Abstract
BACKGROUND
Data from a cardiopulmonary exercise (CPX) test are used to determine prognosis in patients with chronic heart failure (HF). However, few published studies have simultaneously compared the relative prognostic strength of multiple CPX variables.
OBJECTIVES
We sought to describe the strength of the association among variables measured during a CPX test and all-cause mortality in patients with HF with reduced ejection fraction (HFrEF), including the influence of sex and patient effort, as measured by respiratory exchange ratio (RER).
METHODS
Among patients (n = 2,100, 29% women) enrolled in the HF-ACTION (HF-A Controlled Trial Investigating Outcomes of exercise traiNing) trial, 10 CPX test variables measured at baseline (e.g., peak oxygen uptake [VO2], exercise duration, percent predicted peak VO2 [%ppVO2], ventilatory efficiency) were examined.
RESULTS
Over a median follow-up of 32 months, there were 357 deaths. All CPX variables, except RER, were related to all-cause mortality (all p < 0.0001). Both %ppVO2 and exercise duration were equally able to predict (Wald χ2: ~141) and discriminate (c-index: 0.69) mortality. Peak VO2 (mL·kg−1·min−1) was the strongest predictor of mortality among men (Wald χ2: 129) and exercise duration among women (Wald χ2: 41). Multivariable analyses showed that %ppVO2, exercise duration, and peak VO2 (mL·kg−1·min−1) were similarly able to predict and discriminate mortality. In men, a 10% 1-year mortality rate corresponded to a peak VO2 of 10.9 mL·kg−1·min−1 versus 5.3 mlkg−1/min−1 in women.
CONCLUSIONS
Peak VO2, exercise duration, and % ppVO2 carried the strongest ability to predict and discriminate the likelihood of death in patients with HFrEF. The prognosis associated with a given peak V2 differed by sex.
Keywords: peak VO2, respiratory exchange ratio, sex, survival
In patients with chronic heart failure (HF), determining risk for clinical events to help guide clinical decisions can be accomplished using 1 of several multivariable risk prediction models (1–5). These multivariable models are complex yet invaluable for determining prognosis, and several have been externally validated against other datasets (6). However, each model requires time to assemble the needed variables and compute the risk score, which is often completed after the patient and family members are no longer present with the clinician. As a result, such scores are not commonly used in routine clinical practice.
Data measured during a cardiopulmonary exercise (CPX) test (e.g., peak oxygen uptake, [VO2]) are used to supplement the determination of prognosis because they have a strong and independent association with clinical outcomes, avoid having to gather many variables for computation of a risk score, and results can be easily translated to a patient and his/her family. In patients with HF with reduced ejection fraction (HFrEF), other CPX-derived variables strongly associated with clinical outcomes include ventilatory efficiency (minute ventilation-carbon dioxide production [VE -VCO2 slope]), percent predicted peak VO2 (%ppVO2), and exercise oscillatory ventilation (7,8).
However, most published studies have limited their analyses to the prognostic capabilities of just 2 or 3 of these variables, such that few studies have been sufficiently powered or contained enough prospective data to evaluate multiple CPX-derived variables simultaneously. A single large study that compares the prognostic strength of many CPX variables is needed in order to understand their relative importance to prognosis and guide clinical decisions. Also, prior studies addressing the influence of the sex-based differences in exercise capacity (9) on survival suggest that the peak VO2 associated with a similar rate of survival is higher in men (10,11), inferring that the uniform application of “sex-neutral” values when determining risk may not be appropriate.
The primary aim of this study is to determine the strength of the association between multiple variables measured during CPX testing and all-cause mortality in a cohort of patients with HFrEF. Secondary aims were to determine whether: 1) the findings for the entire cohort differed by sex; and 2) the prognostic and discriminatory qualities of CPX-derived variables were influenced by level of patient effort during testing, as measured by peak respiratory exchange ratio (RER).
METHODS
The eligibility characteristics for subjects and the exercise testing protocol used in HF-ACTION (HF-A Controlled Trial Investigating Outcomes of exercise traiNing) have been reported (12,13). HF-ACTION evaluated the effects of exercise training on the combined outcome of all-cause mortality and hospitalization in patients with an ejection fraction ≤35% and New York Heart Association (NYHA) class II to IV symptoms despite guideline-based therapy. The protocol was approved by the institutional review board at each center. After providing informed consent and before randomization, subjects underwent an exercise test with analysis of expired gases.
EXERCISE TESTING
All CPX tests were completed per American College of Cardiology/American Heart Association guidelines (14). There were 2,100 subjects who underwent CPX testing at baseline using a modified Naughton treadmill protocol (15). Tests were terminated due to general/leg fatigue, symptoms (e.g., angina), adverse changes in blood pressure, musculoskeletal complaints, or electrocardiographic evidence of ischemia or arrhythmia. Tests were supervised by a physician or health professional such as a clinical exercise physiologist with medical supervision available. Subjects were asked to take their medications as usual on the day of testing and to take their beta-adrenergic blocking agent between 3 and 10 hours before testing.
Testing personnel were instructed to exercise patients to a Borg rating of perceived exertion level >16 (6 to 20 scale) and/or an RER (VCO2/VO2) >1.1, but not to use achievement of these criteria or heart rate achieved as a reason to stop a test. Two independent reviewers determined peak VO2 and ventilatory-derived anaerobic threshold (VAT). Peak VO2 was defined as the highest VO2, expressed in absolute terms (l/min−1) and adjusted to body weight (mL·kg−1·min−1) for a 15- or 20-second interval within the last 90 seconds of exercise or first 30 seconds of recovery, whichever was higher. VAT was determined using the V-slope method (16) and %ppVO2 calculated using the Wasserman equation (17). Oxygen pulse was computed as absolute VO2 divided by heart rate. VE -VCO2 slope was calculated using data from the entire duration of exercise and used 15- or 20-second averaged data for VCO2 (l/min−1) and VE (l/min−1). Oxygen uptake efficiency slope was calculated using all data and linear regression analysis, with VO2 (ml/min−1) on the y-axis and log VE on the x-axis. Data on exercise oscillatory ventilation or the partial pressure of end-tidal CO2 were not available.
The primary outcome of this secondary analysis from the HF-ACTION trial was all-cause mortality. All deaths were verified by a clinical endpoint committee (18).
STATISTICAL ANALYSIS
Subject characteristics were summarized by percentages for categorical variables and median with 5th and 95th percentiles for continuous variables, computed for the entire cohort and for men and women separately.
Lower and higher risk were defined as 1-year mortality rates of ≤3% and ≥10%, respectively, with the latter based on patients who would, on average, experience better survival at 1 year should they undergo cardiac transplantation (19).
A prior multivariable Cox model from HF-ACTION that included 45 clinical and demographic candidate variables and 3 CPX variables (peak VO2, VE -VCO2 slope, and exercise duration) determined that peak VO2 (mL·kg−1·min−1) and exercise duration were strong and independent predictors of clinical outcomes (2). The current study analyses included the above 3 CPX variables and 7 additional CPX variables (peak VO2 [l/min−1], %ppVO2, RER, heart rate reserve, VO2 at VAT, peak oxygen pulse, oxygen uptake efficiency slope).
Univariable associations with mortality were assessed using Cox proportional hazards model and the corresponding hazard ratio (HR) and 95% confidence interval (CI). The c-index was optimism corrected using the bootstrap method. Multivariable associations were similarly computed, but adjusted for age and sex to mimic what information might be readily available to the clinician at the time they discuss test results with the patient. The proportional hazards assumption of the univariate Cox regressions was assessed by the Wald test for linearity of the log hazard ratio using restricted cubic splines. For the 10 CPX variables, we studied only absolute peak VO2 and oxygen uptake efficiency slope showed statistical evidence of non-linearity. For those 2 variables, modeling with restricted cubic splines did not change the c-index from the value obtained by assuming proportional hazards.
Using the Cox model, 1- and 3-year survival probabilities were plotted by sex as a function of peak VO2. Kaplan-Meier curves were plotted and stratified based on peak VO2 category (<12, 12–18, >18 mL·kg−1·min−1) and peak RER (<1.05, ≥1.05).
RESULTS
Table 1 shows baseline characteristics for all patients and for men (n = 1,493) and women (n = 607) undergoing CPX testing at baseline. In general, men were slightly older and more likely to have an ischemic cardiomyopathy and report race as white. Table 2 shows the responses of patients during CPX testing. Absolute and adjusted peak VO2, exercise duration, and VO2 at VAT were higher in men.
TABLE 1.
Demographic and Clinical Characteristics
| All Patients (N = 2,100) | Men (n = 1,493) | Women (n = 607) | |
|---|---|---|---|
| Age, yrs | 59 (36–78) | 60 (39–79) | 57 (34–78) |
| Race* | |||
| Black | 705 (34) | 420 (28) | 285 (47) |
| White | 1,251 (60) | 970 (65) | 281 (46) |
| Other | 113 (5) | 84 (6) | 29 (2) |
| NYHA class | |||
| II | 1,338 (64) | 960 (64) | 378 (62) |
| III/IV | 762 (36) | 533 (36) | 229 (38) |
| Ischemic etiology | 1,060 (50) | 873 (58) | 187 (31) |
| Left ventricular ejection fraction, % | 25 (14–37) | 24 (14–36) | 25 (13–39) |
| Atrial fibrillation | 441 (21) | 371 (25) | 70 (12) |
| Diabetes | 681 (32) | 504 (34) | 177 (29) |
| Hypertension* | 1,270 (61) | 922 (62) | 348 (58) |
| Systolic blood pressure, mm Hg | 112 (88–146) | 111 (90–146) | 112 (86–148) |
| Diastolic blood pressure, mm Hg | 70 (54–90) | 70 (54–90) | 70 (54–90) |
| Heart rate, beats·min−1 | 70 (54–92) | 70 (54–90) | 71 (54–88) |
| Blood urea nitrogen, mg/dl−1 | 20 (10–49) | 21 (11–53) | 17 (9–43) |
| Creatinine, mg/dl−1 | 1.2 (0.8–2.1) | 1.2 (0.8–2.2) | 1.0 (0.7–1.8) |
| Medications and devices | |||
| ACEI or ARB | 1,982 (94) | 1,408 (94) | 574 (95) |
| Beta-adrenergic blocking agent | 1,980 (94) | 1,408 (94) | 572 (94) |
| Aldosterone receptor antagonist | 935 (45) | 647 (43) | 288 (47) |
| Loop diuretic | 1,628 (78) | 1,165 (78) | 463 (76) |
| Biventricular pacemaker | 373 (18) | 291 (19) | 82 (14) |
Values are median (5th–95th percentiles) or n (%).
Indicates the number of patients (%) with nonmissing data.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; NYHA = New York Heart Association.
TABLE 2.
Cardiopulmonary Exercise Test Responses
| All Patients | Men | Women | |
|---|---|---|---|
| Absolute peak VO2, l/min−1 | 1.34 (1.00–2,40) | 1.45 (0.37–2.52) | 1.11 (6.05–1.81) |
| Relative peak VO2, mL·kg−1·min−1 | 14.6 (8.3–23.7) | 15.2 (8.7–24.3) | 13.4 (7.7–21.0) |
| Percent predicted peak VO2, % | 60 (34–90) | 57 (32–86) | 68 (39–95) |
| VE – VCO2 slope | 32.4 (23.4–52.1) | 32.6 (23.5–53.5) | 31.8 (23.4–48.9) |
| VO2 at VAT, mL·kg−1·min−1 | 10.7 (6.9–15.7) | 10.8 (7.1–15.9) | 10.0 (6.5–14.7) |
| Oxygen uptake efficiency slope | 1655 (846–2800) | 1788 (876–2886) | 1401 (750–2276) |
| Peak oxygen pulse, ml/beat−1 | 11.3 (6.0–18.6) | 12.5 (6.8–19.4) | 9.0 (5.1–13.7) |
| Peak respiratory exchange ratio | 1.08 (0.91–1.27) | 1.09 (0.92–1.28) | 1.06 (0.88–1.24) |
| Exercise duration, min | 9.8 (4.0–17.0) | 10.0 (4.2–17.7) | 9.0 (3.5–15.2) |
| Peak heart rate, beats/min−1 | 120 (86–160) | 118 (85–157) | 125 (89–162) |
| Borg rate of perceived exertion | 17 (13–20) | 17 (13–20) | 17 (13–20) |
| Heart rate reserve (peak-rest), beats/min−1 | 49 (17–86) | 47 (16–85) | 54 (20–90) |
Values are median (5th–95th percentiles).
VAT = ventilatory-derived anaerobic threshold; VE = minute ventilation; VCO2 = carbon dioxide production; VO2 = oxygen uptake.
CLINICAL ENDPOINTS
The median duration of follow-up was 32 (8, 48 [5th, 95th percentile]) months. Overall, there were 357 deaths, with a crude annual mortality rate of 6.6%. Univariable analysis showed that all of the variables measured during CPX testing, except for RER, were strongly (all p < 0.0001) related to all-cause mortality (Table 3). Percent predicted peak VO2 and exercise duration were the 2 strongest predictors of mortality (Wald χ2: 141 and 142, respectively) and equal relative to their ability to discriminate mortality (c-index: 0.69 for both). Peak VO2 (mL·kg−1·min−1) and VE -VCO2 slope were also strong predictors of mortality (Wald χ2: 130 and 133, respectively).
TABLE 3.
Association between Variables Measured during CPX Testing and Mortality (Univariate Analyses)
| Parameters | Hazard Ratio (95% Confidence Interval) | Wald chi-square* | c-index (Optimism Corrected) |
|---|---|---|---|
|
| |||
| Percent predicted peak VO2, per 5% change | |||
| All | 0.81 (0.78–0.84) | 141 | 0.690 (0.661– 0.719) |
| Men | 0.81 (0.78–0.85) | 100 | 0.678 (0.646–0.712) |
| Women | 0.82 (0.76–0.89) | 30 | 0.690 (0.628–0.753) |
|
| |||
| Exercise duration, min | |||
| All | 0.83 (0.80–0.85) | 142 | 0.689 (0.660–0.718) |
| Men | 0.83 (0.80–0.85) | 117 | 0.700 (0.669–0.731) |
| Women | 0.78 (0.73–0.84) | 41 | 0.710 (0.641–0.779) |
|
| |||
| Relative peak VO2, mL·kg−1·min−1 | |||
| All | 0.85 (0.83–0.87) | 130 | 0.684 (0.655–0.713) |
| Men | 0.83 (0.81–0.86) | 129 | 0.708 (0.676–0.740) |
| Women | 0.84 (0.78–0.90) | 25 | 0.673 (0.605–0.740) |
|
| |||
| Absolute peak VO2, per 100 ml/min−1 | |||
| All | 0.88 (0.86–0.90) | 96 | 0.663 (0.632–0.694) |
| Men | 0.86 (0.84–0.89) | 109 | 0.689 (0.656–0.722) |
| Women | 0.79 (0.73–0.86) | 30 | 0.700 (0.634–0.766) |
|
| |||
| Peak respiratory exchange ratio, per 0.1 units | |||
| All | 0.97 (0.88–1.07) | 1 (p=0.50) | 0.501 (0.469–0.533) |
| Men | 0.96 (0.86–1.07) | 1 (p=0.47) | 0.507 (0.472–0.543) |
| Women | 0.84 (0.68–1.05) | 2 (p=0.12) | 0.527 (0.453–0.601) |
|
| |||
| Ventilatory efficiency (VE – VCO2 slope), per 5 units | |||
| All | 1.29 (1.23–1.34) | 133 | 0.648 (0.616–0.68) |
| Men | 1.27 (1.21–1.33) | 99 | 0.641 (0.605–0.677) |
| Women | 1.34 (1.20–1.49) | 28 | 0.668 (0.592–0.743) |
|
| |||
| Heart rate reserve, per 5 beats/min−1 | |||
| All | 0.87 (0.85–0.90) | 103 | 0.660 (0.630–0.689) |
| Men | 0.88 (0.85–0.90) | 73 | 0.656 (0.623–0.688) |
| Women | 0.87 (0.82–0.92) | 22 | 0.643 (0.567–0.719) |
|
| |||
| VO2 at VAT, mL·kg−1·min−1 | |||
| All | 0.83 (0.78–0.87) | 56 | 0.640 (0.606–0.674) |
| Men | 0.80 (0.76–0.85) | 60 | 0.659 (0.621–0.697) |
| Women | 0.83 (0.72–0.95) | 8 (p=0.005) | 0.627 (0.545–0.710) |
|
| |||
| Peak oxygen pulse, ml/beat−1 | |||
| All | 0.90 (0.88–0.93) | 43 | 0.611 (0.580–0.643) |
| Men | 0.86 (0.83–0.89) | 72 | 0.653 (0.618–0.687) |
| Women | 0.83 (0.75–0.92) | 13 | 0.637 (0.572–0.703) |
|
| |||
| Oxygen uptake efficiency slope (all data), per 100 units | |||
| All | 0.94 (0.90–0.93) | 75 | 0.639 (0.607–0.671) |
| Men | 0.90 (0.89–0.92) | 84 | 0.657 (0.622–0.693) |
| Women | 0.85 (0.80–0.91) | 25 | 0.698 (0.635–0.761) |
p values significant at p< 0.0001 except where otherwise indicated.
CPX = cardiopulmonary exercise; other abbreviations as in Table 2.
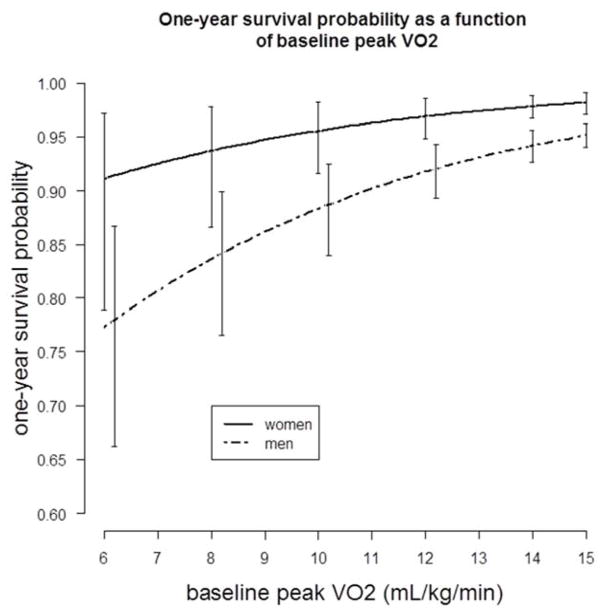
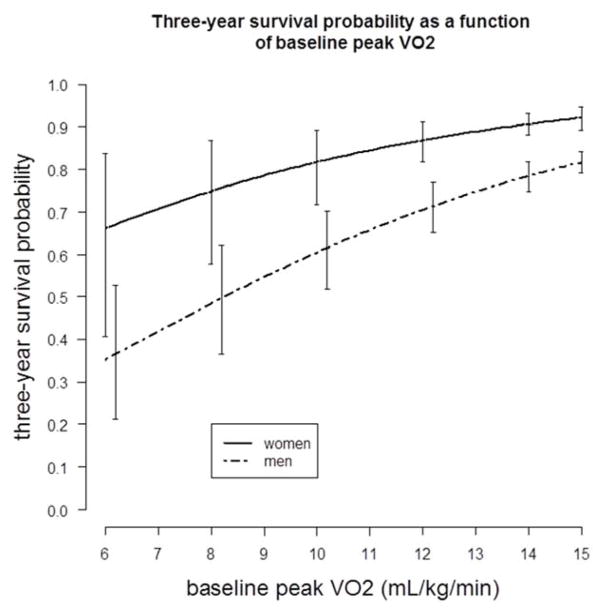
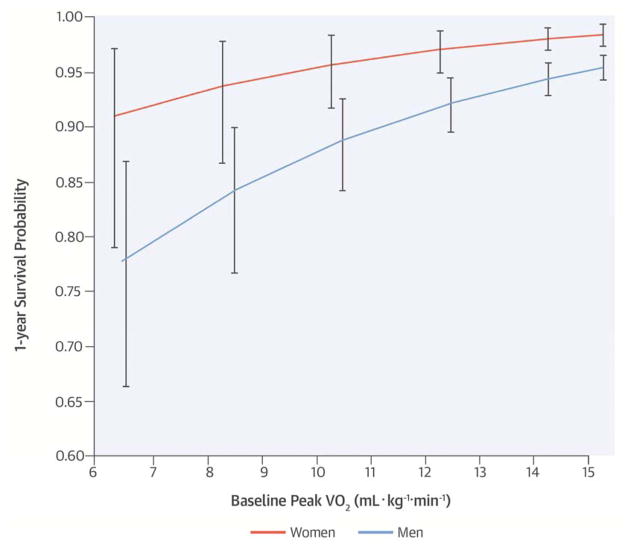
Peak VO2 was the strongest predictor of mortality among men (Wald χ2: 129; p < 0.0001) whereas in women, exercise duration was the strongest (Wald χ2: 41; p < 0.0001). Peak VO2 in men and exercise duration in women similarly discriminated mortality (c-index: 0.708 and 0.710, respectively). Sex-specific 1-year and 3-year survival probability curves for peak VO2 are presented in the Central Illustration and Figure 1, respectively. At a peak VO2 of 11 mL·kg−1·min−1 the estimated 1-year mortality rate is <4% in women versus ~10% in men.
FIGURE 1. Three-year Survival and Baseline Peak VO2.
Using a Cox proportional hazards model, 3-year survival probabilities as a function of baseline peak oxygen uptake (peak VO2) in men and women are similar to those seen at 1 year (Central Illustration). Vertical bars represent 95% confidence intervals.
In the multivariable analyses for the 10 CPX test-derived variables, each adjusted for age and sex, all CPX variables were significant (p <0.0001) predictors of mortality, except for RER (Table 4). As evidenced by the Wald chi-square test and the c-index, %ppVO2, exercise duration, and adjusted peak VO2 were all able to similarly predict and discriminate all-cause mortality. The association between VE -VCO2 slope and mortality remained significant, but the strength of the association was reduced. For every 1 mL·kg−1·min−1lower peak VO2, there was ~16% greater risk. Similarly, for every 5 percentage point lower %ppVO2, there was a corresponding ~19% higher risk. Table 5 shows the %ppVO2, exercise duration, and peak VO2 values that correspond to lower-risk (1-year mortality = 3%) and higher-risk (1-year mortality = 10%) patients. A 10% 1-year mortality rate corresponded to a peak VO2 of 10.9 mL·kg−1·min−1 in men versus a value of 5.3 mL·kg−1·min−1 in women.
Table 4.
Association between Variables Measured during CPX Testing and Mortality* (Multivariable Analyses)
| Parameters | Hazard Ratio (95% Confidence Interval) | Multivariate Wald chi-square value† | Overall Model c-index (Optimism Corrected) |
|---|---|---|---|
| Percent predicted peak VO2, per 5% change | 0.81 (0.78–0.84) | 133 | 0.707 (0.678–0.736) |
| Exercise duration, min | 0.84 (0.82–0.87) | 131 | 0.712 (0.681–0.744) |
| Relative peak VO2, mL·kg−1·min−1 | 0.84 (0.82–0.87) | 133 | 0.711 (0.682–0.740) |
| Absolute peak VO2, per 100 ml/min−1 | 0.86 (0.84–0.88) | 112 | 0.699 (0.670–0.723) |
| Peak RER, per 0.1 units | 0.94 (0.86–1.04) | 1 (p=0.24) | 0.612 (0.581–0.643) |
| Ventilatory efficiency (VE – VCO2 slope), per 5 units | 1.25 (1.19–1.31) | 90 | 0.666 (0.635–0.696) |
| Heart rate reserve, per 5 beats/min−1 | 0.89 (0.86–0.91) | 74 | 0.673 (0.644–0.703) |
| VO2 at VAT, mL·kg−1·min−1 | 0.82 (0.78–0.86) | 56 | 0.674 (0.640–0.707) |
| Peak oxygen pulse, ml.beat−1 | 0.87 (0.84–0.90) | 59 | 0.662 (0.632–0.692) |
| Oxygen uptake efficiency slope (all data), per 100 units | 0.91 (0.89–0.93) | 78 | 0.670 (0.639–0.700) |
TABLE 5.
Variables Corresponding to Lower- and Higher-risk Patients at 1-year Follow-up
| Lower Risk* | Higher Risk* | |||||
|---|---|---|---|---|---|---|
| All | Men | Women | All | Men | Women | |
| Peak oxygen uptake, mL·kg−1·min−1 | 16.9 | 17.7 | 12.2 | 9.2 | 10.9 | 5.3 |
| Percent predicted peak oxygen uptake, % | 68 | 69 | 61 | 38 | 39 | 29 |
| Exercise duration, min | 11.4 | 12.2 | 8.1 | 4.9 | 5.7 | 3.0 |
Lower risk defined as 3% 1-year mortality rate and higher risk as 10% 1-year mortality rate.
Cox modeling involving peak VO2, age, and sex showed that beta-adrenergic blocking agent, aldosterone antagonist, NYHA class (II versus III/IV), and biventricular pacemaker did not modify the relationship between peak VO2 and mortality (i.e., no interaction effect, p values not significant). The interaction term was significant for loop diuretic (p = 0.01; yes = HR: 0.83; 95% CI: 0.81 to 0.86; no = HR: 0.91; 95% CI: 0.85 to 0.97) and for angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker (p = 0.02; yes = HR: 0.83; 95% CI: 0.81 to 0.86; no = HR: 0.92; 95% CI: 0.84 to 1.01).
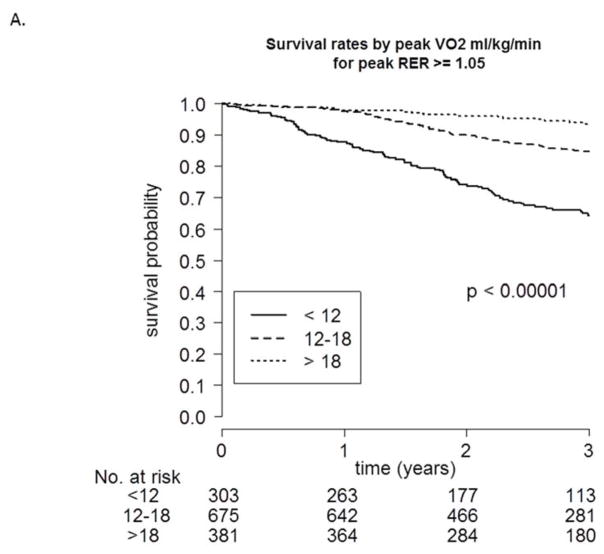
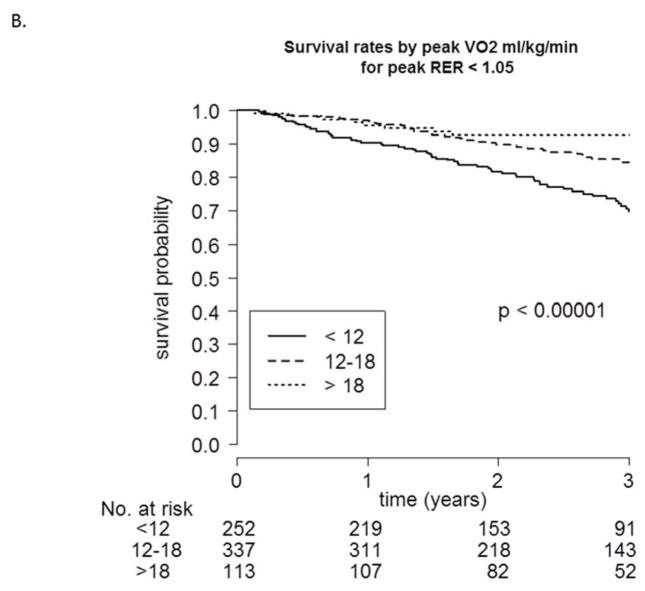
To evaluate the potential influence of achieved peak RER on predicting mortality, we first investigated survival probability during follow-up for 3 different categories of peak VO2 both above (Figure 2A) and below (Figure 2B) an RER of 1.05. As shown in both figures, a peak VO2 <12 mL·kg−1·min−1 was associated with poorer survival when compared to the other 2 categories. Table 6 shows the c-index values, adjusted for age and sex, for peak VO2, exercise duration, and VE -VCO2 slope for 5 different RER categories. In general, at an RER >0.95, adjusted peak VO2 shared a generally similar ability to discriminate events (c-indexes: 0.696 to 0.730).
FIGURE 2. Survival Rates Categorized by Peak VO2.
Kaplan-Meier survival curves stratified based on peak VO2 category (<12, 12–18, >18 mL·kg−1·min−1), both greater than or equal to (A) and less than (B) a respiratory exchange ratio (RER) of 1.05.
TABLE 6.
Mortality Discrimination based on c-index per RER Categories
| Peak RER | Overall Model c-index | ||
|---|---|---|---|
| Peak Oxygen Uptake (Peak VO2) | Exercise Duration | Ventilatory Efficiency (VE-VCO2 Slope) | |
| ≤0.95 (n = 230) | 0.600 (0.501–0.691) | 0.562 (0.468–0.656) | 0.532 (0.428–0.638) |
| >0.95 to ≤1.00 (n = 234) | 0.717 (0.639–0.795) | 0.755 (0.679–0.831) | 0.707 (0.611–0.804) |
| >1.00 to ≤1.05 (n = 317) | 0.703 (0.633–0.774) | 0.700 (0.621–0.778) | 0.676 (0.604–0.747) |
| >1.05 to ≤1.10 (n = 430) | 0.696 (0.624–0.768) | 0.691 (0.608–0.774) | 0.652 (0.582–0.722) |
| >1.10 (n = 850) | 0.730 (0.689–0.771) | 0.737 (0.693–0.781) | 0.683 (0.634–0.732) |
DISCUSSION
In a multicenter study involving a demographically diverse cohort of patients with HFrEF, multiple CPX variables were significantly related to survival, with %ppVO2 and exercise duration demonstrating the strongest association (Wald χ2 ~141, c-index ~ 0.690). After adjusting for age and sex, peak VO2, %ppVO2, and exercise duration were the strongest predictors of survival (Wald χ2 ~132, c-index ~0.710).
This information is unique and important because it is derived from an analysis that evaluated 10 CPX test variables in the same large cohort of patients with HFrEF to compare the relative strength of each variable’s independent relationship with mortality. Although there is extensive literature describing the relationship between various gas exchange variables and clinical outcomes in HF, most prior studies focused on comparing a then novel variable to 1 to 3 other traditional variables such as peak VO2 or VE -VCO2 slope. Two smaller or single-site studies also evaluated the association between multiple CPX variables and clinical events such as mortality, and reported results consistent with our current findings (20,21).
In our previously published predictive risk model that involved 48 demographic, clinical, and CPX-derived candidate variables, exercise duration and adjusted peak VO2 were 2 of the strongest variables associated with increased risk for clinical outcomes (2). In the present study, exercise duration was again 1 of the strongest predictors of mortality, which might lead one to rely on this variable alone for clinical risk stratification. Certainly, exercise duration is readily available to clinicians and avoids the costs for gas exchange equipment and staff needed to conduct CPX testing. Since the potential for colinearity exists between exercise duration and peak VO2 we examined for this and found that a significant correlation existed between the 2 variables (r = 0.80). To explore this association further, we fit a Cox model that included both peak VO2 and exercise duration, still adjusted for age and sex. We found that peak VO2 and exercise duration remained independently predictive of mortality (p < 0.0001). This indicates that both variables carry unique and important information about prognosis and as such, can be interpreted independently.
We offer 3 notes of caution relative to the use of exercise duration to determine risk. First, the exercise tests completed in this study used the same modified Naughton treadmill protocol; therefore, the predictive power of exercise duration if measured using a different protocol is unclear. Second, exercise duration was measured during a CPX test, from which attainment of peak effort (and therefore exercise duration) was likely influenced by the testers’ “real-time” interpretation of RER achieved. Third, exercise duration is influenced by subject familiarization, such that serial testing results in a significantly greater exercise duration with repeat testing, independent of any significant change in measured peak VO2 (22). If exercise testing is used as an adjunctive strategy to stratify risk in patients with HFrEF, the concurrent analysis of expired gases can provide an “RER-guided” exercise duration and a means to collect other important information that can help further parse risk (23). If regular exercise testing without gas exchange is performed, using the modified Naughton protocol and striving to achieve true symptom-limited maximum effort on initial and all subsequent testing will allow for comparison of results to data derived from HF-ACTION. In men, an exercise duration of 12.2 min was associated with a 1-year mortality rate of 3% (lower risk) and an exercise duration of 5.7 min was associated with a 1-year mortality rate of 10% (higher risk) (Table 5). In women, exercise durations of 8.3 min and 3.0 min were associated with 3% and 10% mortality risk at 1-year, respectively.
Ventilatory efficiency is also a strong or potentially stronger predictor of prognosis in patients with HFrEF (8). In our study VE –VCO2 slope was associated with mortality but the strength of the association (based on Wald chi-square test) was better for %ppVO2 and exercise duration (and peak VO2 in our multivariable analysis). Perhaps the sample size, severity of illness, and diverse demographics of our cohort, which was derived from dozens of CPX laboratories, diminished the prognostic characteristics of VE -VCO2 slope in our study.
SEX-BASED DIFFERENCES IN EXERCISE CAPACITY AND MORTALITY
We explored whether known sex-based differences in exercise capacity (9) influenced the prognostic qualities of the CPX-derived variables (Table 3, Central Illustration, Figure 1). Among men in our cohort, a 10% 1-year mortality rate corresponded to a peak VO2 of 10.9 mL·kg−1·min−1, versus a value of 5.3 mL·kg−1·min−1 in women (Table 5). These findings are consistent with prior studies that also showed that the adjusted peak VO2 associated with a similar rate of survival is higher in men than women with HFrEF (10,11). The data suggest that the traditional peak VO2 “cut-point” values used to determine risk and need for advanced HF therapies likely differs by sex, a point that is currently considered in only 1 of the professional guideline statements (24).
Across 5 categories of RER, the ability of peak VO2 to discriminate mortality (c-index) was similar for all categories higher than a peak RER of 0.95 (Table 6). Additionally, a peak VO2 <12 mL·kg−1·min−1 identified patients with a poor outcome over 3 years of follow-up, regardless of whether the RER is greater or lesser than 1.05 (Figure 2). Thus, clinicians should strive to achieve the highest RER possible during testing but not dismiss the potential prognostic and risk stratification capabilities associated with adjusted peak VO2 if the achieved RER is <1.05 (18).
CLINICAL IMPLICATIONS
Since Mancini et al. identified a peak VO2 <14 mL·kg−1·min−1 as the cutoff for increased mortality compared to cardiac transplantation (25), other therapies that do not change exercise capacity (e.g., beta-blockers) but do reduce mortality have become part of guideline-based care in patients with HF. When evaluating patients for advanced therapy such as cardiac transplantation, deciding which CPX test-derived variables to use in conjunction with other risk stratification tools is, to some extent, up to the personal preferences of the individual clinician. Observations in this analysis suggested that using either %ppVO2 or adjusted peak VO2 (mL·kg−1·min−1) might be optimal in that both: are cited in professional statements; are not influenced by subject familiarization to exercise testing; are currently used by many clinicians; have a substantive body of evidence supporting their use; and are easily explained to patients (7,26–31). Table 5 summarizes sex-specific cut-points that clinicians might use relative to helping guide when to refer a patient to an advanced heart failure program (i.e., 1-year risk for mortality >3%) and when to evaluate for cardiac transplantation (i.e., 1-year risk = 10%), with the latter representing patients at high risk who would, on average, experience better survival should they undergo cardiac transplantation (19).
In reality, more than 1 CPX-derived variable is often used to determine or parse risk (27,32,33) and the variable(s) used may vary depending on the patient. For example, when testing older women or obese patients, using %ppVO2 has limitations because of the relatively few number of women and obese patients included in the studies that developed the prediction equations. Conversely, a 35-year-old man with a peak VO2 of 19 mL·kg−1·min−1 might appear to be at a lower risk for mortality; however, this may not be the case when his exercise tolerance is expressed as %ppVO2 (e.g., 45%).
STUDY LIMITATIONS
The current analysis represents a post hoc analysis of prospectively collected data and, therefore, has limitations. First, some CPX-derived candidate variables (e.g., exercise oscillatory ventilation) were not available. Guazzi et al. observed a high prevalence (36%) of exercise oscillatory ventilation, which was a strong predictor of outcome in their study (33). More recently, when compared to 30 other CPX test-related variables, the strength of exercise oscillatory ventilation’s association with clinical events was lower (18). This notwithstanding, most other CPX-derived variables that are strong independent predictors of outcome were included in our analysis. Second, although we assessed a large cohort of ambulatory patients with HFrEF on evidence-based therapies, including many female and nonwhite subjects, our findings should not be extended to patients with HF with preserved ejection fraction. Third, use of the modified Naughton treadmill protocol (15) is likely more common in the United States than other countries; therefore, the generalizability of our findings for exercise duration and possibly determination of ventricular tachycardia may be affected if other modalities or protocols for testing are used. Peak VO2 values are known to be 5% to 20% higher if measured using a treadmill versus a cycle ergometer (9). Finally, our cohort is younger (median = 59 years) than the general population of patients with HFrEF, with 41% of patients ≥62 years of age.
CONCLUSIONS
We assessed and compared multiple CPX-derived variables for their association with mortality in a large cohort of patients with HFrEF and showed that the relationship for all variables, except for RER, was highly significant. The 2 variables that demonstrated the strongest ability to predict risk and discriminate likelihood of all-cause mortality were %ppVO2 and exercise duration. Multivariable adjusted analyses identified these same 2 variables and peak VO2 (mL·kg−1·min−1) as having the strongest ability to predict risk and discriminate likelihood of mortality. RER had little impact on the ability of peak VO2 or exercise duration to discriminate mortality. Substantial sex differences were found and future research and consensus thinking is needed to determine if the level of importance placed on variables measured during CPX testing for the purposes of risk stratification and prognosis should differ based on sex.
FIGURE 3. CENTRAL ILLUSTRATION One-year Survival and Baseline Peak VO2.
This study examined the strength of the association among variables measured during a cardiopulmonary exercise test and all-cause mortality in patients with heart failure with reduced ejection fraction. Peak oxygen uptake (peak VO2) was the strongest predictor of mortality among men and exercise duration among women. When comparing baseline peak VO2, 1-year probability of survival curve as determined using a Cox proportional hazards model varies between men and women. At a peak VO2 of 11 mL·kg−1·min−1, the estimated 1-year mortality rate is <4% in women versus ~10% in men. Vertical bars represent 95% confidence intervals.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
In patients with heart failure and reduced ejection fraction undergoing cardiopulmonary exercise testing with standard protocols, the exercise duration, peak oxygen uptake, and percent predicted peak oxygen uptake correlate more closely with mortality than other measured variables. A peak VO2 of ~11 mL·kg−1·min−1 in men and ~ 5 mL·kg−1·min−1 in women is associated with approximately a 10% risk of mortality within 1 year.
TRANSITIONAL OUTLOOK
Further studies are needed to determine the incremental value of incorporating these exercise measurements into existing clinical risk scores to guide referral of patients to a specialized heart failure program for consideration of advanced therapies including cardiac transplantation.
Acknowledgments
Funding: This work was supported by the National Heart, Lung and Blood Institute (NHLBI), National Institutes of Health (NIH), Bethesda, Maryland.
ABBREVIATIONS AND ACRONYMS
- CPX
cardiopulmonary exercise
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- RER
respiratory exchange ratio
- VAT
ventilatory-derived anaerobic threshold
- VE -VCO2 slope
ventilatory efficiency (minute ventilation-carbon dioxide production)
- VO2
oxygen uptake
- %ppVO2
percent predicted peak VO2
Footnotes
Disclaimer: The contents of this manuscript are entirely the responsibility of the authors and do not necessarily reflect the views of the National Institutes of Health or the Department of Health and Human Services.
Relationship with industry: Keteyian (none); Patel (none); Kraus (none); Ehrman (none); McConnell (none); Piña (none); Leifer (none); Fleg (none); Blackburn (none); Fonarow (none); Chase (none); Piner (none); Vest (none); O’Connor (none); Brawner (none); Walsh (none); Ewald (none); Bensimhon (none); Russell (none)
Clinical Trial: NCT00047437
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agostoni P, Corra U, Cattadori G, et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: A multiparametric approach to heart failure prognosis. Int J Cardiol. 2013;167:2710–8. doi: 10.1016/j.ijcard.2012.06.113. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor CM, Whellan DJ, Wojdyla D, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2012;5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chyu J, Fonarow GC, Tseng CH, Horwich TB. Four-variable risk model in men and women with heart failure. Circ Heart Fail. 2014;7:88–95. doi: 10.1161/CIRCHEARTFAILURE.113.000404. [DOI] [PubMed] [Google Scholar]
- 4.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 5.Aaronson KD, Schwartz JS, Chen TM, et al. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–7. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 6.Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–74. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balady GJ, Arena R, Sietsema K, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 8.Arena R1, Guazzi M, Cahalin LP, Myers J. Revisiting cardiopulmonary exercise testing applications in heart failure: aligning evidence with clinical practice. Exerc Sport Sci Rev. 2014;42:153–60. doi: 10.1249/JES.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 9.Foss ML, Keteyian SJ. Fox’s Physiological Basis for Exercise and Sport. 6. Boston, MA: WCB McGraw-Hill; 1998. p. 88. [Google Scholar]
- 10.Corra U, Mezzani A, Giordano A, et al. Peak oxygen consumption and prognosis in heart failure: 14 mL/kg/min is not a “gender-neutral” reference. Int J Cardiol. 2013;167:157–61. doi: 10.1016/j.ijcard.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 11.Elmariah S, Goldberg LR, Allen MT, Kao A. Effects of gender on peak oxygen consumption and the timing of cardiac transplantation. J Am Coll Cardiol. 2006;47:2237–42. doi: 10.1016/j.jacc.2005.11.089. [DOI] [PubMed] [Google Scholar]
- 12.Whellan DJ, O’Connor CM, Lee KL, et al. HF-ACTION Trial Investigators. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Bensimhon DR, Leifer ES, Ellis SJ, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing) Am J Cardiol. 2008;102:712–7. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J Am Coll Cardiol. 2002;40:1531–40. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 15.American College of Sports Medicine. Clinical Exercise Testing, in ACSM’s Guidelines for Exercise Testing and Prescription. 9. Chapter 5. Philadelphia, PA: Lippincott, Williams & Wilkens; 2014. p. 125. [Google Scholar]
- 16.Sue DY, Wasserman K, Moricca RB, Casaburi R. Metabolic acidosis during exercise in patients with chronic obstructive pulmonary disease. Use of the V-slope method for anaerobic threshold determination. Chest. 1988;94:931–8. doi: 10.1378/chest.94.5.931. [DOI] [PubMed] [Google Scholar]
- 17.Wasserman K, Hansen D, Sue DY, et al. Principles of Exercise Testing and Interpretation. Philadelphia, PA: Lippincott Williams and Wilkins; 1999. p. 148. [Google Scholar]
- 18.O’Connor CM, Whellan DJ, Lee KL, et al. HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The International Society for Heart & Lung Transplantation. Registries, heart/lung registries, quarterly data report. [Accessed July 3, 2015];Survival rates for transplants perfomed between April 1, 2010 and March 31, 2014. Available at: http://www.ishlt.org/registries/quarterlyDataReportResults.asp?organ=HR&rptType=recip_p_surv&continent=4.
- 20.Corrà U, Giordano A, Mezzani A, et al. Cardiopulmonary exercise testing and prognosis in heart failure due to systolic left ventricular dysfunction: a validation study of the European Society of Cardiology Guidelines and Recommendations (2008) and further developments. Eur J Prev Cardiol. 2012;19:32–40. doi: 10.1177/1741826710393994. [DOI] [PubMed] [Google Scholar]
- 21.Brawner CA, Shafiq A, Aldred HA, et al. Comprehensive analysis of cardiopulmonary exercise testing and mortality in patients with systolic heart failure: the Henry Ford HospITal cardioPulmonary eXercise testing (FIT-CPX) project. J Cardiac Fail. 2015;21:710–8. doi: 10.1016/j.cardfail.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Russell SD, McNeer FR, Beere PA, et al. Improvement in the mechanical efficiency of walking: an explanation for the “placebo effect” seen during repeated exercise testing of patients with heart failure. Duke University Clinical Cardiology Studies (DUCCS) Exercise Group. Am Heart J. 1998;135:107–14. doi: 10.1016/s0002-8703(98)70350-3. [DOI] [PubMed] [Google Scholar]
- 23.Guazzi M, Myers J, Abella J, et al. The added prognostic value of ventilatory efficiency to the Weber classification system in patients with heart failure. Int J Cardiol. 2008;129:86–92. doi: 10.1016/j.ijcard.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant. 2006;25:1024–42. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Mancini DM, Eisen H, Kussmaul W, et al. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 26.Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–74. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira AM, Tabet JY, Frankenstein L, et al. Ventilatory efficiency and the selection of patients for heart transplantation. Circ Heart Fail. 2010;3:378–86. doi: 10.1161/CIRCHEARTFAILURE.108.847392. [DOI] [PubMed] [Google Scholar]
- 28.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29:2388–442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 29.Stelken AM, Younis LT, Jennison SH, et al. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J Am Coll Cardiol. 1996;27:345–52. doi: 10.1016/0735-1097(95)00464-5. [DOI] [PubMed] [Google Scholar]
- 30.Osada N, Chaitman BR, Miller LW, et al. Cardiopulmonary exercise testing identifies low risk patients with heart failure and severely impaired exercise capacity considered for heart transplantation. J Am Coll Cardiol. 1998;31:577–82. doi: 10.1016/s0735-1097(97)00533-0. [DOI] [PubMed] [Google Scholar]
- 31.Arena R, Myers J, Abella J, et al. Determining the preferred percent-predicted equation for peak oxygen consumption in patients with heart failure. Circ Heart Fail. 2009;2:113–20. doi: 10.1161/CIRCHEARTFAILURE.108.834168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arena R, Myers J, Abella J, et al. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115:2410–7. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 33.Guazzi M, Boracchi P, Arena R, et al. Development of a cardiopulmonary exercise prognostic score for optimizing risk stratification in heart failure: the (P)e(R)i(O)dic (B)reathing during (E)xercise (PROBE) study. J Card Fail. 2010;16:799–805. doi: 10.1016/j.cardfail.2010.04.014. [DOI] [PubMed] [Google Scholar]