Abstract
The causes of autism spectrum disorder (ASD) are uncertain and there are no pharmacological treatments for core symptoms of this condition. However, new evidence suggests that the brain cells in people with ASD may be poorly defended against harmful by-products, called ‘reactive oxygen species’ or ROS. The anti-oxidant Glutathione (GSH) produced by our cells helps protect against the damage caused by ROS. Some studies have shown that the amount of GSH in blood is lower in people with ASD. However, it is not clear whether this is also true inside the brain. Here we compared GSH levels in the brain of adult men with and without ASD using a safe and painless scanning technique called magnetic resonance spectroscopy (MRS). We found no differences in GSH between the people with ASD and those without; nor were GSH levels related to symptom severity in ASD. These findings suggest that brain antioxidant defences are not, in fact, weakened in this particular group of adult men with ASD.
Keywords: Autism, Magnetic Resonance Spectroscopy, Glutathione, Oxidative stress, Redox
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by repetitive behaviours and impairments in reciprocal social interactions and communication (American Psychiatric Association, 2013), affecting about 1% of the population (Baron-Cohen et al., 2009).
The aetiology of ASD remains unclear, though various genetic, environmental and immunological factors are thought to influence the development of the disorder (R. Deth, Muratore, Benzecry, Power-Charnitsky, & Waly, 2008). Among these, oxidative stress - particularly that arising from toxic environmental exposures - has been proposed to play a central role in its pathogenesis (Chauhan & Chauhan, 2006; R. Deth, et al., 2008; R. C. Deth, 2013; Theoharides, Kempuraj, & Redwood, 2009). This ‘Redox Hypothesis’ is supported by reports of increased markers of oxidative stress and lipid peroxidation in individuals with ASD, including elevated red blood cell nitric oxide, decreased plasma levels of antioxidant proteins, and an imbalance between the reduced and oxidised forms of glutathione (GSH) (Chauhan, Chauhan, Brown, & Cohen, 2004; Kern & Jones, 2006).
GSH is the major endogenous cellular antioxidant. It protects against lipid peroxidation and oxidative stress, by maintaining the balance between production and removal of reactive oxygen species (ROS) (Shimizu et al., 1998). Lower levels of GSH and higher levels of its oxidized metabolite, GSSG, have been reported in the plasma of individuals with ASD and this has been suggested to indicate greater oxidative stress (Frustaci et al., 2012; Geier et al., 2009; James et al., 2004; Main, Angley, O'Doherty, Thomas, & Fenech, 2012). However, it is unclear whether this decrease in peripheral GSH also occurs in the brain. Decreased GSH, increased GSSG, and a decreased GSH:GSSG ratio have been reported in the cerebellum and temporal cortex of subjects with ASD post-mortem (Chauhan, Audhya, & Chauhan, 2012). However post-mortem studies are limited by the quality of protein preservation (Ferrer, Martinez, Boluda, Parchi, & Barrachina, 2008), therefore GSH concentrations in the ASD brain also need to be researched in vivo.
Brain GSH can be measured in vivo using the technique of proton magnetic resonance spectroscopy ([1H]MRS). This approach has been applied to individuals with various neuropsychiatric disorders using a 3T scanner. For instance, temporal cortex GSH levels have been reported to be higher in people with psychosis, whereas no difference in brain GSH has been found in those with bipolar disorder (Godlewska, Yip, Near, Goodwin, & Cowen, 2014; Lagopoulos et al., 2013; Wood et al., 2009). However to date, no-one has examined [1H]MRS measures of GSH in ASD.
Therefore, in this study, we compared brain GSH in unmedicated adult men with ASD and healthy controls of similar age and IQ using [1H]MRS. [1H]MRS spectra were acquired in the basal ganglia (BG) and in the dorsomedial prefrontal cortex (DMPFC). These regions were chosen because they have been consistently been reported to have both structural abnormalities and functional deficits (relevant to the core symptoms) in ASD (e.g. see (Haznedar et al., 2006; Hollander et al., 2005; Horder et al., 2013; Schmitz, Daly, & Murphy, 2007). For instance, anomalies in the BG are thought to be responsible for repetitive behaviours (Hollander, et al., 2005; Naaijen, Lythgoe, Amiri, Buitelaar, & Glennon, 2015), while frontal network deficits have been implicated in social symptoms (Bernhardt et al., 2014; Watanabe et al., 2012; Wicker et al., 2008). We tested the hypotheses that men with ASD have lower GSH than controls and that this would correlate with the severity of ASD symptoms [assessed using the Autism Observation Schedule (ADOS) (Lord, Rutter, DiLavore, & Risi, 1999) and the Autism Diagnostic Interview - Revised (ADI) (Lord, Rutter, & Le Couteur, 1994) for current and past symptoms respectively]. As co-morbid symptoms of anxiety, depression and ADHD are common in ASD (Matson & Cervantes, 2014; Mazzone, Ruta, & Reale, 2012), we also explored potential correlations between GSH levels and symptoms rated using the State-Trait Anxiety Inventory (STAI) (Ferreira & Murray, 1983), the Beck depression inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and the Barkley self-reported childhood and adulthood scales (BSSR-Child and BSSR-Adult) (Barkley & Murphy, 1998) respectively.
Materials and methods
Participants
21 male participants with ASD and 29 healthy men who did not differ in IQ or age were included in this study. Patients were aged 18 to 50. All participants were right handed and had not been taking psychoactive medication for at least six weeks prior to the study. Full scale IQ was assessed by a trained researcher using the Wechsler Abbreviated Scale of Intelligence (Rudie et al.). Only participants with IQ>80 were included in this study. Participants with ASD were recruited from the Behavioural Genetics Clinic at the Maudsley Hospital, a specialist adult autism diagnostic clinic. All had been diagnosed with autism by experienced psychiatrists according to the ICD-10 criteria (2009). The diagnosis was confirmed using the ADI and/or ADOS. We excluded individuals with a comorbid psychotic illness, epilepsy, a history of head injury, or a genetic disorder (e.g. Fragile X syndrome) known to be associated with ASD. Symptoms of anxiety, depression and attention deficit and hyperactivity disorder (ADHD) were measured using the STAI (Ferreira & Murray, 1983), the BDI and the BSSR-Child and BSSR-Adult scales respectively. All participants provided written informed consent to participate. This study was approved by Essex 2 National Research Ethics Committee.
[1H]MRS data acquisition and processing
[1H] MRS data were acquired on a 3T GE HDx MRI scanner (GE Medical Systems, Milwaukee, WI, USA). A structural MRI scan for subsequent voxel positioning was acquired using a 3D fast inversion-recovery prepared gradient echo acquisition with TI = 450 ms, TR = 7 ms, TE = 2.8 ms, matrix = 256 × 256 over a 240 × 240 mm field of view, giving a 0.9375 × 0.9375 mm in plane voxel size, 124 × 1.1 mm slices (partitions).
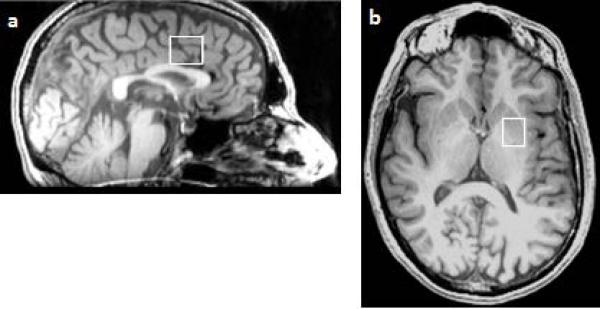
[1H]MRS spectra were acquired using point-resolved spectroscopy (PRESS) (Bottomley, 1987). One voxel (20 × 20 × 15 mm3) was placed in the left basal ganglia (BG), including the head of the caudate, the anterior putamen, and the internal capsule. Another voxel (16 × 24 × 20 mm3) was placed in the left dorsomedial prefrontal cortex (DMPFC) (see Figure 1). PRESS parameters were: TR = 3000 ms, TE = 30 ms. 128 averages were collected in the BG voxel and 96 in the DMPFC voxel. [1H]MRS spectra were processed using LCModel software version 6.3-0I (Provencher, 1993).
Figure 1.
Example of the location of the proton magnetic resonance spectroscopy voxel in the dorsomedial prefrontal cortex (a), and in the basal ganglia (b)
Data were analysed with a basis set optimized for the detection of glutathione, which also included the following metabolites: alanine, aspartate, creatine, gamma-aminobutyric acid (GABA), glutamine, glutamate, GSH, glucose, glycerophosphocholine, phosphocholine, phosphocreatine, myo-inositol (mI), lactate, N-acetyl-aspartate (NAA), N-acetyl-aspartylglutamate (NAAG), scyllo-inositol, and taurine. However, only GSH was considered here.
Spectra were first reviewed visually to verify that spectra were not qualitatively abnormal. Poorly fitted metabolite estimates, defined as Cramer-Rao lower bounds (CRLB) >20%, were excluded from further analysis.
Calculation of GSH concentrations
To control for inter-individual differences in voxel tissue composition, the proportion of grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) in each voxel was calculated. This method provides a useful alternative to reporting metabolite concentrations as ratios to Creatine content (Brooks et al., 2001; Gussew, Erdtel, Hiepe, Rzanny, & Reichenbach, 2012). The structural MRI was segmented into GM, WM and CSF using Statistical Parametric Mapping software (SPM2; http://spm.ion.ucl.ac.uk) and percent tissue class of each voxel was calculated using in-house software as previously described (Horder, et al., 2013). Raw metabolite values were corrected for CSF as follows:
Where ProportionCSF ranged between 0 and 1, and was calculated individually for each voxel. Raw metabolite values corrected for CSF are referred to as corrected GSH concentrations in the rest of this paper.
Statistical analysis
All data were analysed in IBM SPSS 21. Group differences in demographic variables and symptom severity were determined using independent sample t-tests. To investigate potential differences in GSH between groups, we used a general linear model with corrected GSH concentrations as the dependent variable, group as a between subjects factor, and age as covariate, to account for any GSH variation with age (Currais & Maher, 2013; Maurya & Rizvi, 2010).
The relationship between GSH and the severity of current ASD symptoms measured using the three subscales of the ADOS, of past ASD symptoms (three subscales of the ADI) and of anxiety, depression and ADHD symptoms (STAI, BDI, BSSR child and adult scales) was assessed using Pearson correlation coefficient or Spearman's rank correlation coefficient, depending on the data distribution. To account for multiple comparisons in these analyses, we used a ‘family wise’ error correction. The p-value threshold of 0.05 was divided by 3 for analyses of correlations with three subscales of the ADOS; it was also divided by 3 for analyses of correlations with three subscales of the ADI; and it was divided by 4 for analyses of correlations with four co-morbid symptom ratings.
Results
Participants
There was no significant difference in age or IQ between the ASD and control groups. As expected, the participants with ASD scored higher than controls on the questionnaire measures of depression, anxiety, and attention-deficit disorder symptoms (all F > 4.7, all p < 0.04). Participant characteristics are summarised in Table 1. After excluding spectra where the uncertainty (% CRLB) of the GSH estimates were greater than 20% the sample size was 13 patients and 19 controls for the DMPFC voxel, and 16 patients and 18 controls for the BG voxel.
Table 1.
Participant characteristics.
| Characteristic | ASD Patients | Healthy controls | |
|---|---|---|---|
| Age | 31.82 (8.63) | 26.94 (7.39) | |
| ADOS | Social behaviour | 16.47 (5.97) | Not applicable |
| Communication | 12.49 (7.26) | Not applicable | |
| ADI | Social behaviour | 16.47 (5.97) | Not applicable |
| Communication | 12.49 (7.26) | Not applicable | |
| Repetitive behaviour | 4.67 (2.94) | Not applicable | |
| BDI | 8.97 (10.23) | 3.00 (4.43) | |
| STAI | 52.19 (12.90) | 35.29 (8.88) | |
| BSSR Childhood scale | 6.35 (5.04) | 1.95 (2.01) | |
| BSSR Adulthood scale | 3.60 (3.62) | 1.50 (1.58) | |
ADOS, Autism Diagnostic Observation Schedule; ADI, Autism Diagnostic Interview; BDI, Beck Depression Inventory; STAI, State Trait Anxiety Inventory; BSSR, Barkley Self-Reported Scale. Values are reported as mean (standard deviation).
Glutathione
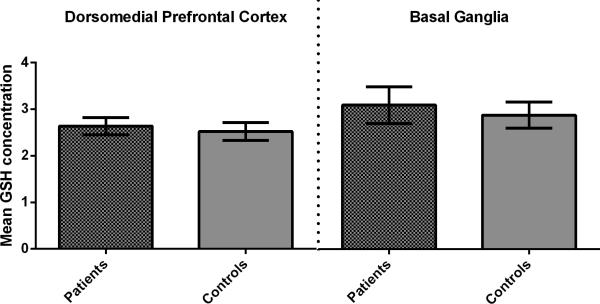
There was no significant effect of group on corrected GSH concentrations in either voxel (F < 0.9, p > 0.1); neither was there a main effect of age, or an interaction between group and age. The results were unchanged when GSH was examined as a ratio to total Creatine (all p > 0.3). Mean corrected GSH concentrations are shown in Figure 2.
Figure 2.
Mean corrected glutathione concentrations in the dorsomedial prefrontal cortex and the basal ganglia did not differ significantly between groups. Error bars represent the standard error of the mean. (GSH, Glutathione; BG, Basal Ganglia; DMPFC, Dorsomedial Prefrontal Cortex)
Correlations with symptom measures
In the ASD group there were no significant correlations between GSH concentrations in either voxel and scores on the ADOS, the ADI, the BDI, the STAI and the BSSR scales.
Tissue composition and data quality
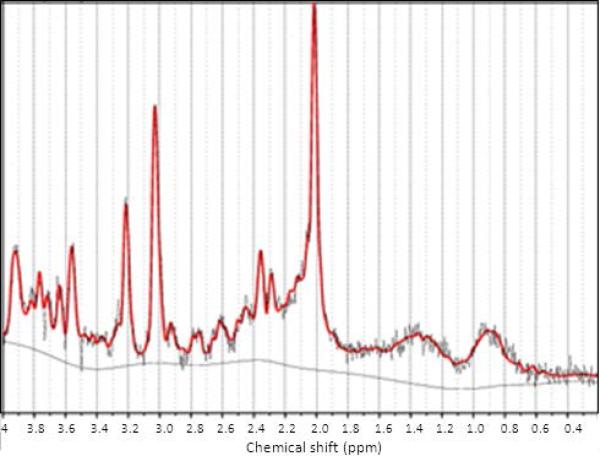
There was no significant group difference in percentage of grey matter, white matter or CSF in either voxel. Figure 3 shows an example of [1H]MRS spectrum after LCModel 6.3-0I fitting.
Figure 3.
Example of a proton magnetic resonance spectroscopy spectrum after LCModel 6.3-0I fitting.
Discussion
This is the first study to quantify glutathione (GSH) brain concentrations in vivo in unmedicated adult men with ASD compared to healthy controls. We observed no group differences in GSH concentrations in either the basal ganglia or the dorsomedial prefrontal cortex and no relationship between GSH levels and clinical symptoms.
Our finding contrasts with prior investigations that have reported decreased GSH in blood plasma in ASD (James, et al., 2004), however it is consistent with post-mortem evidence reporting no difference in GSH in the frontal cortex of individuals with ASD (Chauhan, et al., 2012). Thus, our findings do not support the hypothesis that neuronal damage, secondary to oxidative stress caused by deficient GSH contributes to ASD pathogenesis (R. Deth, et al., 2008; Main, et al., 2012).
We emphasize that our study included only right handed males with normal intelligence. Therefore, it remains possible that GSH abnormalities exist in other subgroups of individuals with ASD, e.g. women, or those with intellectual disability. Age may be especially important to consider in this context because GSH levels have been reported to vary in other age groups (Currais & Maher, 2013; Maurya & Rizvi, 2010). For example, prior investigations have found that GSH is significantly lower in children with ASD (Chauhan, et al., 2012; Frustaci, et al., 2012; James, et al., 2004). Future longitudinal studies are needed to address this.
Alternatively, it is possible that a difference in GSH concentrations does exist in the population that we studied, but that we were unable to detect it (Type II error). Our sample size was modest, reflecting our decision to limit our sample to those individuals who were not taking any medications that might affect brain chemistry. However our sample size was similar to that of Wood et al. (2009) who found a robust 22% difference in GSH concentrations at threshold of p<0.05 using similar approaches in individuals with schizophrenia, and larger than that of Godlewska et al. (2014) who found no difference in GSH concentrations between bipolar participants and healthy controls. In addition, a post-hoc power calculation based on the present data confirmed that if any group differences do exist these are likely to be extremely small and therefore arguably trivial; as a total sample size of at least n = 1200 would be necessary for detection (80% power, at p<0.05).
Our study has a number of other limitations. We did not exclude participants with co-morbid anxiety, depression and ADHD symptoms, but we measured these symptoms using validated clinical scales. We found that ASD participants had higher levels of symptoms than controls. However this was not unexpected. These are very common comorbidities in ASD and sample without any comorbid disorders would be quite unrepresentative for the spectrum (Mazzone, et al., 2012).
From a methodological perspective, GSH is often quantified using a J-edited MEGAPRESS sequence with a longer echo time (TE), but we employed a short TE PRESS sequence to quantify GSH. However, others have shown that GSH can be measured using PRESS at similar echo times (Lagopoulos, et al., 2013; Wood, et al., 2009). While the test-retest reliability of PRESS GSH estimates have never been specifically investigated, PRESS is known to provide reliable quantification for other common metabolites (Fayed, Modrego, & Medrano, 2009).
A further consideration is that we investigated only the basal ganglia and the dorsomedial prefrontal cortex and differences in GSH might yet be found in other brain areas. For example, lower levels of GSH in ASD have been reported in the temporal lobe and cerebellum post-mortem (Chauhan, et al., 2012). Indeed, concentration differences in other brain metabolites are known to be regionally specific in ASD (Horder, et al., 2013). Finally, we did not measure the oxidised counterpart of GSH, GSSG. Hence we cannot exclude the possibility that GSSG levels are altered in our ASD sample, resulting in GSH redox imbalance.
In summary, the data presented here suggest that oxidative stress resulting from low levels of GSH is not a feature of adult men with normal IQ and ASD. However, we cannot exclude the possibility that further exploration of GSH in other subgroups of ASD (for example, those with low IQ), and at different ages, may help identify individuals who may benefit from anti-oxidant treatments.
Acknowledgements
A.M.S.D and J.H. contributed equally to this work. The MRI and MRS data acquisition of this work was funded by the Wellcome Trust (091300/Z/10/Z). A.M.S.D and G.M.M. receive support from the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The funders had no role in the study design, data collection, analysis, or decision to submit for publication. C.M., G.M.M. and D.G.M. are supported by the Dr Mortimer D. Sackler Foundation and are members of the EU-AIMS consortium. GJB received honoraria for teaching from General Electric Healthcare during the course of this study, and acts as a consultant for IXICO. Ethical approval for this study was provided by Essex 2 Research Ethics Committee (REC code 04/Q0102/26).
References
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) 2013 [Google Scholar]
- Barkley RA, Murphy KR. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 2nd ed. Guilford Press; New York: 1998. [Google Scholar]
- Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, et al. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry. 2009;194(6):500–509. doi: 10.1192/bjp.bp.108.059345. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Valk SL, Silani G, Bird G, Frith U, Singer T. Selective disruption of sociocognitive structural brain networks in autism and alexithymia. Cereb Cortex. 2014;24(12):3258–3267. doi: 10.1093/cercor/bht182. [DOI] [PubMed] [Google Scholar]
- Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Roberts N, Kemp GJ, Gosney MA, Lye M, Whitehouse GH. A proton magnetic resonance spectroscopy study of age-related changes in frontal lobe metabolite concentrations. Cereb Cortex. 2001;11(7):598–605. doi: 10.1093/cercor/11.7.598. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Audhya T, Chauhan V. Brain region-specific glutathione redox imbalance in autism. Neurochem Res. 2012;37(8):1681–1689. doi: 10.1007/s11064-012-0775-4. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13(3):171–181. doi: 10.1016/j.pathophys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Chauhan V, Brown WT, Cohen I. Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin--the antioxidant proteins. Life Sci. 2004;75(21):2539–2549. doi: 10.1016/j.lfs.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Currais A, Maher P. Functional consequences of age-dependent changes in glutathione status in the brain. Antioxid Redox Signal. 2013;19(8):813–822. doi: 10.1089/ars.2012.4996. [DOI] [PubMed] [Google Scholar]
- Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology. 2008;29(1):190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Deth RC. Autism: a redox/methylation disorder. Glob Adv Health Med. 2013;2(6):68–73. doi: 10.7453/gahmj.2013.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayed N, Modrego PJ, Medrano J. Comparative test-retest reliability of metabolite values assessed with magnetic resonance spectroscopy of the brain. The LCModel versus the manufacturer software. Neurol Res. 2009;31(5):472–477. doi: 10.1179/174313209X395481. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Murray J. Spielberger's State-Trait Anxiety Inventory: Measuring anxiety with and without an audience during performance on a stabilometer. Percept Mot Skills. 1983;57(1):15–18. doi: 10.2466/pms.1983.57.1.15. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Martinez A, Boluda S, Parchi P, Barrachina M. Brain banks: benefits, limitations and cautions concerning the use of post-mortem brain tissue for molecular studies. Cell Tissue Bank. 2008;9(3):181–194. doi: 10.1007/s10561-008-9077-0. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B, et al. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med. 2012;52(10):2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Geier DA, Kern JK, Garver CR, Adams JB, Audhya T, Nataf R, et al. Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci. 2009;280(1-2):101–108. doi: 10.1016/j.jns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Godlewska BR, Yip SW, Near J, Goodwin GM, Cowen PJ. Cortical glutathione levels in young people with bipolar disorder: a pilot study using magnetic resonance spectroscopy. Psychopharmacology (Berl) 2014;231(2):327–332. doi: 10.1007/s00213-013-3244-0. [DOI] [PubMed] [Google Scholar]
- Gussew A, Erdtel M, Hiepe P, Rzanny R, Reichenbach JR. Absolute quantitation of brain metabolites with respect to heterogeneous tissue compositions in (1)H-MR spectroscopic volumes. MAGMA. 2012;25(5):321–333. doi: 10.1007/s10334-012-0305-z. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. Am J Psychiatry. 2006;163(7):1252–1263. doi: 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, et al. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psychiatry. 2005;58(3):226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Horder J, Lavender T, Mendez MA, O'Gorman R, Daly E, Craig MC, et al. Reduced subcortical glutamate/glutamine in adults with autism spectrum disorders: a [(1)H]MRS study. Transl Psychiatry. 2013;3:e279. doi: 10.1038/tp.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80(6):1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- Kern JK, Jones AM. Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B Crit Rev. 2006;9(6):485–499. doi: 10.1080/10937400600882079. [DOI] [PubMed] [Google Scholar]
- Lagopoulos J, Hermens DF, Tobias-Webb J, Duffy S, Naismith SL, White D, et al. In vivo glutathione levels in young persons with bipolar disorder: a magnetic resonance spectroscopy study. J Psychiatr Res. 2013;47(3):412–417. doi: 10.1016/j.jpsychires.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles, California: 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Main PA, Angley MT, O'Doherty CE, Thomas P, Fenech M. The potential role of the antioxidant and detoxification properties of glutathione in autism spectrum disorders: a systematic review and meta-analysis. Nutr Metab (Lond) 2012;9:35. doi: 10.1186/1743-7075-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JL, Cervantes PE. Commonly studied comorbid psychopathologies among persons with autism spectrum disorder. Res Dev Disabil. 2014;35(5):952–962. doi: 10.1016/j.ridd.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Maurya PK, Rizvi SI. Age-dependent changes in glutathione-s-transferase: correlation with total plasma antioxidant potential and red cell intracellular glutathione. Indian J Clin Biochem. 2010;25(4):398–400. doi: 10.1007/s12291-010-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone L, Ruta L, Reale L. Psychiatric comorbidities in asperger syndrome and high functioning autism: diagnostic challenges. Ann Gen Psychiatry. 2012;11(1):16. doi: 10.1186/1744-859X-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaijen J, Lythgoe DJ, Amiri H, Buitelaar JK, Glennon JC. Fronto-striatal glutamatergic compounds in compulsive and impulsive syndromes: A review of magnetic resonance spectroscopy studies. Neurosci Biobehav Rev. 2015;52:74–88. doi: 10.1016/j.neubiorev.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic resonance in medicine. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Pugliese L, Catani M, Ameis S, Dell'Acqua F, Thiebaut de Schotten M, Murphy C, et al. The anatomy of extended limbic pathways in Asperger syndrome: a preliminary diffusion tensor imaging tractography study. Neuroimage. 2009;47(2):427–434. doi: 10.1016/j.neuroimage.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Rudie JD, Brown JA, Beck-Pancer D, Hernandez LM, Dennis EL, Thompson PM, et al. Altered functional and structural brain network organization in autism. Neuroimage Clin. 2:79–94. doi: 10.1016/j.nicl.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Daly E, Murphy D. Frontal anatomy and reaction time in Autism. Neurosci Lett. 2007;412(1):12–17. doi: 10.1016/j.neulet.2006.07.077. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Iwanaga M, Yasunaga A, Urata Y, Goto S, Shibata S, et al. Protective role of glutathione synthesis on radiation-induced DNA damage in rabbit brain. Cell Mol Neurobiol. 1998;18(3):299–310. doi: 10.1023/A:1022525214871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Kempuraj D, Redwood L. Autism: an emerging ‘neuroimmune disorder’ in search of therapy. Expert Opin Pharmacother. 2009;10(13):2127–2143. doi: 10.1517/14656560903107789. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yahata N, Abe O, Kuwabara H, Inoue H, Takano Y, et al. Diminished medial prefrontal activity behind autistic social judgments of incongruent information. PLoS One. 2012;7(6):e39561. doi: 10.1371/journal.pone.0039561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Soc Cogn Affect Neurosci. 2008;3(2):135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Berger GE, Wellard RM, Proffitt TM, McConchie M, Berk M, et al. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiol Dis. 2009;33(3):354–357. doi: 10.1016/j.nbd.2008.11.018. [DOI] [PubMed] [Google Scholar]