Abstract
Allergic diseases and conditions are widespread and their incidence is on the increase. They are characterized by the activation of mast cells resident in tissues and the consequent infiltration and stimulation of several inflammatory cells, predominantly eosinophils. Cell–cell cross‐talk and the release of mediators are responsible for the symptoms and for the modulation of the response. The gold standard of therapeutic intervention is still glucocorticosteroids, although they are not effective in all patients and may cause numerous side effects. Symptomatic medications are also widespread. As research has led to deeper insights into the mechanisms governing the diseases, new avenues have been opened resulting in recent years in the development of monoclonal antibodies (mAbs) such as anti‐IgE mAbs (omalizumab) and others still undergoing clinical trials aimed to specifically target molecules involved in the migration and stimulation of inflammatory cells. In this review, we summarize new developments in the field of anti‐allergic mAbs with special emphasis on the treatment of asthma, particularly severe forms of this condition, and atopic dermatitis, which are two unmet clinical needs.
Abbreviations
- AD
atopic dermatitis
- AEU
allergic effector unit
- AHR
airway hyper‐responsiveness
- AI
allergic inflammation
- COPD
chronic obstructive pulmonary disease
- Eos
eosinophils
- GCs
glucocorticosteroids
- GM‐CSF
granulocyte–macrophage colony‐stimulating factor
- LTs
leukotrienes
- mAbs
monoclonal antibodies
- MCs
mast cells
- Th2
T‐helper type‐2 cells
- TSLP
thymic stromal lymphopoietin
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Catalytic receptors b |
| CCR3 | GM‐CSF receptor |
| CXCR2 | IL‐1 receptor‐like 1 (ST2) |
| NPSR1 | IL‐4 receptor, α |
| Enzymes c | IL‐9 receptor |
| ADAM33 | IL‐13 receptor, α1 |
| Carboxypeptidase A3 | IL‐17A receptor |
| IL‐31 receptor, β | |
| TSLP receptor |
These Tables list key protein targets and ligands described in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,cAlexander et al., 2015a, 2015b, 2015c).
Introduction
The story of monoclonal antibodies (mAbs) began with the discovery that serum from patients recovering from infectious diseases contained immunoglobulins capable of curing the diseases of other people. Thus started the research to replace human immunoglobulins that, although successful in their applications, presented several limitations in availability and potency (Yamada, 2011). Many of these limitations are now at least partially resolved by mAbs that were first produced (Kohler and Milstein, 1975) by fusing B‐cells from immunized mice with lymphoma cells. However, murine mAbs caused immune reactions. More recently, recombinant technology has produced chimeric, humanized and fully human mAbs (Harding et al., 2010) in which partial or complete replacement with human sequences has resulted in less immunogenicity and this has contributed to the explosion of mAbs now available (Ecker et al., 2015). Currently, mAbs‐based formulations are in development and are being produced using different approaches, ranging from transgenic mouse technologies and the use of human hybridoma and transformed cells (Nelson et al., 2010) to phage‐display technology (Hammers and Stanley, 2014). Several hundred mAbs (Razinkov et al., 2015) have been produced with the majority being devoted to the treatment of autoimmune diseases and tumours (Oldham and Dillman, 2008).
Regardless of their specific target disease, mAbs have both positive and negative aspects. Compared with conventional drugs, mAbs are highly specific therapies characterized by a long t 1/2 (up to 4 weeks thus not requiring frequent dosing) and slow distribution into the tissues (Hansel et al., 2010). Disadvantages associated with mAbs are their large size, which might be responsible for an uneven penetration into the tissue, the need for parental administration and the complexity of the structure of the protein, which may result in difficulties in cloning procedures and the need for considerable resources to optimize their production (Razinkov et al., 2015).
The toxicity of mAbs can result from either target or off‐target effects. Toxic target‐associated effects of mAbs are the result of their ‘exaggerated pharmacology’ and are specifically associated with the blocking or increased effect of the target molecule on the target cells or tissues, for example, immunosuppression and the risk of infection from diseases with TNF‐specific mAbs. In contrast, off‐target effects can result from the binding of mAbs to target antigens at sites not relevant for their therapeutic effect (Brennan et al., 2010). Specifically, immunomodulatory mAbs have been reported to produce hypersensitivity, acute anaphylaxis (IgE‐mediated), pseudoallergic reactions (IgE‐unrelated reactions possibly due to immune cell and complement activation) and cytokine release syndrome.
In this review, we discuss some of the most recent mAbs that have been approved for use and are in clinical trials for the treatment of allergic diseases/allergic inflammation (AI) (Figure 1), in particular asthma and atopic dermatitis (AD), which are currently unmet clinical needs.
Figure 1.
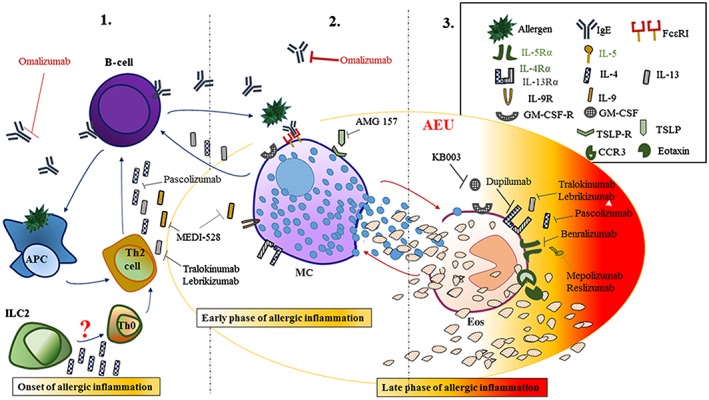
Schematic view of the updated targets for mAb therapy in the initiation and amplification of AI.
In the allergic inflammatory response, several soluble and cellular targets are the feasible targets for mAbs‐based drugs at its onset (1), and then at the early (2) and the late and chronic stages (3). (Figure 1).
The allergic inflammatory response
Allergic diseases that comprise allergic asthma, rhinitis and conjunctivitis, AD, food and drug allergies are widespread conditions affecting ~15% of the world global population. Their incidence is on the increase and thus has a negative impact on the quality of life, as they sometimes lead to life‐threatening conditions such as fatal asthma and anaphylaxis (Pawankar, 2014).
Atopy is the genetic predisposition of certain individuals to develop hypersensitivity reactions to innocuous substances and is influenced by environmental factors (Sengler et al., 2002). Several genes have been hypothesized to have a key role such as DPP10, PCDH1, HLAG, NPSR1, PHF11, PLAUR, ADAM33 (reviewed in Portelli et al., 2015) and, most recently, S100A4 (Bruhn et al., 2014). Most of these genes have been identified by studying the association between variants such as single nucleotide polymorphisms (SNPs) in the major pathways known to take part in AI and asthma (reviewed in Vercelli, 2008). Nutrition and environmental changes such as outdoor and indoor pollution, climate change and reduced biodiversity are likely to contribute to the rise in the prevalence of allergic diseases (Pawankar, 2014). Allergic diseases are caused by exposures of atopic individuals to allergens (Platts‐Mills and Woodfolk, 2011), which trigger sensitization mechanisms with the production of cytokines by mostly T‐helper type‐2 cells (Th2) (i.e. IL‐4, IL‐5 and IL‐13) thus stimulating the production of specific IgE antibodies from B‐cells (Nielsen et al., 2002). IgE binds to the high‐affinity receptor FcεRI expressed by resident mast cells (MCs) in tissues giving rise to the early phase of AI that is the result of activation of multiple signalling pathways and consequent degranulation and release of preformed granular mediators such as histamine, neutral proteases, proteoglycans and the synthesis/release of lipid‐derived mediators (reviewed in (Moon et al., 2014). These substances together with several cytokines and chemokines produced at later time points by MCs have a role in the generation of the late phase in which other cell types such as eosinophils (Eos), lymphocytes, macrophages and basophils are recruited into the tissue (Shakoory et al., 2004). The dominant cells of late phase responses and chronic allergy are the Eos, whose activation results in the release of highly basic specific proteins from cytosolic granules, reactive oxygen species and cysteinyl leukotrienes (LTs) as well as an array of chemokines and cytokines. These mediators influence MCs to further modulate inflammation and tissue damage (Landolina et al., 2015). Moreover, MCs continue to retain the potential to release mediators, to have a soluble cross‐talk and to hold a physical cross‐talk mediated by couples of ligands/receptors with Eos. This cross‐talk that we have named the allergic effector unit (AEU) enhances the effects of these two cells thus amplifying the late phase and chronic outcomes of AI (Elishmereni et al., 2011). Moreover, other inflammatory cells infiltrated into the tissue and resident MCs can also interact to enhance the inflammatory response (Landolina et al., 2015). Importantly, the AEU and other cellular interactions indicate a non‐IgE mediated activation of MCs and its consequences, a process that has to be taken into consideration for an efficient anti‐allergic therapy in addition to the targeting of IgE‐dependent mechanisms.
The general mechanisms of allergic diseases, in spite of the different tissues and organs in which these events take place, are very similar, with the main symptoms due to MCs and inflammatory cell activation. Nevertheless, some local variations in cell populations and pathways do exist.
Asthma is a highly heterogeneous disease characterized by airway hyper‐responsiveness (AHR), smooth muscle contraction and inflammation, which, if persistent, can lead to structural changes, all resulting in narrowing of the airway, obstruction and consequent restricted airflow and shortness of breath (Ishmael, 2011). Asthma affects 300 million people worldwide (Trevor and Deshane, 2014) with severe asthma exacerbations responsible for 250000 deaths annually (Pawankar, 2014). Asthma is characterized by different clinical/inflammatory phenotypes: allergic/eosinophilic asthma, non‐allergic/neutrophilic asthma and paucigranulocytic asthma distinguished according to the number of the sputum granulocytes induced (Simpson et al., 2006) with different biomarkers. Distinct phenotypes and new classifications of asthma are still being proposed (Wenzel, 2012).
Allergic asthma is a Th2‐driven inflammatory process characterized by the infiltration of Eos into the airways (Lambrecht and Hammad, 2015) and a high production of the potent Eos chemoattractant, eotaxin, which is generated also by MCs and its production stimulated by IL‐4 and IL‐13 (Hart, 2001). Neutrophilic asthma is correlated with high numbers of both neutrophils and Th17 cytokines such as IL‐17A, IL‐17F and IL‐22, which have been reported to be present in the sputum (Moore et al., 2014) and respiratory tract of these patients respectively (Newcomb and Peebles, 2013). Different biomarkers derived from induced sputum samples have been shown to be useful to distinguish the inflammatory phenotypes of asthma and possibly predict the responsiveness of patients to certain therapies (i.e. glucocorticosteroids (GCs)). The expression of the genes Charcot–Leydon crystal protein, carboxypeptidase A3 and deoxyribonuclease I‐like 3 have been reported to be increased in patients with eosinophilic asthma, whereas the expression of IL‐1β, alkaline phosphatase, tissue non‐specific isozyme and chemokine receptor 2 (CXCR2) are higher in patients with neutrophilic asthma (Baines et al., 2014).
On the other hand, different candidates have been proposed as possible biomarkers for eosinophilic asthma including fractional exhaled air NO, serum periostin (reviewed in Kim et al., 2014) and chloride channel accessory 1 in airway epithelial brushings of asthmatic individuals (Peters et al., 2014).
In addition to asthma, high numbers of Eos together with granulocyte–macrophage colony‐stimulating factor (GM‐CSF), IL‐5 and IL‐13 also distinguish other allergic inflammatory conditions of the upper airways such as chronic rhinosinusitis (Kato, 2015) where both IL‐5 and IL‐13 are directly/indirectly correlated and promote Eos functions. Specifically, IL‐5 controls all the checkpoints of Eos life span from their expansion in the bone marrow, their release into the blood, enhanced adhesion to endothelial cells, to final maturation, survival and activation (Landolina and Levi‐Schaffer, 2014). IL‐13 activates macrophages, B‐cells and epithelial cells, which trigger different events such as the recruitment of Eos and Th2 cells, IgE‐dependent reactions and remodelling phenomena (Kato, 2015).
AD, also known as atopic eczema, is one of the most common inflammatory skin diseases characterized by pruritic skin lesions and mainly affecting paediatric patients. Barrier dysfunction partly due to filaggrin mutation/defective function, a decrease in antimicrobial peptides and enhanced IL‐22 expression characterize this disease (reviewed in (Miyagaki and Sugaya, 2015)).
It is evident that single checkpoints in the AI loop, that is, cytokines as well as their receptors, can be targeted to avoid the next step, to impede the involvement of other cells and to thus prevent the development of the chronic stages of AI and additional detrimental events.
Current treatment of allergic diseases: from symptoms to mechanism
Current management of allergic diseases includes GCs, antihistamines, LT antagonists, MC stabilizers, anticholinergics, β‐agonists and the potentially disease‐modifying allergen‐specific immunotherapy. GCs (Trevor and Deshane, 2014) and antihistamines (Cataldi et al., 2014) are gold standard treatments for allergic diseases as a result, respectively, of their anti‐inflammatory and symptom relief properties. However, these drugs are not the solution for the treatment of allergic diseases as the antihistamines are mostly symptomatic medications and the GCs often cause several severe side effects and are not efficacious for all patients (Quax et al., 2013). Thus, there is a need to strike out with new pathways and different targets especially for the treatment of asthma and AD by exploring the new possibility of using mAbs.
As discussed above, IgE‐mediated stimulation of MCs is a critical event for the initiation of AI followed by downstream events that culminate in the recruitment/activation of Eos to mark the late and chronic stages of allergy. Moreover, there is an abundance of literature supporting the concept of Eos as multifunctional leukocytes playing a pivotal role in infections and in different airway disorders and a specific decrease in their number is one of the final goals of therapy (Landolina and Levi‐Schaffer, 2014). The initiation and amplification of AI that selectively target MCs, IgE, pro‐inflammatory chemokines and cytokines taking part in intercellular communication networks and Eos have been targeted by mAbs, focusing on the specificity their effects.
Most up‐to‐date targets in allergy: from the molecule to the disease
Targeting IgE in allergic diseases
IgE has been identified as an effective diagnostic biomarker, capable of potently switching on the machinery of allergic reactions within minutes of allergen exposure, and is a clinically efficient therapeutic target for allergic diseases (Licari et al., 2015). Once released from plasma cells, IgE binds principally to FcεRI on MCs triggering various effector responses including the release of mediators leading to AI reactions.
Omalizumab is a commercially available recombinant humanized anti‐IgE mAb (IgG1κ) (Xolair) (Table 1) that specifically binds serum‐free IgE at its CH3 domain in the proximity of the binding site for FcεRI (Jensen et al., 2015), thereby blocking its interaction with FcεRI on MCs, basophils, antigen‐presenting cells (APCs) and other inflammatory cells. This results in (1) a reduction in free IgE, (2) subsequent down‐regulation of FcεRI on the key inflammatory cells and interruption of the allergic cascade, (3) a reduction in the levels of peripheral and bronchial tissue Eos, GM‐CSF, IL‐2, IL‐4 and IL‐13 with relative attenuation of the inflammation, (4) decreased allergen presentation to T‐cells and the production of Th2 cytokines (Holgate et al., 2009). Much effort has been made also to target B‐cell‐associated membrane IgE to eradicate a priori the IgE‐expressing B‐cells so that they will not differentiate into IgE‐secreting plasma cells, thus reducing the amount of total free IgE (Chen et al., 2010; Chowdhury et al., 2012). However, because these Abs, currently in clinical trials, bind to a specific region proximal to the membrane, do not neutralize soluble, circulating IgE and do not block the interaction between IgE and FcεRI, no real interruption of the allergic cascade will take place, thus showing that membrane IgE is not worth targeting (Nyborg et al., 2015).
Table 1.
A list of mAbs for asthma and AD discussed in the review are shown with some of their characteristics
| Name of the mAb | Trade name/company | Target of the mAb | Status/Effective | Indicaton |
|---|---|---|---|---|
| Omalizumab | Xolair (Genetech/Novartis) | IgE (Cε3 domain) | Commercially available | severe, persistent allergic asthma * chronic idiopathic urticaria § |
| QGE031 (ligelizumab) | Tanox/Novartis | IgE (Cε3 domain) | Phase II | allergic asthma (NCT01703312) ¶ AD (NCT01552629) ¶ chronic spontaneous urticaria (NCT02477332) ¶ |
| Dupilumab | Regeneron Pharmaceuticals/Sanofi‐Aventis | IL‐4 α receptor | Phase III | allergic asthma (NCT02414854) ¶ AD (NCT02277769) ¶ |
| Pascolizumab | GlaxoSmithLKline | IL‐4 | Phase II | symptomatic steriod‐naive asthma (NCT00024544) ¶ |
| Tralokinumab | Cambridge Antibody Technology/MedImmune | IL‐13 | Phase III Phase II | Uncontrolled asthma (NCT02161757) ¶ AD (NCT02347176) ¶ |
| Lebrikizumab | Tanox/Chugai Pharmaceutical | IL‐13 | Phase III Phase II | Uncontrolled asthma (NCT01987492) ¶ AD (NCT02340234) ¶ |
| Mepolizumab | Nucala (GlaxoSmithKline) | IL‐5 | Phase III terminated | Uncontrolled eosinophilic asthma (Mukherjee et al., 2014) (NCT01691521) ¶ |
| Reslizumab | Cinquil (Celltech R& D/Teva Pharmaceutical Industries) | IL‐5 | Phase III terminated | Uncontrolled eosinophilic asthma (NCT01285323) ¶ |
| Benralizumab | Kyowa Hakko/AstraZeneca; BioWa; MedImmune | IL‐5 α receptor | Phase III | Eosinophilic asthma (NCT02322775) ¶ |
| KB003 | KaloBios Pharmaceuticals | GM‐CSF | Phase II | Moderate‐to‐severe asthma (NCT01603277) ¶ |
| MEDI‐528 | Genaera Corporation; Ludwig Institute for Cancer Research/MedImmune | IL‐9 | Phase II | Uncontrolled asthma (Oh et al., 2013). |
| Brodalumab | Amgen Inc.; AstraZeneca/MedImmune | IL‐17A receptor | Phase II | Moderate‐to‐severe asthma (Busse et al., 2013) (NCT01199289) ¶ |
| AIN457 (secukinumab) | Novartis | IL‐17A | Phase II | Uncontrolled asthma (NCT01478360) ¶ |
| Ustekinumab | Centocor/Janssen‐Cilag | p40 submit of both IL‐12/IL‐23 | Phase II | AD (NCT01806662) ¶ |
| ILV‐094 | Pfizer/Wyeth | IL‐22 | Phase II | AD (NCT01941537) ¶ |
| BMS‐981164 | Bristol‐Myers Squibb; ZymoGenetics | IL‐31 | Phase I | AD (NCT01614756) ¶ |
| AMG282 | Amgen | IL‐33 | Phase I | Mild atopic asthma (NCT01928368) ¶ |
| AMG157 | Amgen/AstraZeneca | TSLP | Phase II Phase I | Uncontrolled asthma (NCT02054130) ¶ AD (NCT0075042) ¶ |
https://clinicaltrials.gov/(ClinicalTrials.gov. Identifier).
Omalizumab is already in use as add‐on therapy for moderate‐to‐severe persistent allergic asthma reducing the incidence of asthma exacerbations with a long‐term efficacy and an excellent safety profile. In the study of Lai et al. (2015, there were no drug‐related adverse events associated with its use except for sporadic and mild local reactions. However, other studies have reported ‘an incidence of 0.2% of anaphylaxis in 57,300 patients’, type‐III hypersensitive reactions (serum‐sickness‐like) such as fever, arthritis/arthralgia, rash and lymphadenopathy (Galvao and Castells, 2015). This is possibly due to anti‐allotypic or anti‐idiotypic Abs (IgE or IgG) against this reagent that were either pre‐existing, or developed after initial exposures or generated as a response to the aggregated preparations of Xolair (Cox et al., 2007). In addition to allergic asthma, omalizumab is the only licensed therapy for chronic spontaneous urticaria unmanageable with H1receptor antihistamines (Zuberbier and Maurer, 2015) with a good safety/efficacy profile (Sussman et al., 2014) for administration lasting more than 1 year (Har et al., 2015). Furthermore, omalizumab has been under recent investigations for the treatment of perennial and seasonal allergic rhinitis (Vashisht and Casale, 2013), food allergy (Bauer et al., 2015) (Umetsu et al., 2015), chronic rhinosinusitis (Tsabouri et al., 2014), AD (Yalcin, 2014) and so on.
In the case of asthma, rhinitis and AD, their coexistence in paediatric patients both in the presence and absence of IgE sensitization questions the concept of the real dominance of IgE as a causal mechanism (Pinart et al., 2014).
In his attempt to predict which patient might benefit mostly from omalizumab treatment, Bousquet et al. (2007 showed that there is no reliable indication within pretreatment baseline variables in asthmatic patients.
Although different schools of thought are present and more questions are being raised, omalizumab has proven to be a major success as a treatment for both asthma and chronic urticaria with a new generation of anti‐IgE Abs currently under development, such as the humanized QGE031 (ligelizumab) (IgG1) in Phase II trials for allergic asthma (https://clinicaltrials.gov NCT01703312), AD (https://clinicaltrials.gov NCT01552629) and chronic spontaneous urticaria (https://clinicaltrials.gov NCT02477332) (Table 1).
Targeting Th2‐associated cytokines. IL‐4, IL‐5, eotaxin, GM‐CSF, IL‐9, IL‐13 and their receptors
IL‐4 and IL‐13 have been considered for a long time the most important players in airway AI as they are (1) ‘promoters’ of both Ig class switching to the IgE isotype and differentiation to Ab‐producing plasma cells; (2) ‘recruiters’ of Eos to the airways via their shared IL‐4 α receptor, –IL‐13α1 receptor, expressed on Eos (Myrtek et al., 2004); and (3) ‘stimulators’ of other cells such as MCs and structural cells. Additionally, IL‐13 stimulates airway fibrosis and mucus hypersecretion in asthma (Hershey, 2003).
Dupilumab, a human anti‐IL‐4 α receptor mAb (IgG4) was shown to reduce asthma exacerbations, improve lung functions and reduce Th2‐associated inflammatory markers in patients with persistent, moderate‐to‐severe asthma (Wenzel et al., 2013). In AD patients, dupilumab showed rapid improvement of the AD molecular signature (Hamilton et al., 2014) thus encouraging Phase III clinical trials to investigate its efficacy and safety in monotherapy in moderate‐to‐severe AD patients (http://clinicaltrials.gov NCT02277769). Nevertheless, the Phase III study that is still ongoing shows promising effects of the mAb (Table 1).
Pascolizumab, a humanized anti‐IL‐4 mAb (IgG1) showed good potential in preclinical studies (Hart et al., 2002) with ongoing Phase II trials to test its clinical efficacy in asthma (http://clinicaltrials.gov NCT00024544) (Table 1).
Tralokinumab, a human IL‐13‐neutralizing mAb (IgG4) inhibited AHR and bronchoalveolar lavage eosinophilia in antigen‐challenged animal models (May et al., 2012), and its efficacy/safety profile is now being evaluated in a Phase III study in uncontrolled asthma (http://clinicaltrials.gov NCT02161757) and in a Phase IIb trial in AD (http://clinicaltrials.gov NCT02347176) (Table 1).
Another humanized anti‐IL‐13 mAb (IgG4), lebrikizumab, improved lung functions and provided benefit in the treatment of severe uncontrolled asthma (Scheerens et al., 2014). Its efficacy is currently under evaluation in patients with severe GCs‐dependent asthma (http://clinicaltrials.gov NCT01987492), and Phase II studies are underway to assess its safety/adequacy profile in persistent, moderate‐to‐severe AD (http://clinicaltrials.gov NCT02340234) (Table 1). Apparently the IL‐13–IL‐4 axis has a huge potential for the treatment of asthma with clinically encouraging results for both anti‐IL‐4 α receptor, and anti‐IL‐13 mAbs in asthmatic patients with measurable type‐2 signatures, stressing the importance of the differentiation of asthma phenotype before treatment choice. Regarding this, serum periostin, fractional exhaled air NO and blood Eos have been shown to represent promising predictive and pharmacodynamic biomarkers for patients undergoing therapies with anti‐IL‐13, anti‐IL‐5 and anti‐IgE, possibly correlating with a clinical benefit from these treatments (Arron et al., 2013). In addition to type‐2 high asthma, specific inhibition of this pathway has shown positive outcomes in AD patients (Fajt and Wenzel, 2015).
IL‐5 masterminds most of the Eos functions from expansion to maturation, survival and activation. To selectively block IL‐5 activities (and not other Th2 cytokines), anti‐IL‐5 neutralizing mAbs (mepolizumab and reslizumab) and Abs that block IL‐5 α receptor, (benralizumab) have been developed (reviewed in Landolina and Levi‐Schaffer (2014)) and investigated in clinical trials in mild atopic, moderate persistent and eosinophilic asthma (Table 1). Mepolizumab is a humanized mAb (IgG1) currently in Phase III trial for severe uncontrolled refractory asthma (study terminated http://clinicaltrials.gov NCT01691521), which, through its high affinity binding to free IL‐5, prevents the activation of the IL‐5 α receptor, (Mukherjee et al., 2014).
Mepolizumab effectively depletes Eos numbers in the airways, bone marrow and blood and reduces asthma exacerbation frequencies but, puzzlingly, has no effects on the signs of clinical asthma (Pavord et al., 2012). Reslizumab is another humanized mAb (IgG4/k) against IL‐5 that has recently completed a Phase III trial (http://clinicaltrials.gov NCT01285323), showing a reduction in sputum Eos and significant improvement in lung function (Castro et al., 2011). Benralizumab (MEDI‐563) is a humanized, afucosylated mAb (IgG1κ) targeting the IL‐5 α receptor, that is expressed by both mature Eos and their progenitors (Rothenberg and Hogan, 2006). In patients affected by uncontrolled Eos asthma, benralizumab reduced exacerbations and Eos blood count (Castro et al., 2014) and improved lung functions (Mukherjee et al., 2014). Benralizumab is currently in a Phase III clinical trial (http://clinicaltrials.gov NCT02138916) to assess whether it also reduces chronic obstructive pulmonary disease (COPD) exacerbation rate in patients with moderate to very severe COPD. In general, the approach targeting IL‐5 or its receptor has been successful in reducing asthma exacerbations as well as its symptoms and effects on airway function. However, whether the first strategy (target IL‐5) is preferable to the second strategy (target IL‐5 receptor) for treating asthma and possibly other Eos disorders still needs to be determined.
The ‘Th2‐like chemokine’ eotaxins (eotaxin 1, eotaxin 2 and eotaxin 3) but especially their high‐affinity receptor C‐C chemokine receptor type 3 (CCR3) have been recently targeted by mAbs to impede Eos migration and activation and, therefore, indicated for asthma treatment. In view of the poor performance of these candidates in monotherapy, their combination with IL‐5‐targeted treatment has been suggested as a better option for inhibiting AI (Landolina and Levi‐Schaffer, 2014). Additionally, the growth factor GM‐CSF has a critical role in Eos differentiation and survival (Landolina and Levi‐Schaffer, 2014). Animal studies (murine model of allergic asthma) showed that specifically targeting it using anti‐mouse GM‐CSF polyclonal Ab inhibited airway inflammation, mucus generation and bronchial hyper‐responsiveness (Yamashita et al., 2002) thus suggesting it might be an appealing target in asthma. KB003 (KaloBios) is a humanized mAb (IgG1) that directly binds to GM‐CSF thus blocking its binding to the GM‐CSF receptor and is now in Phase II trial for moderate‐to‐severe asthma (http://clinicaltrials.gov NCT01603277) (Table 1).
IL‐9 is a pleiotropic cytokine supporting the growth/activity of MCs, increasing IgE production by B‐cells/up‐regulating FcεRI and thus is associated with atopic diseases (Oh et al., 2011). The expression of IL‐9 and its receptor is increased in the airways of asthmatic patients (Shimbara et al., 2000) and correlates with bronchoconstriction, mucus secretion and mucosal oedema and the tendency to develop AHR (Farahani et al., 2014). In murine models of acute and chronic AI, the reduced production of IL‐9 corresponded to diminished tissue MC numbers and expression of proteases, making this cytokine an intriguing target (Sehra et al., 2015). However, MEDI‐528, a humanized mAb (IgG1) binding to IL‐9, showed no beneficial effects in asthma exacerbations or health‐related quality of life in patients with uncontrolled asthma (Oh et al., 2013) (Table 1). This is an example of how promising observations in animal models do not always translate into therapeutic successes in patients.
Targeting Th17, Th22 and associated cytokines
Recently, the recognition that Th2 is involved in AI has extended to Th1 and to a new population of Th17/Th22 producing IL‐17/IL‐22. However, the relative role of these T‐helper cells is still being investigated especially with regard to AD.
IL‐17A together with IL‐17F are ‘guardians’ of host defence and mucosal barriers against pathogen invasion and ‘recruiters of the pro‐inflammatory battalion’ (cytokines, antimicrobial peptides and chemokines), which recruit ‘major force’ innate immune cells (neutrophils and macrophages) to the site of infection leading to the elimination of the pathogen (Reynolds et al., 2010). IL‐17A has a major role in the pathogenesis of severe asthma (structural modification of epithelial cells and smooth muscle contraction) and is associated with the appearance of its symptoms (high levels in induced sputum and bronchial biopsies from patients suffering from severe asthma) (Chesne et al., 2014).
MAbs against IL‐17A as well as its receptor are currently under development (Table 1). The effectiveness of selective neutralization of IL‐17A through the human mAb AIN457 (secukinumab) (IgG1k) is currently being evaluated in patients with uncontrolled asthma in Phase II trials (http://clinicaltrials.gov NCT01478360). Blocking of the IL‐17A receptor and its signalling using the human mAb (IgG2) brodalumab has been used to treat the development and persistence of moderate‐to‐severe asthma; however, no beneficial effect has been found (www.clinicaltrials.gov NCT01199289) (Busse et al., 2013).
Moreover, IL‐17A is implicated in AD as its deficiency in two murine models of AD reduced the spontaneous development of AD‐like conditions (Nakajima et al., 2014), and an increase in its levels in acute AD lesions compared with non‐lesional skin was reported in patients (Gittler et al., 2012).
Ustekinumab, a fully human mAb (IgG1k) targeting the p40 subunit of both IL‐12 and IL‐23 (this cytokine is specifically valuable for the maintenance of Th17 cells) was used in an off‐label manner to treat AD patients not responding to other systemic treatments (www.clinicaltrials.gov NCT01806662) (Table 1). It was postulated that the neutralization of IL‐12/IL‐23 by ustekinumab might preclude the up‐regulation of Th17 cells in AD. Although a positive response accompanied by a safe profile were reported, additional studies are needed to establish the real validity of ustekinumab as a treatment for AD (Agusti‐Mejias et al., 2013).
IL‐22 is a T‐cell‐derived cytokine playing a critical role in skin homeostasis, coordinating keratinocyte proliferation/differentiation and the production of antimicrobial peptides (Fujita, 2013). Strong activation of IL‐22 is associated with the pathogenesis of psoriasis and AD in which MCs have been recently found to produce this cytokine (Mashiko et al., 2015). Phase II clinical trials are currently evaluating the clinical efficacy and mechanism of action of the human anti‐IL‐22 mAb (IgGIA) ILV‐094 in patients with AD (www.clinicaltrials.gov NCT01941537). Because this cytokine functionally inhibits the production of barrier proteins and antimicrobial peptides, its neutralization is expected to reverse the disease.
IL‐31 is a helical cytokine, a member of the gp130/IL‐6 cytokine family, and is preferentially expressed by activated Th2 CD4+ T‐cells. IL‐31 has numerous effects on the immune system, which are mediated through the heterodimeric receptor complex IL‐31 β receptor, (oncostatin M‐specific receptor, β subunit; OSMR β) and have recently received much attention. In particular, IL‐31 has a role in the pathogenesis of AD with higher levels of the cytokine present in AD patients biopsy specimens compared with healthy individuals (reviewed in Zhang et al., 2008). The most attractive feature of IL‐31 is probably its ability to induce itching/scratching behaviour in an animal model of AD (Arai et al., 2013). In AD patients, IL‐31 determined late onset itch responses rather than immediate ones raising the question of whether its pruritic effects are direct or indirect (Hawro et al., 2014). Phase I studies using the human IL‐31 mAb BMS‐981164 (IgG) have recently been completed in AD patients, but no results have been reported as yet (www.clinicaltrials.gov NCT01614756) (Table 1).
Targeting the three epithelial‐derived type‐2 inflammation‐associated cytokines: IL‐25, TSLP and IL‐33
The three cytokines IL‐25, IL‐33 and thymic stromal lymphopoietin (TSLP) are known to be generated mainly by epithelial cells and to be important promoters of Th2 immune responses.
IL‐25 is a cytokine belonging to the family of IL‐17 (IL‐17E) whose increased expression has been found in the asthmatic bronchial mucosa and dermis of sensitized atopic subjects after allergen stimulation (Corrigan et al., 2011). Together with IL‐33, IL‐25 has been shown to play a key role in the induction of Th2 cytokine‐mediated allergic airway eosinophilia in an experimental mouse model of allergic airway inflammation (Morita et al., 2015). IL‐25 is a potential therapeutic target for treatment in allergic asthma (Knolle et al., 2015) and in chronic rhinosinusitis (Shin et al., 2015), but no clinical studies have been reported yet.
IL‐33 is a nuclear‐associated cytokine belonging to the IL‐1 family having multiple roles from tissue homeostasis, to pathological fibrotic reactions and the setting of inflammation (reviewed in Molofsky et al., 2015). Once released, it immediately provokes immune responses mediated by its receptor ST2. Stimulation of the receptor by this cytokine in the extracellular environment has been described as a ‘molecular clock’ mechanism, capable of self‐regulating thus limiting receptor‐mediated immunological responses to airway stimuli (Cohen et al., 2015).
AMG 282 is a human mAb that prevents the binding of IL‐33 to the ST2 receptor. It is currently being investigated in Phase I as a treatment for mild atopic asthma (http://www.clinicaltrials.gov NCT01928368) (Table 1).
TSLP is mainly an epithelial cell‐derived cytokine having a role in initiating AI through the activation of MCs and is strongly associated with asthma and AD, in which levels of TSLP in skin are associated with symptoms and severity of the disease (Leyva‐Castillo et al., 2013). TSLP‐targeted therapy has been evaluated in allergen‐induced airway responses and persistent airway inflammation in patients with allergic asthma using human anti‐TSLP mAb (IgG2λ) AMG 157 that binds TSLP and prevents its binding to the receptor (Gauvreau et al., 2014). Treatment with AMG 157 reduced allergen‐induced bronchoconstriction, blood/sputum Eos numbers and indexes of airway inflammation before and after allergen challenge (www.clinicaltrials.gov NCT01405963).
AMG 157 is currently under Phase II trials for inadequately controlled, severe asthma (www.clinicaltrials.gov NCT02054130) and in a Phase I study for subjects with moderate‐to‐severe AD (www.clinicaltrials.gov NCT00757042) (Table 1).
IL‐25, IL‐33 and TSLP in airway tissue or plasma of asthmatic individuals are currently under investigation as biomarkers to predict the response of these patients to inhaled GCs (http://www.clinicaltrials.gov NCT01973751). Their detection as well as their potential target might be important to choose the optimal treatment for asthmatic patients or to find another option to overcome the lack of improvements with conventional therapy.
Conclusions
The field of mAbs is constantly developing as new evidence emerges on how this strategy is a powerful tool to target different inflammatory processes connected with asthma and AD. Decades of preclinical research have supported the role of IgE and type‐2 inflammation including the Th2 cell types and cytokines in the pathogenesis of asthma. Anti‐IgE therapy specifically is an excellent example as a treatment for this disease. Although mAbs targeting Th2 cytokines and others targeting Eos have not yet achieved a comparable success, they do show a consistent efficacy and hold good promise. Much progress has also been made in both the understanding and treatment of AD with anti‐IL‐22 in trials and in near future directions targeting IL‐31 as a strategy to inhibit the itching characteristic of this disease. Other targets are still under evaluation.
Because mAbs are characterized by high production costs, it is extremely important to have the proper characterization of the ‘right patient’ with specific diagnostic markers. This is also the case of the specific phenotype of asthmatic patients carrying the Th2 signature who can greatly benefit from treatment with mAbs targeting Th2 cytokines and Eos. The efficacy of mAbs has been shown, at least for the anticancer drugs, to be substantially enhanced using combination therapy, and this might be advisable also with regard to using mAbs to treat allergic diseases. In particular, combinations of mAbs with ‘the classical weapons to fight allergy’ – anti‐inflammatory or antihistamine drugs or bronchodilators in the case of asthma – might meet the challenge and be valuable to improve the health of the patient. Importantly, possible pharmacodynamic synergistic or additional effects of mAbs with these drugs can emerge. Another point for consideration as regards the mAbs themselves: efforts should be made to create drugs characterized by a higher t1/2 that could increase the patient's compliance. Last but not least, the use of mAbs that target inhibitory signals rather than blocking the activating ones might be an additional strategy to the ones already explored to down‐regulate the allergic response.
As we continue to elucidate allergy and further characterize signalling pathways and multiple phenotypes of the disease, it becomes more and more evident that the universal key for favourable outcome of therapy is the selection of the appropriate drug for a particular patient. Although disappointing results are frequent and significant failures are commonly reported in the translation between experimental observations in animal models to studies in patients, mAbs present a promising beacon to pursue in the treatment of allergic diseases.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
F. L‐S. acknowledges grant support from The Israel Science Foundation (grant number 472/15), The Aimwell Charitable Trust Foundation (UK). F. L‐S. is a member of the Dr Adolph and Klara Brettler Centre for Research in Molecular Pharmacology and Therapeutics, and the David R. Bloom Center for Pharmacy at The Hebrew University of Jerusalem's School of Pharmacy and Arthur Guttermann Funds (USA). NC‐IUPHAR receives financial support from the Wellcome Trust.
Landolina, N. , and Levi‐Schaffer, F. (2016) Monoclonal antibodies: the new magic bullets for allergy: IUPHAR Review 17. British Journal of Pharmacology, 173: 793–803. doi: 10.1111/bph.13396.
This article is an NC‐IUPHAR review. The authors are the Chairperson (F. Levi‐Schaffer) and a member (N. Landolina) of ImmuPhar, the Immunopharmacology Section of the International Union of Basic and Clinical Pharmacology (IUPHAR).
References
- Agusti‐Mejias A, Messeguer F, Garcia R, Febrer I (2013). Severe refractory atopic dermatitis in an adolescent patient successfully treated with ustekinumab. Ann Dermatol 25: 368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G Protein‐Coupled Receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai I, Tsuji M, Takeda H, Akiyama N, Saito S (2013). A single dose of interleukin‐31 (IL‐31) causes continuous itch‐associated scratching behaviour in mice. Exp Dermatol 22: 669–671. [DOI] [PubMed] [Google Scholar]
- Arron JR, Choy DF, Scheerens H, Matthews JG (2013). Noninvasive biomarkers that predict treatment benefit from biologic therapies in asthma. Ann Am Thorac Soc 10 (Suppl): S206–S213. [DOI] [PubMed] [Google Scholar]
- Baines KJ, Simpson JL, Wood LG, Scott RJ, Fibbens NL, Powell H, et al (2014). Sputum gene expression signature of 6 biomarkers discriminates asthma inflammatory phenotypes. J Allergy Clin Immunol 133: 997–1007. [DOI] [PubMed] [Google Scholar]
- Bauer RN, Manohar M, Singh AM, Jay DC, Nadeau KC (2015). The future of biologics: applications for food allergy. J Allergy Clin Immunol 135: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al (2007). Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med 101: 1483–1492. [DOI] [PubMed] [Google Scholar]
- Brennan FR, Morton LD, Spindeldreher S, Kiessling A, Allenspach R, Hey A, et al (2010). Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs 2: 233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn S, Fang Y, Barrenas F, Gustafsson M, Zhang H, Konstantinell A, et al (2014). A generally applicable translational strategy identifies S100A4 as a candidate gene in allergy. Sci Transl Med 6: 218ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al (2013). Randomized, double‐blind, placebo‐controlled study of brodalumab, a human anti‐IL‐17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 188: 1294–1302. [DOI] [PubMed] [Google Scholar]
- Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al (2011). Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo‐controlled study. Am J Respir Crit Care Med 184: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al (2014). Benralizumab, an anti‐interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose‐ranging study. Lancet Respir Med 2: 879–890. [DOI] [PubMed] [Google Scholar]
- Cataldi M, Borriello F, Granata F, Annunziato L, Marone G (2014). Histamine receptors and antihistamines: from discovery to clinical applications. Chem Immunol Allergy 100: 214–226. [DOI] [PubMed] [Google Scholar]
- Chen JB, Wu PC, Hung AF, Chu CY, Tsai TF, Yu HM, et al (2010). Unique epitopes on C epsilon mX in IgE‐B cell receptors are potentially applicable for targeting IgE‐committed B cells. J Immunol 184: 1748–1756. [DOI] [PubMed] [Google Scholar]
- Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A (2014). IL‐17 in severe asthma. Where do we stand? Am J Respir Crit Care Med 190: 1094–1101. [DOI] [PubMed] [Google Scholar]
- Chowdhury PS, Chen Y, Yang C, Cook KE, Nyborg AC, Ettinger R, et al (2012). Targeting the junction of CεmX and ε‐migis for the specific depletion of mIgE‐expressing B cells. Mol Immunol 52: 279–288. [DOI] [PubMed] [Google Scholar]
- Cohen ES, Scott IC, Majithiya JB, Rapley L, Kemp BP, England E, et al (2015). Oxidation of the alarmin IL‐33 regulates ST2‐dependent inflammation. Nat Commun 6: 8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, et al (2011). Allergen‐induced of IL‐25 and IL‐25 receptor in atopic asthmatic airways and late‐phase cutaneous responses. J Allergy Clin Immunol 128: 116–124. [DOI] [PubMed] [Google Scholar]
- Cox L, Platts‐Mills TA, Finegold I, Schwartz LB, Simons FE, Wallace DV, et al (2007). A merican Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force report on omalizumab‐associated anaphylaxis. J Allergy Clin Immunol 120: 1373–1377. [DOI] [PubMed] [Google Scholar]
- Ecker DM, Jones SD, Levine HL (2015). The therapeutic monoclonal antibody market. MAbs 7: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elishmereni M, Alenius HT, Bradding P, Mizrahi S, Shikotra A, Minai‐Fleminger Y, et al (2011). Physical interactions between mast cells and eosinophils: a novel mechanism enhancing eosinophil survival in vitro . Allergy 66: 376–385. [DOI] [PubMed] [Google Scholar]
- Fajt ML, Wenzel SE (2015). Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol 135: 299–310 quiz 311. [DOI] [PubMed] [Google Scholar]
- Farahani R, Sherkat R, Hakemi MG, Eskandari N, Yazdani R (2014). Cytokines (interleukin‐9, IL‐17, IL‐22, IL‐25 and IL‐33) and asthma. Adv Biomed Res 3: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H (2013). The role of IL‐22 and Th22 cells in human skin diseases. J Dermatol Sci 72: 3–8. [DOI] [PubMed] [Google Scholar]
- Galvao VR, Castells MC (2015). Hypersensitivity to biological agents‐updated diagnosis, management, and treatment. J Allergy Clin Immunol pract 3: 175–185 quiz 186. [DOI] [PubMed] [Google Scholar]
- Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al (2014). Effects of an anti‐TSLP antibody on allergen‐induced asthmatic responses. New Engl J Med 370: 2102–2110. [DOI] [PubMed] [Google Scholar]
- Gittler JK, Shemer A, Suarez‐Farinas M, Fuentes‐Duculan J, Gulewicz KJ, Wang CQ, et al (2012). Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 130: 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JD, Suarez‐Farinas M, Dhingra N, Cardinale I, Li X, Kostic A, et al (2014). Dupilumab improves the molecular signature in skin of patients with moderate‐to‐severe atopic dermatitis. J Allergy Clin Immunol 134: 1293–1300. [DOI] [PubMed] [Google Scholar]
- Hammers CM, Stanley JR (2014). Antibody phage display: technique and applications. J Invest Dermatol 134: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ (2010). The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 9: 325–338. [DOI] [PubMed] [Google Scholar]
- Har D, Patel S, Khan DA (2015). Outcomes of using omalizumab for more than 1 year in refractory chronic urticaria. Ann Allergy Asthma Immunol 115: 126–129. [DOI] [PubMed] [Google Scholar]
- Harding FA, Stickler MM, Razo J, DuBridge RB (2010). The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs 2: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PH (2001). Regulation of the inflammatory response in asthma by mast cell products. Immunol Cell Biol 79: 149–153. [DOI] [PubMed] [Google Scholar]
- Hart TK, Blackburn MN, Brigham‐Burke M, Dede K, Al‐Mahdi N, Zia‐Amirhosseini P, et al (2002). Preclinical efficacy and safety of pascolizumab (SB 240683): a humanized anti‐interleukin‐4 antibody with therapeutic potential in asthma. Clin Exp Immunol 130: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawro T, Saluja R, Weller K, Altrichter S, Metz M, Maurer M (2014). Interleukin‐31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challange. Allergy 69: 113–117. [DOI] [PubMed] [Google Scholar]
- Hershey GK (2003). IL‐13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol 111: 677–690 quiz 691. [DOI] [PubMed] [Google Scholar]
- Holgate S, Smith N, Massanari M, Jimenez P (2009). Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy 64: 1728–1736. [DOI] [PubMed] [Google Scholar]
- Ishmael FT (2011). The inflammatory response in the pathogenesis of asthma. J Am Osteopath Assoc 111 (11 Suppl 7): S11–S17. [PubMed] [Google Scholar]
- Jensen RK, Plum M, Tjerrild L, Jakob T, Spillner E, Andersen GR (2015). Structure of the omalizumab Fab. Acta Crystallogr F Struct Biol Commun 71 (Pt 4): 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A (2015). Immunopathology of chronic rhinosinusitis. Allergol Int 64: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MA, Shin YS, Pham le D, Park HS (2014). Adult asthma biomarkers. Curr Opin Allergy Clin Immunol 14: 49–54. [DOI] [PubMed] [Google Scholar]
- Knolle MD, Rana BM, McKenzie AN (2015). IL‐25 as a potential therapeutic target in allergic asthma. Immunotherapy 7: 607–610. [DOI] [PubMed] [Google Scholar]
- Kohler G, Milstein C (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256: 495–497. [DOI] [PubMed] [Google Scholar]
- Lai T, Wang S, Xu Z, Zhang C, Zhao Y, Hu Y, et al (2015). Long‐term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta‐analysis. Sci Rep 5: 8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H (2015). The immunology of asthma. Nat Immunol 16: 45–56. [DOI] [PubMed] [Google Scholar]
- Landolina N, Gangwar RS, Levi‐Schaffer F (2015). Mast cells' integrated actions with eosinophils and fibroblasts in allergic inflammation: implications for therapy. Adv Immunol 125: 41–85. [DOI] [PubMed] [Google Scholar]
- Landolina NA, Levi‐Schaffer F (2014). Eosinophils as a pharmacological target for the treatment of allergic diseases. Curr Opin Pharmacol 17C: 71–80. [DOI] [PubMed] [Google Scholar]
- Leyva‐Castillo JM, Hener P, Jiang H, Li M (2013). TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol 133: 154–163. [DOI] [PubMed] [Google Scholar]
- Licari A, Marseglia G, Castagnoli R, Marseglia A, Ciprandi G (2015). The discovery and development of omalizumab for the treatment of asthma. Expert Opin Drug Discov 15: 1–10[E‐pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M (2015). Human mast cells are major IL‐22 producers in patients with psoriasis and atopic dermatitis. J Allergy Clin Immunol . doi:10.1016/j.jaci.2015.01.033. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- May RD, Monk PD, Cohen ES, Manuel D, Dempsey F, Davis NH, et al (2012). Preclinical development of CAT‐354, an IL‐13 neutralizing antibody, for the treatment of severe uncontrolled asthma. Br J Pharmacol 166: 177–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagaki T, Sugaya M (2015). Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci 78: 89–94. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Savage AK, Locksley RM (2015). Interleukin‐33 in tissue homeostasis, injury, and inflammation. Immunity 42: 1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon TC, Befus AD, Kulka M (2014). Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol 5: 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al (2014). Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 133: 1557–1563 e1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Arae K, Unno H, Toyama S, Motomura K, Matsuda A, et al (2015). IL‐25 and IL‐33 contribute to development of eosinophilic airway inflammation In epicutaneously antigen‐sensitized mice. PLoS One 10: e0134226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee M, Sehmi R, Nair P (2014). Anti‐IL5 therapy for asthma and beyond. World Allergy Organ J 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrtek D, Knoll M, Matthiesen T, Krause S, Lohrmann J, Schillinger D, et al (2004). Expression of interleukin‐13 receptor alpha 1‐subunit on peripheral blood eosinophils is regulated by cytokines. Immunology 112: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Kitoh A, Egawa G, Natsuaki Y, Nakamizo S, Moniaga CS, et al (2014). IL‐17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J Invest Dermatol 134: 2122–2130. [DOI] [PubMed] [Google Scholar]
- Nelson AL, Dhimolea E, Reichert JM (2010). Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 9: 767–774. [DOI] [PubMed] [Google Scholar]
- Newcomb DC, Peebles RS Jr (2013). Th17‐mediated inflammation in asthma. Curr Opin Immunol 25: 755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen GD, Hansen JS, Lund RM, Bergqvist M, Larsen ST, Clausen SK, et al (2002). IgE‐mediated asthma and rhinitis I: a role of allergen exposure? Pharmacol Toxicol 90: 231–242. [DOI] [PubMed] [Google Scholar]
- Nyborg AC, Zacco A, Ettinger R, Jack Borrok M, Zhu J, Martin T, et al (2015). Development of an antibody that neutralizes soluble IgE and eliminates IgE expressing B cells. Cell Mol Immunol . doi:10.1038/cmi.2015.19. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh CK, Leigh R, McLaurin KK, Kim K, Hultquist M, Molfino NA (2013). A randomized, controlled trial to evaluate the effect of an anti‐interleukin‐9 monoclonal antibody in adults with uncontrolled asthma. Respir Res 14: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh CK, Raible D, Geba GP, Molfino NA (2011). Biology of the interleukin‐9 pathway and its therapeutic potential for the treatment of asthma. Inflamm Allergy Drug Targets 10: 180–186. [DOI] [PubMed] [Google Scholar]
- Oldham RK, Dillman RO (2008). Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol 26: 1774–1777. [DOI] [PubMed] [Google Scholar]
- Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al (2012). Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet 380: 651–659. [DOI] [PubMed] [Google Scholar]
- Pawankar R (2014). Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV (2014). Measures of gene expression in sputum cells can identify TH2‐high and TH2‐low subtypes of asthma. J Allergy Clin Immunol 133: 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinart M, Benet M, Annesi‐Maesano I, von Berg A, Berdel D, Carlsen KC, et al (2014). Comorbidity of eczema, rhinitis, and asthma in IgE‐sensitised and non‐IgE‐sensitised children in MeDALL: a population‐based cohort study. Lancet Respir Med 2: 131–140. [DOI] [PubMed] [Google Scholar]
- Platts‐Mills TA, Woodfolk JA (2011). Allergens and their role in the allergic immune response. Immunol Rev 242: 51–68. [DOI] [PubMed] [Google Scholar]
- Portelli MA, Hodge E, Sayers I (2015). Genetic risk factors for the development of allergic disease identified by genome‐wide association. Clin Exp Allergy 45: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quax RA, Manenschijn L, Koper JW, Hazes JM, Lamberts SW, van Rossum EF, et al (2013). Glucocorticoid sensitivity in health and disease. Nat Rev Endocrinol 9: 670–686. [DOI] [PubMed] [Google Scholar]
- Razinkov VI, Treuheit MJ, Becker GW (2015). Accelerated formulation development of monoclonal antibodies (mAbs) and mAb‐based modalities: review of methods and tools. J Biomol Screen 20: 468–483. [DOI] [PubMed] [Google Scholar]
- Reynolds JM, Angkasekwinai P, Dong C (2010). IL‐17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev 21: 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP (2006). The eosinophil. Annu Rev Immunol 24: 147–174. [DOI] [PubMed] [Google Scholar]
- Scheerens H, Arron JR, Zheng Y, Putnam WS, Erickson RW, Choy DF, et al (2014). The effects of lebrikizumab in patients with mild asthma following whole lung allergen challenge. Clin Exp Allergy 44: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehra S, Yao W, Nguyen ET, Glosson‐Byers NL, Akhtar N, Zhou B, et al (2015). T9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol . doi:10.1016/j.jaci.2015.01.021. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengler C, Lau S, Wahn U, Nickel R (2002). Interactions between genes and environmental factors in asthma and atopy: new developments. Respir Res 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G (2004). The role of human mast cell‐derived cytokines in eosinophil biology. J Interferon Cytokine Res 24: 271–281. [DOI] [PubMed] [Google Scholar]
- Shin HW, Kim DK, Park MH, Eun KM, Lee M, So D, et al (2015). IL‐25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol 135: 1476–1485. [DOI] [PubMed] [Google Scholar]
- Shimbara A, Christodoulopoulos P, Soussi‐Gounni A, Olivenstein R, Nakamura Y, Levitt RC, et al (2000). IL‐9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J Allergy Clin Immunol 105 (1 Pt 1): 108–115. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Scott R, Boyle MJ, Gibson PG (2006). Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 11: 54–61. [DOI] [PubMed] [Google Scholar]
- Sussman G, Hebert J, Barron C, Bian J, Caron‐Guay RM, Laflamme S, et al (2014). Real‐life experiences with omalizumab for the treatment of chronic urticaria. Ann Allergy Asthma Immunol 112: 170–174. [DOI] [PubMed] [Google Scholar]
- Trevor JL, Deshane JS (2014). Refractory asthma: mechanisms, targets, and therapy. Allergy 69: 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsabouri S, Tseretopoulou X, Priftis K, Ntzani EE (2014). Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systemic review and meta‐analysis of randomized clinical trials. J Allergy Clin Immunol Pract 3: 332–340. [DOI] [PubMed] [Google Scholar]
- Umetsu DT, Rachid R, Schneider LC (2015). Oral immunotherapy and anti‐IgE antibody treatment for food allergy. World Allergy Organ J 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashisht P, Casale T (2013). Omalizumab for treatment of allergic rhinitis. Expert Opin Biol Ther 13: 933–945. [DOI] [PubMed] [Google Scholar]
- Vercelli D (2008). Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol 8: 169–182. [DOI] [PubMed] [Google Scholar]
- Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al (2013). Dupilumab in persistent asthma with elevated eosinophil levels. New Engl J Med 368: 2455–2466. [DOI] [PubMed] [Google Scholar]
- Wenzel SE (2012). Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 18: 716–725. [DOI] [PubMed] [Google Scholar]
- Yalcin AD (2014). An overview of the effects of anti‐IgE therapies. Med Sci Monit 20: 1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T (2011). Therapeutic monoclonal antibodies. Keio J Med 60: 37–46. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Tashimo H, Ishida H, Kaneko F, Nakano J, Kato H, et al (2002). Attenuation of airway hyperresponsiveness in a murine asthma model by neutralization of granulocyte‐macrophage colony‐stimulating factor (GM‐CSF). Cell Immunol 219: 92–97. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W (2008). Structures and biological functions of IL‐31 and IL‐31 receptors. Cytokine Growth Factor Rev 19: 347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberbier T, Maurer M (2015). Omalizumab for the treatment of chronic urticaria. Expert Rev Clin Immunol 11: 171–180. [DOI] [PubMed] [Google Scholar]


