Abstract
Apolipoprotein E (apoE) plays a critical role in maintaining synaptic integrity by transporting cholesterol to neurons through the low-density lipoprotein receptor related protein-1 (LRP1). Bexarotene, a retinoid X receptor (RXR) agonist, has been reported to have potential beneficial effects on cognition by increasing brain apoE levels and lipidation. To investigate the effects of bexarotene on aging-related synapse loss and the contribution of neuronal LRP1 to the pathway, forebrain neuron-specific LRP1 knockout (nLrp1−/−) and littermate control mice were administered with bexarotene-formulated diet (100 mg/kg/day) or control diet at the age of 20-24 months for 8 weeks. Upon bexarotene treatment, levels of brain apoE and ATP-binding cassette sub-family A member 1 (ABCA1) were significantly increased in both mice. While levels of PSD95, glutamate receptor 1 (GluR1), and N-methyl-D-aspartate receptor NR1 subunit (NR1), which are key postsynaptic proteins that regulate synaptic plasticity, were decreased with aging, they were restored by bexarotene treatment in the brains of control but not nLrp1−/− mice. These results indicate that the beneficial effects of bexarotene on synaptic integrity depend on the presence of neuronal LRP1. However, we also found that bexarotene treatment led to the activation of glial cells, weight loss and hepatomegaly, which are likely due to hepatic failure. Taken together, our results demonstrate that apoE-targeted treatment through the RXR pathway has a potential beneficial effect on synapses during aging; however, the therapeutic application of bexarotene requires extreme caution due to its toxic side effects.
Keywords: aging, apoE, synapse, retinoid, nuclear receptor, LRP1, RXR agonist, bexarotene
1. INTRODUCTION
Currently, the aging population has been expanding; the elderly over 60 years of age exceeds 20% of the total population in the world (WHO, 2014). Aging substantially affects the nervous system by altering synaptic transmission/plasticity through increased neuroinflammation (Perry, 2010; Sparkman and Johnson, 2008), modified dendritic spine morphologies (Dumitriu et al., 2010; von Bohlen und Halbach et al., 2006), loss of synaptic proteins (Bahr et al., 1992; Clayton et al., 2002; Sonntag et al., 2000), impaired endomembrane recycling and disrupted calcium homeostasis in synapses (Bezprozvanny and Hiesinger, 2013). In fact, cognitive performance likely starts to decline as early as 55 years of age, even in healthy individuals (Elgamal et al., 2011; Hedden and Gabrieli, 2004). Thus, it is urgent to develop efficient therapeutic interventions for aging-related cognitive decline.
Cholesterol, a major component of neuronal membranes, is essential for synaptic formation and repair. In fact, cholesterol levels are decreased in human brains with aging (Ledesma et al., 2012; van Vliet, 2012). Thus, aging-dependent cognitive decline is likely accompanied with brain cholesterol reductions. In the central nervous system, apolipoprotein E (apoE) mediates the cholesterol supply to neurons through cell surface receptors, in particular the low-density lipoprotein receptor related protein-1 (LRP1) (Mauch et al., 2001). ApoE is mainly produced by astrocytes in the brain, where cholesterol and other lipids are loaded through ATP-binding cassette sub-family A member 1 (ABCA1) to form lipoprotein particles (Bu, 2009). Thus, an ideal pharmacological approach is to increase apoE levels and/or its lipidation to possibly restore the synaptic damages in pathological conditions.
Retinoid X receptor (RXR) and liver X receptor (LXR) are potential targets for apoE-based therapy to treat aging-related neurodegenerative diseases. Both are nuclear receptors which regulate the transcription of apoE and ABCA1 (Liang et al., 2004; Zhao et al., 2014) by forming heterodimers with each other, or with other nuclear receptors such as retinoid acid receptor (RAR), peroxisome proliferator-activated receptor γ (PPARγ), thyroid hormone receptor (TR) or vitamin D receptor (VDR) as transcriptional co-regulators (Chawla et al., 2001; Evans and Mangelsdorf, 2014; Sussman and de Lera, 2005; Szanto et al., 2004). Of importance, bexarotene, an RXR agonist, has been reported to improve cognitive function as well as reduce brain amyloid-β (Aβ) levels in an Alzheimer’s disease (AD) mouse model (Cramer et al., 2012), although it was originally approved by the U.S. Food and Drug Administration (FDA) to treat cutaneous T-cell lymphoma (Gregoriou et al., 2013; Mehta et al., 2012; Scarisbrick et al., 2013; Vakeva et al., 2012). However, the effects of bexarotene on AD are controversial due to conflicting results from different investigators (Fitz et al., 2013; LaClair et al., 2013; Price et al., 2013; Tesseur et al., 2013; Veeraraghavalu et al., 2013). Furthermore, it remains unclear if bexarotene treatment restores aging-dependent loss of synaptic proteins by increasing apoE and whether LRP1 contributes to the pathway.
Therefore, in this study we administered bexarotene-formulated diet or normal control diet to forebrain neuron-specific LRP1 knockout mice and littermate control mice at the age of 20-24 months for 8 weeks to investigate the effects on aging-related changes of synapses and other health-related outcomes.
2. MATERIAL AND METHODS
2.1 Reagents
Goat polyclonal anti-apoE antibody labeled with biotin was purchased from Meridian Life science (Memphis, TN). Mouse monoclonal anti-ABCA1 antibody was purchased from Abcam. Rabbit monoclonal anti-PSD95 antibody was purchased from Cell Signaling. Rabbit polyclonal anti-glutamate receptor (GluR) 1, rabbit polyclonal anti-N-methyl-D-aspartate receptor (NMDAR) NR1 subunit (NR1), rabbit monoclonal anti-NMDAR NR2A subunit (NR2A), rabbit polyclonal anti-NMDAR NR2B subunit (NR2B), and mouse monoclonal anti-GluR2 antibodies were purchased from Millipore, and mouse monoclonal anti-β actin antibody was from Sigma-Aldrich. Rabbit polyclonal anti-LRP1 antibody was produced in our laboratory (Liu et al., 2007).
2.2 Animals
Forebrain neuron-specific LRP1 knockout (nLrp1−/−) mice were generated as previously described (Liu et al., 2010). Briefly, αCamKII driven Cre recombinase mice were bred with Lrp1 loxP mice. Littermates of male nLrp1−/− mice (Lrp1flox/flox; αCamKII-Cre+/−) or controls (Lrp1flox/flox; αCamKII-Cre−/−) were used for the analysis at the age of 20-24 months. Bexarotene-containing diet was formulated by impregnating normal chow with bexarotene (Cayman) (800 μg/g diet; Harlan laboratories). While the mice had ad libitum access to bexarotene containing diet for 8 weeks, the dose was calculated to be 100 mg/kg/day when mice take on average of 125 g/kg/day of the diet, which is the same dose used in a previous publication (Boehm-Cagan and Michaelson, 2014; Cramer et al., 2012; Fitz et al., 2013; LaClair et al., 2013; Price et al., 2013; Tai et al., 2014; Tesseur et al., 2013; Veeraraghavalu et al., 2013). Our preliminary analysis confirmed that the administration of mice with bexarotene-formulated diet (100 mg/kg/day) for 3 weeks was needed to sufficiently increase brain apoE levels at 3 months of age (data not shown). After transcardial perfusion with PBS, brain tissues were dissected and one hemisphere of each mouse was kept frozen at −80°C until further analysis. The other hemisphere was fixed in 10% neutralized formalin and used for histological analysis. Blood samples were collected under anesthesia from inferior vena cava before perfusion. All animal experiments were approved by the Institutional Animal Care and Use Committee of Mayo Clinic and followed the National Institute of Health guidelines for the care and use of laboratory animals.
2.3 Western blot and densitometry analysis
Samples were lysed in RIPA (Millipore) containing 0.1% SDS, protease inhibitor mixture and phosphatase inhibitor (Roche). Protein concentration was measured in each sample using a protein assay kit from Bio-Rad. An equal amount of protein from each sample was used for SDS-PAGE. The immunoreactivity was detected and quantified using Odyssey Infrared Imaging System (Li-COR Biosciences).
2.4 ELISA
Samples were homogenized in RIPA containing 0.1% SDS, protein inhibitor cocktail, and phosphatase inhibitor and used for the analysis. To measure mouse apoE, plates were blocked with 1% milk and incubated with samples for overnight at 4°C. After washing with PBS, a biotinylated antibody HJ6.3 was used as a detection antibody (Ulrich et al., 2013). The HJ6.3 antibody was kindly provided by Dr. David M. Holtzman at Washington University School of Medicine. Synaptophysin, PSD95, GFAP and CD11b in mouse brains were measured by ELISA (Shinohara et al., 2013). For synaptophysin and PSD95, capture antibodies were anti-rat/mouse rabbit-polyclonal synaptophysin (Osenses, Keswick, Australia) and rabbit anti-N-terminal region of PSD95 (Osenses) antibody, and detector antibodies were mouse monoclonal biotinylated antibody to SY38 (ACRIS, SanDiego, CA) and a biotinylated K28/43 from NeuroMab, respectively. For GFAP ELISA, a rabbit anti-GFAP capture antibody (US Biological, Swampscott, MA, USA) and biotinylated GA-5 detector antibody (Abcam, Cambridge, MA, USA) were used. For mouse CD11b ELISA, a rat monoclonal anti-mouse Integrin alpha M/CD11b antibody was used as a capture antibody (R&D systems, Minneapolis, MN) and a biotinylated rat anti-mouse CD11b (5C6) antibody (AbD Serotec, Raleigh, NC) was used as a detector antibody. Following incubation with horseradish peroxidase-streptavidin (Vector), the ELISA plates were developed with tetramethylbenzidine (Sigma-Aldrich) and read at 650 nm with a Bio-Tek 600 plate reader.
2.5 Immunohistochemical analysis
Paraffin embedded brain sections were immunostained for neuron (NeuN), astrocytes (GFAP) and microglia (Iba-1). The stained sections were visualized with DAB, scanned through Aperio AT 2 slide scanner (Aperio Technologies). To quantify the density of NeuN-positive neurons in the cortex, a region of interest (ROI) was set to include all layers above the hippocampus in each image. For hippocampal region CA1, a circular ROI (1.2 × 104 μm2) was selected in each image for the quantification. Three slices from each mouse were used for the analysis. The ratio of area covered by NeuN-staining was measured using the positive pixel count program in Image Scope software (Aperio Technlogies). The analysis was performed in a blinded manner.
2.6 Liver function analysis
Alanine aminotransferase (ALT) activity was measured using plasma samples by ALT activity assay kit (BioAssay Systems, Hayward, CA). To examine lipid synthesis in the liver, the triglyceride and total cholesterol levels in plasma were measured using specific kits from WAKO (Tokyo, Japan).
2.7 Statistical analysis
Quantified data were analyzed by two-tailed two-way ANOVA with a Tukey’s post-hoc test, one-way ANOVA followed by Tukey-Kramer’s post-hoc analysis, or Student’s t-test according to the properties of independent variables. All statistical analyses were performed on JMP 10.0 software (SAS Institute Inc.). Statistical significance was set to p<0.05.
3. RESULTS
3.1 Postsynaptic proteins are decreased in aged mice
We first investigated the aging-related changes of postsynaptic protein levels as well as LRP1 levels in the cortex by comparing young (3-6 months of age) and old (20-24 months of age) mice. Consistent with our previous findings (Liu et al., 2010; Liu et al., 2007), Western blot analyses revealed that the levels of LRP1 were significantly decreased in aged nLRP1−/− mice (Fig. 1A). The residual LRP1 observed in aged nLRP1−/− mice likely represents the expression within glial cells and/or cerebrovasculature (Liu et al., 2010; Liu et al., 2007). Postsynaptic proteins, PSD95 (Fig. 1B), GluR1 (Fig. 1C), and NR1 (Fig. 1D) were significantly lower in aged control mice than in young control mice. These postsynaptic protein levels were further decreased in aged nLrp1−/− mice compared to aged control mice (Fig. 1B, C, D), which are consistent with previous reports (Liu et al., 2010). Other postsynaptic proteins, GluR2 (Fig. 1E), NR2A (Fig. 1F) and NR2B (Fig. 1G) were not significantly decreased in aged mice in our samples when analyzed by one-way ANOVA, while there was a tendency to decrease. In agreement with previous reports (Liu et al., 2010), the levels of GluR2, NR2A, and NR2B were not affected by deletion of neuronal LRP1. Young nLrp1−/− mice were not analyzed, since LRP1 is gradually reduced in this mouse model and its deletion is not completed at their young age (Liu et al., 2010).
FIGURE 1. Synaptic proteins are decreased in the brains of aged mice.
A. LRP1 level was analyzed in the cortex from young control (male, 3-6 months), old control (male, 20-24 months), and old nLrp1−/− mice (male; 20-24 months) by Western blotting. B-G. The levels of postsynaptic markers PSD95 (B), GluR1 (C) NR1 (D), GluR2 (E), NR2A (F), and NR2B (G) were analyzed in the cortex from the above described mice (N=4-6/group). Data are plotted as mean ± SEM. *p<0.05, **p<0.01 by Tukey-Kramer’s post-hoc analysis after one-way ANOVA. n.s., not significant.
3.2 Bexarotene increases the levels of apoE and ABCA1 in mouse brains
To examine the effects of bexarotene on apoE and ABCA1 expression in the brain, aged control and nLrp1−/− mice were fed with bexarotene-formulated diet for 8 weeks. To avoid the potential stresses caused by oral gavage, we used bexarotene-containing diet for drug administration. ABCA1 expression levels in the cortex of bexarotene-treated mice were significantly higher than those of non-treated mice regardless of their genotypes (Fig. 2A). Bexarotene treatment also significantly increased endogenous mouse apoE levels in the cortex of both mice approximately two-fold of non-treated mice when analyzed by Western blot (Fig. 2B) and ELISA (Fig. 2C), while LRP1 levels were unaffected by treatment (Fig. 2D). These results indicate that formulating bexarotene into the diet is an effective approach to administer this drug to experimental animals.
FIGURE 2. Bexarotene treatment increases the levels of ABCA1 and apoE, but not LRP1, in the cortex of aged mice.
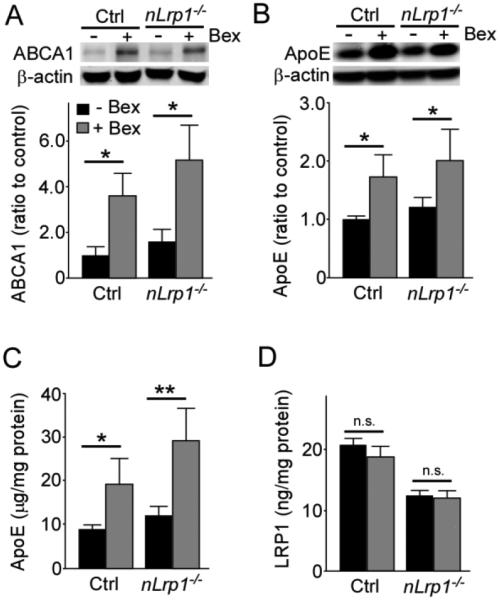
A. The levels of ABCA1 in the cortex of old control and nLrp1−/− mice (male, 20-24 months) with or without bexarotene treatment (100 mg/kg/day) for 8 weeks were analyzed by Western blotting. B, C. The levels of apoE in the cortex were detected by Western blotting (B) and ELISA (C). D. LRP1 level was analyzed in the cortex from the above described mice by ELISA (N=4-6/group). Data are plotted as mean ± SEM. *p<0.05, **p<0.01 by Tukey’s post-hoc analysis of two-way ANOVA. n.s., not significant.
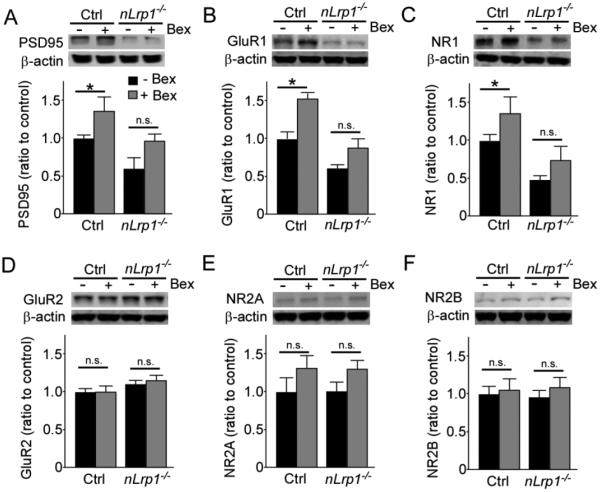
3.3 Bexarotene treatment rescues the age-dependent decrease in postsynaptic proteins through neuronal LRP1
Next, we investigated the effect of bexarotene on synaptic proteins in the aged mice. Bexarotene treatment significantly increased PSD95 (Fig. 3A), GluR1 (Fig. 3B) and NR1 (Fig. 3C) levels in the cortex of aged control mice, although there was no significant effect of bexarotene on these postsynaptic protein levels in aged nLrp1−/− mice (Fig. 3A-C), when analyzed by Western blot. These protein levels were also decreased significantly in nLRP1−/− mice compared to control mice regardless of treatment (Fig. 3A-C). These results indicate that neuronal LRP1 is required for the bexarotene-mediated increase of these postsynaptic proteins. Nonetheless, bexarotene treatment did not influence the expression levels of GluR2 (Fig. 3D), NR2A (Fig. 3E) and NR2B (Fig. 3F) in the cortex in aged mice regardless of genotype, while they were also not affected by either aging or deficiency of neuronal LRP1 (Fig. 1). In addition, we also found that the expression levels of PSD95 (Fig. 4A), GluR1 (Fig. 4B), and NR1 (Fig. 4C) in the hippocampus were significantly increased by bexarotene in aged control mice by Western blot. No significant effects of bexarotene were detected in aged nLrp1−/− mice, while these postsynaptic proteins were decreased in aged nLrp1−/− mice (Fig. 4A-C). Consistent with the results from Western blot, ELISA also detected the up-regulation of PSD95 by the bexarotene administration in the hippocampus of aged control mice, but not nLrp1−/− mice (Fig. 4D). We also found that the expression levels of synaptophysin, a presynaptic marker, were increased by bexarotene treatment in the hippocampus of aged control mice (Fig. 4E). These results suggest that bexarotene treatment has beneficial effects on both pre- and post-synapses in a neuronal LRP1-dependent manner.
FIGURE 3. Bexarotene treatment increases postsynaptic proteins in the cortex of aged mice in a neuronal LRP1-dependent manner.
The levels of postsynaptic proteins PSD95 (A), GluR1 (B), NR1 (C), GluR2 (D), NR2A (E), and NR2B (F) in the cortex of old control and nLrp1−/− mice (male, 20-24 months) with or without bexarotene treatment (100 mg/kg/day) for 8 weeks were analyzed by Western blotting (N=4-6/group). Data are plotted as mean ± SEM. *p<0.05 by Tukey’s post-hoc analysis of two-way ANOVA. n.s., not significant.
FIGURE 4. Bexarotene treatment increases synaptic proteins in the hippocampus of aged mice in a neuronal LRP1-dependent manner.
A-C. The levels of postsynaptic proteins PSD95 (A), GluR1 (B) and NR1 (C) in the hippocampus of aged control and nLrp1−/− mice (male, 20-24 months) with or without bexarotene treatment (100 mg/kg/day) for 8 weeks were analyzed by Western blotting. D, E. The levels of PSD95 (D) and synaptophysin (E) in the hippocampus of the above described mice were analyzed by ELISA (N=4-6/group). Data are plotted as mean ± SEM. *p<0.05 by Tukey’s post-hoc analysis after two-way ANOVA. n.s., not significant.
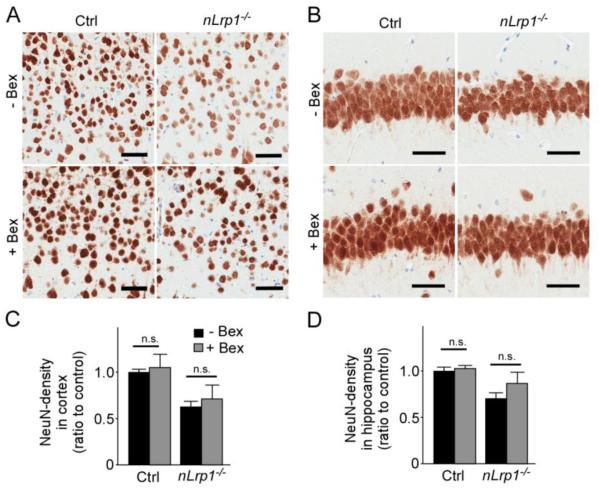
3.4 Bexarotene does not influence the density of NeuN-positive neurons in cortex and hippocampus
To further assess the effects of bexarotene on neurons, we quantified the density of NeuN-positive neurons in the cortex (Fig. 5A) and hippocampus (Fig. 5B) of aged control and nLrp1−/− mice by immunohistochemical analysis. Bexarotene treatment had no significant effect on the density of neurons in either region of control and nLrp1−/− mice (Fig. 5C and D). Deletion of neuronal LRP1 resulted in significant neuronal loss (Fig. 5C and D) consistent with our previous report (Liu et al., 2010); however, bexarotene failed to restore this deficit (Fig. 5C and D). Taken together, these results indicate that bexarotene treatment upregulates the levels of synaptic proteins without increasing the density of neurons.
FIGURE 5. Densities of NeuN-positive neurons are not altered by bexarotene treatment in the brains of aged mice.
A,B. NeuN immunostaining of cortex (A) and hippocampus (B) from aged control and nLrp1−/− mice (male, 20-24 months) with or without bexarotene treatment (100 mg/kg/day) for 8 weeks. Scale bars = 50 μm. (B). C, D. The ratio of area covered by NeuN-staining was measured in cortex (C) and hippocampus (D) (N=5/group). Data are plotted as mean ± SEM. n.s., not significant.
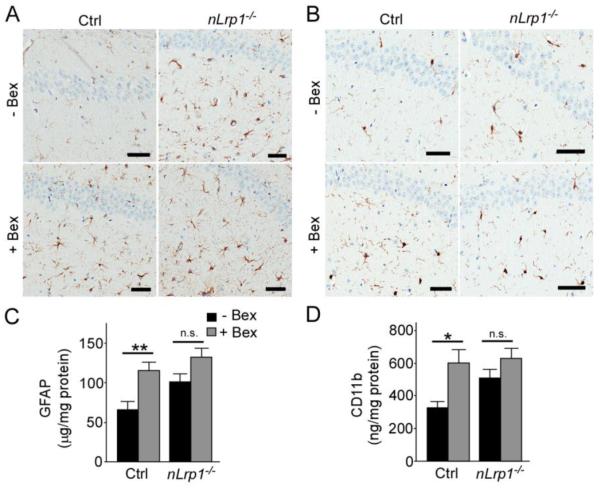
3.5 Bexarotene treatment activates astrocytes and microglia
To investigate whether bexarotene influences glial cell activation, we performed immunostaining for an activated astrocyte marker, GFAP (Fig. 6A), and an activated microglia marker, Iba-1 (Fig. 6B), in aged control and nLrp1−/− mice with or without bexarotene treatment. Bexarotene significantly increased GFAP-positive astrocytes and Iba-1-positive microglia in the hippocampi of control mice (Fig. 6A and B). When the levels of GFAP and another microglia marker CD11b in the hippocampus were analyzed by ELISA, we also found that bexarotene-treated control mice showed higher GFAP and CD11b levels than non-treated control mice (Fig. 6C and D). These results indicate that administration of bexarotene leads to activation of glial cells in the brains of aged mice. The effects of bexarotene were not significant in aged nLrp1−/− mice, despite an increase in activated astrocytes and microglia compared to age-matched control mice (Fig. 6C and D).
FIGURE 6. Glial activation is induced by bexarotene treatment in the hippocampus of aged mice.
A, B. Hippocampus from aged control and nLrp1−/− mice (male, 20-24 months) with or without bexarotene treatment (100 mg/kg/day) for 8 weeks were analyzed by immunostaining for GFAP (A) and Iba-1 (B). Scale bars = 50 μm. C, D. The levels of GFAP (C) and CD11b (D) in the hippocampus of the above described mice were analyzed by ELISA (N=4-6/group). Data are plotted as mean ± SEM. *p<0.05, **p<0.01 by Tukey’s post-hoc analysis after two-way ANOVA. n.s., not significant.
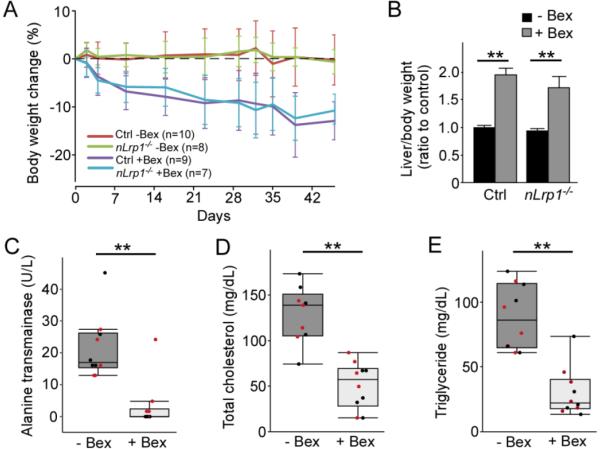
3.6 Long-term bexarotene treatment causes weight loss, hepatomegaly and liver dysfunction in aged mice
During the administration period, we observed gradual weight loss by 10-15% of initial body weight in bexarotene-treated cohorts in both genotypes, while mice fed normal chow did not have any significant changes in body weight (Fig. 7A). Since bexarotene has been reported to cause liver dysfunction (Duvic et al., 2001; Scarisbrick et al., 2013), liver weight and plasma ALT, triglyceride and cholesterol levels were measured at the end of treatment. Liver weight, which was adjusted to initial body weight before treatment, was significantly increased in bexarotene-treated mice to 1.5-2.0 folds to that of non-treated mice, regardless of genotype (Fig. 7B). Furthermore, plasma ALT (Fig. 7C), triglyceride (Fig. 7D) and cholesterol (Fig. 7E) levels were significantly lower in bexarotene-treated mice than in non-treated mice, suggesting the exhaustion of hepatic function. These results indicate that long-term bexarotene treatment causes severe liver damage in aged mice, which likely results in hepatic failure.
FIGURE 7. Bexarotene treatment causes body-weight loss, hepatomegaly and liver dysfunction in aged mice.
A. Body weight of the aged control and nLrp1−/− mice (male, 20-24 months) with or without bexarotene treatment (100 mg/kg/day) was monitored at least once a week throughout the treatment period. Body weight change of each mouse was calculated by ratio to the initial body weight at the beginning of the treatment. Data are plotted as mean ± SEM (N=7-10/group). B. Liver weight of the mice was measured at the end of treatment, and normalized by the initial body weight for each mouse (N=7-10/group). **p<0.01 by Tukey’s post-hoc analysis after two-way ANOVA. C-E. The levels of alanine transaminase (ALT) (C), total cholesterol (D) and triglyceride (E) were measured in plasma from the mice (N=9-11/group). Black and red dots indicate control and nLRP1−/− mice, respectively. Data are plotted as mean ± SD. **p<0.01 by Student’s t-test.
4. DISCUSSION
APOE ε4 is the strongest genetic risk factor for AD among its three polymorphic alleles (ε2, ε3, ε4) (Bu, 2009; Liu et al., 2013). Furthermore, APOE4 carriers display reduced cognitive function compared to non-carriers during aging in healthy individuals (Reinvang et al., 2010; Zehnder et al., 2009). ApoE likely contributes to the maintenance of brain homeostasis through multiple pathways depending on the isoform (Bu, 2009; Huang and Mucke, 2012). ApoE plays critical roles in regulating cholesterol transport, synaptic plasticity, neurogenesis, inflammatory responses, Aβ metabolism, and glucose metabolism, as well as in maintaining blood brain barrier (BBB) integrity (Bu, 2009; Liu et al., 2013). While apoE4 levels in the cerebrospinal fluid (CSF) and plasma are lower than those of apoE2 or apoE3 (Cruchaga et al., 2012), it is not fully understood whether increasing brain apoE levels is beneficial to neuronal functions. Accumulating evidence has shown that increasing apoE levels and lipidation through pharmacological approaches improves cognitive function and reduces brain Aβ accumulation in amyloid mouse models (Bu, 2009; Kanekiyo et al., 2014). In particular, therapeutic effects of a FDA-approved RXR agonist, bexarotene, on AD has been an intense focus since Cramer et. al. reported accelerated Aβ clearance and decreased amyloid plaque burden as well as improved memory function in amyloid mouse models after short-term treatment (Cramer et al., 2012). Although the effects of bexarotene on Aβ metabolism and plaque burdens are still controversial (Fitz et al., 2013; LaClair et al., 2013; Price et al., 2013; Tesseur et al., 2013; Veeraraghavalu et al., 2013), the improvements of cognitive function through bexarotene administration have been confirmed by other groups (Boehm-Cagan and Michaelson, 2014; Fitz et al., 2013; Tesseur et al., 2013).
In this study, we have demonstrated beneficial effects of bexarotene on synapses upon formulation in diet. In particular, bexarotene treatment restored the levels of postsynaptic proteins PSD95, GluR1 and NR1, as well as a presynaptic protein synaptophysin, likely by increasing brain levels of apoE and ABCA1, and related apoE lipidation. While it remains unclear if bexarotene treatment increases these synaptic proteins beyond physiological levels in young healthy mice, recent studies demonstrated that bexarotene increased the presynaptic protein vGluT1 in apoE4-targeted replacement (TR) mice (Boehm-Cagan and Michaelson, 2014) and increased postsynaptic PSD95 levels in an amyloid AD mouse model with apoE4-TR background (Tai et al., 2014) at young age, 4 months and 6 months, respectively. Importantly, our findings showed increased synaptic proteins by bexarotene in old mice at the age of 20-24 months. Aging has been associated with a reduction of synaptic proteins (Bahr et al., 1992; Clayton et al., 2002; VanGuilder et al., 2010), changes in morphology and structure of the synapses (Dumitriu et al., 2010; Pereira et al., 2014; Petralia et al., 2014; von Bohlen und Halbach et al., 2006) and neuroinflammation (Perry, 2010; Sparkman and Johnson, 2008), which contribute to cognitive decline. Whereas therapeutic approaches to treat age-related cognitive impairments by modulating glutamatergic activity (Brothers et al., 2013) or neuroinflammation (Bardou et al., 2013) have been reported, our results demonstrate that apoE is also a promising therapeutic target to repair impaired synapses under pathological conditions during aging. Restoring synaptic protein levels might be beneficial to prevent and/or cure age-dependent cognitive decline, although how excessively increased synaptic proteins influence synaptic functions needs to be further investigated. In addition, more caution should be exercised when using such an approach to treat APOE4 carriers as increasing apoE4 may gain toxic effects (Kanekiyo et al., 2014).
Given that the density of NeuN-positive neurons were not changed by bexarotene administration, the effects on synaptic proteins are likely due to facilitated synaptogenesis or modification of synaptic structures, rather than neurogenesis or prevention of neurodegeneration. Indeed, substantial amounts of cholesterol delivered by apoE-lipoprotein particles are necessary for synaptogenesis (Mauch et al., 2001). In contrast, under stress conditions, the reduction of cholesterol levels in neurons appear to be advantageous for cell survival (Ledesma et al., 2012; Sodero et al., 2011). Thus, the efficient turnover of neuronal cholesterol may be more important than the amount, per se, to preserve cognitive function during aging. Interestingly, apoE not only provides cholesterol to neurons but also suppresses cholesterol release from neurons (Gong et al., 2007), which may affect the exchange of cholesterol at synapses. Further studies are needed to determine the influence of increased apoE through administrations with RXR agonists.
We also found that bexarotene treatment failed to restore synaptic proteins in aged nLrp1−/− mice. ApoE receptors in the brain belong to the low-density lipoprotein receptor (LDLR) family which includes LDLR, LRP1, very-low-density lipoprotein receptor (VLDLR) and apoE receptor 2 (apoER2) (Bu, 2009; Kanekiyo and Bu, 2014). Among them, LRP1 is abundantly expressed in neurons, where it mediates the endocytosis of a variety of ligands including apoE and Aβ (Kanekiyo and Bu, 2014). Furthermore, neuronal LRP1 is critical for brain lipid metabolism, where its deletion results in global disturbances of brain lipid homeostasis (Liu et al., 2010). Thus, neuronal LRP1 may be specifically required in apoE-mediated cholesterol delivery as a transport receptor. Alternatively, it is also possible that deficiency of neuronal LRP1 leads to irreversible damage on synapses which cannot be repaired by apoE, because LRP1 may directly affect the levels of PSD95, GluR1 or NR1 (Gan et al., 2014; May et al., 2004; Nakajima et al., 2013). Further investigation is needed to clarify the molecular mechanism underlying apoE-LRP1 pathway in maintaining synaptic homeostasis during aging.
In spite of the beneficial effects on synapses, bexarotene treatment induced severe systemic side effects in aged mice, which include body weight loss (Tesseur and De Strooper, 2013), irritability (Tesseur and De Strooper, 2013), hepatomegaly (Price et al., 2013; Tai et al., 2014) and dyslipidemia (Price et al., 2013) as reported. Since these symptoms were also induced by the administration of bexarotene through oral gavage or formulating into the drinking water/hydrogel, it is unlikely that the delivery using the drug-formulated diet specifically caused these side effects. Furthermore, our results demonstrated that long-term administration of bexarotene exacerbated glial cell activations in aged mice. In contrast to our results, RXR agonists have been shown to have anti-inflammatory effects (Gonzalez et al., 2009; Kang et al., 2000; Lefterov et al., 2015; Uchimura et al., 2001). One plausible explanation for the conflicting results may be due to hepatic neuropathy caused by liver dysfunction, in which astrocytosis and microglial activation are characteristic pathological changes (Butterworth, 2013; Jayakumar et al., 2015). Although we did not assess the effects of lower doses of bexarotene treatment to potentially reduce these deleterious effects in the current study, the clinical application of this drug in aged patients to treat aging-associated cognitive decline requires great caution.
In conclusion, we have demonstrated that bexarotene rescues aging-related declines of synaptic proteins through a neural LRP1-medtated pathway presumably by increasing apoE levels and lipidation. Thus, the apoE-LRP1 pathway could be a potential therapeutic target for aging-related cognitive decline. However, bexarotene is unlikely a suitable compound due to its strong side-effects. Identification of alternative compounds that can increase apoE levels and lipidation in the brain might be a promising strategy for prevention and treatment of cognitive decline in normal aging or neurodegenerative diseases.
Highlights.
An RXR agonist bexarotene significantly increases brain apoE levels in aged mice.
Bexarotene administration rescues aging-related synapse loss.
Neuronal apoE receptor LRP1 is required for the beneficial effects of bexarotene on synapses.
Severe hepatic dysfunctions were observed upon bexarotene treatment in aged mice.
ACKNOWLEDGMENTS
This work was supported by NIH grants R01AG035355, R01AG046205, R01AG027924, P50AG016574 and P01NS074969 (to G.B.), and a New Investigator Research Grant from the Alzheimer's Association (to T. K.). The authors thank Mary D. Davis for careful reading of the manuscript.
ABBREVIATIONS
- ABCA1
ATP binding cassette, subfamily A, member 1
- AD
Alzheimer’s disease
- Aβ
amyloid-β
- apoE
apolipoprotein E
- Bex
bexarotene
- ELISA
enzyme-linked immune-sorbent assay
- GluR1
glutamate receptor 1
- LRP1
low-density lipoprotein receptor-related protein 1
- LXR
liver X receptor
- NR1
N-methyl-D-aspartate receptor NR1 subunit
- RXR
retinoid X receptor
- TR
targeted replacement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with the content of this article.
AUTHOR CONTRIBUTION
M.T., G.B. and T.K. conceived the study. M.T., M.S., Y.Y., C.L. and J.R. collected data, and M.T., M.S., Y.Y., C.L., J.R., G.B. and T.K. analyzed data. M.T., G.B. and T.K. wrote first draft. All authors contributed in writing the final manuscript. All authors have approved the final version of the manuscript.
REFERENCES
- Bahr BA, Godshall AC, Hall RA, Lynch G. Mouse telencephalon exhibits an age-related decrease in glutamate (AMPA) receptors but no change in nerve terminal markers. Brain Res. 1992;589:320–326. doi: 10.1016/0006-8993(92)91293-n. [DOI] [PubMed] [Google Scholar]
- Bardou I, Brothers HM, Kaercher RM, Hopp SC, Wenk GL. Differential effects of duration and age on the consequences of neuroinflammation in the hippocampus. Neurobiol Aging. 2013;34:2293–2301. doi: 10.1016/j.neurobiolaging.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Hiesinger PR. The synaptic maintenance problem: membrane recycling, Ca2+ homeostasis and late onset degeneration. Mol Neurodegener. 2013;8:23. doi: 10.1186/1750-1326-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm-Cagan A, Michaelson DM. Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J Neurosci. 2014;34:7293–7301. doi: 10.1523/JNEUROSCI.5198-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers HM, Bardou I, Hopp SC, Kaercher RM, Corona AW, Fenn AM, Godbout JP, Wenk GL. Riluzole partially rescues age-associated, but not LPS-induced, loss of glutamate transporters and spatial memory. J Neuroimmune Pharmacol. 2013;8:1098–1105. doi: 10.1007/s11481-013-9476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth RF. The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol. 2013;10:522–528. doi: 10.1038/nrgastro.2013.99. [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Grosshans DR, Browning MD. Aging and surface expression of hippocampal NMDA receptors. J Biol Chem. 2002;277:14367–14369. doi: 10.1074/jbc.C200074200. [DOI] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Kauwe JS, Nowotny P, Bales K, Pickering EH, Mayo K, Bertelsen S, Hinrichs A, Fagan AM, Holtzman DM, Morris JC, Goate AM. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer's disease. Hum Mol Genet. 2012;21:4558–4571. doi: 10.1093/hmg/dds296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, Crowley C, Yocum RC. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- Elgamal SA, Roy EA, Sharratt MT. Age and verbal fluency: the mediating effect of speed of processing. Can Geriatr J. 2011;14:66–72. doi: 10.5770/cgj.v14i3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Cronican AA, Lefterov I, Koldamova R. Comment on "ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models". Science. 2013;340:924-c. doi: 10.1126/science.1235809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan M, Jiang P, McLean P, Kanekiyo T, Bu G. Low-density lipoprotein receptor-related protein 1 (LRP1) regulates the stability and function of GluA1 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor in neurons. PLoS One. 2014;9:e113237. doi: 10.1371/journal.pone.0113237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JS, Morita SY, Kobayashi M, Handa T, Fujita SC, Yanagisawa K, Michikawa M. Novel action of apolipoprotein E (ApoE): ApoE isoform specifically inhibits lipid-particle-mediated cholesterol release from neurons. Mol Neurodegener. 2007;2:9. doi: 10.1186/1750-1326-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AN, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou S, Rigopoulos D, Stamou C, Nikolaou V, Kontochristopoulos G. Treatment of mycosis fungoides with bexarotene results in remission of diffuse plane xanthomas. J Cutan Med Surg. 2013;17:52–54. doi: 10.2310/7750.2012.12022. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Rama Rao KV, Norenberg MD. Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol. 2015;5:S21–28. doi: 10.1016/j.jceh.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Bu G. The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer's disease. Front Aging Neurosci. 2014;6:93. doi: 10.3389/fnagi.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Xu H, Bu G. ApoE and Abeta in Alzheimer's disease: accidental encounters or partners? Neuron. 2014;81:740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BY, Chung SW, Kim SH, Kang SN, Choe YK, Kim TS. Retinoid-mediated inhibition of interleukin-12 production in mouse macrophages suppresses Th1 cytokine profile in CD4(+) T cells. Br J Pharmacol. 2000;130:581–586. doi: 10.1038/sj.bjp.0703345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaClair KD, Manaye KF, Lee DL, Allard JS, Savonenko AV, Troncoso JC, Wong PC. Treatment with bexarotene, a compound that increases apolipoprotein-E, provides no cognitive benefit in mutant APP/PS1 mice. Mol Neurodegener. 2013;8:18. doi: 10.1186/1750-1326-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma MD, Martin MG, Dotti CG. Lipid changes in the aged brain: effect on synaptic function and neuronal survival. Prog Lipid Res. 2012;51:23–35. doi: 10.1016/j.plipres.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Lefterov I, Schug J, Mounier A, Nam KN, Fitz NF, Koldamova R. RNA-sequencing reveals transcriptional up-regulation of Trem2 in response to bexarotene treatment. Neurobiol Dis. 2015;82:132–140. doi: 10.1016/j.nbd.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Lin S, Beyer TP, Zhang Y, Wu X, Bales KR, DeMattos RB, May PC, Li SD, Jiang XC, Eacho PI, Cao G, Paul SM. A liver X receptor and retinoid X receptor heterodimer mediates apolipoprotein E expression, secretion and cholesterol homeostasis in astrocytes. J Neurochem. 2004;88:623–634. doi: 10.1111/j.1471-4159.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Trotter J, Zhang J, Peters MM, Cheng H, Bao J, Han X, Weeber EJ, Bu G. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J Neurosci. 2010;30:17068–17078. doi: 10.1523/JNEUROSCI.4067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, Herz J, Muglia L, Bu G. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, Noebels JL, Beffert U, Sweatt JD, Weeber EJ, Herz J. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N, Wayne AS, Kim YH, Hale GA, Alvarado CS, Myskowski P, Jaffe ES, Busam KJ, Pulitzer M, Zwerner J, Horwitz S. Bexarotene is active against subcutaneous panniculitis-like T-cell lymphoma in adult and pediatric populations. Clin Lymphoma Myeloma Leuk. 2012;12:20–25. doi: 10.1016/j.clml.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima C, Kulik A, Frotscher M, Herz J, Schafer M, Bock HH, May P. Low density lipoprotein receptor-related protein 1 (LRP1) modulates N-methyl-D-aspartate (NMDA) receptor-dependent intracellular signaling and NMDA-induced regulation of postsynaptic protein complexes. J Biol Chem. 2013;288:21909–21923. doi: 10.1074/jbc.M112.444364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Lambert HK, Grossman YS, Dumitriu D, Waldman R, Jannetty SK, Calakos K, Janssen WG, McEwen BS, Morrison JH. Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc Natl Acad Sci U S A. 2014;111:18733–18738. doi: 10.1073/pnas.1421285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277–286. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Mattson MP, Yao PJ. Communication breakdown: the impact of ageing on synapse structure. Ageing Res Rev. 2014;14:31–42. doi: 10.1016/j.arr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AR, Xu G, Siemienski ZB, Smithson LA, Borchelt DR, Golde TE, Felsenstein KM. Comment on "ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models". Science. 2013;340:924-d. doi: 10.1126/science.1234089. [DOI] [PubMed] [Google Scholar]
- Reinvang I, Winjevoll IL, Rootwelt H, Espeseth T. Working memory deficits in healthy APOE epsilon 4 carriers. Neuropsychologia. 2010;48:566–573. doi: 10.1016/j.neuropsychologia.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Scarisbrick JJ, Morris S, Azurdia R, Illidge T, Parry E, Graham-Brown R, Cowan R, Gallop-Evans E, Wachsmuth R, Eagle M, Wierzbicki AS, Soran H, Whittaker S, Wain EM. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:192–200. doi: 10.1111/bjd.12042. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Petersen RC, Dickson DW, Bu G. Brain regional correlation of amyloid-beta with synapses and apolipoprotein E in non-demented individuals: potential mechanisms underlying regional vulnerability to amyloid-beta accumulation. Acta Neuropathol. 2013;125:535–547. doi: 10.1007/s00401-013-1086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodero AO, Trovò L, Iannilli F, Van Veldhoven P, Dotti CG, Martin MG. Regulation of tyrosine kinase B activity by the Cyp46/cholesterol loss pathway in mature hippocampal neurons: relevance for neuronal survival under stress and in aging. Journal of Neurochemistry. 2011;116:747–755. doi: 10.1111/j.1471-4159.2010.07079.x. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman F, de Lera AR. Ligand recognition by RAR and RXR receptors: binding and selectivity. J Med Chem. 2005;48:6212–6219. doi: 10.1021/jm050285w. [DOI] [PubMed] [Google Scholar]
- Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004;11(Suppl 2):S126–143. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- Tai LM, Koster KP, Luo J, Lee SH, Wang YT, Collins NC, Ben Aissa M, Thatcher GR, LaDu MJ. Amyloid-beta pathology and APOE genotype modulate retinoid X receptor agonist activity in vivo. J Biol Chem. 2014;289:30538–30555. doi: 10.1074/jbc.M114.600833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, De Strooper B. When the dust settles: what did we learn from the bexarotene discussion? Alzheimers Res Ther. 2013;5:54. doi: 10.1186/alzrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Lo AC, Roberfroid A, Dietvorst S, Van Broeck B, Borgers M, Gijsen H, Moechars D, Mercken M, Kemp J, D'Hooge R, De Strooper B. Comment on "ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models". Science. 2013;340:924-e. doi: 10.1126/science.1233937. [DOI] [PubMed] [Google Scholar]
- Uchimura K, Nakamuta M, Enjoji M, Irie T, Sugimoto R, Muta T, Iwamoto H, Nawata H. Activation of retinoic X receptor and peroxisome proliferator-activated receptor-gamma inhibits nitric oxide and tumor necrosis factor-alpha production in rat Kupffer cells. Hepatology. 2001;33:91–99. doi: 10.1053/jhep.2001.21145. [DOI] [PubMed] [Google Scholar]
- Ulrich JD, Burchett JM, Restivo JL, Schuler DR, Verghese PB, Mahan TE, Landreth GE, Castellano JM, Jiang H, Cirrito JR, Holtzman DM. In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Mol Neurodegener. 2013;8:13. doi: 10.1186/1750-1326-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakeva L, Ranki A, Hahtola S. Ten-year experience of bexarotene therapy for cutaneous T-cell lymphoma in Finland. Acta Derm Venereol. 2012;92:258–263. doi: 10.2340/00015555-1359. [DOI] [PubMed] [Google Scholar]
- van Vliet P. Cholesterol and late-life cognitive decline. J Alzheimers Dis. 2012;30:2011–111028. doi: 10.3233/JAD-2011-111028. [DOI] [PubMed] [Google Scholar]
- VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem. 2010;113:1577–1588. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavalu K, Zhang C, Miller S, Hefendehl JK, Rajapaksha TW, Ulrich J, Jucker M, Holtzman DM, Tanzi RE, Vassar R, Sisodia SS. Comment on "ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models". Science. 2013;340:924-f. doi: 10.1126/science.1235505. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res. 2006;83:525–531. doi: 10.1002/jnr.20759. [DOI] [PubMed] [Google Scholar]
- WHO World Health Statistics 2014. 2014.
- Zehnder AE, Blasi S, Berres M, Monsch AU, Stahelin HB, Spiegel R. Impact of APOE status on cognitive maintenance in healthy elderly persons. Int J Geriatr Psychiatry. 2009;24:132–141. doi: 10.1002/gps.2080. [DOI] [PubMed] [Google Scholar]
- Zhao J, Fu Y, Liu CC, Shinohara M, Nielsen HM, Dong Q, Kanekiyo T, Bu G. Retinoic acid isomers facilitate apolipoprotein E production and lipidation in astrocytes through the retinoid X receptor/retinoic acid receptor pathway. J Biol Chem. 2014;289:11282–11292. doi: 10.1074/jbc.M113.526095. [DOI] [PMC free article] [PubMed] [Google Scholar]