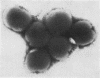
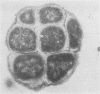
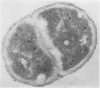
Abstract
In a 12-month period, oxidase-positive, Gram-negative cocci showing similar characteristics in biochemical tests have been isolated from the urogenital tract of 39 male and female patients. Although these organisms superficially resemble Neisseria gonorrhoeae, biochemical characterization and the results of DNA base composition analysis indicate that they do not belong to the genus Neisseria. The relationship of these organisms to the genera Neisseria, Achromobacter, and Pseudomonas is discussed.
Full text
PDF


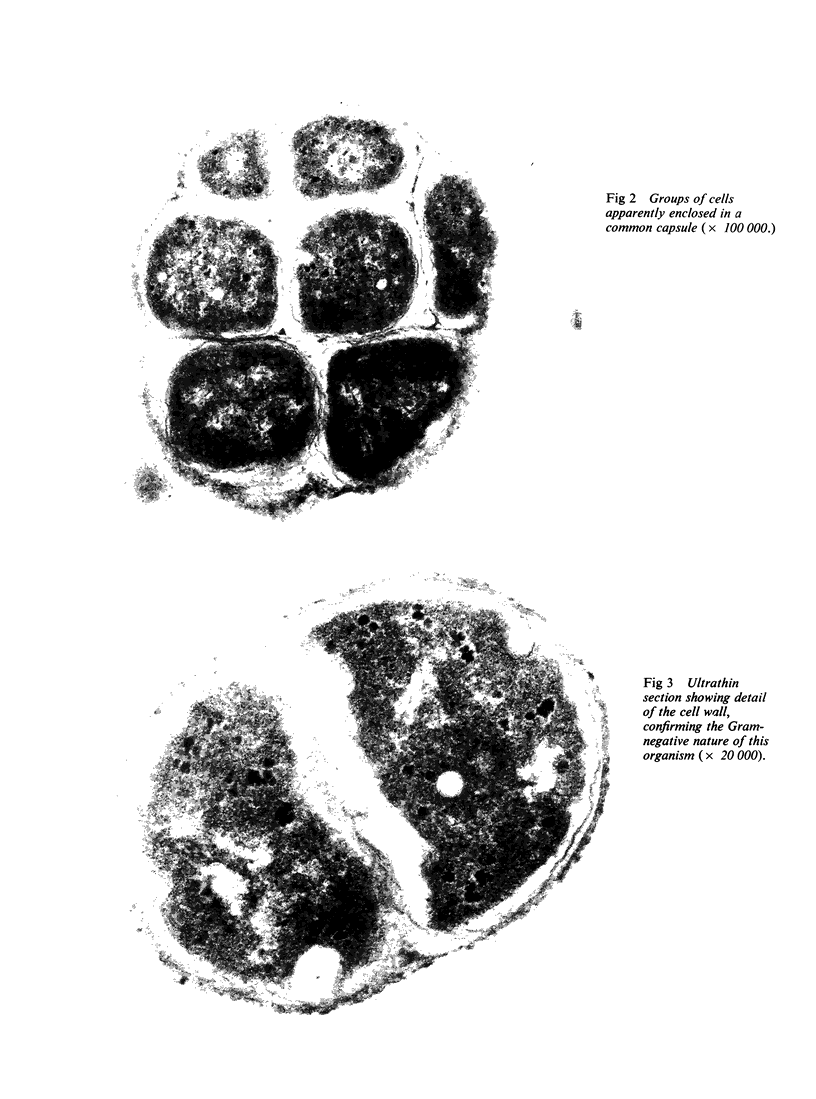


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck A., Fluker J. L., Platt D. J. Neisseria meningitidis in urogenital infection. Br J Vener Dis. 1974 Oct;50(5):367–369. doi: 10.1136/sti.50.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COURTOIS G., THYS A., VERSELDER R. Une nouvelle Neisseria, agent causal de méningite. Ann Soc Belg Med Trop (1920) 1954 Jan 28;34(1):13–20. [PubMed] [Google Scholar]
- Jephcott A. E., Morton R. S. Isolation of neisseria lactamicus from a genital site. Lancet. 1972 Oct 7;2(7780):739–740. doi: 10.1016/s0140-6736(72)92027-2. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Hill L. R., Lapage S. P. Determination of DNA base compositions from melting profiles in dilute buffers. Biopolymers. 1969;7(4):503–516. doi: 10.1002/bip.1969.360070408. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Snell J. J. Comparison of group IIf with Flavobacterium and Moraxella. Antonie Van Leeuwenhoek. 1973;39(3):473–480. doi: 10.1007/BF02578890. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Rhodopseudomonas acidophila, sp. n., a new species of the budding purple nonsulfur bacteria. J Bacteriol. 1969 Aug;99(2):597–602. doi: 10.1128/jb.99.2.597-602.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell R. H., Buck A. C. Trimethoprim as an additional selective agent in media for the isolation of N. gonorrhoeae. J Clin Pathol. 1970 Sep;23(6):481–483. doi: 10.1136/jcp.23.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell J. J., Davey P. A comparison of methods for the detection of phenylalanine deamination by Moraxella species. J Gen Microbiol. 1971 Jun;66(3):371–373. doi: 10.1099/00221287-66-3-371. [DOI] [PubMed] [Google Scholar]
- Snell J. J., Hill L. R., Lapage S. P. Identification and characterization of Moraxella phenylpyruvica. J Clin Pathol. 1972 Nov;25(11):959–965. doi: 10.1136/jcp.25.11.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk J., Kraus S. J. Asymptomatic meningococcal urethritis. Possible protective value against gonococcal infection by bacteriocin production. Br J Vener Dis. 1973 Dec;49(6):511–512. doi: 10.1136/sti.49.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON A. E. Occurrence of neisseria other than the gonococcus in the genital tract. Br J Vener Dis. 1952 Mar;28(1):24–27. doi: 10.1136/sti.28.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]