Abstract
Objectives
To assess the Massachusetts Benchmark ‘Study’ (MBS) that the tobacco companies presented to the Massachusetts Department of Public Health (MDPH) in 1999 in response to ingredient disclosure regulations in the state. This case study can inform future ingredient disclosure regulations, including implementation of Articles 9 and 10 of the WHO Framework Convention on Tobacco Control (FCTC).
Methods
We analysed documents available at http://legacy.library.ucsf.edu to identify internal communications regarding the design and execution of the MBS and internal studies on the relationship between tar, nicotine and carbon monoxide and smoke constituents and reviewed publications that further evaluated data published as part of the MBS.
Results
The companies conducted extensive studies of cigarette design factors and ingredients that significantly impacted the levels of constituents. While this study asserted that by-brand emissions could be estimated reliably from published tar, nicotine, and carbon monoxide levels, the tobacco companies were well aware that factors beyond tar, nicotine and carbon monoxide influenced levels of constituents included in the study. This severely limited the potential usefulness of the MBS predictor equations.
Conclusions
Despite promises to provide data that would allow regulators to predict constituent data for all brands on the market, the final MBS results offered no useful predictive information to inform regulators, the scientific community or consumers. When implementing FCTC Articles 9 and 10, regulatory agencies should demand detailed by-brand information on tobacco product constituents and toxin deliveries to users.
INTRODUCTION
Tobacco companies have long blocked regulatory agencies from disclosing information about the ingredients in their products.1–4 Ingredient and emission disclosure is a continuing issue for tobacco regulators today, including the US Food and Drug Administration and comparable organisations in countries that have ratified the WHO Framework Convention on Tobacco Control (FCTC), which includes Article 9 on the regulation of the contents of tobacco products and Article 10 on the regulation of tobacco product disclosures.5 In 1996 Massachusetts enacted “An Act Providing for Disclosure of Certain Information Relating to Tobacco Products Sold in the Commonwealth” that required the tobacco companies to provide detailed by-brand information on the ingredients and nicotine delivery of their products to the Massachusetts Department of Public Health6 (MDPH) and granted MDPH authority to release this information to the public.6
The companies immediately sued to block the Act7,8 and in 1999 developed a new strategy of appearing to be more open to regulations by offering the state the ‘Massachusetts Benchmark Study’ (MBS) with the assertion that it was possible for regression equations based on publicly available tar, nicotine, and carbon monoxide (CO) to accurately predict the 41 constituents in smoke for different brands without providing the actual information on specific brands that Massachusetts was seeking.22 In April 1999, in the midst of the litigation, MDPH agreed to the MBS, which the companies summarised in a presentation to MDPH in February 200010 and delivered in July 2000.9 The MBS allowed the companies to prevent effective disclosure of constituent information on a brand-by-brand basis.
Despite the tobacco companies’ claim that the MBS would allow reliable estimation for all brands from published tar, nicotine and CO levels of a specific cigarette’s constituents, they knew smoke chemistry could be manipulated in a way not predicted by these published measurements. The companies have also promoted benchmarking in Australia, the UK and Canada. Examining the process of the MBS and the validity of the approach informs the studies completed in these nations as well as challenges countries may face as they implement ingredient assessment and disclosure specified in the Partial Guidelines for Articles 9 and 10.11
METHODS
We searched the University of California San Francisco Legacy Tobacco Documents Library (LTDL: http://legacy.library.ucsf.edu) between February 2013 and August 2014 for documents related to the MBS12 beginning with ‘Massachusetts Benchmark Study,’ ‘Massachusetts and ingredients’ and ‘predictor equation and ingredients. ‘Internal company research studies were located by searching for specific constituents measured in the MBS prior to the industry proposing the MBS to MDPH together with the words ‘predictor,’ ‘regression’ and ‘smoke chemistry’. In addition, reports of product changes that manipulated the constituents measured in the MBS were found by searching for constituent with ‘reduction, ’ ‘reducing’ and ‘decrease’ and by searching for scientists who worked on the MBS with phrases related to altering constituents (eg, formaldehyde). We searched for documents related to benchmark studies conducted in other countries including Canada, Australia and the UK. We evaluated implementing guidelines for FCTC Articles 9 and 1011 to compare their ingredient disclosure recommendations to those attempted in Massachusetts.
RESULTS
Legal maneuvering
In 1996 Brown and Williamson (BW), Lorillard, Philip Morris (PM) and RJ Reynolds (RJR) sued Massachusetts in federal district court claiming that the Massachusetts Disclosure Act was preempted by the Federal Cigarette Labelling and Advertising Act and Smokeless Tobacco Act.7 The companies followed with a second suit in 1997 alleging that the Disclosure Act violated the Constitution’s Commerce, Fourteenth Amendment Due Process, and Takings clauses.8 The first case was heard in June 1997, and in August 1997 the court ruled against the companies on pre-emption without addressing the other claims.7 In December 1997 the court granted a preliminary injunction blocking enforcement of the Act, which was upheld by the Court of Appeals in November 1998.8 In January 2002, the Court of Appeals found that the Disclosure Act was an unconstitutional taking that deprived companies of property without due process.3
Meanwhile, in August 1998, the Massachusetts governor announced new regulations (independent of the still-being litigated Disclosure Act) that would require companies that had a national market share of larger than 3% to disclose the amount of constituents of mainstream and sidestream smoke by brand to MDPH and authorised MDPH to release the information to the public.13,14 The first list of constituents was due in July 2001.
The birth of the Massachusetts Benchmark Study
In December 1998, scientists from the tobacco companies met with MDPH and the US Centers for Disease Control and Prevention (CDC) to discuss the revised regulations and suggested they would be willing to provide ‘benchmark’15 information if MDPH agreed to delay further regulations until information was provided. The ‘benchmarking’ the companies proposed involved the companies providing a set of regression equations that would allow MDPH to reliably estimate 41 constituents in the mainstream and sidestream smoke for different brands of cigarettes using published levels of tar, nicotine and CO that the companies were required to report to the Federal Trade Commission (FTC).16 The four companies, BW, Lorillard, RJ Reynolds and PM met in January 1999 to select 25 brands to measure the levels of tar, nicotine, CO and the constituents MDPH specified in mainstream smoke. In a subset of 12 brands the companies would measure sidestream smoke under two different conditions. The first, was the FTC method, a standardised procedure recognised as unreliable measures of toxin exposure based on actual human smoking patterns,17 and the second condition was designed by the MDPH to better reflect human smoking behaviour.18 Each of the four companies developed their own lists of selected brands.19–21 After the companies developed their individual lists, they selected the brands to include in the MBS at the December 1999 meeting. (The available documents do not indicate why or how the companies made their individual or join brand selections).
The companies then proposed the MBS to MDPH,22 assuring MDPH that the selected brands reflected the range of products on the market across manufacturers, price tier, FTC tar yield, filter characteristics (eg, whether made of cellulose acetate or recessed charcoal), cigarette circumference, cigarette length, paper porosity, menthol, tobacco weight, packaging and market share. The MDPH did not explicitly ask for information on all of these features to be included. MDPH had asked how menthol, tar and nicotine were correlated with the 41 chemicals in the smoke that would be measured, and requested information on cigarette circumference, ventilation and percentage of sheet (reconstituted tobacco) during the December meeting with the companies.15 MDPH also requested information on blends and high level additives; the companies refused this information on the grounds of trade secret protection.23
In March 1999 MDPH agreed to suspend regulations until the MBS was completed 6–8 months later.24 Over the next year the companies worked together in an equal partnership and used the long-time tobacco industry law firm Covington and Burling to communicate study updates to the MDPH.10,23 The companies presented a summary of the results of the MBS to MDPH in February 200026 and the full report in July 2000.9
Consistent with the companies’ assertion in their December 1998 meeting with MDPH, the MBS concluded that, “(1) Mainstream smoke particulate phase constituent yields when cigarettes are smoked with the Massachusetts smoking regimen can be most reliably estimated from either a cigarette’s nicotine or ‘tar’ yields” and “(2) Mainstream smoke vapour phase constituent yields can be estimated from a cigarette’s CO yields under either FTC or Massachusetts smoking conditions”9.
Study concept
Following the December 1998 meeting between MDPH and the companies where the concept of a benchmark study was discussed, a senior RJR scientist wrote a colleague that he was concerned that MDPH was focused on novel technologies, ingredients and processed tobacco that could impact constituent yields not predicted by tar and that the MBS did not address these issues.27
In January 1999, an RJR author of the MBS prepared a draft proposal that stated the brands selected for sidestream analysis were “significantly skewed to lower tobacco weights when compared to the distribution of tobacco weights typically found in the marketplace.” 18 This meant the MBS design was biased towards lower levels of SHS estimates than is true in the market as a whole, but this sentence was omitted when the MBS was formally submitted to MDPH on 29 January 1999. Instead, the proposal stated, “The weight of tobacco in a particular cigarette configuration is, as we discussed, a design feature that is expected to affect sidestream smoke yields. The twelve brand styles we recommend using for sidestream smoke analysis represent a wide range of cigarette weights.” 22 This change is important because the weight of the product (ie, the amount of material burned) is an important determinant of the amount of secondhand smoke produced.28
On March 22, 1999 MDPH accepted the proposed design and the companies started work.24 In contrast to the assurances the companies made to MDPH during these negotiations and in the final MBS report,22,29 they knew that cigarette design parameters, ingredients and blends could impact the constituents of mainstream smoke in ways not predicted by tar, nicotine and CO (table 1).
Table 1.
Research conducted by the tobacco companies on smoke composition that contradict the conclusions of the MBS
| Company, year | Study | Finding (per cigarette unless noted) | Contradiction |
|---|---|---|---|
| Brown and Williamson 195530 | Evaluated phenol in different kinds of tobacco | Burley tobacco 0.37 mg of phenol and 13.72 mg nicotine per litre Bright tobacco 0.508 mg phenol and 9.72 mg nicotine |
Tobacco types have differences in phenol not predicted by nicotine |
| Brown and Williamson 197031 | Addition of urea to cigarettes | A Viceroy cigarette with 5% added urea had 2.06 mg nicotine, 23.1 mg tar and 279 μg acetaldehyde A Viceroy water treated control (no urea) had 1.98 mg nicotine, 24.4 mg tar and 509 μg acetaldehyde |
Urea can be used to make aceltaldehyde not predicted by nicotine or tar |
| PM 197132 | Study of single blend cigarettes (eg, 100% burley) | Turkish tobacco 1.63 mg of nicotine 0.92 mg of acetaldehyde and −0.19 mg HCN Bright tobacco 3.07 mg of nicotine, 0.97 mg acetaldehyde and 0.22 mg HCN |
Constituent levels not accurately predicted by nicotine |
| RJR 197333 | Comparing smoke chemistry to RJR and PM reconstituted tobacco | RJR reconstituted tobacco 0.59 mg, 810 μg acetaldehyde and 41.5 μg formaldehyde Marlboro reconstituted tobacco 2.2 mg nicotine, 840 μg acetaldehyde and 22.9 μg |
Smoke composition not accurately predicted by nicotine for cigarettes with different kinds of reconstituted tobacco |
| PM 198134 | Discussing use of regression equation for predicting acrolein | PM scientists acknowledge an equation that includes phosphorous, sugar and scopeletin (a coumarin) is best for predicting acrolein levels | Equation including other ingredients besides nicotine are best for predicting smoke chemistry contradicts hypothesis they can be predicted from nicotine alone |
| PM 198335 | Fertiliser impact on 100% Burley cigarettes | Burley with special fertiliser (0.36–0.64 mg) NO and (1.57–3.88 mg) nicotine Burley normal fertiliser (0.10–0.14 mg/cig NO and 1.74– 3.10 mg nicotine) |
Presence of fertiliser results in differences in NO in ways not predicted by nicotine |
| RJR 198536 | Addition of a specific kind of salt (MENSA) to RJR brands | MENSA reduced formaldehyde by 25%, acrolein by 15% and other aldehydes by 17% with no significant impact on CO | Special salt on filter changes smoke chemistry in ways not predicted by CO |
| PM 198737 | Glycerol and/or propylene glycol added to filler of cigarette | Increase of 5.4% glycerol resulted in 50% increase of acrolein and 350% increase in formaldehyde sidestream smoke with no changes in co, nicotine or dry particulate matter | Glycerin and propylene glycol change sidestream smoke deliveries in way not predicted by CO, nicotine or tar |
| Lorillard 198938 | Evaluating the impact of urea on smoke chemistry | A Viceroy cigarette with 5% added urea had 2.06 mg nicotine, 23.1 mg tar and 279 μg acetaldehyde A Viceroy water treated control (no urea) had 1.98 mg nicotine, 24.4 mg tar and 509 μg acetaldehyde |
Urea changes aceltaldehyde in ways not predicted by nicotine or tar |
| Lorillard 199239 | Evaluation of adding magnesium nitrate on smoke | Addition of 5% magnesium nitrate had 9 μg hydroquinone and 10 μg catechol compared to a control cigarette with 109 μg hydroquinone and 100 μg catechol with no changes in tar | Magnesium nitrate additives change smoke chemistry in ways not predicted by tar |
HCN, hydrogen cyanide; MBS, Massachusetts Benchmark Study; NO, nitric oxide; PM, Philip Morris; RJR, RJ Reynolds.
Tobacco companies internal research on constituents
Table 1 lists 10 illustrative examples of the tobacco companies’ extensive history of measuring and altering the constituent levels of their own products using reconstituted tobacco, filters, filter additives, and other novel technologies without changing the levels of tar, nicotine or CO.30–39 We were also unable to find any examples of the companies using benchmarking or regression equations to estimate constituent levels on their own or their competitors’ products. This section briefly summarises some of the internal research conducted by the tobacco companies (table 1) that produced results that conflicted with the conclusions in the MBS.
Blends and reconstituted tobacco
The companies have long researched the impact of different blends on smoke chemistry in ways not predicted by tar or nicotine. Even though different types of tobacco have different levels of carcinogenic tobacco specific nitrosamines and yield different levels of polycyclic aromatic hydrocarbons (carcinogens that can damage DNA) in smoke,40 the companies refused to provide MDPH information on the amounts or kind of tobacco in their products.10
BW found bright tobacco had significantly higher phenol levels in smoke than burley tobacco in smoke despite having less nicotine.30 PM compared the gas phase components of cigarettes made with 100% burley, bright and Turkish Tobacco.32 Bright and Turkish tobacco had similar levels of acetaldehyde and hydrogen cyanide despite Turkish having significantly less nicotine. These results contradict the MBS conclusion that smoke composition could be accurately predicted by nicotine levels.
In 1983 a PM study approved by Jerry Whidby, a PM author of the MBS, analysed the gas phase components of cigarettes filled with 100% burley and bright tobaccos treated with different fertilisers.35 Nitric oxide (NO) in the smoke was significantly lower for burley treated with a fertiliser traditionally used on bright tobacco compared to normal burley tobacco despite similar nicotine levels in the smoke. The cigarettes were described as a promising approach for NO reduction that maintained good subjective characteristics when tested by a panel of smokers. An additional study completed in 1973 by RJR33 provided evidence of how reconstituted tobacco impacted smoke chemistry in ways not predicted by nicotine.
Additives
The companies experimented with additives that affected the level of specific smoke constituents while not impacting tar, nicotine or CO. BW found adding urea could substantially decrease acetaldehyde and increase nitrogen oxides without significant changes in tar and nicotine,31 and Lorillard also reported lower levels of acetaldehyde for cigarettes with added urea without changes in nicotine.38 PM Europe37 found adding glycerol and/ or propylene glycol to the filter of a cigarette could increase side-stream deliveries of acrolein and formaldehyde without impacting puff count, CO, nicotine or dry particulate matter. RJR found placing a special sodium salt in a cavity in the filter could reduce aceteldehyde and acrolein without reductions in tar or nicotine.36 These findings show that additives and humectants impact toxic constituents in mainstream and sidestream smoke in ways not predicted by tar, nicotine or CO levels.
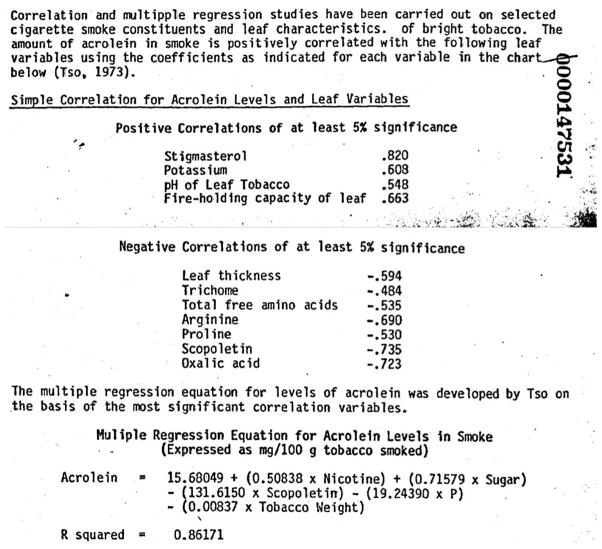
There is also evidence of the companies acknowledging design factors and ingredients that influence smoke chemistry. For example, internal 1981 PM correspondence included lists of ingredients that impact acrolein in cigarette smoke, including highlighting a regression equation developed by the US Department of Agriculture that included nicotine, sugar, phosphorous tobacco weight and scopoletin (a coumarin) to predict acrolein in smoke34 (figure 1). PM scientists described this equation as the best approximation for acrolein levels because it gives coefficients for the most significant variables in the reaction that turns glycerol into acrolein. Although the regression equation in this document is not a strong predictor for acrolein (It is similar to the MBS regression equation for acrolein), it shows that PM was aware of factors that impacted acrolein formation beyond nicotine, tar and CO. The fact that the best regression equation that incorporates more complex factors is still not very accurate underscores the fact that regression equations should not be used to predict constituents in cigarettes and that the individual brands need to be measured.
Figure 1.
An internal 1981 Philip Morris scientific report34 used the multiple regression equation developed by the US Department of Agriculture to determine the amount of acrolein in smoke, showing that Philip Morris was aware that factors beyond tar, nicotine and CO can impact acrolein.
International benchmark studies
Australia
In 1999, the Australian Federal Department of Health and Aging proposed annual emissions testing for all brand varieties with a market share greater than 3%, which British American Tobacco, Imperial Tobacco, and PM blocked such testing in court.41 In July 1999, while the MBS was underway, E. Windholz, director of corporate affairs of PM Australia/New Zealand, wrote the director of the Department’s Tobacco and Alcohol Strategies Section describing the MDPH letter agreeing to forego further regulations until the MBS was completed.42 Later, the director of Imperial Tobacco Australia, Nick Cannaar, told the Department that there was considerable evidence in the scientific public literature that the proportions of emissions in smoke per milligram of tar are essentially fixed for cigarettes with the same tobacco type.43 Confidential emails withheld on grounds of attorney-client privilege indicate that Windholz communicated with PM USA officials involved with the MBS regarding an Australian benchmark study.44
In 2001, the companies agreed on a one-time basis45 to provide the Department cigarette actual emissions results for 41 constituents in mainstream and sidestream smoke for 15 Australian brands the companies selected.46
United Kingdom
In 2001, the UK Department of Health designed a constituents study protocol to be executed by the Tobacco Manufacturers’ Association, the tobacco companies’ trade association.47 The companies selected 25 brands that represented 58% of the marketplace and included a variety of tar yields, filter ventilation, paper permeability, cigarette circumference, length and blends. The brands were smoked under ISO machine smoking conditions and measured for the yields of different chemicals in the smoke.47 The study design and data delivered were similar to the MBS. Both studies provided the constituent data for the individual brands and used the entire data set to develop regression equations for tar and CO. The UK study did not develop a predictor equation for nicotine. Similar to the MBS, the UK study concluded, “For most analytes there is evidence of a linear relationship between ‘tar’ and carbon monoxide yields and the yield of the analyte being measured.” 47
Canada
In June 1998, British Columbia (BC) Health Minister Penny Priddy announced new regulations requiring tobacco companies to report cigarette additives and ingredients and 44 toxic emissions.48,49 In July 1998, the BC government released a fact sheet based on the Health Canada-commissioned research on levels of 44 constituents in the three most purchased cigarette brands in BC.50 In developing similar regulations, Health Canada accepted proposals from the industry to reduce the number of tests required by using ‘benchmarking’51 in June 2000.52 In June 2000, Michael Borgerding, an RJR author on the MBS, provided a copy of a study called the ‘1999 Canadian Benchmark Study’ to the Bureau of Tobacco Control for Health Canada, and asked if the report would satisfy an exemption to the existing Tobacco Products Information Regulations.53 Health Canada accepted benchmarking as an alternative to annual emissions disclosures of 38 specific constituents.41 A subsequent report prepared by the Canadian Tobacco Control Programme concluded that the Canadian Benchmark study may have provided more accurate predictions than the MBS because the cigarettes in the sample had the same tobacco blends, filter materials, paper and additives.54 However, the Canadian benchmarking has the same fundamental flaws of all benchmarking systems in that the values presented are not actual values of cigarette constituents and smoke products and provide an incentive for the tobacco companies to modify products in ways not reflected in the reported tar, nicotine and CO levels.
Worldwide benchmark study
In 2004, PM scientists, including two who worked on the MBS, published a worldwide benchmark study55 in the pro-industry journal Regulatory Toxicology and Pharmacology that used tar, nicotine and CO to predict 42 other constituents under the ISO machine smoking conditions. They reported results for 48 PM brands the company selected from the USA, Latin America, Asia Pacific, Japan, European Union, Central Europe and the Middle East. PM concluded that more than 90% of the brands included in the study had smoke chemistry yields that were within the 95% prediction intervals using the benchmark regressions, and suggested that benchmarking had the potential to provide reliable predicted smoke constituent yield information.
DISCUSSION
In response to proposed regulations that would force tobacco companies to disclose information about the ingredients and constituents in their products, the companies provided MDPH with limited information about constituents in a select group of products and successfully delayed further regulatory action while the study was conducted. The limited information ultimately provided by the companies was of no use to MDPH or the general public and subsequent research into internal documents of the tobacco companies reveals that benchmarking is not a scientific method used in any of the tobacco companies’ internal research. Our recent4 analysis of 100 industry reverse engineering studies of the amounts of ingredients and constituents in their own and competitors’ products did not contain any examples in which the companies used benchmarking of tar, nicotine or CO to predict the constituents.4 If benchmarking produced reliable estimates of constituent levels, presumably the companies would have used it internally.
Evaluation of the validity of benchmarking
The MDPH asked the CDC Office on Smoking and Health and Massachusetts Institute of Technology (MIT) to evaluate the MBS; subsequent analysis of the Canadian and Australian studies was completed by Australia’s VicHealth Centre for Tobacco Control.41,56,57
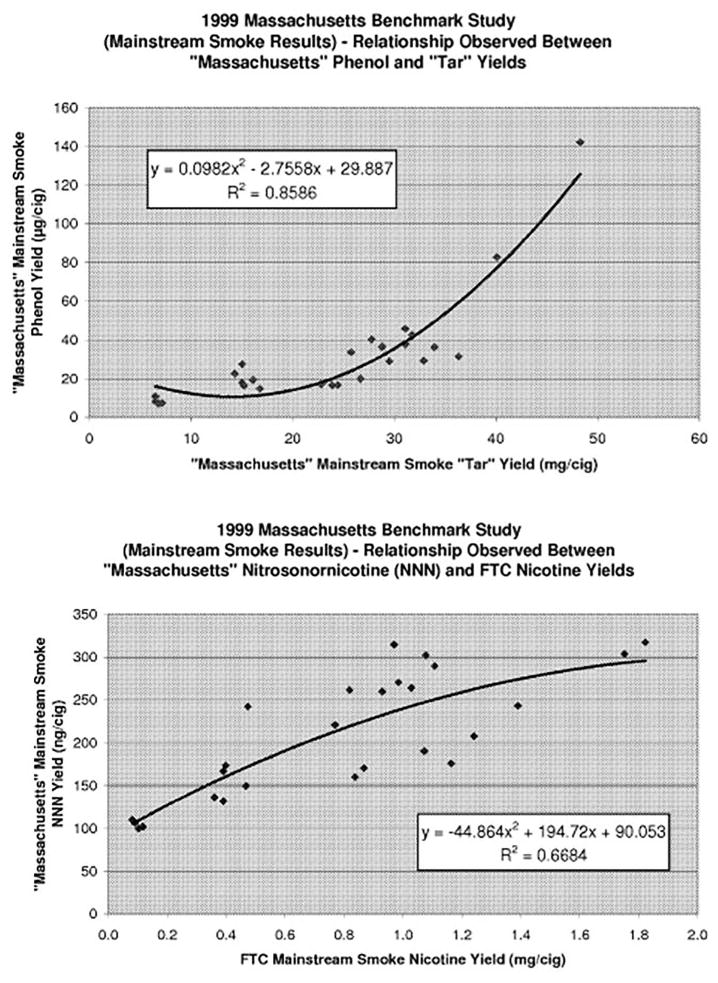
The CDC reported “developing a predictor model using the benchmark approach (ie, the MBS) for particulate and gas phase constituents in the mainstream and sidestream smoke of cigarettes will not provide much useful information to public health researchers and consumers.” 56 The CDC observed there was a lack of a highly correlated functional relationship between the predicted values for specific compounds as presented on the best-fit curves presented in the report. For example, for phenol, the data are correlated best with the measured tar levels (R2=0.86), but in the tar range of 5–25 mg/cig the best-fit line of the benchmark model is essentially flat even though the measured phenol increased by 311% from 9 to 28 μg/cig (figure 2). The CDC also observed that the benchmark model gave much poorer fits for tobacco specific nitrosamines with NNN, NAT and NAB all having R2 between 0.64 and 0.70. NNN levels correlated best with nicotine but for an FTC delivery of 1.0 mg of nicotine NNN levels varied from 150 to 300 ng/cig.58 The CDC recommended direct product testing (what MDPH wanted in the first place) as a better approach.56
Figure 2.
Regression equations from the MBS did not provide accurate predictions for the level of constituents based on tar or nicotine.9 In tar ranges of 5–25 mg/cig the predicted values for phenol ranged by up to 300% despite a relatively flat best fit line (top). There is wide variation in actual NNN from those predicted by FTC nicotine yields (bottom).9 FTC, Federal Trade Commission; MBS, Massachusetts Benchmark Study.
Harris analysed the relationship between the levels of tobacco specific nitrosamines and FTC tar, nicotine and CO yields for the brands included in the MBS using the data in the MBS.57 In a second analysis he added the manufacturer of the brand as an independent variable and found significantly better predictions for nitrosamines. Including the manufacturer increased the coefficient of determination (R2) from 0.38 to 0.78 for NNN, from 0.46 to 0.88 for NNK, and from 0.49 to 0.81 for NAT. In fact, the cigarette manufacturer was a stronger predictor of nitrosamine levels than nicotine and CO. Harris concluded “manufacturers’ blending and processing of tobacco may significantly influence a cigarette’s yield of carcinogenic nitrosamines” and “the present findings contradict the hypothesis (that is, the MBS) that the FTC tar level of a cigarette brand is, by itself, an adequate indicator of the yields of all other harmful smoke constituents.” 57 Consistent with the company studies (table 1), Harris concluded that variables controlled by the manufacturer, including tobacco blends and the methods for processing tobacco, have a stronger impact on nitrosamines yields than tar alone.
Likewise, in 2007 Australia’a VicHealth Centre for Tobacco Control41 examined the efficacy of benchmarking for 13 chemicals with the highest toxicological risk by completing multivariate analysis on data that the tobacco companies submitted to Australia and Canada. Like Harris, they found that tar, nicotine and CO and filtration efficiency were poor predictors for most of the 13 chemicals. Country and manufacturer improved the predictions for all chemicals and strongly improved predictions for some, which they attributed to differences in tobacco sourcing, processing and blending.
All three of these analyses41,56,57 confirm the fact that the companies’ statement in the MBS that tar, nicotine and CO could be used to predict the levels of toxic constituents in different brands of cigarettes is not accurate.
While this subsequent data did provide some useful information to regulators in learning that manufacturing type has a powerful impact on nitrosamine levels, this fact should be not accepted as an argument to allow tobacco companies to evade full disclosure of product and smoke constituents on the grounds that providing some ingredient or constituent information is better than none. In addition, the tobacco companies have a history of manipulating study designs and misrepresenting information to the scientific community.59–62 The MBS, together with these other examples, underscore the danger of allowing the tobacco companies to control how they make ingredient and smoke constituent data available to regulators and the public. Indeed, the experience with the MBS adds to the case for open access to all brand testing and other scientific data on the grounds that such data would be valuable to regulators and the public even in the absence of other disclosure requirements.
Relevance to implementing the FCTC ingredient disclosure provisions
The lessons learned from the MBS are important to regulatory agencies around the world as they work to implement Partial Guidelines for Implementation of Articles 9 and 10 of the WHO Framework Convention on Tobacco Control.11 The disclosure regulations Massachusetts sought to implement are very similar to these guidelines, with one notable exception (table 2). The Massachusetts regulations did not address trade secrets; the Partial Guidelines for the FCTC states that “Governmental authorities should apply appropriate rules in accordance with their national laws when collecting information claimed to be confidential by tobacco manufacturers and importers in order to prevent unauthorised use and/or dissemination of this information.11 During the MBS the tobacco companies used the trade secret argument to avoid disclosing blend and additive information. Parties to the FCTC should be able to prevent the tobacco companies from using this trade secret argument if they have plans in place to prevent unauthorised use or dissemination of blend and additive information.
Table 2.
Comparison of the proposed Massachusetts Ingredient Disclosure regulations and the partial guidelines for implementation of articles 9 and 10 of the FCTC
| Organization | Massachusetts Department of Public Health (1997) | FCTC (2012 onward) |
|---|---|---|
| Legal requirement | An Act Providing for Disclosure of Certain Information Relating to Tobacco Products Sold in the Commonwealth6 | Articles 9 and 1011 |
| Trade secrets | Not addressed | Parties should not accept claims from the tobacco industry concerning the confidentiality of information that would prevent governmental authorities from receiving information about the contents and emissions of tobacco products. Governmental authorities should apply appropriate rules in accordance with their national laws when collecting information claimed to be confidential by tobacco manufacturers and importers in order to prevent unauthorised use and/or dissemination of this information11 |
| Form of disclosure to government | On a brand-by-brand basis all added constituents in the cigarette or smokeless tobacco product listed in descending order by its weight, measure or numerical count to MDPH6 | Manufacturers and importers of tobacco products disclose the ingredients used in products at specified intervals, by product type and for each brand within a brand family. Disclosing on a brand-by-brand basis and in a standardised format provide opportunities to analyse trends in product composition and keep track of subtle changes in the market11 |
| Disclosure to the public | If the department determines it is in the public interest to do so information received as part of a tobacco company disclosure may be released or distributed by the department to the public6 | Parties should disclose information about the toxic constituents and emissions of tobacco products to the public in a meaningful way. Parties may determine in accordance with their national laws the information that should not be disclosed to the public11 |
FCTC, Framework Convention on Tobacco Control; MDPH, Massachusetts Department of Public Health.
In addition, the Partial Guidelines of the FCTC state that Parties should specify the specific methods for the reporting of cigarette design features and that laboratories conducting testing for emission and ingredient disclosure should not be tobacco industry owned or controlled.11 The results of the MBS validate this concern as the tobacco companies selected the brands, provided the cigarettes and conducted all the testing, measurements and statistical analysis. As demonstrated in the Results, the companies selected cigarettes whose weights were skewed towards lighter brands,18 which has an impact on sidestream smoke yields, a point withheld from MDPH.22 RJR scientists also wanted to keep MDPH from focusing on novel technologies,27 which has direct relevance in 2015 as the tobacco companies are introducing a wide range of novel products, including e-cigarettes and heat-not-burn products. The companies’ control of testing and selection of brands also left MDPH at a serious disadvantage because MDPH had no control over how the assays were performed. As new products are introduced into the market countries need to be aware of the limitation of benchmarking.
The development of the WHO Tobacco Free Initiative’s TobLabNet,63 a global network of government, academic, and independent laboratories established to strengthen national and regional capacity for testing tobacco products, including cigarette contents and emission products, provides countries the capacity to test tobacco products independent of the tobacco companies. TobLabNet eliminates the arguments the tobacco companies used to justify their control of testing for the MBS. Countries with limited technical or financial resources can tap TobLabNet resources to facilitate testing of ingredient and emission for regulatory decisions and disclosures without having to rely on the tobacco companies to do and interpret the measurements.64 The development of TobLabNet resources will also provide regulators with control over what is measured in cigarettes and why. In addition, implementing these testing facilities will provide accurate measurements of toxicants in commercial cigarettes and may help regulatory agencies analyse emerging technologies that the tobacco companies tried to divert attention from during the MBS process. Unfortunately, as of July 2015 there was no explicit mechanism for sustained funding to support development of ToblabNet facilities to support Parties’ implementation of the Partial Guidelines for Implementations of Articles 9 or 1011 of the FCTC. To ensure independence from tobacco companies for information on ingredients testing, as per the Partial Guidelines, the Conference of the Parties should consider the development of such a funding mechanism for TobLabNet.
The MBS experience, as well as other benchmarking and emissions based studies in Australia, the UK and Canada, provide information on how tobacco companies around the world may respond to the recommended ingredient disclosure regulations to implement FCTC Articles 9 and 10, particularly information about the tobacco blends used in their products. In February 1999 the director of MDPH requested that the MBS include information on the blends of tobacco and percentage of reconstituted tobacco used in the cigarettes in the MBS; the law firm representing the tobacco companies refused.23 At the conclusion of the study, MDPH again asked for blend information in coded form to protect the trade secrets.10 Again the request was denied with the tobacco company responding, “Providing blend specification, even in coded form, would permit anyone with access to the full data set, including the four companies who are active competitors to match those specifications to a specific product, thus destroying the value of the trade secret.10 These responses represent obstacles that regulators may face when attempting to enforce the best practices recommended by the FCTC for ingredient disclosure despite the fact that information claimed to be trade secret protected is routinely measured by rival companies and may not meet the definition of trade secrets.4 In addition, the development of TobLabNet laboratories with increased independent analytical capabilities bypasses this problem because it permits cigarettes to be tested independently to determine blend information. The resulting data from cigarettes and other emerging novel tobacco products around the world can then be used to conduct independent evaluation of the relationships between blend type and toxic carcinogenic emissions.
Limitations
This paper was limited to the internal documents made available in LTDL up to when the tobacco companies designed and executed the MBS. We screened thousands of documents (the search term ‘Massachusetts Benchmark’ yielded 3658 documents and ‘Massachusetts and Borgerding’ yielded 4671 documents). However, since the ingredient disclosure regulations were heavily litigated, the company lawyers were included on communications related to the MBS and 345 documents remain confidential under claims of attorney client privilege. The available documents do not explain why the companies selected the brands that they did or how they selected the characteristics that they were willing to report in the MBS. The withheld documents may provide additional information about how cigarettes were selected for the study and how or why specific information was or was not presented to MDPH. In 1999, the US Department of Justice sued the major tobacco companies (including those involved in the MBS) for fraudulent and unlawful conduct under the Racketeer Influenced and Corrupt Organisations Act65 which may have led the companies to be more guarded about their communications regarding the MBS. Since it is not known how the cigarettes for the MBS were selected, the fact that they were selected by the tobacco companies raises the question of whether brands were selected with design features and technologies not representative of the majority of products on the market to predict low constituent yields.
CONCLUSIONS
The Massachusetts Benchmark Study was a large scale study that served to delay policy actions in the state of Massachusetts and ultimately provided no useful information to the scientific community or consumers of tobacco products. The companies knew there were a large number of variables that independently affected the constituents measured in the MBS, and used the study design to avoid providing by-brand information about the constituents in their product.
The fact that this study design was proposed and published by tobacco companies in response to regulations around the world demonstrates that the tobacco companies have widely used this approach as a way to avoid accurate reporting about the toxicity of their products. It also demonstrates the importance of communication between regulatory bodies when considering policy changes regarding tobacco products, particularly as they implement the product regulation provisions of the WHO Framework Convention on Tobacco Control.5 When considering tobacco policy, regulators should not negotiate with the tobacco companies or allow tobacco companies to conduct research as a means of influencing policy in a way that allows them to provide misleading information. These agencies should demand the same detailed by-brand information on tobacco product constituents and toxin deliveries to users that Massachusetts sought in the beginning.
What this paper adds.
The tobacco companies had conducted studies confirming that physical design features, technologies and ingredients impacted the chemical yields of cigarettes in ways not predicted by tar, nicotine and carbon monoxide ‘benchmarking’.
‘Benchmarking’ is not an accurate way to determine deliveries of a wide range of toxins in cigarette smoke and should be rejected by public health authorities.
In implementing Framework Convention on Tobacco Control (FCTC) Articles 9 and 10, parties should reject studies conducted or controlled by the tobacco industry.
Acknowledgments
Funding This work was supported by National Cancer Institute Grant CA-087472. The funding agency played no role in the selection of the specific research topic, conduct of the research or preparation of the manuscript.
Footnotes
Contributors SG had the idea for the study. CV collected the data and prepared the first draft of the paper. SA-B contributed information about the relevance to implementation of FCTC Articles 9 and 10. All authors collaborated to prepare the final manuscript.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement All source materials for this paper are publicly available. The tobacco documents are all available at the UCSF Legacy Tobacco Documents Library, http://legacy.library.ucsf.edu.
References
- 1.MacKenzie R, Collin J, Sriwongcharoen K, et al. “If we can just ‘stall’ new unfriendly legislations, the scoreboard is already in our favour”: transnational tobacco companies and ingredients disclosure in Thailand. Tob Control. 2004;13(Suppl 2):ii79–87. doi: 10.1136/tc.2004.009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman S. “Keep a low profile”: pesticide residue, additives, and freon use in Australian tobacco manufacturing. Tob Control. 2003;12(Suppl 3):iii45–53. doi: 10.1136/tc.12.suppl_3.iii45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US District Court. Philip Morris, Inc V. Reilly, 312 F.3d 24 (1st Cir. 2002). 2002.
- 4.Velicer C, Lempert LK, Glantz S. Cigarette Company Trade Secrets Are Not Secret: An Analysis of Reverse Engineering Reports in Internal Tobacco Industry Documents Released as a Result of Litigation. Tob Control. 2014 doi: 10.1136/tobaccocontrol-2014-051571. Published Online First: 2014 Jun 11. pii: tobaccocontrol-2014-051571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. [accessed 12 Aug 2014];Geneva Who Framework on Tobacco Control. 2003 http://www.who.int/fctc/en/
- 6.Massachusetts Acts, 1996 Chapter 0234. An Act Providing For Disclosure Of Certain Information Relating To Tobacco Products Sold In The Commonwealth. (1996) http://archives.lib.state.ma.us/actsResolves/1996/1996acts0234.pdf
- 7.Philip Morris, Inc. v. Harshberger, 957 F. Supp. 327 (D. Mass. 1997).
- 8.Philip Morris, Inc. v. Harshberger, 159 F. 3d 670 (1st Cir. 1998).
- 9.Bodnar J, Borgerding M, Hsu F, et al. The 1999 Massachusetts Benchmark Study Final Report. Philip Morris; Jul 24, 2000. [accessed 11 Jul 2014]. http://legacy.library.ucsf.edu/tid/spt10j00. [Google Scholar]
- 10.Barald P. Benchmark study data: response to your letter of 20000223. Philip Morris; 2000. [accessed 06 Mar 2014]. http://legacy.library.ucsf.edu/tid/yso95c00. [Google Scholar]
- 11.World Health Organization. Partial guidelines for implementation of Articles 9 and 10 of the WHO Framework Convention on Tobacco Control. Regulation of the contents of tobacco products and regulation of tobacco product disclosures) Adopted 2010, amended 2012. http://www.who.int/fctc/guidelines/Guideliness_Articles_9_10_rev_240613.pdf.
- 12.Anderson SJ. Menthol cigarettes and smoking cessation behaviour: a review of tobacco industry documents. Tob Control. 2011;20(Suppl 2):ii49–56. doi: 10.1136/tc.2010.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinger H. Proposed constituent disclosure regulations. Philip Morris; 1998. [accessed 26 Mar 2013]. http://legacy.library.ucsf.edu/tid/czn42c00. [Google Scholar]
- 14.Office of the Governor. [accessed 04 Jun 2014];Cellucci Makes Moves to Smoke out Tobacco Use. (Press Release 18 August 1998). http://legacy.library.ucsf.edu/tid/bzn42c00.
- 15.Murphy V. Meeting with representatives of Massachusetts department of public health and the US center for disease control. Philip Morris; 1998. [accessed 10 Mar 2014]. http://legacy.library.ucsf.edu/tid/exe69h00. [Google Scholar]
- 16.Borgerding M, Norman V, Podraza K, et al. Massachusetts constituents testing study protocol. Brown & Williamson; 1999. [accessed 29 Jul 2014]. http://legacy.library.ucsf.edu/tid/qfl17j00. [Google Scholar]
- 17.National Cancer Institute. Smoking and tobacco control mongraphs: monograph 13: risks associated with smoking cigarettes with low tar machine-measured yields of tar and nicotine. Bethesda, MD: National Institutes of Health; 2001. [Google Scholar]
- 18.Borgerding M. A meeting/teleconference was held this past Tuesday, 19 January 1999 in Charlotte, NC and Richmond, Va to discuss the “Benchmarking Study” proposed by the major tobacco companies. RJ Reynolds; 1999. [accessed 18 Mar 2014]. http://legacy.library.ucsf.edu/tid/ngm21j00. [Google Scholar]
- 19.Dwyer R. Brand selections. Philip Morris; 1999. [accessed 19 Jul 2015]. https://industrydocuments.library.ucsf.edu/tobacco/docs/hmyf0062. [Google Scholar]
- 20.Roy’s List of 25 Brands. Philip Morris; Mar, 1998. [accessed 19 July 2015]. https://industrydocuments.library.ucsf.edu/tobacco/docs/kmyf0062. [Google Scholar]
- 21.Leslie’s List of 25 Brands. Philip Morris; Jan, 1999. [accessed 19 Jul 2015]. https://industrydocuments.library.ucsf.edu/tobacco/docs/jmyf0062. [Google Scholar]
- 22.Ely C. Philip Morris; Jan 29, 1999. [accessed 07 Apr 2014]. http://legacy.library.ucsf.edu/tid/ijc95g00. [Google Scholar]
- 23.Barald P. Massachusetts benchmark study. RJ Reynolds; 1999. [accessed 06 Mar 2014]. http://legacy.library.ucsf.edu/tid/fqt82a00. [Google Scholar]
- 24.Connolly G. Brown & Williamson; Mar 22, 1999. [accessed 19 Mar 2014]. No Title. http://legacy.library.ucsf.edu/tid/iwy11c00. [Google Scholar]
- 25.Barald P. Advisory Committee Meeting” Proposed by Dr. Greg Connolly”; [Confidential Memorandum from Joint Defense Counsel to Philip Morris in-House Counsel, Joint Defense Counsel, Philip Morris Employee and Joint Defense Employee, Copied to Philip Morris Employee and Philip Morris General Counsel, and Forwarded to Philip Morris in-House Counsel Outlining Legal Strategy Regarding Proposed Advisory Committee Meeting] Philip Morris; 1999. http://legacy.library.ucsf.edu/tid/usj42a00. [Google Scholar]
- 26. [accessed 20 Mar 2014];1999 Massachusetts Benchmark Study—Summary of Results. 2000 Feb 16; Author unknown. http://legacy.library.ucsf.edu/tid/gcy13j00.
- 27.Swauger J. Industry meeting with Dr. Greg connolly (Massachusetts department of public health (Dph)—18 December 1998. RJ Reynolds; 1998. [accessed 11 Apr 2014]. http://legacy.library.ucsf.edu/tid/zso44a00. [Google Scholar]
- 28.Repace J. Exposure to secondhand smoke. In: Ott W, Steinemann C, Wallace L, editors. Exposure analysis. Chapter 9. Boca Raton, FL: CRC Press; 2007. pp. 201–35. [Google Scholar]
- 29.Borgerding MF, Bodnar JA, Wingate DE. The 1999 Massachusetts benchmark study; final report. Brown & Williamson; 2000. [accessed 25 Feb 2013]. http://legacy.library.ucsf.edu/tid/yek21c00. [Google Scholar]
- 30.Tucker I. A Discussion of Tobacco Smoke Constituents. [accessed 11 Jul 2014];Research. 1955 Jun 03; http://legacy.library.ucsf.edu/tid/fre56b00.
- 31.Newton R. Alkaline Tobacco Smoke: Effect on Urea and Urea/Urease on Smoke Chemistry. [accessed 24 Jun 2014];Research. 1970 Aug 06; http://legacy.library.ucsf.edu/tid/cpn66b00.
- 32.Wickham J. Ratio gas phase components to tar and nicotine. Philip Morris; 1971. [accessed 09 Jun 2014]. http://legacy.library.ucsf.edu/tid/iqj44e00. [Google Scholar]
- 33.Young H, Bernasek E, Giles D, et al. Laboratory duplication of Marlboro reconstituted tobacco. RJ Reynolds; 1973. [accessed 05 Aug 2014]. http://legacy.library.ucsf.edu/tid/pyc79d00. [Google Scholar]
- 34.John J. Quanitative Determination of Product-Precursor Relationships for the Dehydration Reactions of Two Humectants-Glycerol and Triethyleneglycol. [accessed 11 Apr 2014];Research. 1981 May 19; http://legacy.library.ucsf.edu/tid/gcj46b00.
- 35.Teng D, Burley Whidby J. Low-Nitrogen Burley and Air-Cured Bright from Greeneville, Tennessee (820000 Study) Period Covered: 821100–830600. Philip Morris; Jul 20, 1983. [accessed 02 May 2014]. http://legacy.library.ucsf.edu/tid/bww34e00. [Google Scholar]
- 36.Perfetti P. Mesna test results. RJ Reynolds; 1985. [accessed 09 Jun 2014]. http://legacy.library.ucsf.edu/tid/lil92i00. [Google Scholar]
- 37.Blake C, Bosch V, Droz A, et al. The effect of the humectants glycerol and propylene glycol on mainstream and sidestream smoke deliveries of acrolein, formaldehyde, acetaldehyde, acetone and propionaldehyde. Philip Morris; 1987. [accessed 09 Jun 2014]. http://legacy.library.ucsf.edu/tid/yyd59e00. [Google Scholar]
- 38.Douglas J. Project B-451, ‘Urea’. [accessed 16 Jul 2014];Research. 1989 Dec 18; http://legacy.library.ucsf.edu/tid/fnr46b00.
- 39.Prakash A. Anaylsis of phenols in the remade experimental cigarette samples Tbb34 and Tbb35 with nitrate additive (project B 451) Lorillard; 1992. [accessed 04 Dec 2014]. http://legacy.library.ucsf.edu/tid/bav30e00. [Google Scholar]
- 40.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health. 1997;50:307–64. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 41.King B, Borland R, Fowles J. Mainstream smoke emissions of Australian and Canadian Cigarettes. Nicotine Tob Res. 2007;9:835–44. doi: 10.1080/14622200701485109. [DOI] [PubMed] [Google Scholar]
- 42.Windholz E. Philip Morris; Jul 30, 1999. [accessed 09 Apr 2014]. http://legacy.library.ucsf.edu/tid/jmm59h00. [Google Scholar]
- 43.Cannar N. Draft voluntary agreement for ingredient disclosure. Philip Morris; 2000. [accessed 08 Jul 2014]. http://legacy.library.ucsf.edu/tid/mjq56c00. [Google Scholar]
- 44.Carcich C, Prince J, Windholz E. Emails between Philip Morris in-house counsel and Philip Morris employees requesting information for the purpose of and providing legal advice concerning Australian disclosure of ingredients and emissions of cigarettes; Fw: draft revised Australia disclosure agreement. Philip Morris; 2000. [accessed 09 Jul 2014]. http://legacy.library.ucsf.edu/tid/hcr42b00. [Google Scholar]
- 45.Australian Department of Health. [accessed 7 Jul];Australian Cigarette Emissions Data. 2002 http://www.health.gov.au/internet/main/publishing.nsf/Content/tobacco-emis.
- 46.Imperial Tobacco. Emissions Report. 2001 http://www.health.gov.au/internet/main/publishing.nsf/Content/File/imperial.pdf.
- 47.Arista Laboratories. Uk Smoke Constituents Study. 2003 http://www.the-tma.org.uk/benchmark/benchmarkresources/final_report.pdf.
- 48.Massachusetts State. Tobacco Sales Amendment Act. 1998 [Google Scholar]
- 49.Rooks S. British Columbia ingredients regulations. British American Tobacco; 1998. [accessed 18 Jul 2014]. http://legacy.library.ucsf.edu/tid/nqv70a99. [Google Scholar]
- 50.British Columbia Ministry of Health and Ministry Responsible for Seniors. [accessed 18 Jul 2014];Smoke Poisons: Test Results for Three Leading Cigarette Brands. 1998 http://legacy.library.ucsf.edu/tid/rkp93a99.
- 51.Health Canada. [accessed 13 Aug 2014];Tobacco Reporting Regulations (Sor/2000-273) 2000 http://laws.justice.gc.ca/eng/regulations/SOR-2000-273/
- 52.Government of Canada. [accessed 13 Aug 2014];Ottawa Canada Gazette Part Ii, Statutory Instruments. 2000 http://publications.gc.ca/gazette/archives/p2/2000/2000-07-19/pdf/g2-13415.pdf.
- 53.Borgerding M. I am enclosing a copy of a compact disc called the “1999 Canadian benchmark study”. RJ Reynolds; 2000. [accessed 18 Jul 2014]. http://legacy.library.ucsf.edu/tid/nia06j00. [Google Scholar]
- 54.Kaiserman M. The Canadian Benchmark Study: Myths and Realities. Ottawa: Health Canada; 2003. [Google Scholar]
- 55.Counts ME, Hsu FS, Laffoon SW, et al. Mainstream smoke constituent yields and predicting relationships from a worldwide market sample of cigarette brands: iso smoking conditions. Regul Toxicol Pharmacol. 2004;39:111–34. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Eriksen M. We commend the Massachusetts department of public health for your efforts to reduce the harm caused by smoking. RJ Reynolds; 2000. [accessed 28 May 2014]. http://legacy.library.ucsf.edu/tid/hvx50d00. [Google Scholar]
- 57.Harris JE. Smoke yields of tobacco-specific nitrosamines in relation to Ftc tar level and cigarette manufacturer: analysis of the Massachusetts Benchmark Study. Public Health Rep. 2001;116:336–43. doi: 10.1093/phr/116.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Center for Environmental Health, National Center for Chronic Disease Prevention and Health Promotion’s Office on Smoking and Health. National center for environmental health and national center for chronic disease prevention and health promotion’s office on smoking and health comments on the tobacco manufacturers benchmarking study. RJ Reynolds; 2000. [accessed 25 Jun 2014]. http://legacy.library.ucsf.edu/tid/idz40d00. [Google Scholar]
- 59.Wertz MS, Kyriss T, Paranjape S, et al. The toxic effects of cigarette additives. Philip Morris’ project mix reconsidered: an analysis of documents released through litigation. PLoS Med. 2011;8:e1001145. doi: 10.1371/journal.pmed.1001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes RL, Hammond SK, Glantz SA. The tobacco industry’s role in the 16 cities study of Secondhand tobacco smoke: do the data support the stated conclusions? Environ Health Perspect. 2006;114:1890–7. doi: 10.1289/ehp.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnes RL, Glantz SA. Endotoxins in tobacco smoke: shifting tobacco industry positions. Nicotine Tob Res. 2007;9:995–1004. doi: 10.1080/14622200701488392. [DOI] [PubMed] [Google Scholar]
- 62.Neilsen K, Glantz SA. A Tobacco industry study of airline cabin air quality: dropping inconvenient findings. Tob Control. 2004;13(Suppl 1):i20–9. doi: 10.1136/tc.2003.004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.WHO. Who Tobacco Laboratory Network (Toblabnet) 2015 http://www.who.int/tobacco/industry/product_regulation/toblabnet/en/
- 64.WHO Tobacco Free Initiative. The First Meeting of the Who Tobacco Laboratory Network (Toblabnet); 28 & 29 April 2005; the Hague, the Netherlands. 2005. [accessed 2 Apr]. http://www.who.int/tobacco/global_interaction/tobreg/laboratory/en/ [Google Scholar]
- 65.United States v. Philip Morris USA Inc., 449 F. Supp. 2d 1 (D.D.C. 2006), aff’d in part & vacated in part, 566 F.3d 1095 (D.C. Cir. 2009) (per curiam), cert. denied, 130 S. Ct. 3501 (2010). http://publichealthlawcenter.org/sites/default/files/resources/doj-final-opinion.pdf