Abstract
Objective
To evaluate long-term clinical and economic outcomes of naproxen, ibuprofen, celecoxib or tramadol for OA patients with cardiovascular disease (CVD) and diabetes.
Design
We used the Osteoarthritis Policy Model to examine treatment with these analgesics after standard of care -- acetaminophen and corticosteroid injections -- failed to control pain. NSAID regimens were evaluated with and without proton pump inhibitors (PPIs). We evaluated over-the-counter (OTC) regimens where available. Estimates of treatment efficacy (pain reduction, occurring in ~ 57% of patients on all regimens) and toxicity (major cardiac or gastrointestinal toxicity or fractures, risk ranging from 1.09% with celecoxib to 5.62% with tramadol) were derived from published literature. Annual costs came from Red Book Online®. Outcomes were discounted at 3%/year and included costs, quality-adjusted life expectancy, and incremental cost-effectiveness ratios (ICERs). Key input parameters were varied in sensitivity analyses.
Results
Adding ibuprofen to standard of care was cost saving, increasing QALYs by 0.07 while decreasing cost by $800. Incorporating OTC naproxen rather than ibuprofen added 0.01 QALYs and increased costs by $300, resulting in an ICER of $54,800/QALY. Using prescription naproxen with OTC PPIs led to an ICER of $76,700/QALY, while use of prescription naproxen with prescription PPIs resulted in an ICER of $252,300/QALY. Regimens including tramadol or celecoxib cost more but added fewer QALYs and thus were dominated by several of the naproxen-containing regimens.
Conclusions
In patients with multiple comorbidities, naproxen- and ibuprofen-containing regimens are more effective and cost-effective in managing OA pain than opioids, celecoxib or standard of care.
Keywords: osteoarthritis, opioids, nonsteroidal anti-inflammatory drugs, NSAIDS, cost-effectiveness
INTRODUCTION
Symptomatic knee osteoarthritis (OA) affects over 9.3 million adults, leading to $27 billion in health care expenditures annually in the US.1, 2 Pharmacologic treatment for knee OA generally begins with acetaminophen and proceeds to non-steroidal anti-inflammatory drugs (NSAIDs) and intra-articular corticosteroid injections.3-6 Physicians are cautious about using NSAIDs and cyclooxygenase 2 (COX-2) inhibitors in patients with multiple comorbidities because of these agents’ toxicities.3, 7-9 Yet, despite concerns about toxicity and illicit diversion,10-12 opioid use for knee OA has increased significantly in the past decade.11, 13
Comorbidities affect up to 40% of knee OA patients13 and increase toxicity of NSAIDs and opioids.3, 14 Thus, clinicians are unsure whether to prescribe these medications to patients with OA and comorbidities. Nevertheless, over $500 million is spent annually in the US on opioids for such OA patients.1, 13, 15 OA treatment guidelines are ambiguous on the role of NSAIDs and opioids in this setting.3-5 Osteoarthritis Research Society International (OARSI) guidelines classify use of oral NSAIDs as “uncertain” in patients with moderate comorbidities and “inappropriate” in those with severe comorbidities. These guidelines classify opioid use as “uncertain” in all OA patients.3 The American Academy of Orthopedic Surgeons (AAOS) OA treatment guidelines provide a strong recommendation for NSAIDS and tramadol, but an inconclusive recommendation for acetaminophen.4 The American College of Rheumatology OA treatment guidelines provisionally recommend tramadol and provisionally do not recommend other opiates.5 Neither the AAOS nor American College of Rheumatology distinguishes recommendations for older patients.
We evaluated long-term clinical and economic outcomes of incorporating naproxen, ibuprofen, celecoxib or tramadol in the treatment of patients with knee OA and multiple comorbidities. Additionally, since ibuprofen and naproxen are available over-the-counter (OTC) and proton pump inhibitors (PPIs) are often used in high-risk patients, we evaluated long-term clinical benefits and costs associated with OTC use of ibuprofen and naproxen and with addition of OTC and prescription PPIs to regimens involving NSAIDs.
METHODS
Analytic Overview
We used the Osteoarthritis Policy (OAPol) Model 16, 17 to examine the cost-effectiveness of prescription and OTC naproxen with and without PPIs, prescription and OTC ibuprofen with and without PPIs, celecoxib with and without PPIs and tramadol in persons with knee OA, cardiovascular disease (CVD) and diabetes. The primary outcomes were quality-adjusted life years (QALYs) and lifetime medical costs. The cost-effectiveness of each strategy was assessed as recommended by the US Panel on Cost-Effectiveness in Health and Medicine.18 We defined incremental cost-effectiveness ratios (ICERs) as the ratio of change in costs to change in QALYs of two consecutive strategies, ranked by cost. We discounted costs and QALYs by 3% annually.18 Treatment strategies that decreased QALYs while increasing costs relative to an alternative strategy were termed dominated. We compared ICERs to a threshold societal willingness to pay (WTP) for medical interventions of $100,000/QALY.19-21
The OAPol Model
The OAPol Model is a Monte Carlo simulation model of the natural history and management of knee OA.16, 17 It forms simulated cohorts of knee OA patients based on distributions of demographic and clinical characteristics, including OA severity, obesity, and comorbidities (CVD, diabetes mellitus, chronic obstructive pulmonary disease and other musculoskeletal disorders).22-24 These are the most common comorbidities that have an influence on life expectancy and quality of life.25, 26 Pain severity in the OAPol Model is assessed by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain subscale (range 0-100, with 100 worst) and is subsequently categorized into 5 groups: no pain (0-1), mild pain (2-15), moderate pain (16-40), severe pain (41-70), and extreme pain (71-100).27-29 Upon OA development, subjects are assigned a pain severity value and corresponding pain group. Each year subjects may develop new comorbidities, progress in pain severity, increase BMI, and/or die. The model follows each subject until death, allowing subjects to transition between health states and to accumulate medical costs (OA and non-OA related) and quality of life (QoL) decrements. Factors governing these transitions are detailed in prior publications and the Technical Appendix.2, 16 QoL decrements (utilities) reflect preference valuation for particular health states, estimated from 0.0 to 1.0 where 1.0 represents perfect health and 0.0 death.30 QoL utilities are assigned based on obesity, pain severity, age, and comorbidities. Annual underlying costs are incorporated to capture non-OA related management and are assigned based on age, obesity status, and number of comorbidities.31-33 Each treatment is characterized by its pain relief, toxicity and cost profile. Toxicities are classified as major or minor, each carrying specific costs and QoL decrements. Major toxicities lead to treatment discontinuation and carry a probability of death.
Treatment Strategies
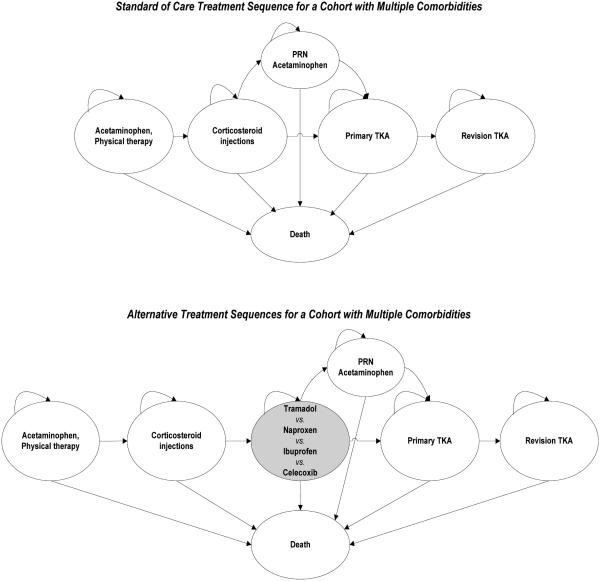
We examined fourteen treatment strategies. The first is standard of care (SOC), which, reflecting treatment guidelines,3, 5 includes physical therapy, acetaminophen and corticosteroid injections. The remaining strategies are implemented if SOC does not relieve pain (Figure 1). Naproxen-containing strategies include: prescription naproxen (naproxen Rx), over-the-counter naproxen (naproxen OTC), naproxen with a proton pump inhibitor (naproxen Rx +PPI Rx), naproxen OTC + PPI OTC and naproxen Rx + PPI OTC. Ibuprofen strategies include: ibuprofen Rx, ibuprofen OTC, ibuprofen Rx + PPI Rx, ibuprofen OTC + PPI OTC, and ibuprofen Rx + PPI OTC. We tested two celecoxib strategies (celecoxib Rx and celecoxib Rx + PPI Rx) and one tramadol strategy. We chose ibuprofen and naproxen because they are the most commonly used NSAIDs among patients with knee OA; celecoxib because of its frequency of use and putatively lower gastrointestinal toxicity; and tramadol because of its frequency of use in this high-risk setting.3, 13, 34 The doses of each agent are provided in Section 3.2 of the Technical Appendix.
Figure 1. Treatment Sequences for OA Management.
This figure depicts the treatment sequences evaluated. Shaded cells indicate a contrast to the standard of care sequence. For each regimen assessing NSAIDs (naproxen, ibuprofen, and celecoxib), an additional regimen incorporating PPIs was evaluated. Further, for naproxen and ibuprofen regimens, over-the-counter based sequences were assessed.
Subjects could exit pharmacologic regimens via three pathways: 1) lack of analgesic efficacy, 2) major toxicity, or 3) voluntary discontinuation for other reasons including minor toxicity (nausea, constipation, somnolence, and dyspepsia). Lack of efficacy in the first year was defined as failure to decrease pain by at least one pain group. In all subsequent years, lack of efficacy was defined as failing to maintain the pain relief achieved in the first year on the regimen. Major toxicities included cardiovascular (CV) events (myocardial infarction, stroke, heart failure), gastrointestinal (GI) events (upper or lower GI bleed, bowel obstruction) and fractures (hip, upper or lower extremities).35 Each category of major toxicity was associated with a unique cost, mortality, and QoL decrement; major toxicity also led to discontinuation. If the treatment strategy did not alleviate pain or was associated with major toxicity, patients were treated with medications to try to manage pain and became eligible for total knee arthroplasty (TKA). If the patient experiences toxicity or lack of efficacy with one of the regimens, he or she is treated with acetaminophen as needed until the patient is offered TKR. Because this is a high risk population, we do not switch the patient from one NSAID to another or to tramadol, as one might in a lower risk group. This period on acetaminophen as needed generally lasts about 13 years.2
TKA recipients were at risk for revision if the primary TKA failed. Eligibility for TKA was governed by radiographic and symptomatic OA severity. The proportion of eligible patients offered a regimen and the proportion of those offered who accepted were stratified by subjects’ age, race, and sex.
Input Data
Cohort characteristics
The cohort had mean age 74 (SD 12), 62% were female, and 47% had BMI > 30 kg/m2. Race/ethnicity, sex, and obesity distributions were derived from the National Health Interview Survey (NHIS) 2012.36 Cohort demographics, costs, and QoL utilities are presented in Table 1. Costs are presented in 2013 US dollars – the most recent year in which all costs are available.
Table 1.
Model Inputs
| Parameter | Estimate | Data Source Used in Derivations |
|||||
|---|---|---|---|---|---|---|---|
| Cohort Characteristics | |||||||
|
| |||||||
| Demographics | Mean (SD) | Assumption US Census Bureau 201272 NHIS 201236 |
|||||
| Mean Age | 74 (12) | ||||||
| Percent Female | 62% | ||||||
| WOMAC Pain | 60 (10) | ||||||
|
| |||||||
| Nonobese/Obese | Osteoarthritis Initiative73
Brazier et al. 200430 |
||||||
| Quality of Life Utilities | WOMAC Pain (0-100) | ||||||
| Age Group | 16 - 40 | 41 - 70 | 71 - 100 | ||||
|
|
|||||||
| 65-74 | 0.762/ 0.751 |
0.665/ 0.654 |
0.530/ 0.519 |
||||
| 75+ | 0.745/ 0.734 |
0.648/ 0.637 |
0.513/ 0.502 |
||||
|
| |||||||
|
Underlying Medical
Costs* |
Comorbidities | MCBS 200934
NHANES 2009-201074 Red Book Online®15 CPI54 |
|||||
| Age group | 0-1 | 2-3 | 4+ | ||||
|
|
|||||||
| 70-74 | $5,300 | $10,700 | $16,200 | ||||
| 75-79 | $6,200 | $11,600 | $17,100 | ||||
| 80+ | $8,200 | $13,500 | $19,100 | ||||
|
| |||||||
| Treatment Characteristics | |||||||
|
| |||||||
| Tramadol | Naproxen | Ibuprofen | Celecoxib |
Data Source Used in
Derivations |
|||
| Rx | OTC | Rx | OTC | ||||
|
| |||||||
| Annual Cost * | $900 | $900 | $400 | $400 | $400 | $3,700 | Red Book Online®15, CPI54, Levinson et al. 200543, IMS 201144 Bronnenberg et al. 201355 Medicare Physician Fee Schedule 2012B53 Medicare Clinical Diagnostic Laboratory Fee Schedule 201275 Bhala et al. 199945 Bensen et al. 199942 Scott et al. 200041 Silverstein et al. 200048 Chan et al. 201049 Schnitzer et al. 200452 Cannon et al. 200650 Lisse et al. 200351 Miller et al. 201156 |
| Efficacy | |||||||
| Mean (SD) WOMAC Pain Reduction in First Year of Treatment |
21 (21) | 20 (17) | 18 (17) | 20 (17) | 18 (17) | 20 (17) | |
| Adverse Effects | |||||||
| Minor Toxicity | 73.62% | 63.00% | 51.19% | 63.00% | 51.19% | 63.00% | |
| Major Toxicity | 5.62% | 1.59% | 1.39% | 2.39% | 2.08% | 1.09% | |
| Gastrointestinal | - | 1.59% | 1.39% | 1.49% | 1.30% | 0.40% | |
| Cardiovascular | - | - | - | 0.90% | 0.78% | 0.70% | |
| Fracture | 5.62% | - | - | - | - | - | |
| Discontinuation due to minor toxicity |
22.00% | 11.28% | 10.42% | 11.28% | 10.42% | 11.28% | |
|
| |||||||
| Proton Pump Inhibitors | |||||||
| Rx | OTC | ||||||
| Annual Cost* | $700 | $300 | Red Book Online®15, Bronnenberg et al. 201355 Rostom et al. 200047 |
||||
| Efficacy | |||||||
| Percent Reduction in NSAID-induced GI Events |
65% | 60% | |||||
Costs are in 2013 US Dollars.
Abbreviations: OA, osteoarthritis; K-L Kellgren-Lawrence grade; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; PPIs, Proton Pump Inhibitors; NHIS, National Health Interview Survey; NHANES National Health and Nutrition Examination Survey; MCBS, Medicare Current Beneficiary Survey; CPI, Consumer Price Index; Rx, prescription; OTC, over-the-counter
Analgesics’ characteristics
The efficacy of each regimen was defined by the reduction in pain achieved by subjects on the regimen in the first year of treatment. Pain reduction was represented by the absolute decrease in pain severity over the course of the first year as assessed by WOMAC Pain subscale (range 0-100, with 100 worst).27 If the absolute reduction in pain severity led a subject to decrease in pain group, the regimen was considered efficacious.
This efficacy parameter was stratified by pain severity upon entering the regimen. Following review of the relevant published literature, efficacy was derived using meta-analytic techniques.37 Major toxicities were classified as cardiovascular (CV), gastrointestinal (GI), or fracture events. Lacking long-term data, we assumed the rates of major toxicity events in subsequent years of treatment were one-half the rate in the first year, reflecting removal of those experiencing toxicities in the first year.35, 38 We tested this assumption in sensitivity analyses. Minor toxicities varied by drug class and included nausea, somnolence, constipation, and dyspepsia.39-42
To estimate the annual cost of each drug we converted Average Wholesale Prices (AWPs) listed in Red Book Online® to Average Sales Prices (ASPs), by reducing brand name and generic drug costs by 26% and 68% respectively.15, 43 The final drug cost was estimated by weighting ASPs of brand name drugs as 7% and generic drugs as 93%, reflecting the proportion of prescriptions filled with branded and generic drugs respectively.44 Additional details on derivations of efficacy, toxicity, and cost attributable to each regimen are presented in the Technical Appendix. The next paragraphs summarize specific characteristics for each of the analgesics.
NSAIDs
Efficacy
We used meta-analytic techniques to derive a mean WOMAC Pain change of 20.0 points for all NSAID regimens. Further details on the derivation of pain decrements associated with NSAIDs are presented in Section 2 of the Technical Appendix. Because OTC analgesics are not available in the same doses as their prescription counterparts, we reduced the efficacy of OTC regimens in a dose-dependent fashion, resulting in a 12% reduction from 20.0 points and resulting in a mean change in WOMAC Pain of 17.6 points. All NSAIDs were associated with a 24% likelihood of failing to maintain pain relief in subsequent years of treatment.17, 41
Toxicity
Prescription naproxen major toxicity included GI events with a likelihood of 1.59% in the first year of treatment and associated mortality of 11.60%.45, 46 The addition of PPIs reduced the probability of major GI events to 0.56%.45, 47 Major toxicity of celecoxib included CV and GI events, with likelihoods of 0.70% and 0.40%, respectively, in the first year.45 Adding PPIs reduced celecoxib’s major first year GI toxicity to 0.14%.45, 47 Celecoxib major toxicity was associated with mortality of 20.03%.45, 46
Prescription ibuprofen was associated with CV and GI events with first year probabilities of 0.90% and 1.49% respectively.45 Incorporating PPIs reduced first year GI toxicity to 0.53%.45, 47 For prescription-based NSAID regimens, we applied a 63% likelihood of experiencing a minor toxicity in all treatment years.41, 42 Minor toxicities did not lead to death or discontinuation. We reduced the toxicity of OTC NSAID regimens by 12% in the base case. Based on data from multiple randomized trials of NSAIDs, we derived an 11.28% probability of discontinuation (for minor toxicity) within the first year of all prescription NSAID regimens.48-52
Cost
Costs of $900, $400, and $3,700 were applied annually for prescription naproxen, prescription ibuprofen, and celecoxib respectively, which included costs of the analgesic, complete blood count, electrolyte tests and office visits.15, 43, 44, 53, 54 We included two office visits with laboratories, reflecting the need to monitor patients with comorbidities closely. We added $700 to the cost of each regimen with prescription PPIs.15, 43, 44 OTC naproxen and ibuprofen regimens were associated with an annual cost of $400, with an additional $300 added to regimens incorporating OTC PPIs.15, 55
Tramadol
Efficacy
Estimated mean WOMAC pain change for tramadol was 21.2 points. Further details on the derivation of pain decrements associated with tramadol are presented in Section 2 of the Technical Appendix. We assigned a 24% likelihood of failing to maintain pain relief in subsequent years (similar to that of NSAIDs).41
Toxicity
Tramadol treatment was associated with major (fractures) and minor toxicities (nausea, constipation, and somnolence). We applied a 5.62% likelihood of developing a fracture within the first year and 2.85% in subsequent years for the base case.56 Fractures were associated with mortality of 8.88%.56-58 The risks, costs and QOL decrements associated with tramadol minor toxicities are provided in the Technical Appendix. We assumed a 22% likelihood of tramadol discontinuation due to minor toxicity, applied in the first year of treatment.59, 60 Because prior literature documents that opioid-treated subjects have worse TKA outcomes than subjects not using opioids prior to TKA,61, 62 we assigned a 10% relative increase in the probability of early revision and 10% relative reduction in pain relief in the first year following surgery for opioid treated persons.61, 62
Cost
We derived annual costs of $900 for the tramadol regimen, which included the cost of tramadol and biannual office visits.15, 43, 44, 53, 54
Sensitivity Analyses
We performed one- and two-way sensitivity analyses to assess the impact of uncertainty in essentially all efficacy and toxicity parameters on cost-effectiveness estimates. We also varied the cost of prescription naproxen by varying the proportion of analgesic cost due to brand name agents. The base case is 7%, and we varied the proportion from 0 to 10%. We simultaneously varied the efficacy and toxicity of OTC-based regimens from 20% reduction to 20% increase relative to the prescription-based regimens. We further assessed the impact of truncating efficacy for analgesic regimens at 3 years. We used probabilistic sensitivity analyses to evaluate the effects of simultaneously varying the efficacy and major toxicity of all regimens. Results of the probabilistic sensitivity analysis are depicted by cost-effectiveness acceptability curves, which illustrate the probability that alternative treatment strategies are cost-effective at various WTP thresholds.63, 64
RESULTS
Base Case Analysis
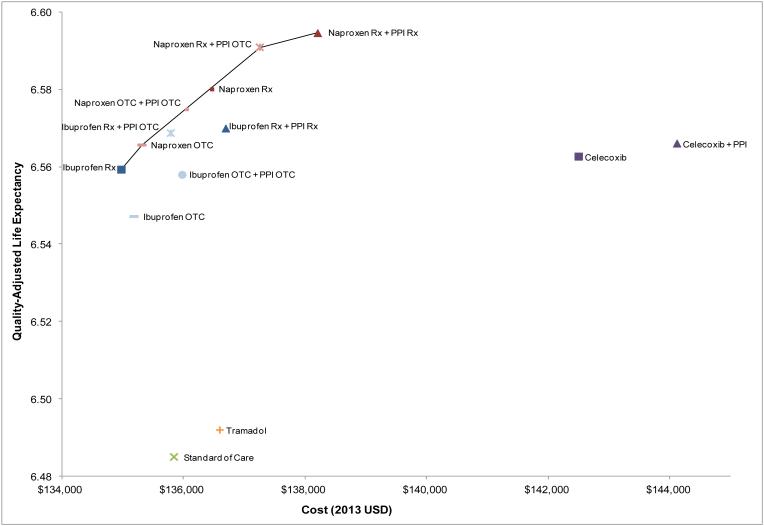
Figure 2 shows the relationship between total lifetime costs and discounted quality-adjusted life expectancy (QALE) for all 14 strategies. Strategies involving naproxen were consistently more effective and more cost-effective than those involving tramadol and celecoxib and most involving ibuprofen. These points are illustrated by the efficiency frontier (Figure 2), which depicts the greatest QALE that can be achieved for any given outlay. Strategies that lie along this frontier are termed ‘efficient.’ The tramadol- and celecoxib-based strategies were dominated. That is, they cost more and led to lower clinical benefits than one or more of the naproxen-based strategies.
Figure 2. Cost-effectiveness of all treatment strategies.
This figure portrays the cost-effectiveness of alternative prescription- and over-the-counter-based treatment strategies for OA patients with multiple comorbidities. The efficiency frontier (solid line) defines the greatest QALE that can be achieved for any given outlay. Strategies that lie along this frontier are termed ‘efficient.’
Table 2 presents costs, QALE and proportion of patients undergoing TKA for each of 14 strategies. The four non-dominated (efficient) strategies are highlighted in grey. SOC led to an estimated discounted QALE of 6.49 QALYs with an associated discounted lifetime cost of $135,800. Addition of prescription ibuprofen was cost saving compared to SOC, increasing the QALE to 6.56 QALYs and decreasing cost to $135,000. OTC naproxen led to a QALE of 6.57 QALYs and a discounted lifetime cost of $135,300, resulting in an ICER of $54,800/QALY compared to prescription ibuprofen. Using prescription naproxen with OTC PPIs increased the QALE to 6.59 and cost to $137,300. As compared with the costs and QALE associated with naproxen OTC, the ICER was $76,700/QALY. The table also shows that the lifetime risk of TKA associated with these strategies was generally inversely proportional to the QALE; strategies with the worst QALE (such as standard of care) had the highest TKA rates (38%) while the NSAID and PPI regimens had the highest QALE and lowest TKA rates (~ 32%).
Table 2.
Cost-effectiveness of prescription- and OTC-based regimens
| Regimen | QALE | COST | ICER* | %TKA Utilization |
|---|---|---|---|---|
| Standard of Care | 6.485 | $135,800 | 37.56% | |
|
| ||||
| Ibuprofen Rx | 6.559 | $135,000 | Cost Saving | 31.53% |
|
| ||||
| Ibuprofen OTC | 6.547 | $135,200 | Dominated | 31.78% |
|
| ||||
| Naproxen OTC | 6.566 | $135,300 | $54,800 | 31.81% |
|
| ||||
| Ibuprofen Rx + PPI OTC | 6.569 | $135,800 | Extended Dominance | 31.49% |
| Ibuprofen OTC + PPI OTC | 6.558 | $136,000 | Dominated | 31.76% |
| Naproxen OTC + PPI OTC | 6.575 | $136,100 | Extended Dominance | 31.76% |
| Naproxen Rx | 6.580 | $136,500 | Extended Dominance | 31.57% |
| Tramadol | 6.492 | $136,600 | Dominated | 32.15% |
| Ibuprofen Rx + PPI Rx | 6.570 | $136,700 | Dominated | 31.51% |
|
| ||||
| Naproxen Rx + PPI OTC | 6.591 | $137,300 | $76,700 | 31.54% |
| Naproxen Rx + PPI Rx | 6.595 | $138,200 | $252,300 | 31.51% |
|
| ||||
| Celecoxib | 6.563 | $142,500 | Dominated | 32.11% |
| Celecoxib + PPI | 6.566 | $144,100 | Dominated | 32.11% |
This table shows the quality adjusted life expectancy (QALE), cost, incremental cost effectiveness ratios (ICERs) and utilization of TKA in each cohort. Strategies are ordered by cost. The ICER is calculated as the difference in cost between two strategies divided by the difference in quality adjusted life expectancy. These comparisons are made between a given strategy and the prior, non-dominated strategy. By convention, a strategy is labeled “dominated” if it costs more and delivers fewer benefits than some other strategy; a strategy is labeled “weakly dominated” (also called extended dominance) if it costs more and delivers fewer benefits than a combination of two other strategies. For example, the ICER for Naproxen Rx + PPI OTC is calculated as the difference in cost /difference in QALE between that strategy and Naproxen OTC. The QALE and cost data presented in the table are rounded while the ICERs are calculated precisely using unrounded parameters. Thus, the ICER’s presented are slightly different from those derived from the QALE and cost data in the table.
ICERs reported as incremental costs in 2013 USD per QALY gained compared to the alternative treatment.
Abbreviations: QALE, quality adjusted life expectancy; ICER, incremental cost-effectiveness ratio; TKA, total knee arthroplasty; PPI, proton pump inhibitor; Rx, prescription; OTC, over-the-counter
Sensitivity Analyses
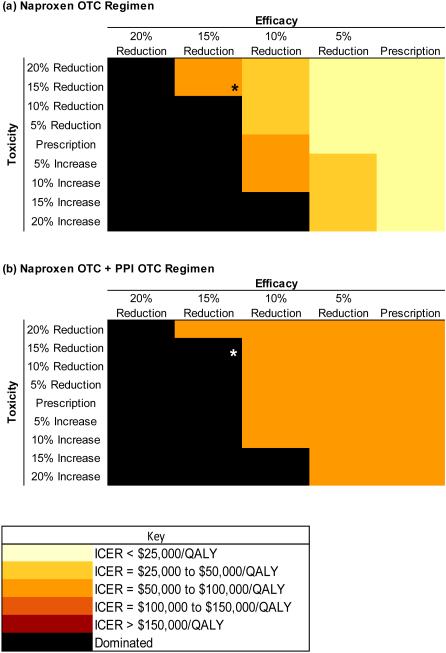
All regimens were evaluated under a range of toxicity and efficacy values (Figures 3a and b). The base case assumed a 12% reduction in efficacy and toxicity of OTC naproxen relative to prescription naproxen. Assuming a 20% reduction in efficacy of naproxen OTC (vs. prescription) led to a reduction in QALE of the naproxen OTC regimen relative to prescription ibuprofen. Naproxen OTC with these characteristics was dominated by prescription ibuprofen (Figure 3a). When we applied a 10% relative reduction in naproxen OTC (vs. prescription) efficacy, the ICER for naproxen OTC was less than $50,000/QALY as long as the toxicity was less than that of prescription naproxen. The ICER of naproxen OTC with OTC PPIs regimen remained less than $100,000/QALY when the toxicity was greater than prescription naproxen and the efficacy remained within 10% of the prescription-based regimen (Figure 3b). Truncating efficacy at 3 years had no impact on cost-effectiveness estimates.
Figure 3. Incremental Cost-Effectiveness Ratios of Over-the-Counter Regimens of (a) Naproxen and (b) Naproxen + PPI.
Figure 3 shows the results of a “two-way sensitivity analysis.” This figure illustrates the ICERs calculated for (a) the naproxen over-the-counter (OTC) regimen and (b) OTC naproxen with PPIs regimens, under a range of values for regimen efficacy and toxicity. The purpose of the analysis is to examine the effect on cost-effectiveness (measured in ICERs) of differences in efficacy and toxicity of OTC vs. prescription naproxen. The horizontal axis shows changes in efficacy of OTC naproxen as compared with prescription naproxen. The vertical axis shows changes (increases or reductions) in toxicity of the regimen compared to prescription naproxen. The shading denotes the ICER, ranging from light yellow (ICERs < $25,000/QALY) to black (dominated, indicating greater cost and lower efficacy than the comparator). In panel (a) the OTC naproxen regimen is compared to prescription ibuprofen. In panel (b) OTC naproxen with PPIs is compared to OTC naproxen (without PPIs). In both instances, the base case, using the initial input assumptions, is marked with an asterisk.
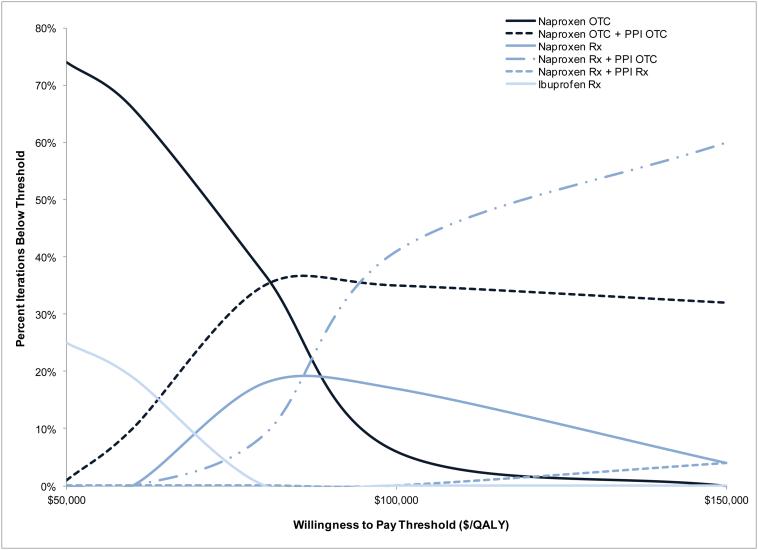
We evaluated the effects of simultaneously varying efficacy, major toxicity, and relative decrement in efficacy and toxicity for OTC analgesics through probabilistic sensitivity analyses. At a WTP of $50,000/QALY, naproxen OTC had 74% likelihood of being the cost-effective regimen. At a WTP of $100,000/QALY, prescription naproxen with PPI OTC had 41% likelihood of being the most cost-effective treatment strategy, naproxen OTC and PPI OTC had 35% likelihood and prescription naproxen alone had a 17% likelihood. Increasing the WTP threshold to $150,000/QALY resulted in prescription naproxen with PPI OTC being the most cost-effective strategy 60% of the time, and naproxen with PPI OTC 32% of the time (Figure 4).
Figure 4. Probability of cost-effectiveness of alternative treatment strategies at each WTP threshold.
The probability of cost-effectiveness of prescription naproxen with either prescription or OTC PPIs is shown. These results are based on 100 iterations, varying the efficacy of all regimens, probability of prescription naproxen major toxicity, and the toxicity of OTC agents. The probability of cost-effectiveness of prescription naproxen with OTC PPIs reaches 60% if the WTP threshold is $150,000/QALY.
DISCUSSION
Pain management in knee OA patients with multiple comorbidities is costly and requires a delicate balance of pain relief and toxicity. We used the OAPol Model to evaluate the cost effectiveness of incorporating naproxen, ibuprofen, celecoxib or tramadol into the treatment of these patients. At a willingness-to-pay threshold of $100,000/QALY, prescription naproxen with PPI OTC appears cost-effective with an ICER of $76,700/QALY. This finding persists even when naproxen toxicities are assumed to be 20% higher than those documented in published studies. Probabilistic sensitivity analyses show that at a WTP threshold of $100,000/QALY, prescription naproxen and naproxen OTC with PPI OTC also appear attractive from a cost-effectiveness standpoint. More generally, we found that several naproxen-based regimens are cost-effective, while tramadol, celecoxib and standard of care are not.
Tramadol’s higher rates of toxicity and discontinuation led to smaller gains in QALYs than NSAID regimens while Celecoxib’s higher cardiac toxicity led to smaller gains than most naproxen regimens. Celecoxib was the most costly regimen. Consequently, both tramadol and celecoxib were dominated by NSAID-based regimens. Naproxen’s lack of cardiac toxicity45 resulted in its providing greater clinical benefit and cost-effectiveness than ibuprofen.
To our knowledge, this contribution is unique. It is the first cost-effectiveness analysis of analgesic strategies in knee OA patients with comorbidities and the first to incorporate opioids or evaluate OTC regimens. A cost-utility analysis of celecoxib and rofecoxib in patients with OA or rheumatoid arthritis reported that in high-risk patients ibuprofen with PPIs was dominated by celecoxib while diclofenac with PPIs had an ICER of $298,400/QALY.54, 65, 66 This analysis was conducted with a five-year perspective, excluded a standard of care comparator and did not consider cardiovascular toxicity, an important factor in our analysis. A comparison of COX-2 selective and non-selective NSAIDs, and non-selective NSAIDs with PPIs, concluded that in average-risk patients, NSAIDs with PPIs are unlikely to be cost-effective, while in patients at high risk for ulcers, NSAIDs with PPIs were preferred.67
We acknowledge several limitations. Since analgesic trial duration ranges from 2-12 weeks, our estimates of longer-term efficacy and toxicity relied upon expert clinician opinion. We did not address acetaminophen toxicity,68 as it is substantially lower than NSAID and opioids toxicity and thus would influence our results minimally.3 The findings we present pertain to older OA patients with multiple comorbidities and should not be generalized to the broader OA population. We focus on tramadol because of its wide use and lower toxicity than more potent opioids. Our findings should not be generalized to the entire class of opioids. We acknowledge that clinicians may switch from agent to agent in some patients due to inefficacy or toxicity. Our findings can be useful in prioritizing the order of medications in these circumstances. Finally, we note that more potent opioids may be diverted to illicit use, leading to societal costs exceeding $63 billion across all users.10, 69 Since tramadol contributes little to opioid abuse, we did not consider diversion.70, 71
This work presents important implications for research, policy, and practice. To overcome the limitations of short term studies, we urgently need studies on the long-term efficacy, toxicity and costs of analgesics. From a policy perspective, over $500 million is spent on opioids in patients with OA and comorbidities.1, 13, 15 Our results suggest that for older individuals with comorbidities, tramadol provides less benefit than naproxen-containing regimens. Policy makers could consider limiting tramadol use in this setting to patients with contraindications, intolerance or failure to respond to NSAIDs. From a clinical perspective, our findings challenge the reluctance of physicians to use non-selective NSAIDs in patients with multiple comorbidities. Our findings indicate that no treatment, tramadol and celecoxib are poor choices in this setting, whereas naproxen (prescription or OTC) with PPI OTC or prescription naproxen alone can be recommended as cost-effective regimens in older patients with knee OA and cardiovascular disease and diabetes.
Supplementary Material
ACKNOWLEDGEMENTS
ROLE OF FUNDING SOURCE:
Supported by: National Institute of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 AR064320, K24 AR057827. The funding source had no role in the study design, collection, analysis and interpretation of the data, drafting of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Dr. Katz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conception and design: Katz, Losina
Collection and assembly of data: Katz, Smith, Losina
Analysis and interpretation of the data: Katz, Smith, Collins, Solomon, Jordan, Hunter, Suter, Yelin, Paltiel, Losina
Statistical expertise: Losina, Collins
Drafting of the article: Katz, Smith, Losina
Critical revision of the article for important intellectual content: Katz, Smith, Collins, Solomon, Jordan, Hunter, Suter, Yelin, Paltiel, Losina
Final approval of the article: Katz, Smith, Collins, Solomon, Jordan, Hunter, Suter, Yelin, Paltiel, Losina
Obtaining of funding: Losina
COMPETING INTEREST STATEMENT
Dr. Solomon serves without pay on the Executive Committee of a Pfizer-sponsored trial examining the safety of NSAIDs. He also has received a research grant from Pfizer on an unrelated topic.
REFERENCES
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losina E, Paltiel AD, Weinstein AM, Yelin E, Hunter DJ, Chen SP, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken) 2015;67:203–215. doi: 10.1002/acr.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21:571–576. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 6.Hepper CT, Halvorson JJ, Duncan ST, Gregory AJ, Dunn WR, Spindler KP. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg. 2009;17:638–646. doi: 10.5435/00124635-200910000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH, Schneeweiss S, Glynn RJ, Kiyota Y, Levin R, Mogun H, et al. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation. 2004;109:2068–2073. doi: 10.1161/01.CIR.0000127578.21885.3E. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 9.Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ. 2005;330:1366. doi: 10.1136/bmj.330.7504.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- 11.The DAWN Report Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on the Drug-Related Emergency Department Visits. Drug Abuse Warning Network (DAWN), Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality; Rockville, MD: 2011. [PubMed] [Google Scholar]
- 12.Dhalla IA, Persaud N, Juurlink DN. Facing up to the prescription opioid crisis. BMJ. 2011;343:d5142. doi: 10.1136/bmj.d5142. [DOI] [PubMed] [Google Scholar]
- 13.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in Prescription of Opioids from 2003-2009 in Persons with Knee Osteoarthritis. Arthritis Care Res (Hoboken) 2014 doi: 10.1002/acr.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon DH, Gurwitz JH. Toxicity of nonsteroidal anti-inflammatory drugs in the elderly: is advanced age a risk factor? Am J Med. 1997;102:208–215. doi: 10.1016/s0002-9343(96)00380-4. [DOI] [PubMed] [Google Scholar]
- 15.Red Book Online®. Truven Health Analytics Inc.; 2013. [Google Scholar]
- 16.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med. 2011;154:217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Losina E, Daigle ME, Suter LG, Hunter DJ, Solomon DH, Walensky RP, et al. Disease-modifying drugs for knee osteoarthritis: can they be cost-effective? Osteoarthritis Cartilage. 2013;21:655–667. doi: 10.1016/j.joca.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–1341. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 19.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 20.Hidden Costs, Value Lost: Uninsurance in America: Institute of Medicine of the National Academies. 2003. [PubMed]
- 21.Ryen L, Svensson M. The Willingness to Pay for a Quality Adjusted Life Year: A Review of the Empirical Literature. Health Econ. 2014 doi: 10.1002/hec.3085. [DOI] [PubMed] [Google Scholar]
- 22.Brandt KD, Fife RS, Braunstein EM, Katz B. Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum. 1991;34:1381–1386. doi: 10.1002/art.1780341106. [DOI] [PubMed] [Google Scholar]
- 23.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 24.Holt HL, Katz JN, Reichmann WM, Gerlovin H, Wright EA, Hunter DJ, et al. Forecasting the burden of advanced knee osteoarthritis over a 10-year period in a cohort of 60-64 year-old US adults. Osteoarthritis Cartilage. 2011;19:44–50. doi: 10.1016/j.joca.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy LBJ, Helmick CG, Brady TJ. Comorbidities Are Very Common Among People with Arthritis. Poster 43. 20th National Conference on Chronic Disease Prevention and Control; CDC; 2009. CDC. [Google Scholar]
- 26.van Dijk GM, Veenhof C, Schellevis F, Hulsmans H, Bakker JP, Arwert H, et al. Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord. 2008;9:95. doi: 10.1186/1471-2474-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 28.Kapstad H, Hanestad BR, Langeland N, Rustoen T, Stavem K. Cutpoints for mild, moderate and severe pain in patients with osteoarthritis of the hip or knee ready for joint replacement surgery. BMC Musculoskelet Disord. 2008;9:55. doi: 10.1186/1471-2474-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zelman DC, Hoffman DL, Seifeldin R, Dukes EM. Development of a metric for a day of manageable pain control: derivation of pain severity cut-points for low back pain and osteoarthritis. Pain. 2003;106:35–42. doi: 10.1016/s0304-3959(03)00274-4. [DOI] [PubMed] [Google Scholar]
- 30.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) 2005-2006 National Health and Nutrition Examination Survey (NHANES) Data. National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services; Hyattsville, MD: 2006. [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) 2007-2008 National Health and Nutrition Examination Survey (NHANES) Data. National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services; Hyattsville, MD: 2008. [Google Scholar]
- 33.Pope GC, Kautter J, Ellis RP, Ash AS, Ayanian JZ, Lezzoni LI, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 34.Medicare Current Beneficiary Survey. Centers for Medicare & Medicaid Services; 2009. [Google Scholar]
- 35.Solomon DH, Rassen JA, Glynn RJ, Garneau K, Levin R, Lee J, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170:1979–1986. doi: 10.1001/archinternmed.2010.450. [DOI] [PubMed] [Google Scholar]
- 36.National Health Interview Survey (NHIS) Centers for Disease Control and Prevention, National Center for Health Statistics; 2012. [Google Scholar]
- 37.Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Abstract: Comparative efficacy of oral NSAIDs and opioids for knee OA: meta-analysis and systematic review. Osteoarthritis Research Society International (OARSI) 2015 [Google Scholar]
- 38.Portenoy RK, Farrar JT, Backonja MM, Cleeland CS, Yang K, Friedman M, et al. Long-term use of controlled-release oxycodone for noncancer pain: results of a 3-year registry study. Clin J Pain. 2007;23:287–299. doi: 10.1097/AJP.0b013e31802b582f. [DOI] [PubMed] [Google Scholar]
- 39.Ultram ER® (tramadol HCl) Extended-Release Tablets Prescribing Information. 2009.
- 40.Anastassopoulos KP, Chow W, Tapia CI, Baik R, Ackerman SJ, Biondi D, et al. Economic study on the impact of side effects in patients taking oxycodone controlled-release for noncancer pain. J Manag Care Pharm. 2012;18:615–626. doi: 10.18553/jmcp.2012.18.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott DL, Berry H, Capell H, Coppock J, Daymond T, Doyle DV, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford) 2000;39:1095–1101. doi: 10.1093/rheumatology/39.10.1095. [DOI] [PubMed] [Google Scholar]
- 42.Bensen WG, Fiechtner JJ, McMillen JI, Zhao WW, Yu SS, Woods EM, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc. 1999;74:1095–1105. doi: 10.4065/74.11.1095. [DOI] [PubMed] [Google Scholar]
- 43.Levinson DR. Medicaid Drug Price Comparison: Average Sales Price to Average Wholsale Price. Office of the Inspector General: Department of Health and Human Serives; 2005. [Google Scholar]
- 44.IMS Institute for Healthcare Informatics The Use of Medicines in the United States: Review of 2010. 2011.
- 45.Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Straube S, Tramer MR, Moore RA, Derry S, McQuay HJ. Mortality with upper gastrointestinal bleeding and perforation: effects of time and NSAID use. BMC Gastroenterol. 2009;9:41. doi: 10.1186/1471-230X-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. 2002:CD002296. doi: 10.1002/14651858.CD002296. [DOI] [PubMed] [Google Scholar]
- 48.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 49.Chan FK, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet. 2010;376:173–179. doi: 10.1016/S0140-6736(10)60673-3. [DOI] [PubMed] [Google Scholar]
- 50.Cannon CP, Curtis SP, FitzGerald GA, Krum H, Kaur A, Bolognese JA, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet. 2006;368:1771–1781. doi: 10.1016/S0140-6736(06)69666-9. [DOI] [PubMed] [Google Scholar]
- 51.Lisse JR, Perlman M, Johansson G, Shoemaker JR, Schechtman J, Skalky CS, et al. Gastrointestinal tolerability and effectiveness of rofecoxib versus naproxen in the treatment of osteoarthritis: a randomized, controlled trial. Ann Intern Med. 2003;139:539–546. doi: 10.7326/0003-4819-139-7-200310070-00005. [DOI] [PubMed] [Google Scholar]
- 52.Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 2004;364:665–674. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- 53.Medicare Fee Schedules. Centers for Medicare & Medicaid Services (CMS); 2012. [Google Scholar]
- 54.Consumer Price Index (CPI) National Bureau of Labor Statistics; 2014. [Google Scholar]
- 55.Bronnenberg B, Dube J-P, Gentzkow M, Shapiro JM. Do Pharmacists Buy Bayer? Sophosticated Shopers and the Brand Premium. Capital Ideas. 2013 [Google Scholar]
- 56.Miller M, Sturmer T, Azrael D, Levin R, Solomon DH. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59:430–438. doi: 10.1111/j.1532-5415.2011.03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of fracture in adults: a nested case-control study using the general practice research database. Am J Epidemiol. 2013;178:559–569. doi: 10.1093/aje/kwt013. [DOI] [PubMed] [Google Scholar]
- 58.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 60.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7:R1046–1051. doi: 10.1186/ar1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patanwala AE, Jarzyna DL, Miller MD, Erstad BL. Comparison of opioid requirements and analgesic response in opioid-tolerant versus opioid-naive patients after total knee arthroplasty. Pharmacotherapy. 2008;28:1453–1460. doi: 10.1592/phco.28.12.1453. [DOI] [PubMed] [Google Scholar]
- 62.Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988–1993. doi: 10.2106/JBJS.J.01473. [DOI] [PubMed] [Google Scholar]
- 63.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32:722–732. doi: 10.1177/0272989X12458348. [DOI] [PubMed] [Google Scholar]
- 64.Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford University Press; Oxford: 2006. [Google Scholar]
- 65.Maetzel A, Krahn M, Naglie G. The cost effectiveness of rofecoxib and celecoxib in patients with osteoarthritis or rheumatoid arthritis. Arthritis Rheum. 2003;49:283–292. doi: 10.1002/art.11121. [DOI] [PubMed] [Google Scholar]
- 66.Daily Currency Converter. Bank of Canada; 2015. [Google Scholar]
- 67.Spiegel BM, Chiou CF, Ofman JJ. Minimizing complications from nonsteroidal antiinflammatory drugs: cost-effectiveness of competing strategies in varying risk groups. Arthritis Rheum. 2005;53:185–197. doi: 10.1002/art.21065. [DOI] [PubMed] [Google Scholar]
- 68.Towheed TE, Maxwell L, Judd MG, Catton M, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006:CD004257. doi: 10.1002/14651858.CD004257.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention (CDC) Prescription Painkiller Overdoses in the US. CDC Vital Signs [Google Scholar]
- 70.Adams EH, Breiner S, Cicero TJ, Geller A, Inciardi JA, Schnoll SH, et al. A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. J Pain Symptom Manage. 2006;31:465–476. doi: 10.1016/j.jpainsymman.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 71.Inciardi JA, Cicero TJ, Munoz A, Adams EH, Geller A, Senay EC, et al. The Diversion of Ultram, Ultracet, and generic tramadol HCL. J Addict Dis. 2006;25:53–58. doi: 10.1300/J069v25n02_08. [DOI] [PubMed] [Google Scholar]
- 72.Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States, States, Counties, and Puerto Rico Common Wealth and Municipios: April 1, 2010 to July 1, 2012. June 2012 U.S Census Bureau Population Division; 2012. [Google Scholar]
- 73.Osteoarthritis Initiative (OAI) University of California; San Francisco: 2013. [Google Scholar]
- 74.Centers for Disease Control and Prevention (CDC) 2009-2010 National Health and Nutrition Examination Survey (NHANES) Data. National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services; Hyattsville, MD: 2010. [Google Scholar]
- 75.Medicare Clinical Diagnostic Laboratory Fee Schedule 2012. Centers for Medicare & Medicaid Services; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.