Abstract
Over the past decade, the genetics of external genital development has begun to be understood. Male and female external genitalia develop from the genital tubercle. The early tubercle has a superficial resemblance to the limb bud, but an important distinction is that the limb consists only of mesoderm and ectoderm, whereas the genital tubercle also has an endodermal component, the urethral epithelium. Urethral epithelium, which expresses Sonic hedgehog, acts as a signaling region that controls outgrowth and pattern formation, and ultimately differentiates into the urethral tube. While there are intriguing parallels between limb and genital development, recent studies have identified some key differences, including the role of Fgf signaling. Our understanding of the mechanisms of genital development still lags far behind the limb, and major questions remain to be answered, including the molecular nature of the signals that initiate genital budding, sustain outgrowth, induce tissue polarity and orchestrate urethral tubulogenesis.
Keywords: limb bud, genital tubercle, appendages, urethra, digits
Introduction
Limb development has been a powerful system for identifying the mechanisms that construct complex appendages. Different types of outgrowths utilize the same fundamental genetic programs to initiate budding, polarize tissues in three dimensions, and coordinate these processes with proximodistal outgrowth. Application of the limb model has been particularly useful for studies of external genital development. The genital tubercle is the embryonic precursor of the penis and clitoris, and much of its early development (through approximately E15 in the mouse) is indistinguishable between males and females. Building external genitalia requires that the embryo solve many of the same problems it faces during limb formation, including specification of a field of competence, induction of a budding (the genital swellings), maintenance of outgrowth (the genital tubercle) and coordination of outgrowth with dorsoventral and proximodistal patterning of the tissues (Fig. 1). Surgical manipulations of the genital tubercle identified cell populations with functions that appear to be analogous to the AER and ZPA. Moreover, comparisons of some of the initial gene expression patterns of Hox genes, Fgfs and Sonic hedgehog suggested that these parallels extend to the molecular level, which led to the hypothesis that the genital tubercle may be, in essence, a limb bud that develops in a different context. However, as functional studies have progressed in the area of external genital development, the parallels with the limb have been shown to be less extensive than initially thought. Below I discuss recent progress in the molecular mechanisms of external genital development and address the similarities and differences with the vertebrate limb bud.
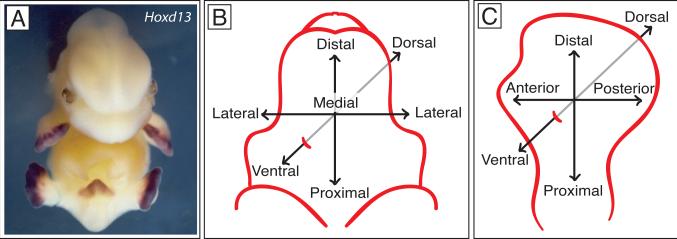
Fig. 1. Comparative organization of the genital tubercle and limb bud.
A. Mouse embryo at E13.5 after in situ hybridization with Hoxd13 antisense RNA probe. Purple stain shows Hoxd13 expression in the handplates, footplates and genital tubercle. B. Major axes of the genital tubercle. C. Major axes of the limb.
Initiation of external genital budding
Although the genital tubercle resembles a limb bud at early stages of development, it originates as a pair of buds, the genital swellings, on either side of the cloacal membrane (Perriton et al., 2002). These swellings are first detected in the mouse at embryonic day (E) 10.5, and by E11.5 the paired swellings have merged to form a single genital tubercle. Whereas the limb bud is composed of mesoderm contained within a jacket of surface ectoderm, the genital tubercle is derived from all three germ layers. The bulk of the tubercle is composed of mesoderm surrounded by ectoderm, like the limb, however the genital tubercle also contains an extension of the cloacal endoderm, which forms the urethral plate epithelium (Fig. 2A, C). This endoderm extends ventral to the urogenital sinus as an open tube that closes distally to form a bilaminar urethral plate within the genital tubercle (Fig. 2C). It was previously thought that the urethral plate endoderm gave rise to the proximal part of the penile urethra, and that surface ectoderm at the tip of the tubercle invaginated to form the urethra of the glans (Larson, 2001). However, recent fate mapping studies overturned this idea by showing that the entire penile urethra is derived from the endodermal urethral plate (Seifert et al., 2008)
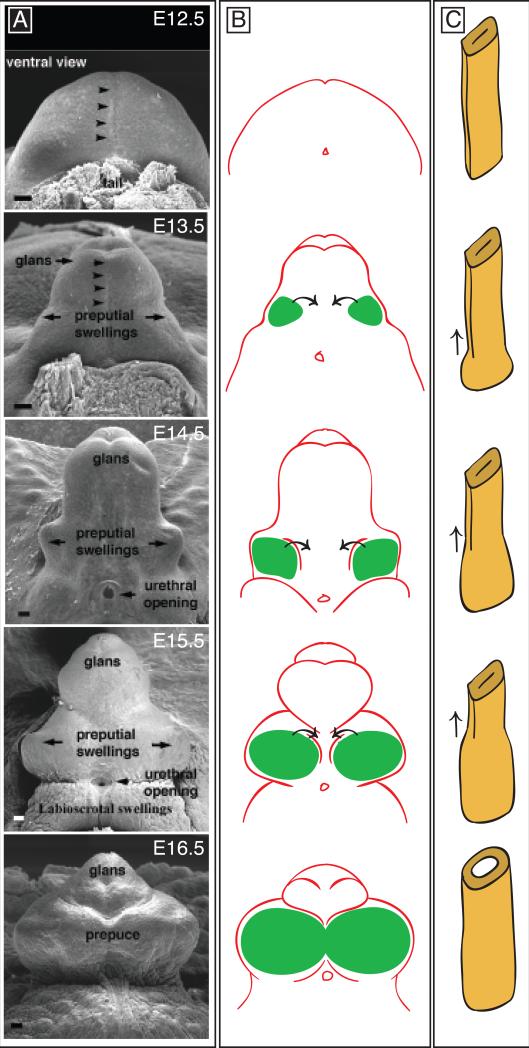
Fig. 2. Development of the external genitalia.
A. Scanning electron micrographs of mouse genital tubercles from E12.5-E16.5. All panels are ventral views. Arrowheads mark the position of the urethral plate along the ventral midline. Modified from Perriton et al., 2002. B. Development of the prepuce. Paired preputial swellings are shown in green. Ventral and lateral growth of the preputial swellings results in development of a prepuce that surrounds the glans of the penis or clitoris. C. Schematic diagram of urethral tube formation. At E12.5, the endodermally-derived urethral epithelium is a bilaminar plate without a lumen. Tubulogenesis then progresses from proximal to distal (arrows), resulting in conversion of the closed plate to an open tube.
Although good progress has been made identifying the molecules involved in the initiation of limb budding, the initiation of genital outgrowth is not well understood. At the time of this writing, the only reported examples of mutants that fail to initiate genital budding are the subset of Wnt5a mutants in which the urorectal septum endoderm fails to contact the cloacal ectoderm (Seifert et al., 2009b). This suggested that an interaction between endoderm and ectoderm at the cloacal membrane may be an important step in induction of budding. Wnt5a mutants have a variably penetrant phenotype, which has complicated its use as a model study early genital development (see below), but the correlation between absence of genital budding and failure to form a cloacal membrane (endodermal and ectodermal components) is intriguing and warrants further investigation.
The first gene found to be involved in genital outgrowth was Hoxd13, which is expressed both in the genital tubercle and the autopod (digit forming region) of the limb (Dolle et al., 1991). Hoxd13 expression in these two positions is controlled by the same enhancer element within a Global Control Region (GCR), approximately 160kb from the Hoxd13 locus (Spitz et al., 2003). The paralogous Hoxa13 gene is also co-expressed in the autopod and genital tubercle, and homozygous null mutants for either Hoxd13 or Hoxa13 have patterning defects of the external genitalia and limbs (described below), while Hoxa13;Hoxd13 double knockouts lack external genitalia and autopodia altogether (Kondo et al., 1997; Warot et al., 1997). Careful examination of these mutants at initiation stages needs to be done in order to determine whether Hox13 genes are required for the earliest phase of genital outgrowth.
Fgf signaling initiates limb budding, and Fgf10 is both necessary and sufficient for induction of complete limbs (Cohn et al., 1995; Min et al., 1998). The T-box transcription factor Tbx5 directly regulates expression of Fgf10 at the forelimb level (Agarwal et al., 2003). Tbx5 (as well as Tbx2, Tbx3 and Tbx4) expression has been described in the genitalia (Chapman et al., 1996), however the roles of these T-box genes in genital development have not been reported. In the genitalia, Fgf10 is involved in urethral tube closure but is not required for outgrowth (Haraguchi et al., 2000; Petiot et al., 2005). Thus, while there are similarities in expression patterns of many genes between limb buds and genital tubercles, it appears that different molecular mechanisms are involved in the initiation of budding in these two organs.
In chick embryos, Wnt2b and Wnt8c are expressed in the presumptive limb fields and, like Fgfs, are sufficient to initiate limb bud formation (Ng et al., 2002), although studies of mouse embryos failed to detect either of these genes during limb initiation (Agarwal et al., 2003). During initiation of external genital budding, canonical Wnt signaling is active, which raised the possibility that the genital initiation factor could be a Wnt protein. Conditional deletion of B-catenin results in absence of external genitalia (Lin et al., 2009), however initial budding of the paired genital swellings occurs in B-catenin mutants (L. Ma, personal communication). Sonic hedgehog (Shh) is expressed in the cloacal endoderm prior to the onset of budding and, although Shh knockouts fail to develop a genital tubercle, they do form the initial paired genital swellings (Haraguchi et al., 2001; Perriton et al., 2002). Although neither Hedgehog nor B-catenin signaling is required for initiation of genital outgrowth, both are necessary for sustained outgrowth and formation of a tubercle (Lin et al., 2008; see below). Thus, the nature of the signal that initiates outgrowth of the genital tubercle is unknown at present.
Maintenance of genital tubercle outgrowth: the role of urethral epithelium
Twenty-five years ago, it was shown that proximodistal development of the external genitalia is dependent on the epithelial component of the genital tubercle. Removal of the epithelium from rat genital tubercles resulted in stage-dependent truncation of the baculum (penian bone) and erectile tissues (Murakami and Mizuno, 1986). Although these manipulations were done at relatively late stages of outgrowth, the observation was reminiscent of the permissive role of the apical ectodermal ridge in the limb bud and suggested that conserved mechanisms could be responsible for maintenance of outgrowth of both structures.
Fgf8 is not involved in genital outgrowth
More recently, it was shown that Fgf8 is expressed in the distal tip of the urethral epithelium in a pattern that bears a striking resemblance to its expression in the AER at the distal tip of the limb bud (Haraguchi et al., 2000; Perriton et al., 2002). Surprisingly, genetic deletion of Fgf8 in the genital tubercle has no effect on external genital development (Seifert et al., 2009b). Indeed, analysis of Fgf8 target genes and the distribution of Fgf8 protein showed that Fgf8 is not even translated in the urethral epithelium (Seifert et al., 2009b). Although previous in vitro experiments had suggested a role for Fgf8 in the genital tubercle (Haraguchi et al., 2001), genetic studies in vivo showed that Fgf8 is not involved in genital development (Seifert et al., 2009b). Additionally, the other AER-associated Fgfs either are not expressed at any stage of genital development (Fgf4, Fgf17) or are not detectable during the initiation of genital outgrowth (Fgf9) (A. Seifert, M. Gredler and M. J. C, unpublished data). These findings highlight a key difference in the role of Fgf signaling between limbs and genitalia.
Wnt signaling
Wnt genes are good candidates for the proximodistal outgrowth signal, and Wnt2, Wnt3, Wnt4, Wnt5a, Wnt9b and Wnt11 are expressed by E11.5, one day after initiation of budding (Lin et al., 2008). Conditional deletion of B-catenin in endodermal cells (using ShhGfpCre) results in failure to progress beyond the initiation of genital swellings (Lin et al., 2008). Removal of B-catenin activity results in diminished expression of a suite of genes, including Shh (described below), which led Lin et al to conclude that canonical Wnt signaling is essential for genital tubercle outgrowth (Lin et al., 2008). It should be noted that Wnt proteins are not the only factors that signal through B-catenin and, therefore, experimental modulation of B-catenin does not necessarily implicate Wnts in external genital development. Nonetheless, the role of Wnt/B-catenin is supported by two additional discoveries; (1) deletion of Lrp6, a co-receptor for canonical Wnt/B-catenin signaling, results in agenesis of external genitalia (Zhou et al., 2010), and (2) Dkk1, a negative regulator of canonical Wnt signaling, is expressed in the distal urethral epithelium and contains non-coding elements that can drive reporter gene expression in the genital tubercle (Lieven et al., 2010).
The role of non-canonical Wnt signaling in external genital development is less clear. Wnt5a was initially thought to be essential for genital tubercle outgrowth, however the penetrance of Wnt5a null allele is variable, and null mutants have been recovered with phenotypes ranging from rudimentary genital swellings to complete genital tubercles (Yamaguchi et al., 1999; Seifert et al., 2009b). Consistent with Wnt5a playing a role is the discovery that inactivation of Ror2, a Wnt5a receptor that mediates non-canonical Wnt signaling, results in underdevelopment of the external genitalia (Schwabe et al., 2004). Wnt11, which encodes another non-canonical Wnt signal, is expressed at the appropriate stages in external genital development to play a role in outgrowth, but its function is unknown (Lin et al., 2008).
Dynamic role of Sonic hedgehog in outgrowth and patterning
Before the onset of genital outgrowth, Shh is expressed in the cloacal epithelium, which gives rise to the urethral epithelium, and expression persists in urethral cells during the outgrowth period (Haraguchi et al., 2001; Perriton et al., 2002; Seifert et al., 2008). Although Shh mutant embryos do form the initial paired genital swellings, these buds arrest immediately after initiation and fail to give rise to a genital tubercle, which implicates Shh as having an essential role in the maintenance of genital outgrowth (Perriton et al., 2002). Interestingly, the Shh null phenotype can be partially rescued by over-expression of B-catenin in urethral epithelial cells, indicating that although B-catenin signaling maintains expression of Shh in these cells, it can also promote limited outgrowth in the absence of Shh (Lin et al., 2009).
Conditional removal of Shh at different stages of genital tubercle development has provided a detailed picture of Shh function at daily intervals, from the initiation of budding through to stages of sexual differentiation (Lin et al., 2009; Miyagawa et al., 2009a; Seifert et al., 2009a). Shh regulates outgrowth by acting both as a proliferative cue and as a cell survival factor (Haraguchi et al., 2001; Perriton et al., 2002; Lin et al., 2009; Miyagawa et al., 2009a; Seifert et al., 2009a). Temporally controlled deletions showed that the extent of genital tubercle outgrowth is determined by the duration of Shh signaling, with earlier removals resulting in more severe truncations (Lin et al., 2009; Miyagawa et al., 2009a; Seifert et al., 2009a).
In addition to controlling proximodistal outgrowth, Shh is required for development of a closed urethral tube and for correct positioning of the urethral opening, as deletion of Shh up to E16.5 results in hypospadias, an ectopic opening of the urethra on the ventral side of the phallus (Lin et al., 2009; Miyagawa et al., 2009a; Seifert et al., 2009a). If Shh is removed prior to E13.5, mutants also develop a persistent cloaca, in which the embryonic cloaca fails to subdivide into anorectal and genitourinary sinuses. Removal of Shh after E13.5 does not affect division of the anorectal and genitourinary sinises, but does result in severe hypospadias, which causes the genitalia to appear feminized (Seifert et al., 2009a).
Given that the genital tubercle consists of cells from endoderm, ectoderm and mesoderm, which cells respond directly to Shh? Removal of Smoothened (Smo), which is required for activation of the hedgehog transduction pathway in response to hedgehog ligand, from either the genital mesenchyme or ectoderm disrupts genital development, whereas removal of Smo from the urethral epithelium has no effect on external genital morphology (Lin et al., 2009; Seifert et al., 2009a). These studies show that Shh does not act in an autocrine manner on Shh-expressing cells of the urethra, which is consistent with the absence of Patched (Ptch) expression in these cells (Perriton et al., 2002; Lin et al., 2009; Seifert et al., 2009a). When Smo is removed from the mesenchyme, mice have severely underdeveloped genitalia and develop hypospadias (Lin et al., 2009). Removal of Smo from the ectoderm causes a loss of epithelial integrity ventral to the urethral plate, which results in an ectopic opening of the urethra along the ventral margin of the phallus (Seifert et al., 2009a). Thus, Shh signals directly to ectoderm and mesenchyme of the genital tubercle, and activation of the hedgehog pathway in both tissues is required for normal development of the external genitalia.
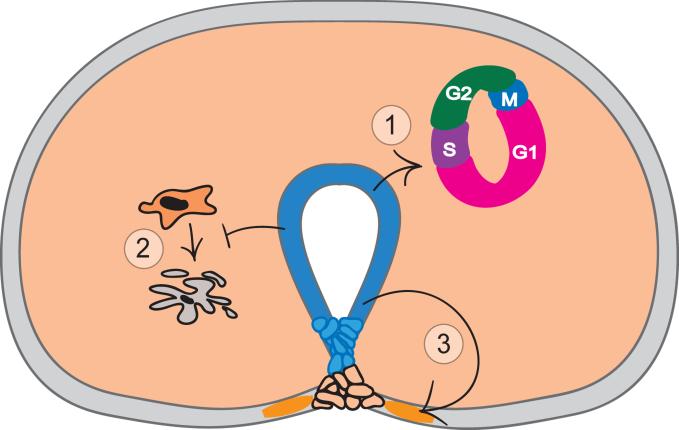
Whereas the role of Shh in genital ectoderm is to maintain structural integrity of the surface epithelium overlying the urethra, its major role in the mesenchyme is to inhibit apoptosis and to stimulate cell proliferation (Fig. 3). Shh promotes mesenchymal proliferation and tubercle outgrowth by controlling the duration of the cell cycle in the genital tubercle mesenchymal cells (Seifert et al., 2010). Deletion of Shh causes the mesenchymal cell cycle to slow to almost half-speed, which leads to grossly underdeveloped genitalia. Specifically, in Shh conditional mutants, mesenchymal cells spend an extended period in G1-phase before progressing to S-phase, which causes total cell cycle time to slow from 8.5 to 14.4 hours (Seifert et al., 2010). Shh directly and indirectly regulates a number of genes that govern cell cycle kinetics (Seifert et al., 2010) as well as pattern formation (Lin et al., 2009; Miyagawa et al., 2009a; Seifert et al., 2009a). The dual role of Shh – specification of early pattern and subsequent elaboration of pattern via growth regulation – appears to be common to limb and genital development (Towers et al., 2008; Zhu et al., 2008; Seifert et al., 2010). Given that integration of pattern formation and growth must occur for normal development of the limb and genital tubercle, this role of Shh may be necessary for outgrowth in many types of appendages.
Fig. 3. Roles of Sonic hedgehog in the genital tubercle.
Sonic hedgehog (Shh) expression in urethral epithelial cells is shown in blue. Mesodermal and surface ectodermal cells that respond directly to Shh signaling are shown in orange. Shh signals directly to the adjacent mesenchyme to positively regulate cell cycle progression (1) and to inhibit apoptosis (2). Shh signals directly to the ventral ectoderm to maintain the structural integrity of the epithelium (3), which is essential for maintenance of a closed urethral tube.
The upstream regulation of Shh expression has been well characterized in the limb (Lettice et al., 2003; Galli et al., 2010), but remains poorly understood in the genital tubercle. By contrast to the limb, in which Shh and Fgf8 maintain each other in a positive feedback loop, loss of Fgf8 in the genital tubercle does not affect Shh expression. Fgf8 expression in the distal urethra is reduced in B-catenin and Shh mutants, although the discovery that Fgf8 is not involved in genital development indicates that Fgf8 transcription is simply a readout, rather than a cause, of genital outgrowth (Seifert et al., 2009b). Further analysis of Wnt gene function in early genital development should cast additional light on the interactions between specific Wnts and Sonic hedgehog.
Polarizing the genital tubercle
Like the limb, the genital tubercle is polarized along three axes (Fig .1). Both structures have proximodistal axes, but the top-to-bottom axis of the genital tubercle is referred to as dorsoventral rather than anteroposterior, and Shh is expressed ventrally (Fig. 1B, C and Fig. 3). As a midline structure, the tubercle also has a mediolateral polarity that exhibits bilateral symmetry (Fig. 1B). Differentiation of the erectile and connective tissues of the tubercle is organized around the urethral plate, which led Perriton et al to suggest that the urethral plate could be a signaling region that confers polarity to the adjacent mesenchyme (Perriton et al., 2002). Transplantation of urethral plate cells to the anterior margin of the limb bud induces mirror image duplication of the digits, and the resultant duplicated wings also develop an epithelial tube that resembles the urethra, complete with a surrounding mesenchymal condensation that differentiates into muscle (Perriton et al., 2002). These experiments identified the urethral plate as an organizing region that can induce tissue polarity as well as epithelial tubulogenesis. It is important to note that these conclusions were based on transplantations of genital cells to the limb bud, and it remains unclear whether the urethral plate (or Shh) induces dorsoventral polarity in the genital tubercle itself. This question is further complicated by the multifunctional role of Shh in coordinating growth and pattern, as the truncations of Shh mutant genitalia have made it difficult to isolate a role in dorsoventral patterning from a role in outgrowth. Further experiments focusing on dorsoventrally restricted gene expression patterns and on the identification of additional signaling regions will be required to determine the mechanisms of dorsovental patterning in the genital tubercle.
One aspect of dorsoventral patterning in which considerable progress has been made is closure of the urethral tube. Several pathways in addition to Shh have been implicated in urethral tube closure, including Fgf10-FgfR2, EphB2-EphrinB2, Hoxa13, and Dlx5/6 (Haraguchi et al., 2000; Morgan et al., 2003; Dravis et al., 2004; Petiot et al., 2005; Suzuki et al., 2008). Two critical morphogenetic processes are involved in urethral tube closure; ventro-lateral growth of the preputial swellings to form the prepuce (or foreskin) that surrounds the glans (Fig. 2B), and remodeling of the bilaminar urethral plate into an epithelial tube (Fig. 2C). Hypospadias can occur when either of these processes is disrupted. FgfR2 is expressed in the ectoderm overlying the preputial swellings and in the urethral epithelium, and its ligand, Fgf10, is expressed in mesenchyme adjacent to the urethral cells. Detailed characterization of Fgf10 and FgfR2iiib knockout mice revealed that both urethral epithelial maturation and ventral growth of the preputial swellings fail to occur when this pathway is inactivated (Petiot et al., 2005). FgfR2 mutants develop dorsal genital structures, but the ventral aspect of the genital tubercle is grossly underdeveloped, which suggests that ventral growth of the prepuce is required for development of a closed urethral tube.
Hoxa13 is expressed in the urethral plate and in the surrounding mesenchyme, and deletion of Hoxa13 also results in hypospadias (Morgan et al., 2003). Interestingly, Hoxa13 mutants exhibit decreases both in mesenchymal cell proliferation and in apoptosis of the urethral plate and mesenchyme (Morgan et al., 2003). Hoxa13 also regulates transcription of Bmp7, which is expressed ventrally in the urethral plate, mesenchyme and prepuce, and dorsally in the genital mesenchyme (Morgan et al., 2003; Wu et al., 2009). Functional studies have implicated Bmp7 in the development of a closed urethral tube and elongation of the urethral plate, as well as septation of the cloaca (Wu et al., 2009).
Analyses of the ontogeny of hypospadias in these mouse mutants raise the intriguing possibility that urethral tube defects may result from failure to maintain the ventral junction of the urethral plate, or its interface with the adjacent surface ectoderm. This implies that cell adhesion is an important feature of urethral tube closure, particularly in light of the observation that the tube begins as a closed, bilaminar epithelial plate that has to open a lumen while maintaining epithelial integrity at the dorsal and ventral margins. It is interesting, therefore, that Eph-Ephrin signaling has been found to be essential for development of a ventrally closed urethra, and appears to function specifically in epithelial cells of the cloaca and urethra (Dravis et al., 2004; Yucel et al., 2007). Loss of either EphrinB2 or the receptors EphB2 and EphB3 leads to severe hypospadias and persistance of the cloaca (Yucel et al., 2007). In addition, disruption of B-catenin or Smo activity in the ventral ectoderm results in loss of epithelial integrity and hypospadias (Lin et al., 2008; Seifert et al., 2009a).
Most of the aforementioned pathways are active in multiple cell types within the genitalia, and while knockouts have been important for identifying genes with roles in genital tubercle development, their complex expression patterns have made it particularly challenging to interpret the underlying cause(s) of the phenotypes. For a gene that is expressed in the urethral plate and prepuce, like FgfR2, global deletion makes it impossible to determine whether the hypospadias results from loss in one or both of these cell types. As the field progresses, spatially and temporally controlled deletions, a well as localized gain of function experiments, will be important for dissecting the mechanisms of dorsoventral patterning, including urethral tube closure.
Local responses to global cues: the role of sex steroids
Androgens and estrogen are typically associated with sexual differentiation of the genitalia and secondary sex characters. Patterning of genital tubercle appears to be under similar genetic control in males and females until approximately E15 in the mouse, when the first signs of sexual differentiation of the external genitalia are seen. It is well known that disruption of androgen signaling can result in feminization of male genitalia, and excess androgen can cause virilization of the female genitalia. Clinically, the former often results in hypospadias or micropenis, whereas the latter can present as cliteromegaly or pseudo-phallus. Complete or partial sex reversal of the genitalia is often part of a condition known as intersex, which can result from mutations in sex steroid pathway genes, exposure to environmental endocrine disruptors, or arise as secondary effects of other congenital anomalies such as congenital adrenal hyperplasia (CAH), in which the adrenal produces excess levels of androgen.
Androgens (testosterone and dihydrotestosterone) and estradiol signal via androgen receptor (AR) and estrogen receptors (ERα and ERβ), respectively. When activated by ligand-binding, ER and AR translocate from the cytoplasm to the nucleus, where they can bind DNA directly to affect transcription of target genes. Molecular phylogenetics show that all members of the steroid receptor (SR) family, which includes AR, ERα and ERβ, mineralocorticoid receptor (MR), glucocorticoid receptor (GR), and progestagen receptor (PR), evolved from a single ancestral SR gene that was present in the common ancestor of protostomes and deuterostomes and was ER-like in its structure and function (Eick and Thornton, 2010). Although the longstanding dogma is that the sexually indifferent genital tubercle is masculinized by androgens and that feminization is a default state that occurs in the absence of androgen activity, recent studies of ER mutants have falsified this hypothesis. Homozygous null mutants for ERα have an elongated clitoris that contains a bone (os clitoris) several times longer than that of wild type females (Yang et al., 2010). Thus, in the absence of ERα activity, female external genitalia are partially masculinized, suggesting that estrogen is required for inhibition of clitoral growth in females. This raises the intriguing possibility that basal levels of androgen in females can lead to masculinization of the genital tubercle, and estrogen is required to counter the influence of androgen.
Mutations in the androgen receptor (as in the testicular feminization or Tfm mutation), by contrast, cause feminization of the external genitalia, such that Tfm mutant male genitalia are indistinguishable from wild type female genitalia (Yang et al., 2010). Mutations in the gene that encodes 5α-reductase 2 (SRD5A2), which converts testosterone to dihydrotestosterone, also disrupt masculinization of the genital tubercle and cause defects ranging from hypospadias and micropenis to complete feminization of the external genitalia (Sinnecker et al., 1996). Together these results suggest that the balance of androgens to estrogen is a critical factor in determining sexual differentiation of the genitalia.
Little is known about the interactions between these systemically circulating hormones and the locally expressed genes involved in patterning of the genital tubercle, yet the phenotypes that result from disruption of sex steroid signaling are consistent with these factors regulating genes that control cell proliferation, apotosis, tubulogenesis and cell adhesion. Although the developmental genetics of external genitalia is only beginning to be understood, evidence that sex steroid receptors can regulate the genetic pathways that govern pattern formation is already emerging. Petiot et al. showed that FgfR2, which is essential for urethral tube closure, contains an androgen response element in its promoter, and antagonism of AR results in down-regulation of FgfR2 in the urethral plate and prepuce (Petiot et al., 2005). Thus, loss of FgfR2 function either by genetic mutation or by disruption of endocrine signaling results in a similar phenotypic outcome, hypospadias. Lorenzo et al. found that culturing female genital tubercles with DHT, which virilizes the genitalia, also up-regulates EphrinB2 (mutations in EphrinB2 lead to hypospadias; Lorenzo et al., 2003). The Wnt pathway is also susceptible to regulation by sex steroids. For example, Dkk2, a Wnt antagonist, can be negatively regulated by testosterone and up-regulated by antiandrogen treatment (Miyagawa et al., 2009b). These examples highlight the potential for hard-wired genetic pathways to be influenced by sex steroids, opening new avenues for the modulation of pattern formation by systemic factors. Therefore, mutations in developmental control genes or their cis regulatory elements are not required to alter their expression patterns during external genital development. Indeed, it seems likely that the same phenotypes (e.g., hypospadias) can be induced either by exposure to endocrine disrupting chemicals or by mutations, but the former may leave no genetic signature. It will be interesting to take these lessons back to the limb and investigate whether the gene networks that pattern the limb bud are susceptible to regulation by systemic and environmental endocrine signals.
Although progress in external genital development owes much to studies of the limb, the emerging picture is that the parallels between these appendages are not as extensive as initially suspected. Given what we now know about the role of endoderm in external genital development, this complex structure may have more in common with the gut than with the limb.
Acknowledgments
I thank Marissa Gredler for preparing the figures, Dr. Brooke Armfield, Marissa Gredler and Dr. Ashley Seifert for comments on the manuscript, and Dr. Liang Ma for discussion of data. Work in the author's lab is supported by HHMI, NIEHS (ES017099), NICHD (HD54554) and NSF (IOB 0843590).
References
- Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, Grosschedl R, Bruneau BG. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Izpisúa-Belmonte JC, Abud H, Heath JK, Tickle C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell. 1995;80:739–746. doi: 10.1016/0092-8674(95)90352-6. [DOI] [PubMed] [Google Scholar]
- Dolle P, Izpisua-Belmonte JC, Brown JM, Tickle C, Duboule D. HOX-4 genes and the morphogenesis of mammalian genitalia. Genes Dev. 1991;5:1767–1767. doi: 10.1101/gad.5.10.1767. [DOI] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Eick GN, Thornton JW. Evolution of steroid receptors from an estrogen-sensitive ancestral receptor. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Galli A, Robay D, Osterwalder M, Bao X, Benazet JD, Tariq M, Paro R, Mackem S, Zeller R. Distinct roles of Hand2 in initiating polarity and posterior Shh expression during the onset of mouse limb bud development. PLoS Genet. 2010;6:e1000901. doi: 10.1371/journal.pgen.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N, Yamada G. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127:2471–2479. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]
- Kondo T, Zákany J, Innis JW, Duboule D. Of fingers, toes and penises. Nature. 1997;390:29. doi: 10.1038/36234. [DOI] [PubMed] [Google Scholar]
- Larson WJ. Human Embryology. 3rd edition Churchill Livingstone; New York: 2001. [Google Scholar]
- Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E. A long-range Shh enhancer regulates expression in theeveloping limb and fin and is associated with preaxial polydactyly. Hum MolGenet. 2003;12:1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- Lieven O, Knobloch J, Ruther U. The regulation of Dkk1 expression during embryonic development. Dev Biol. 2010;340:256–268. doi: 10.1016/j.ydbio.2010.01.037. [DOI] [PubMed] [Google Scholar]
- Lin C, Yin Y, Long F, Ma L. Tissue-specific requirements of beta-catenin in external enitalia development. Development. 2008;135:2815–2825. doi: 10.1242/dev.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development. 2009;136:3959–3967. doi: 10.1242/dev.039768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo AJ, Nguyen MT, Sozubir S, Henkemeyer M, Baker LA. Dihydrotestesterone induction of EPHB2 expression in the female genitaltubercle mimics male pattern of expression during embryogenesis. J Urol. 2003;170:1618–1623. doi: 10.1097/01.ju.0000087423.89813.64. discussion 1623. [DOI] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development andexhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S, Moon A, Haraguchi R, Inoue C, Harada M, Nakahara C, Suzuki K, Matsumaru D, Kaneko T, Matsuo I, Yang L, Taketo MM, Iguchi T, Evans SM, Yamada G. Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program. Development. 2009a;136:3969–3978. doi: 10.1242/dev.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S, Satoh Y, Haraguchi R, Suzuki K, Iguchi T, Taketo MM, Nakagata N, Matsumoto T, Takeyama K, Kato S, Yamada G. Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol Endocrinol. 2009b;23:871–880. doi: 10.1210/me.2008-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EA, Nguyen SB, Scott V, Stadler HS. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development. 2003;130:3095–3109. doi: 10.1242/dev.00530. [DOI] [PubMed] [Google Scholar]
- Murakami R, Mizuno T. Proximal-distal sequence of development of the skeletal tissues in the penis of rat and the inductive effect of epithelium. J Embr ol Ex Mor ol. 1986;92:133–143. [PubMed] [Google Scholar]
- Ng JK, Kawakami Y, Buscher D, Raya A, Itoh T, Koth CM, Rodriguez Esteban C, Rodriguez-Leon J, Garrity DM, Fishman MC, Izpisua Belmonte JC. The limb identity gene Tbx5 promotes limb initiation by interacting with Wnt2b and Fgf10. Development. 2002;129:5161–5170. doi: 10.1242/dev.129.22.5161. [DOI] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehogsignaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132:2441–2450. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- Schwabe GC, Trepczik B, Suring K, Brieske N, Tucker AS, Sharpe PT, Minami Y, Mundlos S. Ror2 knockout mouse as a model for the developmentalpathology of autosomal recessive Robinow syndrome. Dev Dyn. 2004;229:400–410. doi: 10.1002/dvdy.10466. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Bouldin CW, Choi K-S, Harfe BD, Cohn MJ. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development. 2009a;136:3949–3957. doi: 10.1242/dev.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol. 2008;318:143–152. doi: 10.1016/j.ydbio.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Yamaguchi T, Cohn MJ. Functional and phylogenetic an alysis shows that Fgf8 is a marker of genital induction in mammals but is not required for external genital development. Development. 2009b;136:2643–2651. doi: 10.1242/dev.036830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Zheng Z, Ormerod BK, Cohn MJ. Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat Commun. 2010;1:1–9. doi: 10.1038/ncomms1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnecker GH, Hiort O, Dibbelt L, Albers N, Dorr HG, Hauss H, Heinrich U, Hemminghaus M, Hoepffner W, Holder M, Schnabel D, Kruse K. Phenotypic classification of male pseudohermaphroditism due to steroid 5 alpha-reductase 2 deficiency. Am J Med Genet. 1996;63:223–230. doi: 10.1002/(SICI)1096-8628(19960503)63:1<223::AID-AJMG39>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Spitz F, Gonzalez F, Duboule D. A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell. 2003;113:405–417. doi: 10.1016/s0092-8674(03)00310-6. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Haraguchi R, Ogata T, Barbieri O, Alegria O, Vieux-Rochas M, Nakagata N, Ito M, Mills AA, Kurita T, Levi G, Yamada G. Abnormal urethra formation in mouse models of split-hand/split-foot malformation type 1 and type 4. Eur J Hum Genet. 2008;16:36–44. doi: 10.1038/sj.ejhg.5201925. [DOI] [PubMed] [Google Scholar]
- Towers M, Mahood R, Yin Y, Tickle C. Integration of growth and specification in chick wing digit-patterning. Nature. 2008;452:882–886. doi: 10.1038/nature06718. [DOI] [PubMed] [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dollé P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- Wu X, Ferrara C, Shapiro E, Grishina I. Bmp7 expression and null phenotype in the urogenital system suggest a role in re-organization of the urethral epithelium. Gene Expr Patterns. 2009;9:224–230. doi: 10.1016/j.gep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yang JH, Menshenina J, Cunha GR, Place N, Baskin LS. Morphology of mo external genitalia: implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. J Urol. 2010;184:1604–1609. doi: 10.1016/j.juro.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel S, Dravis C, Garcia N, Henkemeyer M, Baker LA. Hypospadias and anorectal malformations mediated by Eph/ephrin signaling. J Pediatr Urol. 2007;3:354–363. doi: 10.1016/j.jpurol.2007.01.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Wang YZ, Yamagami T, Zhao T, Song L, Wang K. Generation of Lrp6 conditional gene-targeting mouse line for modeling and dissecting multi lebirth defects/congenital anomalies. Dev Dyn. 2010;239:318–326. doi: 10.1002/dvdy.22054. [DOI] [PubMed] [Google Scholar]
- Zhu J, Nakamura E, Nguyen MT, Bao X, Akiyama H, Mackem S. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14:624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]