Abstract
Objectives
Sleep disturbance, pain, anxiety, depression and low energy/fatigue, the SPADE pentad, are the most prevalent and co-occurring symptoms in the general population and clinical practice. Co-occurrence of SPADE symptoms may produce additive impairment and negatively affect treatment response, potentially undermining patients' health and functioning. The purpose of this paper is to determine: (1) prevalence and comorbidity (i.e., clustering) of SPADE symptoms; (2) internal reliability and construct validity of a composite SPADE symptom score derived from the Patient-Reported Outcomes Measurement Information System (PROMIS) measures; and (3) whether improvement in somatic symptom burden represented by a composite score predicted subsequent measures of functional status at 3 and 12 months follow-up.
Methods
Secondary analysis of data from the Stepped Care to Optimize Pain care Effectiveness study, a randomized trial of a collaborative care intervention for Veterans with chronic pain.
Results
Most patients had multiple SPADE symptoms; only 9.6% of patients were monosymptomatic. The composite PROMIS symptom score had good internal reliability (Cronbach's alpha = 0.86) and construct validity and strongly correlated with multiple measures of functional status; improvement in the composite score significantly correlated with higher scores for five of six functional status outcomes. The standardized error of measurement for the raw composite score was 2.84, suggesting a 3-point difference in an individual's composite score may be clinically meaningful.
Discussion
Brief PROMIS measures may be useful in evaluating SPADE symptoms and overall symptom burden. Because symptom burden may predict functional status outcomes, better identification and management of comorbid symptoms may be warranted.
Keywords: chronic pain, depression, anxiety, fatigue, sleep, psychometrics
Introduction
More than half of all outpatient visits are attributed to physical symptoms, which translates to over 400 million outpatient visits annually.1 The complexity in symptom science research is burgeoning2 as the need to address symptom clusters in clinical practice has become increasingly evident.3, 4 Research to date suggests that symptoms may not be independent entities, but rather symptoms interacting synergistically.3, 4 Thus, co-occurring symptoms (i.e., symptom clusters) are a current research priority because they have a greater adverse impact on outcomes than individual symptoms alone.3 Sleep disturbance, pain, anxiety, depression, and low energy/fatigue (the SPADE pentad) comprise five of the most prevalent, chronic, disabling, and under-treated symptoms in both the general population5 and in clinical practice.5-10 Pain and depression co-occur with rates of 30-50%,11, 12 and a majority of patients seeking treatment for pain report sleep disturbance of a severity warranting attention; sleep disturbance can aggravate pain and inflammatory processes, reduce endogenous pain inhibitory responses, and increase emotional distress and reduce well-being.13 The co-occurrence of SPADE symptoms can produce an additive impairment, negatively affect treatment response, and undermine patients' general health and physical functioning. Thus, focusing solely on one symptom, while ignoring other comorbid symptoms, may not be an optimal approach. Rather, targeting multiple symptoms may be preferable.14
The Patient-Reported Outcomes Measurement Information System (PROMIS)2, 15 establishes a national resource for highly reliable and precise measurement of patient-reported symptoms, functioning, and health-related quality of life in chronic disease.2, 15 These can be used as primary or secondary outcomes in research as well as clinical practice.16 There are PROMIS profiles with four-, six-, and eight-item scales to assess seven domains including depression, anxiety, pain, fatigue, sleep, physical functioning, and social role satisfaction.15 Consistent use of common, standardized measures across research studies will better position researchers and clinicians to ask and answer complex questions about the nature of individual symptoms and symptom clusters.
In this paper, data is analyzed from a clinical trial involving primary care Veteran patients with chronic musculoskeletal pain. Specific aims were to determine: (1) the prevalence and comorbidity (i.e., clustering) of SPADE symptoms; (2) the internal reliability and construct validity of a composite SPADE symptom score derived from PROMIS measures; and (3) whether improvement in somatic symptom burden represented by a composite score predicted subsequent measures of functional status at 3 and 12 months follow-up.
Materials and Methods
Design and Study Participants
This was a secondary analysis of data from the Stepped Care to Optimize Pain care Effectiveness (SCOPE) study, a 12-month randomized controlled effectiveness trial of a telecare collaborative care intervention for primary care patients with chronic musculoskeletal pain. The study design and sample characteristics of the SCOPE trial have been described in detail elsewhere.17, 18 SCOPE participants were primary care patients aged 18 to 65 years enrolled from five general medicine clinics at a single Veterans Affairs (VA) medical center. Patients were eligible if they had pain meeting pre-specified criteria including: (a) musculoskeletal, defined as regional (joints, limbs, back, neck) or generalized (fibromyalgia or persistent widespread) pain; (b) moderately severe pain, defined as a Brief Pain Inventory intensity item score of 5 or higher for either “average” or “worst” pain in the past week (this threshold was selected since scores of 4 to 6 on this 0-10 point scale represent moderate pain and selecting 5 allowed for some regression to the mean); and (c) persistent (i.e., ≥ 3 months) pain despite trying at least one analgesic medication. Excluded were patients who had a pending pain-related disability claim (because their motivation to improve during a trial may be less), dementia, schizophrenia, bipolar disorder, illicit drug use, active suicidal ideation, or an anticipated life expectancy of less than 12 months. Letters were mailed to 940 patients with ICD-10 musculoskeletal pain codes, of whom 311 expressed potential interest and were contacted by telephone. Among these, 10 refused the eligibility interview, 29 were ineligible, and 22 were eligible but not interested in participating. Thus, 250 patients were randomized to either a telecare intervention arm that optimized analgesic therapy or a usual care control arm. The study was approved by the university institutional review board and VA Medical Center research review committee.
Measures
As part of the SCOPE trial, study participants completed PROMIS four-item measures for each of the five SPADE symptoms (i.e., sleep, pain, anxiety, depression, and low energy/fatigue). Each of the four-item scales are part of the PROMIS-29 profile.15 Individual items for each of the five symptom scales use five response options (e.g., 1 = not at all to 5 = very much), with scale scores ranging from 4 to 20 and higher scores indicating worse symptom severity.15 Raw scores can be converted to T-scores (using conversion tables available at www.nihpromis.org)16 which are derived from item-response therapy and are standardized so that a scale score of 50 represents the mean of the general population, based on large-sample normative data.19 Each 10-point change represents one SD (e.g., a score of 60 is 1 SD worse and a score of 40 is 1 SD better than the general population mean). The advantages of the T-score are that the severity of different symptoms can be compared (e.g., a T-score of 60 for pain and 55 for fatigue would mean than an individual's pain is relatively worse than their fatigue).
Several other measures completed by SCOPE participants were used to assess construct and predictive validity of the SPADE symptoms. Somatic symptom burden was assessed with the 14-item Patient Health Questionnaire (PHQ) somatization scale, which is identical to the PHQ-15 except for deletion of the infrequently endorsed sexual dysfunction item; the PHQ-15 is among the best validated and widely used measures of somatization.20, 21 Functional status was assessed with the SF-12 (which provides both Physical Component Summary and Mental Component Summary scores), plus additional items from the SF-36 to provide the general health and social functioning scores.22, 23 SF-12 and SF-36 scores range from 0 to 100 with lower scores representing worse health-related quality of life. Health-related disability days were assessed by asking patients for the total number of days, during the past 4 weeks, that they reduced their usual activities for one-half day or more because of physical health or emotional problems. Scores range from 0 to 28 days. Patients were also asked how effective they had been at their job during the past 2 weeks from 0% (“not at all effective”) to 100% (“completely effective”). These measures had high reliability in SCOPE as well as several previous trials.17, 24-25
Statistical Analysis
Data were analyzed using SAS (version 9.1). PROMIS raw and T-scores were calculated for each of the five SPADE symptoms. Composite PROMIS SPADE raw and T-scores were calculated by taking the sum of the individual symptom scale scores and dividing by 5. For operational purposes, a T-score ≥ 55 was considered a clinically significant threshold since this represents a score that is ≥ 0.5 SD worse than the general population, which in turn represents a moderate effect size.26 Internal reliability of the composite score was estimated by Cronbach's alpha. The standard error of measurement (SEM) for the raw composite SPADE score was calculated as the SD of the baseline score for that measure, multiplied by the square root of one minus the Cronbach's alpha.27 The SEM can be regarded as the SD of a person's individual score, and either 1 or 2 SEMs have been considered one approach to estimating the minimally important difference for a scale.28, 29
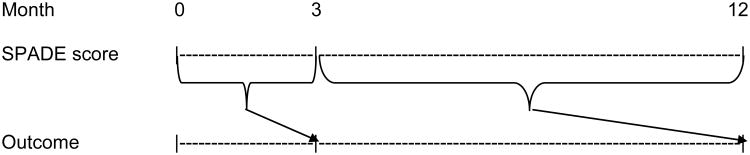
Descriptive statistics were used to evaluate the prevalence and co-occurrence of SPADE symptoms as well as associations with sociodemographic factors. Construct validity was examined by determining the correlations of the PROMIS individual and composite symptom scale scores with measures of somatization and functional status. Predictive validity was examined by using linear mixed effects models for repeated measures (MMRM) analysis to determine if antecedent changes in somatic symptom burden represented by the composite PROMIS SPADE score predicted subsequent measures of functional status at 3 and 12 months follow-up (Figure 1). MMRM models were adjusted for age, sex, medical comorbidity, and socioeconomic status (SES). Medical comorbidity (scored 0 to 9) was assessed using a checklist of nine common medical conditions shown to predict hospitalization and mortality.30 SES was assessed with the Socioeconomic Disadvantage Index (scored 0 to 3) which assigns one point each for low education (high school or less), unemployment, and low income reported as “just enough” or “not enough to make ends meet.”31
Figure 1.
Longitudinal analysis framework using mixed effects model for repeated measures (MMRM) analysis for examining whether antecedent improvement in PROMIS composite SPADE score (i.e., somatic symptom burden) predicts subsequent outcomes.
Results
Prevalence and Comorbidity of SPADE Symptoms
Table 1 shows the mean raw and T-scores of the PROMIS four-item measures for each of the five SPADE symptoms. Of the 250 patients, 80.8% had a pain T-score ≥ 55 and thus met the operational threshold criteria for a potential clinically significant symptom. Furthermore, 65.6% met the clinical threshold for fatigue, 52.8% for sleep disturbance, 37.2% for anxiety, and 31.6% for depression. The proportion of study patients exceeding the PROMIS pain score threshold was less than 100% since eligibility for the SCOPE trial required endorsement of only a single BPI item (average or worst pain in the past week) as 5 or greater on a 0-10 scale, whereas the PROMIS score was derived from 4 items measuring pain interference, and the clinical threshold was set to be of a moderate level and comparable across all 5 symptoms. Most patients had multiple symptoms; 9.6% (n = 24) of patients had no threshold symptoms, 20% (n = 50) had one, 15.6% (n = 39) had two, 22.8% (n = 57) had three, 11.6% (n = 29) had four, and 20.4% (n = 51) reported having all five SPADE symptoms at or above threshold.
Table 1. Baseline PROMIS SPADE Symptom Scores in 250 Primary Care Patients with Chronic Pain.
| PROMIS 4-item scale | Cronbach's alpha | Raw Score Mean (SD) | T-score Mean (SD) | T-score ≥ 55 N (%) |
|---|---|---|---|---|
| Pain | 0.88 | 11.3 (4.2) | 59.9 (7.1) | 202 (80.8) |
| Fatigue | 0.93 | 12.4 (4.3) | 57.2 (9.8) | 164 (65.6) |
| Sleep | 0.88 | 12.7 (4.3) | 55.4 (9.4) | 132 (52.8) |
| Anxiety | 0.89 | 7.2 (3.7) | 51.1 (10.1) | 93 (37.2) |
| Depression | 0.93 | 7.0 (4.1) | 50.2 (10.1) | 79 (31.6) |
Table 2 highlights the degree of clustering within each symptom subgroup where, as previously mentioned, a T-score of ≥ 55 is operationally defined as a “clinical symptom.” Comorbidity of all symptoms was high in each symptom group. For example, of the 202 patients with threshold-level pain (i.e., T-score ≥ 55), the proportion with threshold-level fatigue, sleep disturbance, anxiety, and depression was 73.3%, 60.4%, 42.6%, and 37.5%, respectively. A similar degree of clustering was seen in each of the other four symptom groups. The clustering was also exemplified by the number of comorbid symptoms in each symptom group. For example, of the 202 patients with threshold-level pain, the proportion who had one, two, three, or all four of the other symptoms was 25.3%, 28.2%, 31.8%, and 13.9%, respectively. Indeed, for any given symptom, the likelihood of having no other symptoms was uncommon, ranging from 7.6% to 12.9% in each of the five symptom groups. Thus, most patients were polysymptomatic rather than monosymptomatic.
Table 2. Clustering of SPADE Symptoms above Threshold (T-score ≥ 55)*.
| Pain (n = 202) |
Fatigue (n = 164) |
Sleep (n = 132) |
Anxiety (n = 93) |
Depression (n = 79) |
|
|---|---|---|---|---|---|
| Pain, n (%) | 148 (90.2) | 122 (92.4) | 86 (92.5) | 75 (94.9) | |
| Fatigue, n (%) | 148 (73.3) | 113 (85.6) | 83 (89.3) | 73 (92.4) | |
| Sleep, n (%) | 122 (60.4) | 113 (68.9) | 66 (71.0) | 57 (72.2) | |
| Anxiety, n (%) | 86 (42.6) | 83 (50.6) | 66 (50.0) | 71 (89.9) | |
| Depression, n (%) | 75 (37.1) | 73 (44.5) | 57 (43.2) | 71 (76.3) | |
| No. Comorbid Symptoms, n (%) | |||||
| 0 | 22 (10.9) | 17 (10.4) | 17 (12.9) | 9 (9.7) | 6 (7.6) |
| 1 | 51 (25.3) | 36 (22.0) | 28 (21.2) | 21 (22.6) | 16 (20.3) |
| 2 | 57 (28.2) | 48 (29.3) | 35 (26.5) | 27 (29.0) | 22 (27.9) |
| 3 | 44 (21.8) | 37 (22.6) | 35 (26.5) | 20 (21.5) | 20 (25.3) |
| 4 | 28 (13.9) | 26 (15.9) | 17 (12.9) | 16 (17.2) | 15 (19.0) |
Each symptom group is represented in a column and the totals of all 5 symptom groups exceeds 250 because of high co-occurrence rates. Proportions should be read down each column. For example, of the 202 patients with threshold-level pain, the proportion with fatigue, sleep, anxiety, and depression is 73.3%, 60.4%, 42.6%, and 37.1%, respectively. Likewise, the proportion with pain only, or 1, 2, 3, or 4 additional comorbid symptoms is 10.9%, 25.3%, 28.2%, 21.8%, and 13.9%, respectively.
Reliability and Validity of the Composite Symptom Score
The SPADE composite T-score had a mean of 54.8 (SD = 7.5) and a Cronbach's alpha of 0.86; 118 (47.2%) of the patients had a composite T-score ≥ 55. The SEM for the composite T-score was 2.84, suggesting that for an individual person a difference in the SPADE composite T-score of 3 points or more might represent a clinically meaningful difference.
Table 3 highlights the strong associations between both the composite and individual PROMIS symptom scale scores and all seven measures of construct validity. Among the PROMIS symptom measures, the composite score had the highest correlation with four of the construct validators (social functioning, general health, work effectiveness, and somatization) and the second highest correlation with two of the construct validators (mental component summary score and health-related disability days). With respect to sociodemographic factors, the composite symptom score was significantly worse (i.e., higher) for those with lower socioeconomic status (r2 = .323; p < .0001) and higher medical comorbidity (r2 = .224; p = .0004). Women also had a significantly higher mean composite PROMIS symptom score compared to men (58.0 vs. 54.1, p = .002). The composite score was not associated with age, race, or marital status.
Table 3. Correlations Between PROMIS T-scores and Measures of Construct Validity.
| Construct Validator | Cronbach's alpha* | Correlations with PROMIS T-scores† | |||||
|---|---|---|---|---|---|---|---|
| SPADE Com-posite | Pain | Fatigue | Sleep | Depression | Anxiety | ||
| SF-12 Mental Component | 0.90 | -.785 | -.517 | -.552 | -.445 | -.821 | -.761 |
| SF-12 Physical Component | 0.90 | -.417 | -.610 | -.456 | -.332 | -.186 | -.172 |
| Social functioning, SF-36 | 0.85 | -.702 | -.662 | -.595 | -.430 | -.588 | -.559 |
| General health item, SF-12 | n/a | -.508 | -.425 | -.461 | -.301 | -.429 | -.415 |
| Health-related disability days | n/a | .519 | .549 | .435 | .391 | .372 | .369 |
| Work effectiveness, percent | n/a | -.473 | -.372 | -.366 | -.288 | -.433 | -.427 |
| Somatization, PHQ-14 | 0.77 | .681 | .530 | .629 | .558 | .512 | .500 |
n/a = not applicable for this measure since it only consists of a single item or question
For all correlations that are negative, higher scores on the construct validator measure reflect better functioning or quality of life. Thus, a negative correlation means that as the PROMIS symptom scores get higher (i.e., worse), the construct validator also gets worse (i.e., lower)
Somatic Symptom Burden as a Predictor of Functional Status Outcomes
Table 4 shows that improvement in the composite symptom T-score is a significant predictor of improvement in five of the six functional status outcomes measured (i.e., all except work effectiveness). Specifically, an antecedent improvement in symptom burden is significantly associated with better mental (MCS), physical (PCS) and social functioning, better self-rated general health, and fewer health-related disability days at subsequent points of follow-up. The magnitude of improvement in terms of effect size ranged from 0.07 to 0.24, with the largest effect sizes for the mental component summary score (0.24), social functioning (0.22), and health-related disability days (0.18).
Table 4. Antecedent Change in Composite PROMIS SPADE T Score as a Predictor of Subsequent Functional Status Outcomes over 12 Months*.
| Functional Status Outcome | Beta coefficient (SE) | P-value | Change in outcome per 5-point improvement in PROMIS composite score | Effect size† |
|---|---|---|---|---|
| Mental component summary score (MCS), SF-12 | 0.586 (.060) | <.0001 | 2.9 point increased (improved) score | 0.24 |
| Social functioning, SF-36 | 1.200 (.151) | <.0001 | 6.0 point increased (improved) score | 0.22 |
| Health-related disability days in past 4 weeks | -0.302 (.053) | <.0001 | 1.51 less disability days in past 4 weeks | 0.18 |
| Physical component summary score (PCS), SF-12 | 0.172 (.053) | .0014 | 0.9 point increased (improved) score | 0.10 |
| General health item, SF-12 | 0.534 (.148) | .0004 | 2.7 point increased (improved) score | 0.09 |
| Percent work effectiveness | 0.318 (.182) | .083 | 1.6% increased (improved) score | 0.07 |
From mixed effects models for repeated measures (MMRM) with: (a) predictor variable being change in PROMIS composite score from baseline to 3 months and from 3 months to 12 months, (b) dependent variable being the functional status outcome, and (c) covariates adjusted for in the model being age, sex, medical comorbidity, and socioeconomic disadvantage index.
Effect size = change in outcome per 5-point improvement in PROMIS composite score divided by the standard deviation of the outcome at baseline.
Discussion
Our study has three important findings. First, the SPADE symptoms commonly cluster, with the norm being a polysymptomatic patient, whereas only about 1 in 10 patients are monosymptomatic. Second, a composite SPADE symptom score demonstrates strong internal reliability as well as construct validity. Third, antecedent improvement in somatic symptom burden represented by a composite PROMIS SPADE score longitudinally predicts subsequent improvement in functional status at follow-up.
Symptom cluster research initially focused on cancer patients;3, 4 however, a high co-occurrence of symptoms seems to exist across a variety of diseases.1, 14 Although the five SPADE symptoms have not been specifically evaluated as one unique symptom cluster, much research has supported the existence of some of these symptoms as co-occurring in pairs (e.g., anxiety and depression or sleep and pain). Pain and depression co-occur with rates of 30-50%.11, 12 In addition, many primary care patients seeking treatment for pain report significantly impaired sleep; which is known to aggravate pain and inflammatory processes, reduce endogenous pain inhibitory responses, and increase emotional distress and reduce well-being.13 Previous research has demonstrated the adverse effect that symptom clusters have on functional outcomes3, and our longitudinal analysis strengthens these findings by demonstrating that a reduction in SPADE symptom cluster severity predicts improvement in multiple functional outcomes.
Our study provides preliminary data regarding use of the four-item PROMIS measures for sleep disturbance, pain, anxiety, depression, and fatigue to calculate a composite SPADE symptom score. Whereas individual symptom scores can be used to assess and monitor specific symptoms, the composite score may serve a complementary role in measuring and tracking overall symptom burden. Some treatments may be effective across multiple SPADE symptoms (e.g., cognitive-behavioral therapy, exercise, certain types of antidepressants, self-management programs)1, 32 and may be considered for polysymptomatic patients as either primary or adjunctive therapies, particularly in individuals not responding to symptom-specific treatments. In such cases, tracking both individual and composite symptom scores may be useful in assessing clinical response and adjusting treatment. Our study findings may also be useful in communicating with patients that clustering of symptoms is common, polypharmacy for concurrent symptoms (e.g., analgesics for pain, sedatives for sleep, psychostimulants for fatigue) may not always be the best approach, and monitoring response to treatment of one symptom may warrant attention to other symptoms as well.
A statistical approach to estimating a minimally important clinical difference is the SEM, which was estimated to be approximately a 3-point change on the raw composite SPADE score in our sample. Because SEMs may vary among clinical populations, this 3-point estimate should be considered a preliminary finding. Furthermore, sensitivity to change with treatment may be an even better metric of responsiveness which requires testing changes in the PROMIS score in trials that target multiple SPADE symptoms.
The finding that antecedent improvement in somatic symptom burden as measured by the PROMIS composite score predicts subsequent improvement in a number of functional status outcomes has important implications. The longitudinal nature of the MMRM analysis provides stronger evidence of a causal role for somatic symptoms influencing functional status. Also, it suggests that treatments effectively targeting SPADE symptoms may have beneficial effects on functional status and quality of life. Since pain, depressive, and anxiety disorders account for six of the nine most disabling chronic disorders from a population standpoint,10 our findings add to the public health importance of greater attention to common symptoms.
Three caveats regarding our finding that SPADE improvement predicts improvement in functional outcomes should be noted. First, two of the measures in Table 4 (MCS and PCS) share several constructs in common with the SPADE composite score, in which case the predictive effect of SPADE improvement for MCS and PCS improvement may be overestimated due to shared variance. However, the MCS and PCS also include constructs that are not captured by the SPADE composite score; moreover, the latter also predicted improvement in multiple outcomes in Table 4 with which it did not share any constructs. Second, the effect sizes were larger for outcomes more influenced by mental (i.e., MCS and social functioning) than by physical (i.e., PCS) health. This may be because 2 of the 5 SPADE symptoms are depression and anxiety, or because symptoms in general have stronger effects on these outcomes. It is worth noting that there was also a moderate effect size for health-related disability, a construct not wedded specifically to mental or physical health. Third, because our data was drawn from a clinical trial in which patients were randomized to either a treatment group or usual care group, some of the improvement in functional outcomes predicted by SPADE improvement may have been due in part to differential treatments in the clinical trial.
Our study has several limitations. Although our sample was reasonably sized (N = 250), it was comprised of primary care patients with chronic musculoskeletal pain. Because PROMIS symptom scores may differ in non-pain or other clinical populations, our findings should be replicated in other samples. Second, our study enrolled predominantly male Veterans. Because women in our study and in other studies33, 34 typically have higher symptom scores, extending our research to samples including non-Veterans as well as more women is warranted.
The use of PROMIS profiles and other standardized patient-reported outcome measures among researchers and clinical practitioners is well-aligned with initiatives set forth by the National Institutes of Health,15, 16 and other groups35, 36 which seek to advance knowledge of symptom science and improve clinical outcomes among symptomatic patients. Additionally, determining the optimal way to display the scores to clinicians and patients as well as incorporating the scores into electronic medical records will facilitate future implementation.37, 38 Furthermore, efficient and cost-effective systems-based strategies to facilitate the clinical management of symptoms in busy practice settings should be implemented since previous research has shown that simple feedback of scores alone may be inadequate to improve outcomes.1, 39 Finally, there has been a movement in recent years (further catalyzed by the 2011 Institute of Medicine report) to view chronic pain not as merely a symptom, but rather as a chronic illness in and of itself.40 Measuring and addressing not only the cardinal symptom in patients with chronic pain but also the burdensome co-occurring symptoms has the potential of optimizing quality of life and other health outcomes.
Acknowledgments
Source of Funding: Department of Veterans Affairs Health Services Research and Development Merit Review award to Dr. Kroenke (IIR 07-119); VA Career Development Award to Dr. Kean (CDA IK2RX000879); National Institute of Arthritis and Musculoskeletal Disorders R01 award to Dr. Monahan (R01 AR064081); and a Doctoral Degree Scholarship in Cancer Nursing from the American Cancer Society [DSCN-14-080-01-SCN]; a Predoctoral Fellowship from the Behavioral Cooperative Oncology Group, Walther Program for Cancer Care Research; a Research Doctorate Scholarship from the Oncology Nursing Society Foundation; and Research Incentive Funding from the Indiana University School of Nursing awarded to Ms. Davis.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Trial Registration: Clinicaltrials.gov Identifier: NCT00926588
References
- 1.Kroenke K. A practical and evidence-based approach to common symptoms: a narrative review. Ann Intern Med. 2014;161(8):579–586. doi: 10.7326/M14-0461. [DOI] [PubMed] [Google Scholar]
- 2.Corwin EJ, Berg JA, Armstrong TS, et al. Envisioning the future in symptom science. Nurs Outlook. 2014;62(5):346–351. doi: 10.1016/j.outlook.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Aktas A. Cancer symptom clusters: current concepts and controversies. Curr Opin Support Palliat Care. 2013;7(1):38–44. doi: 10.1097/SPC.0b013e32835def5b. [DOI] [PubMed] [Google Scholar]
- 4.Barsevick A, Aktas A. Cancer symptom cluster research: new perspectives and tools. Curr Opin Support Palliat Care. 2013;7(1):36–37. doi: 10.1097/SPC.0b013e32835defac. [DOI] [PubMed] [Google Scholar]
- 5.Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153(21):2474–2480. [PubMed] [Google Scholar]
- 6.Kroenke K, Mangelsdorff AD. Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Am J Med. 1989;86(3):262–266. doi: 10.1016/0002-9343(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 7.Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150(8):1685–1689. doi: 10.1001/archinte.150.8.1685. [DOI] [PubMed] [Google Scholar]
- 8.Kroenke K, Spitzer RL, Williams JB, et al. Physical symptoms in primary care. Predictors of psychiatric disorders and functional impairment. Arch Fam Med. 1994;3(9):774–779. doi: 10.1001/archfami.3.9.774. [DOI] [PubMed] [Google Scholar]
- 9.Khan AA, Khan A, Harezlak J, Tu W, Kroenke K. Somatic symptoms in primary care: etiology and outcome. Psychosomatics. 2003;44(6):471–478. doi: 10.1176/appi.psy.44.6.471. [DOI] [PubMed] [Google Scholar]
- 10.US Burden of Disease Collaborators. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12(9):964–973. doi: 10.1016/j.jpain.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301(20):2099–2110. doi: 10.1001/jama.2009.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang NK, McBeth J, Jordan KP, Blagojevic-Bucknall M, Croft P, Wilkie R. Impact of musculoskeletal pain on insomnia onset: a prospective cohort study. Rheumatology. 2014 doi: 10.1093/rheumatology/keu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aktas A, Walsh D, Rybicki L. Symptom clusters: myth or reality? Palliat Med. 2010;24(4):373–385. doi: 10.1177/0269216310367842. [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PROMIS. About PROMIS. [Accessed April 7, 2015]; Available at: http://www.nihpromis.org/about/abouthome.
- 17.Kroenke K, Krebs E, Wu J, et al. Stepped Care to Optimize Pain care Effectiveness (SCOPE) trial study design and sample characteristics. Contemp Clin Trials. 2013;34(2):270–281. doi: 10.1016/j.cct.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Krebs EE, Wu J, Yu Z, Chumbler NR, Bair MJ. Telecare collaborative management of chronic pain in primary care: a randomized clinical trial. JAMA. 2014;312(3):240–248. doi: 10.1001/jama.2014.7689. [DOI] [PubMed] [Google Scholar]
- 19.PROMIS. Dynamic Tools to Measure Health Outcomes From the Patient Persepctive - Pain Intensity: A Brief Guide to the PROMIS Pain Intensity Instrument. [Accessed April 7, 2015];2014 Apr 28; Available at: https://www.assessmentcenter.net/documents/PROMIS%20Pain%20Intensity%20Scoring%20Manual.pdf.
- 20.Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Zijlema WL, Stolk RP, Lowe B, Rief W, White PD, Rosmalen JG. How to assess common somatic symptoms in large-scale studies: a systematic review of questionnaires. J Psychosom Res. 2013;74(6):459–468. doi: 10.1016/j.jpsychores.2013.03.093. [DOI] [PubMed] [Google Scholar]
- 22.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 23.McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Bair M, Damush T, et al. Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) study Design and practical implications of an intervention for comorbid pain and depression. Gen Hosp Psychiatry. 2007;29(6):506–517. doi: 10.1016/j.genhosppsych.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Theobald D, Norton K, et al. Indiana Cancer Pain and Depression (INCPAD) Trial: design of a telecare management intervention for cancer-related symptoms and baseline characteristics of enrolled participants. Gen Hosp Psychiatry. 2009;31(3):240–253. doi: 10.1016/j.genhosppsych.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27:S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 27.Krebs EE, Bair MJ, Wu J, Damush TM, Tu W, Kroenke K. Comparative responsiveness of pain outcome measures among primary care patients with musculoskeletal pain. Med Care. 2010;48:1007–1014. doi: 10.1097/MLR.0b013e3181eaf835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52(9):861–873. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 29.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42(12):1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Perkins AJ, Kroenke K, Unutzer J, et al. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol. 2004;57:1040–1048. doi: 10.1016/j.jclinepi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Chumbler NR, Kroenke K, Outcalt S, et al. Association between sense of coherence and health-related quality of life among primary care patients with chronic musculoskeletal pain. Health Qual Life Outcomes. 2013;11(1):216. doi: 10.1186/1477-7525-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson JL, O'Malley PG, Kroenke K. Antidepressants and cognitive-behavioral therapy for symptom syndromes. CNS Spectr. 2006;11(3):212–222. doi: 10.1017/s1092852900014383. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med. 1998;60(2):150–155. doi: 10.1097/00006842-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16:266–275. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22:1889–1905. doi: 10.1007/s11136-012-0344-y. [DOI] [PubMed] [Google Scholar]
- 36.Snyder CF, Aaronson NK, Chouchair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res. 2012;21:1305–1314. doi: 10.1007/s11136-011-0054-x. [DOI] [PubMed] [Google Scholar]
- 37.Wu AW, Kharrazi H, Boulware L, Snyder CF. Measure once, cut twice-adding patient-reported outcome measures to the electronic health record for comparative effectiveness research. J Clin Epidemiol. 2013;8:S12–S20. doi: 10.1016/j.jclinepi.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glasgow RE, Kaplan RM, Ockene JK, Fisher EB, Emmons KM. Patient-reported measures of psychosocial issues and health behavior should be added to electronic health records. Health Aff. 2012;31:497–504. doi: 10.1377/hlthaff.2010.1295. [DOI] [PubMed] [Google Scholar]
- 39.Gilbody S, Sheldon T, House A. Screening and case-finding instruments for depression: a meta-analysis. CMAJ. 2008;178(8):997–1003. doi: 10.1503/cmaj.070281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academy of Sciences; Washington DC: 2011. [Google Scholar]