Abstract
Background/Aims
The mechanisms by which hepatocyte exposure to alcohol activates inflammatory cells such as macrophages in alcoholic liver disease (ALD) are unclear. The role of released nano-sized membrane vesicles, termed extracellular vesicles (EV), in cell-to-cell communication has become increasingly recognized. We tested the hypothesis that hepatocytes exposed to alcohol may increase EV release to elicit macrophage activation.
Methods
Primary hepatocytes or HepG2 hepatocyte cell lines overexpressing ethanol-metabolizing enzymes Alcohol dehydrogenase (HepG2ADH) or cytochrome P450 2E1 (HepG2Cyp2E1) were treated with ethanol and EV release was quantified with nanoparticle tracking analysis (NTA). EV mediated macrophage activation was monitored by analyzing inflammatory cytokines and macrophage associated mRNA expression, immunohistochemistry, biochemical serum ALT and triglycerides analysis in our in vitro macrophage activation and in vivo murine ethanol feeding studies.
Results
Ethanol significantly increased EV release by 3.3 fold from HepG2Cyp2E1 cells and was associated with activation of caspase-3. Blockade of caspase activation with pharmacological or genetic approaches abrogated alcohol induced EV release. EV stimulated macrophage activation and inflammatory cytokine induction. An unbiased microarray-based approach and antibody neutralization experiments demonstrated a critical role of CD40 ligand (CD40L) in EV mediated macrophage activation. In vivo, wild-type (WT) mice receiving a pan-caspase, Rho kinase inhibitor or with genetic deletion of CD40 (CD40−/−) or the caspase-activating TRAIL receptor (TR−/−), were protected from alcohol-induced injury and associated macrophage infiltration. Moreover, serum from patients with alcoholic hepatitis (AH) showed increased levels of CD40L enriched EV.
Conclusion
In conclusion, hepatocytes release CD40L containing EV in a caspase dependent manner in response to alcohol exposure which promotes macrophage activation, contributing to inflammation in ALD.
Keywords: Alcoholic hepatitis, Caspase inhibitor, Liver, Inflammation, TNF-α, CD40L, Hepatocyte, Macrophage, Cyp2E1, Alcohol, Exosomes
INTRODUCTION
Alcoholic liver disease (ALD) is an important cause of preventable death with a potential for 30–40% 1-month mortality among those with alcoholic hepatitis [1]. Current concepts in ALD pathogenesis include enhanced ethanol induced intestinal permeability leading to hepatic delivery of gut derived lipopolysaccharide (LPS) [2, 3] as well as direct hepatic effects of ethanol on hepatocytes [4]. While alcohol is metabolized primarily by hepatocyte ADH [2], chronic exposure and/or at higher alcohol doses lead to metabolism from the hepatic microsomal mono-oxygenases, particularly cytochrome P4502E1 [5]. Alcohol metabolism contributes to liver injury via acetaldehyde generation and ROS production [2]. However, there is lack of information pertaining to the signals that connect hepatocyte injury with macrophage activation in this process.
Recent studies show that cells undergoing stress or injury may release nano-sized vesicles such as microparticles or exosomes, referred to here as extracellular vesicles (EV) [6, 7]. These EV contain molecules that may transfer signals from one cell to another through contents that include mRNA, miRNA, lipids, DNA or proteins. This mechanism could conceivably link epithelial cell injury with its associated inflammatory response. However, the role of EV in alcohol-induced liver injury has not been expansively explored previously.
We sought to identify mechanisms by which hepatocyte exposure to alcohol could lead to macrophage activation by addressing several specific questions: 1) Does alcohol exposure to hepatocytes lead to EV production? 2) If so, then what mechanisms mediate EV release? 3) Does EV release lead to macrophage activation and inflammation? 4) If so, then how? We show here that upon ethanol exposure and metabolism, hepatocytes release EV. Both in vitro and in vivo studies show that this process requires TRAIL receptor (TR) and caspase-3 activation. We further show that CD40L contained within EV contributes to macrophage activation associated with alcohol induced injury. Thus, the studies mechanistically extend existing paradigms relating to alcohol-induced liver inflammation by showing a role for caspase-dependent EV production and CD40L that mediate pathobiological paracrine signaling.
METHODS (Additional methods are provided in Supplement Section)
Cell culture
HCC cell lines HepG2, HepG2ADH and HepG2Cyp2E1 were routinely cultured in EV reduced DMEM, supplemented with 10% FBS. The HepG2Cyp2E1 cells were kind gift from Dr. A. Cederbaum, (Mount Sinai School of Medicine, New York) [5] and HepADH cells were a kind gift from Dr. Mark McNiven (Mayo Clinic) [8]. Murine primary hepatocytes were isolated as described earlier [9]. Cells were cultured in reduced EV medium and stimulated with ethanol (0–100 mM) as indicated in individual experiments. For in vitro studies, a pan-caspase inhibitor (IDN-7314, 20 μM), diallyl sulphide (DAS; 100μM), cyanamide (10 μmol/l), or a rho kinase inhibitor (Fasudil; 50μM) were added prior to addition of ethanol.
Isolation of EV
EV were isolated as described earlier [10]. The pellets obtained were resuspended in a large volume of PBS and washed three times by centrifugation for 2 h at 110,000g, 4 °C. EV numbers were quantified and characterized using nanoparticle tracking analysis (NTA); (NS300, Malvern Instruments, Malvern, UK). Reduced EV media was prepared by ultracentrifugation of 20% FBS containing DMEM for 2 hours in a SW32Ti Rotor at 100,000g, 4 °C in Optima XPN-80 ultracentrifuge. Murine serum EV was isolated using TLA100 rotor in an Optima TLX Ultracentrifuge. Reduced EV medium was further used for culture at final 10% FBS concentration.
Caspase Assay
Caspase activation in response to ethanol was monitored using methods described earlier using Synergy H1 multi plate reader (Bio-Tek Instruments) [11].
Adhesion Assay
Cell adhesion was assessed by a 96-well format CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay reagent (Promega) as per the manufacturer’s instruction and as described earlier [12]. The cells were treated with 1 × 1011 amounts of EV or EV-free culture supernatant as control [13].
Quantitative real-time PCR (RT-PCR)
The macrophages were treated with EV or EV free medium; following treatment total RNA was isolated from cells or whole livers using TRIzol (Invitrogen, Carlsbad, CA) and RNeasy kit (Qiagen) with modifications as described [14, 15]. RT-PCR was performed using ABI7500 Real-Time PCR Detection System (Applied Biosystems, Foster City, CA) and the CT values were normalized to GAPDH or β-actin. The primers used in real-time PCR are listed in Supplementary Table 1.
Chemokine/Cytokine arrays
EV isolated from control or ethanol treated HepG2Cyp2E1 cells and alcoholic hepatitis patient samples were subjected to human cytokines and chemokines antibody-based array (ARY05; R&D Systems, USA) according to manufacturer’s instructions and quantitated using ImageJ-software (NIH).
Mouse model of chronic plus single binge ethanol consumption
All animal experiments followed protocols approved by Mayo Clinic Institutional Animal Care and Use Committee. For in vivo ethanol feeding studies, chronic-binge ethanol feeding (Gao-Binge model), with 12–13 week old wild-type (WT), TR−/−, CD40−/− C57 / BL6 background female mice was used [16]. The WT & CD40−/− mice were purchased from Jackson Laboratories, and the TR−/− mice were kindly provided by Wafik S El-Deiry [17, 18]. WT mice received control, ethanol with daily oral administration of compound (IDN-7314; 5 mg/kg in 0.5% carboxy-methyl-cellulose), Rho kinase inhibitor (ROCK1); 50 mg/kg or vehicle alone. The TR−/− or CD40−/− mice were similarly subjected to pair fed control or ethanol diet regimen (n = 5–10 per group).
For details on methodology, please see the Supplementary Methods.
RESULTS
Hepatocytes release EV in response to alcohol exposure
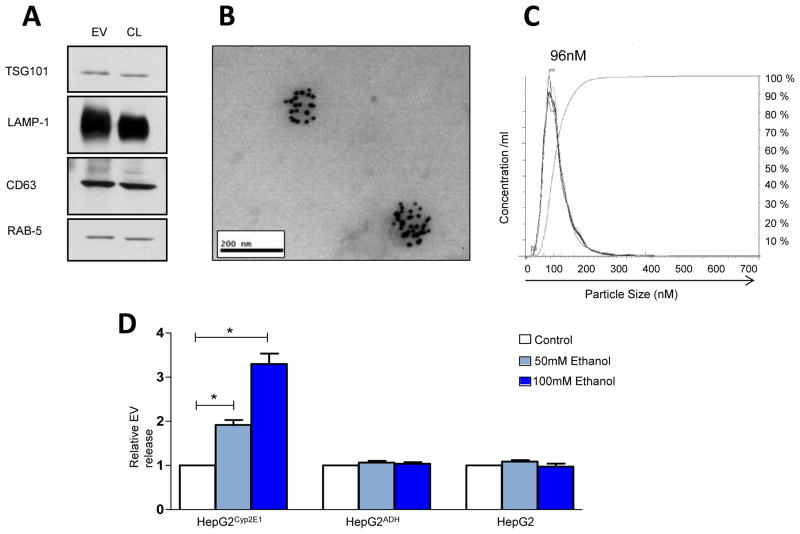
Recent work has shown that hepatocytes can generate EV in response to diverse stimuli [19]. As an initial step to explore this model in pathogenesis of alcohol-related liver injury, EV were isolated from the conditioned media of HepG2 cells using a sequential ultra-centrifugation method described for EV isolation and characterized using biochemical and morphological approaches. Electron microscopy (EM), NTA, and immunoblot analysis of EV compared to cell lysates (CL) confirmed morphology, size, and presence of TSG-101, LAMP-1, CD63, and RAB5 which are typical marker proteins for EV (Fig. 1A) [20, 21]. EM revealed the presence of vesicles morphologically resembling EV and which were positively immuno-labeled with TSG-101 specific gold particle-conjugated antibodies (Fig. 1B). The particle size and distribution measured using NTA showed a mean particle size of 110 nm and mode of 96 nm (Fig. 1C) in our preparations consistent with EV. With this characterization in hand, we explored effects of ethanol on EV production using this in vitro model system.
Figure 1. Characterization and quantification of EV produced by hepatocytes in response to alcohol treatment.
(A) Western blot analyses for membrane vesicle markers from EV or cell lysates (CL) from HePG2Cyp2E1 (30 μg) were analyzed in parallel. Four different membrane vesicles associated markers (TSG101, LAMP-1, CD63 and RAB5) are shown. (B) Representative image of EM analysis shows morphology size and presence of EV-associated marker, TSG101. (C) Representative plot showing size distribution of EV was carried out using NTA. (D) Quantification of EV was done using NTA. EV release was significantly increased in HepG2Cyp2E1 cells upon ethanol stimulation (50,100 mM), whereas no increase in EV release was observed in HepG2ADH or in control HepG2 cells stimulated with ethanol (n = 3, *p<0.05).
Ethanol metabolism by CYP2E1 promotes EV release
To examine the impact of ethanol and its oxidative metabolism by CYP2E1 and ADH, HepG2, HepG2ADH or HepG2Cyp2E1, stably transfected cell lines were treated with 0, 50 or 100 mM ethanol. The concentration of ethanol used mimics the heavy alcohol consumption seen in heavy drinkers after binge drinking, and levels observed in the chronic-binge murine alcohol feeding models [16, 22]. After 24 hr, EV were isolated from cell culture supernatant. The fractions harvested from, ethanol-treated HepG2Cyp2E1 cells demonstrated a significant, 1.9 and 3.3-fold higher EV yield with 50 and 100mM ethanol, respectively when quantified for EV number, whereas ADH-mediated ethanol metabolism did not result in enhanced EV release in response to ethanol stimulation; similar to that observed with native HepG2 cells (Fig. 1D). Furthermore, inhibition of CYP2E1 with DAS significantly reduced ethanol induced EV release, whereas no reduction in ethanol induced EV release was observed upon aldehyde dehydrogenase (ALDH) inhibition using cyanamide (Fig. 2A). Collectively these data indicate that ethanol metabolism by CYP2E1 promotes ethanol-induced EV release.
Figure 2. Caspase activation contributes to ethanol induced EV release from HepG2Cyp2E1.
(A) Bar graph shows that EV release in ethanol exposed cells is blocked by inhibition of Cyp2E1 with DAS but not with inhibition of ALDH with cyanamide (n=3: *p,0.05). (B) Significant increase in caspase activity was observed in response to 50,100 mM ethanol in HepG2Cyp2E1 cells, while only non-significant caspase activation was observed in HepG2ADH or HepG2 cells (n = 3; *p<0.05). (C) Bar graph shows relative suppression of ethanol induced EV release in response to pan-caspase inhibitor, IDN-7314 in HepG2Cyp2E1 cells. (n = 3; *p<0.05). (D) Non-targeted (NT) or two different caspase-3 lentiviral shRNA constructs (sh1 or sh2) with the respective knockdown efficiency (>90%) are shown as determined by western blot analysis (N=2). The histogram represents the relative EV production as determined by enumerating the EV using NTA from the non-targeted shRNA or two different Caspase-3 shRNA transfected HepG2Cyp2E1 cells (n = 3 *p<0.05). (E) Reduced ethanol induced EV release was observed with ROCK-1 inhibition using fasudil (*p<0.05). (F) Reduced ethanol induced EV release in primary hepatocytes was observed with caspase inhibitor IDN-7314 (*p<0.05).
Ethanol induced EV release requires caspase activation but occurs independent of apoptosis per se
ALD is associated with hepatocyte apoptosis thereby providing a rational basis to evaluate caspase inhibitors as potential therapies in preclinical studies and in patients with chronic liver diseases [23–27]. Furthermore, caspase activation has been implicated in the ROCK-1 associated actin-based machinery theoretically required for EV exocytosis [28]. We therefore explored the role of caspase activation in our in vitro experimental model of alcohol-mediated EV release. HepG2, HepG2Cyp2E1 and HepG2ADH cells were treated with 0–100 mM concentrations of ethanol for 24 h and activation of caspase 3/7 was measured using a sensitive spectrofluorometric method. Interestingly, HepG2Cyp2E1 cells showed significantly increased caspase 3/7 activity both with 50 and 100 mM concentration of ethanol (Fig. 2B). No caspase activation was observed in HepG2ADH cells; similar to what we reported previously in HepG2 cells [11]. To further elucidate whether increased caspase-3 activation in our in vitro studies was part of a cascade leading to increased programmed cell death, we next performed an in situ fluorometric cell death detection assay using TUNEL stain where HepG2Cyp2E1 cells were treated with 100 mM ethanol for 24 hours. TUNEL assay revealed no increase in cell death compared to control cells, indicating that while alcohol stimulates caspase activation, it does not lead to overt cell death in our experimental model system (Supplementary Fig. 1).
Based on these results, we next explored the potential of a caspase inhibitor, IDN-7314, to limit EV release in response to ethanol. The caspase inhibitor significantly reduced ethanol-induced EV release from HepG2Cyp2E1 cells (Fig. 2C), however an inhibitor of necroapoptosis (Necrostatin-1) did not reduced ethanol induced EV release (Supplementary Fig 5C). To further confirm specificity of effect seen with pharmacological caspase inhibition, we used a lentivirus based caspase-3 shRNA targeting approach. Caspase-3 shRNA or non-targeted shRNA were packaged into lentivirus and transfected into HepG2Cyp2E1. Immunoblot for caspase-3 from lentiviral based caspase-3 shRNAs transfected cell lysate is depicted in Fig. 2D, in which two shRNAs showed more than 90% knockdown efficiency in HepG2Cyp2E1 cells; these were used for subsequent EV release experiments. EV from conditioned medium of cells in respective groups was isolated and quantified using NTA which revealed that caspase-3 knockdown significantly attenuated ethanol-induced EV release (Fig. 2D), compared to non-targeted lentiviral-based shRNA control construct. Moreover, inhibition of Rho kinase (ROCK1), a molecule associated with actin polymerization, membrane blebbing, and implicated in exosome release, with a pharmacological inhibitor (Fasudil), also significantly reduced ethanol induced EV release (Fig 2E). These cell line based studies were corroborated with murine primary hepatocytes (Fig. 2F). Thus, ethanol metabolism stimulates EV release in a caspase-3 axis dependent mechanism.
Hepatocyte derived EV activate macrophage to M1 type inflammatory phenotype
Kupffer cells are strongly implicated in the inflammation process that defines alcoholic hepatitis in both humans and in experimental preclinical models [29]. We therefore explored the potential effect of hepatocyte-derived EV on macrophage activation. Cellular crosstalk between hepatocytes and macrophages mediated by EV was investigated by using vehicle (Con-EV) or alcohol-treated HepG2Cyp2E1 derived EV (Alc-EV) incubation on pdTHP-1 cell line and primary macrophages. THP-1 cells become adherent upon activation; we exploited this characteristic to ascertain macrophage activation in response to EV. THP-1 cells were cultured in presence of EV derived from control or alcohol treated HepG2Cyp2E1 cells for 24 hours. The potency of EV to activate THP-1 cell adhesion was detected using a colorimetric MTS assay. The Alc-EV activated THP-1 cells by 3.7 fold (Fig. 3A) whereas no significant change was observed with Con-EV or EV free medium alone. Incubation of the fluorescent labeled Alc-EV with THP-1 cells showed internalization of labeled EV as early as 1 hour after incubation based on confocal microscopy imaging (Supplementary Fig. 2), indicating that these cells may indeed respond to EV. These findings support the concept that EV from alcohol-stimulated hepatocytes promotes macrophage activation.
Figure 3. Activation of macrophages to M1 phenotype in response to Alc-EV or Con-EV.
(A) THP-1 cell activation was monitored by quantifying adhesion to plastic (n = 3; *p<0.05). (B) qPCR mRNA analysis showing elevated M1 macrophage associated pro-inflammatory cytokine markers TNF-α, IL-1β, IL-6 and NOS-2 from PMA-differentiated THP-1 cells upon incubation with Alc-EV, compared to Con-EV, or EV free medium for 12 hours (n = 3; *p<0.05). No change in M2 macrophage associated marker, CD206 and IL-10 levels was observed. (C) Histograms shows increased mRNA expression of M1 macrophage associated markers TNF-α, IL-1β and NOS-2 with Alc-EV relative to Con-EV or EV free medium from Kupffer cells (n=3; *p<0.05). No change in M2 macrophage associated markers was observed. (D) Histogram depicts Alc-EV significantly elevated mRNA levels of cell adhesion and migration associated molecule ICAM-1, M1 macrophage associated / pro-inflammatory cytokine markers TNF-α, IL-1β and IL-6, compared with control groups (n = 3; *p<0.05). No change was observed in M2 macrophage associated markers. (E) Protein levels of TNFα, IL-1β and IL-6 from MDM were quantified using quantitative ELISA. Significantly increased release of these pro-inflammatory cytokines was observed with Alc-EV compared with Con-EV or medium control only (n = 3; *p<0.05).
We also explored whether increased macrophage adhesion was associated with increased cytokine production from macrophages in response to EV stimulation. Indeed, pdTHP-1 cells stimulated with Alc-EV for 12 hours showed significantly increased mRNA levels of TNF-α, IL-1β, IL-6 and NOS-2, suggestive of M1 polarization (M2 markers, CD206 and IL-10, did not change; Fig. 3B). Similar results were observed in primary murine kupffer cells and primary human monocyte derived macropahges (MDM; Fig. 3C–D). These observations indicate that hepatocyte-derived EV stimulated by ethanol, lead to macrophage activation. Additionally, protein levels of cytokines TNFα, IL-1β and IL-6 from MDM were also quantified using quantitative ELISA, which showed a significant release of these pro-inflammatory cytokines with Alc-EV compared with Con-EV or medium control only (Fig. 3E).
Hepatocyte derived EV contain CD40L which mediates macrophage activation
To identify potential macrophage activating components within HepG2Cyp2E1 derived EV, we subjected EV protein lysates from control or ethanol treated cells to a low-density 36 target Chemokine/Cytokine array. From the results that were obtained (Supplementary Table 2), one target that was highly expressed on Alc-EV, CD40L (TNFSF5), a member of the TNF family and previously implicated in macrophage activation was validated by western blot and EM analysis (Fig. 4A), and then explored in further detail. Since CD40L was localized on EV surface (Fig. 4A), involvement of CD40L was mechanistically explored further by utilizing a CD40L-neutralizing antibody. Neutralization of CD40L from Alc-EV was associated with a significant reduction in ability of these EV to induce pro-inflammatory cytokine production from THP-1 cells (Fig. 4B) and in primary macrophages (Fig. 4C). This was also examined in a signaling context whereby CD40L neutralization attenuated EV-induced ERK activation with ERK being a prototypical signal required for pro-inflammatory cytokine production (Supplementary Fig. 3).
Figure 4. CD40L on EV contributes to macrophage activation.
(A) Immunoblot using CD-40L and LAMP-1 antibody using 30 μg of EV is shown. EM analysis depicts presence of CD40L on surface of EV. (B) CD40L on EV was neutralized by treating ethanol exposed HepG2Cyp2E1 cells derived EV with control IgG or with CD40L neutralizing antibody. Significant reduction in pro-inflammatory cytokines TNF-α, IL-1β and IL-6 mRNA expression upon CD40L neutralization was observed, compared to non-neutralized EV or with control IgG treated EV (n = 3; *p<0.05). (C) Reduced pro-inflammatory cytokine mRNA levels from primary macrophages were observed when EV were subjected to CD40L neutralization corroborating results of THP-1 macrophage cell line (n =3; *p<0.05).
Caspase-3 inhibition attenuates alcohol induced liver injury in vivo
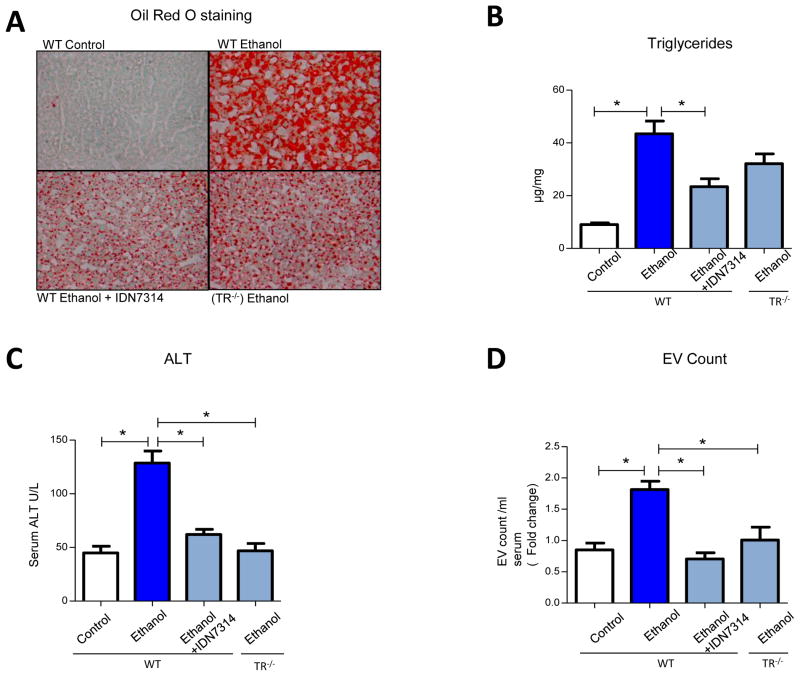
Our in vitro findings suggested that alcohol-induced activation of caspases leads to EV production and macrophage activation. We further explored this concept in vivo using a binge alcohol feeding model [16] which was administered to WT mice, mice receiving the pan-caspase inhibitor (IDN-7314), or TR−/− mice, as TR−/− is molecularly situated upstream from caspase-3 activation. These mice evidence reduced apoptosis and caspase-3 activation when subjected to diverse apoptotic stimuli including the TR ligand (TRAIL), dexamethasone, ionizing radiation or nutrient excess [17, 30, 31]. Quantitative real-time PCR analysis for the expression of transcripts encoding pro-inflammatory cytokines TNF-α, IL-1β revealed significantly increased mRNA levels from liver of ethanol-fed mice (Fig. 5A&B). The increased macrophage infiltration in liver tissues from alcohol fed mice was evidenced by increased macrophage specific F4/80 mRNA expression analysis (Fig. 5C) and by immunohistochemistry analysis using Mac-2 as a macrophage marker (Fig. 5D). However, these parameters were attenuated in ethanol-fed mice administered the caspase inhibitor or in TR− /− mice (Fig. 5A–D). Parameters of steatosis and liver injury were also performed; liver sections from ethanol-fed mice showed higher steatosis as observed by Oil Red-O staining and significantly high liver triglycerides content compared to control diet-fed mice (Fig. 6A&B). This was attenuated in ethanol fed mice receiving the caspase inhibitor and in genetically modified mice lacking TR indicating this pathway is required for steatosis. Finally, serum ALT levels were also reduced in mice administered the caspase inhibitor or in TR−/− mice (Fig. 6C). Serum, bile acid (BA) and alkaline phosphatase (Alk-Phos) levels in ethanol-fed mice were significantly increased, whereas this increase was attenuated in ethanol-fed mice treated with the pan-caspase inhibitor or in TR−/− mice. No significant change in cholesterol, bilirubin, albumin, blood urea nitrogen (BUN), diet consumption, α-SMA mRNA levels, or LY6G neutrophil marker mRNA levels were observed between the ethanol fed and caspase inhibition (IDN7314 and TR−/−) groups (Supplementary Fig. 4 and 5A). TUNEL positive cells were increased in ethanol group compared with caspase inhibition groups (Supplementary Fig. 5B).
Figure 5. Disruption of caspase activation reduces ethanol induced hepatic pro-inflammatory cytokine mRNA levels and macrophage infiltration.
(A–C) mRNA levels of TNF-α, IL-1β, and F4/80 in WT mice with control diet (n = 9), ethanol diet (n = 10), ethanol with once daily oral gavaged with IDN-7314 (n = 10) and ethanol fed TR−/− (n = 5) mice is shown. Significantly reduced pro-inflammatory cytokines mRNA and macrophage infiltration was observed in IDN-7314 administered and in TR−/− mice (*p<0.05). (D) Depicted image (20X) shows macrophage infiltration as determined by immunohistochemistry for Mac-2 in response to alcohol and its resolution upon treatment with IDN-7314 or in TR−/− mice. Quantification is depicted graphically using Metamorph software (*p<0.05).
Figure 6. Disruption of caspase activation ameliorates liver injury markers and EV release.
(A) Photomicrograph depicting reduction in steatosis by Oil-red-O staining from frozen liver sections (20X). (B) Hepatic triglycerides (TG) μg/mg of liver tissue (*p<0.05). (C) Serum EV enumeration using NTA for all groups of mice (*p<0.05). (D) Serum biochemical analysis for ALT is depicted (*p<0.05). For panels A–D, reduced hepatic injury markers were observed in the IDN-7314 administered or TR−/− ethanol fed group of mice, compared to WT mice on ethanol alone. Groups consists of WT mice with control diet (n = 9), ethanol diet (n = 10), ethanol with IDN-7314 (n = 10) and in TR−/− ethanol fed (n = 5) (*p<0.05).
To examine whether alcohol feeding affects total serum EV numbers, serum EV from WT mice fed with control diet, alcohol diet or from mice administered caspase inhibitor along with alcohol feeding, and from the TR−/− mice on ethanol diet were isolated and quantified. Serum from alcohol-fed mice contained significantly higher EV levels compared to serum from control diet-fed mice, with this increase being abolished in mice administered the caspase inhibitor as well as in TR−/− mice (Fig. 6D). These findings are in agreement with our in vitro studies suggesting involvement of increased EV for mediating ethanol-induced hepatic inflammation and injury. These observations indicate that disruption of caspase activation by genetic or pharmacologic approaches can attenuate EV production, alcohol-induced steatosis and inflammation in vivo.
Rho kinase inhibition protects mice from ethanol induced liver injury
To more directly test if hepatocyte derived exosomes were responsible for macrophage activation, we extended our in vitro studies with ROCK1 inhibition by administering fausadil to mice in vivo during ethanol feeding. Ethanol fed mice receiving fasudil showed lower macrophage activation markers (IL-1β, IL-6) and hepatic injury (serum ALT levels) compared to ethanol fed mice receiving control compound (Fig. 7A–C). These in vivo observations in combination with the earlier in vitro studies with fausadil highlight the direct role of hepatocyte derived EVs for promoting macrophage activation associated inflammation and injury and support our data obtained with caspase inhibition (Fig. 5 & 6).
Figure 7. Inhibition of EV release with fasudil or EV mediated macrophage activation with CD40−/ − mice is associated with protection from ethanol induced hepatic injury and inflammation.
(A–C) Biochemical ALT and mRNA analysis for proinflammatory cytokine levels from liver tissue of mice receiving fasudil or control groups are shown. Significant reduction of ALT, IL-1β, and IL-6 was observed in fausadil group (n=5–6 mice each group *p<0.05). (D–G) Bar plot representing mRNA levels of TNF-α, IL-1β, and macrophage specific F4/80 marker from the WT and CD40−/− control or ethanol diet fed mice is shown. Reduced alcohol induced inflammatory and macrophage activation markers were observed in CD40−/− mice compared to control groups (n = 6 each group; *p<0.05) (D) Serum biochemical ALT analysis in control diet, ethanol diet fed WT or CD40−/− mice are depicted (n = 6 mice each group; *p<0.05).
Disruption of CD40L signaling attenuates alcohol induced liver injury in vivo
To build on the role of CD40L uncovered from our in vitro studies, we utilized CD40−/− and age-matched wild type WT mice for additional chronic-binge ethanol feeding studies. In ethanol-fed, WT mice, we found a significant increase in RNA levels of inflammation associated cytokines, TNF-α and IL-1β (Fig. 7D&E), as well as hepatic macrophage infiltration as assessed by F4/80 mRNA (Fig. 7F). Alcohol-fed CD40−/− mice were protected from ethanol-induced damage as evidenced by reduced markers of inflammation (TNF-α, IL-1β) and reduced macrophage infiltration (Fig. 7D–F). Biochemical analysis for serum ALT also showed reduced ALT levels in ethanol fed CD40−/− mice, compared to WT mice (Fig. 7G). Together these findings support the essential role of hepatocyte-derived EV associated CD40L for inducing ethanol mediated inflammation and subsequent hepatic injury.
Role of EV derived CD40L in human alcoholic hepatitis
To recapitulate the findings of elevated ethanol-induced EV in human samples, especially in patients with AH, EV were isolated from serum derived from healthy volunteers, heavy drinker controls, or from patients with alcoholic hepatitis. EV numbers (though not size) were significantly higher in the serum from alcoholic hepatitis patients compared to other groups (Fig. 8A&B). Similar results have been observed in patients with NASH compared to obese control patients (unpublished data-HM). Additional biochemical analyses were performed by pooling EV from patients with alcoholic hepatitis which revealed that CD40L was highly enriched in these EV further supporting a critical role of CD40L in human alcohol related liver injury (Fig. 8C).
Figure 8. Increased CD40L rich EV in alcoholic hepatitis patients.
(A) Quantification of EV numbers from the serum of healthy controls (n=3), heavy alcoholics (n=5) and alcoholic hepatitis patients (n=6; *p<0.05). (B) The histogram depicts size distribution of EV derived from different groups of individuals. (C) Histogram represents background subtracted pixel intensity for the cytokines and chemokine expression profile in the pooled plasma (n=5) derived EV of alcoholic patients. Abundance of CD40L (diagonal hash bar) containing EVs in plasma is observed.
DISCUSSION
Significant advances have been made in identifying mechanisms of alcohol-induced liver damage; however, many facets of this process remain undefined. In the present study, we investigated how ethanol affects the release of EV by hepatocytes and how this process leads to macrophage activation. The work identifies novel mechanistic insights by which this regulatory process occurs including 1) ethanol metabolism stimulates EV release, 2) molecular machinery associated with the caspase-3 pathway contributes to EV release, 3) CD40L (TNFSF5) resides within EV and contributes to pro-inflammatory macrophage activation, and 4) disruption of these pathways in murine models in vivo protects from ethanol induced hepatic injury. Furthermore, the link to innate immune cell activation connects hepatocyte alcohol exposure to inflammation in the process of alcoholic liver injury. Complementary human studies buttress the pathobiological relevance of our observations.
Cells have developed diverse mechanisms of paracrine communication. Emerging evidence indicates that cells can communicate with each other through EV by virtue of their cargo, such as, mRNA, miRNA, proteins, lipids, and DNA that are internalized by cells in the surrounding microenvironment [7]. Conceptually, EV have several advantages over traditional intercellular communication involving direct release of soluble proteins since the coated transport carrier can theoretically provide stability to the intraluminal contents from proteases within the blood. This process could facilitate transport of signaling proteins to a greater distance, and provide a mechanism for intracellular uptake by the target cells [7]. Indeed, increased EV are now linked with various pathobiological conditions including in liver and alcoholic-related disorders outside the liver, with different putative molecules contained within EV in various disease models [19, 32]. We have extended these concepts to cover crosstalk between ethanol-induced hepatocyte EV release to link hepatocyte stress from alcohol with innate immune cell activation.
To elucidate the mechanism associated with increased EV release in response to ethanol, we interrogated the role of intracellular caspase activation since caspases are implicated in cell injury, inflammasome activation, and for the actin remodeling that can mediate EV release [33]. While, we observed caspase activation in hepatocytes stimulated with alcohol, overt cell death was not detected. In the present studies, pharmacological administration of a caspase inhibitor or lentivirus shRNA-mediated knock down of caspase-3 abrogated EV release suggesting that early apoptotic cell injury responses may lead to EV release. However, the role of caspase-mediated actin remodeling and inflammasome signaling [33] requires further investigation. These results and implications are of particular interest given recent preclinical and human studies aimed at exploring how caspase activation contributes to alcoholic liver disease and determining if inhibition of this process confers therapeutic benefit [34–36].
Next, we used primary hepatocytes or relevant cell lines with reconstituted expression of ADH and Cyp2E1 to elucidate the relative roles of these enzyme systems [2]. The present work highlights the salient role of Cyp2E1 enzymatic activity in the process by which alcohol stimulates EV production and macrophage activation. Cyp2E1-mediated metabolism of ethanol leads to production of reactive oxygen species, which in some studies has been implicated in the injurious effects of alcohol [2]. Interestingly, ADH metabolism of ethanol was less potent in its ability to promote EV release and macrophage activation thus indirectly implicating Cyp2E1 mediated ethanol metabolism, including ROS production in the process of EV release.
Although alcoholic liver disease is histologically defined by PMN infiltrates, increasing evidence implicates the role of innate immune cell activation in this process with particular focus on how hepatocyte injury leads to Kupffer cell activation [37–39]. This inflammatory response parallels the extent of injury and fibrosis [40]. In the present study, hepatocyte-derived EV activated macrophages (both primary and cell lines) based on a number of readouts including macrophage adhesion and pro-inflammatory cytokines mRNA and protein levels. Interestingly, recent studies suggest that these responses may also be necessary for beneficial regenerative responses necessary for recovery [41].
Nonalcoholic steatohepatitis (NASH) and alcohol-induced steatohepatitis share many histopathologic features suggesting common mechanisms of liver injury. However, identifying these common mechanisms has been difficult. Our current studies implicate TR signaling in the pathogenesis of alcoholic induced liver toxicity as injury was attenuated in TR−/− animals; an observation also consistent with the salutary effects of the caspase inhibitor as TR initiates caspase-dependent proapoptotic signaling cascades. In prior studies, we have also implicated TR receptor signaling in the pathogenesis of NASH and caspase inhibitors are also beneficial in preclinical models of this disease [31, 42–44]. Taken together, these observations suggest TR receptor proapoptotic signaling cascades contribute to both forms of hepatic steatosis, suggesting a unifying mechanism contributing to liver injury in different forms of steatohepatitis.
CD40L is a member of the tumor necrosis factor (TNF) protein family, whose cognate receptor is constitutively expressed on macrophages [45]. Direct involvement of EV derived CD40L for promoting hepatic inflammation and subsequent liver injury was mechanistically validated using CD40−/− mice which showed attenuated inflammatory changes in response to ethanol. While more characterized in hematopoietic cells, epithelial-derived cells such as HepG2 upregulate expression of CD40L in response to inflammatory conditions [46] Our finding were also supported by prior high fat diet studies done with CD40−/− mice, showing reduced hepatic inflammatory cytokine expression in CD40−/− mice [47]. Previous findings showed deleterious effects of CD40L in liver injury where ligand overexpression induced severe liver cell damage [48]. These experimental findings taken together provide evidence for our hypothesis that EV associated CD40L play a critical role in ethanol induced macrophage activation, inflammation and subsequent liver injury.
In summary, the present studies highlight a new and important role for hepatocyte-derived EV in the process that links hepatocyte injury with inflammation. The mechanistic role of intracellular caspases and EV associated CD40L highlight potentially new and unrecognized actions of these molecules and identify them as potential therapeutic targets that may be amenable to modulation in alcoholic liver disease.
Supplementary Material
Acknowledgments
GRANT SUPPORT: Supported by National Institutes of Health (NIH) U01 AA 21788 and R01 AA21171 (VHS), DK97178 (HM), DK41876 (GJG), and DK P30 084567 (Clinical Core).
The authors thank Theresa J. Johnson, Usman Yaqoob, Mary Drinane and Robert C. Huebert for their contributions to this work. Authors acknowledge fellow members of TREAT Consortium for their support (D. Crabb, N. Chalasani, A. Sanyal). Authors acknowledge Bin Gao for advice on the alcohol feeding model.
Footnotes
Conflict of Interest: The authors having nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12:555–564. doi: 10.1016/j.cgh.2013.06.013. quiz e531–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391:1249–1264. doi: 10.1515/BC.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014;5:e996. doi: 10.1038/cddis.2013.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu D, Cederbaum AI. Ethanol cytotoxicity to a transfected HepG2 cell line expressing human cytochrome P4502E1. J Biol Chem. 1996;271:23914–23919. doi: 10.1074/jbc.271.39.23914. [DOI] [PubMed] [Google Scholar]
- 6.Lemoinne S, Cadoret A, Rautou PE, El Mourabit H, Ratziu V, Corpechot C, et al. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology. 2015;61:1041–1055. doi: 10.1002/hep.27318. [DOI] [PubMed] [Google Scholar]
- 7.Lemoinne S, Thabut D, Housset C, Moreau R, Valla D, Boulanger CM, et al. The emerging roles of microvesicles in liver diseases. Nat Rev Gastroenterol Hepatol. 2014;11:350–361. doi: 10.1038/nrgastro.2014.7. [DOI] [PubMed] [Google Scholar]
- 8.Clemens DL, Forman A, Jerrells TR, Sorrell MF, Tuma DJ. Relationship between acetaldehyde levels and cell survival in ethanol-metabolizing hepatoma cells. Hepatology. 2002;35:1196–1204. doi: 10.1053/jhep.2002.32668. [DOI] [PubMed] [Google Scholar]
- 9.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 11.Seo YS, Kwon JH, Yaqoob U, Yang L, De Assuncao TM, Simonetto DA, et al. HMGB1 recruits hepatic stellate cells and liver endothelial cells to sites of ethanol-induced parenchymal cell injury. Am J Physiol Gastrointest Liver Physiol. 2013;305:G838–848. doi: 10.1152/ajpgi.00151.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691–36702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine. 2011;7:780–788. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trebicka J, Racz I, Siegmund SV, Cara E, Granzow M, Schierwagen R, et al. Role of cannabinoid receptors in alcoholic hepatic injury: steatosis and fibrogenesis are increased in CB2 receptor-deficient mice and decreased in CB1 receptor knockouts. Liver Int. 2011;31:860–870. doi: 10.1111/j.1478-3231.2011.02496.x. [DOI] [PubMed] [Google Scholar]
- 15.Verma VK, Taneja V, Jaiswal A, Sharma S, Behera D, Sreenivas V, et al. Prevalence, distribution and functional significance of the -237C to T polymorphism in the IL-12Rbeta2 promoter in Indian tuberculosis patients. PLoS One. 2012;7:e34355. doi: 10.1371/journal.pone.0034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, Kim SH, et al. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol Cell Biol. 2005;25:2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–2775. [PubMed] [Google Scholar]
- 19.Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6:ra88. doi: 10.1126/scisignal.2004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee TH, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular ‘debris’. Semin Immunopathol. 2011;33:455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 22.Henzel K, Thorborg C, Hofmann M, Zimmer G, Leuschner U. Toxicity of ethanol and acetaldehyde in hepatocytes treated with ursodeoxycholic or tauroursodeoxycholic acid. Biochim Biophys Acta. 2004;1644:37–45. doi: 10.1016/j.bbamcr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Pockros PJ, Schiff ER, Shiffman ML, McHutchison JG, Gish RG, Afdhal NH, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324–329. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]
- 24.Shiffman ML, Pockros P, McHutchison JG, Schiff ER, Morris M, Burgess G. Clinical trial: the efficacy and safety of oral PF-03491390, a pancaspase inhibitor - a randomized placebo-controlled study in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2010;31:969–978. doi: 10.1111/j.1365-2036.2010.04264.x. [DOI] [PubMed] [Google Scholar]
- 25.Ratziu V, Sheikh MY, Sanyal AJ, Lim JK, Conjeevaram H, Chalasani N, et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55:419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barreyro FJ, Holod S, Finocchietto PV, Camino AM, Aquino JB, Avagnina A, et al. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2014 doi: 10.1111/liv.12570. [DOI] [PubMed] [Google Scholar]
- 27.Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci. 2005;10:3093–3099. doi: 10.2741/1765. [DOI] [PubMed] [Google Scholar]
- 28.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, et al. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci U S A. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, et al. Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345:98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idrissova L, Malhi H, Werneburg NW, LeBrasseur NK, Bronk SF, Fingas C, et al. Trail Receptor Deletion in Mice Suppresses the Inflammation of Nutrient Excess. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, et al. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol. 2013;304:H954–965. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- 34.Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34:248–253. doi: 10.1016/s0168-8278(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 35.Roychowdhury S, Chiang DJ, Mandal P, McMullen MR, Liu X, Cohen JI, et al. Inhibition of apoptosis protects mice from ethanol-mediated acceleration of early markers of CCl4 -induced fibrosis but not steatosis or inflammation. Alcohol Clin Exp Res. 2012;36:1139–1147. doi: 10.1111/j.1530-0277.2011.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maricic I, Sheng H, Marrero I, Seki E, Kisseleva T, Chaturvedi S, et al. Inhibition of type I natural killer T cells by retinoids or following sulfatide-mediated activation of type II natural killer T cells attenuates alcoholic liver disease in mice. Hepatology. 2015;61:1357–1369. doi: 10.1002/hep.27632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28(Suppl 1):77–84. doi: 10.1111/jgh.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 41.Dubuquoy L, Louvet A, Lassailly G, Truant S, Boleslawski E, Artru F, et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015 doi: 10.1136/gutjnl-2014-308410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 43.Anstee QM, Concas D, Kudo H, Levene A, Pollard J, Charlton P, et al. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J Hepatol. 2010;53:542–550. doi: 10.1016/j.jhep.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Mundt B, Wirth T, Zender L, Waltemathe M, Trautwein C, Manns MP, et al. Tumour necrosis factor related apoptosis inducing ligand (TRAIL) induces hepatic steatosis in viral hepatitis and after alcohol intake. Gut. 2005;54:1590–1596. doi: 10.1136/gut.2004.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melino M, Gadd VL, Walker GV, Skoien R, Barrie HD, Jothimani D, et al. Macrophage secretory products induce an inflammatory phenotype in hepatocytes. World J Gastroenterol. 2012;18:1732–1744. doi: 10.3748/wjg.v18.i15.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo CA, Kogan S, Amano SU, Wang M, Dagdeviren S, Friedline RH, et al. CD40 deficiency in mice exacerbates obesity-induced adipose tissue inflammation, hepatic steatosis, and insulin resistance. Am J Physiol Endocrinol Metab. 2013;304:E951–963. doi: 10.1152/ajpendo.00514.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz V, Dombrowski F, Prieto J, Qian C, Diehl L, Knolle P, et al. Induction of murine liver damage by overexpression of CD40 ligand provides an experimental model to study fulminant hepatic failure. Hepatology. 2006;44:430–439. doi: 10.1002/hep.21274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.