Abstract
Objective
Bilateral tubal ligation (BTL) is a common form of birth control in the United States. There is limited, contradictory data examining BTL and the risk of endometrial cancer and none examining type I and type II cancers separately. We investigated the association between BTL and endometrial cancer risk utilizing the Women’s Health Initiative (WHI) Observational (OS) and Dietary Modification (DM) Studies.
Methods
Demographic information and history of BTL were obtained from the baseline questionnaires from 76,483 WHI participants in the OS and DM. Univariable and multivariable models were used to examine the association of BTL with type I and type II endometrial cancers.
Results
1,137 women were diagnosed with incident endometrial cancer (972 type I and 128 type II) over a mean follow-up of 11.3 years. Overall, 14,499 women (19%) had undergone BTL. There were no statistically significant associations noted between BTL or age at BTL for type I or type II cancers.
Conclusion
We examined the largest patient cohort to date in an effort to determine the impact of BTL on endometrial cancer risk. In the WHI trial we observed no overall effect of BTL on the risk of type I or type II endometrial cancer, suggesting that patients undergoing this popular birth-control method likely do not have an associated change in their baseline risk for endometrial cancer.
Keywords: Tubal Ligation, Endometrial Cancer, Women’s Health Initiative
Introduction
Bilateral tubal ligation (BTL) is one of the common methods of birth control utilized in the United States, estimated to be used by more than 10 million women in 2002 (1). Endometrial cancer is the most common cancer of the female genital tract and fourth most common of cancer among women in the United States, with more than 47,000 new cases and 8,000 deaths annually (2). Risk of type I endometrial cancer is associated with hormone balance and estrogen excess; however, the underlying risk factors for type II cancers may be fundamentally different and are not thought to be as strongly associated with hormonal imbalance, although this role has not been completely excluded (3–5). Type II endometrial carcinomas act in a manner more synonymous to ovarian cancers with regards to spread, recurrence and survival, accounting for their higher rates of recurrence and mortality.
The link between tubal ligation and endometrial cancer has not been clearly defined either in biological or population based studies. Four studies have examined a combined total of 1,000 women with endometrial cancer and have reported mixed conclusions, possibly due to insufficient power (6–9). Furthermore, endometrial cancer was evaluated as a homogeneous outcome rather than as individual subtypes. The limited and contradictory data on the relationship between BTL and endometrial cancer risk in the epidemiologic literature, specifically the differential risk by sub-type, suggests the need for further investigation. We investigated the association between BTL and endometrial cancer risk utilizing data collected from the Women’s Health Initiative (WHI) Observational Study (OS) and Dietary Modification (DM) Study.
Materials and Methods
Study Population
Details of the Women’s Health Initiative OS and DM including the design, recruitment, and exposure assessments have been previously published.(10–12) The current study is a secondary analysis of this data approved by the Women’s Health Initiative. The OS enrolled a cohort of 93,676 women and the DM enrolled 48,835 women from October 1993 through December 1998 including 40 clinical centers in 24 states and the District of Columbia. At baseline, women were eligible for inclusion in the OS and DM studies if they were between the ages of 50 and 79, postmenopausal, and planning to reside in the same area for at least 3 years. Women were excluded if they were participating in another clinical trial, were unlikely to survive 3 years due to medical comorbidities, or had conditions such as dementia, drug dependency, or alcoholism that could interfere with study participation. For the DM, women were further excluded if they: were on a low-fat diet (<32% energy from fat), had dietary needs incompatible with the intervention program, ate 10 or more meals per week outside the home, could not complete a 4-day food record, had Type I diabetes mellitus, colon cancer, or any gastrointestinal conditions that contraindicated a high-fiber diet, or had a bilateral prophylactic mastectomy.
The initial cohort of 134,461 women included 93,676 participants from the observational study (OS) and 40,785 from the dietary modification (DM) study. For the purposes of this study, we excluded 8,050 women from the DM who were also enrolled in the WHI’s hormone therapy trial (HT). From this initial cohort, we then excluded women who reported a hysterectomy at baseline (n=56,960), were missing information regarding BTL status or age at BTL (n=516), or reported a history of endometrial carcinoma at enrollment (n=68). Next, we excluded women whose endometrial carcinoma was unclassified regarding type (n=68). The definitions of type I and type II endometrial cancers used in the WHI were based on the SEER EOD-88, 2nd edition of the SEER coding scheme. Type I was classified as follows: endometrioid (8380/3), adenocarcinoma with squamous metaplasia (8570/3), and adenocarcinoma NOS (8140/3). Type II was classified as follows: clear cell (8310/3), serous (8441/3), papillary serous (8460/3) or papillary carcinoma NOS (8050/3), papillary adenocarcinoma (8260/3), adenosquamous (8530/3), and small cell carcinoma (8041/3) or mullerian mixed tumor (8950/3) or carcinosarcoma NOS (8980/3). Finally, women with missing or zero event time for which follow-up risk could not be calculated (n=366) were excluded from the analysis. After our exclusions, a total of 76,483 women (53,737 women from the OS and 22,746 women from the DM) remained eligible as our final analysis cohort.
Diagnosis of Endometrial Cancer
Details of the outcome assessments for both the OS and the DM have also been previously published (13). As noted, endometrial cancers were initially self-reported by participants in the trial arms on follow-up questionnaires. Reports of new cancers were then adjudicated by local physicians and ultimately by the central coordinating center and/or central adjudicators, with endometrial cancer subtype (endometrioid, serous, etc.) recorded by the central coordinating center. Follow up for the main study continued until March 2005. After that time, participants were re-consented and data collection for the first extension study was updated in an identical fashion through September 2010. In our analysis cohort, the average follow-up time was 11.4 years (SD=3.3 years).
Data Collection
Data were extracted from the original WHI study baseline questionnaires which were completed by the participants in the study. Tubal ligation status was assessed in the initial questionnaires by asking whether the participant had a tubal ligation and the age of tubal ligation with age ranges: <30 years-old, 30–34 years-old, 35–39 years-old, 40–44 years-old and >45 years-old. No information regarding type of tubal ligation was included in the questionnaires. Information regarding reproductive history, medical history including hormone use, and demographics were also collected by self-report. Physical measurements to calculate body mass index (BMI) and waist-to-hip ratio were documented at the baseline enrollment visit.
Statistical analysis
Demographic characteristics of the women were summarized by cancer types and tubal ligation status. In order to assess the relationships between both BTL (yes/no) and age at tubal ligation (<40, 40+, no tubal ligation) and endometrial cancer, separate Cox proportional hazards models were fit for type I and type II cancers separately. For all models, membership in the WHI (OS, DM control arm, DM intervention arm) was used as a stratification variable, and the participant’s age at cancer diagnosis was used as the time scale, with the participant’s age at screening as the left truncation point. Left truncation was used due to the delay from the time when a woman becomes at risk for endometrial cancer and actual study entry (minimum age 50) which cannot be accounted for by simply adjusting for age at enrollment or screening (14, 15).
The unadjusted hazard ratios (HR) with 95% confidence intervals (CI) from univariate Cox models were initially calculated. Next, minimally adjusted models were created using age stratum at randomization and region as covariates. Finally, a more fully adjusted model was considered with the covariates: age stratum at randomization, region, ethnicity, BMI category, parity, history of diabetes at screening, age at menopause, age at menarche, oral contraceptive use and unopposed estrogen use. The additional factors in the multivariate model were chosen a priori based on their clinical relevance. All two-way interactions between each covariate and BTL were calculated; those with p < 0.01 were considered significant effect modifiers. The assumption of proportional hazards was assessed for all models using scaled Schoenfeld residuals as well as including interactions with age in the model. All analyses were performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp, College Station, TX).
Results
Table 1 describes the general categorization of tubal ligation, age at tubal ligation and type of endometrial cancer per study arm. In our final analytic cohort, 19% of the study population had a tubal ligation with 62% (8,951/14,499) of these women reporting tubal ligation between the ages of 30–39. There were 1,137 women who were diagnosed with endometrial cancer (~1% in the study population), with the majority of these type I (n=972, 85%) as compared to type II cancers (n=165, 15%). Table 2 shows the distribution of demographic and clinical variables stratified by history of BTL and by type of endometrial cancer.
Table 1. Characteristics of Women in the WHI Cohort by Tubal Ligation Status and History of Endometrial Cancer.
Characteristics of the women in the OS (Observational Study Arm) and DM (Dietary Modification Study Arm) are noted in (A) as well as endometrial cancers noted and tubal ligation status. In (B) the relationship between tubal ligation and endometrial cancers in the study cohort are noted.
| A.
| |||
|---|---|---|---|
| Demographic | OS (n=53,737) | DM (n=22,746) | OS + DM (n=76,483) |
|
| |||
| N (%) | N (%) | N (%) | |
|
| |||
| Ever had tubes tied? | |||
| No | 43,781 (81%) | 18,203 (80%) | 61,984 (81%) |
| Yes | 9,956 (19%) | 4,543 (20%) | 14,499 (19%) |
|
| |||
| Age when tubes tied: | |||
| < 30 | 1,082 (11%) | 455 (10%) | 1,537 (11%) |
| 30 – 34 | 2,530 (25%) | 1,149 (25%) | 3,679 (25%) |
| 35 – 39 | 3,640 (37%) | 1,632 (36%) | 5,272 (36%) |
| 40 – 44 | 2,165 (22%) | 1,080 (24%) | 3,245 (22%) |
| ≥45 | 539 (5%) | 227 (5%) | 766 (5%) |
|
| |||
| Endometrial cancer: | |||
| No | 52,977 (99%) | 22,369 (98%) | 75,346 (99%) |
| Yes | 760 (1%) | 377 (2%) | 1,137 (1%) |
|
| |||
| Cancer Type: | |||
| I | 649 (85%) | 323 (86%) | 972 (85%) |
| II | 111 (15%) | 54 (14%) | 165 (15%) |
| B.
| |||
|---|---|---|---|
| Age at tubal ligation | No endometrial cancer (n=75,346) | Type I (n=972) | Type II (n=165) |
|
| |||
| N (%) | N (%) | N (%) | |
|
| |||
| No tubal ligation | 61,039 (81%) | 810 (83%) | 135 (82%) |
| Age < 40 | 10,352 (14%) | 115 (12%) | 21 (13%) |
| Age ≥40 | 3,955 (5%) | 47 (5%) | 9 (5%) |
OS=Observational Study Arm DM=Dietary Modification Study Arm
Table 2.
Demographic and Clinical Characteristics by Tubal Ligation Status and Type of Endometrial Cancer. Characteristics of women in the study cohort comparing women with and without tubal ligation and with and without endometrial carcinoma. Characteristics ultimately utilized in multivariate models.
| Demographic | No Tubal Ligation (n=61,984) | Tubal Ligation (n=14,499) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No Endometrial Cancer (n=61,039) | Type I (n=810) | Type II (n=135) | No Endometrial Cancer (n=14,307) | Type I (n=162) | Type II (n=30) | |
|
| ||||||
| Region : | ||||||
| Northeast | 16755 (27%) | 226 (28%) | 38 (28%) | 3308 (23%) | 41 (25%) | 8 (27%) |
| South | 13207 (22%) | 173 (21%) | 30 (22%) | 3805 (27%) | 39 (24%) | 4 (13%) |
| Midwest | 13492 (22%) | 177 (22%) | 33 (24%) | 3412 (24%) | 34 (21%) | 5 (17%) |
| West | 17585 (29%) | 234 (29%) | 34 (25%) | 3782 (26%) | 48 (30%) | 13 (43%) |
|
| ||||||
| Age stratum at randomization or enrollment: | ||||||
| 50 – 54 | 6652 (11%) | 70 (9%) | 10 (7%) | 3715 (26%) | 33 (20%) | 1 (3%) |
| 55 – 59 | 11065 (18%) | 148 (18%) | 27 (20%) | 4810 (34%) | 52 (32%) | 9 (30%) |
| 60 – 69 | 28370 (46%) | 390 (48%) | 56 (41%) | 4908 (34%) | 68 (42%) | 17 (57%) |
| 70 – 79 | 14952 (24%) | 202 (25%) | 42 (31%) | 874 (6%) | 9 (6%) | 3 (10%) |
|
| ||||||
| Ethnicity : | ||||||
| Asian or Pacific Islander | 1840 (3%) | 8 (1%) | 2 (1%) | 483 (3%) | 4 (2%) | 1 (3%) |
| Black or African-American | 3821 (6%) | 28 (3%) | 10 (7%) | 1358 (9%) | 7 (4%) | 1 (3%) |
| Hispanic/Latino | 1882 (3%) | 7 (1%) | 4 (3%) | 682 (5%) | 5 (3%) | 1 (3%) |
| White (not of Hispanic origin) | 52453 (86%) | 752 (93%) | 118 (87%) | 11522 (81%) | 141 (87%) | 27 (90%) |
| American Indian or Alaskan | 862 (1%) | 11 (1%) | 1 (1%) | 234 (2%) | 5 (3%) | 0 |
| Native /Other Missing | 181 (<1%) | 4 (<1%) | 0 | 28 (<1%) | 0 | 0 |
|
| ||||||
| Age at menarche: | ||||||
| < 12 | 12444 (20%) | 180 (22%) | 15 (11%) | 3235 (23%) | 53 (33%) | 1 (3%) |
| 12 – 13 | 34077 (56%) | 496 (61%) | 66 (49%) | 7813 (55%) | 75 (46%) | 19 (63%) |
| 14+ | 14384 (24%) | 131 (16%) | 22 (16%) | 3230 (23%) | 34 (21%) | 5 (17%) |
| Missing | 134 (<1%) | 3 (<1%) | 32 (24%) | 29 (<1%) | 0 | 5 (17%) |
|
| ||||||
| Age at menopause: Mean (SD)(min, max) | 50.4 (SD=4.9) (20, 60) | 51 (SD=4.6) (30, 60) | 51.8(SD=4.6) (36, 60) | 50 (SD=4.4) (20, 60) | 50.1 (SD=5.1) (34, 60) | 52 (SD=4.4) (42, 60) |
|
| ||||||
| Number of term pregnancies: | ||||||
| Never pregnant | 7604 (12%) | 124 (15%) | 16 (12%) | 370 (3%) | 6 (4%) | 0 |
| No term pregnancy | 1910 (3%) | 29 (4%) | 6 (4%) | 243 (2%) | 4 (2%) | 0 |
| 1 | 5790 (9%) | 74 (9%) | 11 (8%) | 949 (7%) | 12 (7%) | 4 (13%) |
| 2 | 15560 (25%) | 218 (27%) | 33 (24%) | 3872 (27%) | 39 (24%) | 4 (13%) |
| 3 | 14037 (23%) | 199 (25%) | 28 (21%) | 3956 (28%) | 56 (35%) | 7 (23%) |
| 4 | 8300 (14%) | 96 (12%) | 24 (18%) | 2558 (18%) | 22 (14%) | 9 (30%) |
| 5+ | 7564 (12%) | 68 (8%) | 16 (12%) | 2291 (16%) | 22 (14%) | 6 (20%) |
| Missing | 274 (<1%) | 2 (<1%) | 1 (1%) | 68 (<1%) | 1 (1%) | 0 |
|
| ||||||
| Oral contraceptive use ever at screening: | ||||||
| No | 37537 (61%) | 522 (64%) | 85 (63%) | 5909 (41%) | 75 (46%) | 16 (53%) |
| Yes | 23502 (39%) | 288 (36%) | 50 (37%) | 8398 (59%) | 87 (54%) | 14 (47%) |
|
| ||||||
| Unopposed estrogen usage status at screening: | ||||||
| Never used | 53604 (88%) | 646 (80%) | 119 (88%) | 12855 (90%) | 133 (82%) | 25 (83%) |
| Past user | 5940 (10%) | 117 (14%) | 12 (9%) | 1037 (7%) | 18 (11%) | 4 (13%) |
| Current user | 1476 (2%) | 46 (6%) | 4 (3%) | 410 (3%) | 11 (7%) | 1 (3%) |
| Missing | 19 (<1%) | 1 (<1%) | 0 | 5 (<1%) | 0 | 0 |
|
| ||||||
| Diabetes ever at screening: | ||||||
| No | 57986 (95%) | 766 (95%) | 123 (91%) | 13660 (95%) | 153 (94%) | 30 (100%) |
| Yes | 3013 (5%) | 44 (5%) | 12 (9%) | 634 (4%) | 9 (6%) | 0 |
| Missing | 40 | 0 | 0 | 13 (<1%) | 0 | 0 |
|
| ||||||
| Diabetes now at screening: | ||||||
| No | 641 (22%) | 10 (23%) | 10 (23%) | 144 (23%) | 2 (22%) | 0 |
| Yes | 2327 (78%) | 34 (77%) | 34 (77%) | 483 (77%) | 7 (78%) | 0 |
|
| ||||||
| Bilateral oophorectomy at screening: | ||||||
| No | 60495 (99%) | 803 (99%) | 134 (99%) | 14205 (99%) | 161 (99%) | 30 (100%) |
| Yes | 392 (1%) | 6 (1%) | 0 | 60 | 1 (1%) | 0 |
| Missing | 152 (<1%) | 1 (<1%) | 1 (1%) | 42 (<1%) | 0 | 0 |
|
| ||||||
| BMI: Mean (SD)(min, max) | 27.3 (SD=5.8) (11.9, 69.6) | 29.2 (SD=7.1) (12.9, 66.2) | 28.7 (SD=6.2) (18, 50) | 27.7 (SD=6) (12, 69.9) | 29.5 (SD=7.6) (18.4, 59.1) | 27.8 (SD=5.5) (21, 41.3) |
| BMI at screening, categorized: | ||||||
| Underweight (<18.5) | 722 (1%) | 6 (1%) | 1 (1%) | 130 (1%) | 1 (1%) | 0 |
| Normal (18.5 – 24.9) | 23784 (39%) | 266 (33%) | 46 (34%) | 5280 (37%) | 51 (31%) | 12 (40%) |
| Overweight (25.0 – 29.9) | 20801 (34%) | 224 (28%) | 38 (28%) | 4833 (34%) | 48 (30%) | 10 (33%) |
| Obesity I (30.0 – 34.9) | 9883 (16%) | 165 (20%) | 28 (21%) | 2467 (17%) | 30 (19%) | 4 (13%) |
| Obesity II (35.0 – 39.9) | 3760 (6%) | 83 (10%) | 17 (13%) | 1001 (7%) | 17 (10%) | 3 (10%) |
| Obesity III (≥40) | 1984 (3%) | 65 (8%) | 5 (4%) | 571 (4%) | 15 (9%) | 1 (3%) |
| Missing | 105 (<1%) | 1 (<1%) | 0 | 25 (<1%) | 0 | 0 |
In order to assess the generalizability of our study cohort, we examined the relationships between known risk factors and endometrial cancer from Table 2. As shown in Supplementary Table 1, increasing BMI was associated with a higher hazard for type I cancer in a univariable model (HR=1.29; 95% CI: 1.24, 1.35). In addition, the hazard for type I cancer was higher for women with diabetes, albeit not statistically significant (HR=1.11; 95% CI: 0.83, 1.45). Increased parity and smoking were also associated with lower hazards of type I cancer, however the results for smoking were not statistically significant. The relationship between oral contraceptive use and type I cancer depended on the age of the participant in our cohort: for younger women (age 50–59 at screening), there was a protective, albeit not statistically significant effect of OC (HR=0.84; 95% CI: 0.67, 1.06). As the age at screening increased, this protective trend disappeared (Supplementary Table 2). Overall, 38% of patients with no BTL and 59% of patients with BTL had used oral contraceptives at baseline.
The results of univariable and multivariable models describing the relationship between BTL and risk of type I endometrial cancer are shown in Table 3; cumulative hazard plots are shown in Figure 1A–B. Overall, there were no statistically significant associations noted between either the presence of BTL or the age at which the BTL was performed and type I endometrial cancer.
Table 3.
Univariate and Multivariate Models: Risk of Tubal Ligation and Type I Endometrial Cancer Stratified by Age at Tubal Ligation. Results of the univariate and multivariate models for Type I endometrial carcinomas in the study cohort for both history of tubal ligation (A) and age at tubal ligation in those women who had a prior tubal ligation (B).
| A Primary predictor: Tubes tied (yes vs. no) | |||
|---|---|---|---|
| Model Type | Estimated HR | 95% CI | p-value |
| Unadjusted (univariable) | 0.87 | (0.75, 1.05) | 0.173 |
| Adjusted for age stratum at randomization and region | 0.90 | (0.76, 1.07) | 0.240 |
| Adjusted for age stratum at randomization, region, race, BMI, parity, diabetes, age at menopause, age at menarche, unopposed estrogen use, oral contraceptive use. | 0.97 | (0.811, 1.17) | 0.780 |
| B. Primary predictor: Age at tubal ligation | |||
|---|---|---|---|
|
| |||
| Model Type | Estimated HR | 95% CI | p-value |
|
| |||
| Unadjusted (univariable): | |||
| Age < 40 vs. No tubal ligation | 0.89 | (0.73, 1.08) | 0.239 |
| 40+ vs. No tubal ligation | 0.89 | (0.66, 1.19) | 0.423 |
|
| |||
| Adjusted for age stratum at randomization and region: | |||
| Age < 40 vs. No tubal ligation | 0.90 | (0.74, 1.10) | 0.313 |
| 40+ vs. No tubal ligation | 0.90 | (0.67, 1.21) | 0.482 |
|
| |||
| Adjusted for age stratum at randomization, region, ethnicity, parity, diabetes, age at menopause, age at menarche, unopposed estrogen use, oral contraceptive use. | |||
| Age < 40 vs. No tubal ligation | 0.99 | (0.80, 1.23) | 0.956 |
| 40+ vs. No tubal ligation | 0.96 | (0.71, 1.30) | 0.781 |
Figure 1. Cumulative Hazard Ratios Plots for Type I Endometrial Cancers and Tubal Ligation.
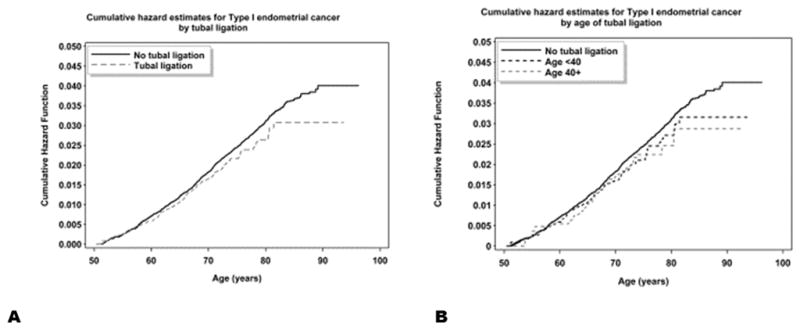
Cumulative hazard plots for Type I endometrial cancers and tubal ligation are shown for predictors: tubal ligation yes vs. no (A) and for tubal ligation by age <40 and >40 vs. no tubal ligation (B).
The analysis of type II cancers was limited due to the overall smaller number of events. In particular, we were only able to examine the presence/absence of tubal ligation and were unable to stratify based on age, as there were only nine women with type II cancer who also had tubal ligations after the age of 40. An examination of the cumulative hazard plot shows strong evidence of non-proportional hazards (Figure 2), with the curves crossing around age 80 and then an apparent large separation in curves at later ages. However, given the fact that there are only 20 total women with type II endometrial cancer past age 80, and only four women with tubal ligation and type II cancer past age 80, we cannot draw any conclusions about these later events. Restricting our analysis to type II endometrial cancers occurring before age 80, we found no statistically significant association between tubal ligation and endometrial cancer risk (estimated HR = 1.14 from the multivariable model; 95% CI: 0.69, 1.90) (Table 4).
Figure 2. Cumulative Hazard Ratios Plot for Type II Endometrial Cancers and Tubal Ligation.
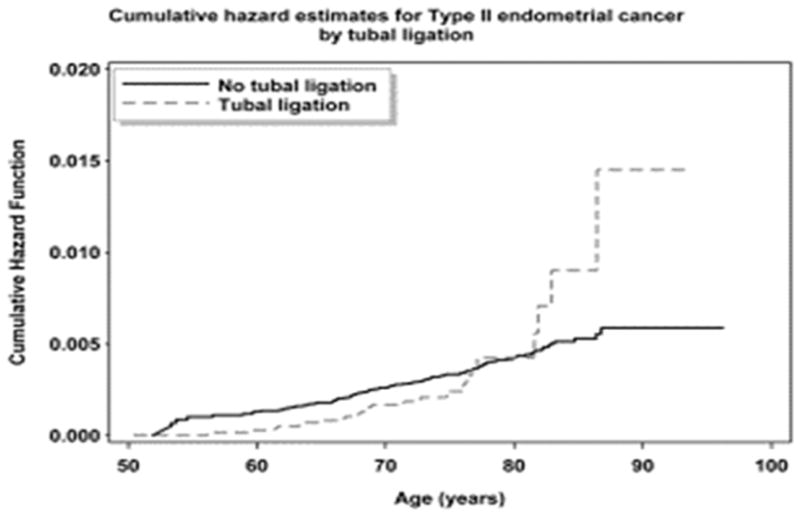
Cumulative hazard plot for tubal ligation in type II endometrial cancers for primary predictor: tubal ligation yes vs. no. Given the cohort size of patients with type II cancers we were unable to further stratify by age at tubal ligation.
Table 4.
Type II Endometrial Cancer and Tubal Ligation. Risk of type II endometrial cancers following tubal ligation using unadjusted, minimally adjusted and full multivariate models. Model results are restricted to events occurring before age 80.
| Model Type | Estimated HR | 95% CI | p-value |
|---|---|---|---|
| Unadjusted (univariable) | 0.96 | (0.62, 1.49) | 0.871 |
| Adjusted for age stratum at randomization and region | 0.97 | (0.63, 1.51) | 0.900 |
| Adjusted for age stratum at randomization, region, BMI, parity, diabetes, age at menopause, ethnicity (white vs. others) age at menarche, unopposed estrogen use, oral contraceptive use. | 1.14 | (0.69, 1.90) | 0.601 |
Discussion
The impact of BTL on endometrial cancer risk is uncertain. To date, only four studies have examined the link between BTL and endometrial cancer with mixed results. Castellsague et al. (9), using the Cancer and Steroid Hormone (CASH) study population (n=437 cases), reported a decreased risk of endometrial cancer among women with BTL in univariate analysis (OR=0.58, 95% CI: 0.43–0.78) which was not significant in their multivariate analysis (OR=0.87, 95% CI: 0.63–1.20). Another study by Rosenblatt et al. (6) utilized the WHO Collaborative Study of Neoplasia and Steroid Contraceptives database and found an increased risk of endometrial cancer associated with history of BTL; their analysis, however included only 135 total endometrial cancer cases of all types. Lacey et al. (7) reported no significant association between BTL and endometrial cancer comparing 405 women with endometrial cancer and 297 controls. A record linkage study from a Danish population database showed a non-significant reduction in risk of endometrial cancer associated with BTL (SIR=0.66, 95% CI: 0.5–1.0); however, this analysis was based on only 30 women with endometrial cancer (8).
Tubal ligation has been studied in the physical spread and overall progression of endometrial carcinomas. Ayeni et al. demonstrated a decrease in intraabdominal spread but a potential increase in lymphatic spread of type II carcinomas with a nonsignificant decrease in progression free survival and overall survival (16). Felix et al. in a recently published review of GOG 210 data suggested an overall decrease in stage and improved survival in patients who had previously undergone BTL (17). Authors hypothesize that the physical blockage of the tube affects the spread of disease perhaps limiting progression of disease via peritoneal dissemination. In the most recent, larger study type II carcinomas were not found to have higher mortality. As noted, a physical linkage of tubal ligation to endometrial cancer risk is not easily identified. Biologic hypotheses have been suggested for both increased or decreased risk of type I cancers based on estrogen and progesterone changes following tubal ligation. However, recent studies have demonstrated conclusively that there are no long term changes in hormone levels or ovarian blood flow associated with BTL (18–21). The biologic basis for any change in type II cancer risk is more tenuous. One hypothesis exists that the origin of uterine serous carcinoma might, as with a large portion of ovarian cancers arise in the fallopian tube. Recent data, however, suggests these cancers likely develop from nests of cells with p53 mutations within the endometrial cavity itself (22–27).
Based on these observations and on the above epidemiologic studies it was hypothesized that any increase or decrease in endometrial cancer risk in relationship to BTL might be based on random variation and chance. Our findings support these conclusions suggesting that there is no association seen between BTL and either an increased or decreased risk for either type I or type II endometrial cancers.
The current study is one of the largest cohort studies examining a potential link between BTL and risk of endometrial cancer with over 76,000 women included in the analysis and 1,137 cancers identified. Given the large sample size, we were able to examine the association between both type I and type II cancers which had not been examined previously. An additional strength of our study was that all of the diagnoses of endometrial cancer in the WHI were centrally adjudicated. One limitation of the WHI analysis is that monitoring for a diagnosis of endometrial cancer began only after enrollment to the study between the ages of 50 and 79, which could have been many years after a presumed cancer initiating event. Another limitation is that the primary risk factor of interest, BTL, and the age at which it occurred, was based on self-reported data alone, rather than medical record review. Therefore, it is possible that this exposure could be misclassified and ultimately incorporates recall bias into the analysis. Lastly, women who reported hysterectomy prior to enrollment were excluded from the study thereby introducing a component of selection bias.
Despite the listed limitations, the findings from the analysis of WHI data are of interest as they add to the growing evidence that choice of BTL as a contraceptive method should not directly affect an individual’s risk for endometrial cancer.
Supplementary Material
Acknowledgments
Funding: Women's Health Initiative: Funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
The Women's Health Initiative, specifically the following:
Program Office: Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Fred Hutchinson Cancer Center, Seattle, WA Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
WHI Investigators/Academic Centers: Brigham and Women's Hospital, Harvard Medical School, Boston, MA-JoAnn E. Manson; MedStar Health Research Institute/Howard University, Washington, DC- Barbara V. Howard; Stanford Prevention Research Center, Stanford, CA- Marcia L. Stefanick; The Ohio State University, Columbus, OH-Rebecca Jackson; University of Arizona, Tucson/Phoenix, AZ-Cynthia A. Thomson; University at Buffalo, Buffalo, NY- Jean Wactawski-Wende; University of Florida, Gainesville/Jacksonville, FL- Marian Limacher; University of Iowa, Iowa City/Davenport, IA-Robert Wallace; University of Pittsburgh, Pittsburgh, PA-Lewis Kuller; Wake Forest University School of Medicine, Winston-Salem, NC-Sally Shumaker
Footnotes
The authors have no conflicts of interest to disclose for the current report.
Contributor Information
Ira Winer, Division of Gynecologic Oncology, Department of Oncology, Karmanos Cancer Institute and Wayne State University, Detroit, MI.
Amy Lehman, Center for Biostatistics, Ohio State University, Columbus, OH
Jean Wactawski-Wende, Department of Epidemiology and Environmental Health, University at Buffalo, Buffalo, New York.
Randall Robinson, Division of Reproductive Endocrinology and Infertility, University of Texas, Health Sciences Center, San Antonio, Texas.
Michael Simon, Department Of Oncology, Karmanos Cancer Institute and Wayne State University, Detroit, MI.
Michele Cote, Population Studies and Disparities Program, Karmanos Cancer Institute, Detroit, MI.
References
- 1.Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004;(350):1–36. [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Tung KH, Goodman MT, Wu AH, McDuffie K, Wilkens LR, Kolonel LN, et al. Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. Am J Epidemiol. 2003;158(7):629–38. doi: 10.1093/aje/kwg177. [DOI] [PubMed] [Google Scholar]
- 4.Massuger L, Roelofsen T, Ham M, Bulten J. The origin of serous ovarian cancer may be found in the uterus: a novel hypothesis. Med Hypotheses. 2010;74(5):859–61. doi: 10.1016/j.mehy.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Cibula D, Widschwendter M, Majek O, Dusek L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update. 2011;17(1):55–67. doi: 10.1093/humupd/dmq030. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblatt K, Thomas D. Association between tubal ligation and endometrial cancer. Int J Cancer. 1997;71(1):129–30. doi: 10.1002/(sici)1097-0215(19970328)71:1<129::aid-ijc22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Lacey JV, Jr, Brinton LA, Mortel R, Berman ML, Wilbanks GD, Twiggs LB, et al. Tubal sterilization and risk of cancer of the endometrium. Gynecol Oncol. 2000;79(3):482–4. doi: 10.1006/gyno.2000.5970. [DOI] [PubMed] [Google Scholar]
- 8.Kjaer SK, Mellemkjaer L, Brinton LA, Johansen C, Gridley G, Olsen JH. Tubal sterilization and risk of ovarian, endometrial and cervical cancer. A Danish population-based follow-up study of more than 65 000 sterilized women. Int J Epidemiol. 2004;33(3):596–602. doi: 10.1093/ije/dyh046. [DOI] [PubMed] [Google Scholar]
- 9.Castellsague X, Thompson WD, Dubrow R. Tubal sterilization and the risk of endometrial cancer. Int J Cancer. 1996;65(5):607–12. doi: 10.1002/(SICI)1097-0215(19960301)65:5<607::AID-IJC9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled clinical trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 11.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Annals of epidemiology. 2003;13(9 Suppl):S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 12.Ritenbaugh C, Patterson RE, Chlebowski RT, Caan B, Fels-Tinker L, Howard B, et al. The Women's Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Annals of epidemiology. 2003;13(9 Suppl):S87–97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 13.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA : the journal of the American Medical Association. 2004;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 14.Thiebaut AC, Benichou J. Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study. Statistics in medicine. 2004;23(24):3803–20. doi: 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- 15.Thiebaut R, Jacqmin-Gadda H. Mixed models for longitudinal left-censored repeated measures. Computer methods and programs in biomedicine. 2004;74(3):255–60. doi: 10.1016/j.cmpb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Ayeni TA, Bakkum-Gamez JN, Mariani A, McGree ME, Weaver AL, Alhilli MM, et al. Impact of tubal ligation on routes of dissemination and overall survival in uterine serous carcinoma. Gynecologic oncology. 2013;128(1):71–6. doi: 10.1016/j.ygyno.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Felix AS, Brinton LA, McMeekin DS, Creasman WT, Mutch D, Cohn DE, et al. Relationships of Tubal Ligation to Endometrial Carcinoma Stage and Mortality in the NRG Oncology/ Gynecologic Oncology Group 210 Trial. J Natl Cancer Inst. 2015;107(9) doi: 10.1093/jnci/djv158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelekci S, Yilmaz B, Yakut Y, Yasar L, Savan K, Sonmez S. Hormonal and ovarian stromal blood supply changes after laparoscopic tubal sterilization: a prospective controlled study. Contraception. 2006;73(3):279–83. doi: 10.1016/j.contraception.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Kelekci S, Yilmaz B, Yasar L, Savan K, Sonmez S, Kart C. Ovarian reserve and ovarian stromal blood supply after tubal ligation by the Pomeroy technique: comparison with controls. Gynecol Endocrinol. 2005;20(5):279–83. doi: 10.1080/09513590500097192. [DOI] [PubMed] [Google Scholar]
- 20.Nelson DB, Sammel MD, Freeman EW, Gracia CR, Liu L, Langan E. Tubal ligation does not affect hormonal changes during the early menopausal transition. Contraception. 2005;71(2):104–10. doi: 10.1016/j.contraception.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Wu E, Xiao B, Yan W, Li H, Wu B. Hormonal profile of the menstrual cycle in Chinese women after tubal sterilization. Contraception. 1992;45(6):583–93. doi: 10.1016/0010-7824(92)90109-7. [DOI] [PubMed] [Google Scholar]
- 22.Pathiraja P, Dhar S, Haldar K. Serous endometrial intraepithelial carcinoma: a case series and literature review. Cancer management and research. 2013;5:117–22. doi: 10.2147/CMAR.S45141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia L, Liu Y, Yi X, Miron A, Crum CP, Kong B, et al. Endometrial glandular dysplasia with frequent p53 gene mutation: a genetic evidence supporting its precancer nature for endometrial serous carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(8):2263–9. doi: 10.1158/1078-0432.CCR-07-4837. [DOI] [PubMed] [Google Scholar]
- 24.Zheng W, Schwartz PE. Serous EIC as an early form of uterine papillary serous carcinoma: recent progress in understanding its pathogenesis and current opinions regarding pathologic and clinical management. Gynecologic oncology. 2005;96(3):579–82. doi: 10.1016/j.ygyno.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 25.Tashiro H, Isacson C, Levine R, Kurman RJ, Cho KR, Hedrick L. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. The American journal of pathology. 1997;150(1):177–85. [PMC free article] [PubMed] [Google Scholar]
- 26.Sherman ME, Bur ME, Kurman RJ. p53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumorigenesis. Human pathology. 1995;26(11):1268–74. doi: 10.1016/0046-8177(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 27.Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Human pathology. 1995;26(11):1260–7. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


