Abstract
Animal models of decision-making are some of the most highly regarded psychological process models; however, there remains a disconnection between how these models are used for pre-clinical applications and the resulting treatment outcomes. This may be due to untested assumptions that different species recruit the same neural or psychological mechanisms. We propose a novel human foraging paradigm (Web-Surf Task) that we translated from a rat foraging paradigm (Restaurant Row) to evaluate cross-species decision-making similarities. We examined behavioral parallels in human and nonhuman animals using the respective tasks. We also compared two variants of the human task, one using videos and the other using photos as rewards, by correlating revealed and stated preferences. We demonstrate similarities in choice behaviors and decision reaction times in human and rat subjects. Findings also indicate that videos yielded more reliable and valid results. The joint use of the Web-Surf Task and Restaurant Row is therefore a promising approach for functional translational research, aiming to bridge pre-clinical and clinical lines of research using analogous tasks.
Keywords: Functional Translation, Decision-Making, Human, Rat, Impulsivity
Background
Animal models of impulsivity are regarded as being among the most well developed representations of human psychopathology (Kalivas, Peters, & Knackstedt, 2006; Madden & Bickel, 2010), and have been key contributors to our understanding of human psychopathologies, such as addiction (Madden & Bickel, 2010; O'Brien & Gardner, 2005). Nonetheless, there remains a gap between model validity and the efficacy of human treatments based on these animal models (Hall, De Serrano, Rodd, & Tropepe, 2014; Kalivas et al., 2006). Prior research suggests this gap may stem from untested assumptions that humans and nonhuman animals recruit the same cognitive systems (Demeter, Sarter, & Lustig, 2008). Coordinating human and non-human animal research to model the same behaviors is therefore critical to elucidating the behavioral and neurobiological mechanisms that underlie many psychopathologies (Belzung & Lemoine, 2011; Potenza, 2009). However, this functional approach to translation requires parallel tasks that access similar functional constructs. Here, we present a novel experiential human foraging task translated from a rat food foraging paradigm (Steiner & Redish, 2014). Instead of food, humans foraged for information through an internet-like interface, as a naturalistic analogue to the food rewards used with nonhuman animals (Pirolli, 2005). Our results suggest these tasks captured behavioral parallels in human and rat decision-making.
The Foraging Model of Decision-Making
New theories posit that many psychopathologies are fundamentally problems with decision-making. This notion implies that understanding the causes (and improving treatments) depends on understanding how those decision-making systems work and break down (Montague, Dolan, Friston, & Dayan, 2012; Rangel, Camerer, & Montague, 2008; Redish, Jensen, & Johnson, 2008; Redish, 2013). Foraging models of decision-making provide a computational account of how humans and nonhuman animals allocate scarce resources (e.g. time) when searching for valuable resources like food, money, or drugs (Stephens, 2008). Sociological observations of drug-users suggest that users are seen as “foraging” for drugs in a “patchy” world of opportunities; for example, smokers looking for the cheapest cigarettes (Feighery, Schleicher, Boley Cruz, & Unger, 2008; Grossman & Chaloupka, 1998), gamblers looking for video poker machines (Schüll, 2012), or heroin addicts looking for narcotics (Hoffer, Bobashev, & Morris, 2009). Thus, foraging paradigms may be a promising approach for examining the complex decision-making systems that underlie addiction or other psychopathological disorders.
Foraging models advance historical intertemporal choice models of decision-making, during which subjects make binary choices between rewards of different value that are available at disparate time delays (often referred to as ‘delay-discounting’ paradigms). Delay-discounting tasks have been widely used to assess impulsive decision-making among addicted human and nonhuman animals. However, multi-option foraging paradigms may be more akin to real-world scenarios where humans are cognizant of other options or alternatives in the background when making a decision. Moreover, researchers have posited that stay/skip serial foraging choices may better characterize naturalistic decision-making (Stephens, 2008; Wikenheiser, Stephens, & Redish, 2013). As a result, researchers are using foraging models at an increasing rate in both human and nonhuman animal studies (Hayden, Pearson, & Platt, 2011; Kolling, Behrens, Mars, & Rushworth, 2012; Shenhav, Straccia, Cohen, & Botvinick, 2014; Steiner & Redish, 2014; Wikenheiser et al., 2013). A logical next step is to develop a foraging model that translates across species; researchers could then use this foraging model to examine cross-species parallels in the maladaptive decision-making behaviors that support psychopathologies like addiction. This type of translation requires a bridging of research branches, which have typically produced methodologically divergent decision-making paradigms.
Current Challenges in Functional Translation
Decision-making tasks for nonhuman animals are experiential, in that they typically entail a rat physically running through a maze or pressing a lever, waiting through real-time delays, and receiving primary reinforcers as reward, like food (Mazur, 1987; Papale, Stott, Powell, Regier, & Redish, 2012). In contrast, efforts to produce comparable human experiential paradigms generally result in one of three approaches for reward stimuli: i) secondary reinforcers like tallied points that may eventually convert to money (Kolling et al., 2012; Reynolds & Schiffbauer, 2004; Shenhav et al., 2014), ii) secondary reinforcers like coins that are dispensed during the task (Krishnan-sarin et al., 2007; Reynolds, 2006b; Voon et al., 2010), or iii) primary rewards like juice or candy (Kool & Botvinick, 2014; McClure, Ericson, Laibson, Loewenstein, & Cohen, 2007). These methodological differences may impact the underlying reward systems evoked in humans. For example, in scenarios where i) points/money are summated over the session or ii) the subject randomly receives one or several of their choices at the end (the latter called real-reward measures; Reynolds, 2006a), each poor decision may be salient. This is because either: i) each choice influences the ultimate gain or ii) the subject does not know which final outcome the subject will receive (thus, making each decision important). On the contrary, in real-time measures, the subject consumes rewards at the end of each trial (Reynolds, 2006a). As a result, poor choices may be less salient as individual choices do not influence a post-session outcome. Given these distinctions, it is also possible that the same reward systems observed in rats are not evoked during human tasks that lack comparable real-time consummatory rewards.
Researchers have identified and addressed similar methodological gaps with respect to visuospatial paradigms. For example, human studies historically assessed spatial ability via paper and pencil tests, whereas non-human studies assessed spatial navigation via maze-learning tasks (Moffat, Hampson, & Hatzipantelis, 1998). In response, many human studies adopted virtual reality radial mazes and Morris water tasks and successfully identified cross-species behavioral and neural parallels (Bohbot, Lerch, Thorndycraft, Iaria, & Zijdenbos, 2007; Hamilton, Driscoll, & Sutherland, 2002; Hamilton, Kodituwakku, Sutherland, & Savage, 2003; Iaria, Petrides, Dagher, Pike, & Bohbot, 2003). Despite the success of these virtual reality paradigms, these particular tasks did not address the issue of primary versus secondary reinforcement that may be pertinent to decision making (as subjects solely received monetary compensation at the study conclusion). Thus, experiential human foraging models with primary reinforcement are needed to fill this gap in external validity and provide a link to the animal decision-making literature.
Primary Reinforcement for Humans
Considering how humans interface with the world on a daily basis while seeking rewards or entertainment may improve insight into the processes underlying decision-making, which in turn, may guide task development. Information Foraging Theory suggests that humans seek and acquire information using the Internet (Pirolli & Card, 1999). More specifically, individuals perform ongoing cost-benefit analyses as they navigate through websites, making stay/skip foraging choices to remain on the current site or move on to the next (Pirolli, 2005). Humans also forage the Internet for rewarding stimuli, frequently presented in the form of video segments or images – each of which we can feasibly incorporate into an experimental paradigm. Such internet-found stimuli may even yield natural reinforcement that is comparable to drugs or food. Recent findings that Internet-addicted individuals exhibited functional and structural brain similarities to drug-addicted individuals bolster this claim (Ding et al., 2013; Kuss & Griffiths, 2012; Weinstein & Lejoyeux, 2013). The combined feasibility and primary reinforcement possible from using videos or photos as reward makes their use a compelling option for human task development.
Study Aims
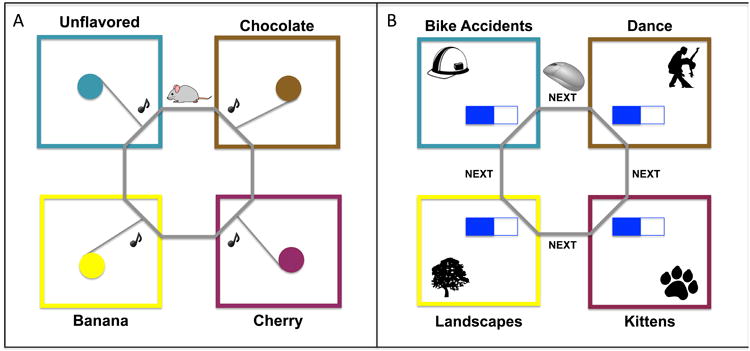
The current study advances available experiential tasks for humans by developing a foraging paradigm that i) translates across species, ii) includes primary reinforcement and realtime delays, and iii) integrates natural human ethology into the design. We translated the proposed task directly from a novel stay/skip foraging paradigm, called “Restaurant Row” (Steiner & Redish, 2014), during which a rat had a fixed amount of time to traverse a circular track and collect food rewards from four feeders. Each feeder (or “restaurant”) provided a different flavor of food pellet after a random time delay (see Figure 1A). We drew from Information Foraging Theory and the burgeoning Internet-addiction literature to develop the human variant of Restaurant Row, which we call the “Web-Surf Task” (see Figure 1B). During this task, humans made a series of stay/skip decisions while traveling between galleries that contain primary rewards (videos or photos), which were presented after real-time delays. In this paper, we illustrate the external and face validity of the Web-Surf Task, as well as cross-species behavioral parallels using the Web-Surf Task and Restaurant Row.
Figure 1.
Methods and Materials
Web-Surf Task in Humans
Sample Demographics
The total sample included 64 University of Minnesota undergraduates (72% female, mean age = 20.5), who received extra credit toward a psychology course. The initial round of data collection included both the video (N = 22) and photo versions (N = 15); the second round of data collection included only the video version (N = 27). This resulted in a total of 49 subjects who completed the video version. The University of Minnesota's Institutional Review Board approved the study, and all subjects provided written informed consent.
Web-Surf Task Design
Subjects had 30 minutes to “surf” (or forage) through four galleries (see Figure 2 for decision flow-diagram) presented using PsychoPy (Peirce, 2009). In the video variant, each gallery presented a video clip from one of four categories (kittens, dance, bike-accident, landscapes) as a reward. In the photo variant, each gallery presented an image from one of four categories (kittens, desserts, female faces, or landscapes); we note that we transformed the still images using the Ken Burns panning and zooming effect to parallel the video variant. We selected these particular categories in consideration of future functional neuroimaging data collection and neural decoding analyses. More specifically, we expect these categories will map onto separable neural substrates, as prior evidence indicates unique correlates for faces, bodies, animals, natural scenes, tools/objects, and animals (Bradley, Katherine, Dylan, Daphna, & Nathaniel, 2015; Haxby et al., 2001, 1999; Peelen & Downing, 2007; Walther, Caddigan, Fei-Fei, & Beck, 2009). For additional details on the anticipated decoding analyses see Steiner and Redish (2014).
Figure 2.
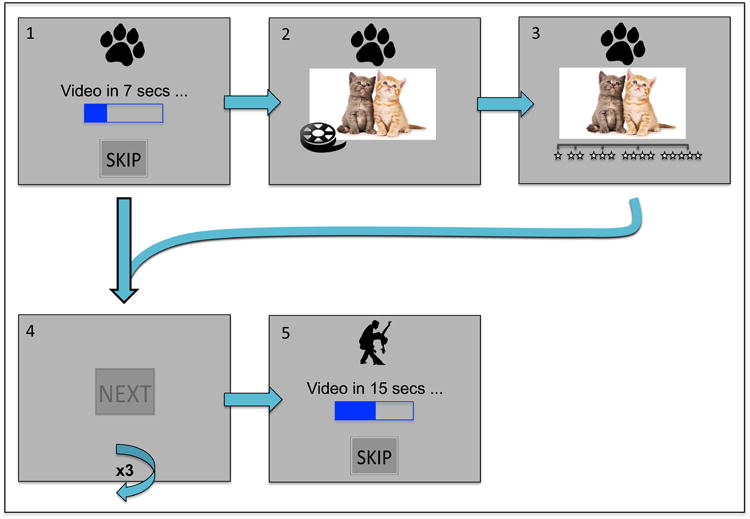
We laid out the task as follows: Upon arrival at a gallery, the subject was informed of the random delay time before video presentation. Delay time was displayed using text and a progress bar similar to those located on an Internet webpage. The subject was given the option to stay and wait for the current reward or skip and continue on to the next gallery. If the subject decided to stay, the subject viewed the stimulus for four seconds and then rated it using a 5-star rating system (1 star = extremely dislike, 5 stars = extremely like). If the subject decided to skip, the subject pushed the “SKIP” button located at the bottom of the screen. After leaving the gallery, the subject “surfed” to the next gallery that presented a new offer (i.e. new video or photo after a new random delay). The subject then completed a series of “NEXT” screens when traveling between galleries, regardless of the decision to stay or skip; this entailed finding and clicking 3 or 5 “NEXT” buttons that were randomly positioned on the screen. We intended the “NEXT” to serve as an analogue to the rats physically running a track to travel between feeders. We designed the buttons to blend into the background to increase the cost for locating them around the screen. 22 subjects completed the task with 5 “NEXT” screens between galleries; 27 subjects completed the task with 3 “NEXT” screens between galleries.
As preliminary training, subjects completed two forced practice trials, during which we instructed them to push the “SKIP” button for trial one and to stay and wait for trial two. We created this structure to illustrate the two choice options as well as the transition between the galleries. Subjects then completed eight practice trials where they could decide whether to stay or skip.
Restaurant Row in Rats
Sample Characteristics
We used eight adult Brown-Norway rats in this experiment. Our methods were consistent with Steiner and Redish (2014), as we aimed to replicate behavioral findings in a new sample. Our study protocol complied with the National Institute of Health guidelines for animal care, and the Institutional Animal Care and Use Committee at the University of Minnesota approved the protocol.
Restaurant Row Design
Restaurant Row consisted of a circular track with four spokes leading off to food-reward sites (restaurants) as illustrated in Figure 1A. Each food-reward site provided a different flavor of food (cherry, chocolate, banana, and unflavored/plain sugar). The rat proceeded around the circle encountering each offer serially. When the rat entered the offer zone, a tone sounded, with pitch indicating the delay (1-30s). The tone counted down once per second (change = 250 Hz) until it reached the base tone (1 kHz), at which time the two pellets of the flavor for that restaurant were delivered. If the rat left the offer zone before the delay finished counting down, the tone stopped, the offer was rescinded, and the rat had to proceed to the next restaurant to get food. Because zones were only triggered in a clockwise serial manner, rodents quickly learned to run in one direction. Essentially, the animal made a series of stay/skip decisions, such as − Is it worth waiting 25 seconds for two banana-flavored food pellets? In this task, we gave rodents 60 minutes to collect food for the day. The 60-minute time limit means that the encounters were not independent of each other − time spent waiting at one restaurant was time that could not be spent waiting at another. This means that an animal using an economically intelligent strategy should have waited longer for more preferred flavors. Rats' preferences were “revealed” by an increased willingness to wait out a longer delay for a favored flavor of pellet. Each rat completed 9 or 10 sessions in total.
We trained rats in four phases. In the first phase (5-7 days, twice daily), rats completed 30-minute sessions of habituation. The delay at every feeder was 1s and the reward was 2 pellets. During this phase the rat became accustomed to the task, whereby it learned the correct direction of travel and the flavor available at each feeder site. The rat moved on to the next training phase after it reliably ran clockwise around the loop. In the second phase (4 days, twice daily), the 30-minute sessions had increasingly longer delays. The delays began with a range of 1-2 seconds, then 1-3 seconds, and continued to increase by 1 second each day until the rat achieved a maximum of 5 seconds (this trained the rat to wait). In the third phase (10 days, twice daily), the rat completed 30-minute sessions with the full delay set (1-30 seconds). In the fourth phase (5-10 days, once daily), the rat completed 60-minute sessions with the full delay set (1-30 seconds). By the end of this final training phase, the rats typically showed delay thresholds (by visual inspection), skipped high tones, and left the feeder site after reward receipt. From this evidence, we concluded that the rats understood the task and commenced the experimental testing portion.
Task-Derived Decision Metrics
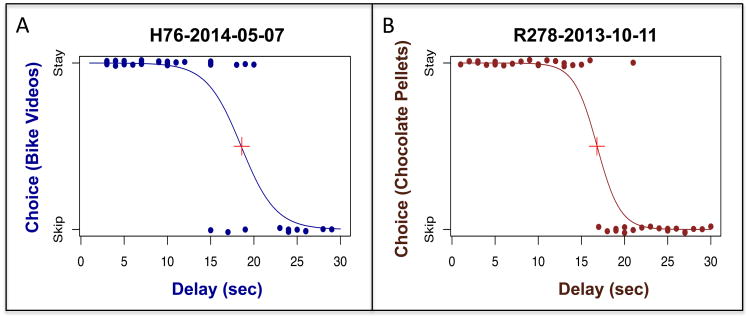
To examine behavioral parallels across species we used three decision metrics: i) revealed preferences, ii) stated preferences, and iii) decision consistency. We calculated revealed preferences for each category via a logistic fit function for human and nonhuman animals (see Figure 3). These values reflect the delay time (or delay threshold) at which a subject reliably began to skip offers for the respective category. In other words, the inflection point equates to the delay threshold at which a subject had a 50% probability of staying (or skipping). We computed inflection points according to the following equation (one per category, per subject):
Figure 3.
| (1) |
where p is the desired probability (50% in this case), β0 is the intercept, β1 is the slope, and x is the delay threshold. Notice that the function on the left of equation (1) is the logit of p, and the function on the right of equation (1) represents a linear regression model with a single predictor. We then rearranged the equation to solve for x:
| (2) |
We considered favored galleries to be those where a subject consistently waited longer for the reward (equating to a higher delay threshold). We acquired stated preferences for human subjects only, which included average ratings for each category, as well as post-test category rankings, 1 to 4.
We also measured decision consistency in both species, which indicated the extent to which subjects cohered to category-specific strategies (i.e. stayed for trials below threshold, and skipped trials above threshold). Given the economically normative assumption that subjects had a subjective valuation of a particular category and a fixed time constraint, subjects should have stayed when the subjective valuation of a category (reward) was larger than the offered delay (cost). Subjects that deviated from this economically normative model made “economic errors” in that they sacrificed time that could be spent in a preferred location (e.g. time spent waiting for a bike-accident video was time that could not be spent waiting for a [potentially preferred] kitten video). To derive the decision consistency metric we computed the proportion of error trials for each subject (number of error trials divided by the total number of trials).
Analyses
Our first set of analyses assessed behavioral cross-species parallels using the respective tasks. First, we identified evidence of revealed preferences in both species. Next, we evaluated within-subject consistency. For human subjects, we examined the correspondence between a human subjects' revealed and stated preferences; this analysis also provided a measure of the Web-Surf task's external validity. For rats, we evaluated the consistency of the rats' revealed preferences (i.e. delay thresholds) across sessions because we could not ask a rat to explicitly state its preference. Third, we investigated whether humans and nonhuman animals exhibited similarities in decision consistency. Fourth, we analyzed decision times to determine whether subjects made quick decisions or waited for cues; we used this measurement to evaluate the face validity of each task. Finally, we examined within-session dynamics to determine whether humans and rats behaved similarly as the session proceeded.
In the second set of analyses, we compared the two variants of the Web-Surf task (video versus photo stimuli). We conducted these analyses to determine which type of stimuli provided the most reliable and valid results, while also considering category homogeneity across stimuli types (rats always received the same reward at a given feeder). We also assessed within-session dynamics and gender differences across the two task variants.
Results
Humans versus Rats: Revealed Preferences
First, we determined whether human and non-human animal subjects showed evidence of revealed preferences. Figure 3 illustrates a side-by-side comparison for a single human and single rat session, where each curve depicts choices for a particular category. In both species, we visually (and statistically) identified a clear inflection point at which the subject had a 50% chance of staying or skipping on to the next offer. The distributions in Figure 3 are typical of those we observed from other human and non-human animal subjects (see Steiner and Redish, 2014, for additional rodent examples).
Humans versus Rats: Evaluating Within-Subject Consistency
Next, we evaluated the extent to which both species displayed consistent within-subject preferences. For the human sample (N = 49), we computed two correlations per subject: i) the correlation between delay thresholds and average category ratings (4 values for each) and ii) the correlation between delay thresholds and post-test category rankings (4 values for each). We found that delay thresholds corresponded with average category ratings, with 69% of correlations above 0.50. Similarly, 73% of correlations between delay thresholds and post-test category rankings were above 0.50. We did not detect significant differences between subjects who completed the 3 versus 5-NEXT versions for rating (t47 = 0.51, p = 0.61) or ranking (t38 = 0.08, p = 0.94) validity correlations.
To determine within-subject consistency for rats, we evaluated delay thresholds across sessions using a repeated-measures analysis of variance (ANOVA). We constructed a model that included delay thresholds as the dependent variable, and zone (i.e. feeder site) and session number as the predictor variables. As shown in Table 1, we observed a significant main effect for zone (F3,231 = 4.58, p = 0.004) but not session number (F70,231 = 0.59, p = 0.99), thus indicating that rats had detectable and stable flavor preferences. These results are consistent with across-session rat performance in Steiner and Redish (2014).
Table 1. Across-Session Threshold Consistency (Rat Subjects, N = 8).
| Source | df | Sum Sq | Mean Sq | F-Value | p-value |
|---|---|---|---|---|---|
|
| |||||
| Zone | 3 | 523 | 174.27 | 4.58 | 0.004 |
| Session Number | 70 | 1581 | 22.59 | 0.59 | 0.99 |
| Residuals | 231 | 8788 | 38.04 | ||
Humans versus Rats: Decision Consistency
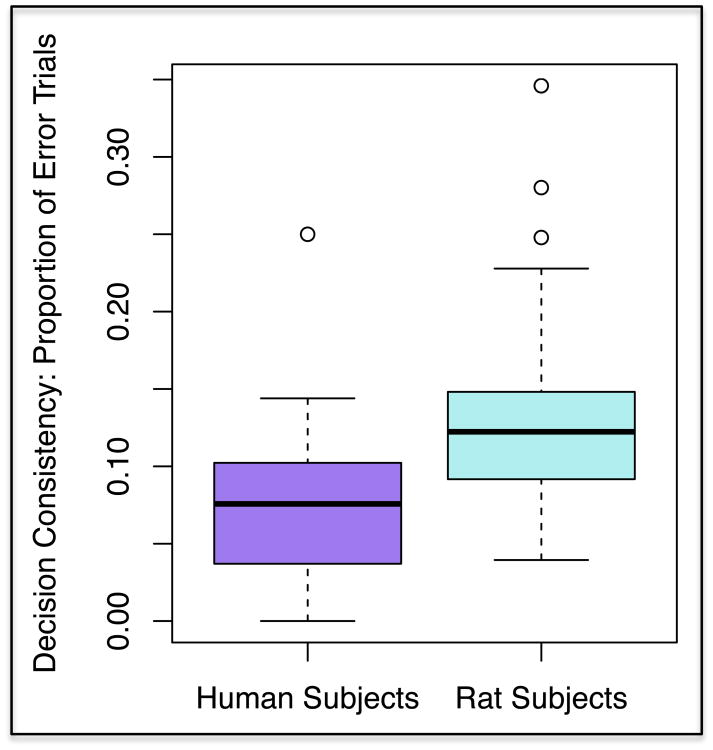
Third, we examined parallels in decision consistency for humans and nonhuman animals (see Figure 4). In particular, we found that rat subjects exhibited greater decision instability (mean = 0.12, median = 0.12), compared to human subjects (mean = 0.07, median = 0.08). Several human subjects also had no error trials, which was not the case for rats. We were also interested in the spread of this decision consistency metric within each species, as we hope to capture a comparable range of threshold error variance using the respective tasks. To this end, we used an F-test to investigate differences in decision consistency variance between species and found no significant differences (F49, 77 = 1.15, p = 0.60). Thus, although humans had less mean decision instability, the spread of this metric was consistent in humans and rats.
Figure 4.
Humans versus Rats: Decision Times
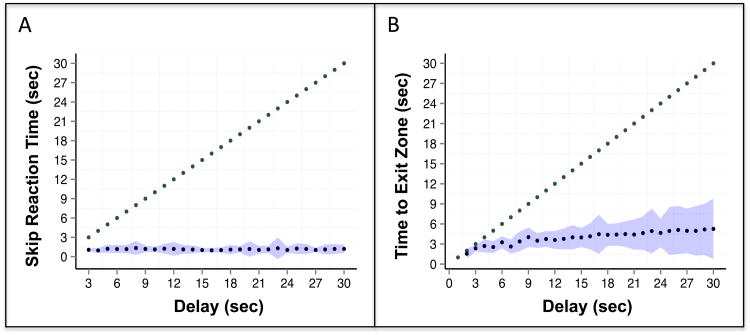
Fourth, we evaluated the association between choice reaction time and delay to determine whether delay times influenced the speed at which subjects made a decision. This analysis included a subset of human video subjects (N = 27) for whom the task recorded skip reactions times. The plots in Figure 5 illustrate the relation between choice reaction time and delay for all human and rat subjects separately. Stay trials are represented as the full delay time (points along diagonal) and mean times for skip trials are represented as the points parallel to the x-axis. The blue shaded bands indicate skip time standard deviations around each possible delay time. Humans and rats made decisions shortly after arrival at a gallery, with decisions made within 3 seconds or less for the majority human trials and within 5 seconds or less for the majority of rat trials. In other words, when presented with a less valuable offer, subjects did not hesitate to skip and travel towards other potential offers. Instead, both species efficiently decided whether an offer was preferable or not. This supports the face validity of each task, where neither species waited for a specific cue to decide but made a quick choice and remained engaged in the task. For example, the rats did not appear to wait for a specific tone before deciding to leave.
Figure 5.
Humans versus Rats: Within-Session Dynamics
As a final cross-species comparison, we assessed whether human and rat behaviors showed comparable fluctuations throughout the session. To this end, we used repeated measures ANOVAs with choice as the dependent variable, and category/zone and trial number as the predictor variables. We were particularly interested in the trial number term as an indicator of reward satiation, where a significant effect would suggest a change in stay/skip tendencies over the session. Table 2A reveals a significant main effect for category (F3,5587 = 110.78, p < 0.001) but not trial number (F1,5587 = 2.03, p = 0.16) for human subjects. Table 2B reveals comparable findings for rat subjects, including a significant main effect for zone (F3,13856 = 18.69, p < 0.001) but not trial number (F1,13856 = 1.28, p = 0.25). This suggests that, regardless of species, subjects exhibited differential choice patterns across reward sites but not trial number. In other words, neither species appeared to satiate during the session.
Table 2A. Choice as a Function of Category and Trial N (Video Subjects, N = 49).
| Source | df | Sum Sq | Mean Sq | F-Value | p-value |
|---|---|---|---|---|---|
|
| |||||
| Category | 3 | 65.70 | 21.91 | 110.78 | <0.001 |
| Trial Number | 1 | 0.40 | 0.40 | 2.03 | 0.16 |
| Residuals | 5587 | 1104.90 | 0.20 | ||
|
| |||||
| Table 2B. Choice as a Function of Zone and Trial N (Rat Subjects, N = 8) | |||||
| Source | df | Sum Sq | Mean Sq | F-Value | p-value |
|---|---|---|---|---|---|
|
| |||||
| Zone | 3 | 12.20 | 4.07 | 18.69 | <0.001 |
| Trial Number | 1 | 0.30 | 0.28 | 1.28 | 0.25 |
| Residuals | 13856 | 3015.80 | 0.22 | ||
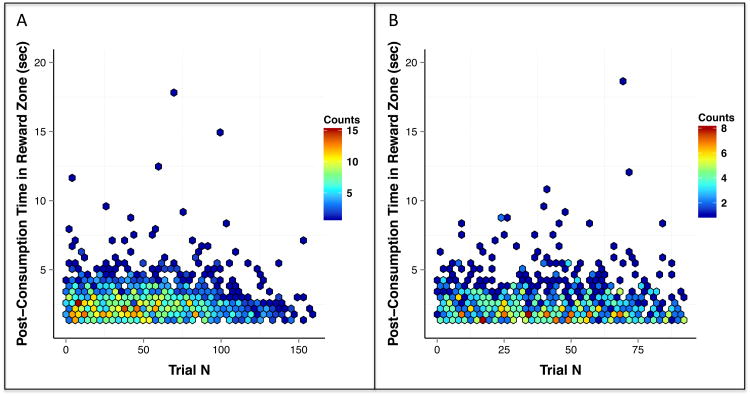
We also assessed the amount of time spent in the reward zone following reward consumption as a function of trial number. In human subjects this equated to the time after viewing a stimulus but before providing a rating (as the subject could not advance to the next gallery before rating the video or photo). In rat subjects this equated to the time after eating but before running to the next zone. A t-test indicated that rats spent significantly more time lingering in the reward zone than humans, t12207 = 46.23, p < 0.001 (see Figures 6A and 6B).
Figure 6.
Videos versus Photos: Selecting Optimal Stimuli
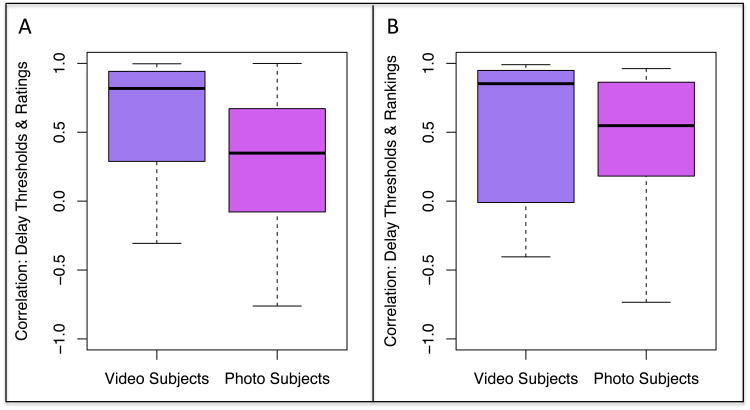
To compare the task variants, we first computed correlations between revealed and stated preferences for the photo (N = 15) and a subset of the video subject (N = 22) whose data we collected during the same period. For average category ratings, 68% of video subjects had correlations of 0.50 or above versus 40% of photo subjects. As depicted in Figure 7A, video subject correlations for average category ratings ranged from -0.31 to 1.00, with a mean of 0.62 and a median of 0.82. The photo subject correlations spanned a comparable range from -0.76 to 1.00. However, the mean and median correlations for the photo subjects were lower, with values of 0.27 and 0.35, respectively. A t-test revealed a significant difference between the groups (t35 = -2.10, p = 0.04).
Figure 7.
A similar pattern emerged for the post-test category rankings, where 72% of video subjects had correlations 0.50 or above versus 64% of photo subjects (See Figure 7B). Video subject correlations for category rankings ranged -0.40 to 0.99, with a mean of 0.56 and a median of 0.85. The photo subject correlations covered an even larger range, with bounds of -0.73 and 0.96. As a result, the mean correlation for these subjects was 0.41 and the median 0.55. Although video subjects generally had higher validity correlations, this difference was not significant (t27 = -0.73, p = 0.47); 8 subjects did not have ranking data, hence the reduced degrees of freedom.
We also calculated decision consistency metrics for photo subjects and the same subset of 22 video subjects. As shown in Figure 8, video and photo subjects did not exhibit mean differences in their proportion of error trials (t35 = -0.65, p = 0.52). Similar to the human and rat comparison, we also assessed for differences in decision consistency variance. Here we did find a significant difference (F21, 14 = 0.19, p < 0.001), whereby video subjects exhibited a more homogenous pattern of decision instability.
Figure 8.
Videos versus Photos: Within-Session Dynamics
Next, we assessed within-session choice behaviors for the two task variants using repeated measures ANOVAs. This entailed two models with choice as the dependent variable, and category and trial number as the predictor variables (i.e. separate models for the initial 22 video subjects and the 15 photo subjects). Table 3A reveals a significant main effect for category (F3,2382 = 64.35, p < 0.001) but not trial number (F1,2382 = 0.12, p = 0.73) for video subjects. Table 3B reveals similar findings for photo subjects, including a significant main effect for zone (F3,1241 = 8.85, p < 0.001) but not trial number (F1,1241 = 1.72, p = 0.19). Thus, subjects showed significant choice differences as a function of category but not trial duration.
Table 3A. Choice as a Function of Category and Trial N (Video Subjects, N = 22).
| Source | df | Sum Sq | Mean Sq | F-Value | p-value |
|---|---|---|---|---|---|
|
| |||||
| Category | 3 | 37.90 | 12.63 | 64.35 | <0.001 |
| Trial Number | 1 | 0.00 | 0.02 | 0.12 | 0.73 |
| Residuals | 2382 | 467.40 | 0.20 | ||
|
| |||||
| Table 3B. Choice as a Function of Category and Trial N (Photo Subjects, N = 15) | |||||
| Source | df | Sum Sq | Mean Sq | F-Value | p-value |
|---|---|---|---|---|---|
|
| |||||
| Category | 3 | 4.84 | 1.61 | 8.85 | <0.001 |
| Trial Number | 1 | 0.31 | 0.31 | 1.72 | 0.19 |
| Residuals | 1241 | 226.24 | 0.18 | ||
We also investigated whether video and photo subjects differed in the amount of time spent in the reward zone following consumption (after viewing a stimulus but before rating it). As shown in Figures 9A and 9B, we observed similar patterns using the two task variants (t2272 = 0.85, p = 0.39), as human subjects generally rated the videos and exited the reward zone in 5 seconds or less (with comparable variation extending into the 5 – 18 second range).
Figure 9.
Videos versus Photos: Gender Differences
Lastly, we built linear mixed models to assess gender differences in category preference; this approach uses restricted maximum likelihood to obtain parameters estimates and can thus accommodate unbalanced designs (i.e. missing data). We constructed two models per task variant (four models total) that included either delay thresholds or average category ratings as the dependent variable, and gender, category, and a gender × category interaction term as the predictor variables. We were particularly interested in the interaction term as an indicator of preference differences across gender. We observed non-significant interactions in all models (see Supplemental Tables 1A-2B). However, a trend-level gender × category interaction for average category ratings in the video subjects (N = 22) and a subsequent power analysis encouraged us to re-assess for significant interactions using the complete video sample (N = 49). Here, we found significant gender × category interactions for delay thresholds (F3,141 = 2.77, p = 0.04) and average category ratings (F3,138 = 6.12, p < 0.001; see Supplemental Tables 3A and 3B). Follow-up tests revealed that gender differences were most prominent for the bike-accident and landscape categories. We refer readers to the Supplementary Materials for further details.
Discussion
The current study proposes a novel experiential foraging paradigm for humans called the Web-Surf Task. We designed this paradigm to assess similarities in decision-making systems in humans and rats. The Web-Surf Task involves individuals making a series of stay/skip foraging decisions as they cycle through four galleries. This task builds on available decision-making paradigms in several ways: i) its experiential design includes primary reinforcement and realtime delays, ii) it entails serial stay/skip offers and is therefore more akin to real-world choices, and iii) it was designed as a direct analogue to a rat foraging task. This last point is particularly salient in the context of psychopathology research, where translational models are critical for developing successful treatments. Our preliminary findings demonstrate both the external and face validity of the Web-Surf task, as well as cross-species behavioral parallels using the analogous tasks. Therefore, the complementary use of the Web-Surf or Restaurant Row tasks could be a step forward for bridging pre-clinical and clinical lines of research.
We first examined cross-species parallels using data from both the Web-Surf Task and Restaurant Row. Our results showed that each task captured individual differences in preference as evidenced by delay thresholds, as well as within-subject consistency in humans and non-human animals. We also found evidence that both species actively made decisions as they traversed through their respective tasks, where each offer (combination of delay length with specific gallery or flavor) represented a certain value that fit within a given subjects' strategic framework. Moreover, we detected cross-species parallels in reward satiation rates, as tendencies to stay versus skip remained relatively stable throughout the session. We did observe cross-species divergences with respect to decision consistency, where rat subjects exhibited more deviations, on average, from the ideal strategy. However, the spread of decision instability was similar using the analogous tasks. The tasks also diverged according to post-consumption reward time. In particular, we found that rat subjects spent more time lingering in the reward zone partaking in leisure activities such as grooming.
We then compared two variants of the Web-Surf Task: one that included video stimuli as reward and a second that included photo stimuli. Our primary intention was to empirically assess which type of internet-available reward stimuli yielded more reliable and valid results. We found that subjects who completed the video version showed greater correspondence between revealed and stated preferences. We also observed a tighter range of decision stability in the video subjects. Hence, although photo categories may appear more homogenous, the data suggest that video rewards yielded more reliable results. These discrepancies may reflect the notion that videos are inherently more rewarding to humans. Comparable findings have been reported in macaques, where animated movies had considerably more reward value than static pictures (Blatter & Schultz, 2006). We also compared within-session dynamics across the two task variants, which indicated similar stay proportions and post-reward consumption times. As a last step, we explored gender differences in category preference. These results suggested that males tended to wait longer for landscape and bike-accident videos, and also rated these categories more highly (although the bike-accident ratings did not attain significance). We were unable to detect gender differences using the photo version, further suggesting an increased sensitivity in the video version.
Previous studies have demonstrated the utility of multi-option foraging models for investigating natural foraging behaviors in human and nonhuman animals; however, ours is the first to compare these processes across species. The Web-Surf Task provides a novel combination of primary reinforcement, real-time delays, and serial stay/skip foraging choices that parallels Restaurant Row.
Future Directions
Despite the promising overlap of the described tasks, functional translation is a dynamic and evolving process that benefits from ongoing modifications at both the pre-clinical and clinical ends. We therefore suggest several avenues to further reduce cross-species divergences. First, future studies could assess whether decision-making parameters derived from the Web-Surf Task are stable via repeated sessions. This approach would not only foster design parallels with nonhuman animal studies (which typically entail multiple sessions) but also elucidate whether the Web-Surf Task captures state and/or trait-like effects. Some researchers argue that experiential decision-making tasks better capture acute state changes (e.g. drug effects), whereas questionnaire based tasks may tap into stable, trait-like impulsivity (Reynolds, 2006a). Nonetheless, empirical research is needed to assess if the Web-Surf Task, which we consider an experiential measure, can measure state-level fluctuations in a similar fashion to Restaurant Row. Second, researchers could investigate the extent to which various experimental design manipulations (e.g. increasing delay lengths, adjusting distance/effort to travel between feeders or galleries) similarly influence human and rodent behavior. Third, future endeavors could modify the stimuli sets to address specific psychopathology questions (e.g. food pictures or videos for obesity hypotheses, drug paraphernalia for addiction hypotheses, etc.). In effect, such stimuli would serve as a combination of primary and conditioned reinforcement. Researchers might then investigate whether satiation rates for these stimuli differ from other primary rewards. Fourth, researchers may examine relations between error trials and psychopathology, particularly the influence errors might have on trial-by-trial behavior. For example, a subject might encounter an unfavorable scenario where one skips an offer below threshold (where they should have stayed) only to encounter a less favorable offer on the next trial (termed “regret” when seen in rats by Steiner and Redish, 2014). The manner in which a subject uses this experience to guide subsequent decisions may reflect pathological processes. For instance, addicted individuals may continue to deviate from strategy despite negative feelings or repercussions.
Another pertinent avenue for future endeavors is to explore the underlying neural systems evoked during the analogous tasks. Although we restricted the current study to behavioral methods, prior investigations have identified the rodent neural systems recruited during Restaurant Row (Breton, Schmidt, & Redish, 2014; Schmidt, Breton, & Redish, 2014; Steiner & Redish, 2014). Steiner and Redish (2014) found that representations in both orbitofrontal cortex (OFC) and ventral striatum (vStr) reliably tracked choices and preferences (e.g. neuronal signals in these areas differentiated between feeders during reward receipt). Breton et al. (2014) found that compromising OFC with DREADD-driven pyramidal-cell inhibition led to a disruption in flavor preferences, while Schmidt et al. (2014) found that compromising medial prefrontal areas (prelimbic, PL and infralimbic, IL) led to a disruption in hesitation during difficult decisions. Although the homologies between rat and human prefrontal areas remain controversial (Preuss, 1995; Uylings, Groenewegen, & Kolb, 2003), these findings suggest that it would be extremely interesting to compare human neuroimaging findings and rodent neurophysiological findings on these parallel tasks.
For example, these findings are consistent with human neuroimaging findings, whereby studies have shown that medial OFC activation scales proportional to expected reward value (Rushworth, Kolling, Sallet, & Mars, 2012), the ventromedial prefrontal cortex (suggested to parallel rodent OFC; Ongür & Price, 2000; Schoenbaum, Roesch, & Stalnaker, 2006) reflects rewards and decisions (Balleine & O'Doherty, 2010; Gläscher, Hampton, & O'Doherty, 2009; Hampton, Bossaerts, & O'Doherty, 2006), and the dorsolateral prefrontal cortex (suggested to parallel rodent mPFC; Ongür & Price, 2000; Seamans, Lapish, & Durstewitz, 2008) links with deliberative decision processes (Krawczyk, 2002). One might also anticipate cross-species parallels in recruitment of the anterior cingulate cortex (ACC; Kolling, Behrens, Mars, & Rushworth, 2012). For example, evidence suggests that the ACC may monitor performance, such as the yield of foraging decisions. In particular, the ACC is sensitive to situations where the alternative value is deemed greater than the current option, thus leading the subject to skip. Lastly, the anterior insula is an additional target region for tracking reward responsiveness during the Web-Surf Task, as this area is closely linked to the salience network and has been shown to activate more strongly in response to primary than secondary rewards in humans (Sescousse, Caldú, Segura, & Dreher, 2013).
Conclusions
Collectively, our findings support the use of the Web-Surf Task as an effective experiential human foraging paradigm. Many decision-making tasks are concerned with modeling the motivation (or aversion) to reward and punishment as a means to characterize impulse-related psychopathology. To effectively model reward requires that a given task capture the natural ethology of a species – a reason that has led many to utilize monetary questionnaire or point-based delay-discounting paradigms. However, money is only symbolically rewarding to humans, and is not a comparable primary reinforcer as the food rewards used in rodent paradigms. Our results demonstrate that video stimuli provide a compelling counterpart to food that can be easily incorporated into an experimental setup. Moreover, the multi-option design enables researchers to evaluate individual differences in preference. This feature may be valuable for researchers interested in mapping various behavioral parameters with other marks of impulsivity (e.g. self-report, neural activation, etc.). Therefore, this research lays the foundation for a stream of functional translational research that seeks to narrow the gap between pre-clinical and clinical research via parallel tasks.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (NIDA) to Samantha Abram (F31-DA040335-01), Brandy Schmidt (F32-DA038392-01A1 and R01-DA030672S1), and A. David Redish (R01-DA030672). Brandy Schmidt's contributions to this study were also supported by the Society for Neuroscience's (SfN) Neuroscience Scholars Program.
References
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biology of Mood & Anxiety Disorders. 2011;1(1):9. doi: 10.1186/2045-5380-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter K, Schultz W. Rewarding properties of visual stimuli. Experimental Brain Research. 2006;168:541–546. doi: 10.1007/s00221-005-0114-y. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Lerch J, Thorndycraft B, Iaria G, Zijdenbos AP. Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. The Journal of Neuroscience. 2007;27:10078–10083. doi: 10.1523/JNEUROSCI.1763-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DB, Katherine DD, Dylan SA, Daphna S, Nathaniel DD. Model-based choices involve prospective neural activity. Nature Neuroscience. 2015;18(5):767–772. doi: 10.1038/nn.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton Y, Schmidt B, Redish A. Society for Neuroscience. Washington, DC: 2014. Orbitofrontal inactivation blunts flavor preferences in the Restaurant Row task. [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and Humans Paying Attention. Neuropsychology. 2008;22(6):787–799. doi: 10.1037/a0013712.Rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WN, Sun JH, Sun YW, Zhou Y, Li L, Xu J rong, Du Y song. Altered Default Network Resting-State Functional Connectivity in Adolescents with Internet Gaming Addiction. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0059902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighery EC, Schleicher NC, Boley Cruz T, Unger JB. An examination of trends in amount and type of cigarette advertising and sales promotions in California stores, 2002-2005. Tobacco Control. 2008;17:93–98. doi: 10.1136/tc.2007.022046. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Hampton AN, O'Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cerebral Cortex. 2009;19:483–495. doi: 10.1093/cercor/bhn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Chaloupka FJ. The demand for cocaine by young adults: A rational addiction approach. Journal of Health Economics. 1998;17:427–474. doi: 10.1016/S0167-6296(97)00046-5. [DOI] [PubMed] [Google Scholar]
- Hall ZJ, De Serrano AR, Rodd FH, Tropepe V. Casting a wider fish net on animal models in neuropsychiatric research. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2014;55:7–15. doi: 10.1016/j.pnpbp.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Driscoll I, Sutherland RJ. Human place learning in a virtual Morris water task: Some important constraints on the flexibility of place navigation. Behavioural Brain Research. 2002;129:159–170. doi: 10.1016/S0166-4328(01)00343-6. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behavioural Brain Research. 2003;143:85–94. doi: 10.1016/S0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hampton AN, Bossaerts P, O'Doherty JP. The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans. The Journal of Neuroscience. 2006;26:8360–8367. doi: 10.1523/JNEUROSCI.1010-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and Overlapping Representations of Faces and Objects in Ventral Temporal Cortex. Science. 2001;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, Martin A. The effect of face inversion on activity in human neural systems for face and object perception. Neuron. 1999;22:189–199. doi: 10.1016/S0896-6273(00)80690-X. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Pearson JM, Platt ML. Neuronal basis of sequential foraging decisions in a patchy environment. Nature Neuroscience. 2011;14:933–939. doi: 10.1038/nn.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer LD, Bobashev G, Morris RJ. Researching a local heroin market as a complex adaptive system. American Journal of Community Psychology. 2009;44:273–286. doi: 10.1007/s10464-009-9268-2. [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. The Journal of Neuroscience. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. doi:23/13/5945[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Peters J, Knackstedt L. Animal Models and Brain Circuits in Drug Addiction. Molecular Intervention. 2006;6(6):339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- Kolling N, Behrens TEJ, Mars RB, Rushworth MFS. Neural Mechanisms of Foraging. Science. 2012;336:95–98. doi: 10.1126/science.1216930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool W, Botvinick MM. A labor/leisure tradeoff in cognitive control. Journal of Experimental Psychology General. 2014;143(1):131–141. doi: 10.1037/a0031048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neuroscience and Biobehavioral Reviews. 2002;26:631–664. doi: 10.1016/S0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Potenza MN. Cessation Program for Adolescent Smokers. Drug and Alcohol Dependence. 2007;88(1):79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss DJ, Griffiths MD. Internet Gaming Addiction: A Systematic Review of Empirical Research. International Journal of Mental Health and Addiction. 2012;10:278–296. doi: 10.1007/s11469-011-9318-5. [DOI] [Google Scholar]
- Madden GJ, Bickel WK, editors. Impulsivity: The Behavioral and Neurological Science of Discounting. Washington, DC: American Psychological Association; 2010. [DOI] [Google Scholar]
- Mazur J. An Adjusting Procedure for Studying Delayed Reinforcement. In: Commons M, Mazur JE, Nevin JA, Rachlin H, editors. The effect of delay and intervening events on reinforcement value. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [DOI] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time Discounting for Primary Rewards. The Journal of Neuroscience. 2007;27(21):5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Hatzipantelis M. Navigation in a “Virtual” Maze: Sex Differences and Correlation With Psychometric Measures of Spatial Ability in Humans. Evolution and Human Behavior. 1998;19:73–87. doi: 10.1016/S1090-5138(97)00104-9. [DOI] [Google Scholar]
- Montague PR, Dolan RJ, Friston KJ, Dayan P. Computational psychiatry. Trends in Cognitive Sciences. 2012;16:72–80. doi: 10.1016/j.tics.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacology & Therapeutics. 2005;108(1):18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Papale AE, Stott JJ, Powell NJ, Regier PS, Redish AD. Interactions between deliberation and delay-discounting in rats. Cognitive, Affective, & Behavioral Neuroscience. 2012;12:513–526. doi: 10.3758/s13415-012-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Downing PE. The neural basis of visual body perception. Nature Reviews Neuroscience. 2007;8:636–648. doi: 10.1038/nrn2195. [DOI] [PubMed] [Google Scholar]
- Peirce JW. Generating Stimuli for Neuroscience Using PsychoPy. Frontiers in Neuroinformatics. 2009;2:10. doi: 10.3389/neuro.11.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirolli P. Rational Analyses of Information Foraging on the Web. Cognitive Science. 2005;29:343–373. doi: 10.1207/s15516709cog0000_20. [DOI] [PubMed] [Google Scholar]
- Pirolli P, Card S. Information foraging. Psychological Review. 1999;106:643–675. doi: 10.1037/0033-295X.106.4.643. [DOI] [Google Scholar]
- Potenza MN. The Importance of Animal Models of Decision Making, Gambling, and Related Behaviors: Implications for Translational Research in Addiction. Neuropsychopharmacology. 2009;34:2623–2624. doi: 10.1038/npp.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM. Do Rats Have Prefrontal Cortex? The Rose-Woolsey-Akert Program Reconsidered. Journal of Cognitive Neuroscience. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience. 2008;9(7):545–56. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. In: The Mind Within the Brain: How We Make Decisions and How Those Decisions Go Wrong. 1st. Press OU, editor. New York: 2013. p. 392. [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. The Behavioral and Brain Sciences. 2008;31:415–437. doi: 10.1017/S0140525X08004986. discussion 437–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behavioural Pharmacology. 2006a;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B. The Experiential Discounting Task is sensitive to cigarette- smoking status and correlates with a measure of delay discounting. Behavioural Pharmacology. 2006b;17:133–142. doi: 10.1097/01.fbp.0000190684.77360.c0. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: An experiential discounting task. Behavioural Processes. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Kolling N, Sallet J, Mars RB. Valuation and decision-making in frontal cortex: one or many serial or parallel systems? Current Opinion in Neurobiology. 2012;22:1–10. doi: 10.1016/j.conb.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Breton Y, Redish A. Society for Neuroscience. Washington, DC: 2014. Silencing the rat medial prefrontal cortex decreases hesitation and impairs vicarious trial and error (VTE) behavior on the Restaurant Row task. [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decisionmaking and drug addiction. Trends in Neuro sciences. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüll N. Addiction by design: machine gambling in Las Vegas. The Annals of the American Academy of Political and Social Science. 2012;597:65–81. doi: 10.2307/j.ctt12f4d0. [DOI] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: Insights from electrophysiology. Neurotoxicity Research. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher JC. Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2013;37:681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Straccia MA, Cohen JD, Botvinick MM. Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nature Neuroscience. 2014;17:1249–1254. doi: 10.1038/nn.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AP, Redish AD. Behavioral and neurophysiological correlates of regret in rat decision-making on a neuroeconomic task. Nature Neuroscience. 2014;17(7):995–1002. doi: 10.1038/nn.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. Decision ecology: Foraging and the ecology of animal decision making. Cognitive, Affective & Behavioral Neuroscience. 2008;8(4):475–484. doi: 10.3758/CABN.8.4.475. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Voon V, Reynolds B, Brezing C, Gallea C, Skaljic M, Ekanayake V, et al. Hallett M. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology. 2010;207:645–659. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DB, Caddigan E, Fei-Fei L, Beck DM. Natural scene categories revealed in distributed patterns of activity in the human brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:10573–10581. doi: 10.1523/JNEUROSCI.0559-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein A, Lejoyeux M. New developments on the neurobiological and pharmaco-genetic mechanisms underlying internet and videogame addiction. American Journal on Addictions. 2013 doi: 10.1111/j.1521-0391.2013.12110.x. [DOI] [PubMed] [Google Scholar]
- Wikenheiser AM, Stephens DW, Redish AD. Subjective costs drive overly patient foraging strategies in rats on an intertemporal foraging task. Proceedings of the National Academy of Sciences. 2013;110(20):8308–8313. doi: 10.1073/pnas.1220738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.