Abstract
Objective
To determine clinical outcomes in patients with stage IA polyp-limited versus endometrium-limited high-grade (type II) endometrial carcinoma (EC).
Methods
We identified all cases of stage IA polyp-limited or endometrium-limited high-grade EC (FIGO Grade 3 endometrioid, Serous, Clear Cell, or Mixed) who underwent simple hysterectomy, bilateral salpingo-oophorectomy, peritoneal washings, omental biopsy, and pelvic and paraaortic lymph node dissection and received adjuvant treatment at our institution from 10/1995–11/2012. Progression-free survival (PFS) and overall survival (OS) by histology, adjuvant therapy, and polyp-limited vs endometrium-limited disease status were determined using log-rank test. We analyzed three treatment groups: patients who received chemotherapy with or without Radiation Therapy (RT) (intravaginal or pelvic); patients who received RT (intravaginal RT or pelvic RT) alone; and patients who received no adjuvant treatment.
Results
In all, 85 women underwent hysterectomy/salpingo-oophorectomy; all were surgically staged with lymph node assessment and had stage IA EC with no lymphovascular or myometrial invasion. Median follow-up for survivors was 46.5 months (range, 1.98–188.8 months). Forty-nine patients (57.6%) had polyp-limited disease and 36 (42.4%) had endometrium-limited disease. There were no significant differences in clinicopathologic characteristics between patients within the three treatment groups with regards to age at diagnosis, mean BMI, ECOG performance status, polyp-limited, endometrium-limited disease, diabetes, or race. The 3-year PFS rate was 94.9% and the 3-year OS rate was 98.8%. Univariate PFS and OS analysis revealed that age was a relevant prognostic factor [(PFS:HR (95%CI):1.13(1.02–1.25); P=0.022 and OS HR (95%CI):1.19(1.02–1.38); P=0.03]. Adjuvant treatment did not impact outcomes.
Conclusions
Clinical outcomes of surgical stage IA, type II polyp- or endometrium-limited high-grade epithelial EC are equally favorable regardless of histologic subtype or adjuvant therapy received. The benefit of adjuvant therapy in this select group remains to be determined.
Introduction
Endometrial carcinoma (EC) is the most common gynecologic malignancy in the US, estimated to affect 54,870 women and to result in 10,170 deaths in 2015 [1]. On the basis of clinical characteristics and molecular features, two types of endometrial cancer have been classically recognized. Type I EC, representing approximately 80% of primary surgical cases, are characterized by estrogen and progesterone receptor expression and by alterations of the phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) signaling pathway [2–5]. Type I EC also evinces microsatellite instability, is often diagnosed at an early stage, and portends an excellent long-term prognosis. Type II ECs represent approximately 20% of primary cases of EC, are characterized by P53 mutations and HER2/neu amplification [2, 6], and are associated with poorer long-term survival. Type II EC includes the International Federation of Gynecology and Obstetrics (FIGO) grade 3 endometrioid adenocarcinoma, uterine papillary serous (UPSC), and clear cell carcinoma (CC) histologic subtypes.
Stage remains the most important prognostic factor for EC [7, 8]. Additional factors used to assess recurrence risk and inform adjuvant therapy decisions include lymph node status, tumor histologic subtype, grade, depth of myometrial invasion (MMI), and the presence of lymphovascular invasion (LVI) [8–11]. Adjuvant therapy for patients with early-stage disease is tailored according to the estimated recurrence risk, which is defined according to FIGO stage and compiled histological factors [13]. However, no standard treatment approach or regimen exists for early-stage, high-grade endometrial carcinoma.
Optimal management of stage IA polyp-limited versus endometrium-limited epithelial high-grade (Grade 3) EC with no LVI or MMI is even more unclear and warrants further investigation. At this time, sparse data in the form of case reports and small heterogeneous case series have been published, but none have specifically evaluated the management and clinical outcomes of surgically staged type II polyp-limited versus endometrium-limited ECs that do not have the additional risk factors associated with increased recurrence. Hence, we sought to retrospectively evaluate this specific patient population for which no treatment consensus exists, and which is unlikely to be prospectively evaluated given its rare incidence. Here we present a retrospective study analyzing the outcomes of surgical stage IA type II ECs, which were either endometrial-limited or polyp-limited. In our study, we investigated the effects of adjuvant therapy, uterine cancer histology, and other clinicopathologic characteristics on the survival of type II endometrial-limited versus polyp-limited uterine carcinoma.
METHODS
Patient eligibility
Memorial Sloan Kettering Cancer Center (MSK) electronic medical records from 10/1995–11/2012 were reviewed for patient age, diagnosis date, type of primary surgery, residual disease at completion of primary surgery, stage, treatment (chemotherapy and radiation therapy [RT]), dates of progression and death, site(s) of first recurrence, and toxic side effects. Only patients with high-grade (FIGO Grade 3 Endometrioid, Serous, Clear Cell, or Mixed) histology with polyp-confined or endometrium-confined disease were eligible. In an effort to only evaluate high-grade patients within these two cohorts who had no additional risk factors, no LVI or MMI were allowed. For all patients, the initial diagnosis of endometrial cancer was made by endometrial biopsy or curettage. Surgery consisted of simple hysterectomy, bilateral salpingo-oophorectomy, peritoneal washings, omental biopsy, and pelvic and/or paraaortic lymph node dissection. Experienced gynecologic pathologists reviewed all presurgical and surgical pathology specimens. All patients underwent hysterectomy/salpingo-oophorectomy and lymph node assessment at MSK, and had stage IA EC based on the revised FIGO 2009 criteria. For cases obtained from 2009–2012, no Stage IA cases with any myometrial invasion were included. As all patients underwent complete pathology review of their presurgical and surgical specimens, a formal pathology re-review was not undertaken. Treatment had to have been initiated within 2 months following the initial surgery. Patients who received chemotherapy with or without RT (intravaginal [IVRT] or pelvic), RT alone (IVRT or pelvic RT), or no adjuvant treatment were eligible.
A waiver of authorization was obtained and approved by the MSK IRB committee prior to conducting this retrospective analysis.
Statistical considerations
Patients were categorized into and analyzed as three groups: chemotherapy with or without RT (IVRT or Pelvic RT), RT alone (IVRT or Pelvic RT), or no adjuvant therapy. Sites of first recurrence were classified as vaginal, pelvic, abdomen/peritoneum, lung, lymph node, or other distant sites and patients with multiple sites of first recurrence were counted once for each site. Adverse events of treatment were also evaluated and were graded based on the Common Terminology Criteria for Adverse Events v 4.0 (CTCAE). The associations between clinicopathological characteristics were compared to the adjuvant treatment groups (Table 1) by using Fisher-Exact test for the categorical variables and Kruskal-Wallis test for the continuous variables.
Table 1.
Clinicopatholigic Characteristics and Associations
| Treatment Groups |
|||||
|---|---|---|---|---|---|
| Variable | All | None | RT Only | Chemo+/−RT | p- value* |
| All | 85 | 20 | 21 | 44 | |
| Age at Diagnosis | |||||
| Median(Mean) | 65(65.1) | 68(67.1) | 64(62.8) | 65(65.3) | 0.114 |
| Range | 46~82 | 54~77 | 49~82 | 46~80 | |
| BMI(2 missing) | |||||
| Median(Mean) | 31.9(32.1) | 30.9(30.9) | 31.7(32.5) | 32.8(32.4) | 0.826 |
| Range | 19.8~55.7 | 24.1~41.3 | 22.3~55.7 | 19.8~53.3 | |
| Normal(<25) | 14(16.9%) | 1(7.1%) | 3(21.4%) | 10(71.4%) | 0.28 |
| Overweight(25–30) | 21(25.3%) | 8(38.1%) | 4(19%) | 9(42.9%) | |
| Obese(>=30) | 48(57.8%) | 10(20.8%) | 13(27.1%) | 25(52.1%) | |
| Histology | |||||
| Serous (pure) | 56(65.9%) | 13(23.2%) | 11(19.6%) | 32(57.1%) | 0.022 |
| Clear Cell Carcinoma | 14(16.5%) | 5(35.7%) | 7(50%) | 2(14.3%) | |
| Mixed | 15(17.6%) | 2(13.3%) | 3(20%) | 10(66.7%) | |
| Polyp | |||||
| No | 36(42.4%) | 5(13.9%) | 9(25%) | 22(61.1%) | 0.175 |
| Yes | 49(57.6%) | 15(30.6%) | 12(24.5%) | 22(44.9%) | |
| Smoking Status | |||||
| No | 53(62.4%) | 13(24.5%) | 14(26.4%) | 26(49.1%) | 0.836 |
| Yes | 32(37.6%) | 7(21.9%) | 7(21.9%) | 18(56.3%) | |
| Diabetes | |||||
| No | 65(76.5%) | 16(24.6%) | 18(27.7%) | 31(47.7%) | 0.418 |
| Yes | 20(23.5%) | 4(20%) | 3(15%) | 13(65%) | |
| Race | |||||
| White | 54(63.5%) | 15(27.8%) | 12(22.2%) | 27(50%) | 0.443 |
| Black | 21(24.7%) | 2(9.5%) | 6(28.6%) | 13(61.9%) | |
| Asian/Hispanic | 10(11.8%) | 3(30%) | 3(30%) | 4(40%) | |
| CA125(14 missing) | |||||
| Median(Mean) | 10(14.8) | 9(17.7) | 11(19.2) | 9.5(12) | 0.492 |
| Range | 3~101 | 4~74 | 4~101 | 3~49 | |
| ECOG | |||||
| 0 | 38(44.7%) | 9(23.7%) | 10(26.3%) | 19(50%) | 0.958 |
| 1 or 2 (only 1 ECOG=2 pt) | 47(55.3%) | 11(23.4%) | 11(23.4%) | 25(53.2%) | |
p-value for continuous variables are obtained using Kruskal-Wallis test and other p-values are obtained using Fisher-Exact test.
Abbreviations: RT, radiation therapy; BMI, body mass index; chemo, chemotherapy; ECOG, Eastern Cooperative Oncology Group.
Overall Survival (OS) was defined as the time from surgery to either death date or the last follow-up date. Progression-free survival (PFS) was defined as the time from surgery date to either recurrence or death or to the last follow-up date, whichever occurred first. Due to the time-dependent nature of the adjuvant therapy variable, landmark analysis was conducted using 2 months after surgery as the chosen landmark time. The OS and PFS rates were estimated by the Kaplan–Meier method. The univariate P-values in survival analysis were obtained by using the log-rank test. The hazard ratios were calculated by applying the Cox regression model. All analyses were performed by using SAS 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Patient characteristics
Patient characteristics are detailed in Table 1. Eighty-five patients were included in study analysis (91 patients were identified; 5 were excluded for not having lymph node dissection; 1 for short follow-up time). Endometrial biopsy was performed in 61 patients (72%), and endometrial curettage was performed in 34 patients (40%). Twenty-four (48.9%) of 49 patients with polyp-limited disease and 14 (38.9%) of 36 patients with endometrium-limited disease underwent a laparoscopic-assisted vaginal hysterectomy and bilateral salpingo-oophorectomy (LAVH/BSO) and peritoneal washings, omental biopsy, and pelvic and/or paraaortic lymph node dissection. The remaining patients in both cohorts underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO) and peritoneal washings, omental biopsy, and pelvic and/or paraaortic lymph node dissection. Pelvic lymph nodes were dissected in 85 patients (100%), with an average of 15 dissected lymph nodes per patient. Paraaortic lymph nodes were dissected in 53 patients (62.3%), with an average of 5 paraaortic lymph nodes dissected per patient. Sentinel lymph node (SLN) mapping using the cervical injection approach based on the MSK SLN algorithm [14] was performed in 23/85 patients (27.1%). Omental sampling was performed in 70/85 (82.3%) patients. All patients had a negative pelvic wash. Forty-nine out of 85 patients had polyp-limited disease (18 located in the lower uterine segment and 31 located in the uterine fundus) and 36/85 patients had endometrium-limited disease (11 located in the lower uterine segment and 25 located in the uterine fundus). The median age was 65.1 years (range, 46–82). Histologic subtypes were: 56/85 serous, 14/85 clear cell, and 15/85 mixed histology (serous/endometrioid and serous/clear cell). No patients had pure grade 3 endometrioid histology. Patient race distribution was as follows: 54 (63.5%) white, 21 (24.7%) Black, 10 (11.8%) Asian/Hispanic. ECOG performance status range was as follows: ECOG 0, 38 (44.7%); ECOG 1/2, 47 (55.3%). 57.8% of patients were obese, and 23.5% had diabetes. Finally, 12.9% of patients in our analysis had a prior history of estrogen receptor positive breast cancer and received tamoxifen therapy for 3–5 years, and 7.0% of patients had a prior history of colorectal adenocarcinoma.
There were no significant differences in clinicopathologic characteristics between patients within the three treatment groups with regards to the following: age at diagnosis, mean BMI, ECOG performance status, polyp-limited, endometrium-limited disease, diabetes, or race (Table 1). Univariate PFS and OS analysis revealed that age is a relevant prognostic factor [(PFS: HR (95%CI):1.13(1.02–1.25); P = 0.022 and OS HR (95%CI):1.19(1.02–1.38); P = 0.03], and adjuvant treatment did not impact outcomes (Tables 2 and 3).
Table 2.
Univariate Progression-Free Survival Analysis
| Variables | All | Progress/Death# | 3 Year PFS Rate (95%CI) | HR(95%CI) | P-Value* |
|---|---|---|---|---|---|
| All | 85 | 9 | 94.9%(87–98.1%) | ||
| Age at Diagnosis | 1.13(1.02–1.25) | 0.022 | |||
| BMI | |||||
| Normal(<25) | 14 | 1 | 100% | 1 | 0.931 |
| Overweight(25–30) | 21 | 2 | 95%(69.5–99.3%) | 0.91(0.08–10.51) | |
| Obese(>=30) | 48 | 6 | 93.3%(80.7–97.8%) | 1.22(0.14–10.33) | |
| Histology | |||||
| Serous (pure) | 56 | 5 | 96.1%(85.1–99%) | 1 | 0.712 |
| Clear Cell Carcinoma | 14 | 3 | 92.9%(59.1–99%) | 1.7(0.4–7.28) | |
| Mixed | 15 | 1 | 92.9%(59.1–99%) | 0.81(0.09–7.19) | |
| Polyp | |||||
| No | 36 | 5 | 93.9%(77.7–98.4%) | 1 | 0.576 |
| Yes | 49 | 4 | 95.5%(83.3–98.9%) | 0.68(0.18–2.6) | |
| Smoking Status | |||||
| No | 53 | 6 | 96%(85–99%) | 1 | 0.845 |
| Yes | 32 | 3 | 92.9%(74.3–98.2%) | 1.15(0.27–4.85) | |
| Diabetes | |||||
| No | 65 | 7 | 95.1%(85.5–98.4%) | 1 | 0.987 |
| Yes | 20 | 2 | 94.1%(65–99.1%) | 1.01(0.2–5.05) | |
|
Landmark Analysis: by pushing the starting time 2.04 months later* 1 pt is removed from the following analysis due to short follow-up time |
|||||
| Adjuvant Treatment | |||||
| None | 20 | 2 | 100% | 1 | 0.618 |
| RT Only | 21 | 3 | 90.2%(66.2–97.5%) | 2.99(0.31–28.81) | |
| Chemo+/−RT | 43 | 4 | 94.9%(81–98.7%) | 2.31(0.26–20.94) | |
P-value for age is obtained using CoxPH model; while other p-values are obtained through Log-Rank test.
Abbreviations: #, number; PFS, progression-free survival; HR, hazard ratio; RT, radiation therapy; Chemo, chemotherapy.
Table 3.
Univariate Overall Survival Analysis
| Variables | All | Death# | 3 Year OS Rate (95%CI) |
HR(95%CI) | P- Value |
|---|---|---|---|---|---|
| All | 85 | 6 | 98.8%(91.8–99.8%) | ||
| Age at Diagnosis | 1.19(1.02–1.38) | 0.03 | |||
| BMI | |||||
| Normal(<25) | 14 | 0 | 100% | - | |
| Overweight(25–30) | 21 | 2 | 100% | ||
| Obese(>=30) | 48 | 4 | 97.9%(86.1–99.7%) | ||
| Histology | |||||
| Serous (pure) | 56 | 2 | 100% | 1 | 0.385 |
| Clear Cell Carcinoma | 14 | 3 | 92.9%(59.1–99%) | 3.43(0.53–22.07) | |
| Mixed | 15 | 1 | 100% | 1.74(0.14–21.82) | |
| Polyp | |||||
| No | 36 | 4 | 97.1%(81.4–99.6%) | 1 | 0.349 |
| Yes | 49 | 2 | 100% | 0.45(0.08–2.49) | |
| Smoking Status | |||||
| No | 53 | 3 | 100% | 1 | 0.245 |
| Yes | 32 | 3 | 96.8%(79.2–99.5%) | 2.77(0.46–16.68) | |
| Diabetes | |||||
| No | 65 | 4 | 98.4%(89.4–99.8%) | 1 | 0.484 |
| Yes | 20 | 2 | 100% | 1.88(0.31–11.29) | |
|
Landmark Analysis: by pushing the starting time 2.04 months later* 1 pt is removed from the following analysis due to short follow-up time |
|||||
| Adjuvant Treatment | |||||
| None | 20 | 2 | 100% | 1 | 0.836 |
| RT Only | 21 | 2 | 95.2%(70.7–99.3%) | 2.01(0.18–22.28) | |
| Chemo+/−RT | 43 | 2 | 100% | 1.38(0.12–15.36) | |
P-value for age is obtained using CoxPH model; while other p-values are obtained through Log-Rank test.
Abbreviations: #, number; OS, overall survival; HR, hazard ratio; BMI, body mass index; RT, radiation therapy; Chemo, chemotherapy
Adjuvant Treatment
Forty-four (51.8%) patients received chemotherapy +/− RT (38-chemo/IVRT, 5 chemotherapy only, and 1 chemotherapy/pelvic RT). Twenty (23.5%) patients received no adjuvant therapy (physician decision in 11 cases and patient refusal in 9 cases), and 21 (24.7%) patients received IVRT only. Of the 9 patient refusal cases, adjuvant treatment was recommended in each of these cases (chemotherapy/IVRT in 6 cases and IVRT only in 3 cases). Patients who received IVRT were administered between 1500 and 3000cGy of IVRT; and of these 59 patients, 35 received a dose of 2100cGy, 21 received 1800cGy, 2 received 1500cGy, and 1 received 3000cGy of IVRT. IVRT was typically given to 5cm length and 0.5cm depth. The 1 patient who received Pelvic RT received a dose of 5040cGy. Of the patients who received no adjuvant therapy based on physician decision, 8 were serous and polyp-limited, 2 were clear cell and polyp-limited, 1 was mixed and endometrium-limited. Of the patients who received no adjuvant therapy based on patient decision, 6 were serous and polyp-limited, 2 were clear cell and polyp-limited, and 1 was clear cell and endometrium-limited.
Among the 44 patients who received chemotherapy, treatment regimens were as follows: 38 received paclitaxel-carboplatin, 3 received docetaxel-carboplatin (2 patients had baseline neuropathy, 1 patient had paclitaxel-carboplatin for cycle 1 and docetaxel-carboplatin for cycles 2–6 due to paclitaxel hypersensitivity reaction), 2 received carboplatin only, and 1 received cisplatin only.
Paclitaxel 175 mg/m2 administered as a 3-hour infusion with carboplatin AUC 5–6 was given approximately every 3 weeks for 5–6 cycles. Docetaxel 75mg/m2 as a 1-hour infusion with carboplatin AUC 5–6 was administered approximately every 3 weeks for 5–6 cycles. Cisplatin 50mg/m2 as a 1-hour infusion was administered on days 1 and 29.
Adverse effects of treatment
Overall, both chemotherapy with or without RT and RT alone were well tolerated. More treatment-related toxicities were noted in the chemotherapy with or without RT group than in the RT alone group. Only 1 grade 3 hypersensitivity reaction occurred in a patient who received the first cycle of paclitaxel. No other grade 3 event occurred. Grade 2 adverse events in the chemotherapy +/− RT group included fatigue (6/44), neuropathy (3/44), bone marrow suppression (8/44), and constipation (11/44). The RT only group had no grade 2 or 3 adverse events, and grade 1 fatigue (8/21), dysuria (8/21), and nausea (6/21) were the most common adverse events in this group. Only 5 patients experienced recurrence. Sites of recurrence included: lung-2, lymph node-2, peritoneum-1, other (bone)-1.
Progression-free survival and overall survival
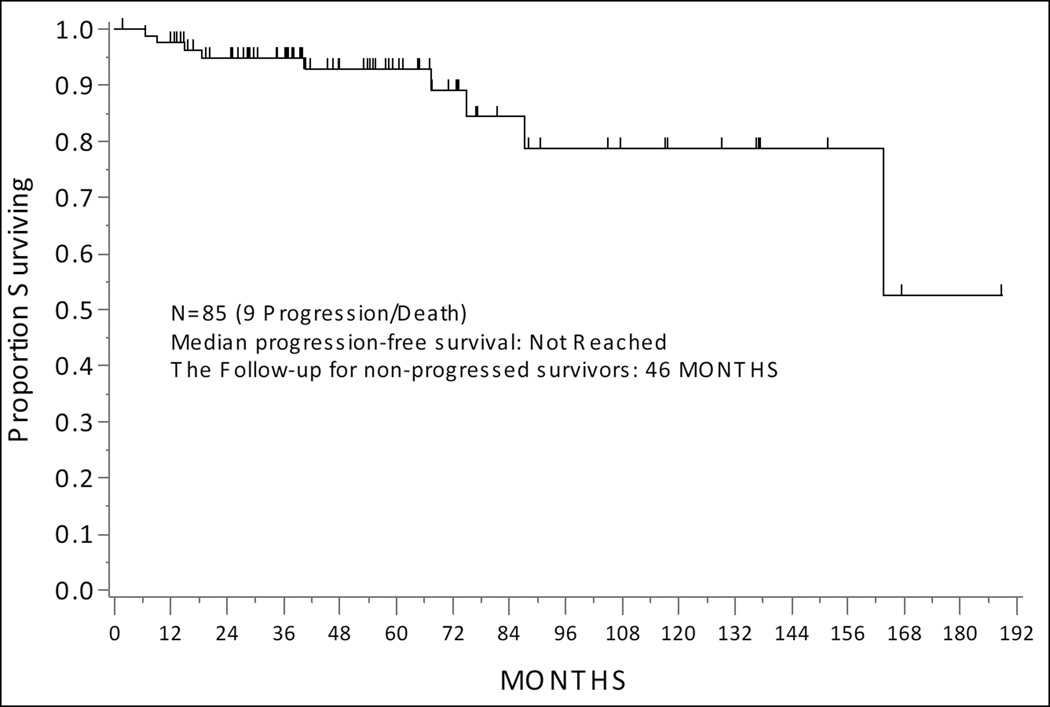
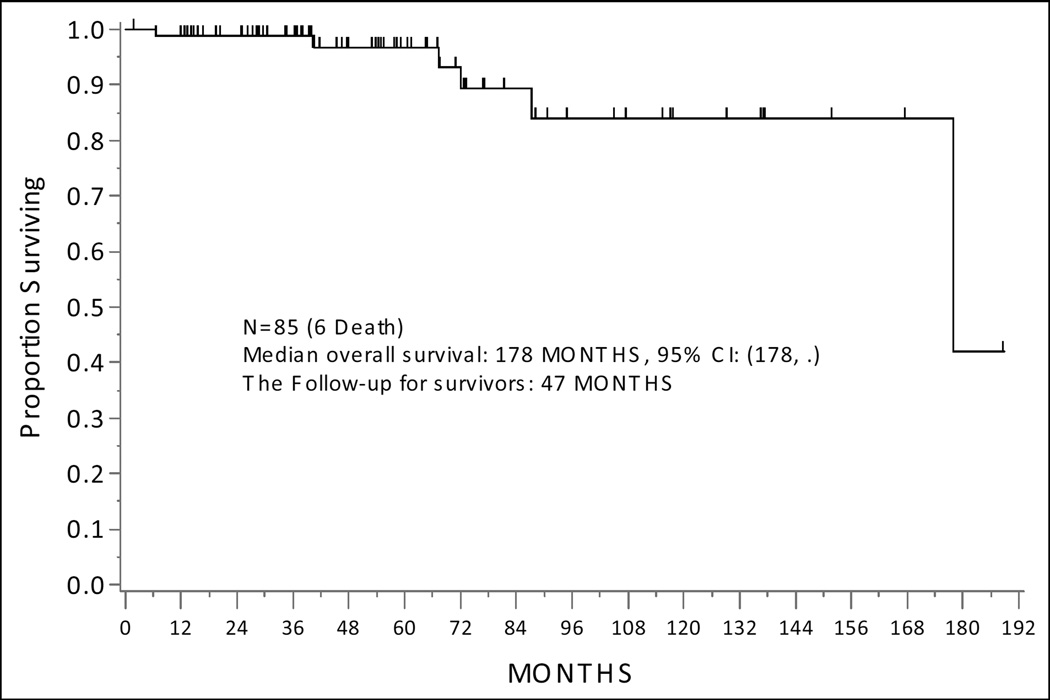
At the time of analysis, 5 patients had progressed (4/5 patients with recurrence received adjuvant therapy [chemo+IVRT-3 and IVRT only-1], and among them 1 died of disease, 1 died of other causes, and 3 were alive with disease. Additionally, 4 patients died of other causes without progression. No patients developed new gynecologic malignancies; 1 patient developed a stage IVB Non-Small Cell Lung Cancer and 1 patient developed a hormone-positive stage I breast cancer following completion of adjuvant therapy for EC (both patients received IVRT). Median follow-up for survivors was 46.5 months (range, 1.98–188.8 months). The median PFS for the entire cohort has not been reached (Figure 1a). There was no difference in PFS by histology, polyp-limited vs endometrium-limited disease, or adjuvant treatment (Table 2). Median OS for the entire cohort was 178 months, 95% CI: (178, Not Estimable) (Figure 2a). There was no difference in PFS by histology, polyp-limited vs endometrium-limited disease, or adjuvant treatment (Table 3).
Figure 1.
Progression-Free Survival of the Entire Cohort
Figure 2.
Overall Survival of the Entire Cohort
CONCLUSION
Optimal management of stage IA polyp-limited vs endometrium-limited epithelial high-grade (Grade 3) EC with no lymphovascular invasion or myometrial invasion is unclear, and at this time, no treatment consensus exists. To our knowledge, this is the largest series to report on polyp-limited vs endometrium-limited stage IA high-grade endometrial cancer where no LVI or MMI exists, and where all patients underwent complete surgical resection, adjuvant therapy, and follow-up at a single institution. In this 85-patient study with a median follow-up for survivors of 46.5 months, there were no significant differences in clinicopathologic characteristics between patients within the three treatment groups with regards to age at diagnosis, mean BMI, ECOG performance status, polyp-limited vs endometrium-limited disease, diabetes, or race. The time to recurrence for the 5 observed cases ranged from 6.4 to 163.7 months (Table 4). The 3-year PFS rate for the cohort is 94.9% and the 3-year OS rate is 98.8%, and univariate PFS and OS analyses revealed that age is a relevant prognostic factor [(PFS: HR (95%CI):1.13(1.02–1.25); P = 0.022 and OS HR (95%CI):1.19(1.02–1.38); P = 0.03]. We also showed that adjuvant treatment did not impact outcomes in this polyp-limited/endometrium-limited cohort.
Table 4.
Characteristics of Patients with Recurrence
| Age | PL/EL | Histology | # Pelvic LN | #PA LN |
Adjuvant tx | FR | TTR (months*) |
Status |
|---|---|---|---|---|---|---|---|---|
| 76 | PL | serous/endo. | 15 | 6 | chemo/IVRT | Lung | 9.9 | DOO |
| 69 | PL | serous | 17 | 4 | IVRT | Med LN | 6.4 | AWD |
| 63 | PL | serous | 11 | 4 | chemo/IVRT | Hilar/AP LN | 69.8 | AWD |
| 70 | EL | serous | 10 | 12 | chemo/IVRT | peritoneum | 13.8 | AWD |
| 68 | EL | clear cell | 11 | 6 | none | rib and lung | 163.7 | DOD |
TTR is calculated from the end of the treatment date to the progression date
all of these patients had omental biopsy
Abbreviations: PL, polyp-limited; EL, endometrium limited; LN, lymph node; tx, treatment; FR, first Recurrence; TTR, time to recurrence; endo, endometrioid; chemo, chemotherapy; IVRT, intravaginal radiation therapy; DOO, died of other causes; Med, mediastinal; AWD, alive with disease; AP, aorto-Pulmonary; DOD, died of disease.
The vast majority of tumors in this study were serous or mixed serous/clear cell or serous/endometrioid. Our survival outcomes are higher than those reported in a recent retrospective study by Chang-Halpenny et al [15]. In their study of 51 patients with stage IA serous or clear cell carcinoma arising from or associated with a polyp (where + cytology and MMI were allowed), the Kaplan-Meier 5-year OS estimate was 80.6%. In our study, 3-year OS was 98.5%. Our study’s OS is comparable to that reported in a recent study by Kiess et al, in which stages I-II UPSC were evaluated and the 5-year OS rate was 94.3% in stage IA patients (with and without LVI or MMI) [16]. Only 20% of patients in the Chang-Halpenny et al study received adjuvant treatment compared to 76.2% of patients in our study, and 85% of patients in the Kiess et al study who completed six cycles of adjuvant paclitaxel-carboplatin chemotherapy and 3 fractions of IVRT; this could have contributed to the more inferior survival in the Chang-Halpenny study.
Specifically, in our study, 20 (24%) patients received no treatment, 21 (25%) received RT only, and 43 (51%) received chemo +/− RT compared to 80% of patients in the Chang-Halpenny et al [15] who received no adjuvant therapy, 18% who received chemo+/− RT, and 2% who received IVRT only. This variability in treatment methodology further reflects the lack of consensus regarding the management of such patients.
In our study 12.9% of patients had a diagnosis of hormone positive breast cancer for which they received 3–5 years of tamoxifen therapy that pre-dated their uterine cancer diagnosis. While it is not known whether tamoxifen use is associated with high-grade cancer in a polyp, it is known that endometrial polyps are common after postmenopausal tamoxifen exposure, and this can result in a 3% rate of malignant transformation compared to 0.48% of the control population [17]. Seven percent of patients had a diagnosis of colorectal cancer that predated diagnosis of endometrial cancer. Of the 16 patients who underwent genetics testing, none were positive for a genetic predisposition to endometrial cancer. Interestingly, 17 patients (20%) were non-insulin dependent diabetics treated with biguanide Metformin; 3 patients (3.5%) were insulin dependent diabetics who were treated with insulin +/− metformin, and 3/5 patients with recurrent disease were non-insulin dependent diabetics.
While our study is limited by it retrospective nature, we attempted to define our clinical question clearly by strictly limiting our study to patients who had staging, workup, and all treatment and follow-up at a single institution. The study is still limited by small sample size, rarity of this disease state, and variable treatments and follow-up. There exists a paucity of data regarding the optimal approach for patients with type II polyp-limited and endometrium-limited EC, and we believe that this study meaningfully contributes to literature regarding this rare clinical entity. We conclude that patients with both high-grade polyp-limited and endometrium-limited EC have a favorable prognosis. Additionally, we corroborate findings of other studies of adjuvant therapy for stage I UPSC [16,18,19] and polyp-confined EC [15]. While >50% of patients in this study received adjuvant therapy, we show that following complete surgical staging, surveillance alone also appears to be an appropriate management approach for patients with stage IA high-grade EC with disease limited to a polyp or the endometrium who have no MMI and negative washings. We recognize that there is a small subset of patients who may recur, and for this cohort of patients, molecular profiling and subsequent management with rationally targeted therapeutics should be pursued.
REFERENCES
- 1.Siegel R, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16:8–13. doi: 10.1177/107327480901600102. [DOI] [PubMed] [Google Scholar]
- 3.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 4.Urick ME, Rudd ML, Godwin AK, et al. PIK3R1 (p85{alpha}) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 2011;71:4061–4067. doi: 10.1158/0008-5472.CAN-11-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oda K, Stokoe D, Takentani Y, et al. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65:10669–10673. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosary CL. Chapter 15: Cancer of the corpus uteri. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001, Patient and Tumor Characteristics. Bethesda, MD: National Cancer Institute, SEER Program; 2007. NIH Pub. No. 07-6215. [Google Scholar]
- 8.Tejerizo-Garcia A, Jimenez-Lopez JS, Munoz-Gonzalez JL, et al. Overall survival and disease-free survival in endometrial cancer: prognostic factors in 276 patients. Onco Targets Ther. 2013;9:1305–1313. doi: 10.2147/OTT.S51532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aalders J, Abeler V, Kolstad P, et al. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419–427. [PubMed] [Google Scholar]
- 10.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial: PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 11.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 12.Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 13.Kong A, Johnson N, Kitchener HC, et al. Adjuvant radiotherapy for stage I endometrial cancer. Cochrane Database Syst Rev. 2012;104:1625–1634. doi: 10.1002/14651858.CD003916.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Leitao MM, Jr, Khoury-Collado F, Gardner G, et al. Impact of incorporating an algorithm that utilizes sentinel lymph node mapping during minimally invasive procedures on the detection of stage IIIC endometrial cancer. Gynecol Oncol. 2013;129:138. doi: 10.1016/j.ygyno.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Chang-Halpenny CN1, Natarajan S, Hwang-Graziano J. Early stage papillary serous or clear cell carcinoma confined to or involving an endometrial polyp: outcomes with and without adjuvant therapy. Gynecol Oncol. 2013;131:598–603. doi: 10.1016/j.ygyno.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Kiess AP, Damast S, Makker V, et al. Five-year outcomes of adjuvant carboplatin/paclitaxel chemotherapy and intravaginal radiation for stage I–II papillary serous endometrial cancer. Gynecol Oncol. 2012;127:321–325. doi: 10.1016/j.ygyno.2012.07.112. [DOI] [PubMed] [Google Scholar]
- 17.Cohen I, Azaria R, Bernheim J, et al. Risk factors of endometrial polyps resected from postmenopausal patients with breast carcinoma treated with tamoxifen. Cancer. 2001;92:1151–1155. doi: 10.1002/1097-0142(20010901)92:5<1151::aid-cncr1433>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Kelly MG, O'Malley DM, Hui P, et al. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol. 2005;98:353–359. doi: 10.1016/j.ygyno.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 19.van der Putten LJ, Hoskins P, Tinker A, et al. Population-based treatment and outcomes of Stage I uterine serous carcinoma. Gynecol Oncol. 2014;132:61–64. doi: 10.1016/j.ygyno.2013.11.002. [DOI] [PubMed] [Google Scholar]