Abstract
Background
Metastases to the skin are found with increased frequency at certain sites, such as the scalp, but the biological factors that influence this distribution are not understood.
Objective
We aimed to compare the proportional frequency of metastases at various cutaneous locations with the immunologic microenvironments at those sites.
Methods
We retrospectively identified all biopsies of cutaneous metastases diagnosed at our institution from 1991 to 2014 (n = 1984) and mapped their anatomic distribution while controlling for regional surface area. Using a separate, mapped cohort of normal skin samples (n = 140), we measured the density of regulatory T cells (Tregs), CD4+ effector T cells, and CD8+ T cells by flow cytometry.
Results
Per unit surface area, cutaneous metastases arise most commonly on the head and neck, followed by the trunk, upper extremities, and lower extremities, respectively. Sites with more frequent metastases tend to contain a greater density of Tregs and a lower proportion of CD8+ T cells (p < 0.05).
Limitations
Immunologic factors were only assessed in control tissue and were not measured from patients with metastatic disease in this correlative single-center study.
Conclusion
The distribution of cutaneous metastases follows the distribution of regulatory and effector T cells in skin. Further studies are required to prove a mechanistic association between local immunologic factors and the development of cutaneous metastases.
Keywords: metastatic cancer, cutaneous metastasis, anatomic distribution, immunology, regulatory T cells, effector T cells
Introduction
The skin is an organ commonly affected by metastatic cancer. Approximately 10% of all metastases are cutaneous, and roughly 1–2% of all internal malignancies will eventually metastasize to the skin.1,2 For some types of metastatic cancer, cutaneous spread is particularly frequent: about half of all melanoma metastases and roughly one-third of breast cancer metastases involve the skin.1 Accordingly, dermatologists and dermatopathologists are often in a position to diagnose and monitor metastatic disease.
Despite the frequency with which metastases involve the skin, little is known about the biologic mechanisms that underlie their development. Early studies of cutaneous metastasis focused mostly on epidemiology, primary tumor types, and the anatomic regions of the skin most commonly involved.1,3 Among men, lung cancer, colon cancer, and melanoma have been the tumors that most frequently metastasize to skin; whereas for women, the overwhelming majority of metastases are of breast origin.3,4 In both genders, the trunk has been identified as a site of predilection, with the head and neck region commonly involved as well.1,4 However, these data have not been controlled for regional surface area, thereby limiting comparison among sites.
Recent advances in immunology have highlighted the importance of immune surveillance in tumor development and progression. In this study, we seek to integrate local immunologic factors into a model of cutaneous metastasis. Using the largest cohort to date, we map, in controlled fashion, the distribution of cutaneous metastases. By correlating these maps with tissue-specific immunologic data, we propose that the immunologic milieu of the skin plays a critical role in the distribution of cutaneous metastasis.
Methods
Metastasis data collection and mapping
The archival database of the University of California, San Francisco (UCSF) Dermatopathology Service was searched for all cases of cutaneous metastasis from solid-organ tumors diagnosed between January 1, 1991, and November 17, 2014, after institutional review board approval. All diagnoses were made by a board-certified dermatopathologist and supported by a combination of clinical, histopathologic, and immunohistochemical features indicative of metastasis. Exclusion criteria were non-cutaneous biopsy site, unspecified cutaneous biopsy site, and inconclusive evidence of metastasis. When multiple biopsies were obtained from the same patient on the same day from the same anatomic site, those biopsies were counted as a single metastasis. For each case, the anatomic location of the metastasis and the tumor type from which it originated, if known, were recorded. All data were recorded and analyzed in an anonymized fashion.
Proportional frequency of metastasis at a given anatomic site was calculated by dividing the number of metastases at that site by the total number of all metastases, and then dividing again by the percent body surface area occupied by that anatomic site. Percent body surface area was estimated using the “rule of nines.”5 Heat maps were created by assigning a color to each anatomic region based on the region’s proportional frequency of metastasis, using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA) to arbitrarily assign red to the highest proportional frequency, yellow to the median, and green to the lowest, with intermediate colors determined proportionately.
Flow cytometric immunophenotyping of normal skin
Immune cell subsets were quantified from normal skin using flow cytometry as previously described.6 Briefly, normal human skin was obtained from patients at UCSF undergoing elective surgery, in which healthy skin was discarded as a routine procedure. Subcutaneous fat and hair were removed, skin was minced finely with dissection scissors and mixed in a 6-well plate with 3mL of digestion buffer consisting of 0.8mg/mL Collagenase Type 4 (4188, Worthington, Lakewood, NJ), 0.02mg/mL DNAse (DN25-1G, Sigma-Aldrich, St. Louis, MO), 10% FBS, 1% Hepes and 1% Penicillin/Streptavidin in RPMI medium. Samples were incubated overnight in 5% CO2 and harvested with wash buffer (2% FBS, 1% Penicillin/Streptavidin in RPMI medium) and filtered through a 100µm filter, centrifuged, counted and stained for flow cytometry. The following antibodies were used for flow cytometry: anti117 hCD3 (-FITC, eBioscience, San Diego, CA), anti-hCD4 (-PerCP-eFluor710, eBioscience), anti-CD8 (-BV605, BD, San Jose, CA), anti-hFoxP3 (-eFluor 450, eBioscience), and LIVE/DEAD Fixable Aqua Dead Cell Stain (Life Technologies, South San Francisco, CA). Data was acquired by an LSRFortessa (BD Bioscienes) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Statistical analysis
Immunophenotyping data followed a Gaussian distribution and variation was similar between groups for each group. Significance was assessed using the unpaired Student’s t test. In all dot plots, the mean value ± SEM is visually depicted. Statistical analysis was done using GraphPad Prism software (GraphPad, San Diego, CA).
Results
Distribution of cutaneous metastases
A total of 1984 cases of cutaneous metastasis were identified, including 1292 melanoma metastases and 692 metastases of non-melanoma tumors. Breast cancers were the most common non-melanoma tumors of origin (n = 317). The anatomic distribution of the most common cutaneous metastases, as indicated by the proportional frequency of metastasis to each anatomic site, is summarized in Table I (complete distribution data is provided in Table II, Supplemental materials). In general, the sites with the highest proportional frequencies of cutaneous metastasis were the scalp, ear, face, axilla, and groin; although for breast cancer the chest was the site most commonly involved. In aggregate for all cutaneous metastases, the trunk had the greatest absolute tumor burden, but the head and neck region had the highest proportional frequency of involvement, followed by the trunk, upper extremities, and lower extremities, respectively. Selected tumor-specific and aggregated distributions are illustrated in Figure 1.
Table I.
Proportional rates of metastasis to various cutaneous regions.
| Primary cancer Type |
n | Scalp | Ear | Face | Neck | Chest | Axilla | Back | Abdomen | Groin | Upper extremity |
Lower extremity |
Buttock |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanoma | 1292 | 3.26 | 3.29 | 2.53 | 2.19 | 1.07 | 4.10 | 0.67 | 0.42 | 2.94 | 0.73 | 0.78 | 0.27 |
| Breast | 317 | 2.55 | - | 0.38 | 0.95 | 7.22 | 5.36 | 0.51 | 0.42 | - | 0.22 | 0.02 | - |
| Cutaneous SCC | 53 | 4.04 | 4.72 | 10.86 | 5.03 | 1.26 | - | 0.15 | - | 3.77 | 0.44 | 0.11 | 0.47 |
| Renal | 36 | 11.24 | - | 8.42 | 0.93 | 0.31 | 2.78 | 0.64 | 0.31 | - | 0.33 | - | - |
| Lung | 26 | 5.49 | 9.62 | 1.17 | 3.85 | 2.56 | - | 0.59 | 0.42 | 3.85 | 0.68 | 0.22 | - |
| Colon | 22 | 7.58 | - | 2.75 | - | 0.51 | - | 0.35 | 3.00 | 9.09 | - | - | 3.41 |
| Neuroendocrine | 18 | 5.29 | 13.89 | 1.68 | - | 1.85 | 5.56 | 0.85 | - | - | 0.33 | 0.79 | - |
| Ovarian | 15 | - | - | - | 4.44 | 2.22 | - | - | 3.66 | 20.00 | - | 0.19 | 1.67 |
| Thyroid | 11 | 10.82 | - | 5.51 | 6.06 | 2.02 | - | - | - | - | - | - | - |
| All cancers | 1984 | 3.70 | 2.90 | 2.69 | 2.15 | 2.09 | 4.03 | 0.61 | 0.56 | 2.57 | 0.57 | 0.54 | 0.24 |
| All cancers, excluding melanoma |
692 | 4.51 | 2.17 | 2.98 | 2.07 | 3.98 | 5.35 | 0.50 | 0.84 | 1.88 | 0.26 | 0.09 | 0.18 |
SCC, Squamous cell carcinoma; –, no metastases identified
Figure 1.
Heat maps illustrating the proportional frequency of metastasis to the skin. (A) Melanoma; (B) Breast cancer; (C) Cutaneous squamous cell carcinoma; (D) Renal cell carcinoma; (E) All metastases.
Anatomic variation of T cell subsets
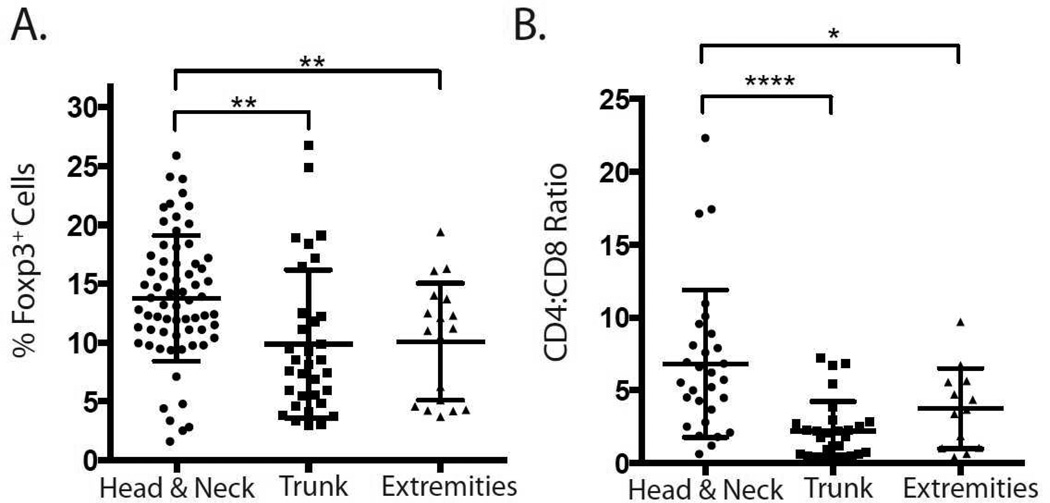
To evaluate the immunologic environment of normal skin, we measured percentages of FoxP3-expressing regulatory T cells (Tregs) within the CD3+ T cell compartment, as well as the effector (i.e., non-Treg) CD4+ to CD8+ T cell ratio in 140 control skin samples from known anatomic sites (Figure 2). It has been previously shown that all FoxP3-expressing CD4+ T cells in normal human skin are bona fide Tregs.6 Treg percentages were significantly higher in specimens from the head and neck when compared to both the trunk and extremities (Figure 2A). In addition, CD4:CD8 ratios followed a similar trend, with the highest ratios found in the head and neck compared to both the trunk and extremities (Figure 2B).
Figure 2.
Quantification of cutaneous immune cell subsets based on skin location. (A) Flow cytometric quantification of percentage of FoxP3+ regulatory T cells (Tregs) as a proportion of total skin-resident CD3+ T cells. (B) Ratio of effector CD4+ to CD8+ T cells as determined by flow cytometry. * p≤0.05, ** p≤0.01, **** p≤0.0001.
Discussion
The distribution of cutaneous metastases has long been known to be nonrandom.3 However, previous studies have not controlled for the surface area of anatomic regions when reporting the distribution of cutaneous metastases, limiting the utility of these historical data. By controlling for regional surface area, direct comparison between anatomic sites is possible, and this permits a more accurate understanding of the behavior of cutaneous metastases and the identification of sites that are most permissive to metastasis.
In particular, using the largest study cohort published to date, we confirm that the head and neck are especially common sites of cutaneous metastasis per unit surface area. These data are consistent with prior reports noting the frequency with which metastases involve the scalp.7 Furthermore, they emphasize the importance of careful scalp examination when evaluating patients with a history of malignancy.
Additionally, based on our heat maps of tumor-specific and overall metastasis, several potential factors that influence the distribution of cutaneous metastasis can be deduced. First, it is apparent that metastasis can occur contiguous to the site of a primary tumor. Breast cancer metastases, for example, frequently involve the skin of the chest and axilla. By contrast, colon cancer metastasizes more commonly to the abdomen, buttocks, and groin.
Second, cutaneous metastases may develop by extension from underlying lymph node basins. The most striking example of this phenomenon appears to occur with melanoma metastases, which exhibit a relative predilection for the neck, axilla, and groin.
However, these two factors cannot fully explain the tendency of many tumors to metastasize to the head and neck. We hypothesized that variation in the local immunologic environment may provide an explanation for this tendency, and because T cell function can modulate tumor progression, we focused specifically on the balance between effector and regulatory T cells in the skin. Unopposed Treg activity has been shown in animal models to promote distant metastasis, and human studies have confirmed that increased Treg density in tumor tissue is associated with adverse outcomes in breast, colorectal, hepatocellular, papillary thyroid, gastric, and renal cell carcinomas.8–14 Moreover, elevated Treg density in lymph node basins is associated with an increased risk of metastasis in cervical, gastric, and papillary thyroid carcinomas, suggesting that T cell function in a recipient tissue, and not just within a primary tumor, affects the incidence with which a tumor metastasizes.13,15–18
Our data from normal skin samples demonstrate that Treg density and CD4:CD8 ratio (the latter of which is an indirect measure of the abundance of cytotoxic T cells) vary across different regions of skin. Remarkably, these patterns of variation roughly correlate with the overall distribution of cutaneous metastases. Areas of the skin with relatively high Treg density and increased CD4:CD8 ratio (head and neck), which would be predicted to be most permissive to tumor growth, are the areas affected most frequently by cutaneous metastasis per unit surface area. We therefore propose that the immunologic milieu of normal skin may determine, at least in part, the relative rate of cutaneous metastasis. This proposal could be viewed as an extension on observations that sites affected by trauma, lymphedema, or other factors that potentially alter the local immunologic balance may be at increased risk for primary malignancy.19 Additionally, given that Tregs preferentially localize to hair follicles in human skin,6 it is interesting to speculate that areas of increased hair follicle density (i.e., scalp) are more permissive to tumor growth as a result of increased Treg density.
There are several limitations to our study. First, our results are correlative, and while a mechanistic association between local immunologic factors and the development of metastases may be plausible, additional controlled experiments would be needed to substantiate this hypothesis. Second, all of our data regarding metastases was taken from a database of histopathologic samples, and we did not perform additional chart reviews to verify patients’ histories of malignancy. However, in all cases, the diagnosis of cutaneous metastasis was rendered only if a history of prior malignancy was provided in conjunction with the tissue specimen and the histopathologic features of that specimen were consistent with the known primary malignancy, or if the histopathologic and immunohistochemical features of the specimen were convincingly those of a metastasis when a history of prior malignancy was not known. Third, our data were anonymized, and individuals with biopsies performed at multiple time points would have been counted multiply, but as our unit of measure was a metastasis and not a patient with metastatic disease, this method of counting would not be expected to alter significantly our overall analysis. Fourth, immunologic data were not collected from patients with metastatic disease, and it is possible that data from control subjects cannot be generalized to patients affected by metastasis.
Despite these limitations, our results suggest a compelling explanatory model for the distribution of cutaneous metastases. These findings also highlight the importance of immunologic factors in regulating tumor progression and metastasis. Recent advances in immunotherapy have proven successful in treating both primary malignancies and metastatic disease, and our data suggest that modulating the immune system may additionally be an attractive target for primary prevention of metastatic spread.
Supplementary Material
Capsule Summary.
-
-
Cutaneous metastases are distributed in nonrandom fashion.
-
-
The distribution of cutaneous metastases can be predicted by variations in T cell populations within the skin.
-
-
Interventions that modulate the immune system hold the potential to influence metastatic spread.
Acknowledgments
We thank Aaron Wu for assistance with graphic design and three-dimensional rendering.
Statement of funding sources: This study was supported in part by grant DP2AR068130 from the National Institutes of Health and grant BWF1010934 from the Burroughs Wellcome Fund (Dr. Rosenblum).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of IRB approval: This study has been approved by the University of California, San Francisco, Committee on Human Research.
Conflict of interest statement: The authors report no conflicts of interest.
References
- 1.Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 Pt 1):228–236. doi: 10.1016/0190-9622(93)70173-q. [DOI] [PubMed] [Google Scholar]
- 2.Hu SC, Chen GS, Wu CS, Chai CY, Chen WT, Lan CC. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60(3):279–287. doi: 10.1016/j.jaad.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105(6):862–868. [PubMed] [Google Scholar]
- 4.Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419–430. doi: 10.1111/j.0303-6987.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 5.Knaysi GA, Crikelair GF, Cosman B. The rule of nines: its history and accuracy. Plast Reconstr Surg. 1968;41(6):560–563. [PubMed] [Google Scholar]
- 6.Sanchez Rodriguez R, Pauli ML, Neuhaus IM, et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124(3):1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu CS, Lin CY, Kuo TT, et al. Malignant cutaneous tumors of the scalp: a study of demographic characteristics and histologic distributions of 398 Taiwanese patients. J Am Acad Dermatol. 2007;56(3):448–452. doi: 10.1016/j.jaad.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 8.Olkhanud PB, Baatar D, Bodogai M, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69(14):5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demir L, Yigit S, Ellidokuz H, et al. Predictive and prognostic factors in locally advanced breast cancer: effect of intratumoral FOXP3+ Tregs. Clin Exp Metastasis. 2013;30(8):1047–1062. doi: 10.1007/s10585-013-9602-9. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Huang Q, Liu G, et al. Presence of FOXP3(+)Treg cells is correlated with colorectal cancer progression. Int J Clin Exp Med. 2014;7(7):1781–1785. [PMC free article] [PubMed] [Google Scholar]
- 11.Mathai AM, Kapadia MJ, Alexander J, Kernochan LE, Swanson PE, Yeh MM. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol. 2012;36(7):980–986. doi: 10.1097/PAS.0b013e31824e9b7c. [DOI] [PubMed] [Google Scholar]
- 12.French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab. 2010;95(5):2325–2333. doi: 10.1210/jc.2009-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen Z, Zhou S, Wang Y, et al. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136(10):1585–1595. doi: 10.1007/s00432-010-0816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang MJ, Kim KM, Bae JS, et al. Tumor-infiltrating PD1-positive lymphocytes and FoxP3-positive regulatory T cells predict distant metastatic relapse and survival of clear cell renal cell carcinoma. Transl Oncol. 2013;6(3):282–289. doi: 10.1593/tlo.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Shima T, Saeki A, et al. Expression of indoleamine 2,3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98(6):874–881. doi: 10.1111/j.1349-7006.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heeren AM, Koster BD, Samuels S, et al. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastastic lymph nodes from patients with cervical cancer. Cancer Immunol Res. 2015;3(1):48–58. doi: 10.1158/2326-6066.CIR-14-0149. [DOI] [PubMed] [Google Scholar]
- 17.Lee HE, Park DJ, Kim WH, Kim HH, Lee HS. High FOXP3+ regulatory T-cell density in the sentinel lymph node is associated with downstream non-sentinel lymph-node metastasis in gastric cancer. Br J Cancer. 2011;105(3):413–419. doi: 10.1038/bjc.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French JD, Kotnis GR, Said S, et al. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97(6):E934–E943. doi: 10.1210/jc.2011-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruocco V, Ruocco E, Piccolo V, Brunetti G, Guerrera LP, Wolf R. The immunocompromised district in dermatology: A unifying pathogenic view of the regional immune dysregulation. Clin Dermatol. 2014;32(5):569–576. doi: 10.1016/j.clindermatol.2014.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.