Abstract
Steroids modulate the transcription of a multitude of genes and ultimately influence numerous aspects of reproductive behaviors. Our research investigates how one single steroid, testosterone, is able to trigger this vast number of physiological and behavioral responses. Testosterone potency can be changed locally via aromatization into 17β-estradiol which then activates estrogen receptors of the alpha and beta sub-types. We demonstrated that the independent activation of either receptor activates different aspects of male sexual behavior in Japanese quail. In addition, several studies suggest that the specificity of testosterone action on target genes transcription is related to the recruitment of specific steroid receptor coactivators. We demonstrated that the specific down-regulation of the coactivators SRC-1 or SRC-2 in the medial preoptic nucleus by antisense techniques significantly inhibits steroid-dependent male-typical copulatory behavior and the underlying neuroplasticity. In conclusion, our results demonstrate that the interaction between several steroid metabolizing enzymes, steroid receptors and their coactivators plays a key role in the control of steroid-dependent male sexual behavior and the associated neuroplasticity in quail.
Keywords: Estradiol, estrogen receptor, steroid receptor coactivator, Japanese quail, medial preoptic nucleus
1. INTRODUCTION
The steroid hormone testosterone (T) plays a critical role in the regulation of development, physiology and behavior in nearly all vertebrate species investigated to date. Although a vast array of studies have demonstrated the rapid action of steroid hormones at the membrane and cytoplasm levels [35, 43, 46, 91], many of the biological effects of steroids are mediated through the activation of their respective nuclear receptors and rapid, non-genomic effects will not be discussed here. When exposed to the steroid, the steroid receptors dimerize, bind to a specific response element, and regulate gene transcription. Modulation of gene transcription will affect the cell physiology, and ultimately could affect the response of an organism to its environment and the expression of a specific behavior in response to a defined social stimulus. Exposure to T, mainly produced in the gonads, but also in the adrenals and in the brain, will significantly modulate brain physiology and will increase the probability that the males perform male-typical behaviors, such as, in birds, singing, aggression and territoriality or copulation, depending on the social context they face. For example, intact male Japanese quail confronted to another male will display various forms of aggressive behavior [82, 83, 90], while presentation of a sexually mature female will trigger the display of a full copulatory sequence, including neck grab, mount attempt, mount and cloacal contact movement [3]. These behaviors are testosterone-dependent since castration completely abolishes aggression and copulation while exogenous testosterone treatment fully restores these activities [2, 24]. In addition, Japanese quail are an excellent model species to investigate the neuronal mechanisms underlying the activation of testosterone-dependent male sexual behavior. The medial preoptic nucleus (POM) was shown to be significantly larger in males compared to females [95] and, unlike most sexually dimorphic preoptic nuclei in mammals, its volume, defined by various markers including Nissl staining or aromatase immunohistochemistry, is reduced after castration in adulthood and fully restored to values observed in intact males after treatment with exogenous testosterone [7, 10, 22, 75, 77]. Importantly, functional experiments demonstrated that the action of T in this nucleus is necessary and sufficient to activate male sexual behavior in males. Lesions of the POM, but not in the surrounding area, completely suppress male sexual behavior while stereotaxic implants filled with testosterone activate all aspects of copulatory behavior if the tip of the implant is located within the boundaries of the POM [21].
2. GENOMIC ACTION OF TESTOSTERONE IS UNEXPECTEDLY FAST
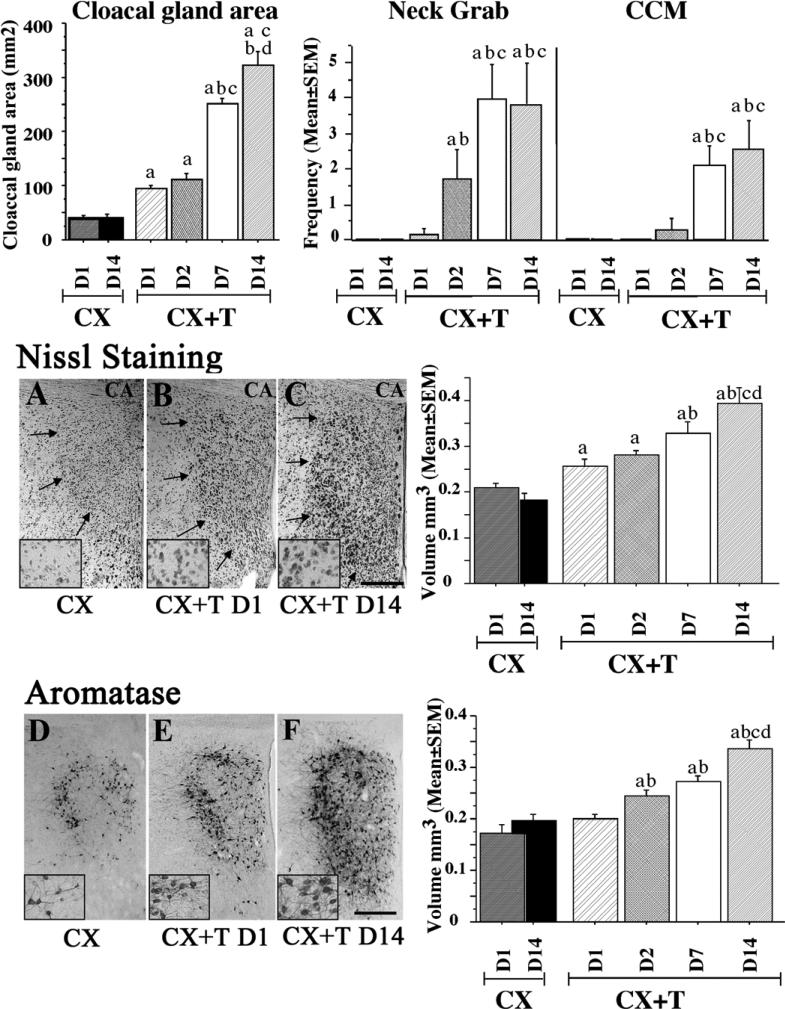
While previous studies investigated the effect of T two weeks or more after the beginning of the treatment, we recently investigated in more detail the time course of T action on male sexual behavior and on the changes in POM morphology [28]. We showed that only 2 days of exposure to T were sufficient to significantly affect the initial stages of the copulatory sequence, i.e. neck grab and mount attempts, but 7 days of steroid exposure were necessary to activate the full sequence, including the cloacal contact movements (see figure 1). Groups of subjects were killed at different times after the beginning of the T treatment to investigate potential changes of POM volume, defined by two different techniques. We showed that the entire volume defined by Nissl staining was already significantly increased after 2 days of T exposure. Nissl staining highlights mainly the ribosomal RNA and this increase in ribosomal content likely mirrors major changes in general protein synthesis activity within this region. In agreement with this idea, aromatase expression was also significantly increased within this brain nucleus. This change was observed after only 24 hours of T treatment, likely due to the higher sensitivity of aromatase detection by immunohistochemistry as compared to a Nissl stain. This was to our knowledge the most rapid increase ever reported that affects the global volume of a brain nucleus (see figure 1). Increases in global volume of steroid-sensitive brain nuclei such as POM in quail, but also in the postero-dorsal medial amygdala in mammals [34], have been related to changes in neuronal size, size of the non-neuronal compartment (glial cells, blood vessels), cell spacing, volume of the neuronal dendritic arborization, and in the case of the song control nucleus HVC in oscine songbirds [25, 26, 54] the incorporation and survival of new neurons.
Figure 1.
Bar graphs illustrating the size of the cloacal gland (in mm2), and the frequencies of neck grabs and cloacal contact movements (CCM). Data were recorded just before brain collection in castrated birds killed on day 1 (CXD1) and day 14 (CXD14) and in castrated birds treated with exogenous testosterone for 1 day (CX+T D1), 2 days (CX+T D2), 7 days (CX+T D7) or 14 days (CX+T D14). a: p<0.05 vs pooled CX birds; b: p<0.05 vs CX+T D1; c: p<0.05 vs CX+T D2; d: p<0.05 vs CX+T D7. Photomicrographs illustrating the POM as defined by Nissl staining (A–C) and ARO-ir cells (D–F) in castrates (A, D) and castrates treated with exogenous testosterone for 1 day (B, E) or for 14 days (C, F). The inserts represent higher magnification photomicrographs in the dorso-lateral part of the POM. CA: anterior commissure. The scale bar located in F represents 200 μm in the 6 main panels and 50 μm in the 6 inserts at higher magnification. Associated to the photomicrographs are bar graphs illustrating the measurement of POM volume defined by Nissl staining and aromatase immunoreactivity.
In addition to these major structural changes, T also strongly affects cell phenotypes and modulates the expression of specific enzymes (aromatase, neural nitric oxide synthase, Catechol-O-methyl transferase,...) and neuropeptides (vasotocin, galanin, substance P, somatostatin, neurotensin to name a few) in various regions of the brain (see for review: [13, 74]). T thus has pleiotropic, albeit specific, effects on the neural structure and transcriptome. This large array of changes induced by the presence of T raises the question of how one single steroid hormone can trigger hundreds, if not thousands, of cellular and physiological responses in an anatomically specific manner? There have to be mechanisms that specifically modulate how one tissue or even one cell will appropriately respond to the presence of T! Over the past decades, it has become clear that T action can be fine-tuned in vivo by three groups of distinct processes.
One way to modulate T action is to change its availability and effective concentration through the binding to specific binding proteins such as alpha-fetoprotein (AFP), sex hormone binding globulin (SHBG), or corticosteroid binding globulin (CBG) [101]. According to the “free hormone hypothesis”, only free steroids not bound to globulins or binding proteins can bind to nuclear receptors in target tissues [63]. Based on this concept, it has been suggested that binding globulins can store steroid hormones and then release them when needed [47, 55]. This release of steroids from binding globulins can affect all tissues, or it can be targeted at specific sites [49, 56]. Because more than 50% of circulating steroids may be bound to binding globulins in plasma, it is important to consider the storage of steroids available under different physiological or environmental conditions. The importance of binding globulins and their role in the modulation of T action has been reviewed recently and will not be further discussed here [48, 58].
Another way to change T action is to modify the steroid identity through local metabolism and activation of different receptors or finally
to modulate T action at the level of the target genes (increase or decrease of transcription) via the recruitment by the steroid receptor of defined transcriptional coregulators, i.e. coactivators or corepressors. These two aspects have recently been investigated in our laboratory and will be further considered here.
3. TESTOSTERONE METABOLITES
Testosterone can be metabolized into 5α- or 5β-dihydrotestosterone by 5α- or 5β-reductases respectively. 5α-dihydrotestosterone activates androgen receptors, similarly to testosterone, while 5β- dihydrotestosterone is essentially an inactive metabolite [1, 36, 86] although see [19, 38]. The avian brain contains a significant amount of 5β-reductase activity [37], suggesting a strong modulation of testosterone action via inactivation. It should be noted that the exact neuroanatomical localization of the enzyme has not been studied in detail and its specific contribution to the control of testosterone action remains to be tested.
More importantly, the androgen T can be aromatized into its estrogenic metabolite 17β-estradiol (E2) by the enzyme aromatase (CYP19A) and this metabolism plays a critical role in the behavioral effects of T in numerous species, including the Japanese quail. In this species, high levels of aromatase activity have been measured in those brain areas that are implicated in the activation of male copulatory behavior, especially in the POM (for a review see [9, 12, 78]). This high level of aromatase expression is usually linked to an elevated local concentration of E2 [32, 33]. The treatment of quail with aromatase inhibitors also prevents T from activating male sexual behavior [15, 41]. Importantly, it has been demonstrated that the behavioral effects of T on sexual behavior can be mimicked by E2 or by the synthetic estrogenic compound, diethylstilbestrol. In addition, the blockade of estrogen receptors by antiestrogens such as tamoxifen or CI-628 blocks the activational effects of T on male copulatory behavior [4, 20]. Subsequent studies based on the stereotaxic implantation of steroids, steroid antagonists and steroid metabolism inhibitors demonstrated that T must be aromatized and the resulting estrogens must act within the POM to activate sexual behavior [16, 21, 98, 99].
This metabolism of androgenic to estrogenic compounds is functionally important since it allows T to not only activate androgen receptors and but also estrogen receptors (ER) and the related signaling pathways. Interestingly, the POM contains androgen receptors and the 2 isoforms of ER, namely the ERα and ERβ [17, 18, 42, 97].
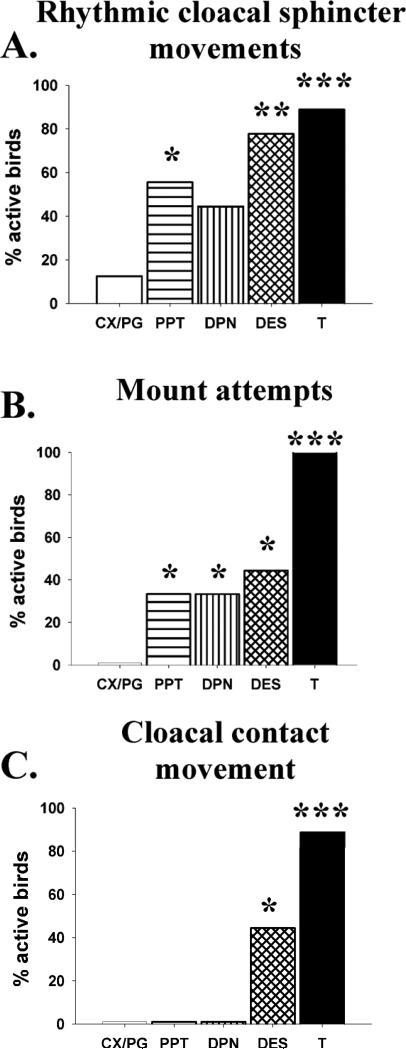
While numerous studies have confirmed the importance of E2 in activating male quail sexual behavior, the contribution of each ER was unknown until recently. Selective agonists for each receptor are now commercially available and we thus used them in an attempt to define the specific involvement of both ERα or ERβ in the activation of male sexual behavior [85]. Castrated male Japanese quail were daily injected with the general ER agonist diethylstilbestrol (DES), with propyl-pyrazole-triol (PPT), an ERα specific agonist, or with diarylpropionitrile (DPN), an ERβ specific agonist, and they were tested for activation of both appetitive and consummatory aspects of male sexual behavior (see figure 2).
Figure 2.
Specific activation of estrogen receptor α and β modulates appetitive or consumatory aspects of male sexual behaviors. Bar graph representing the percentage of birds that displayed at least one female-induced rhythmic cloacal sphincter movement (RCSM) (A) and percentage of birds that displayed at least one mount attempt (B) or one cloacal contact movement (C). Castrated male Japanese quail were daily injected with the general ER agonist diethylstilbestrol (DES), with propyl-pyrazole-triol (PPT), an ERα specific agonist, with diarylpropionitrile (DPN), an ERβ specific agonist, with the vehicle propyleneglycol (PG) or received an empty implant (CX) or implant filled with crystalline testosterone (T). *: p<0.05, **: p<0.01 and ***: p<0.001.
Appetitive sexual behavior (ASB) consists of behaviors directed toward a female in anticipation of copulation (e.g., searching or approaching a female), whereas consummatory sexual behavior (CSB) consists of direct sexual contacts with the female, including mounting and copulation [13, 23, 79]. Both aspects of behavior are, to a large extent, under the control of T-derived estrogens though the site of steroid action in activating each component of male behavior appears to be distinct to some degree, involving mostly the anterior part of the POM for ASB and the posterior part of the nucleus for CSB [11, 14, 88].
The general estrogen receptor agonist, DES, significantly increased the number of castrated males displaying rhythmic cloacal sphincter movements (RCSM) in response to the visual presentation of a female, one aspect of appetitive behavior (figure 2A). The effects of DES were largely mimicked by injections of the ERα agonist PPT (increase in percentage of active birds but very low frequency of contractions), suggesting that the activation of ERα is required to trigger RCSM in response to the female. In contrast, no significant effect of the ERβ agonist DPN was observed on this behavior, suggesting that this receptor is possibly not implicated in the activation of this form of appetitive behavior [85]. The frequency of the cloacal contractions after the activation of ERα was extremely low in comparison with birds treated with T, suggesting that other aspects of T action are likely to be critical for the activation of this behavior, such as the combined activation of estrogen and androgen receptors. Interestingly, it seemed that both ER isoforms were involved in the control of the consummatory aspects of male sexual behavior [85]. Indeed, both ERα and ERβ agonists, separately or together, enhanced the performance of the initial behavior patterns (NG and MA) in the copulatory sequence (see figure 2B). It should however be noted that behavior activation concerned a smaller percentage of subjects that displayed the behaviors with much lower frequencies than T treated birds, as was the case in preceding studies [4]. The two specific ER agonists activated NG and MA roughly to the same extent as DES (64% of birds active but with low frequencies) but had absolutely no effect on the expression of cloacal contact movement, CCM (see figure 2C).
Somewhat surprisingly, we observed only minimal effects of DES administration on the POM volume defined by aromatase-immunoreactive neurons and PPT or DPN had absolutely no effect on this measure while T-treated birds showed, as expected, a significant enlargement of the nucleus. Similarly, the vasotocinergic innervation of the POM, a well-established estrogen-dependent response [93], was not significantly affected by the general estrogen receptor agonist DES although the relative optical density was numerically higher in this group as compared to controls. The independent activation of each receptor by specific agonists also did not result in any increase of this measure. This would suggest that both ER subtypes are playing a similar role in the activation of consummatory behavior but the low level and incomplete activation of the copulatory sequence, as well as the absence of marked changes in the POM neurochemical features (aromatase and vasotocin) prevent us from drawing final conclusions. The relatively weak activation of the behaviors observed here might be due to the use of insufficient doses of agonists, to a requirement for synergistic activation of both ERα and ERβ or to differences between avian and mammalian receptors resulting in a differential binding of PPT and DPN. These potential interpretations are discussed in more detail in our original publication [85].
We recently performed an additional study to define with more detail the genes regulated by the specific ER isoforms. We confirmed that both ER agonists independently affect male sexual behavior. The hypothalamus/preoptic area region containing the POM was then dissected out after behavioral testing and the transcription level in the different experimental groups was quantified using the chicken Affymetrix™ arrays as previously described [68]. These analyses are still in progress but we found that transcription of numerous functional groups of genes is affected by each agonist, including transcription factors and genes coding for proteins involved in signal transduction and metabolism. Due to the lack of complete information from the chicken genome sequencing, a relatively large percentage of genes (around 20%) were however not defined or could not be assigned any known function. Interestingly, only a small percentage of genes (less than 10%) were activated by more than one of the ligands (PPT vs. DPN), confirming the specificity of each ligand in the activation of their specific ER isoform. Validations using quantitative PCR and precise neuroanatomical localization of these transcriptional controls by in situ hybridization need now to be performed before any firm conclusion can be drawn. Altogether, this set of data shows that T can modulate transcription not only via androgen receptors, but also via the two estrogen receptor isoforms. Although this increases by a factor of three the diversity of pathways by which T can act on physiology and behavior, the increase is still not sufficient to explain the huge diversity of T actions on cell specific phenotypes. It should be added that the 8 exons that constitute the coding region of the canonical ERα, ERβ and AR may generate a number of splice variants with single or multiple exons skipping, exon duplication or partial exon deletion. These splice variants were mostly studied in reproductive organs and in various cancer and cell lines but their potential role in various mental disease such as Schizophrenia and Alzheimer is poorly understood. Currently, up to 64 ERα [53], 5 ERβ [80] and 11 AR [100] variants have been described but their exact function is relatively unknown. To our knowledge, the presence and importance of splice variants has not been studied in birds but their potential expression in the avian brain would likely increase the diversity of response to testosterone.
4. TRANSCRIPTIONAL COREGULATORS
A third way to fine-tune steroid action is identified at the transcriptional level. When exposed to their ligand, steroid receptors dimerize and recruit several proteins implicated in the regulation of transcriptional activation. Initial molecular biology techniques suggested that these proteins acted as transcriptional adaptors whose only purpose was to link the DNA-bound steroid receptor to the general transcription factors [81]. However, it is now clear that nuclear receptor coregulators display an extremely diverse array of enzymatic activities involved in the entire process of transcription and translation. These include ATPase-dependent chromatin remodeling, acetylation and methylation of histones, stabilization of the general transcription and modulation of protein activity and stability, the subcellular localization of nuclear receptors and coactivators and mRNA splicing [57, 60, 62, 72]. The targets for these enzymatic activities are other members of the coactivator family, nuclear receptors, other transcription factors, and components of the basal transcription machinery, as well as the chromatin adjacent to the genes they regulate. It is now evident that coactivators do not act as single entities but are present in the form of preexisting complexes, regulating the sequential steps of transcription/translation processes [59, 61]. Altogether, approximately 300 coactivators and corepressors have been identified to date (see a complete list of nuclear receptors and coregulatory proteins at www.nursa.org) and it is likely that most of them undergo differential posttranslational modifications, adding to the great diversity of control factors [50]. Interestingly, a large number of these coregulators are not specific to one nuclear receptor but are part of transcriptional complexes recruited by different steroid hormone receptors. For example, SRC-1 can interact with ER and AR, but also with glucocorticoid, vitamin D and progesterone receptors [39, 45, 52, 73]. This requirement for the same coactivator results in some cases in a competition, or squelching, between nuclear receptors. For example, ligand-induced activation of PR is reduced by coexpression of ERα, due to squelching or sequestering of shared coactivators and this squelching can be reversed by over-expression of SRC-1 [73]. In another example, the ERα-mediated transcription of the preproenkephalin gene in transient transfection assays is inhibited following the activation of thyroid hormone receptor and restored following the over-expression of SRC-1 [92]. This suggests that coactivators are not only a limiting factor necessary for full transcriptional activation of receptors, but also recruited by different receptors.
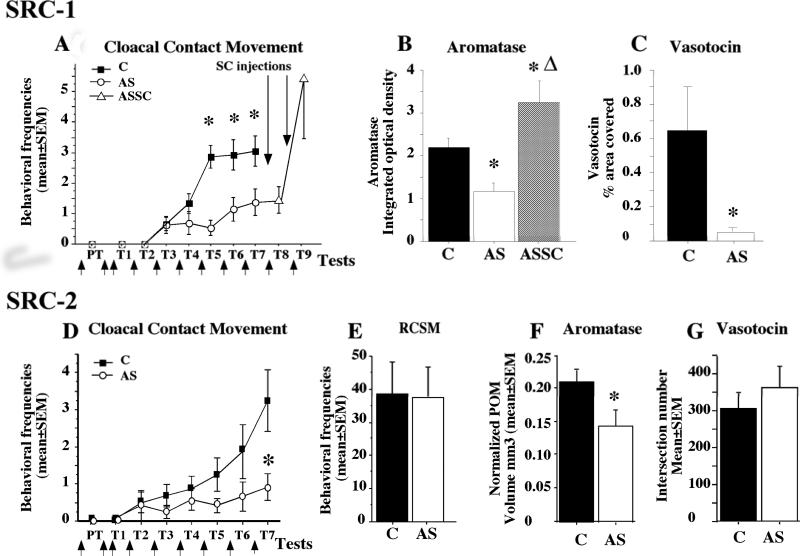
Very few laboratories are investigating the physiological role of coregulators in vivo and a few years ago, our lab initiated studies on the potential role of the first described coactivator, the steroid receptor coactivator 1, SRC-1 [73]. We showed that SRC-1 is present in steroid-sensitive brain regions in Japanese quail, and particularly in the POM [29, 31]. To test the hypothesis that the coactivator could effectively play a significant role in the control of the steroid-dependent male sexual behavior and in the associated neuroplasticity, we used antisense technology to specifically decrease the expression of SRC-1. In a first set of experiments [27], male Japanese quail were daily injected in the third ventricle at the level of the POM with locked nucleic acid (LNA) antisense. Male-typical behaviors such as neck grabs, cloacal contact movements and pre- and post-copulatory displays (struts) were observed in these antisense (AS)-injected birds and in the control groups injected with saline or scrambled oligonucleotides (SC). The birds treated with antisense showed a weaker behavioral response to T, as attested by the low frequencies of neck grabs, cloacal contact movements and struts (see figure 3A). These data therefore confirmed the importance of SRC-1 not only for the activation of aspects of male sexual behavior influenced by estrogens (copulatory behavior per se), but also on a strictly androgen-dependent behavior (strut). All brains were then collected for histological analyses but five birds from the antisense-treated group were kept alive to test whether their behavior would recover after stopping the injections of the antisense and replacing them with control SC injections for two additional days (ASSC group). Males in this group showed an important increase of the behavioral response to T within two days after the interruption of the antisense treatment. Their sexual behaviors were expressed with frequencies exceeding the frequency of the control animals. Western blot analysis of SRC-1 expression in the preoptic area-hypothalamus, containing the POM, confirmed the down-regulation of this protein in subjects injected with antisense LNA. These analyses also showed that the ASSC birds that had been injected with antisense and then treated with SC oligonucleotides had a stronger expression of SRC-1 compared to control animals at the time of sacrifice. This SRC-1 over-expression could explain the very high behavioral response to T observed in these ASSC subjects.
Figure 3.
Effects of the downregulation of SRC-1 (A-C) or SRC-2 (D-G) expression by antisense (AS) on testosterone-dependent responses as compared to control (C) subjects. In the SRC-1 experiment, the AS group was split into two groups after test 7 (T7). One was killed, and the other (ASSC) received scrambled injections (SC) and was behaviorally tested for 2 d (T8–T9). A and D highlight the frequencies of cloacal contact movements and the upward arrows represent the daily injection of antisenses. Bar graphs in B represent the reduction of aromatase expression and in C the vasotocin innervation after SRC-1 antisense injection. E illustrates the absence of effect of SRC-2 reduction on an appetitive sexual behavior, the rhythmic cloacal sphincter movements, whereas F shows the reduction of POM volume defined by aromatase staining and G the absence of effect on vasotocin innervation.
The behavioral inhibition induced by the blockade of SRC-1 expression was associated with major neuroanatomical and neurochemical changes in the preoptic region. In agreement with the idea that SRC-1 is involved in the modulation of steroid action in the brain, we found that the volume of the medial preoptic nucleus as defined by Nissl staining was markedly decreased in birds treated with SRC-1 antisense [27]. In parallel, the volume of the POM defined by the cluster of aromatase-immunoreactive neurons was smaller in the AS group compared to controls and the expression of vasotocin in the POA, was also significantly reduced after antisense treatment (see figure 3C). This confirms that SRC-1 indeed regulates steroid action in the POM. Finally, the integrated density of aromatase in the POM, an index reflecting aromatase content in this nucleus (see figure 3B), was decreased in the AS compared to the control group while the higher expression of SRC-1 in ASSC was associated not only with a major rebound of copulatory behavior but also with an extremely rapid development of the POM volume defined by Nissl staining and by aromatase immunoreactivity and a very high aromatase integrated density [27]. These experiments support the idea that SRC-1 is required in vivo for the behavioral and physiological response to T. A reduction of the expression of the coactivator decreased the estrogen- and androgen-dependent male sexual behavior and the associated T-induced changes in the underlying neural substrate while an over-expression of the coactivator was associated with an augmentation of these steroid-dependent responses.
We also performed an additional experiment [30] to analyze in more detail the rapid increase of behavioral and neurochemical attributes of the HPOA during the recovery period, after interruption of antisense treatment (ASSC condition). In this experiment, birds were first treated for 6 days with the antisense targeting SRC-1 expression and then received for three additional days scrambled injections (ASSC group) while control subjects were injected for the entire duration with either AS or SC. As expected, subjects of the ASSC group increased their behavioral response to T within a day of the cessation of AS injections. However, the histological analysis revealed a lack of differences in POM volume and in various measures of aromatase expression and aromatase activity in the preoptic area between the ASSC and SC birds and more surprisingly between the two groups and the AS group. Unlike what we had observed in a previous experiment, the POM volume defined by Nissl staining or aromatase immunoreactivity as well as the optical density of the aromatase signal and the aromatase index reflecting the enzyme content in the POM were similar in the three groups [30].
This set of data therefore revealed a dissociation between the effects of T on male copulatory behavior and on the anatomical and neurochemical plasticity in the POM. As a consequence, the specific mechanisms mediating the inhibition of male sexual behavior following SRC-1 reduction remain partly unclear. Our first antisense experiment suggested that the inhibition of male sexual behavior was mediated, at least in part, by the decrease of aromatase content in POM. However, sexual behavior can also be inhibited by injections of SRC-1 antisense in the absence of significant changes in measures of brain aromatase. It is likely that other neurochemical systems controlling the expression of the behavior are also altered by the antisense treatment and play a major role in the suppression of behavior. For example, perikarya immunoreactive for neuropeptide Y, substance P, corticotropin releasing factor, galanin or neurotensin (see reviews in [13, 76, 94] are present along the third ventricle in close vicinity to or directly within the POM and their fibers are particularly dense within this nucleus. Many studies have shown that these systems are steroid-dependent and, more specifically, that T modulates their expression in quail or in other vertebrate species (review by [74]. Moreover, these neuropeptides have been directly implicated in the regulation of male sexual behavior. It is therefore very likely that, in quail, SRC-1 depletion is associated with changes in the expression of these different neuropeptides within the POM, which may, in turn, modulate male copulatory behavior. It should also be noted that the importance of SRC-1 in mediating steroid-dependent behaviors was also investigated and confirmed in rodents [5, 8, 65, 66].
While the reduction of SRC-1 expression significantly inhibits T-dependent male sexual behavior, the cell phenotypes that are affected by this reduction remain largely unknown. This raises a large number of questions concerning the exact role of SRC-1 in biochemical and physiological cascades leading to the activation of behavior and the importance of SRC-1 for individual target genes. We need to define whether enzymatic and peptidergic systems, other than aromatase, are involved in male sexual behavior and require SRC-1 for full activation. In addition, several coactivators, other than SRC-1, are very likely part of the physiological and behavioral response to T. Notably, SRC-1 is a member of the SRC family encompassing two other coactivators [104]. Its closely related member, SRC-2, shows a significant homology with SRC-1 and more importantly, exhibits the same functional domains [6, 70]. In vitro and in vivo studies indicate that SRC-2 interacts with numerous nuclear receptors, including ER and androgen receptors (AR), and dramatically enhances the transcriptional activity of these steroid receptors [51, 96, 103]. In addition, the neuroanatomical pattern of SRC-2 expression seems to match the pattern of SRC-1 expression in numerous brain regions in quail, including the POM [70], similarly to what was described in rodents [5]. Not surprisingly then, the functions of SRC-1 and -2 in the brain seem to be redundant and one coactivator seems to easily replace the other [71, 102]. In addition, one study suggests similar functions for SRC-1 and -2 in hormone-dependent female sexual behavior and estradiol induction of progesterone receptors in the hypothalamus [5]. However, distinct physiological functions of SRC-2 have also been identified such as regulation of fertility and ductal branching in mammary gland [44, 67], and bone mass control [64]. We therefore investigated whether SRC-2 could also be involved in the control of steroid-dependent male sexual behavior and tested whether the SRC-2 regulated similar aspects of the neuroplasticity as those controlled by SRC-1.
Using the same methods as for SRC-1, we targeted SRC-2 expression in the POM using the antisense technique and showed that the chronic down-regulation of the coactivator expression led to a significant reduction of all consummatory aspects of male sexual behavior, including neck grabs, mount attempts, mounts (and cloacal contact movements ([70] and see figure 3D). In parallel to the copulatory behavior per se, we also analyzed the appetitive aspect of sexual behavior by comparing the mean numbers of RCSM and showed that SRC-2 depletion did not affect this form of appetitive behavior (figure 3E). The most exciting interpretation of these results is that SRC-2 could be required only for specific aspects of male sexual behavior and this hypothesis will be further investigated. It is also possible that the antisense injections in the third ventricle affected SRC-2 expression in only one part of the POM; SRC-2 could have blocked T action only the posterior part of the nucleus leading to an inhibition of copulatory behavior per se whereas the rostral part of the POM and the expression of RCSM would not have been affected. As indicated above, appetitive sexual behavior was previously shown to be markedly inhibited when the anterior region of the POM is destroyed by an electrolytic lesion while the consummatory aspect seems to be controlled mostly by the posterior part of this nucleus [11, 14]. It should also be noted that the frequency of RCSM is only one of the possible tests used to quantify appetitive behavior in Japanese quail [84, 87, 89] and another specific test based on the measure of the learned social proximity response towards a female could potentially lead to different results [40, 69]. Indeed, the development of these two appetitive behaviors does (social proximity response) or does not (rhythmic sphincter cloacal contact movement) require previous sexual experience. It would therefore be interesting to define whether different aspects of appetitive behavior are equally (un)affected by the reduction of SRC-2 in the preoptic area-hypothalamus.
In addition to the reduced male sexual behavior, the depletion of SRC-2 significantly affected aromatase expression in the POM (figure 3F), while leaving the number of vasotocin fibers intact (figure 3G). It is interesting to note that the effects of SRC-2 antisense on T-dependent male sexual behavior and aromatase expression mirror to a large extent those obtained with SRC-1 knock-down [27, 30]. Similar observations were made in rodents highlighting the importance of both SRC-1 and SRC-2 for the activation of estrogen-dependent female sexual behavior and the induction of progesterone receptors in the ventro-medial nucleus of the hypothalamus [5, 65, 66]. Additional studies will be required to define whether SRC-1 and SRC-2 act on the same or different target genes in vivo to control the complex sexual behavior.
5. CONCLUSIONS
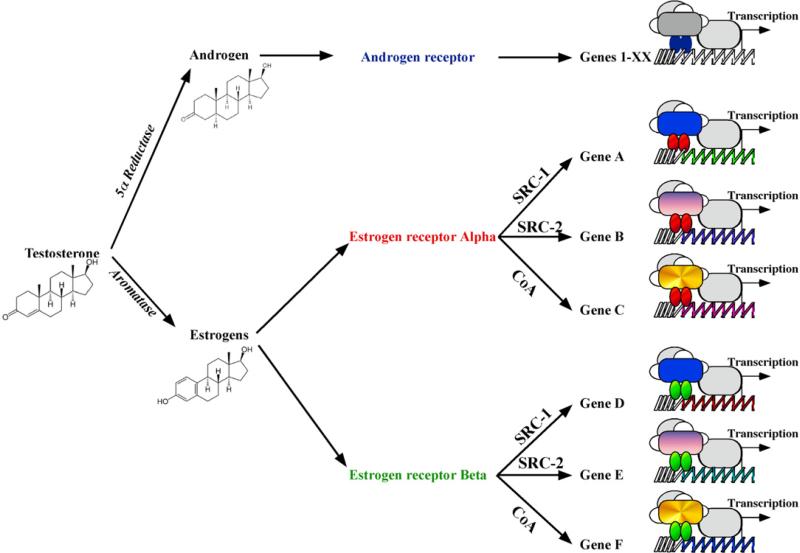
The mechanisms by which T acts in a region-specific, and cell type-specific, manner is a fundamental issue in steroid hormone action in brain. It is now clear that the androgen T can not only act on androgen receptor, but also on two other steroid receptors, the estrogen receptors alpha and beta, via local metabolism by aromatase. Importantly, each of these receptor actions can be modulated by nuclear receptor coregulators. Studies from cell lines have revealed much about the molecular mechanisms of action of these coactivators. Furthermore, work in the brain, as well as other steroid-sensitive tissues, indicates that steroid receptor coregulators are critical in the fine-tuning of steroid responsiveness within individual cells. Coactivators appear to control hormone-responsiveness in specific brain sites. Understanding the mechanisms defining the early configuration of coactivator complexes and their later recruitment to the promoter of specific genes, a process likely to be cell- and tissue-specific, will be critical to understand how hormones, and T in particular, function in the brain to regulate complex behaviors and the underlying neural plasticity. The interest in coactivators is growing exponentially and researchers involved in the study of nuclear receptors cannot neglect the importance of these proteins. While the role of these coregulatory proteins is actively studied at the molecular level, their role in vivo remains largely unknown. In order to understand how hormones function in the brain to regulate complex behaviors, it will be critical to understand the recruitment of different coactivator complexes by each receptor to the promoter, which is likely to determine the cell- and tissue-specificity of steroid action.
Highlights.
-Testosterone is able to trigger a vast number of physiological and behavioral events
- Testosterone can be aromatized to activate estrogen receptors
- Activation of estrogen receptors α or β activates male sexual behavior in quail
- The specificity of testosterone action is related to steroid receptor coactivators
- Steroid metabolizing enzymes, receptors and coactivators interact in a complex manner
Figure 4.
Schematic diagram representing the potential mechanisms regulating the pleiotropic, albeit specific, effects of testosterone on the activation of gene expression.
Acknowledgments
The research from our laboratory described in this paper was supported by grants from NIMH (R01MH50388), the Belgian FRFC (Nbr. 2.4537.9) and the University of Liège (Crédits spéciaux) to Jacques Balthazart. Thierry D. Charlier was a Research Associate at the University of Liège and is currently assistant professor at Ohio University.
Abbreviations
- AR
Androgen receptor
- ASB
Appetitive sexual behavior
- CSB
Consummatory sexual behavior
- DES
diethylstilbestrol
- DPN
diarylpropionitrile ERβ specific agonist
- E2
17β-estradiol
- ER
estrogen receptors
- POM
medial preoptic nucleus
- PPT
propyl-pyrazole-triol
- ERα
specific agonist
- RCSM
rhythmic cloacal sphincter movement
- SRC
steroid receptor coactivator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adkins EK. Effects of diverse androgens on the sexual behavior and morphology of castrated male quail. Horm.Behav. 1977;8:201–207. doi: 10.1016/0018-506x(77)90037-x. [DOI] [PubMed] [Google Scholar]
- 2.Adkins EK, Adler NT. Hormonal control of behavior in the Japanese quail. J.Comp.Physiol.Psychol. 1972;81:27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- 3.Adkins EK, Pniewski EE. Control of reproductive behavior by sex steroids in male quail. J.Comp.Physiol.Psychol. 1978;92:1169–1178. [Google Scholar]
- 4.Alexandre C, Balthazart J. Effects of metabolism inhibitors, antiestrogens and antiandrogens on the androgen and estrogen induced sexual behavior in Japanese quail. Physiol.Behav. 1986;38:581–591. doi: 10.1016/0031-9384(86)90429-4. [DOI] [PubMed] [Google Scholar]
- 5.Apostolakis EM, Ramamurphy M, Zhou D, Onate SA, O'Malley BW. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endocrinol. 2002;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- 6.Arai S, Ogawa K, Yamachika S, Nishihara T, Nishikawa J. Cloning and functional characterization of chicken p160 coactivator family members. Biochim.Biophys.Acta. 2001;1518:7–18. doi: 10.1016/s0167-4781(00)00307-9. [DOI] [PubMed] [Google Scholar]
- 7.Aste N, Panzica GC, Aimar P, Viglietti-Panzica C, Harada N, Foidart A, Balthazart J. Morphometric studies demonstrate that aromatase-immunoreactive cells are the main target of androgens and estrogens in the quail medial preoptic nucleus. Exp.Brain Res. 1994;101:241–252. doi: 10.1007/BF00228744. [DOI] [PubMed] [Google Scholar]
- 8.Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. PNAS. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball GF, Balthazart J. Hormonal regulation of brain circuit mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Ball GF, Nock B, McEwen BS, Balthazart J. Distribution of alpha2-adrenergic receptors in the brain of the Japanese quail as determined by quantitative autoradiography: Implications for the control of sexually dimorphic reproductive processes. Brain Res. 1989;491:68–79. doi: 10.1016/0006-8993(89)90088-7. [DOI] [PubMed] [Google Scholar]
- 11.Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J.Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiology and Behavior. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Balthazart J, Ball GF. The Japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Ann.Rev.Sex Res. 1998;9:96–176. [PubMed] [Google Scholar]
- 14.Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Frontiers in neuroendocrinology. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balthazart J, Evrard L, Surlemont C. Effects of the non-steroidal aromatase inhibitor, R76713 on testosterone-induced sexual behavior in the Japanese quail (Coturnix coturnix japonica) Horm.Behav. 1990;24:510–531. doi: 10.1016/0018-506x(90)90039-z. [DOI] [PubMed] [Google Scholar]
- 16.Balthazart J, Foidart A, Surlemont C, Harada N. Preoptic aromatase in quail: behavioral, biochemical and immunocytochemical studies Hormones, Brain and Behavior in vertebrates. 2. Behavioral activation in males and females - Social interactions and reproductive physiology. In: Balthazart J, editor. Comp. Physiol. Vol. 9. Karger; Basel: 1990. pp. 45–62. [Google Scholar]
- 17.Balthazart J, Foidart A, Wilson EM, Ball GF. Immunocytochemical localization of androgen receptors in the male songbird and quail brain. J.Comp.Neurol. 1992;317:407–420. doi: 10.1002/cne.903170407. [DOI] [PubMed] [Google Scholar]
- 18.Balthazart J, Gahr M, Surlemont C. Distribution of estrogen receptors in the brain of the Japanese quail: an immunocytochemical study. Brain Res. 1989;501:205–214. doi: 10.1016/0006-8993(89)90638-0. [DOI] [PubMed] [Google Scholar]
- 19.Balthazart J, Malacarne G, Deviche P. Stimulatory effects of 5β-dihydrotestosterone on the sexual behavior in the domestic chick. Horm.Behav. 1981;15:246–258. doi: 10.1016/0018-506x(81)90014-3. [DOI] [PubMed] [Google Scholar]
- 20.Balthazart J, Reid J, Absil P, Foidart A, Ball GF. Appetitive as well as consummatory aspects of male sexual behavior in quail are activated by androgens and estrogens. Behav.Neurosci. 1995;109:485–501. [PubMed] [Google Scholar]
- 21.Balthazart J, Surlemont C. Copulatory behavior is controlled by the sexually dimorphic nucleus of the quail POA. Brain Res.Bull. 1990;25:7–14. doi: 10.1016/0361-9230(90)90246-v. [DOI] [PubMed] [Google Scholar]
- 22.Balthazart J, Surlemont C, Harada N. Aromatase as a cellular marker of testosterone action in the preoptic area. Physiol.Behav. 1992;51:395–409. doi: 10.1016/0031-9384(92)90158-x. [DOI] [PubMed] [Google Scholar]
- 23.Beach FA. Characteristics of masculine “sex drive”. Nebraska Symposium on Motivation. 1956;4:1–32. [Google Scholar]
- 24.Beach FA, Inman NG. Effects of castration and androgen replacement on mating in male quail. Proc.Natl.Acad.Sci.USA. 1965;54:1426–1431. doi: 10.1073/pnas.54.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenowitz EA. Plasticity of the adult avian song control system. Annals of the New York Academy of Sciences. 2004;1016:560–585. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- 26.Brown SD, Bottjer SW. Testosterone-induced changes in adult canary brain are reversible. Journal of neurobiology. 1993;24:627–640. doi: 10.1002/neu.480240508. [DOI] [PubMed] [Google Scholar]
- 27.Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J Neurosci. 2005;25:906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlier TD, Ball GF, Balthazart J. Rapid action on neuroplasticity precedes behavioral activation by testosterone. Horm Behav. 2008;54:488–495. doi: 10.1016/j.yhbeh.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charlier TD, Balthazart J, Ball GF. Sex differences in the distribution of the steroid receptor coactivator SRC-1 in the song control nuclei of male and female canaries. Brain Res. 2003;959:263–274. doi: 10.1016/s0006-8993(02)03758-7. [DOI] [PubMed] [Google Scholar]
- 30.Charlier TD, Harada N, Ball GF, Balthazart J. Targeting steroid receptor coactivator-1 expression with locked nucleic acids antisense reveals different thresholds for the hormonal regulation of male sexual behavior in relation to aromatase activity and protein expression. Behav Brain Res. 2006;172:333–343. doi: 10.1016/j.bbr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Charlier TD, Lakaye B, Ball GF, Balthazart J. Steroid receptor coactivator SRC-1 exhibits high expression in steroid-sensitive brain areas regulating reproductive behaviors in the quail brain. Neuroendocrinology. 2002;76:297–315. doi: 10.1159/000066624. [DOI] [PubMed] [Google Scholar]
- 32.Charlier TD, Newman AE, Heimovics SA, Po KW, Saldanha CJ, Soma KK. Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J Neuroendocrinol. 2011;23:742–753. doi: 10.1111/j.1365-2826.2011.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charlier TD, Po KW, Newman AE, Shah AH, Saldanha CJ, Soma KK. 17beta-Estradiol levels in male zebra finch brain: combining Palkovits punch and an ultrasensitive radioimmunoassay. Gen Comp Endocrinol. 2010;167:18–26. doi: 10.1016/j.ygcen.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooke BM, Tabina G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. PNAS. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain-derived estrogens. Frontiers in neuroendocrinology. 2012;33:425–446. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies DT, Massa R, James R. Role of testosterone and of its metabolites in regulating gonadotrophin secretion in the Japanese quail. J.Endocrinol. 1980;84:211–222. doi: 10.1677/joe.0.0840211. [DOI] [PubMed] [Google Scholar]
- 37.Delville Y, Hendrick JC, Sulon J, Balthazart J. Testosterone metabolism and testosterone-dependent characteristics in Japanese quail. Physiol.Behav. 1984;33:817–823. doi: 10.1016/0031-9384(84)90053-2. [DOI] [PubMed] [Google Scholar]
- 38.Deviche P, Bottoni L, Balthazart J. 5beta-dihydrotestosterone is weakly androgenic in the adult Japanese quail (Coturnix coturnix japonica) Gen.Comp.Endocrinol. 1982;48:421–424. doi: 10.1016/0016-6480(82)90176-9. [DOI] [PubMed] [Google Scholar]
- 39.Ding X, Anderson C, Ma H, Hong H, Uht M, Kushner P, Stallcup M. Nuclear receptor-binding sites of coactivators glucocorticoid receptor interacting protein 1 (GRIP1) and steroid receptor coactivator-1 (SRC-1): multiple motifs with different binding specificities. Mol Endo. 1998;12:302–313. doi: 10.1210/mend.12.2.0065. [DOI] [PubMed] [Google Scholar]
- 40.Domjan M, Hall S. Determinants of social proximity in Japanese quail (Coturnix coturnix japonica): male behavior. J Comp Psychol. 1986;100:59–67. [PubMed] [Google Scholar]
- 41.Foidart A, Harada N, Balthazart J. Effects of steroidal and non steroidal aromatase inhibitors on sexual behavior and aromatase-immunoreactive cells and fibers in the quail brain. Brain Res. 1994;657:105–123. doi: 10.1016/0006-8993(94)90958-x. [DOI] [PubMed] [Google Scholar]
- 42.Foidart A, Lakaye B, Grisar T, Ball GF, Balthazart J. Estrogen receptor-beta in quail: Cloning, tissue expression and neuroanatomical distribution. J.Neurobiol. 1999;40:327–342. [PubMed] [Google Scholar]
- 43.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Frontiers in neuroendocrinology. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22:5923–5937. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill RK, Atkins LM, Hollis BW, Bell NH. Mapping the domains of the interaction of the vitamin D receptor and steroid receptor coactivator-1. Mol Endocrinol. 1998;12:57–65. doi: 10.1210/mend.12.1.0048. [DOI] [PubMed] [Google Scholar]
- 46.Hammes SR, Levin ER. Extracellular steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 47.Hammond G. Potential function of plasma steroid-binding proteins. Trends Endocrinol Metab. 1995;6:298–304. doi: 10.1016/1043-2760(95)00162-x. [DOI] [PubMed] [Google Scholar]
- 48.Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biology of reproduction. 2011;85:431–441. doi: 10.1095/biolreprod.111.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammond GL, Smith CL, Paterson NA, Sibbald WJ. A role for corticosteroid-binding globulin in delivery of cortisol to activated neutrophils. J Clin Endocrinol Metab. 1990;71:34–-39. doi: 10.1210/jcem-71-1-34. [DOI] [PubMed] [Google Scholar]
- 50.Han SJ, Lonard DM, O'Malley BW. Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrinol Metab. 2009;20:8–15. doi: 10.1016/j.tem.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoids and vitamin D receptors. Mol Cell Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikonen T, Palvimo JJ, Janne OA. Interaction between the amino- and carboxyl-terminal regions of the rat androgen receptor modulates transcriptional activity and is influenced by nuclear receptor coactivators. Journal of Biological Chemistry. 1997;272:29821–29828. doi: 10.1074/jbc.272.47.29821. [DOI] [PubMed] [Google Scholar]
- 53.Ishunina TA, Swaab DF. Decreased alternative splicing of estrogen receptor-alpha mRNA in the Alzheimer's disease brain. Neurobiology of aging. 2012;33:286–296. e283. doi: 10.1016/j.neurobiolaging.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Johnson F, Bottjer SW. Hormone-induced changes in identified cell populations of the higher vocal center in male canaries. J.Neurobiol. 1993;24:400–418. doi: 10.1002/neu.480240311. [DOI] [PubMed] [Google Scholar]
- 55.Klieber MA, Underhill C, Hammond GL, Muller YA. Corticosteroid-binding globulin, a structural basis for transport and proteinase-triggered release. J Biol Chem. 2007;282:29594–29603. doi: 10.1074/jbc.M705014200. [DOI] [PubMed] [Google Scholar]
- 56.Lin HY, Muller YA, Hammond GL. Molecular and structural basis of steroid hormone binding and release from corticosteroid-binding globulin. Molecular and cellular endocrinology. 2010;316:3–12. doi: 10.1016/j.mce.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 57.Lonard DM, O'Malley BW. Expanding functional diversity of the coactivators. TIBS. 2005;30:126–132. doi: 10.1016/j.tibs.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Malisch JL, Breuner CW. Steroid-binding proteins and free steroids in birds. Molecular and cellular endocrinology. 2010;316:42–52. doi: 10.1016/j.mce.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 59.McKenna N, Nawaz Z, Tsai SY, Tsai M-J, O'Malley BW. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc Natl Acad Sci USA. 1998;95:11697–11702. doi: 10.1073/pnas.95.20.11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKenna NJ, Cooney AJ, DeMayo FJ, Downes M, Glass CK, Lanz RB, Lazar MA, Mangelsdorf DJ, Moore DD, Qin J, Steffen DL, Tsai MJ, Tsai SY, Yu R, Margolis RN, Evans RM, O'Malley BW. Minireview: Evolution of NURSA, the Nuclear Receptor Signaling Atlas. Mol Endocrinol. 2009;23:740–746. doi: 10.1210/me.2009-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 62.McKenna NJ, Xu J, Nawaz Z, Tsai SY, Tsai M-J, O'Malley BW. Nuclear receptor coactivator: multiple enzymes, multiple complexes, multiple functions. J Steroid Bioch Mol Biol. 1999;69:3–12. doi: 10.1016/s0960-0760(98)00144-7. [DOI] [PubMed] [Google Scholar]
- 63.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 64.Modder UI, Monroe DG, Fraser DG, Spelsberg TC, Rosen CJ, Gehin M, Chambon P, O'Malley BW, Khosla S. Skeletal consequences of deletion of steroid receptor coactivator-2/transcription intermediary factor-2. J Biol Chem. 2009;284:18767–18777. doi: 10.1074/jbc.M109.000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ. Nuclear receptor coactivators function in estrogen receptor- and progestin receptor-dependent aspects of sexual behavior in female rats. Horm Behav. 2006;50:383–392. doi: 10.1016/j.yhbeh.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- 67.Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O'Malley BW. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol. 2006;26:6571–6583. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Iigo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S, Ueda HR, Yoshimura T. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- 69.Nash S, Domjan M, Askins M. Sexual-discrimination learning in male japanese quail (Coturnix coturnix japonica) J.Comp.Psychol. 1989;103:347–358. doi: 10.1037/0735-7036.103.4.347. [DOI] [PubMed] [Google Scholar]
- 70.Niessen NA, Balthazart J, Ball GF, Charlier TD. Steroid receptor coactivator 2 modulates steroid-dependent male sexual behavior and neuroplasticity in Japanese quail (Coturnix japonica) Journal of neurochemistry. 2011;119:579–593. doi: 10.1111/j.1471-4159.2011.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishihara E, Yoshida-Komiya H, Chang C-SC, Davis RL, O'Malley BW, Xu J. SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar purkinje cells. J Neurosci. 2003;23:123–222. doi: 10.1523/JNEUROSCI.23-01-00213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Malley BW, Kumar R. Nuclear receptor coregulators in cancer biology. Cancer Res. 2009;69:8217–8222. doi: 10.1158/0008-5472.CAN-09-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oñate SA, Tsai SY, Tsai M-J, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 74.Panzica G, Viglietti-Panzica C, Balthazart J. Sexual dimorphism in the neuronal circuits of the quail preoptic and limbic regions. Microsc Res Tech. 2001;54:364–374. doi: 10.1002/jemt.1149. [DOI] [PubMed] [Google Scholar]
- 75.Panzica GC, Aste N, Viglietti-Panzica C, Ottinger MA. Structural sex differences in the brain: Influence of gonadal steroids and behavioral correlates. J.Endocrinol.Invest. 1995;18:232–252. doi: 10.1007/BF03347808. [DOI] [PubMed] [Google Scholar]
- 76.Panzica GC, De Bernardi W, Coscia A, Fasolo A, Andreone C, Viglietti-Panzica C. An immunocytochemical and cytoarchitectural analysis of the sexually dimorphic medial preoptic nucleus of the Japanese quail. European Journal of Neuroscience. 1989;0(Suppl):0. [Google Scholar]
- 77.Panzica GC, Viglietti-Panzica C, Balthazart J. Sex dimorphism of the avian medial preoptic nucleus: cytoarchitecture, morphometry and hormonal control. Acta Anatomica. 1987;130:70–70. [Google Scholar]
- 78.Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front.Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- 79.Pfaus JG. Revisiting the concept of sexual motivation. Annual review of sex research. 1999;10:120–156. [PubMed] [Google Scholar]
- 80.Price RH, Jr., Lorenzon N, Handa RJ. Differential expression of estrogen receptor beta splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Brain research. Molecular brain research. 2000;80:260–268. doi: 10.1016/s0169-328x(00)00135-2. [DOI] [PubMed] [Google Scholar]
- 81.Ptashne M, Gann AA. Activators and targets. Nature. 1990;346:329–331. doi: 10.1038/346329a0. [DOI] [PubMed] [Google Scholar]
- 82.Schlinger BA, Callard GV. Aggressive behavior in birds: An experimental model for studies of brain-steroid interactions. Comp.Biochem.Physiol.[A] 1990;97A:307–316. doi: 10.1016/0300-9629(90)90616-z. [DOI] [PubMed] [Google Scholar]
- 83.Schlinger BA, Callard GV. Aromatization mediates aggressive behavior in quail. Gen.Comp.Endocrinol. 1990;79:39–53. doi: 10.1016/0016-6480(90)90086-2. [DOI] [PubMed] [Google Scholar]
- 84.Seiwert CM, Adkins-Regan E. The foam production system of the male japanese quail: characterization of structure and function. Brain Behav.Evol. 1998;52:61–80. doi: 10.1159/000006553. [DOI] [PubMed] [Google Scholar]
- 85.Seredynski AL, Ball GF, Balthazart J, Charlier TD. Specific activation of estrogen receptor alpha and beta enhances male sexual behavior and neuroplasticity in male Japanese quail. PloS one. 2011;6:e18627. doi: 10.1371/journal.pone.0018627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steimer T, Hutchison JB. Metabolic control of the behavioral action of androgens in the dove brain: testosterone inactivation by 5b-reduction. Brain Res. 1981;209:189–204. doi: 10.1016/0006-8993(81)91180-x. [DOI] [PubMed] [Google Scholar]
- 87.Taziaux M, Cornil CA, Balthazart J. Aromatase inhibition blocks the expression of sexually-motivated cloacal gland movements in male quail. Behavioural Processes. 2004;67:461–469. doi: 10.1016/j.beproc.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 88.Taziaux M, Cornil CA, Dejace C, Arckens L, Ball GF, Balthazart J. Neuroanatomical specificity in the expression of the immediate early gene c-fos following expression of appetitive and consummatory male sexual behaviour in Japanese quail. The European journal of neuroscience. 2006;23:1869–1887. doi: 10.1111/j.1460-9568.2006.04719.x. [DOI] [PubMed] [Google Scholar]
- 89.Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatum nucleus taeniae in the sexual behavior of male japanese quail (Coturnix japonica): a comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav.Evol. 1998;51:215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- 90.Tsutsui K, Ishii S. Effects of sex steroids on agressive behavior of adult male japanese quail. Gen Comp Endocrinol. 1981;44:480–486. doi: 10.1016/0016-6480(81)90336-1. [DOI] [PubMed] [Google Scholar]
- 91.Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogen in neurobiology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 92.Vasudevan N, Zhu Y-S, Daniel S, Koibuchi N, Chin WW, Pfaff D. Crosstalk between oestrogen receptors and thyroid hormone resceptor isoforms results in differential regulation of preproenkephalin gene. J Neuroendocrinol. 2001;13:779–790. doi: 10.1046/j.1365-2826.2001.00693.x. [DOI] [PubMed] [Google Scholar]
- 93.Viglietti-Panzica C, Balthazart J, Plumari L, Fratesi S, Absil P, Panzica GC. Estradiol mediates effects of testosterone on vasotocin immunoreactivity in the adult quail brain. Horm Behav. 2001;40:445–461. doi: 10.1006/hbeh.2001.1710. [DOI] [PubMed] [Google Scholar]
- 94.Viglietti-Panzica C, Panzica GC. Peptidergic neurons in the avian brain, Annales des Sciences Naturelles. Zoologie, Paris. 1991;12:137–155. [Google Scholar]
- 95.Viglietti-Panzica C, Panzica GC, Fiori MG, Calcagni M, Anselmetti GC, Balthazart J. A sexually dimorphic nucleus in the quail preoptic area. Neurosci.Ltrs. 1986;64:129–134. doi: 10.1016/0304-3940(86)90087-x. [DOI] [PubMed] [Google Scholar]
- 96.Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 97.Voigt C, Ball GF, Balthazart J. Sex differences in the expression of sex steroid receptor mRNA in the quail brain. J Neuroendocrinol. 2009;21:1045–1062. doi: 10.1111/j.1365-2826.2009.01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watson JT, Adkins-Regan E. Activation of sexual behavior by implantation of testosterone propionate and estradiol benzoate into the preoptic area of the male Japanese quail (Coturnix japonica) Horm.Behav. 1989;23:251–268. doi: 10.1016/0018-506x(89)90065-2. [DOI] [PubMed] [Google Scholar]
- 99.Watson JT, Adkins-Regan E. Testosterone implanted in the preoptic area of male Japanese quail must be aromatized to activate copulation. Horm.Behav. 1989;23:432–447. doi: 10.1016/0018-506x(89)90055-x. [DOI] [PubMed] [Google Scholar]
- 100.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively ac tive androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Westphal U. Steroid-protein interaction II. Springer-Verlag; Heidelberg; Berlin: New York, Tokyo: 1986. [Google Scholar]
- 102.Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai M-J, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 103.Ye X, Han SJ, Tsai SY, DeMayo F, Xu J, Tsai M-J, O'Malley BW. Role of steroid receptor coactivator (SRC)-1 and transcriptional intermediary factor (TIF) in androgen receptor activity in mice. PNAS. 2005;102:9487–9492. doi: 10.1073/pnas.0503577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.York B, O'Malley BW. Steroid receptor coactivator (SRC) family: masters of systems biology. J Biol Chem. 2010;285:38743–38750. doi: 10.1074/jbc.R110.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]