Abstract
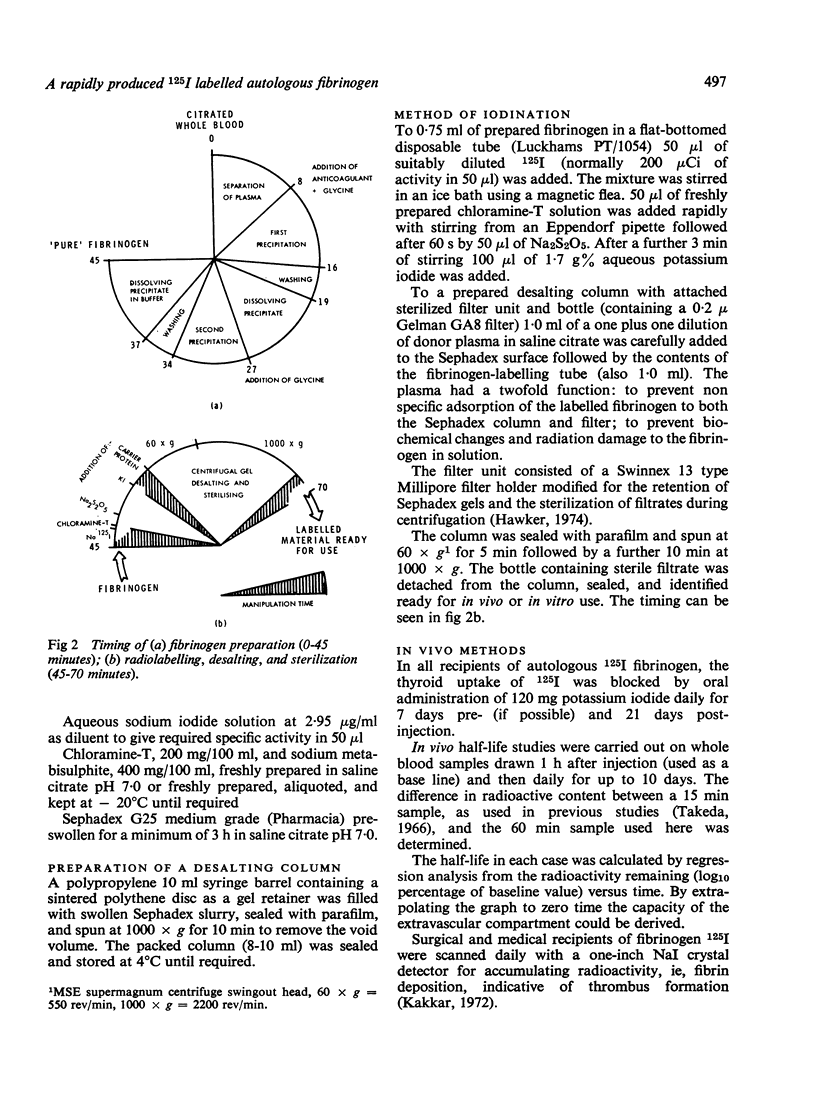
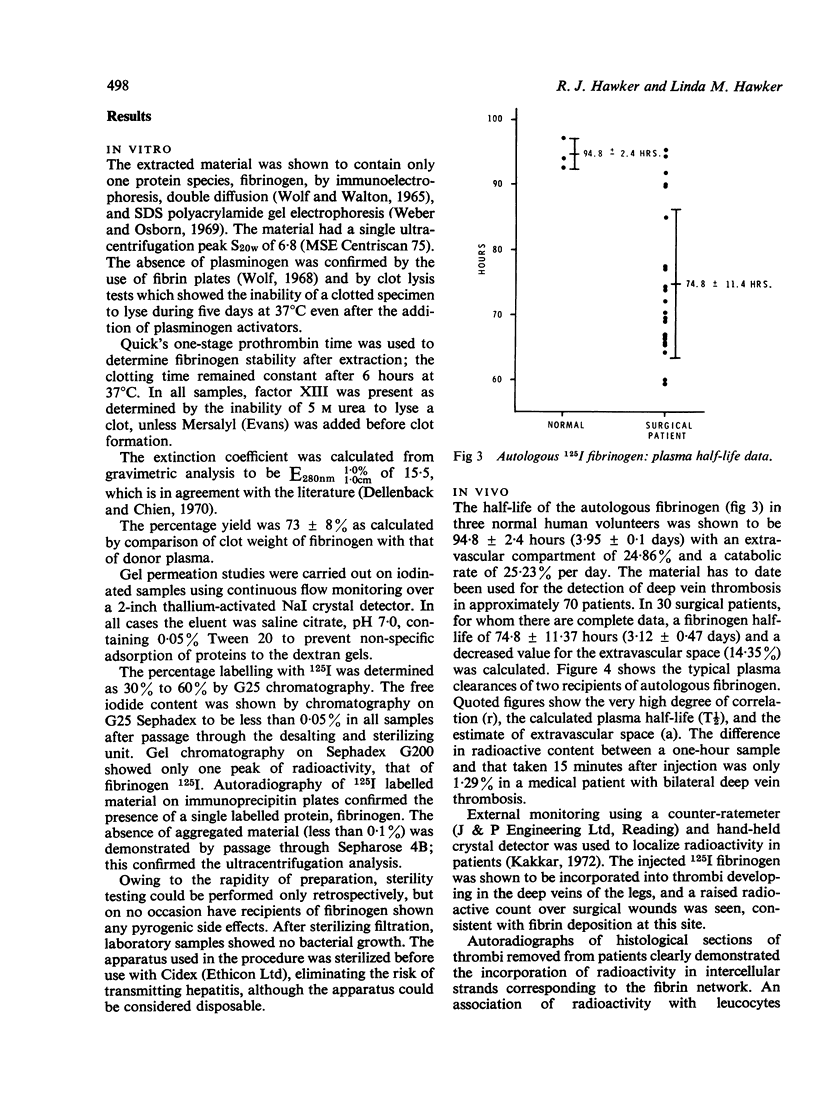
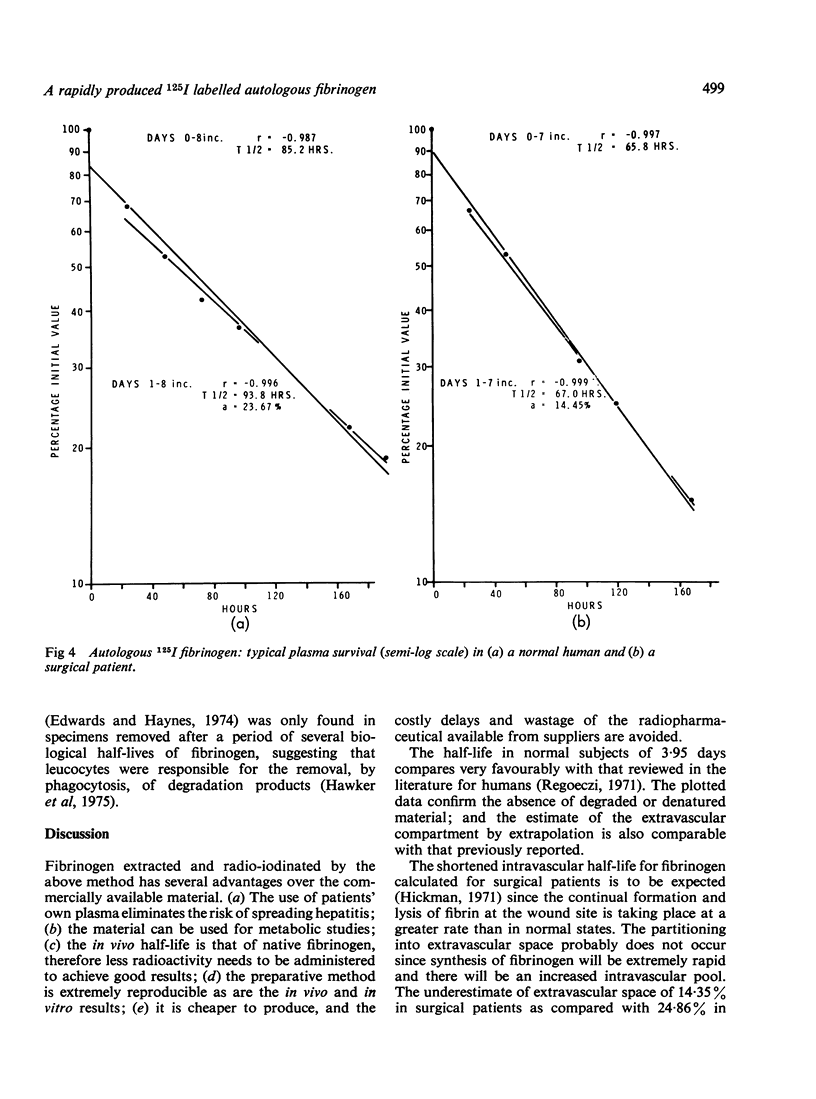
The properties of fibrinogen extracted by a precipitation method using glycine at ambient temperatures near neutral pH are described. The simple and reproducible method gives a 73% yield of high purity plasminogen-free fibrinogen in 45 minutes from small volumes of plasma. The protein extract was labelled with 125I using chloramine-T under conditions optimal for fibrinogen stability. The extraction procedure, radio-iodination, desalting, and sterilization take only 70 minutes for completion from the time donor blood is received in the laboratory. The methods, using a specially developed extraction vessel and desalting/sterilizing column, can be used in a small hospital laboratory. Autologous fibrinogen can thus be extracted from patients' blood, eliminating the risk of transmitting hepatitis when it is re-administered. The autologous material, which is 97% clottable and contains less than 0-05% free iodide, is being routinely used as a diagnostic tool in the detection of deep vein thrombosis. The high purity of the preparation facilitates metabolic studies and in vitro experimental work. In vivo results show a mean half-life in three normal volunteers of 3-95 days and a catabolic rate of 25-23% per day with the extravascular space estimated as 24-86%. In 30 surgical patients an expected reduced half-life in plasma was determined with a mean of 3-1 days.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley J., Hickman J. A. Behaviour of commercially prepared I-125 fibrinogen in metabolic studies. J Clin Pathol. 1975 Jun;28(6):487–493. doi: 10.1136/jcp.28.6.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. E., Krohn K. R., Metzger J. M., Welch M. J., Secker-Walker R. H., Siegel B. A. An in vivo evaluation of I-fibrinogen labeled by four different methods. J Lab Clin Med. 1974 Jun;83(6):977–982. [PubMed] [Google Scholar]
- Collen D., Tytgat G. N., Claeys H., Piessens R. Metabolism and distribution of fibrinogen. I. Fibrinogen turnover in physiological conditions in humans. Br J Haematol. 1972 Jun;22(6):681–700. doi: 10.1111/j.1365-2141.1972.tb05715.x. [DOI] [PubMed] [Google Scholar]
- Dellenback R. J., Chien S. The extinction coefficient of fibrinogen from man, dog, elephant, sheep, and goat at 280 mmu. Proc Soc Exp Biol Med. 1970 May;134(1):353–355. doi: 10.3181/00379727-134-34792. [DOI] [PubMed] [Google Scholar]
- Edwards D. H., Haynes D. W. Letter: Fate of 125I-labelled fibrinogen. Lancet. 1974 Jul 27;2(7874):220–221. doi: 10.1016/s0140-6736(74)91522-0. [DOI] [PubMed] [Google Scholar]
- Goudie R. B., Lindsay M. K., Pettigrew N. M. Letter: Safe use of 125I-fibrinogen not proven. Lancet. 1973 Nov 10;2(7837):1083–1083. doi: 10.1016/s0140-6736(73)92685-8. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hagan P., Loberg M. D., Rhodes B. A., Harrison K., Cooper M. D. "Kit" preparation of radioiodinated autologous fibrinogen using 131I-monochloride. J Nucl Med. 1974 Nov;15(11):974–980. [PubMed] [Google Scholar]
- Hawker R. J., Hawker L. M. Protein losses during sterilizing by filtration. Lab Pract. 1975 Dec;24(12):805-7, 818. [PubMed] [Google Scholar]
- Hawker R. J., Hawker L. M., Wilkinson G. A., Hamer J. D. Letter: Fate of 125I-labelled fibrinogen. Lancet. 1975 Nov 29;2(7944):1099–1100. doi: 10.1016/s0140-6736(75)90483-3. [DOI] [PubMed] [Google Scholar]
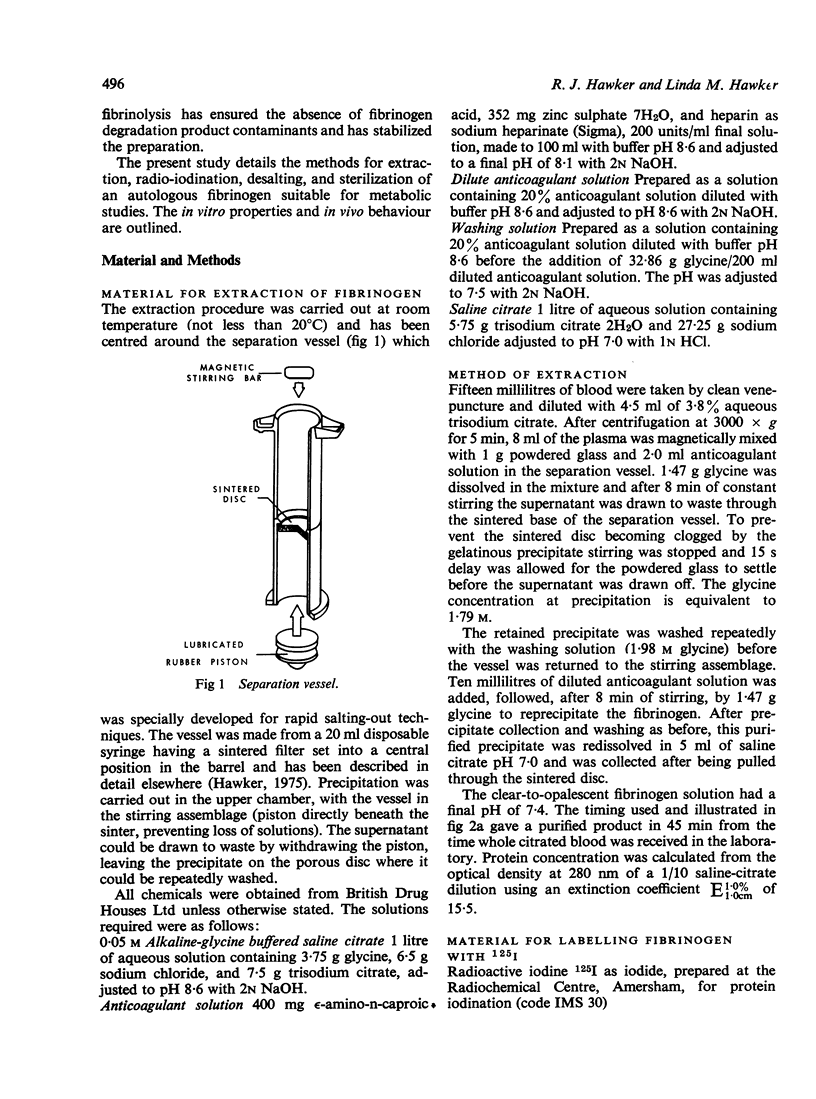
- Hawker R. J. Vessel for the rapid extraction of plasma proteins. Lab Pract. 1975 Jan;24(1):25–25. [PubMed] [Google Scholar]
- Hickman J. A. A study of the metabolism of fibrinogen after surgical operations. Clin Sci. 1971 Aug;41(2):141–152. doi: 10.1042/cs0410141. [DOI] [PubMed] [Google Scholar]
- Hicks B. H., Hazell J. Safe use of 125I-fibrinogen. Lancet. 1973 Oct 27;2(7835):931–934. doi: 10.1016/s0140-6736(73)92596-8. [DOI] [PubMed] [Google Scholar]
- KAZAL L. A., AMSEL S., MILLER O. P., TOCANTINS L. M. THE PREPARATION AND SOME PROPERTIES OF FIBRINOGEN PRECIPITATED FROM HUMAN PLASMA BY GLYCINE. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:989–994. doi: 10.3181/00379727-113-28553. [DOI] [PubMed] [Google Scholar]
- Krohn K., Sherman L., Welch M. Studies of radioiodinated fibrinogen. I. Physicochemical properties of the ICl, chloramine-T, and electrolytic reaction products. Biochim Biophys Acta. 1972 Dec 28;285(2):404–413. doi: 10.1016/0005-2795(72)90327-3. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- McFarlane A. S. IN VIVO BEHAVIOR OF I-FIBRINOGEN. J Clin Invest. 1963 Mar;42(3):346–361. doi: 10.1172/JCI104721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Secker-Walker R. H., Krohn K. A., Welch M. J., Potchen E. J. Unsatisfactory biologic behavior of I-fibrinogen labeled by the chloramine-T method. J Lab Clin Med. 1973 Aug;82(2):267–275. [PubMed] [Google Scholar]
- Peabody R. A., Halse T., Tsapogas M. J. Rapid method for preparation of human fibrinogen labeled with 125I. J Nucl Med. 1974 Mar;15(3):195–197. [PubMed] [Google Scholar]
- Regoeczi E. Iodine-labelled fibrinogen: a review. Br J Haematol. 1971 Jun;20(6):649–663. doi: 10.1111/j.1365-2141.1971.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Roberts R. C., Soonnentag C. O., Frisbie J. H. Rapid preparation of autologous radioiodinated fibrinogen. J Nucl Med. 1972 Nov;13(11):843–846. [PubMed] [Google Scholar]
- Takeda Y. Studies of the metabolism and distribution of fibrinogen in healthy men with autologous 125-I-labeled fibrinogen. J Clin Invest. 1966 Jan;45(1):103–111. doi: 10.1172/JCI105314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF P., WALTON K. W. INVESTIGATION OF A QUANTITATIVE ANOMALY ENCOUNTERED IN THE ASSAY OF FIBRINOGEN BY IMMUNO-DIFFUSION. Immunology. 1965 Jan;8:6–24. [PMC free article] [PubMed] [Google Scholar]
- Walker L., Catlin A. A simplified method for preparation of fibrinogen. Thromb Diath Haemorrh. 1971 Aug 31;26(1):99–102. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wolf P. Modification of the fibrin agar plate for measurements of the components of the fibrinolytic system. I. The measurement of plasminogen (on type I fibrin agar plates). Thromb Diath Haemorrh. 1968 Nov 15;20(1):50–65. [PubMed] [Google Scholar]


