Abstract
Rationale
Long-term heavy cannabis users (cannabis users) who are not acutely intoxicated have diminished subconscious neural responsiveness to affective stimuli.
Objective
This study sought to determine if abnormal processing extends to the conscious evaluation of emotional stimuli.
Methods
Functional Magnetic Resonance Imaging (fMRI) was used to examine brain activity as cannabis users (N=16) and non-cannabis using controls (N=17) evaluated and categorized standardized International Affective Picture System (IAPS) stimuli. Individual judgments were used to isolate activity during the evaluation of emotional (i.e., emotional evaluation) or neutral (i.e., neutral evaluation) stimuli. Within- and between-group analyses were performed.
Results
Both groups judged the same stimuli as emotional and had activations in visual, midbrain, and middle cingulate cortices during emotional evaluation, relative to neutral. Within-group analyses also revealed amygdalar and inferior frontal gyrus activations in controls, but not cannabis users, and medial prefrontal cortex (mPFC) deactivations in cannabis users, but not controls, during emotional evaluation, relative to neutral. Between-group comparisons found that mPFC activity during positive and negative evaluation was significantly hypoactive in cannabis users, relative to controls.
Conclusions
Abnormal neural processing of affective content extends to the level of consciousness in cannabis users. The hypoactive mPFC responses observed resembles the attenuated mPFC responses found during increased non-affective cognitive load in prior research. These findings suggest that abnormal mPFC singling in cannabis users during emotional evaluation might be associated with increased non-affective cognitive load.
Keywords: Human, Cannabis, Emotion, fMRI, medial PFC
Introduction
With increasing legalization and social acceptance of cannabis use, mental health issues related to cannabis use are a growing public health concern (Hall 2009; Hall and Degenhardt 2009; Johnston et al. 2009; Copeland et al. 2013; Johnston et al. 2015; SAMHSA 2014; Hall 2015). There is mounting evidence that cannabis use is associated with dysfunction in affective processes of the brain (i.e., signals that may contribute to emotional experience), which is of interest because of the important role that emotions play in various mental health conditions that impact the welfare of individuals and society. Whether dysfunction in affective processes is a trait or temporary state predisposing individuals to cannabis abuse, is a neurobiological consequence of habitual use, is related to circulating cannabinoids and metabolites that remain in the body after the acute intoxicating effects are no longer detectable (Ellis et al. 1985), or some combination of these and other factors, is unknown and difficult to establish. Nonetheless, data support a link between the cannabinoid system and affective processing, as highlighted below.
Cannabinoid receptor density is highly concentrated in brain areas such as the amygdala, a region well known for its role in emotion (Phelps and LeDoux 2005; Phelps 2006; Olsson et al. 2007; Olsson and Phelps 2007), as well as the medial prefrontal cortex (mPFC), a region where the integration of affective and non-affective neural signals is hypothesized to contribute to normal decision-making (Bechara et al. 2000; Bechara et al. 2001; Simpson et al. 2001a). Consistent with those data, laboratory measures of affective processes are modulated by endogenous cannabinoids and the exogenous phytocannabinoids in cannabis (e.g., Mechoulam and Parker 2013; Pertwee 2008). For example, cannabinoid agonists injected directly into the rodent amygdala prevented the reconsolidation of fear memories (Lin et al. 2006) or extinguished them altogether (Marsicano et al. 2002). These results are consistent with human studies that demonstrated acute administration of Δ9-tetrahydrocannabinol (THC), the primary active compound in cannabis, decreased amygdala reactivity to social signals of threat. For example, Phan et al. (2008) observed that oral THC administered to recreational cannabis users dose-dependently decreased the functional Magnetic Resonance Imaging (fMRI) blood oxygen level dependent (BOLD) signal in the amygdala, but not visual or motor cortices, in response to angry faces. Together, these data demonstrate the ability of cannabinoid agonists to directly impact central mechanisms thought to underlie emotions and showcase the involvement of the endogenous cannabinoid system in affect-related neural processes.
Subsequent investigations have addressed whether affective processes are altered in long-term heavy cannabis users who were not currently experiencing psychoactive cannabis effects. To test this possibility, Gruber et al. (2009) used fMRI to examine subconscious reactivity to masked emotional faces following at least twelve hours of abstinence. Testing at that time avoided acute cannabis intoxication as well as symptom of cannabis withdrawal, which can include changes in affective states (Haney 2005; Budney and Hughes 2006). That time point is clinically relevant because it models next-day working hours after nightly cannabis use. They found that the subconscious presentation of positive and negative stimuli resulted in diminished amygdala and middle cingulate cortex reactivity in cannabis users (Gruber et al. 2009).
The results from the study by Gruber and colleagues have intriguing individual and social implications. As they relate to individuals, these findings suggest that the subconscious neural mechanisms that evolved to quickly detect threats and ensure survival are operating at sub-optimal levels in cannabis users even when these individuals are not intoxicated. From a cannabis-use disorder management perspective, impaired affective processing could feed-forward into continued cannabis use, thereby impeding abstinence attempts in cannabis-dependent individuals seeking treatment (Bonn-Miller et al. 2008). From a social perspective, these data are suggestive of potential problems in jobs that rely on the integration of affective information into ongoing decision-making processing for optimal performance, as well as person-to-person interactions that may rely on concordant affective responsiveness for shared experience and effective communication. What remains unclear, however, is whether affective neural processes remain abnormal in this group as emotional stimuli emerge to the level of conscious awareness. Additionally, it is unknown whether or not perceptual judgments of emotional stimuli are abnormal at this time (i.e., after intoxication but prior to the onset of withdrawal). As with the subconscious processing of emotional stimuli, the conscious evaluation and identification of emotional content has important individual and social implications.
With this in mind, the present study sought to identify whether or not affect-related neural function was altered in recently abstained long-term heavy cannabis users during the evaluation of stimuli consciously judged as emotional. Brain activity and behavior was recorded as individuals evaluated and categorized visual stimuli as emotional or neutral. Brain activity during emotional evaluation was retroactively isolated based on individual judgments. We hypothesized that cannabis users would exhibit diminished neurofunctional responsiveness during conscious emotional evaluation in brain areas involved in affective reactivity (e.g., amygdala) as well as affective and non-affective signal integration during decision-making (e.g., medial prefrontal cortices). Additionally, based on previous data demonstrating impaired emotional perception in a similar experimental group (e.g., Hindocha et al. 2014), we hypothesized that cannabis users would judge fewer stimuli as emotional, relative to noncannabis using controls.
Methods
Participants
Seventeen long-term heavy cannabis users (cannabis users) and 16 non-cannabis using controls (controls) were included (see Table 1). Participants were recruited through local media or flyers and contacted the laboratory by phone. Following an initial phone screen, participants were invited into the laboratory for additional screening and to obtain informed consent. Consent was obtained according to the Declaration of Helsinki, and the Wake Forest School of Medicine Institutional Review Board approved all screening and study procedures. Participants provided urine samples to test for pregnancy and drug use and were administered the Structured Clinical Interview for DSM-IV (First 1997) as well as the Wechsler Abbreviated Scale of Intelligence (Wechsler 1999). Consistent with previous methods (Wesley et al. 2011), participants were required to be right handed, and were excluded if they presented with current or lifetime systemic diseases of the central nervous system, head trauma, neurological disorders, psychosis, mania and / or current drug dependence on substances other than cannabis in cannabis users or nicotine in both groups, or an I.Q. of less than 80. No participants met diagnostic criteria for current or previous alcohol abuse or dependence. Participants were excluded if they reported more than five lifetime episodes of using any category of illicit drugs except for cannabis in cannabis users. Long-term and heavy cannabis use was considered use occurring greater than 5 years at rates more than 2 times a day and 20 days out of a month. This criterion was chosen to screen out recreational cannabis users and to yield demographics consistent with previous studies (Gruber et al. 2009). Participants were required to test negative for the recent use of illicit drugs, except for cannabis in cannabis users. Participants who met inclusion criteria were scheduled for a second laboratory visit to complete the experimental procedures.
Table 1.
Group demographics for non-cannabis using controls (controls) and long-term heavy cannabis users (cannabis users).
| Variable | Controls (N = 16) |
Cannabis Users (N = 17) |
||
|---|---|---|---|---|
| Mean (± SD) | Mean (± SD) | t/X2 Value |
p | |
| Age (years) | 27.1 (6.3) | 25.1 (3.1) | 1.17 | n.s. |
| Full I.Q. | 113.81 (7.4) | 105.59 (15.1) | 1.96 | n.s. |
| Sex | 1.59 | n.s. | ||
| Male | 5 | 9 | ||
| Female | 11 | 8 | ||
| Cigarette Smokers | 12.5 % | 52.9% | 6.07 | 0.03 |
| Caffeine (mg/day) | 103.1 (72.1) | 108.7 (69.5) | 0.98 | n.s. |
| Alcohol AUDIT Score | 3.3 (2.0) | 4.8 (2.7) | 1.76 | n.s. |
| Spielberger State Anxiety | 27.0 (10.0) | 26.9 (6.3) | 0.56 | n.s. |
| Beck’s Depression | 2.5 (3.2) | 4.0 (2.9) | 1.22 | n.s. |
| Cannabis Use: | ||||
| Age of onset (years) | 14.9 (2.0) | |||
| Years of Total Use | 10.2 (3.3) | |||
| Days per month | 29.3 (1.4) | |||
| Times per day | 4.3 (4.4) | |||
| Years at current use level | 5.6 (3.3) | |||
| Hours since last use | 13.0 (1.7) |
Procedure
Cannabis users were asked to abstain from cannabis use starting at midnight before the experimental session. Consistent with previous studies, participants were reminded repeatedly about the importance this abstinence period. Upon arrival for the experimental session, participants once again provided urine samples to test for recent drug use and pregnancy. Cannabis users verbally confirmed abstinence from cannabis. In addition, trained staff monitored for common signs of recent cannabis use, including cannabis odor, bloodshot eyes and impaired perception and motor skills; no evidence of recent cannabis use was found. Because cannabis withdrawal was not anticipated after this limited period of abstinence, a formal assessment was not conducted. However, participants were asked an open-ended question about how they were feeling upon arrival, throughout the experimental session, and before leaving the laboratory; no symptoms of cannabis withdrawal were reported (Budney and Hughes 2006). Participants also completed depression (Beck’s Depression Inventory; Beck et al. 1996) and anxiety (Spielberger Test of Anxiety; Spielberger 1989) inventories at the onset of the experimental session; these instruments also did not reveal evidence of cannabis withdrawal.
Participants performed a shortened training version of the task approximately one hour before entering the scanner. The layout and timing of the training task was identical to the scanner task but used a smaller number of unique visual stimuli (i.e. 10). Training occurred on a standard laptop computer and button box. Approximately 30 minutes before entering the scanner, participants were given a 15-minute break allowing participants the chance to smoke a nicotine cigarette. This was included to help avoid confounds of nicotine withdrawal on brain activity and task performance (Wang et al. 2007; Xu et al. 2007). Three participants smoked a single cigarette.
Modified International Affective Picture System (IAPS) Task
fMRI scanner goggles and a button box placed under the right hand were used to evaluate task stimuli and record categorical judgments. The modified IAPS scanner task consisted of 100 photographic stimuli. IAPS stimuli have been standardized based on scores ranging from 1 to 9 on different dimensions, including valance (pleasant/unpleasant) and arousal (calm/excited) (Lang et al. 2005). For this study, 80 emotional stimuli were chosen based on valence score cutoffs (40 positive = valence > 6; 40 negative = valence < 4). For neuroimaging control purposes, 20 neutral photographs were also included (valence 4 – 6). For both positive and negative stimuli, arousal values ranged from 3 to 7. The task did not include stimuli with arousal values greater than 7, because sex differences have been observed at higher arousal levels (Rupp and Wallen 2008). Neutral control stimuli had arousal scores less than 3. Arousal scores did not significantly differ between positive and negative stimuli (p=0.45), and stimuli were balanced so that each valence category contained an equivalent amount of inanimate objects and people. Before the scanner task, participants visually followed along as task instructions were read aloud and verbally acknowledged task comprehension and readiness to perform the task.
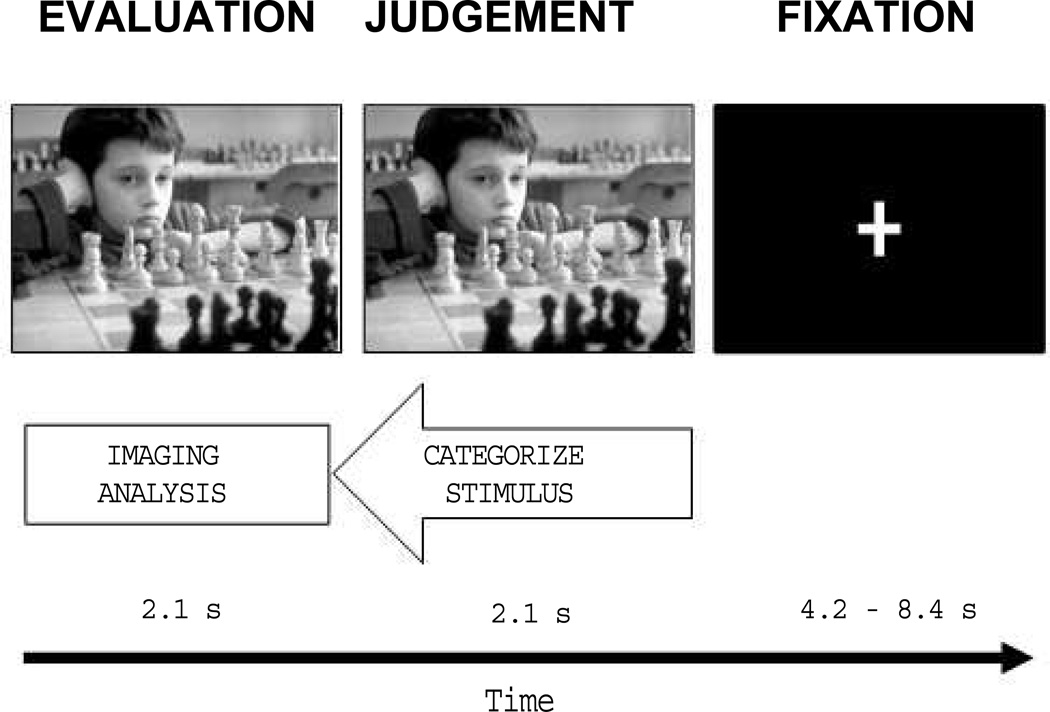
The scanner task consisted of two functional blocks each starting with a 20-second countdown period and each lasting 8.75 min. Each block contained 50 trials in which participants evaluated and categorized pseudorandomized stimuli (Figure 1). First, a stimulus appeared alone on the screen for 2.1 s. During this time participants consciously evaluated its emotional content. Next, the letters POS, NEU, and NEG appeared below the image for 2.1 s as a signal for participants to record their judgment. Stimuli were categorized as positive, neutral, or negative by pressing buttons 1, 2 or 3 on the button box, respectively. A jittered fixation screen lasting between 4.2 and 8.4 s separated stimulus presentations. On average, a trial lasted for 10.5 s.
Figure 1.
An example trial from the modified International Affective Picture System Task (IAPS). First, an IAPS image appeared alone on the task screen and participants consciously evaluated its emotional content. Next, category labels appeared below the image to allow participants to log their emotional judgment. A jittered fixation screen was then presented and lasted until the onset of the next trial. Responses made during the judgment phase were used to retroactively isolate brain activity during the evaluation of stimuli considered to be emotional.
Structural and Functional MRI data acquisition
Images were acquired on a 1.5T GE scanner with a standard quadrature head coil and an advanced nuclear magnetic resonance echoplanar system. The head was positioned along the canthomeatal line and foam inserts limited head motion. High-resolution T1-weighted anatomical images (3D SPGR, TR=10 ms, TE=3 ms, voxel dimensions 1.0×1.0×1.5 mm, 256×256 voxels, 124 slices) were acquired for coregistration and normalization of functional data. Functional data were 250 co-planar images acquired using a gradient echoplanar sequence (TR=2100 ms, TE=40 ms, voxel dimensions 3.75×3.75×5.0 mm, 64×64 voxels, 28 slices). Two radio frequency excitations were performed prior to image acquisition to achieve steady-state transverse relaxation. The scanning planes were oriented parallel to the anterior and posterior commissure line and extended from the superior extent of motor cortex to the base of the cerebellum. Functional volumes acquired during countdown periods were discarded to allow equilibrium.
Statistical Analyses
Demographics and Behavior
Chi-square and independent-samples t-tests tested for differences between groups in categorical and parametric variables, respectively. A 2×2 analysis of variance (ANOVA) was performed with between-subjects group factor (controls or cannabis users) and between-stimulus judgment type (POS or NEG), with Bonferroni post-hoc corrections, to determine if groups differed in the number of stimuli judged as emotional. Due to the fewer number of neutral control stimuli included, it was statistically inappropriate to compare this category to the emotional categories. Instead, these data were analyzed with an independent samples t-test.
Functional MRI preprocessing and data analysis
For each participant, functional data were corrected for acquisition time (slice timing), realigned to the first volume (motion correction), normalized to standardized anatomical space (MNI space), smoothed with an 8 mm Gaussian kernel, and high-pass filtered (128s) to remove low frequency noise. For each participant, an event-related fixed-effects general linear model (Friston et al. 1998) was created to account for all task-relevant events, including screen onset times (evaluation, judgment, or fixation), button press times, and head movement parameters obtained from motion correction. The onset times for evaluation screens were entered as explanatory variables according to each individual’s future categorical judgment that the stimulus was POS, NEU, or NEG. Events were convolved with the hemodynamic response function (HRF). Evaluation events were modeled by a boxcar function lasting the duration of the evaluation screen with all other events modeled as instantaneous delta functions. This design was chosen to allow a 2.1 s temporal window to capture variance associated with the conscious processing of visual stimuli during evaluation, as this event could not be tied to a specific millisecond time point. From fixed-effects models, whole-brain contrasts for each participant isolated activity maps for all emotional evaluation (POS + NEG > NEU) as well as separately for positive and negative emotional evaluation (POS > NEU; NEG > NEU). Resultant maps were entered into a series of second-level whole-brain random-effects analyses comparing emotional evaluation between the controls and cannabis users.
Second-level models included the number of cigarettes an individual smoked per week as a nuisance variable. Within-group analyses utilized voxel-wise one-sample t-tests, whereas between-group analyses utilized voxel-wise independent-samples t-tests. Analyses were performed using voxel-level thresholds of p<.001 with extent thresholds of 25 contiguous voxels and FWE small volume correction (SVC) performed at the cluster-level (p<.05). Functional clusters emerging from between-group analyses were examined further in a region of interest (ROI) analysis. Standard percent signal change calculations were performed for each ROI according to each valence event type using the MarsBaR signal processing toolbox for MATLAB (citations below). MarsBaR uses the HRF and temporal derivative (TD) to calculate the percent signal change from the onset an event of interest, specified here as the 2.1 s evaluation periods for stimuli judged as POS or NEG, to the peak responses for those events. Thus positive and negative percent signal changes reflect increases and decreases in neural activity while evaluating a specific valence type. Percent signal changes were compared with 2×2 ANOVAs with between-subjects group factor (controls or cannabis users) and between-stimulus judgment type (POS or NEG). Bonferroni post-hoc analyses were utilized. Percent signal changes during the evaluation of neutral control stimuli (NEU) were also calculated for display purposes. These events were not included in the ROI analysis, however, as they were previously accounted for in the whole-brain comparisons that yielded functional ROIs.
Imaging analyses were conducted with the Statistical Parametric Mapping software (Wellcome Department of Imaging Neuroscience, London, UK) and locally developed scripts written for and implemented in MATLAB 7.0 (Mathworks, Natick, MA). Time course extractions and percent change calculations were performed with MarsBaR software (Brett et al. 2002). Functional clusters were identified using the wfu_pickatlas software package (Maldjian et al. 2003; Maldjian et al. 2004) and confirmed with Talairach Daemon software (see http://www.talairach.org/), following conversion to Talairach standard space with the mni2tal script for MATLAB. Three-dimensional coordinates presented here correspond to MNI space.
Results
Demographics
Demographics are displayed in Table 1. Controls were 27.1 ± 6.3 years old (mean ± sd). Four controls reported previous experience with cannabis, with use occurring fewer than 50 times and more than 2 years prior to the study. Cannabis users were 25.1 ± 3.1 years old and reported using cannabis 4.3 ± 4.4 times a day, 29.3 ± 1.4 days a month, for 10.2 ± 3.3 years. The average age of first cannabis use was 14.9 ± 2.0 years. All participants tested negative for illicit substances, other than cannabis in cannabis users. All cannabis users tested positive for cannabis metabolites and reported 13.0 ± 1.7 h since last cannabis use. Cigarette smoking significantly differed between groups, with more smokers being cannabis users (52.9%) than controls (12.5%). Consequently, the number of cigarettes smoked per week was entered as nuisance variables in neuroimaging analyses. Groups did not differ in depression or anxiety levels.
IAPS Task Performance
Controls categorized a mean of 70.7 stimuli as emotional while the cannabis users categorized a mean of 69.2 stimuli as emotional. Groups did not differ in the mean (±se) number of stimuli placed into positive or negative valence categories (F(1,24) = 0.276, p = 0.604; [controls: POS = 36.70 ±1.9 and NEG = 34.00 ±1.8]; [cannabis users: POS = 35.47±1.4 and NEG = 33.73±1.0]). Groups also did not differ in the number of control stimuli categorized as neutral (t(31) = 3.59, p = 0.65; controls = 28.00 ± 2.1 and cannabis users = 29.80 ± 1.9).
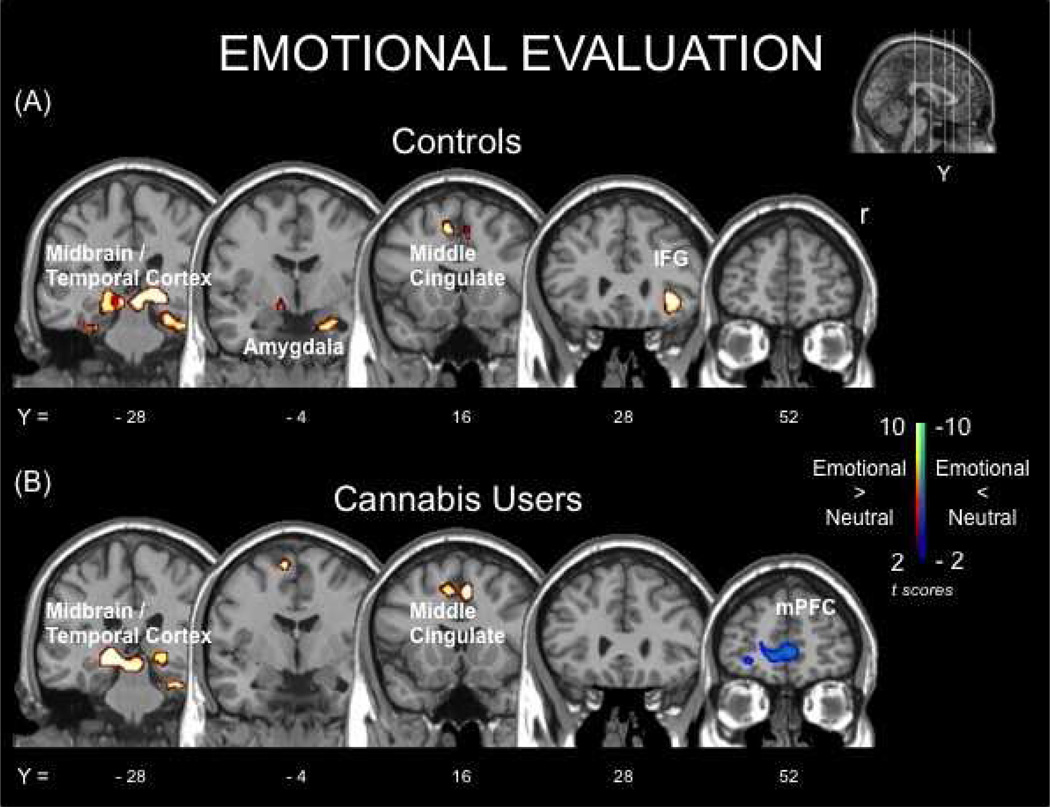
Brain Activity During the Evaluation of Emotional Stimuli
Brain activity associated emotional evaluation is displayed in Figure 2 and Table 2. During emotional evaluation, compared to neutral, both groups displayed increased activity in portions of the thalamus and medial temporal cortex, the middle cingulate gyrus, and portions of secondary visual cortex. Additionally, controls had increased activity in right inferior frontal gyrus and amygdala during emotional evaluation, compared to neutral. Cannabis users, on the other hand, had decreased activity in the medial prefrontal cortex (mPFC) during emotional evaluation, compared to neutral. Of the local maxima within the mPFC cluster, two were spatially coincident with the anterior cingulate cortex (ACC) and one with the ventral medial prefrontal cortex (vmPFC). There were no areas where controls displayed decreased activity during emotional evaluation, compared to neutral.
Figure 2.
Functional brain responses for emotional evaluation, compared to neutral, within groups: (A) non-cannabis using controls (controls) and (B) recently abstained long-term heavy cannabis users (cannabis users). Positive contrasts (hot; red) are clusters where activity was greater for emotional stimuli, compared to neutral. Negative contrasts (cold; blue) are clusters where activity was less for emotional stimuli, compared to neutral.
Table 2.
Clusters of significant BOLD activity during the evaluation of stimuli judged as emotional. Analyses correspond to within-group (Figure 2) and between-group (Figure 3) results for non-cannabis using controls (Controls) and recently abstained long-term heavy cannabis users (Cannabis Users).
| Analysis | H | Anatomical Regions | BA* | MNI Coordinates+ (x, y, z) |
Max z-value |
Ks* | |||
|---|---|---|---|---|---|---|---|---|---|
| Controls Within-group | |||||||||
| Emotional > Neutral | L,R | Occipital Lobe | 17 (18, 19) | −8 | −86 | −2 | 6.91 | 846 | |
| R | Midbrain (amygdala) | (27, 30) | 20 | −28 | −4 | 6.55 | 613 | ||
| R | Inferior Frontal Gyrus (alnsula) |
(47) | 36 | 28 | −4 | 6.13 | 161 | ||
| L,R | Middle Cingulate Cortex | 32 (6) | −8 | 8 | 52 | 5.99 | 263 | ||
| Emotional < Neutral | N/A | no suprathreshold cluster | |||||||
| Cannabis Users Within-group | |||||||||
| Emotional > Neutral | L,R | Occipital Lobe | 17 (18, 19) | −26 | −76 | −10 | 7.16 | 814 | |
| L | Midbrain | (27, 30) | −16 | −30 | −4 | 6.96 | 263 | ||
| L,R | Middle Cingulate Cortex | 32 (6) | −10 | −4 | 66 | 5.73 | 257 | ||
| Emotional < Neutral | L | Medial Prefrontal Cortex (ACC; vmPFC) |
10 (11) | −10 | 52 | −4 | 4.72 | 374 | |
| Emotional Evaluation Between-group | |||||||||
| Cannabis Users > Controls | N/A | no suprathreshold cluster | |||||||
| Cannabis Users < Controls | R,L | Medial Prefrontal Cortex (ACC; vmPFC |
24 (10, 11, 32) | 4 | 36 | 0 | 3.57 | 873 | |
BA, Brodmann areas. Ks, Cluster size. Listed areas correspond to location of the maximum voxel of activation and other locations associated with the activity cluster are in parentheses, H, Hemisphere. +MNI, Montreal Neurological Institute. Regions and coordinates correspond to the maximum voxel of activity within the cluster. Additional Brodmann areas associated with the activity cluster are listed in parentheses. ACC, anterior cingulate cortex. vmPFC, ventral medial prefrontal cortex
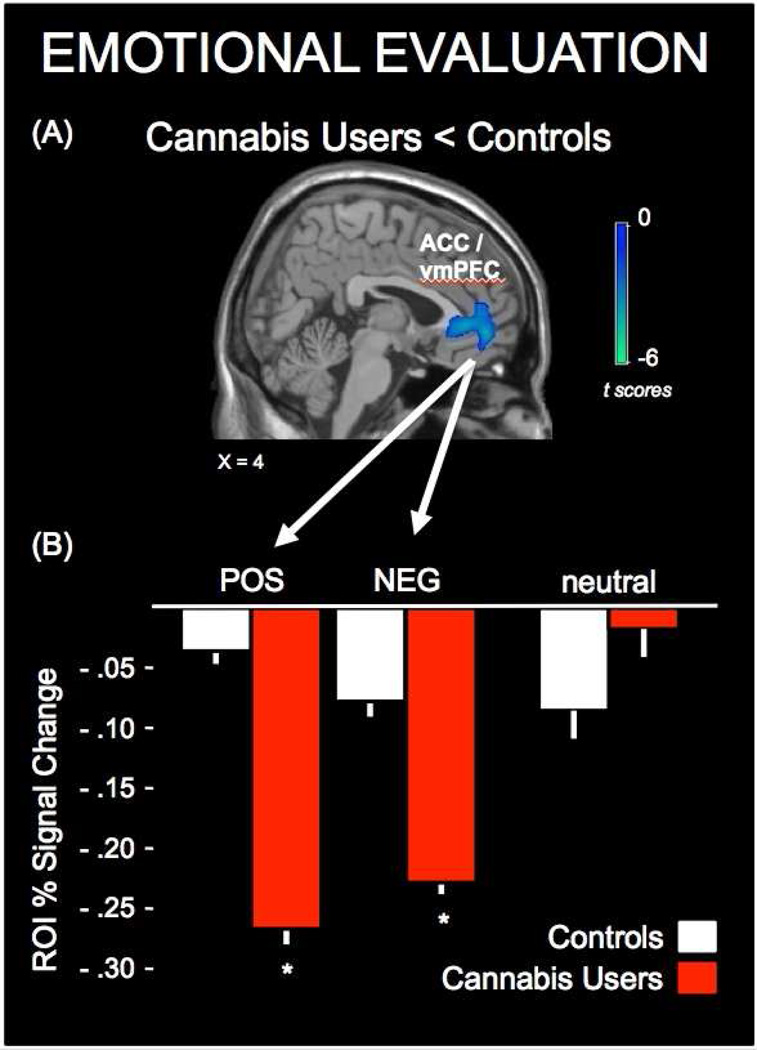
Between group results are presented in Figure 3 and Table 2. This analysis revealed that during emotional evaluation, activity in the mPFC was significantly less in cannabis users, compared to controls. Of the local maxima within this cluster, three were spatially coincident with the ACC and two with the vmPFC. There were no areas where cannabis users had greater activity than controls. Whole-brain analyses examining within-group and between-group activity for positive and negative stimuli independently did not yield significant cluster-corrected results. However, a significant group (controls, cannabis users) by valence (positive, negative) interaction (F(3,31) = 4.29, p = 0.047) was observed in response magnitudes extracted from the mPFC functional ROI (Figure 3B). Post hoc analysis demonstrated that percent signal changes to both positive and negative valence types in cannabis users were significantly hypoactive, relative to that of controls (positive: t(31) = 3.59, p = 0.001; negative t(31) = 2.59, p = 0.01). The mean (± se) percent signal change in controls during positive emotional evaluation was −0.04 ± 0.04, compared to −0.26 ± 0.05 in cannabis users. Whereas the mean percent signal change in controls during negative emotional evaluation was −0.08 ± 0.05, compared to −0.23 ± 0.03 in cannabis users.
Figure 3.
Between-group comparison of brain responses in non-cannabis using controls (controls) and recently abstained long-term heavy cannabis users (cannabis users) during emotional evaluation. (A) Functional cluster where cannabis users had significantly less activity than controls for the evaluation of stimuli judged as emotional. (B) Functional responses magnitudes demonstrating that responses in the medial prefrontal cortex (mPFC) region of interest (ROI) were hypoactive in cannabis users for both positive (POS) or negative (NEG) valence types, compared to controls. Values for neutral control stimuli are presented for visual comparison. * p<.05.
Discussion
The current study examined conscious emotional evaluation in recently abstained long-term heavy cannabis users (i.e., cannabis users) and controls. Counter to one hypothesis, cannabis users did not judge fewer stimuli as emotional, compared to controls, which is inconsistent with data from previous studies demonstrating deficits in the ability of cannabis users to identify emotional faces (Platt et al. 2010; Hindocha et al. 2014). This negative result may relate to experimental design in the current study. Unlike previous studies specifically examining reactivity to social signals of threat (i.e., emotional facial expressions), the current study used generalized emotional stimuli not limited to faces. An additional possibility is that the simple evaluation and judgment scheme used in the current study was not sufficiently sensitive to capture behavioral variance reflective of the neurofunctional differences observed. Future studies should attempt to isolate behavioral manifestations of the neurofunctional abnormalities observed in the current study.
During emotional evaluation, activity in the visual cortex, midbrain, thalamus and middle cingulate cortex of cannabis users mirrored that of controls. This is consistent with previous observations demonstrating that emotional stimuli increased activity in these brain areas in healthy controls (Mathews et al. 2003; Phelps et al. 2006; Todd et al. 2012). Thus, not all activity patterns in cannabis users appear to be abnormal during conscious emotional evaluation. The increased middle cingulate cortex activity in cannabis users is of particular interest because activity in this area was significantly diminished for emotional stimuli presented below the level of consciousness (Gruber et al. 2009). The current data suggest that middle cingulate reactivity to emotional stimuli may return to normal levels during conscious emotional evaluation in cannabis users. Taken together, the within-group results suggest that many affect-related processes appear normal in long-term heavy cannabis users who are not intoxicated during conscious emotional evaluation.
The main result of the current study was that cannabis users displayed significant hypoactive medial prefrontal cortex (mPFC) activity during emotional evaluation, compared to controls. Hypoactive responses were in portions of the anterior cingulate cortex (ACC) and ventral medial prefrontal cortex (vmPFC) while evaluating both positive and negative stimuli. The mPFC is known as an interface for affective and non-affective cognitive processing streams in the brain (Bechara et al. 2000; Bechara et al. 2001; Chambers et al. 2006; Johnson et al. 2008; Brosch et al. 2013). Through connections with the dorsal lateral prefrontal cortex (DLPFC), the mPFC supports executive functions by regulating processes considered to be non-affective and more rational in nature, including the attention and working memory demands of the current task. The mPFC also shares reciprocal connections with the anterior insula, ventral striatum and amygdala, where more somatic and limbic-based affective signals arise (Drevets et al. 2008; Price and Drevets 2010). A large body of neurofunctional data has demonstrated that mPFC function is decreased in healthy controls during the performance of cognitive tasks requiring increased attention (Simpson et al. 2001a,b; see Shulman et al. 1997 for review). However, when affective content is integrated into ongoing cognition, mPFC function is increased, which could result in less hypoactivity (Simpson et al. 2001a,b). Together, these results suggest that cannabis users experience increased cognitive load, including attentional demand, during emotional evaluation, which might interfere with normal affective signaling in the mPFC.
Abnormal affective and non-affective signal integration in the mPFC might explain previous neurofunctional and behavioral abnormalities observed in cannabis users. For example, a previous study found that learning on the Iowa Gambling Task (IGT) was impaired in long-term heavy cannabis users, relative to controls (Wesley et al. 2011). The IGT is a complex decision-making task with high cognitive demand that relies on mPFC function for optimal performance (Bechara et al. 2000). Relative to controls, cannabis users performing the IGT had significantly less mPFC activity while evaluating positive and negative affective feedback on the task. Furthermore, in controls, but not cannabis users, greater mPFC function while evaluating affective feedback predicted greater learning on the task (Wesley et al. 2011). Impaired affective mPFC signal integration may also help explain other reports of impaired learning and mental flexibility in cannabis users (Lundqvist 2005; Kedzior and Martin-Iverson 2006; Solowij and Battisti 2008; Scholes and Martin-Iverson 2009; Battisti et al. 2010a; Battisti et al. 2010b; Broyd et al. 2013).
An alternative interpretation of the current results is that cannabis users and controls have differential deactivation of default mode-related brain activity during emotional and neutral evaluation. Although this is a possibility, especially in light of the known role of the mPFC in default mode functionality, it is unlikely given the current task design. That is, default mode activity is typically measured during, and largely characterized by, the absence of external task demands (Raichle 2015). In the present study, external demands were present throughout the entirety of the experimental task. Sustained attention, working memory and ongoing behavioral output were required while evaluating and categorizing all stimulus valence types. Therefore, future studies utilizing different experimental designs are needed to directly address if and/or how abnormal mPFC function in cannabis users may relate to default mode processes.
How the current results might relate to emotional experiences and social functioning in cannabis users is unknown but important to consider. Clearly, long-term heavy cannabis users are not devoid of emotion and the anxiolytic effects of cannabis, such as a dampening of affective processing under stress, could be medicinally beneficial. Thus, whether abnormal mPFC function can be considered detrimental or beneficial to broader emotional experiences may be context dependent. Nonetheless, given the growing public health concern associated with cannabis use there is an increasing need to understand how cannabis-related abnormalities in affective processing may impact interpersonal and context-dependent social experiences. Indeed, mPFC activity has been positively linked to processing social emotions (Van Overwalle 2009, Etkin et al. 2011) and regulating one’s own emotions (Urry et al. 2009), and patients with mPFC damage have difficulty interpreting social and emotional cues (Hornak et al. 1996; Mah et al. 2004; Heberlein et al. 2008). Related to the current results, increased cognitive load in healthy controls both reduced mPFC activity and the subjective experience of empathy (Morelli and Lieberman 2013). In terms of clinical relevance, individuals seeking treatment for cannabis use disorder had decreased therapeutic alliance and emotional bonding, as reported by both patient and therapist, compared to individuals seeking treatment for alcohol or other drugs (Healey et al. 2013). Furthermore, greater cannabis use severity was associated with less emotional bonding (Healey et al. 2013). Worth noting is that affect regulation training appears to be a promising adjunct for improving treatment outcomes in those individuals for whom emotional dysregulation is identified as a barrier to substance dependence management (Stasiewicz et al. 2013). Based on the current results, future research might focus on understanding how mPFC function in cannabis users relates to emotion-related treatment outcomes in effort to enhance treatment strategies for cannabis use disorder.
Some limitations should be considered when interpreting the results from the current study. First, an uneven distribution of cigarette smokers was enrolled across the groups, although steps were taken to ensure that this did not confound the results. Specifically, participants were permitted to smoke a single tobacco cigarette prior to image data collection and cigarette use was included as a nuisance variable in imaging analyses. Also, an analysis of cigarette smokers (n = 9) and non-smokers (n = 8) within cannabis users revealed no difference in behavioral performance or brain activity. Second, this study used generalized emotional stimuli from a standardized database to probe emotional evaluation. Whether the observed effects would be amplified or diminished using more personalized emotional content, which might be more clinically relevant, is unknown (Cerqueira et al. 2008). Third, whether or not cannabis use contributed to the abnormal neurofunctional response during judgment of emotional content, or was a predisposing factor to use, cannot be determined from the present study design. For example, there were no significant correlations between the cannabis use variables presented in Table 2 and neurofunctional response to IAPS stimuli in cannabis users, which might have been expected if the neurofunctional abnormalities were a direct consequence of cannabis use. Fourth, abstinence was confirmed verbally and visually, but not biochemically, although important to note is that the present methods are consistent with other studies (e.g., Gruber et al. 2009). Further, objective verification of abstinence would have required semi-quantitative urinalysis, which would have added cost and complexity to the study design. Lastly, there were no group differences on the limited psychiatric assessments that were collected (i.e., depression, anxiety) or behavioral task performance, so interpretations of the clinical significance of the present results should be approached carefully.
In conclusion, the present study found that long-term heavy cannabis users who were not experiencing the acute psychoactive effects of cannabis or cannabis withdrawal symptoms displayed abnormal neural activity during the conscious evaluation of emotional stimuli. Compared to controls, cannabis users displayed significant hypoactive responses in the medial prefrontal cortex, which is known to balance and/or integrate affective and non-affective signals during cognition. We propose that this may reflect increases in cognitive load as cannabis users evaluate emotional stimuli and may represent a shift in mPFC function in favor of non-affective signal processing of affective content. This possibility may help explain reported abnormalities executive functioning abilities and emotion-related treatment outcomes in cannabis users and should be studied further.
Acknowledgments
This work was supported by the National Institute of Drug Abuse grants DA007246 (MJW), DA031766 (JAL), DA020074 (LJP) and DA06634 (LJP).
Footnotes
The authors have no conflicts of interest to report.
References
- Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology. 2010a;212:613–624. doi: 10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- Battisti RA, Roodenrys S, Johnstone SJ, Respondek C, Hermens DF, Solowij N. Chronic use of cannabis and poor neural efficiency in verbal memory ability. Psychopharmacology. 2010b;209:319–330. doi: 10.1007/s00213-010-1800-4. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bidwell LC, Metrik J, McGeary J, Palmer RH, Francazio S, Knopik VS. Impulsivity, variation in the cannabinoid receptor (CNR1) and fatty acid amide hydrolase (FAAH) genes, and marijuana-related problems. J Stud Alcohol Drugs. 2013;74:867–878. doi: 10.15288/jsad.2013.74.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Vujanovic AA, Zvolensky MJ. Emotional dysregulation: association with coping-oriented marijuana use motives among current marijuana users. Subst Use Misuse. 2008;43:1653–1665. doi: 10.1080/10826080802241292. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox [abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain. Available on CD-ROM in NeuroImage. 2002;16(2) [Google Scholar]
- Brosch T, Scherer KR, Grandjean D, Sander D. The impact of emotion on perception, attention, memory, and decision-making. Swiss Med Wkly. 2013;143:w13786. doi: 10.4414/smw.2013.13786. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Greenwood LM, Croft RJ, Dalecki A, Todd J, Michie PT, Johnstone SJ, Solowij N. Chronic effects of cannabis on sensory gating. Int J Psychophysiol. 2013;89:381–389. doi: 10.1016/j.ijpsycho.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry. 2006;19:233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Cerqueira CT, Almeida JR, Gorenstein C, Gentil V, Leite CC, Sato JR, Amaro E, Jr, Busatto GF. Engagement of multifocal neural circuits during recall of autobiographical happy events. Braz J Med Biol Res. 2008;41:1076–1085. doi: 10.1590/s0100-879x2008001200006. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, Morris AP, Mattingley JB. Executive "brake failure" following deactivation of human frontal lobe. J Cogn Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Copeland J, Rooke S, Swift W. Changes in cannabis use among young people: impact on mental health. Current OpinPsychiatry. 2013;26:325–329. doi: 10.1097/YCO.0b013e328361eae5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis GM, Jr, Mann MA, Judson BA, Schramm NT, Tashchian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin Pharmacol Ther. 1985;38:572–578. doi: 10.1038/clpt.1985.226. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. Users Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinical Version. American Psychiatric Publishing, Inc; 1997. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend. 2009;105:139–153. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. The adverse health effects of cannabis use: What are they, and what are their implications for policy? Int J Drug Policy. 2009;20:458–466. doi: 10.1016/j.drugpo.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110:19–35. doi: 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- Haney M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr Psychiatry Rep. 2005;7:360–366. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Healey A, Kay-Lambkin F, Bowman J, Childs S. Avoiding emotional bonds: an examination of the dimensions of therapeutic alliance among cannabis users. Front Psychiatry. 2013;4:70. doi: 10.3389/fpsyt.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein AS, Padon AA, Gillihan SJ, Farah MJ, Fellows LK. Ventromedial frontal lobe plays a critical role in facial emotion recognition. J Cogn Neurosci. 2008;20(4):721–733. doi: 10.1162/jocn.2008.20049. [DOI] [PubMed] [Google Scholar]
- Hindocha C, Wollenberg O, Carter Leno V, Alvarez BO, Curran HV, Freeman TP. Emotional processing deficits in chronic cannabis use: a replication and extension. J Psychopharmacol. 2014;28:466–471. doi: 10.1177/0269881114527359. [DOI] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34(4):247–261. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Xiao L, Palmer P, Sun P, Wang Q, Wei Y, Jia Y, Grenard JL, Stacy AW, Bechara A. Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th grade Chinese adolescent binge drinkers. Neuropsychologia. 2008;46:714–726. doi: 10.1016/j.neuropsychologia.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2008. 2009. p. 12. [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 1975–2014: Overview, Key Findings on Adolescent Drug Use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2015. [Google Scholar]
- Kedzior KK, Martin-Iverson MT. Chronic cannabis use is associated with attentionmodulated reduction in prepulse inhibition of the startle reflex in healthy humans. J Psychopharmacol. 2006;20:471–484. doi: 10.1177/0269881105057516. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report. 2005 A–6. [Google Scholar]
- Lin HC, Mao SC, Gean PW. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem. 2006;13:316–321. doi: 10.1101/lm.217006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. Am J Psychiatry. 2004;161(7):1247–1255. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mathews A, Fox E, Yiend J, Calder A. The face of fear: Effects of eye gaze and emotion on visual attention. Vis cogn. 2003;10:823–835. doi: 10.1080/13506280344000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Morelli SA, Lieberman MD. The role of automaticity and attention in neural processes underlying empathy for happiness, sadness, and anxiety. Front Hum Neurosci. 2013;7:160. doi: 10.3389/fnhum.2013.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nat Neurosci. 2007;10(9):1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol Sci. 2006;17:292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B, Kamboj S, Morgan CJ, Curran HV. Processing dynamic facial affect in frequent cannabis-users: evidence of deficits in the speed of identifying emotional expressions. Drug Alcohol Depend. 2010;112:27–32. doi: 10.1016/j.drugalcdep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The Brain's Default Mode Network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Rupp HA, Wallen K. Sex differences in response to visual sexual stimuli: a review. Arch Sex Behav. 2008;37(2):206–218. doi: 10.1007/s10508-007-9217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes KE, Martin-Iverson MT. Alterations to pre-pulse inhibition (PPI) in chronic cannabis users are secondary to sustained attention deficits. Psychopharmacology (Berl) 2009;207:469–484. doi: 10.1007/s00213-009-1679-0. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J Cogn Neurosci. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; HHS Publication No. SMA; 2014. pp. 14–4887. NSDUH Series H-49. [Google Scholar]
- Simpson JR, Jr, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci U S A. 2001a;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotioninduced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A. 2001b;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev. 2008;1:81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory: Bibliography. 2nd. Palo Alto, CA: Consulting Psychologists Press; 1989. [Google Scholar]
- Stasiewicz PR, Bradizza CM, Schlauch RC, Coffey SF, Gulliver SB, Gudleski GD, Bole CW. Affect regulation training (ART) for alcohol use disorders: development of a novel intervention for negative affect drinkers. J Subst Abuse Treat. 2013;45:433–443. doi: 10.1016/j.jsat.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RM, Talmi D, Schmitz TW, Susskind J, Anderson AK. Psychophysical and neural evidence for emotion-enhanced perceptual vividness. J Neurosci. 2012;32:11201–11212. doi: 10.1523/JNEUROSCI.0155-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 2009;47(3):852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30(3):829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsle D. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. 2011;191:51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Jarvik M, Olmstead R, Brody AL, Ernst M, London ED. Effect of cigarette smoking on prefrontal cortical function in nondeprived smokers performing the Stroop Task. Neuropsychopharmacology. 2007;32:1421–1428. doi: 10.1038/sj.npp.1301272. [DOI] [PMC free article] [PubMed] [Google Scholar]