Abstract
Developmental dyslexia is an unexplained inability to acquire accurate or fluent reading that affects approximately 5–17% of children. Dyslexia is associated with structural and functional alterations in various brain regions that support reading. Neuroimaging studies in infants and pre-reading children suggest that these alterations predate reading instruction and reading failure, supporting the hypothesis that variant function in dyslexia susceptibility genes lead to atypical neural migration and/or axonal growth during early, most likely in utero, brain development. Yet, dyslexia is typically not diagnosed until a child has failed to learn to read as expected (usually in second grade or later). There is emerging evidence that neuroimaging measures, when combined with key behavioral measures, can enhance the accuracy of identification of dyslexia risk in prereading children but its sensitivity, specificity, and cost-efficiency is still unclear. Early identification of dyslexia risk carries important implications for dyslexia remediation and the amelioration of the psychosocial consequences commonly associated with reading failure.
INTRODUCTION
Developmental dyslexia (henceforth dyslexia) is an unexpected failure to develop accurate or fluent reading. Dyslexia is a common learning disability affecting 5–17% of children, with prevalence rates varying across studies depending on its definition.1 Familial studies suggest that dyslexia is heritable, occurring in up to 68% of identical twins and up to 40–60% of individuals who have a first-degree relative with dyslexia.2–4 Several candidate genes for dyslexia susceptibility (e.g., ROBO1, DCDC2, DYX1C1, KIAA0319) have been suggested, and all of these to play an important role in brain development (e.g., Refs 5–8).
Dyslexia is a neurobiological condition associated with morphological and functional atypicalities in the brain areas within the complex reading network.9–13 It remains debated which brain characteristics of dyslexia are a result of reduced reading practice (e.g., due to the daily struggle to read) and which predate the onset of reading instruction. A tentative pathway among genetic effects, developmental brain changes, and perceptual/cognitive deficits in dyslexia has been proposed.5 According to this hypothesis, variant function in any number of genes involved in cortical development may lead to subtle cortical malformations involving neural migration and axonal growth, which in turn results in atypical cortico-cortical and cortico-thalamic circuits. Alterations in these circuits may be associated with the range of sensorimotor, perceptual, and cognitive deficits reported in dyslexia.14
Studies in rodents and humans have demonstrated support for this hypothesis (e.g., see Refs 15–21). For instance, experimental interference with the dyslexia susceptibility genes in rodents caused atypical neuronal migration, which in turn resulted in localized gray matter malformations that affect cortical circuitry.15 These malformations were similar to those reported in post-mortem studies of individuals with dyslexia.16 Interestingly, studies have shown that polymorphisms in these genes are associated with temporo-parietal gray and white matter structure during development in humans.17,18 Additionally, in utero disruption of KIAA0319 expression in rats has been shown to result in poor neural representation of speech sounds in the auditory cortex.19 The observed alterations of the neural representations were similar to those in individuals diagnosed with dyslexia,20 especially those who showed KIAA0319 variants.21 This supports a direct relationship between the dyslexia susceptibility genes and function crucial for learning to read.
Typically, dyslexia is not diagnosed until a child has failed to learn to read as expected, usually in second grade or later. As a result, children with dyslexia must often make up a large gap in reading ability and experience to reach the level of their typically reading peers.12,22 Furthermore, targeted interventions are most effective when administered in kindergarten and first grade. Across six studies, after receiving intensive instruction (number of instruction hours ranged from 30 to over 300 across studies), 56–92% of the at-risk beginning readers reached the range of average reading ability.23 A meta-analysis comparing early intervention studies offering at least 100 sessions reported larger effect sizes for intervention studies conducted with kindergarten and first graders than with children in 2nd and 3rd grades25. Thus, to date, dyslexia is generally diagnosed after the most effective time for intervention has passed, which can be termed the ‘dyslexia paradox.’
This ‘dyslexia paradox’ is detrimental to the well-being of children and their families who experience the psychosocial implications of dyslexia for years prior to diagnosis. Self-perception of reading failure and negative response from others leave children with dyslexia vulnerable to feelings of shame, failure, inadequacy, helplessness, depression, and loneliness (e.g., Ref 26). As a result, asocial behaviors may develop and have long-standing consequences.27 Children with dyslexia are less likely to complete high school or pursue higher education and are at an increased risk of entering the juvenile justice system. 28 Early identification of dyslexia is therefore critical for improving reading outcomes in children12,22 and for preventing and ameliorating the socio-emotional problems that accompany reading failure.
While several behavioral measures show promise in predicting which children will develop dyslexia even before reading onset (see a detailed review below), early identification requires a trade-off between specificity (i.e., reducing the rate of false positives) and sensitivity (i.e., reducing the rate of false negatives) of identification, which can often result in high rates of over/under identification and inadequate resource allocation. Alternatively, brain measures, when combined with behavioral assessments, considerably enhance the ability to predict reading outcomes (Table 1) (e.g., Refs 29–35). Two neuroimaging methods commonly applied to the study of dyslexia in young children are magnetic resonance imaging (MRI) and electroencephalography (EEG) because these methods afford high spatial and temporal resolution respectively, without being invasive. Yet, high costs, limited availability, and logistical challenges are some of the obstacles to using these measures in educational settings. Still, these imaging modalities may prove cost-effective in a clinical setting if sensitivity and specificity could be maximized. Regardless of such challenges, the advent of neuroimaging methods in the study of early reading development and dyslexia may offer at least two advancements: (1) understanding the underlying mechanism of dyslexia, its etiological basis, and developmental trajectories; and (2) improving early identification of at-risk children prior to formal reading instruction.
TABLE 1.
Representative Studies that Used Logistic Regression Methods to Predict Future Reading Achievement from Kindergarten Measures or Even Earlier
| Year | First Author | Language | N | Modality | Grade/Age (From-To) | Risk Definition | Combined Predictors | Sensitivity | Specificity | Overall Prediction |
|---|---|---|---|---|---|---|---|---|---|---|
| 1989 | Scarborough | Eng | 62 | beh | K-2nd grade | An IQ-reading discrepancy-based definition (reading is 2 SE below IQ) or 1 year behind in reading | 1) FHD, 2) vocabulary, 3) LNK, 4) PA | 78% | 95% | 82% |
| 1996 | Elbro | Dan | 90 | beh | K-2nd grade | 1 SD below mean on reading measures | 1) LNK, 2) PA, 3) speech production | 57% | 94% | 84% |
| 2000 | Molfese | Eng | 48 | ERP | Newborn-8 years | An IQ-reading discrepancy-based definition or low IQ and low reading | ERPs to speech syllables | 92% | 79% | 81% |
| 2001 | Catts | Eng | 604 | beh | K-2nd grade | 1 SD below mean on reading measures | 1) sentence imitation, 2) PA, 3) LNK, 4) RAN, 4) maternal education | 74% | 91% | 82% |
| 2001 | Pennington | Eng | 134 | beh | K-2nd grade | An IQ-reading discrepancy-based definition (reading is 1.5 SD below IQ) or reading is below 80th percentile on a composite reading score | 1) LNK, 2) speech perception, 3) PA, 4) verbal short-term memory | 49% | 76% | 75% |
| 1) FHD, 2) LNK, 3) speech perception, 4) PA, 5) verbal short-term memory | 74% | 87% | 75% | |||||||
| 2007 | Puolakanaho | Finn | 198 | beh | 3.5 years-2nd grade | 10th percentile on reading measures | 1) FHD, 2) LNK, 3) RAN | 28% | 92% | 77% |
| 4.5 years-2nd grade | 1) FHD, 2) LNK, 3) PA | 37% | 89% | 77% | ||||||
| 5.5 years-2nd grade | 1) FHD, 2) LNK, 3) RAN | 41% | 91% | 80% | ||||||
| 2009 | Maurer | Germ | 44 | ERP | K-2nd grade | 20th percentile on reading measures | 1) FHD+ children only, 2) PA, 3) IQ, 4) MMN to speech | 68% | 80% | 75% |
| 1) FHD+ children only, 2) PA, 3) MMN to speech | 93% | 50% | 81% | |||||||
| 2012 | Wong | Chin | 114 | beh | 5–7 years | 1 SD below mean on reading measures | 1) Risk only 2) RAN, 3) Chinese character recognition | 73% | 72% | 73% |
| 1) Risk only 2) RAN, 3) English letter naming | 78% | 75% | 76% | |||||||
| 1) Risk only, 2) gender, 3) RAN, 4) English letter naming, 5) Chinese character recognition, 6) morphological construction | 74% | 64% | 75% | |||||||
| 2013 | Bach | Germ | 17 | ERP, fMRI | K-2nd grade | 25th percentile on reading measures | 1) RAN, 2) N1 amplitude to words, 3) FG activation to words | 100% | 90% | 94% |
See Ref 24 for an additional review of behavioral studies.
ERP, event-related potentials; FHD, family history of dyslexia (yes, no); LNK, letter-name knowledge; MMN, mismatch negativity; PA, phonological awareness; RAN, rapid automatized naming.
This review focuses on evidence from behavioral and neuroimaging studies of early literacy skills in pre-reading children and the potential of these studies to enhance the identification of children at risk for dyslexia prior to the start of formal reading instruction. We start by summarizing the neurobiology of reading development, and then proceed to integrate evidence from neuroimaging studies that examine infants and children prior to/concomitant with the onset of reading and reading instruction. We conclude by discussing the clinical and societal implications of early identification of at-risk children.
TYPICAL READING DEVELOPMENT
Literacy is a recent development in human evolution and requires the repurposing of neural circuits that are used for other sensory and cognitive functions including auditory, visual, motor, working memory, and language processes.36,37 Reading development relies on phonological awareness, the ability to perceive and manipulate the sounds in one’s language. These sounds are mapped to their orthographic counterparts, the letters that are learned through the process of visual recognition. Mapping letters to sounds to read words is called decoding and forms the basis for word identification and, subsequently, reading. Fluent reading relies on automatic identification of familiar words and the ability to decode unfamiliar words. Fluent reading is additionally governed by global cognitive mechanisms, such as attention and temporal synchronization.38 Reading comprehension requires, among other things, lexical and background knowledge, correct utilization of linguistic cues, and inference and reasoning skills.39 While the constituent processes are more or less the same across different orthographies, their developmental timing and their importance for reading differ across languages. For example, although phonological awareness has an important role in alphabetic languages, in logosyllabic languages like Chinese, visual processing and visual memory are more important.40
An ensemble of sensory and cognitive systems is therefore utilized to form the neural reading circuit (shown in Figure 1). The emergence of this circuit is thought to parallel the developmental trajectory of reading. Pugh et al. proposed that the integration of orthography and phonology occurs in the dorsal or temporo-parietal network that includes the superior temporal, supramarginal and angular gyri.41 Following the development of the dorsal temporo-parietal circuit for linguistic structure, the ventral occipito-temporal circuit becomes specialized for print and rapid word processing; this circuit includes lateral extrastriate, fusiform, and inferior temporal regions hosting the putative visual wordform area; but see Ref 42. The automatization of the ventral circuit for words occurs gradually through the experience of reading.37,43 Finally, the anterior inferio-frontal circuit plays an important, but not well-defined role in reading. It is thought to be involved in phonological processing, speech planning, lexical access, semantics,44 and comprehension, 45,46 as well as in general cognitive functions, such as attention and inhibition.47 This circuit shows increased involvement with age and reading experience.48–51 While the importance of these regions for reading is undisputed, the timing of the specialization of these regions for reading and their roles across development are still under some debate.52,53 For example, there is some recent evidence for the involvement of the ventral occipito-temporal regions in orthographic-phonological conversion early in reading development.54,55 As discussed above, this process has been previously attributed to the dorsal network early in reading acquisition.
FIGURE 1.
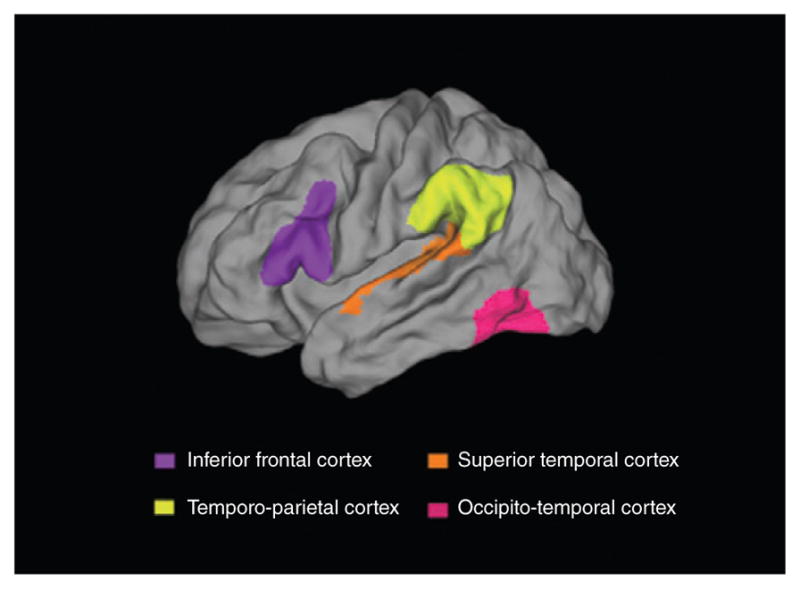
Brain regions important for reading that are commonly found to be associated with atypical function or structure in dyslexia.
Recent technological advances have allowed researchers to examine the white matter tracts that connect the dorsal, ventral, and anterior regions to form the reading circuit (shown in Figure 2). Several pathways in particular have shown an association with reading performance: the posterior corpus callosum (not shown) (connecting inter-hemispheric temporal, occipital, and parietal regions), the arcuate fasciculus (connecting dorsal and anterior regions), inferior fronto-occipital fasciculus (connecting occipital and inferior frontal regions), and the inferior-longitudinal fasciculus (connecting ventral and anterior regions).56,57 White matter development is strongly influenced by environmental experiences such as reading.58,59 Fractional anisotropy (FA), a measure of the degree of directionality of water molecule diffusivity and an indicator of tract integrity, increases with reading experience in the arcuate fasciculus and inferior-longitudinal fasciculus, suggesting that these pathways specialize for reading throughout development.60 Alternatively, the posterior corpus callosum follows the opposite developmental trajectory, decreasing inter-hemispheric connectivity throughout reading development and thereby supporting the left hemispheric lateralization for reading.11
FIGURE 2.
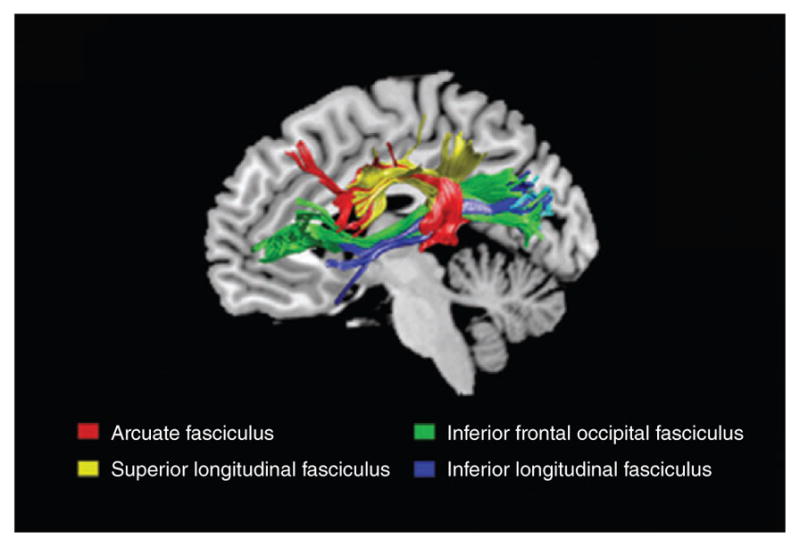
White matter pathways important for reading: arcuate fasciculus (red), superior longitudinal fasciculus (yellow), inferior frontal occipital fasciculus (green), inferior-longitudinal fasciculus (blue), corpus callosum is not shown.
Thus, typical literacy development relies on the emergence of neural circuitry for reading. Understanding the early developmental trajectory of reading, behaviorally and in the brain, will allow for better understanding of the etiological basis of dyslexia and may help inform early identification practices.
BEHAVIORAL PREDICTORS OF DYSLEXIA
Hereditary/Home Environment
Even prior to formal reading instruction in kindergarten, children are exposed to literacy-related activities through interaction with their caregivers. The home literacy environment, often measured by variables such as the number of books at home or the frequency and quality of shared reading between children and their parents, has been shown to contribute to unique variance in the development of early reading skills.61–63 This variance has been estimated at 8% by one meta-analysis.64 While small, home literacy environment’s unique contribution plays an important factor in counteracting genetic or other environmental risks (e.g., Ref 65).
Due to the hereditary nature of dyslexia, family history is one of the strongest risk factors for developing dyslexia. Consequently, the majority of studies aiming to identify pre-literacy precursors of dyslexia have focused their investigation on comparing prereaders with and without familial history of dyslexia (henceforth referred to as FHD+ and FHD−, respectively). Such investigations in infants, for instance, have revealed that the early course of language development differs between high-risk and low-risk groups.24,66–73
Early Language Skills
A relationship between early language development and later reading abilities has been demonstrated in infants as young as 8–30 months old (for a review, see Ref 74). Speech perception and production serve as the foundation for the acquisition of phonology and, subsequently, alphabetic knowledge. Accordingly, slow language development as indicated by delayed onset of talking, short mean length of utterances, lower complexity of syllables produced, and poor receptive or expressive vocabulary, has been associated with poor literacy outcomes.71,75 An important distinction has been made between receptive and expressive language development. While most late-talkers with typical receptive language will develop typical literacy skills, children with language delay in both expressive and receptive realms are more likely to experience persistent language and reading difficulties.75–78
Poor speech perception in infants with FHD+ has been documented as early as 6 months of age.79 FHD+ infants also showed impairment in syllable production by 19 months of age and used fewer words and less grammatically complex structures than their non-risk counterparts by 30 months of age. Further, FHD+ infants demonstrated poorer expressive and receptive vocabulary than their FHD− counterparts.74,80 Importantly, these poor language abilities were associated with poor reading outcomes, and it has been demonstrated that for FHD+ infants with language delay, the risk of facing reading problems at school age is more than the expected 40–60% based solely on genetic risk.24,70–72,78 Yet, speech delay was reported in only 25% of at-risk children with a diagnosis of dyslexia. Comorbidities with other disorders such as specific language impairment, which is also associated with poor language skills, especially speech production, complicate the specificity of dyslexia risk identification and suggest that other indicators are necessary to adequately predict atypical reading outcome.
Across different languages several pre-literacy skills, when measured in kindergarten, have emerged as the most robust predictors of dyslexia.74,81–83 These skills include letter-sound knowledge, phonological awareness, and rapid automatized naming (RAN).
Letter-sound knowledge measured in kindergarten is the most robust, but ephemeral, predictor of reading ability.84,85 Letter-sound knowledge is at the intersection of phonology and written language because it reflects the knowledge of letter names, the sounds they make, and their visual representations. The strong association between letter-sound knowledge and later reading outcome does not persist beyond pre-school years, as children master this skill rapidly, reaching a performance ceiling by late kindergarten.81 Additionally, letter-sound knowledge is thought to be strongly influenced by environmental factors, such as home literacy, and thus may reflect lack of experience rather than a cognitive deficit.86
Poor phonological awareness for spoken words is also an early indicator of dyslexia risk. Across prereaders, there is a moderate correlation between kindergarten performance on tasks such as deciding whether two words rhyme (e.g., ‘bat’ and ‘cat’), blending sounds together to make a word (e.g., /b/ /a/ /t/ to ‘bat’), or segmenting words into parts (e.g., knowing that ‘cat’ is composed of /c/, /a/ and /t/), and reading outcomes in later grades.87 Studies of FHD+ children, however, demonstrated consistently low phonological awareness performance in these children regardless of reading outcomes.87–91 This suggests that phonological awareness may be a more reliable marker of genetic propensity for dyslexia than of actual reading outcome. Additionally, as phonological awareness has a strong reciprocal relationship with reading (e.g., orthographic knowledge of ‘cat’ may enhance the segmentation of the word into sounds), its association with reading, and thereby its predictive value increases in later grades.78,87
RAN is another reliable indicator of literacy outcomes at the pre-reading stage. RAN is the ability to rapidly name an array of high-frequency items (e.g., objects, colors or letters, or a combinations of these) repeated multiple times across several rows.92 It is an especially robust predictor in shallow orthographies, such as Finnish, in which simple letter-to-sound correspondence rules deem phonological awareness an easier skill to acquire than in deep orthographies, such as English.93 As RAN measures the speed required to name serially presented visual stimuli, it is thought to parallel the cognitive and neural demands of fluent reading. Indeed, RAN is a stronger predictor of reading fluency, the ability to read text accurately, quickly, and with appropriate expression/intonation, than of un-timed reading abilities.81 The predictive power of RAN also varies depending on what stimuli are used (e.g., numbers, pictures, or letters), with colors and objects being stronger predictors in earlier grades.94 RAN is a stronger predictor of reading achievement in poor readers than in typical readers and RAN deficits have been shown to persist into adulthood.94
Perceptual Abilities
It has been postulated that deficits in phonological awareness are due to basic abnormalities in lower-level auditory processing in individuals with dyslexia.95–99 Several studies have demonstrated atypical auditory processing in pre-reading children at risk for dyslexia.31,100–102 In particular, sensitivity to slowly varying acoustic signals (i.e., perception of amplitude and frequency modulation) has been important for reading outcomes in studies of preliterate children across languages.100,103–105 Studies have also demonstrated the significance of rapid auditory processing for language development and the atypical perception of rapid auditory transitions in infants and young children with a genetic risk for language-based learning disabilities.106–111 Yet, the role of rapid auditory processing in dyslexia has not been consistent.100,112 Findings of strong links between early musical abilities in young children, particularly processing of rhythm and prosody, and reading outcomes have sparked interest in investigating the shared neural and cognitive systems between music and language and their role in dyslexia.103,113–119 For example, poor beat synchronization was associated with less precise neural encoding of speech and has emerged as a significant predictor of school readiness in pre-school children.103 There is a strong case for the significance of early basic auditory processing cues for the development of phonological skills, but which cues are important is still under debate.
Fluent reading relies on rapid visual processing of serial stimuli. Consequently, the importance of visual-spatial attention processing for reading development has been established by several longitudinal studies in pre-readers.120–122 For example, one study showed that poor visual-spatial attention in Italian kindergarteners strongly predicted reading abilities in 2nd grade, accounting for a unique variance in text reading.120 Two studies to date have examined magnocellular processing in pre-reading children with mixed findings. In one study, magnocellular functioning in pre-reading children was associated with later reading abilities,123 but the other study failed to find this association.124
General Cognitive Abilities
Non-verbal intelligence has been widely considered in the context of dyslexia prediction and diagnosis. 125 Historically, dyslexia has been diagnosed based on a reading achievement and IQ discrepancy model. Recent studies have shown that the core mechanisms of dyslexia are consistent regardless of IQ.126,127 There is some evidence, however, for a stronger genetic contribution to reading difficulties for those with higher IQ.128 IQ has also been suggested to be a protective factor differentiating at-risk readers who will demonstrate persistent reading failure from those who could be successfully remediated. 129 There is little empirical support, however, that IQ can reliably identify dyslexia risk.130,131
A weak but still noteworthy predictor of reading is working memory.132 Broadly, working memory is the memory system that is involved in the storage and active processing of current information. 133 While different components of the working memory system have been implicated in dyslexia (e.g., visual, central executive, and verbal), a deficit in verbal working memory, or the phonological loop, has been most consistently reported culprits.134,135 Yet, due to varied operational definitions of working memory across studies, high frequency of cooccurrence between reading and attentional deficits, and the inherent demand on working memory during most pre-literacy measures, the unique contribution of this system to reading has not consistently been established (for a review, see Refs 133, 136, and 137).
Across studies, behavioral measures are modest predictors of reading in kindergarten and collectively account for about half of the variance in later reading. 74 When genetic risk is entered into the model, the accuracy of prediction reaches a much higher rate of around 80%.74,89 The psychosocial consequences for the 20% of children whose reading outcomes could not be predicted by the model can be detrimental, and highlight the need to turn to alternative measures, such as brain imaging, to possibly enhance the accuracy of early identification.
EARLY BRAIN CHARACTERISTICS IN AT-RISK CHILDREN: EVIDENCE FROM EEG/EVENT-RELATED POTENTIAL AND MISMATCH NEGATIVITY
Event-Related Potentials (ERP)
EEG is a well-suited technique for studying language processes in young children for several reasons: (1) it is simple to use by applying elastic caps with electrodes on the heads of subjects; (2) it acquires brain activity rapidly and therefore it can capture readingrelated cognitive processes as they unfold; and (3) it can capture pre-attentive processes without performance-related constraints and biases.138
Event-related potentials measure brain activation in response to a specific event (e.g. the display of a letter) using EEG technology. ERP studies in children and adults with dyslexia have revealed longer latencies and reduced amplitudes for components ranging from early perceptual processing to higher-level linguistic and cognitive processing across different domains (e.g., visual, auditory, linguistic, and non-linguistic) (for a review, see Ref 139). In young children, several of the early, exogenous components have been identified as important for reading: N1, P2, N2, and mismatch negativity (MMN) (for a summary of these components, see Ref 140). These components are passively elicited by auditory and visual stimuli; the subject is not required to perform a task but simply has to remain alert. As they are not influenced by behavioral and performance-related demands, these evoked responses provide a reliable, objective measure of cortical perceptual function in infants and children.138
Early Event-Related Responses to Speech
In the first series of studies to investigate ERP predictors of dyslexia in infants, Molfese et al.33,141,142 demonstrated that ERPs recorded to speech syllables /bi/ and /gi/ within 36 hours of birth discriminated among newborns who 8 years later would be classified as dyslexic (poor reading and average IQ scores), poor readers (poor reading and poor IQ scores), or regular readers (average IQ and average reading scores). Specifically, three ERP components, N1, P2, and N2, classified the three groups of readers with an 81.25% accuracy.33 These components accounted for a large unique variance (above other environmental and cognitive variables) in reading performance. 143 Right-hemisphere N2 peak amplitudes were largest for the dyslexic group. Furthermore, correlations between the N1 amplitude and latency measured in the same children between ages 1 and 4 and word reading measured at age 8 were significant. 141 Molfese et al. suggested that these differences in neural response to speech reflect deficits in perceptual mechanisms that are important for detecting, processing, and responding to linguistic stimuli during development.
A longitudinal study in the Netherlands further bolstered the importance of the early ERP responses to speech for reading. FHD+ infants demonstrated reduced P2 ERPs to the Dutch word /bak/ at 5 months of age, and delayed P1 and P2 responses to /bAk/ and /dAk/ at 17 months.144 Additionally, while the 17-month-old controls demonstrated similar N2 amplitudes in both hemispheres, the FHD+ group had a larger N2 for the right hemisphere than the left. Importantly, the P2 latency at 5 months explained 18% of variance in verb production at 17 months. The study suggests that poor neural differentiation of phonemes that differ in their second-formant frequency transition is an early precursor of dyslexia.
In a study with Finnish FHD+ infants, ERPs to consonant-vowel syllables (/ba/, /da/, /ga/) were recorded within 36 h after birth.145 As compared with controls, FHD+ infants demonstrated a larger response between 50 and 170 ms and a slower shift in polarity from positivity to negativity in the right hemisphere in response to /ga/. This slower shift in polarity was related to poorer receptive language skills at 2.5 years, poorer verbal memory at 5 years, and low performance on pre-literacy measures at 6.5 years for both groups.30,146
Thus, hemispheric lateralization differences in FHD+ children have been consistent across these ERP studies and have been associated with a myriad of reading outcomes. These differences in lateralization have been interpreted as compensatory mechanisms used by at-risk children during language processing to offset the improper function of the left-hemispheric language areas,144 or as aberrant or delayed lateralization of language areas that leads to perceptual deficits in dyslexia (e.g., Ref 146).
Early Mismatch Negativity Responses to Speech
MMN is a particularly useful paradigm to capture perceptual detection of subtle acoustic cues in language. The MMN is thought to reflect the pre-attentive memory-based comparison processes where each incoming sound is compared with the memory trace based on the regularities of the preceding auditory context.147,148 Subjects are presented with repeated stimuli, and ERPs (commonly present at the fronto-central electrode location) to an occasional deviant sound are recorded. A large amplitude and short latency of MMN response usually reflects the magnitude of the deviance.149–151
Several studies demonstrated differences in MMN response to speech between FHD+ and FHD− infants and young children. Specifically, attenuated MMN responses were shown to contrasts that vary in vowel duration cues (i.e., Finnish /ka//kaa/)68, and attenuated early MMN response and an absent late MMN response to contrasts varying in frequency transition (i.e., Dutch /bAk//dAk/152–154). In particular, FHD− infants demonstrated responses predominantly over the left hemisphere, whereas those of the FHD+ infants were distributed over the right hemisphere, again suggesting early hemispheric differences in auditory speech processing. A retrospective study compared infant MMN to speech at 2 months among 2nd grade FHD− fluent readers, FHD+ fluent readers, and FHD+ poor readers.31 The three groups demonstrated different patterns of MMN response with a frontally positive response in FHD− readers, parietally positive response in FHD+ readers, and absent response in FHD+ poor readers. Thus, MMN studies showed attenuated or absent neural responses to speech in FHD+ infants and the importance of these responses for later literacy outcomes.
Additionally, studies in older FHD+ and FHD− early reading children also reported atypical MMN responses to speech. In one study, FHD+ 6-year-olds showed a reduced MMN for Finnish speech sounds that varied in duration and intensity, but not frequency, as compared with FHD− children.149 In another study, Swiss FHD+ kindergarteners showed a bilateral MMN response to the deviants /da//ta/ embedded in a repeating /ba/, as opposed to the left-lateralized MMN response of their FHD− counterparts. 155 This pattern further discriminated FHD+ children who became typical readers from those who developed dyslexia in later grades. Models that included behavioral and MMN measures accounted for up to 45% of variance in reading outcomes and correctly classified FHD+ children as typical or poor readers with a 75% accuracy.156
Taken together, MMN studies of response to speech demonstrate strong associations with later reading abilities, beyond those of behavioral measures. This suggests that MMN measures are uniquely sensitive to some underlying processing deficits in dyslexia that are not captured by behavioral measures because they measure these deficits more directly, with less influence from attention and decision-making processes.
Event-Related Responses to Non-speech Auditory Stimuli
To evaluate whether early differences in ERPs to speech are due to more basic auditory deficits that are thought to be the primary deficit in dyslexia (e.g., Refs 23, 97, and 157), several studies evaluated MMN responses to tones in FHD+ and FHD− newborns. The results demonstrated attenuated MMN responses to pitch transitions in at-risk newborns, to rapid auditory transitions in FHD+ 17-month-olds, and to amplitude rise time and frequency transitions in FHD+ 41-month-olds.102 MMN responses to rapid transitions were correlated with language comprehension in kindergarten and with reading fluency in 2nd grade.31 The ERPs to amplitude rise time and frequency transitions, however, were not significantly related to later reading outcomes and were instead markers of genetic risk for dyslexia affecting FHD+ children regardless of their eventual reading outcomes.102
A study in Finnish pre-reading children ages 5–6 examined MMN responses to deviant tones that varied in frequency, intensity, and duration.158 As compared with FHD− children, the FHD+ group showed a significantly larger MMN and smaller P1 response to the frequency deviant, but not other deviants. The amplitudes of the MMN and P1 responses correlated with distinct literacy measures. In another study, FHD+ kindergarten children demonstrated a reduced amplitude of the late MMN to tone frequency deviant.155 This MMN to tone frequency was a significant predictor of reading outcomes in 2nd, 3rd, and 5th grades.32
In sum, the results of several longitudinal ERP studies suggest that early auditory processing plays a role in dyslexia, but the specific association between non-speech neural processing and reading outcomes is inconsistent across these studies.
Event-Related Responses to Visual and Lexical Stimuli
While most ERP research on early predictors of reading focused on auditory processing, several recent studies investigated visual and lexical processing in pre-reading kindergarten children. One such study in Swiss children reported that differences in N1 amplitude in response to the visual presentation of words, as compared with symbols, was a significant discriminator between poor and typical readers at 2nd grade.160 This component together with behavioral measures accounted for 67% of variance in later reading performance. Therefore, neural tuning for print, even prior to reading onset, could be an important predictor of dyslexia.
To investigate neural habituation to visual stimuli in dyslexia, ERPs to repeated visual stimuli (i.e., black and white patterns) were recorded in FHD− and FHD+ Dutch kindergartners.159 Comparison at the end of second grade revealed reduced habituation (indicated by decrease in N1 across repetitions) in FHD+ typical readers as compared with FHD− typical readers and reverse habituation (increase in N1 amplitude across trials) in FHD+ poor readers. Reduced and reversed habituation could suggest reduced efficiency in processing new information and may point to an underlying deficit in low-level visual processing as a risk factor for dyslexia.
Interesting findings of higher-level linguistic deficits in young children came from a study in Norway that investigated lexical and semantic priming in FHD− and FHD+ 20- to 24-month-old children.161 Children were presented with pictures (e.g., a chair) and with either the correct name (e.g., ‘chair’), an incorrect but semantically related name (e.g., ‘desk’), or incorrect and unrelated name (e.g., ‘dog’). In response to semantic violations, FHD− children demonstrated a significant incongruency effect in the form of the N400 component (thought to index lexical prediction error). This effect was lacking in FHD+ children. These results indicate higher order linguistic deficits in lexical and semantic processing in FHD+ children that could be due to a more limited vocabulary in this group.
Taken together, early ERP markers of dyslexia include aberrant neural responses at the lower level of phonological, auditory, and orthographic processing, as well as at the higher level of lexical processing. However, due to the limited spatial resolution of EEG, evidence from MRI studies is necessary to localize these patterns in the brain.
EARLY BRAIN CHARACTERISTICS IN AT-RISK CHILDREN: EVIDENCE FROM MAGNETIC RESONANCE IMAGING (MRI), DIFFUSION TENSOR IMAGING (DTI) AND FUNCTIONAL MAGNETIC RESONANCE IMAGING (fMRI)
Magnetic resonance imaging (MRI) studies in children and adults with dyslexia commonly demonstrate hypoactivation in left-hemispheric temporo-parietal, occipito-temporal, and inferior frontal networks (for reviews, see Refs 162–164). Further, reduced functional connectivity among these regions has also been demonstrated.165–167 Children with dyslexia display this pattern of hypoactivation even when compared with younger children with equivalent reading skills,168,169 suggesting that the observed alterations are not due to delayed maturation, but are instead unique brain characteristics of dyslexia. Research studies have also documented hypoactivation in inferior frontal regions in individuals with dyslexia, but these findings have been less consistent (for reviews, see Refs 162,163,170–172). In some cases, individuals with dyslexia also show increased activation in corresponding right-hemispheric regions (e.g., Refs 34,41,173). It is not clear whether increased right-hemispheric activation is a compensatory mechanism underlying remediation or a brain characteristic of dyslexia reflecting failed left-hemispheric lateralization for language.143
Structural gray matter differences in dyslexia tend to co-localize with regions that show functional differences (for a review, see Ref 171), but are also observed in the cerebellum.174 Diffusion tensor imaging (DTI) studies often report low FA or volume in the left arcuate fasciculus and corona radiata fibers.57,175,176 A study in children with dyslexia demonstrated increased FA in a posterior portion of the corpus callosum, suggesting increased interhemispheric connectivity in dyslexia.11,177 While FA is thought to reflect a number of microstructural features, including axonal cell membranes, amount and integrity of myelin around axons, coherence of axonal orientation, and number and size of axons, the underlying neurobiological cause of the reduced FA in dyslexia is still uncertain.57
Structural Alterations in At-Risk Pre-Readers/Beginning Readers: MRI Studies
The first MRI study to examine early structural brain differences related to dyslexia compared regional gray matter volume in FHD+ and FHD− English-speaking children examined during the summer prior to kindergarten.178 Results revealed reduced gray matter indices for FHD+ as compared with FHD− children in the left occipito-temporal regions, bilateral temporo-parietal regions, left fusiform gyrus, and right lingual gyrus. Furthermore, gray matter volume indices in the left temporo-parietal and left occipito-temporal region correlated significantly with RAN performance, an important early predictor of reading abilities. Thus, the result suggests that structural brain alterations of dyslexia may predate reading failure, but the sensitivity and specificity of such alterations remain unclear.
A consequent analysis of the same group of children (with a few additional subjects), examined the interaction between FHD status and a retrospective report of delay in language production.179 Results revealed that FHD+ children with language delay had significantly more pronounced reductions in the regions reported above; namely, occipoto-temporal and temporo-parietal regions. Furthermore, there was a significant association between reduced gray matter volume and early language delay in left-hemispheric middle temporal, occipital, and frontal regions. These findings support behavioral evidence of the cumulative effects of hereditary risk for dyslexia and delayed language development.
A recent study characterized the sulcal patterns (the arrangement, number, and size of primary cortical folds) of the same group of children as well as a new sample of older children with dyslexia.180 Results demonstrated atypical sulcal patterns in temporo-parietal and occipito-temporal regions in FHD+ prereaders as compared with FHD− pre-readers, and the same patterns were observed in older children with dyslexia. Sulcal pattern has been hypothesized to relate to optimal organization of cortical function and white matter connectivity, and is largely determined during prenatal development. Thus, atypical cortical folding patterns in regions important for reading could reflect atypical brain development in dyslexia, as suggested by Galaburda et al.5
A longitudinal study in Norwegian pre-reading children examined FHD+ and FHD− children’s behavioral performance in the beginning of kindergarten and their brain structure in the spring of 1st grade (prior to reading instruction), 3rd grade, and 6th grade.181 Retrospective analysis revealed that 1st graders who eventually received a diagnosis of dyslexia (in 6th grade) had significantly thinner cortex in several sensory regions of the left hemisphere, including Heschl’s gyrus, lingual gyrus, medial frontal gyrus, middle cingulate gyrus, and an area in the right orbitofrontal cortex. In 6th graders, cortical reductions in dyslexia were observed in the temporo-parietal region, visual word-form area of the fusiform gyrus, and inferior frontal gyrus. The only structure in which group differences were consistent across development was the primary auditory cortex. These findings support the notion of underlying auditory deficits in dyslexia and suggest that atypicalities in the reading network only emerge after reading acquisition. However, due to the small group sizes (N = 7, 10), the longitudinal results in this study should be interpreted with caution.
In a longitudinal study of German 1st graders, increased gray matter volume in the left superior temporal gyrus in 1st grade was associated with greater gains in reading proficiency a year later.182 Reading gains were also associated with longitudinal volume reduction in the left inferior parietal lobule, precentral gyri, and postcentral gyri. The former finding suggests that pre-existing brain differences can determine the development potential of reading early in schooling. The latter finding highlights the importance of developmental transformation of the reading circuit for reading acquisition.
A nuanced view of the interaction between brain structure and familial history of dyslexia was provided by a study that compared maternal and paternal risk (measured by a detailed questionnaire) in beginning readers (ages 5–6) using volumetric and cortical thickness measures.183 Results indicated that a higher maternal risk, but not paternal risk, was associated with reduced volume in the bilateral prefrontal and temporo-parietal regions. Interestingly, when the components of gray matter volume were deconstructed to surface and thickness, the severity of maternal reading history was associated with reduced left temporo-parietal region only for cortical surface area (which is thought to reflect more prenatal influences), but not thickness (which is thought to reflect more postnatal influences). These results further indicate that genetic influences, particularly maternal, are important for the neural development that supports reading.
Structural Alterations in At-Risk Pre-Readers/Beginning Readers: DTI Studies
Several recent DTI studies demonstrated the importance of white matter tract integrity for reading development. A study in English-speaking kindergarten children showed a significant positive association between phonological awareness scores and the volume and FA of the left arcuate fasciculus, but not with other tracts.184 This study further suggests that white matter structural differences in dyslexia are a cause rather than a consequence of poor reading development.
Another study replicated these results in Dutch-speaking pre-reading kindergarteners and extended them to include an association with phonological awareness in the ventral inferior fronto-occipital fasciculus bilaterally.185 Importantly, the study demonstrated reduced FA in FHD+ kindergarten students in the ventral inferior fronto-occipital tract in the left hemisphere, but not the right, suggesting that white matter abnormalities in the left reading networks predate reading onset. Furthermore, a recent study has demonstrated that these white matter atypicalities are already present in as early as infancy.186 In this study, FHD+ compared to FHD- infants showed reduced FA in the central portion of the arcuate fasciculus. Furthermore, higher FA in this region was associated with better language skills across all infants.
Rather than looking at static brain measures from just one time point to predict dyslexia, a structural longitudinal study investigated the degree to which white matter development predicts reading outcomes.187 Behavioral and MRI data were collected from English-speaking children in kindergarten and 3rd grade. Reading in 3rd grade was associated with developmental increases in white matter volume in two left temporo-parietal regions. Specifically, white matter volume in the left arcuate fasciculus and corona radiata accounted for a unique 21.6% of variance in 3rd grade reading, even when controlling for environmental factors and pre-literacy performance. Together, the behavioral and brain measures accounted for 59% of variance in 3rd grade reading outcomes. However, this model is notably weaker in predicting reading than the behavioral models reviewed in the previous section.
Thus, the literature so far suggests that structural gray and white matter alterations predate reading onset. Due to the significant impact of environmental factors, such as language and literacy exposure (even prior to reading instruction), on brain development, more MRI studies in infants will be valuable in characterizing the innate brain characteristics of dyslexia and in establishing causality. Structural neuroimaging methods are particularly well-suited for this purpose, as they can be conducted in naturally sleeping infants.178
fMRI Activation to Auditory/Phonological Processing
The first fMRI study to examine functional phonological processing in pre-reading children employed a first-sound matching task (see Figure 3) with FHD− and FHD+ children the summer before formal reading instruction began in kindergarten.188 As compared with FHD− children, FHD+ children demonstrated reduced activation in left occipito-temporal and temporo-parietal regions, and the bilateral cerebellum. These findings complement the evidence from ERP studies demonstrating atypical phonological processing in the brain prior to reading onset. Importantly, this is the first study to show that brain alterations characteristic of school-age children and adults with dyslexia predate the onset of reading instruction.
FIGURE 3.
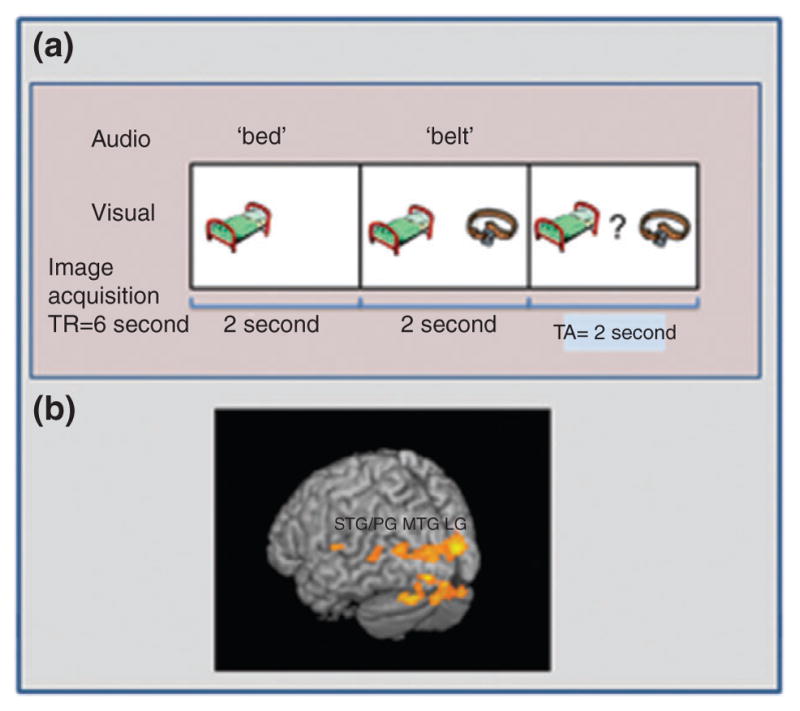
(a) The first-sound matching phonological task implemented by Raschle et al.187 During the task, children heard two consecutively presented common object-words, spoken in a male or female voice, accompanied by corresponding pictures (‘bat,’ ‘ball’) and had to determine whether two words start with the same sound or different sound. In the control task the children had to determine whether two words were spoken with the same voice or different voice (female or male). (b) Brain regions that demonstrated increased activation in FHD− as compared with FHD+ pre-readers for phonological greater than voice processing.
Another experiment evaluated rapid auditory processing abilities in the same FHD+ and FHD− pre-reading children.106 The results revealed reduced activation in a left hemispheric prefrontal region in FHD+ children as compared with FHD− children. Furthermore, activation in this region correlated with phonological awareness scores and with neuronal activation in the posterior dorsal and ventral areas during the phonological task discussed above.187 The role of dorsolateral prefrontal brain regions in processing rapid acoustic features has been reported in previous studies in adults and children (e.g., Refs 189 and 190). Results from both studies are consistent with MRI findings in older children and adults with dyslexia and the ERP findings in at-risk infants, indicating that aberrant neural processing of auditory and phonological information seems to be an important precursor of dyslexia.
fMRI Activation to Orthographic Processing
Reduced activation in brain regions that are important for processing of print has been reported in prereaders at risk for dyslexia. In one such study, English-speaking kindergarten children were assigned into risk and low-risk groups based on their performance on a battery of pre-literacy assessments, and their neural responses during a one-back orthographic task were then compared.191 A comparison between the two groups at the start of kindergarten revealed reduced activation for letters in the at-risk group in several posterior dorsal and anterior frontal regions. Children were then scanned again following a 3-month reading intervention for the at-risk group. At the follow-up, some of these differences were significantly ameliorated. Instead, at-risk children showed increased activation in the right supramarginal gyrus, the left inferior frontal gyrus, and the left precentral gyrus. The left lateralization of the temporo-parietal activation that emerged in the low-risk children at the follow-up was less pronounced in the at-risk group. Thus, typical reading development in this study was associated with initial bilateral recruitment of the dorsal reading network and subsequent disengagement of the right-hemispheric homologous regions. Atypical reading development was associated with reduced recruitment of the bilateral regions and, after intervention, with compensatory recruitment of frontal regions. Importantly, abnormal neural activation to print preceded reading onset, but was malleable to intervention.
In another study, activation to stimuli with different levels of orthographic demands was compared between high-risk and low-risk groups of Norwegian 6-year-old children.192 Risk was defined based on multiple hereditary and developmental factors. As compared with the controls, the high-risk group demonstrated lower activation for the task with the highest orthographic demands (i.e., sight word recognition task) in the bilateral occipito-temporal regions and higher activation in the bilateral insular cortex, right thalamus, and several right-hemispheric temporal regions. These findings suggest that distinct patterns of activation in response to print can be observed as early as kindergarten.
A Swiss study compared activation with words between two groups of kindergartners retrospectively classified as low-risk and high-risk based on 2nd grade reading performance.160 While no significant differences in activation were evident between the groups, activation for words in the occipital and frontal areas (left inferior frontal gyrus, bilateral medial frontal gyrus, bilateral middle temporal gyrus, and left fusiform gyrus) was significant for the non-risk group only. Activation in the visual word-form area of the left fusiform gyrus, when entered into a regression model with behavioral measures and ERP response to words, explained 17% of unique variance in 2nd grade reading performance. All measures combined explained 84% of variance. These findings offer further evidence for the importance of print selectivity in the occipito-temporal region for reading development even prior to formal reading instruction.
CONCLUSION
Neuroimaging studies in pre-reading children and beginning readers have provided ample evidence for the presence of brain alterations early in development and prior to formal reading instruction. These alterations are similar to those observed in older children with a diagnosis of dyslexia. ERP studies have demonstrated alterations in neural activity in infants and young children with a genetic and behavioral risk for dyslexia in response to pre-linguistic and linguistic stimuli, such as categorical syllable perception; changes in speed, duration and structure of speech sounds; rapid auditory processing; and orthographic and lexical processing. Structural MRI studies have demonstrated atypicalities within the dorsal and ventral reading networks and functional MRI studies have shown reduced neural processing of phonological, rapid auditory, and orthographic information in these networks. Across studies, neural differences in FHD+ pre-reading children have been predictive of later language and reading outcomes. Importantly, few MRI studies to date compared prereading children who later developed dyslexia with those who developed typical reading skills160,181. Thus, additional longitudinal MRI studies are necessary to establish the utility of MRI in predicting dyslexia. Furthermore, due to the influence of early language environment on the development of regions comprising the reading network, investigating structural neural alterations in infancy (similarly to the ERP studies reviewed above) may be of particular importance.
Taken together, these research studies, while still limited, suggest that neural alterations in dyslexia predate reading onset and reflect the differential developmental trajectory of reading brain networks as the result of genetic predisposition for dyslexia. Importantly, these alterations may serve as early biomarkers of risk for dyslexia, but their sensitivity and specificity are still unclear. Identifying the neurobiology and the underlying mechanisms of dyslexia, as well as establishing reliable biomarkers for dyslexia, is important for promoting early and reliable diagnosis of dyslexia, thereby allowing for targeted intervention early in schooling and prior to reading failure, which could ultimately resolve the ‘dyslexia paradox.’ Preventing the spiraling effects of reading failure has major implications for children and their families, as well as society at large. Children with dyslexia are less likely to graduate high school and more likely to end up in the juvenile justice system28. Even without these bleak consequences, the impacts of reading failure on the psychological well-being of children and their families can be tremendous.
Despite the progress reviewed above, we are still far from having reliable biomarkers of dyslexia. The limited number of studies, small sample sizes, differences in criteria for defining dyslexia, heterogeneity of symptoms reported for dyslexia, and differences across orthographies are all factors that contribute to the high variance in findings across studies. Furthermore, while neural measures enhance the overall prediction accuracy of behavioral measures, their additional contribution is moderate and may not warrant the high costs and logistical problems associated with using MRI or even EEG with young children (yet).
Nevertheless, we are on the threshold of a more comprehensive understanding of dyslexia. The convergence of decades of behavioral research and the advent of neuroimaging technologies enable us to comprehensively characterize the behavioral, cognitive, and neural patterns of dyslexia and risk for dyslexia. In particular, the characterization of the neural patterns and underlying neural mechanisms will become more fine-grained so that specific hypotheses about subtle cortical malformations involving neural migration and axonal growth, as well as cortico-cortical circuits, may be formed and tested. As cross-disciplinary efforts continue to bear fruit, we are moving closer to the overreaching goal of preventing reading failure for most children, thereby maximizing their intellectual potential and allowing them to discover the joy of reading.
Acknowledgments
This work was supported by NICHD Grant 1RO1HD065762 and 1R01HD067312 to NG and Evans Literacy Fellowship to OOP. Special thanks to Xi Yu for creating the figures for this paper and to Meaghan Mauer, Grace Coviello, Bryce Becker, Mike Figuccio, and Ying Ying Wang for their helpful comments and assistance with the paper.
Footnotes
Conflict of interest: The authors have declared no conflicts of interest for this article.
References
- 1.Elliott JG, Grigorenko EL. The Dyslexia Debate. New York: Cambridge University Press; 2014. p. 14. [Google Scholar]
- 2.Finucci JM, Cilds B. Genetic Aspects of Speech and Language Disorders. New York: Academic Press; 1983. Dyslexia: family studies; pp. 157–167. [Google Scholar]
- 3.Volger GP, DeFries JC, Decker SN. Family history as an indicator of risk for reading disability. J Learn Disabil. 1985;18:419–421. doi: 10.1177/002221948501800711. [DOI] [PubMed] [Google Scholar]
- 4.Grigorenko EL. Genetic bases of developmental dyslexia: a capsule review of heritability estimates. Enfance. 2004;56:273–288. [Google Scholar]
- 5.Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. From genes to behavior in developmental dyslexia. Nat Neurosci. 2006;9:1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- 6.Hannula-Jouppi K, Kaminen-Ahola N, Taipale M, Eklund R, Nopola-Hemmi J, Kaariainen H, Kere J. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet. 2005;1:e50. doi: 10.1371/journal.pgen.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng H, Hager K, Held M, Page GP, Olson RK, Pennington BF, Gruen JR. TDT-association anaylsis of EKN1 and dyslexia in a Colorado twin cohort. Hum Genet. 2005;118:87–90. doi: 10.1007/s00439-005-0017-9. [DOI] [PubMed] [Google Scholar]
- 8.Skiba T, Landi N, Wagner R, Grigorenko EL. In search of the perfect phenotype: an analysis of linkage and association studies of reading and reading-related processes. Behav Genet. 2011;41:6–30. doi: 10.1007/s10519-011-9444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boets B, de Beeck HPO, Vandermosten M, Scott SK, Gillebert CR, Mantini D, Ghesquiere P. Intact but less accessible phonetic representation in adults with dyslexia. Science. 2013;342:1251–1254. doi: 10.1126/science.1244333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ligges C, Ungureanu M, Ligges M. Understanding the time variant connectivity of the language network in developmental dyslexia: new insight using Granger causality. J Neural Transm. 2010;117:529–543. doi: 10.1007/s00702-010-0367-x. [DOI] [PubMed] [Google Scholar]
- 11.Frye RE, Hasan K, Xue L, Strickland D, Malmberg B, Liederman J, Papnicolaou A. Splenium microstucture is related to two dimensons of reading skills. Neuroreport. 2008;19:1627. doi: 10.1097/WNR.0b013e328314b8ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiebert EH, Taylor BM. Beginning reading instruction: research on early interventions. In: Kamil ML, Mosenthal PB, Pearson PD, Barr R, editors. Handbook of Reading Research. Vol. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 455–482. [Google Scholar]
- 13.Finn ES, Shen X, Holahan JM, Scheinost D, Lacadie C, Papdemetris X, Constable RT. Disruption of functional networks in dyslexia: a whole-brain data-driven analysis of connectivity. Biol Psychiatry. 2014;76:397–404. doi: 10.1016/j.biopsych.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pennington BF. From single to multiple deficit models of developmental disorders. Cognition. 2006;101:385–413. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Paracchini S, Thomas A, Castro S, Lai C, Paramasivam M, Wang Y, Keating BJ, Taylor JM, Hacking DF, Scerri T. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum Mol Genet. 2006;15:1659–1666. doi: 10.1093/hmg/ddl089. [DOI] [PubMed] [Google Scholar]
- 16.Galaburda AM, Sherman GF, Rosen GD. Developmental dyslexia: four consecutive patients with cortical anomahes. Reading. 1985;6:1.8. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- 17.Darki F, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. Three dyslexia susceptibility genes, DYX1C1, DCDC2, and KIAA0319, affect temporo-parietal white matter structure. Biol Psychiatry. 2012;72:671–676. doi: 10.1016/j.biopsych.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Jahanshad N, Kohannim O, Hibar DP, Stein JL, McMahon KL, de Zubicaray GI, Medland SE, Montgomery GW, Whitfield JB, Martin NG. Brain structure in healthy adults is related to serum transferrin and the H63D polymorphism in the HFE gene. Proc Natl Acad Sci USA. 2012;109:E851–E859. doi: 10.1073/pnas.1105543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centanni TM, Booker AB, Sloan AM, Chen F, Maher B, Carraway R, Khodaparast N, Rennaker R, LoTurco J, Kilgard M. Knockdown of the dyslexia-associated gene Kiaa0319 impairs temporal responses to speech stimuli in rat primary auditory cortex. Cereb Cortex. 2014;24:1753–1766. doi: 10.1093/cercor/bht028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornickel J, Kraus N. Unstable representation of sound: a biological marker of dyslexia. J Neurosci. 2013;33:3500–3504. doi: 10.1523/JNEUROSCI.4205-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinel P, Fauchereau F, Moreno A, Barbot A, Lathrop M, Zelenika D, Le Bihan D, Poline J-B, Bourgeron T, Dehaene S. Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions. J Neurosci. 2012;32:817–825. doi: 10.1523/JNEUROSCI.5996-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torgesen JK. The prevention of reading difficultues. J Sch Psychol. 2002;40:7–26. [Google Scholar]
- 23.Torgesen JK. Individual differneces in response to early interventions in reading: the lingering problem of treatment resisters. Learn Disabil Res Pract. 2000;15:55–64. [Google Scholar]
- 24.Smith AB, Roberts J, Lambrecht Smith S, Locke JL, Bennett J. Reduced speaking rate as an early predictor of reading disability. Am J Speech Lang Pathol. 2006;15:289–297. doi: 10.1044/1058-0360(2006/027). [DOI] [PubMed] [Google Scholar]
- 25.Wanzek J, Vaughn S. Research-based implications from extensive early reading interventions. School Psych Rev. 2007;36:541. [Google Scholar]
- 26.Valas H. Students with learning disabilities and low-achieving students: peer acceptance, loneliness, self-esteem, and depression. Soc Psychol Educ. 1999;3:173–192. [Google Scholar]
- 27.Baker SF, Ireland JL. The link between dyslexic traits, executive functioning, impulsivity and social self-esteem among an offender and non-offender sample. Int J Law Psychiatry. 2007;30:492–503. doi: 10.1016/j.ijlp.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Grigorenko EL. Learning disabilites in juvenile offenders. Child Adolesc Psychiatr Clin N Am. 2006;15:353–371. doi: 10.1016/j.chc.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Molfese VJ, Modglin A, Molfese DL. The role of environment in the development of reading skills: a longitudinal study of preschool and school-age measures. J Learn Disabil. 2003;36:59–67. doi: 10.1177/00222194030360010701. [DOI] [PubMed] [Google Scholar]
- 30.Guttorm TK, Leppanen PH, Hamalainen JA, Eklund KM, Lyytinen HJ. Newborn event-related potentials predict poorer pre-reading skills in children at risk for dyslexia. J Learn Disabil. 2010;43:391–401. doi: 10.1177/0022219409345005. [DOI] [PubMed] [Google Scholar]
- 31.van Zuijen TL, Plakas A, Maassen BA, Been P, Maurits NM, Krikhaar E, van Driel J, van der Leij A. Temporal auditory processing at 17 months of age is associated with preliterate language comprehension and later word reading fluency: an ERP study. Neurosci Lett. 2012;528:31–35. doi: 10.1016/j.neulet.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 32.Maurer U, Schulz E, Brem S, der Mark S, Bucher K, Martin E, Brandeis D. The development of print tuning in children with dyslexia: evidence from longitudinal ERP data supported by fMRI. Neuroimage. 2011;57:714–722. doi: 10.1016/j.neuroimage.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 33.Molfese DL. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 2000;72:238–245. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- 34.Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, Lyytinen H, Whitfield-Gabrieli S, Glover GH, Reiss AL, et al. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci USA. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Black JM, Myers CA, Hoeft F. The utility of neuroimaging studies for informing educational practice and policy in reading disorders. New Dir Child Adolesc Dev. 2015;147:49–56. doi: 10.1002/cad.20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf M. Proust and the Squid: The Story and Science of the Reading Brain. New York: Cambridge: Icon; 2008. [Google Scholar]
- 37.Dehaene S. Reading in the Brain: The New Science of How We Read. New York: Penguin; 2009. [Google Scholar]
- 38.Breznitz Z. Fluency in Reading: Synchronization of Proccesses. Mahwah, NJ: Routledge; 2006. [Google Scholar]
- 39.Just MA, Carpenter PA. Cognitive Processes in Comprehension. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 2013. [Google Scholar]
- 40.Goswami U. Phonology, reading development, and dyslexia: a cross-linguistic perspective. Ann Dyslexia. 2002;52:141–163. [Google Scholar]
- 41.Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Neurobiological studies of reading and reading disability. J Commun Disord. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- 42.Price CJD, Devlin JT. The interactive account of the ventral occipitotemporal contributions to reading. Trends Cogn Sci. 2011;15:246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dehaene S, Cohen L. The unique role of the visual word from area in reading. Trends Cogn Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Binder JR, Desai RH. The neurobiolgoy of semantic memory. Trends Cogn Sci. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rimrodt SL, Clements-Stephens AM, Pugh KR, Courtney SM, Gaur P, Pekar JJ, Cutting LE. Functional MRI of sentence comprehension in children with dyslexia: beyond word recognition. Cereb Cortex. 2009;19:402–413. doi: 10.1093/cercor/bhn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Bitan TB, Burman DD, Chou TL, Lu D, Cone NE, Cao F, Booth JR. The interaction between orthographic and phonological information in children: an fMRI study. Hum Brain Mapp. 2007;28:880–891. doi: 10.1002/hbm.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelamn DR, Parrish TR, Mesulam MM. The development of specialized brain systems in reading and oral language. Child Neuropsychol. 2001;7:119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- 50.Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- 51.Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- 52.Martin A, Schurz M, Kronbichler M, Richlan F. Reading in the brain of children and adults: a meta-analysis of 40 functional magnetic resonance imaging studies. Hum Brain Mapp. 2015;36:1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon G, Lanoë C, Poirel N, Rossi S, Lubin A, Pineau A, Houdé O. Dynamics of the anatomical changes that occur in the brains of schoolchildren as they learn to read. PLoS One. 2013;8:e81789. doi: 10.1371/journal.pone.0081789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houde O, Rossi S, Lubin A, Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev Sci. 2010;13:876–885. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- 55.Vogel AC, Church JA, Power JD, Miezin FM, Petersen SE, Schlaggar BL. Functional network architecture of reading-related regions across development. Brain Lang. 2013;125:231–243. doi: 10.1016/j.bandl.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wandell BA, Yeatman JD. Biological development of reading circuits. Curr Opin Neurobiol. 2013;23:261–268. doi: 10.1016/j.conb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012;7:e49790. doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. DTI demonstrates non-linear white matter tract development from childhood to adulthood. Proc Intl Soc Mag Reson Med. 2007;10:30. [Google Scholar]
- 60.Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proc Natl Acad Sci USA. 2012;109:E3045–E3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamilton LG. The Role of the Home Literacy Environment in the Early Literacy Development of Children at Family-Risk of Dyslexia. York, England: University of UK; 2013. [Google Scholar]
- 62.Payne AC, Whitehurst GJ, Angell AL. The role of home literacy environment in the development of language ability in preschool children from low-income families. Early Child Res Q. 1994;9:427–440. [Google Scholar]
- 63.Sénéchal M, LeFevre JA. Parental involvement in the development of children’s reading skill: a five year longitudinal study. Child Dev. 2002;73:445–460. doi: 10.1111/1467-8624.00417. [DOI] [PubMed] [Google Scholar]
- 64.Bus AG, van Ijzendoorn MH, Pellegrini AD. Joint book reading makes for success in learning to read: a meta-analysis of intergenerational transmission of literacy. Rev Educ Res. 1995;65:1–22. [Google Scholar]
- 65.Torppa M, Poikkeus AM, Laakso ML, Tolvanen A, Leskinen E, Leppanen PH, Lyytinen H. Modeling the early paths of phonological awareness and factors supporting its development in children with and without familial risk of dyslexia. Sci Stud Read. 2007;11:73–103. [Google Scholar]
- 66.Koster CB, Krikhaar EM, Zwarts F. Differences at 17 months: productive language patterns in infants at familial risk for dyslexia and typically developing infants. J Speech Lang Hear Res. 2010;48:426–438. doi: 10.1044/1092-4388(2005/029). [DOI] [PubMed] [Google Scholar]
- 67.Leppanen PH, Lyytinen H. Auditory event-related potentials in the study of developmental language-related disorders. Audiol Neurootol. 1997;2:308–340. doi: 10.1159/000259254. [DOI] [PubMed] [Google Scholar]
- 68.Leppanen PH, Pihko E, Eklund KM, Lyytinen H. Cortical responses of infants with and without a genetic risk for dyslexia: II Group effects. Neuroreport. 1999;10:969–973. doi: 10.1097/00001756-199904060-00014. [DOI] [PubMed] [Google Scholar]
- 69.Lyytinen H, Ahonen T, Eklund K, Guttorm TK, Laakso ML, Leinonen S, Leppanen PH, Lyytinen P, Poikkeus AM, Puolakanaho A, et al. Developmental pathways of children with and without familial risk for dyslexia during the first years of life. Dev Neuropsychol. 2001;20:535–554. doi: 10.1207/S15326942DN2002_5. [DOI] [PubMed] [Google Scholar]
- 70.Lyytinen H, Guttorm TK, Huttunen T, Hamalainen J, Leppanen PHT, Vesterinen M. Psychophysiology of developmental dyslexia: a review of findings including studies of children at risk for dyslexia. J Neurolinguistics. 2005;18:167–195. [Google Scholar]
- 71.Lyytinen P, Eklund K, Lyytinen H. Language development and literacy skills in late-talking toddlers with and without familial risk for dyslexia. Ann Dyslexia. 2005;55:166–192. doi: 10.1007/s11881-005-0010-y. [DOI] [PubMed] [Google Scholar]
- 72.Smith AB, Smith SL, Locke JL, Bennett J. A longitudinal study of speech timing in young children later found to have reading disability. J Speech Lang Hear Res. 2008;51:1300–1314. doi: 10.1044/1092-4388(2008/06-0193). [DOI] [PubMed] [Google Scholar]
- 73.Scarborough HS. Very early language deficits in dyslexic children. Child Dev. 1990;61:1728–1743. [PubMed] [Google Scholar]
- 74.Scarborough HS. Early identification of children at risk for reading disabilities: phonological awareness and some other promising predictors. In: Shapiro BK, Accardo PJ, Capute AJ, editors. Specific Reading Disability: A View of the Spectrum. Timonium, MD: York Press; 1998. pp. 75–119. [Google Scholar]
- 75.Flax JF, Realpe-Bonilla T, Roesler C, Choudhury N, Benasich A. Using early standardized language measures to predict later language and early reading outcomes in children at high risk for language-learning impairments. J Learn Disabil. 2009;42:61–75. doi: 10.1177/0022219408326215. [DOI] [PubMed] [Google Scholar]
- 76.Lyytinen P, Eklund K, Lyytinen H. Language development and literacy skills in late talking toddlers with and without familial risk for dyslexia. Ann Dyslexia. 2007;55:166–192. doi: 10.1007/s11881-005-0010-y. [DOI] [PubMed] [Google Scholar]
- 77.Rescorla L, Roberts J. Nominal versus verbal morpheme use in late talkers at ages 3 and 4. J Speech Lang Hear Res. 2002;45:1219–1231. doi: 10.1044/1092-4388(2002/098). [DOI] [PubMed] [Google Scholar]
- 78.Scarborough HS, Dobrich W. Development of children with early language delay. J Speech Lang Hear Res. 1990;33:70–83. doi: 10.1044/jshr.3301.70. [DOI] [PubMed] [Google Scholar]
- 79.Richardson U, Leppanen PH, Leiwo M, Lyytinen H. Speech perception of infants with high familial risk for dyslexia differ at the age of 6 months. Dev Neuropsychol. 2003;23:385–397. doi: 10.1207/S15326942DN2303_5. [DOI] [PubMed] [Google Scholar]
- 80.Marchman V, Fernald A. Speed of word recognition and vocabulary knowledge in infancy predict cognitive and language outcomes in later childhood. Dev Sci. 2008;11:F9–F11. doi: 10.1111/j.1467-7687.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schatschneider C, Fletcher JM, Francis DJ, Carlson CD, Foorman BR. Kindergarten prediction of reading skills: a longitudinal comparative analysis. J Educ Psychol. 2004;96:265–282. [Google Scholar]
- 82.de jong PF, van der Leij A. Specific contributions of phonological abilities to early reading acquisition: results from a Dutch latent variable longitudinal study. J Educ Psychol. 1999;91:450–476. [Google Scholar]
- 83.Georgiou GK, Parrila R. Predictors of word decoding and reading fluency across languages varying in orthographic consistency. J Educ Psychol. 2008;100:566–580. [Google Scholar]
- 84.Wagner RK, Torgesen JK, Rashotte CA. Longitudinal studies of phonological processing and reading. J Learn Disabil. 1994;27:276–286. doi: 10.1177/002221949402700503. [DOI] [PubMed] [Google Scholar]
- 85.McBride-Chang C. The ABCs of the ABCs: the development of letter name and letter sound knowledge. Merrill-Palmer Quart. 1999;45:2. [Google Scholar]
- 86.Senechal M, LeFevre J. Parental involvement in development of children’s reading skills: a five year longitudinal study. Child Dev. 2002;73:445–460. doi: 10.1111/1467-8624.00417. [DOI] [PubMed] [Google Scholar]
- 87.Scarborough HS, Dobrich W, Hager M. Preschool literacy experience and later reading achievement. J Learn Disabil. 1991;24:508–511. doi: 10.1177/002221949102400811. [DOI] [PubMed] [Google Scholar]
- 88.Moll KL, Loff A, Snowling MJ. Cognitive endophenotypes of dyslexia. Sci Stud Read. 2013;17:385–597. [Google Scholar]
- 89.Pennington BF, Lefly DL. Early reading development in children at family risk for dyslexia. Child Dev. 2001;72:816–833. doi: 10.1111/1467-8624.00317. [DOI] [PubMed] [Google Scholar]
- 90.Scarborough HS. Prediction of reading disabilites from familial and individual differences. J Educ Psychol. 1989;81:101. [Google Scholar]
- 91.Boets B, Wouters J, van Wieringen A, Ghesquiere P. Auditory processing, speech perception and phonological ability in pre-school children at high-risk for dyslexia: a longitudinal study of the auditory temporal processing theory. Neuropsychologia. 2007;45:1608–1620. doi: 10.1016/j.neuropsychologia.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 92.Wolf M, Denckla MB. RAN/RAS: Rapid Automatized Naming and Rapid Alternating. Austin, TX: PRO-ED, Inc; 2005. [Google Scholar]
- 93.Caravolas M, Lervag A, Mousikou P, Efrim C, Litavsky M, Onochie-Quintanilla E, Salas N, Schoffelova M, Defior S, Mikulajova M, et al. Common patterns of prediction of literacy development in different alphabetic orthographies. Psychol Sci. 2012;23:678–686. doi: 10.1177/0956797611434536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilites. Annu Rev Psychol. 2012;63:427–452. doi: 10.1146/annurev-psych-120710-100431. [DOI] [PubMed] [Google Scholar]
- 95.Goswami U. The neural basis of dyslexia may originate in primary auditory cortex. Brain. 2014;137:3100–3102. doi: 10.1093/brain/awu296. [DOI] [PubMed] [Google Scholar]
- 96.Bishop DV, Carlyon RP, Deeks JM, Bishop SJ. Auditory temporal processing impairment: neither necessary nor sufficient for causing language impairment in children. J Speech Lang Hear Res. 1999;42:1295–1310. doi: 10.1044/jslhr.4206.1295. [DOI] [PubMed] [Google Scholar]
- 97.Tallal P. Improving language and literacy is a matter of time. Nat Rev Neurosci. 2004;5:721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- 98.Tallal P, Gaab N. Dynamic auditory processing, musical experience and language development. Trends Neurosci. 2006;29:382–390. doi: 10.1016/j.tins.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 99.Banai K, Hornickel J, Skoe E, Nicol T, Zecker S, Kraus N. Reading and subcortical auditory function. Cereb Cortex. 2009;19:2699–2707. doi: 10.1093/cercor/bhp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boets B, Vandermosten M, Poelmans H, Luts H, Wouters J, Ghesquiere P. Preschool impairments in auditory processing and speech perception uniquely predict future reading problems. Res Dev Disabil. 2011;32:560–570. doi: 10.1016/j.ridd.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 101.Leppanen PH, Hamalainen JA, Salminen HK, Eklund KM, Guttorm TK, Lohvansuu K, Puolakanaho A, Lyytinen H. Newborn brain event-related potentials revealing atypical processing of sound frequency and the subsequent association with later literacy skills in children with familial dyslexia. Cortex. 2010;46:1362–1376. doi: 10.1016/j.cortex.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Plakas A, van Zuijen T, van Leeuwen T, Thomson JM, van der Leij A. Impaired non-speech auditory processing at a pre-reading age is a risk-factor for dyslexia but not a predictor: an ERP study. Cortex. 2013;49:1034–1045. doi: 10.1016/j.cortex.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 103.Carr KW, White-Schwoch T, Tierney AT, Strait DL, Kraus N. Beat synchronization predicts neural speech encoding and reading readiness in preschoolers. Proc Natl Acad Sci USA. 2014;111:14559–14564. doi: 10.1073/pnas.1406219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Corriveau KH, Goswami U, Thomson JM. Auditory processing and early literature skill in a preschool and kindergarten population. J Learn Disabil. 2010;43:369–382. doi: 10.1177/0022219410369071. [DOI] [PubMed] [Google Scholar]
- 105.Van Lancker DR, Kreiman J, Cummings J. Voice perception deficits: neuroanatomical correlates of phonagnosia. J Clin Exp Neuropsychol. 1989;11:665–674. doi: 10.1080/01688638908400923. [DOI] [PubMed] [Google Scholar]
- 106.Raschle N, Stering PL, Meissner S, Gaab N. Altered neuronal response during rapid auditory processing and its relation to phonological processing in prereading children at familial risk for dyslexia. Cereb Cortex. 2013;24:2489–2501. doi: 10.1093/cercor/bht104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- 108.Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006;44:396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choudhury N, Benasich AA. Maturation of auditory evoked potentials from 6 to 48 months: prediction to 3 and 4 year language and cognitive abilities. Clin Neurophysiol. 2011;122:320–338. doi: 10.1016/j.clinph.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 110.Choudhury N, Leppanen PH, Leevers HJ, Benasich AA. Infant information processing and family history of specific language impairment: converging evidence for RAP deficits from two paradigms. Dev Sci. 2007;10:213–236. doi: 10.1111/j.1467-7687.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Choudhury N, Benasich AA. A family aggregation study: the influence of family history and other risk factors on language development. J Speech Lang Hear Res. 2003;46:261–272. doi: 10.1044/1092-4388(2003/021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.White S, Milne E, Rosen S, Hansen P, Swettenham J, Frith U, Ramus F. The role of sensorimotor impairments in dyslexia: a multiple case study of dyslexic children. Dev Sci. 2006;9:237–269. doi: 10.1111/j.1467-7687.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 113.Moritz C, Sasha Y, Papadelis G, Thomson J, Wolf M. Links between early rhythm skills, musical training, and phonological awareness. Read Writ. 2012;26:739–769. [Google Scholar]
- 114.Anvari SH, Trainor LJ, Woodside J, Levy BA. Relations among musical skills, phonological processing, and early reading ability in preschool children. J Exp Child Psychol. 2002;83:111–130. doi: 10.1016/s0022-0965(02)00124-8. [DOI] [PubMed] [Google Scholar]
- 115.David D, Wade-Woolley L, Kirby J, Smithrim K. Rhythm and reading development in school-age children: a longitudinal study. J Res Read. 2007;30:169–183. [Google Scholar]
- 116.Dellatolas G, Watier L, Le Normand MT, Lubart T, Chevrie-Muller C. Rhythm reproduction in kindergarten, reading performance at second grade, and developmental dyslexia theories. Arch Clin Neuropsychol. 2009;24:555–563. doi: 10.1093/arclin/acp044. [DOI] [PubMed] [Google Scholar]
- 117.Zuk J, Andrade PE, Andrade OVCA, Gardiner MF, Gaab N. Musical, language and reading abilities in early Portuguese readers. Front Psychol. 2013;4:288. doi: 10.3389/fpsyg.2013.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tallal P, Gaab N. Dynamic auditory processing, musical experience and language development. Trends Neurosci. 2006;29:382–390. doi: 10.1016/j.tins.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 119.Gaab N, Tallal P, Kim H, Lakshminarayanan K, Glover GH, Glover GH. Neural correlates of rapid spectro-temporal processing in musicians and nonmusicians. Ann N Y Acad Sci. 2005;1060:82–88. doi: 10.1196/annals.1360.040. [DOI] [PubMed] [Google Scholar]
- 120.Franceschini S, Corradi N, Ruffino M, Gori S, Gianesini T, Facoetti A. Atypical local perception precedence in preschooler poor readers. Perception. 2010;39:79. [Google Scholar]
- 121.Plaza M, Cohen H. The contribution of phonological awareness and visual attention in early reading and spelling. Dyslexia. 2007;13:67–76. doi: 10.1002/dys.330. [DOI] [PubMed] [Google Scholar]
- 122.Ferretti G, Mazzoti S, Brizzolara D. Visual scanning and reading ability in normal and dyslexic children. Behav Neurol. 2008;19:87–92. doi: 10.1155/2008/564561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boets B, Wouters J, van Wieringen A, De Smedt B, Ghesquiere P. Modelling relations between sensory processing, speech perception, orthographic and phonological ability, and literacy achievement. Brain Lang. 2008;106:29–40. doi: 10.1016/j.bandl.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 124.Kevan A, Pammer K. Predicting early reading skills from pre-reading measures of dorsal stream functioning. Neuropsychologia. 2009;47:3174–3181. doi: 10.1016/j.neuropsychologia.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 125.Stanovich KE. Toward a more inclusive definition of dyslexia. Dyslexia. 1996;2:154–166. [Google Scholar]
- 126.Stanovich KE. The future of mistake: will discrepancy measurement continue to make the learning disabilities feild a pseudoscience? Learn Disabil Q. 2005;28:103–106. [Google Scholar]
- 127.Tanaka H, Black JM, Hulme C, Stanley MS, Kesler SR, Whitfield-Gabrieli S, Hoeft F. The brain basis of the phonological deficit in dyslexia is independent of IQ. Psychol Sci. 2011;22:1442–1451. doi: 10.1177/0956797611419521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wadsworth SJ, Olson RK, DeFries JC. Differential genetic etiology of reading difficulties as a function of IQ: an update. Behav Genet. 2010;40:751–758. doi: 10.1007/s10519-010-9349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van der Leij A, van Bergen E, van Zuijen T, de Jong P, Maurits N, Maassen B. Precursors of developmental dyslexia : an overview of the longitudinal dutch dyslexia programme study. Dyslexia. 2013;19:191–213. doi: 10.1002/dys.1463. [DOI] [PubMed] [Google Scholar]
- 130.Francis DJ, Shaywitz SE, Stuebing KK, Shaywitz BA, Fletcher JM. Developmental lag versus deficit models of reading disability: a longitudinal, individual growth curves analysis. J Educ Psychol. 1996;88:3–17. [Google Scholar]
- 131.Velluntino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? J Child Psychol Psychiatry. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- 132.Scarborough H. Predicting the future achievement of second graders with reading disabilities: contributions of phonemic awareness, verbal memory, rapid naming, and IQ. Ann Dyslexia. 1998;48:115–136. [Google Scholar]
- 133.Savage R, Lavers N, Pillay V. Working memory and reading difficulties: what we know and what we don’t know about the relationship. Educ Psychol Rev. 2007;19:185–221. [Google Scholar]
- 134.Johnson C, Goswami U. Phonological awareness, vocabulary, and reading in deaf children with cochlear implants. J Speech Lang Hear Res. 2010;53:237–261. doi: 10.1044/1092-4388(2009/08-0139). [DOI] [PubMed] [Google Scholar]
- 135.Swanson HL, Zheng X, Jerman O. Working memory, short-term memory, and reading disabilites : a selective meta-analysis of the literature. J Learn Disabil. 2009;42:280–287. doi: 10.1177/0022219409331958. [DOI] [PubMed] [Google Scholar]
- 136.Melby-Lervag M. Is working memory training effective? a meta analysis. Dev Psychol. 2011;49:270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- 137.Cowan FM, Alloway T. Development of Working Memory in Childhood. New York: Psychology Press; 2008. [Google Scholar]
- 138.Shaul S. Event-related potentials (ERPs) in the study of dyslexia. In: Breznitz Z, editor. Brain Research in Language. New York: Springer; 2008. pp. 51–92. [Google Scholar]
- 139.Breznitz Z. Brain Research in Language. Vol. 1. New York: Springer; 2008. [Google Scholar]
- 140.Luck SJ. An Introduction to the Event-Related Potential Technique. Vol. 2. Cambridge, MA: MIT Press; 2014. [Google Scholar]
- 141.Espy KA, Molfese DL, Molfese VJ, Modglin A. Development of auditory event related potentials in young children and relations to word level reading abilities at age 8 years. Ann Dyslexia. 2004;54:9–38. doi: 10.1007/s11881-004-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Molfese DL, Molfese VJ, Key S, Modglin A, Kelley S, Terrell S. Reading and cognitive abilities: longitudinal studies of brain and behavior changes in young children. Ann Dyslexia. 2002;52:99–119. [Google Scholar]
- 143.Molfese VJ, Molfese DL, Modgline AA. Newborn and preschool predictors of second-grade reading scores: an evaluation of categorical and continuous scores. J Learn Disabil. 2001;34:545–554. doi: 10.1177/002221940103400607. [DOI] [PubMed] [Google Scholar]
- 144.van Herten M, Pasman J, van Leeuwen TH, Been PH, van der Leij A, Zwarts F, Maassen B. Differences in AERP responses and atypical hemispheric specialization in 17-month-old children at risk of dyslexia. Brain Res. 2008;1201:100–105. doi: 10.1016/j.brainres.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 145.Guttorm TK, Leppanen PHT, Richardson U, Lyytinen H. Event related potentials and consonant differentiation in newborns with familial risk for dyslexia. J Learn Disabil. 2001;34:534–544. doi: 10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- 146.Guttorm TK, Leppanen PH, Poikkeus AM, Eklund KM, Lyytinen P, Lyytinen H. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex. 2005;41:291–303. doi: 10.1016/s0010-9452(08)70267-3. [DOI] [PubMed] [Google Scholar]
- 147.Näätänen R, Winkler I. The concept of auditory stimulus representation in cognitive neuroscience. Psychol Bull. 1999;125:826. doi: 10.1037/0033-2909.125.6.826. [DOI] [PubMed] [Google Scholar]
- 148.Näätänen R, Jacobsen T, Winkler I. Memory based or afferent processes in the mismatched negativity (MMN): a review of the evidence. Psychophysiology. 2005;42:25–32. doi: 10.1111/j.1469-8986.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 149.Lovio R, Näätänen R, Kujala T. Abnormal pattern of cortical speech feature discrimination in 6-year-old children at risk for dyslexia. Brain Res. 2010;1335:53–62. doi: 10.1016/j.brainres.2010.03.097. [DOI] [PubMed] [Google Scholar]
- 150.Pakarinen S, Takegata R, Rinne T, Huotilainen M, Näätänen R. Measurement of extensive auditory discrimination profiles using mismatch negativity of the auditory event-related potential. Clin Neurophysiol. 2007;118:177–185. doi: 10.1016/j.clinph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 151.Rinne T, Särkkä A, Degerman A, Schröger E, Alho K. Two seperate mechanisms underlie auditory change detection and involuntary control of attention. Brain Res. 2006;1077:135–143. doi: 10.1016/j.brainres.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 152.van der Leij A, van Bergen E, van Zuijen T, de Jong P, Maurits N, Maassen B. Precursors of developmental dyslexia: an overview of the longitudinal Dutch dyslexia programme study. Dyslexia. 2013;19:191–213. doi: 10.1002/dys.1463. [DOI] [PubMed] [Google Scholar]
- 153.van Leeuwen T, Been P, van Herten M, Zwarts F, Maassen B, van der Leij A. Two month old infants at risk for dyslexia do not discriminate: a brain mapping study. J Neurolinguist. 2008;21:333–348. [Google Scholar]
- 154.van Leeuwen T, Been P, Kuijpers C, Zwarts F, Maassen B, van der Leij A. Mismatch response is absent in 2-month-old infants at risk for dyslexia. Neuroreport. 2006;17:351–355. doi: 10.1097/01.wnr.0000203624.02082.2d. [DOI] [PubMed] [Google Scholar]
- 155.Maurer U, Bucher K, Brem S, Brandeis D. Altered responses to tone and phoneme mismatch in kindergartners at familial dyslexia risk. Neuroreport. 2003;14:2245–2250. doi: 10.1097/00001756-200312020-00022. [DOI] [PubMed] [Google Scholar]
- 156.Maurer U, Bucher K, Brem S, Benz R, Kranz F, Schulz E, van der Mark S, Steinhausen H-C, Brandeis D. Neurophysiology in preschool improves behavioral prediction of reading ability throughout primary school. Biol Psychiatry. 2009;66:341–348. doi: 10.1016/j.biopsych.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 157.Goswami U. A temporal sampling framework for developmental dyslexia. Trends Cogn Sci. 2011;15:3–10. doi: 10.1016/j.tics.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 158.Leppanen PH, Hamalainen JA, Guttorm TK, Eklund KM, Salminen H, Tanskanen A, Torppa M, Puolakanaho A, Richardson U, Pennala R, et al. Infant brain responses associated with reading-related skills before school and at school age. Neurophysiol Clin. 2012;42:35–41. doi: 10.1016/j.neucli.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 159.Regtvoort AG, van Leeuwen TH, Stoel RD, van der Leij A. Efficiency of visual information processing in children at risk for dyslexia: habituation of single trial ERPs. Brain and Language. 2006;98:319–331. doi: 10.1016/j.bandl.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 160.Bach S, Richardson U, Brandeis D, Martin E, Brem S. Print specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade. Neuroimage. 2013;82:605–616. doi: 10.1016/j.neuroimage.2013.05.062. [DOI] [PubMed] [Google Scholar]
- 161.von Koss TJ, Syversen G, Simonsen HG, Moen I, Lindgren M. Brain responses to lexical semantic priming in children at risk for dyslexia. Brain Lang. 2007;102:243–261. doi: 10.1016/j.bandl.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 162.Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage. 2011;56:1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 164.Norton ES, Beach SD, Gabrieli JD. Neurobiology of dyslexia. Current Opinion in Neurobiology. 2015;30:73–78. doi: 10.1016/j.conb.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hampson M, Olson IR, Leung HC, Skudlarski P, Gore JC. Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport. 2004;15:1315–1319. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- 166.Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment Retard Dev Disabil Res Rev. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 168.Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, et al. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Maisog J, Einbinder E, Flowers D, Turkeltaub P, Eden G. A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- 171.Richlan F, Kronbichler M, Wimmer H. Structural abnormalities in the dyslexic brain: A meta-analysis of voxel-based morphometry studies. Hum Brain Mapp. 2012;34:3055–3065. doi: 10.1002/hbm.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Norton ES, Beach SD, Gabrieli JD. Neurobiolgoy of dyslexia. Curr Opin Neurobiol. 2015;30:73–78. doi: 10.1016/j.conb.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shaywitz S, Shaywitz B, Pugh K, Fulbright R, Constable R, Mencl W, Shankweiler D, Liberman A, Skudlarski P, Fletcher J, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci USA. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stoodley CJ, Stein JF. The cerebellum and dyslexia. Cortex. 2011;47:101–116. doi: 10.1016/j.cortex.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 175.Richards TL, Berninger VW. Abnormal fMRI connectivity in children with dyslexia during a phoneme task: before but not after treatment. J Neurolinguist. 2008;21:294–304. doi: 10.1016/j.jneuroling.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Steinbrink C, Vogt K, Kastrup A, Muller HP, Juengling FD, Kassubek J, Riecker A. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3. 0 T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 177.Hasan K, Molfese D, Walimuni I, Stuebing K, Papanicolaou A, Narayana P, Fletcher J. Diffusion tensor quantification and cognitive correlates of the macrostructure and microstructure of the corpus callosum in typically developing and dyslexic children. NMR Biomed. 2012;25:1263–1270. doi: 10.1002/nbm.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Raschle N, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2011;57:742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Raschle NM, Becker BLC, Smith S, Fehlbaum LV, Wang Y, Gaab N. Investigating the influences of language delay and/or familial risk for dyslexia on brain structure in 5-year-olds. Cereb Cortex. doi: 10.1093/cercor/bhv267. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Im K, Raschle NM, Smith SA, Grant PE, Gaab N. Atypical sulcal pattern in children with developmental dyslexia and at-risk kindergartners. Cereb Cortex. doi: 10.1093/cercor/bhu305. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Clark KA, Helland T, Specht K, Narr KL, Manis FR, Toga AW, Hugdahl K. Neuroanatomical precursors of dyslexia identified from pre-reading through age to 11. Brain. 2014;137(Pt 12):3136–3141. doi: 10.1093/brain/awu229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Linkersdörfer J, Jurcoane A, Lindberg S, Kaiser J, Hasselhorn M, Fiebach CJ, Lonnemann J. The association between gray matter volume and reading proficiency: a longitudinal study of beginning readers. J Cogn Neurosci. doi: 10.1162/jocn_a_00710. In press. [DOI] [PubMed] [Google Scholar]
- 183.Black JM, Tanaka H, Stanley L, Nagmine M, Zakerani N, Thurston A, Hoeft F. Maternal history of reading difficulty is associated with reduced language related gray matter in beginning readers. Neuroimage. 2012;59:3021–3023. doi: 10.1016/j.neuroimage.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Saygin ZM, Norton ES, Osher DE, Beach SD, Cyr AB, Ozernov-Palchik O, Yendiki A, Fischl B, Gaab N, Gabrieli JD. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J Neurosci. 2013;33:13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vandermosten M, Vanderauwera J, Theys C, De Vos A, Vanvooren S, Sunaert S, Wouters J, Ghesquière P. A DTI tractography study in prereaders at risk for dyslexia. Dev Cogn Neurosci. 2015;14:8–15. doi: 10.1016/j.dcn.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Langer N, Peysakhovich B, Zuk J, Drottar M, Sliva DD, Smith S, Becker B, Grant E, Gaab N. White matter alterations in infants at risk for developmental dyslexia. Cereb Cortex. doi: 10.1093/cercor/bhv281. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Myers CA, Vandermosten M, Farris EA, Hancock R, Gimenez P, Black JM, Hoeft F. Structural changes in white matter are uniquely related to children’s reading development. Psychol Sci. 2014;25:1870–1883. doi: 10.1177/0956797614544511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Raschle NM, Zuk J, Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc Natl Acad Sci USA. 2012;109:2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gaab N, Gabrieli JD, Deutsch GK, Tallal P, Temple E. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: an fMRI study. Restor Neurol Neurosci. 2007;25:295–310. [PubMed] [Google Scholar]
- 190.Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JD. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci USA. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yamada Y, Stevens C, Dow M, Harn BA, Chard DJ, Neville HJ. Emergence of the neural network for reading in five-year-old beginning readers of different levels of pre-literacy abilities: an fMRI study. Neuroimage. 2011;57:704–713. doi: 10.1016/j.neuroimage.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Specht K, Hugdahl K, Ofte S, Nygard M, Bjornerud A, Plante E, Helland T. Brain activation on pre-reading tasks reveals at-risk status for dyslexia in 6-year-old children. Scand J Psychol. 2009;50:79–91. doi: 10.1111/j.1467-9450.2008.00688.x. [DOI] [PubMed] [Google Scholar]


