disease, non-variceal acute upper gastrointestinal bleeding, chronic heart failure and post-partum uterine bleeding. Other participants experienced bleeding owing to invasive techniques such as apheresis and haemodialysis.
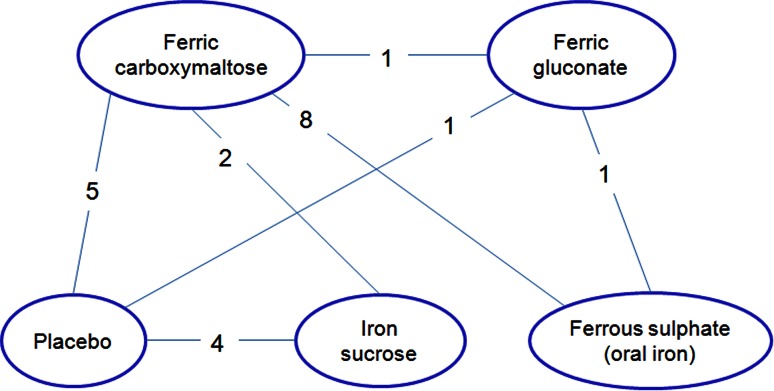
Eight trials [9, 12, 30, 32, 34, 36, 38, 39] compared intravenous ferric carboxymaltose to ferrous sulphate (oral iron) and in one of these studies [13] ferric carboxymaltose was also compared to intravenous standard of care in patients intolerant to oral iron. Since the formulation constituting the ‘standard of care’ was not specified in the article, this comparison was excluded from the analysis. In another trial [30], an additional comparison was performed between ferric carboxymaltose and intravenous placebo. In four articles [8, 33, 40, 42], ferric carboxymaltose was compared to placebo, while in two [28, 31] it was compared to iron sucrose. In two RCTs [27, 29], ferric gluconate was assessed against oral iron (ferrous sulphate) and placebo, respectively; in four studies [6, 26, 37, 41], iron sucrose was compared to placebo. Finally, one study only directly compared ferric carboxymaltose to ferric gluconate [35]. Thus, the overall number of comparisons (n = 22) exceeded the number of studies (n = 21) due to a study [30] investigating more than two formulations (Fig. 2).
Fig. 2.
Schematic representation of comparisons (n = 22) among different iron formulations addressed in the included studies (n = 21)
Efficacy Outcomes
In ten studies comparing any iron formulation versus placebo [6, 8, 26, 27, 30, 33, 37, 40–42], efficacy outcomes were always superior in the treatment group. The only study [35] directly comparing ferric carboxymaltose to ferric gluconate revealed that both iron formulations were safe and effective (only six patients per group experienced non-serious AEs), but the increase in haematological parameters was more substantial and rapid in ferric carboxymaltose. When compared to other iron formulations [9, 13, 28, 30, 31, 34, 36, 38, 39], ferric carboxymaltose performed better in the achievement of a rapid and consistent Hb response with the exception of one study [32], where the difference in median Hb between ferric carboxymaltose and oral ferrous sulphate was not statistically significant. A higher increase in serum ferritin and TSAT levels was also observed in patients receiving ferric carboxymaltose compared to other therapies. The only study assessing the performance of ferric gluconate versus oral ferrous sulphate [29] revealed greater improvements in Hb, serum ferritin and TSAT levels in ferric gluconate-treated chronic kidney disease patients than in the oral iron group.
Safety Outcomes
Overall, ferric carboxymaltose was well tolerated and associated with a minimal risk of AEs, even if some authors [8, 9, 32] reported that AEs occurred in a larger proportion of ferric carboxymaltose-treated patients than those receiving oral iron or placebo; the difference seldom reached the significance level (p < 0.05). Moreover, some studies [9, 32, 34, 36, 38, 39, 42] revealed that patients who were administered ferric carboxymaltose experienced less drug-related gastrointestinal disorders (e.g. constipation, diarrhoea, nausea and vomiting) than those treated with oral ferrous sulphate. At the same time, patients treated with ferric carboxymaltose iron were more likely to develop skin disorders (e.g. rash, dermatitis and pruritus) that usually resolved within a few minutes of the infusion [9, 31, 34, 38]. Other frequent AEs associated with ferric carboxymaltose administration were fatigue, headache and dizziness [8, 32, 34, 39]. No true cases of anaphylactic reactions were reported [9, 31, 35, 42] and no deaths occurred [39, 42] in patients treated with ferric carboxymaltose.
With reference to other intravenous formulations, the safety profile of ferric gluconate seemed to be favourable [29, 35], with few AEs reported after drug injection. Similarly, iron sucrose was well tolerated and symptoms associated with its administration were mild and resolved rapidly [26, 28, 31, 37].
Risk of Bias Assessment
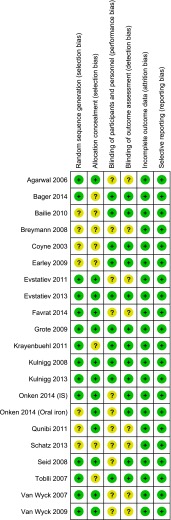
The risk of bias assessment for the included studies is presented in Figs. 3 and 4. Eight studies [9, 13, 29, 31, 34, 36, 38, 39] were open-label RCTs without blinding of patients and personnel. In two cases [28, 35] no indication on blinding was reported while in two other cases [40, 42] blinding was performed for patients only. For the latter, in one case the authors stated that the primary end-point (Hb values) was unlikely to be affected by the study personnel’s awareness of the treatment. All the remaining studies were double-blind RCTs.
Fig. 3.
Risk of bias summary: judgements regarding risks of bias for each study included in the systematic review (n = 21). The symbol ‘+’ represents low risk of bias, while the symbol ‘?’ represents unclear risk of bias
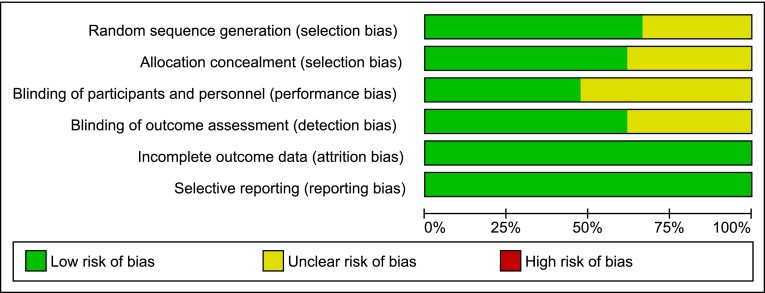
Fig. 4.
Risk of bias graph: judgements regarding risks of bias presented as percentages across all studies included in the systematic review (n = 21). This figure illustrates, for each considered bias domain, the proportion of studies falling in each category of risk (low risk of bias, high risk of bias, unclear risk of bias)
In seven cases the sequence generation process was not described [8, 9, 13, 26, 27, 34, 35], while all the other studies showed low selection biases.
Network Meta-analysis
Due to the high heterogeneity in trials included in the systematic review, the NMA was limited to those adopting a time horizon of 6 weeks (or 43 days), which was the most frequent period reported. Six [6, 29, 33, 36, 38, 39] out of 21 studies were included in the NMA (see Table 1, studies with “a” label). Studies reported a mean iron dose in the range 800—1600 mg for intravenous formulations, while a daily dose of 975 mg (i.e. 325 mg three times daily for 6 weeks) was administered for oral comparators.
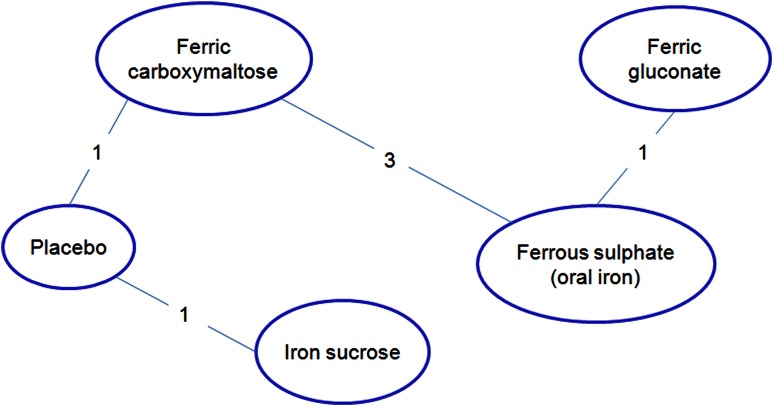
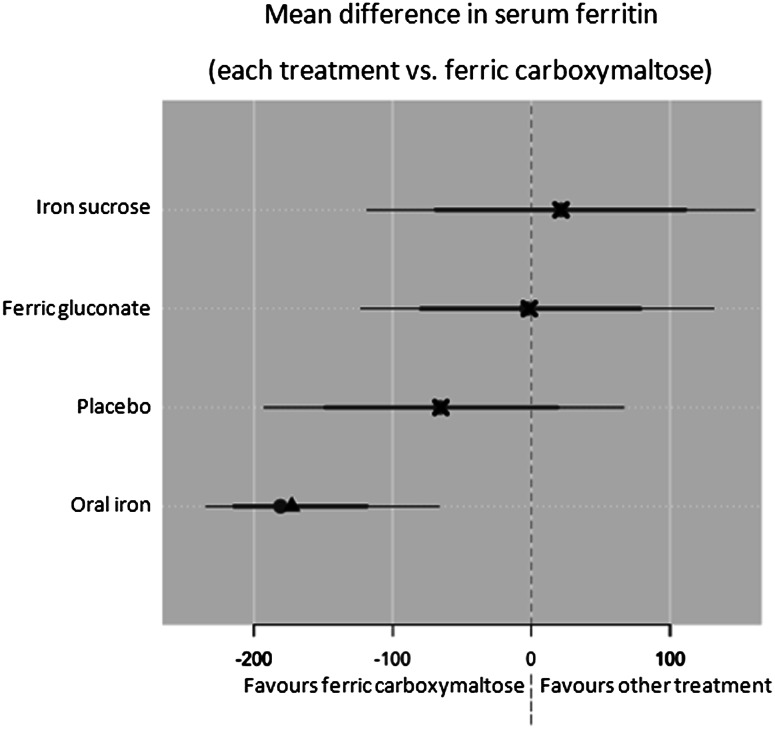
The analyses focused on the increase of Hb and serum ferritin levels after iron administration. In details, the differences between final and initial values of these parameters when using ferric carboxymaltose were compared to the differences obtained with the other iron formulations; the mean difference of these differences (‘delta’) was estimated for each couple of treatments (i.e. ferric carboxymaltose vs. competitors) across the studies.
The network of studies considered is reported in Fig. 5, where numbers indicate how many comparisons were performed across the different formulations. The best performing networks were considered: random effects model (Deviance Information Criterion: 55.86 for random effects model vs. 74.69 for fixed effects model) for the serum ferritin NMA and the fixed effects model (Deviance Information Criterion: 5.50 for fixed effects model vs. 5.91 for random effects model) for the Hb NMA.
Fig. 5.
Network of connected studies
As regards serum ferritin (µg/l), the mean difference over the study period (final value − basal value) was significantly larger for ferric carboxymaltose compared to oral iron (delta 172.76; 95 % CI 66.7–234.4) (see Fig. 6).
Fig. 6.
Network meta-analysis (NMA) results on serum ferritin. Central dots represent posterior medians, triangles and crosses represent posterior means; thin lines are 95 % credible intervals, while thicker ones are 80 % credible intervals; a triangle indicates that ferric carboxymaltose is significantly superior
Ferric carboxymaltose was superior in comparison to placebo and ferric gluconate with a delta of 65.2 (95 % CI −66.5 to 192.5) and 1.5 (95 % CI −131.4 to 122.8), respectively. Iron sucrose was superior to ferric carboxymaltose with a delta of 21.4 (95 % CI −160.7 to 118.4). None of the comparisons was statistically significant.
As regards Hb (g/dl), the mean difference over the study period (final value − basal value) was significantly larger for ferric carboxymaltose compared to ferric gluconate (delta 0.6; 95 % CI 0.2–0.9), placebo (delta 2.1; 95 % CI 1.2–3.0) and oral iron (delta 0.8; 95 % CI 0.6–0.9) (see Fig. 7). Ferric carboxymaltose was superior to iron sucrose with a delta of 1.1 (95 % CI −1.8 to 3.9) but without statistical significance.
Fig. 7.
Network meta-analysis (NMA) results on haemoglobin (Hb). Central dots represent posterior medians, triangles and crosses represent posterior means; thin lines are 95 % credible intervals, while thicker ones are 80 % credible intervals; a triangle indicates that ferric carboxymaltose is significantly superior
Discussion
Iron deficiency is a common nutritional deficit, affecting men and women of all ages, races and ethnic groups. In some cases, iron stores may be depleted so as to lead to anaemia (IDA), a severe condition associated with a number of chronic diseases (mainly of the kidney and the bowel) and particular events in women’s lives (such as pregnancy and significant menstrual blood loss in young women). Patients suffering from IDA and other ID-related conditions can benefit from iron therapy. Oral iron is the first-line treatment for most patients due to its effectiveness, safety and cost; however, this formulation presents a number of disadvantages, such as low absorption of iron and high incidence of gastrointestinal side effects. Moreover, iron stores are replenished most effectively and rapidly when intravenous iron supplementation is administered; therefore, parenteral iron administration was introduced in clinical practice to overcome limitations and risks related to oral iron.
Currently, different iron formulations for intravenous infusion are available. These products are quite similar in terms of safety profile but differ in the content and frequency of the doses administered. The present study aimed at improving the level of evidence to support the indication of the available intravenous iron formulations and, particularly, of the most recent preparation, ferric carboxymaltose, brought into clinical practice.
Twenty-one RCTs involving ferric carboxymaltose, iron sucrose and ferric gluconate were analysed in order to retrieve information on clinical efficacy and safety of the different iron formulations. Ferric carboxymaltose treatment was shown to be superior in improving IDA compared to other iron regimens. Ferric carboxymaltose superiority is justified by the large amount of iron that can be administered through a small number of injections; this leads to quicker iron replacements in the body and consequently a higher success in anaemia correction. A ferric carboxymaltose dosing regimen was also well tolerated, with a low number of AEs reported, most minor.
The present systematic literature review mainly confirms the results already published by other authors [43–45]. The early response obtained with ferric carboxymaltose (from day 7 onwards) implies a substantial improvement in time-to-response and patient convenience and emphasises the clinical relevance of this treatment. From the Patient Blood Management (PBM) perspective [46] that aims at optimising the care of patients who might need blood transfusions, ferric carboxymaltose could represent an effective alternative to transfusions in pre-operative settings or in cases of severe anaemia (Hb <8 g/dl), while in patients with non-dialysis-dependent renal failure, ferric carboxymaltose administration might avoid the use of erythropoietin-stimulating agents. In this way, PBM can improve patient outcomes and reduce healthcare costs, while ensuring that blood components are available for the patients who need them.
Due to the paucity of RCTs comparing ferric carboxymaltose with other parenteral iron formulations, a NMA was performed to combine the highest quality (direct and indirect) evidence regarding the efficacy of the novel formulation. The analyses focused on the increase of Hb and serum ferritin levels after the administration of the iron formulations considering a time horizon of 6 weeks. Mean differences in serum ferritin (µg/l) were significantly larger for ferric carboxymaltose compared to oral iron, and in Hb (g/dl) compared to oral iron, ferric gluconate and placebo. Although ferric carboxymaltose superiority over oral iron and placebo was already demonstrated in several trials [13, 30, 33, 34, 36, 38–40, 42], NMA results supported these findings comparing ferric carboxymaltose also to ferric gluconate in terms of improvements in serum ferritin levels. At present, only one trial directly compared ferric carboxymaltose to ferric gluconate [35]; this study reported a significant difference in serum ferritin levels achieved by the two preparations but over a limited period of treatment (i.e. 4 weeks).
Further RCTs are needed to establish the role of ferric carboxymaltose with respect to the other intravenous formulations in patients with IDA. These studies are essential to provide more direct evidence of the comparisons and should also focus on a short time horizon (i.e. 2 weeks) to highlight the formulations suitable to avoid blood transfusions in pre-operative settings. In the current review, only two studies [32, 36] reported high haematological responses for ferric carboxymaltose at 2 weeks, but both considered placebo as the comparator.
The present study was based on an extensive bibliographical search that entailed the inclusion of all published clinical trials addressing different intravenous formulations considering any ID-related medical condition. However, this review presents a number of limitations, including those typical of systematic searches and indirect comparisons of interventions. First, a selection bias may have occurred, since only four databases were searched and ‘grey’ literature such as unpublished studies and trial registers were omitted; the existence of some publication biases cannot be excluded either. Secondly, clinical outcomes, time horizon and treatment dose were not consistent across the studies and a quantitative synthesis of results was possible for six studies only. Moreover, Hb levels for inclusion criteria and for the assessment of the primary outcome varied a lot in the studies; in some studies, only severe anaemic patients (Hb <10.5 g/dl) were recruited, while in others these cases were excluded on purpose. In some articles, the primary outcome was expressed in terms of difference in Hb levels (g/dl), while in others authors reported the percentage of patients who recovered from IDA, thus performing a comparison of treatment effects was complicated.
In the included studies, no health-related quality-of-life and economic data were available, thus a comparison in terms of cost effectiveness of the different intravenous iron formulations was not feasible. Anyway, some authors showed the cost effectiveness of ferric carboxymaltose compared to placebo in patients with chronic heart failure [47–50], while others showed that treatment with ferric carboxymaltose also improved the quality of life of anaemic patients in the postpartum [38] or in patients with IDA associated with inflammatory bowel disease [32] or heavy uterine bleeding [51]. Moreover, a recent study conducted from an Italian perspective showed that ferric carboxymaltose, due to the low number of infusions needed, might be a cost-saving option from both national health system and hospital points of view when compared to ferric gluconate in the treatment of iron-deficient patients [52].
A systematic literature review also considering cost-effectiveness outcomes would be desirable to give a broader perspective to the analyses.
Despite its appeal in synthesizing all the available evidence for a treatment, NMA clearly has some limitations as well. The most critical one is the potentially high heterogeneity of the studies included in the analysis, both with regard to the reference study populations and to the treatment allocation schedule. This may raise some doubts about the validity of the findings. However, the present analysis incorporates all the currently available evidence about iron treatments for IDA and, to the best of our knowledge, this is the first attempt to systematically and quantitatively review the literature in this field.
Conclusions
Among the different iron formulations available for the treatment of IDA, intravenous ferric carboxymaltose was shown to be superior when compared with other iron regimens. This new formulation can rapidly improve haemoglobin levels and re-establish depleted iron stores in different populations of patients with ID (or IDA) related to a variety of medical conditions (i.e. chronic kidney disease, inflammatory bowel disease, heavy uterine bleeding or postpartum). Moreover, ferric carboxymaltose resulted in a higher increase of serum ferritin levels in comparison to ferric gluconate and showed a high safety profile.
From the Patient Blood Management perspective, ferric carboxymaltose may avoid the use of blood transfusions or other drugs (e.g. erythropoietin-stimulating agents) in patients requiring an iron replacement.
The advantages of ferric carboxymaltose should be further investigated through broader analyses that also include quality-of-life measures and economic outcomes.
Compliance with Ethical Standards
Funding
The present study was funded by Vifor Pharma Italia Srl through an unrestricted grant to CERGAS, Bocconi University, Via Roentgen 1, 20136 Milan, Italy. The authors were solely responsible for carrying out the research project and in writing the manuscript.
Conflict of interest
Dr. Melania Marmifero received a fee from Vifor Pharma Italia Srl to participate in the drafting of this manuscript. None of the other authors have any conflicts of interest to declare.
References
- 1.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. World Health Organization, Geneva (WHO/NMH/NHD/MNM/11.1) (2011). http://www.who.int/vmnis/indicators/haemoglobin/en. Accessed 30th October 2015.
- 3.Umbreit J. Iron deficiency: a concise review. Am J Hematol. 2005;78(3):225–231. doi: 10.1002/ajh.20249. [DOI] [PubMed] [Google Scholar]
- 4.Shander A, Goodnough LT, Javidroozi M, Auerbach M, Carson J, Ershler WB, et al. Iron deficiency anemia: bridging the knowledge and practice gap. Transfus Med Rev. 2014;28(3):156–166. doi: 10.1016/j.tmrv.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24(2):109–116. doi: 10.1097/MEG.0b013e32834f3140. [DOI] [PubMed] [Google Scholar]
- 6.Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritinconcentration. Blood. 2011;118(12):3222–3227. doi: 10.1182/blood-2011-04-346304. [DOI] [PubMed] [Google Scholar]
- 7.Bregman DB, Morris D, Koch TA, He A, Goodnough LT. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Haematol. 2013;88(2):97–101. doi: 10.1002/ajh.23354. [DOI] [PubMed] [Google Scholar]
- 8.Bailie GR, Mason NA, Valaoras TG. Safety and tolerability of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Hemodial Int. 2010;14:47–54. doi: 10.1111/j.1542-4758.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- 9.Breymann C, Gliga F, Bejenariu C, Strizhova N. Comparative efficacy and safety of intravenous ferric carboxymaltose in the treatment of postpartum iron deficiency anemia. Int J Gynaecol Obstet. 2008;101(1):67–73. doi: 10.1016/j.ijgo.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M. Anaemia Working Group España. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011;107(3):477–478. doi: 10.1093/bja/aer242. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz M, García-Erce JA, Cuenca J, Bisbe E, Naveira E, AWGE (Spanish Anaemia Working Group) On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus. 2012;10(1):8–22. doi: 10.2450/2011.0061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematol Am Soc Hematol Educ Program. 2010;2010:338–347. doi: 10.1182/asheducation-2010.1.338. [DOI] [PubMed] [Google Scholar]
- 13.Onken JE, Bregman DB, Harrington RA, Morris D, Acs P, Akright B, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54(2):306–315. doi: 10.1111/trf.12289. [DOI] [PubMed] [Google Scholar]
- 14.http://www.products.sanofi-aventis.us/ferrlecit/ferrlecit.pdf. Accessed 30th October 2015.
- 15.Moore RA, Gaskell H, Rose P, et al. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord. 2011;11:4. doi: 10.1186/1471-2326-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Sutton AJ, Abrams KR, Jones DR, et al. Methods for meta-analysis in medical research. London: Wiley; 2000. [Google Scholar]
- 18.Jansen JP, Crawford B, Bergman G, Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11:956–964. doi: 10.1111/j.1524-4733.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 19.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. doi: 10.1016/S0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 20.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3:80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 21.Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14:417–428. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Donegan S, Williamson P, Gamble C, Tudur-Smith C. Indirect comparisons: a review of reporting and methodological quality. PLoS One. 2010;5:e11054. doi: 10.1371/journal.pone.0011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ. 2009;338:b1147. doi: 10.1136/bmj.b1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 25.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure and extensibility. Stat Comput. 2000;10:325–337. doi: 10.1023/A:1008929526011. [DOI] [Google Scholar]
- 26.Earley CJ, Horská A, Mohamed MA, Barker PB, Beard JL, Allen RP. A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. 2009;10(2):206–211. doi: 10.1016/j.sleep.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coyne DW, Adkinson NF, Nissenson AR, Fishbane S, Agarwal R, Eschbach JW, Michael B, Folkert V, Batlle D, Trout JR, Dahl N, Myirski P, Strobos J, Warnock DG, Ferlecit Investigators Sodium ferric gluconate complex in hemodialysis patients. II. Adverse reactions in iron dextran-sensitive and dextran-tolerant patients. Kidney Int. 2003;63(1):217–224. doi: 10.1046/j.1523-1755.2003.00703.x. [DOI] [PubMed] [Google Scholar]
- 28.Onken JE, Bregman DB, Harrington RA. Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: the REPAIR-IDA trial. Nephrol Dial Transpl. 2014;29:833–842. doi: 10.1093/ndt/gft251. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal R, Rizkala AR, Bastani B, et al. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol. 2006;26(5):445–454. doi: 10.1159/000096174. [DOI] [PubMed] [Google Scholar]
- 30.Bager P, Dahlerup JF. Randomised clinical trial: oral vs. intravenous iron after upper gastrointestinal haemorrhage. A placebo-controlled study. Aliment Pharmacol Ther. 2014;39(2):176–187. doi: 10.1111/apt.12556. [DOI] [PubMed] [Google Scholar]
- 31.Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor: a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846–853. doi: 10.1053/j.gastro.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182–1192. doi: 10.1111/j.1572-0241.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 33.Kulnigg-Dabsch S, Schmid W, Howaldt S, et al. Iron deficiency generates secondary thrombocytosis and platelet activation in IBD: the randomized, controlled thromboVIT trial. Inflamm Bowel Dis. 2013;19(8):1609–1616. doi: 10.1097/MIB.0b013e318281f4db. [DOI] [PubMed] [Google Scholar]
- 34.Qunibi WY, Martinez C, Smith M, Benjamin J, Mangione A, Roger SD. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26(5):1599–1607. doi: 10.1093/ndt/gfq613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schatz U, Arneth B, Siegert G, et al. Iron deficiency and its management in patients undergoing lipoprotein apheresis. Comparison of two parenteral iron formulations. Atheroscler Suppl. 2013;14(1):115–122. doi: 10.1016/j.atherosclerosissup.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Seid MH, Derman RJ, Baker JB, Banach W, Goldberg C, Rogers R. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. Am J Obstet Gynecol 2008;199(4):435.e1–7. [DOI] [PubMed]
- 37.Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50(17):1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 38.Van Wyck DB, Martens MG, Seid MH, et al. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia. A randomized controlled trial. Obstet Gynecol. 2007;110:267–278. doi: 10.1097/01.AOG.0000275286.03283.18. [DOI] [PubMed] [Google Scholar]
- 39.Van Wyck DB, Mangione A, Morrison J, et al. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49(12):2719–2728. doi: 10.1111/j.1537-2995.2009.02327.x. [DOI] [PubMed] [Google Scholar]
- 40.Favrat B, Balck K, Breymann C, Hedenus M, Keller T, Mezzacasa A, Gasche C. Evaluation of a single dose of ferric carboxymaltose in fatigued, iron-deficient women-PREFER a randomized, placebo-controlled study. PLoS One. 2014;9(4):e94217. doi: 10.1371/journal.pone.0094217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grote L, Leissner L, Hedner J, Ulfberg J. A randomized, double-blind, placebo controlled, multi-center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Mov Disord. 2009;24(10):1445–1452. doi: 10.1002/mds.22562. [DOI] [PubMed] [Google Scholar]
- 42.Evstatiev R, Alexeeva O, Bokemeyer B, et al. Ferric carboxymaltose prevents recurrence of anemia in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:269–277. doi: 10.1016/j.cgh.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Lyseng-Williamson KA, Keating GM. Ferric carboxymaltose: a review of its use in iron-deficiency anaemia. Drugs. 2009;69(6):739–756. doi: 10.2165/00003495-200969060-00007. [DOI] [PubMed] [Google Scholar]
- 44.Keating GM. Ferric carboxymaltose: a review of its use in iron deficiency. Drugs. 2015;75(1):101–127. doi: 10.1007/s40265-014-0332-3. [DOI] [PubMed] [Google Scholar]
- 45.Bregman DB, Goodnough LT. Experience with intravenous ferric carboxymaltose in patients with iron deficiency anemia. Ther Adv Hematol. 2014;5(2):48–60. doi: 10.1177/2040620714521127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shander A, Van Aken H, Colomina MJ, Gombotz H, Hofmann A, Krauspe R, et al. Patient blood management in Europe. Br J Anaesth. 2012;109(1):55–68. doi: 10.1093/bja/aes139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hofmarcher T, Borg S. Cost-effectiveness analysis of ferric carboxymaltose in iron-deficient patients with chronic heart failure in Sweden. J Med Econ. 2015;18(7):492–501. doi: 10.3111/13696998.2015.1029491. [DOI] [PubMed] [Google Scholar]
- 48.Comín-Colet J, Rubio-Rodríguez D, Rubio-Terrés C, Enjuanes-Grau C, Gutzwiller FS, Anker SD, Ponikowski P. A cost-effectiveness analysis of ferric carboxymaltose in patients with iron deficiency and chronic heart failure in Spain. Rev Esp Cardiol (Engl Ed). 2015;68(10):846–851. doi: 10.1016/j.recesp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Gutzwiller FS, Schwenkglenks M, Blank PR, Braunhofer PG, Mori C, Szucs TD, et al. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure based on the FAIR-HF trial: an analysis for the UK. Eur J Heart Fail. 2012;14(7):782–790. doi: 10.1093/eurjhf/hfs083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim EA, Sohn HS, Lee H, Choi SE. Cost-utility of ferric carboxymaltose (Ferinject®) for iron-deficiency anemia patients with chronic heart failure in South Korea. Cost Eff Resour Alloc. 2014;10(12):19. doi: 10.1186/1478-7547-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon SS, Hadley PE, Van Wyck DB, et al. Iron carboxymaltose, a new intravenous iron agent for iron deficiency anemia in heavy uterine bleeding. Obstet Gynecol. 2007;109(Suppl 4):108S. [Google Scholar]
- 52.Rognoni C, Tarricone R, Meregaglia M. Impatto economico dell’utilizzo di carbossimaltosio ferrico in pazienti con anemia da carenza di ferro nelle regioni italiane. MECOSAN. Manag Econ Sanit. 2015;93:99–114. [Google Scholar]