Abstract
Rationale
Consistent sex differences are observed in human drug addiction, with females often exceeding males on drug intake. However, there is still a need for animal models for some aspects of addiction such as acquisition of drug self-administration and the subsequent development of drug-seeking.
Objectives
The present study examined sex differences in the acquisition of self-administration of two widely used stimulants, cocaine and nicotine.
Methods
Male and female rats self-administered cocaine (0.4 mg/kg/infusion) or nicotine (0.03 mg/kg/infusion) daily under a fixed-ratio 1 (FR 1) schedule until acquisition criteria were met (maximum of 30 sessions). The self-administration criterion for cocaine was ≥20 infusions in a 2 h session and ≥5 infusions in a 1 h session for nicotine. Sex differences were assessed by examining the percentage of rats that met acquisition criteria, the number of sessions to meet criteria and the number of infusions earned during the maintenance phase.
Results
A significantly higher percentage of male rats acquired both cocaine and nicotine self-administration than females, and males met acquisition criteria in fewer sessions. However, after criteria were met, females self-administered more cocaine than males during the first 5 days of maintenance. There were no sex differences in nicotine infusions post acquisition.
Conclusions
Differences in acquisition amongst sexes can reveal factors that are integral to initiation of drug use, an often overlooked phase of drug addiction.
Keywords: Acquisition, Maintenance, Self-Administration, Sex Differences, Nicotine, Cocaine
Introduction
In humans, consistent sex differences have been reported in many phases of the drug addiction process, from escalation to withdrawal and relapse (Anker and Carroll 2010). Overall, men use more drugs and are 2–3 times more likely to have a drug dependence problem than women; whereas, women show earlier initiation of use and greater escalation of drug taking (Becker et al. 2012). This is especially true for stimulants like cocaine and nicotine for which women show higher rates of cocaine use, a younger age of onset, and maintain abstinence for a shorter time-period than men (DeVito et al. 2014; McCance-Katz et al. 1999; Kosten et al. 1993; for review, see Fattore et al. 2008). However, men take less time to feel the subjective effects of cocaine and report more euphoria than women (Quinones-Jenab and Jenab 2012). Sex differences are seen in many stages of nicotine dependence as well. Women have lower success rates of smoking cessation and experience poorer treatment outcomes; whereas, men maintain higher rates of nicotine use, although this differs across ages and regions (Smith et al. 2015; Jarvis et al. 2013; for reviews, see Pogun and Yararbas 2009; Perkins 2009, 2001).
While sex differences have been reported in all phases of drug addiction, from initiation to relapse, sex differences during certain phases of drug abuse have been relatively understudied in humans (for reviews, see Anker and Carroll 2010, Carroll and Anker 2010, and Becker and Hu 2008). For example, research on initiation or acquisition of drug use is limited by self-report measures typically taken long after initiation of drug use has begun. This is likely due to difficulties in studying drug use in humans, and more specifically, during adolescence, when drug use is most likely to begin (Chambers et al. 2014). The limited research with humans suggests that women take less time than men to become addicted to a variety of drugs including stimulants (see Anker and Carroll 2011; Fattore et al. 2008, 2014 for reviews). As cocaine and nicotine are two of the most commonly abused illicit and licit stimulants, with relatively low cessation rates and high rates of relapse seen with both drugs, examining factors such as sex that influence the initiation of use is important in understanding how to prevent use before it begins.
Significant sex differences in the initiation and development of cocaine and nicotine dependence have been reported. For cocaine, women initiate cocaine use earlier in life (Sartor et al. 2014; DeVito et al. 2014; Lynch et al. 2002) and transition from cocaine use to abuse sooner than men (Lozano et al. 2008; Haas and Peters 2000). Females also take less time to become dependent after initial use of tobacco (DiFranza et al. 2002; for review, see Perkins 2001), while men are more likely to become regular smokers after experimenting with tobacco and have a greater sensitivity to nicotine than women (for review, see Perkins et al. 2009; Waldron 1991). Preclinical research on drug self-administration in rodents is invaluable for examining sex-specific effects of initiation and initial maintenance of stimulant use without the influence of sociocultural or experiential factors.
However, the preclinical literature has relatively few studies of sex differences in acquisition of drug-taking behaviors and even fewer following sex differences from acquisition through maintenance (for review, see Carroll and Anker 2010). In studies on acquisition of cocaine and nicotine self-administration, results have not been consistent between sexes. Regarding cocaine, females acquire self-administration faster than males (Lynch 2008; Jackson et al. 2006; Hu et al. 2004; Lynch and Carroll 1999), although some studies reported that males acquired self-administration faster than females (Caine et al. 2004; for review, see Anker and Carroll 2011). Sex differences in self-administration during the subsequent maintenance phase show higher levels of infusions in females (Peterson et al. 2014; Anker et al. 2011; Cummings et al. 2011; Lynch 2008; Lynch and Carroll 1999; Morse et al. 1993), but others show no differences (Perry et al. 2013; Kosten and Zhang 2008; Jackson et al. 2006; Cosgrove et al. 2002; Roberts et al. 1989). In the literature on sex differences in the acquisition of nicotine self-administration, females acquired nicotine self-administration faster than males (Lynch 2009), but one study indicated no sex differences (Feltenstein et al. 2012). Post-acquisition, females maintained significantly higher levels of nicotine infusions than males during maintenance (Grebenstein et al. 2013; Rezvani et al. 2008; Chaudhri et al. 2005; Donny et al. 2000; but see Feltenstein et al. 2012).
Perhaps one reason for the inconsistent findings is that researchers typically only report data from acquisition or maintenance, leaving the relationship between these phases to be inferred across different studies. Indeed, only a few previous studies (e.g., Feltenstein et al. 2012; Jackson et al. 2006; Lynch and Carroll 1999) reported on the relationship between the rate of acquisition and subsequent number of infusions during maintenance. Given that the transition of initiation of drug use to consistent drug-taking behaviors could inform prevention efforts for drug addiction (Carroll and Meisch 2011), more research is needed on rates of acquisition and subsequent alteration of drug-taking behaviors.
The purpose of the present study was to examine sex differences in the acquisition and maintenance phases of self-administration of two commonly abused substances, cocaine and nicotine, to determine whether sex differences in acquisition transferred to the ensuing maintenance phase. Rates of acquisition, length of time to acquire, and number of infusions during maintenance were compared between separate groups of male and female rats. We hypothesized that a greater percentage of females would acquire cocaine and nicotine self-administration in a shorter period of time than males. Once acquired, it was predicted that females would self-administer greater amounts of both cocaine and nicotine than males.
Materials and Methods
Animals
Male and female drug-naïve Wistar rats (weighing 250–274 grams and 200–224 grams, respectively, on arrival) from Harlan Sprague-Dawley Inc. (Madison, WI, USA) were used throughout all phases of the experiment. Overall, 52 males and 54 females (cocaine) and 28 males and 83 females (nicotine) were used in the analysis of percent of group acquiring. A total of 39 males and 32 female rats (cocaine) and 22 males and 51 females (nicotine) acquired drug self-administration and were used to determine the number of days to acquire data. All rats that acquired cocaine self-administration completed the maintenance sessions but only 19 males and 42 females completed maintenance for nicotine and were used in the nicotine maintenance analysis. Ages ranged from 63 to 77 days upon arrival, and female and male rats were matched for age.
All rats were habituated to the facility for at least 3 days after arrival and allowed ad libitum access to water and food (Teklad 2018, Harlan Laboratories, Madison, WI, USA) until experiments began. Rats used in the nicotine self-administration study were individually housed in hanging stainless steel cages, and in the cocaine study, rats were single-housed in operant conditioning chambers described in the apparatus section post-surgery. The difference in housing conditions was due to previous work in our laboratory showing that nicotine self-administration did not occur when rats were permanently housed in the octagonal operant conditioning chambers, suggesting that a change of stimuli between the home cages and chambers where drug self-administration took place was an important factor for nicotine self-administration. Each rat was transferred to the same designated self-administration chamber daily. Upon start of the experiments, rats were restricted to 16 g (female) or 20 g (male) of food with free access to water, which has been found to maintain rats ~85% of their free-feeding weight. Rats used in the cocaine self-administration studies remained in the octagonal chambers throughout the experiments. All experiments were approved by the Institutional Animal Care and Use Committee (Protocol #1307-30762A), and the research was conducted in accordance with the Principles of Laboratory Animal Care (National Research Council 2011).
Apparatus
Self-administration sessions took place in custom-built octagonal operant conditioning chambers as previously described by Anker et al. (2007). Each chamber was housed inside a sound-attenuating wooden box with a fan to provide ventilation and masking noise. Two standard response levers were located on opposite sides of the chamber with stimulus lights of three colors (4.76 W) above each lever to act as an infusion cue. Infusions of nicotine and cocaine were delivered by an infusion pump (PHM-100, Med Associates. St. Albans, VT) connected to a swivel-tether system (375/22PS, Instech, Plymouth Meeting, PA, USA; C313CS-MN, Plastics One, Roanoke, VA, USA). Rats were then attached to the tether by a harness (CIH95AB, Instech).
Drugs
Cocaine HCl (National Institute of Drug Abuse, Research Triangle Institute, Research Triangle Park, NC) and (−) nicotine tartrate salt (Sigma, St. Louis, MO) were dissolved in sterile saline with additional heparin (5 USP/mL) added to prevent patency loss. Nicotine was adjusted to a pH of 7.0±0.2 with a dilute NaOH solution. The initial concentration of cocaine was 1.6 mg cocaine HCl/1 ml saline that was diluted to 0.4 mg/ml, with each cocaine infusion at the standard 0.4 mg/kg dose used in previous studies. Nicotine was diluted in saline to 0.36 mg/ml and the dose was 0.03 mg/kg. The infusion lengths of nicotine and cocaine were based on the weight of the rat (1s/100 g for cocaine and 0.33 s/100g for nicotine) with an infusion rate of 0.025 ml/s.
Surgical Procedures
All rats were first anesthetized for surgery with ketamine (IP, 90 mg/kg for males; 60 mg/kg for females) and xylazine (10 mg/kg, IP) with additional atropine (0.15 ml, SC) to stabilize respiration. A chronic indwelling silastic single-beaded catheter (15 cm long, Plastics One, Roanoke, VA) was implanted to lead from the right jugular vein to the right atrium. The distal end of the catheter was led subcutaneously to a medial incision attached to a harness. Post-surgery, rats were given buprenorphine (0.05 mg/ml; SC) twice daily for 3 days as an analgesic with additional ibuprofen (PO, 50 mg/kg) in the drinking water. During these recovery days, enrofloxacin (10 mg/kg) and heparinized sterile saline (10 IU/kg) were flushed daily through the catheters, with heparin flushing continuing daily after recovery. Patency was checked weekly with ketamine (10 mg/kg) and midazolam (0.5 mg/kg) mixed in sterile saline (KMS), and patency was determined by the loss of a righting reflex within 10 seconds of the KMS infusion.
Behavioral Procedures
Identical procedures were used on males and females in each study. After recovery from surgery, acquisition of self-administration began. Rats were initially trained to lever-press for either cocaine or nicotine in the operant conditioning chambers. Sessions were conducted daily and initially lasted 6 h for cocaine or 1 h for nicotine. Session length was based on previous data from our laboratory as well as others that showed sex differences during acquisition at these initial lengths (Lynch and Carroll 1999; Chaudhri et al. 2005). For all rats, session onset activated illumination of the house light. Two levers were available on opposite sides of the chamber: one active, one inactive. A lever press on the active lever led to a drug infusion under a fixed-ratio (FR) 1 schedule, and illumination of the tri-colored stimulus lights above the lever for the length of the infusion. For the cocaine rats, a lever-press on the inactive lever resulted in illumination of the stimulus lights above that lever but no infusion; whereas, in the nicotine rats, an inactive lever press produced no consequence similar to the methods used by Harris et al. (2008). Inactive lever presses were used as a general measure of overall activity. Each active lever press and infusion was followed by a 20-second timeout for cocaine or a 15-second timeout for nicotine, during which responses were recorded but had no programmed consequences.
The acquisition training procedure for both cocaine and nicotine consisted of ground food placed on the active lever until the lever-press response was developed and infusion criteria were met. For cocaine acquisition, 3 free infusions were given at the beginning of the session for rats. Once rats met criteria of the maximum of 40 self-administered cocaine infusions in a 6 h session over 2 days, the ground food and free infusions were stopped, session length for cocaine was shortened to 2 h, and the maximum number of infusions was increased to 500/session. Rats were considered to have acquired cocaine self-administration once they reached the criterion of 20+ infusions/session in the absence of food and drug primes. For nicotine acquisition, ground food was placed on the lever until rats self-administered 8 infusions/h over 2 days. Rats were considered to have acquired nicotine self-administration once they reached the criterion of 5+ infusions/session in the absence of food and drug primes. If rats did not meet either cocaine or nicotine criteria within 30 days, they were designated as “did not acquire” and did not continue. The maintenance phase followed acquisition, using the same criteria (e.g. maintenance days consisted of 20 or more infusions for cocaine self-administration and 5 or more infusions for nicotine self-administration).
Statistical Analysis
The dependent measures for acquisition were the percentage of rats that met acquisition requirements and for the rats that acquired, the number of days to meet acquisition criteria. During the 5 maintenance days, the dependent measures were total infusions and activity (measured by inactive lever-presses). The Breslow-Gehan-Wilcoxon survival analysis and rank statistics were used to analyze the rate of acquisition and the percentage of female and male rats acquiring nicotine or cocaine self-administration. A two-way repeated measure ANOVA with sex and days as the variables was conducted to examine differences in activity, drug lever-responding and total number of infusions. A two-proportion z-test was used to compare percentages of rats acquiring. Rats that did not complete all 5 maintenance days were not included in the analysis of infusion numbers. A Pearson correlation between number of days to reach acquisition and average number of infusions during the first 5 maintenance sessions was run to analyze the relationship between acquisition and maintenance. All means are reported as ± standard error of the mean (SEM). An alpha value of p < 0.05 was used to indicate significance.
Results
Cocaine Self-Administration
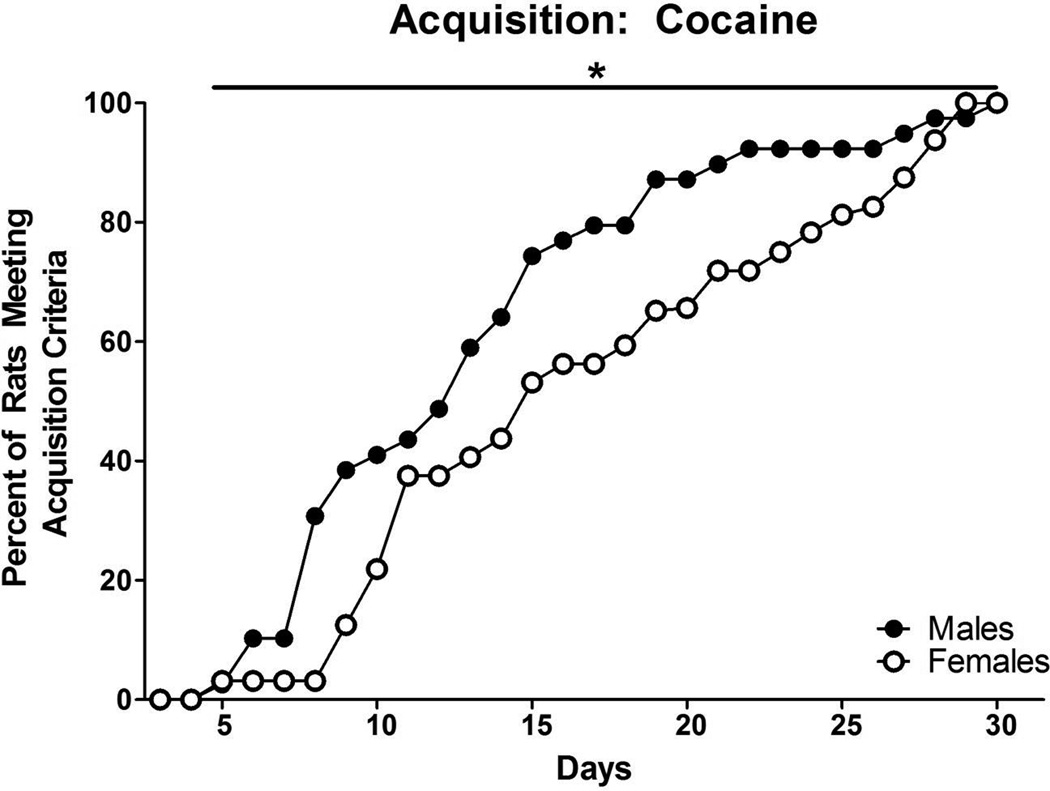
Sex differences were found on all measures for cocaine and are illustrated in Figure 1. Survival analyses indicated that males acquired cocaine self-administration faster than female rats; it took on average 13 (±1.01) days for males to acquire and 17 (±1.26) days for females [χ2 (1, 71) = 4.180, p<0.05]. Additionally, more males (75.0%) than females (59.3%) acquired cocaine self-administration (z = 1.835, p<0.05).
Figure 1.
Acquisition of cocaine self-administration in males and females. Data represent percent of rats acquiring self-administration on each day of the 30-day criterion period (starting at day 3, the first day acquisition was possible based on training). Males acquired cocaine self-administration in fewer days than females. * denotes a significant sex difference (p<0.05)
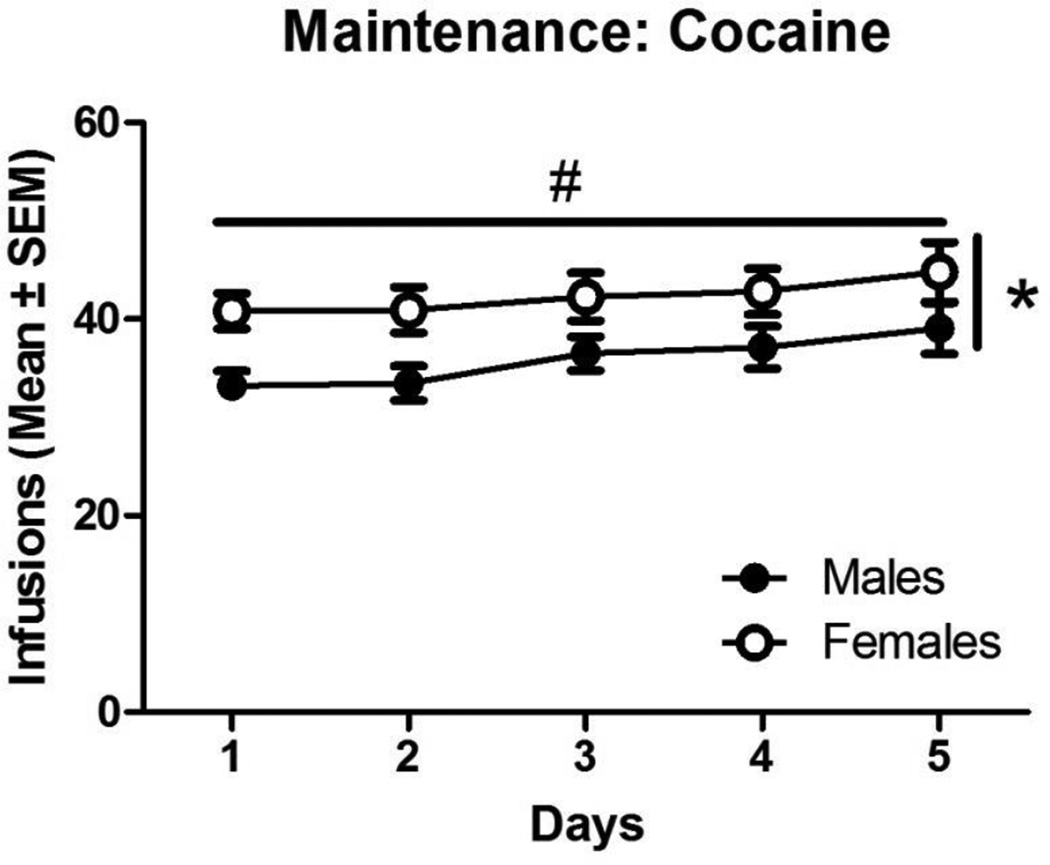
Sex differences in total number of infusions over the first 5 days of maintenance were observed and are illustrated in Figure 2. There was a main effect of sex [F(1,69) = 7.67, p<0.01], and a main effect of time [F(4,276) = 3.25, p<0.05] on the number of cocaine infusions, but there was no significant interaction. Post hoc analyses revealed that females self-administered more cocaine infusions than males (42.3±0.7 and 35.9±1.1 respectively). Number of infusions increased over days in both groups.
Figure 2.
Number of infusions for cocaine self-administration in males and females for the 2 hr sessions. Data represent means ± SEM during the 5-day criterion period. Females self-administered more cocaine than males. # denotes a significant effect of time and * denotes a significant sex difference (p<0.05)
Nicotine Self-administration
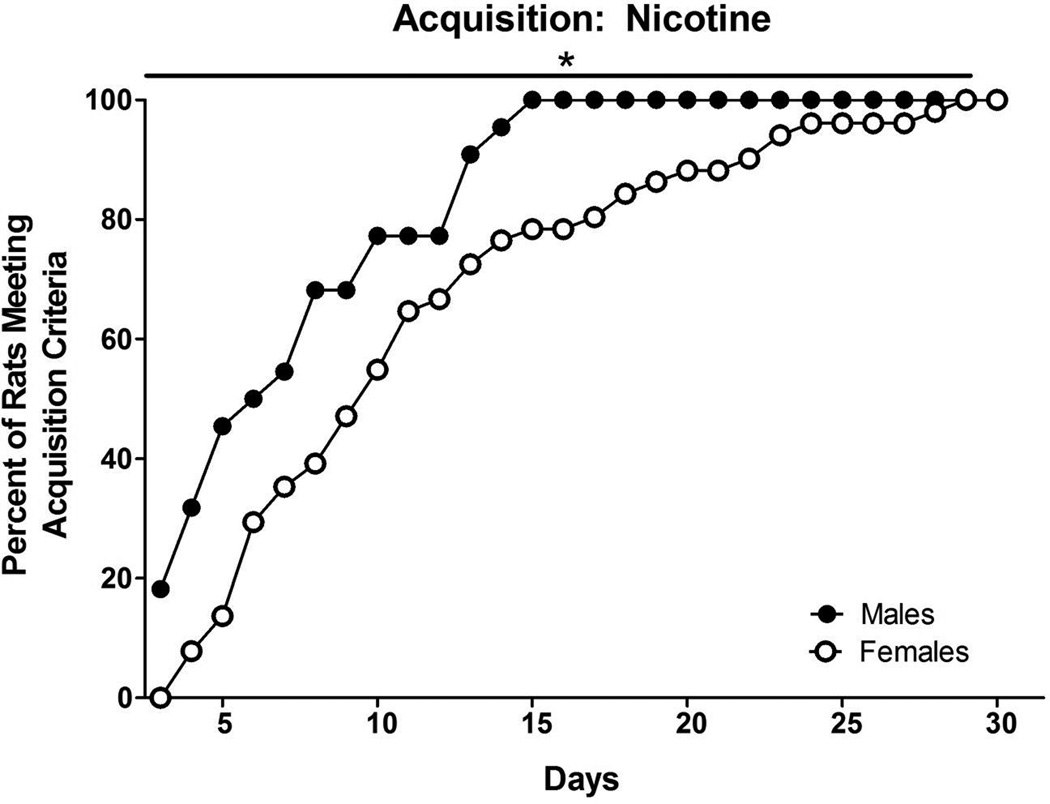
Survival analyses indicated differences between males and females on rates of acquisition and percentage of rats acquiring. Figure 3 shows that males acquired nicotine self-administration faster than females and more males than females reached the acquisition criteria within the 30 days [χ2 (1, 73) = 9.463, p<0.01].
Figure 3.
Acquisition of nicotine self-administration in males and females. Data represent percent of rats acquiring self-administration on each day of the 30-day criterion period (starting at day 3, the first day acquisition was possible based on training). Males acquired nicotine self-administration in fewer days than females. * denotes a significant sex difference (p<0.01)
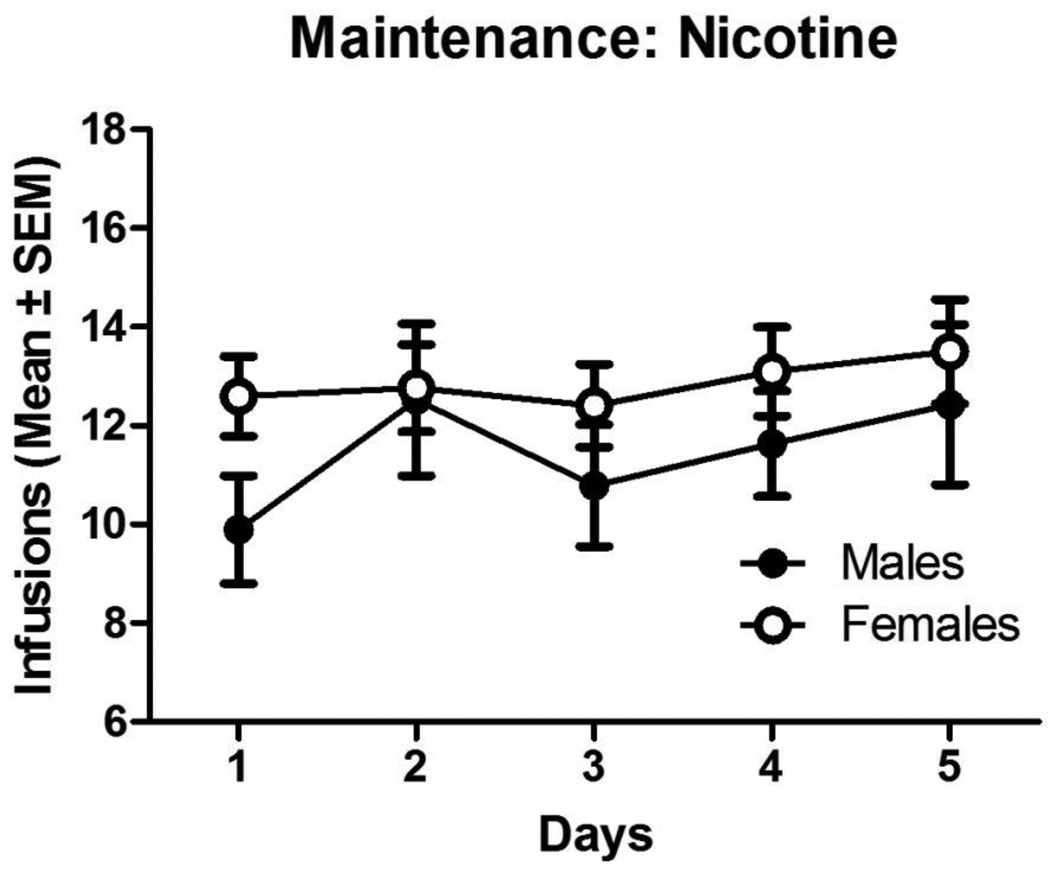
Males acquired nicotine self-administration in a mean of 7 (±0.86) days and females acquired in a mean of 11 (±0.91) days (t(71) = 2.647, p<0.05) and more males (78.6%) than females (60.5%) acquired nicotine self-administration (z=1.90, p<0.05). Figure 4 illustrates total number of infusions over the first 5 days of maintenance for nicotine self-administration. A two-way ANOVA revealed that there was no significant difference in total number of infusions during maintenance and no interaction (ps>0.05).
Figure 4.
Number of infusions for nicotine self-administration in males and females for the 2 hr sessions. Data represent means ± SEM during the 5-day criterion period. There were no significant differences between males and females.
There were no main effects of sex on activity, no differences over days, and no interactions for both nicotine and cocaine self-administration (ps>0.05, data not shown). Overall, inactive lever presses were relatively low. This suggests that any significant differences in acquisition rate or number of infusions are unlikely to be explained by activity.
A Pearson correlation to analyze the relationship between acquisition and maintenance showed no significant correlation between the two phases for cocaine in either males or females (ps>0.05; data not shown). There was no significant correlation between acquisition and maintenance for nicotine self-administration in males (p>0.05), but there was a significant negative relationship in females (r=−0.41, p=0.007; data not shown), suggesting that the longer it took females to acquire nicotine self-administration, the less females administered.
Discussion
This study examined sex differences in acquisition and maintenance of self-administration of two commonly used stimulants, cocaine and nicotine. Significantly more males than females acquired both cocaine and nicotine self-administration according to our 30-day acquisition criteria. In addition, males took fewer days than females to reach criteria for acquisition of cocaine and nicotine. In contrast, once rats acquired cocaine self-administration, mean infusions over the first 5 days of maintenance were significantly higher in females than males. It is unlikely that the differences between males and females during cocaine maintenance can be explained by differences in general activity, as males and females did not differ on this measure. The only correlation between the number of days to acquire self-administration and total maintenance infusions was a negative association seen in females self-administering nicotine, suggesting that there is no strong relationship between the time needed to acquire drug use and general drug-taking behaviors.
Acquisition of Cocaine Self-Administration
Males reached the cocaine acquisition criteria faster, and a greater percentage of the males met criteria than females. Previous studies generally show a faster rate of acquisition in female rats than males (Hu et al. 2004; Lynch and Carroll 1999; for review, see Anker and Carroll 2011), but another study reported that male rats acquired cocaine self-administration more rapidly than females (Caine et al. 2004; Kosten et al. 2004). These differences may be based on dose or the stability criteria used (Kosten and Zhang 2008). At higher doses, male rats acquired self-administration faster and more reliably than females (Kosten et al. 2004). Other variables, such as differences in the number of infusions to reach criteria, could explain variability in sex differences across studies. While the dose used in the present study (0.4 mg/kg cocaine) was higher than that used in the closest corresponding study (0.2 mg/kg; Lynch and Carroll 1999), it is not known whether dose explains the contrary finding of faster female acquisition in Lynch and Carroll (1999) versus faster acquisition in the males in the present study. Dose-dependent findings have been self-reported for measures of euphoria; whereby, a complex interaction occurred between dose and menstrual cycle phase in women (Evans et al. 2002). Given that dose seems to influence responses to cocaine in both rodents and humans, further work on sex differences in the initiation and maintenance of drug-taking is warranted.
Maintenance of Cocaine Self-Administration
During the maintenance phase, the finding that females had more cocaine infusions than males is consistent with rodent studies indicating that females have higher drug self-administration levels than males with a variety of drug classes, such as stimulants (Lynch 2008; Roth and Carroll 2004; Donny et al. 2000; Heppner et al. 1986), opioids (Cicero et al. 2003; Klein et al. 1997; Alexander et al. 1978; Hadaway et al. 1979), cannabinoids (Fattore et al. 2009, 2007) and sedatives (Lancaster and Spiegel 1992). Results are mixed, however, for cocaine maintenance, with a few studies showing no sex-specific effects (Perry et al. 2013; Kosten and Zhang 2008; Jackson et al. 2006; Cosgrove et al. 2002; Roberts et al. 1989). Furthermore, the studies that report sex differences show more infusions earned in females than males (Anker et al. 2011; Cummings et al. 2011; Lynch and Carroll 1999; Morse et al. 1993). The present finding in rats is also consistent with reports that women are more likely to experience higher rates of cocaine use (see Fattore et al. 2007).
While the majority of the work on sex differences has been done in extended access 6 h sessions (Carroll et al. 2002; Campbell et al. 2002; Lynch et al. 2000; Lynch and Carroll 1999; Roberts et al. 1989), the present study showed that shorter access (e.g. 2 h sessions) also resulted in sex differences, with females self-administering more cocaine than males. However, in previous studies, no sex differences were found under short access conditions (Fuchs et al. 2005; Roth and Carroll 2004; Caine et al. 2004; Roberts et al. 1989). A higher dose as well as a larger sample size in the present study might have contributed to the effect seen here with 2-h sessions.
Acquisition of Nicotine Self-Administration
Males acquired nicotine self-administration quicker and with a greater percentage of rats per group than female rats. These results are identical to those found with cocaine, but they are contrary to a few previous studies indicating sex differences in nicotine acquisition (Lynch 2009; Rezvani et al. 2008; Chaudhri et al. 2005; Donny et al. 2000). When the percentage of rats acquiring was examined, no difference were found between males and females at relatively higher doses. However, in a separate study, at a lower dose, a significantly higher percentage of females acquired (Lynch 2009; Donny et al. 2000). In the Lynch (2009) study, males and females took a similar amount of time to acquire nicotine self-administration at a lower dose (5 µg/kg), but no differences were found at a higher infusion dose (10 µg/kg). Significant differences were seen at the lowest dose used in Donny et al. (2000) as well.
Unlike the previously discussed studies, the present study did not employ food training prior to nicotine self-administration, and that factor may underlie the differences in acquisition seen in the present study compared to previous work. The effects of prior food-training on acquisition may be reflected in the number of days to reach acquisition. For example, with prior food training, Lynch (2009) found that rats acquired nicotine self-administration in 2–4 days, while, according to our protocol, rats were only able to reach acquisition on Day 3. On average, the rats in the present work took 7–11 days to reach acquisition. Clemens et al. (2010) found that prior food-training significantly accelerated acquisition of nicotine self-administration but had a relatively weak effect on nicotine-reinforced responding following the acquisition period. A possible explanation is that food-training prior to acquisition may differentially potentiate acquisition in males vs. females while not altering total amount of drug administered. This could explain the faster acquisition rate that was observed in males in the present study, which is contrary to the previous acquisition work (Lynch 2009; Rezvani et al. 2008; Chaudhri et al. 2005; Donny et al. 2000), with the higher infusion rates in females during maintenance. While food training may lead to faster acquisition of self-administration in rodents, this prior training may attenuate the face validity of this model. The present findings indicate that food training may accelerate sex differences in the acquisition of nicotine and cocaine with potentially differential effects in males and females, and these results suggest future research examining the impact of food-training on the acquisition of drug self-administration.
One additional aspect contributing to sex differences in acquisition and maintenance of self-administration is the visual stimuli that were coupled with the infusion. In the present research on nicotine self-administration, an active lever-press produced both an infusion and an associated cue while an inactive lever-press had no consequence. This is contrary to the cocaine self-administration procedure in which both levers produced a cue. Cues play a significant role in developing and maintaining nicotine self-administration, and females are more sensitive to the nicotine-related stimuli or cues than males (Donny et al. 2003; Chaudhri et al. 2005). If the presence of cues on the active lever or absence of cues on the inactive lever were to be a significant contributor to differences between this research and others, it would be expected that females would acquire self-administration faster and would self-administer more nicotine overall. As this is contrary to the present findings, it is relatively unlikely that non-pharmacological stimuli contributed to these results.
Several investigators have identified a number of behavioral and neurobiological factors that contribute to sex differences in acquisition. Behavioral factors such as dose of drug, length of access, and even pair-housing (Westenbroek et al. 2013) or prior access to sweets (Cason and Grigson 2013) may be integral to the development of sex differences in cocaine acquisition. A biological basis for cocaine acquisition has been suggested by Hu and colleagues (2004). Neurobiological mechanisms may also be implicated in the development of drug-taking behavior, such as gonadal hormones (Perry et al. 2013; Lynch 2008, 2009; Hu et al. 2004), and stressors such as neonatal isolation (Kosten et al. 2004). However, factors involved in sex differences in the acquisition of nicotine self-administration have been far less studied. The present results suggest that factors such as dose and prior food-training could differentially affect acquisition of nicotine self-administration in males and females.
Maintenance of Nicotine Self-Administration
In comparison to acquisition, total nicotine infusions did not significantly differ between males and females. Previous studies showed that females self-administered significantly more nicotine and had significantly higher breakpoints on a progressive-ratio schedule than males, suggesting that females are more sensitive to the reinforcing effects of nicotine than males (Rezvani et al. 2008; Chaudhri et al. 2005; Donny et al. 2000; but see Lynch 2009). However, sex differences in these studies were highly dose-dependent. One study showed significant differences only at the lowest dose of 0.02 mg/kg (Donny et al. 2000), and another showed that females self-administered more nicotine than males at higher doses (0.06 and 0.15 mg/kg; Chaudhri et al. 2005). At the dose used in the present work (0.03 mg/kg), our findings are consistent with nicotine self-administration seen by Donny and colleagues (Donny et al. 2000; Chaudhri et al. 2005).
Conclusion
This study showed sex differences in acquisition and maintenance of self-administration of two commonly used stimulants, nicotine and cocaine. Males acquired self-administration of both drugs faster than females, and a higher percentage of males acquired self-administration of both drugs, suggesting that sex-specific prevention efforts relative to both cocaine and nicotine addiction may be useful. Once acquired, female rats self-administered more cocaine than males during maintenance. However, with nicotine, males and females had similar levels of drug use during the maintenance phase. These results suggest that differences between sexes and types of drugs were important factors during transition phases of addiction for both drugs, such as acquisition and early maintenance. However, sex differences in acquisition did not transfer to similar sex differences in the maintenance phase, as sex-specific effects in cocaine use were contrary dependent on the phase and sex differences seen in acquisition of nicotine use were eliminated during maintenance. The finding that females exceeded males in the early maintenance phase of cocaine use is consistent with other phases of addiction (e.g. escalation and relapse; for review, see Carroll and Anker 2011). Taken together, these findings suggest a sex-specific vulnerability to different phases of addiction that may vary with the drug self-administered.
Acknowledgements
The authors are grateful to Dr. Natalie Zlebnik and Heather Veglahn for their assistance with data collection. This study was supported by NIH/NIDA P50 DA033942 (MEC) and NIDA training grant T32 DA007097 (JRS; Dr. Thomas Molitor, PI).
References
- Alexander BK, Coambs RB, Hadaway PF. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology. 1978;58(2):175–179. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Biological Basis of Sex Differences in Psychopharmacology. Berlin Heidelberg: Springer; 2011. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones; pp. 73–96. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Navin SF, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology. 2011;215(4):785–799. doi: 10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobeh Rev. 2010;35(2):315–333. doi: 10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15(5):472. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3(1):1–35. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacol. 2004;29(5):929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Dep. 2002;66(1):61–69. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Animal Models of Drug Addiction. Humana Press; 2011. Acquisition of drug self-administration; pp. 237–265. [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58(1):44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161(3):304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Cason AM, Grigson PS. Prior access to a sweet is more protective against cocaine self- administration in female rats than in male rats. Physiol Behav. 2013;112:96–103. doi: 10.1016/j.physbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2014;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology. 2005;180(2):258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74(3):541–549. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caillé S, Cador M. The effects of response operandum and prior food training on intravenous nicotine self-administration in rats. Psychopharmacology. 2010;211(1):43–54. doi: 10.1007/s00213-010-1866-z. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73(3):663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2011;2(3) doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacol. 2014;39(6):1431–1440. doi: 10.1038/npp.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, Coleman M, Wood C. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob Control. 2002;11(3):228–235. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169(1):68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Fattore L, Melis M, Fadda P, Fratta W. Sex differences in addictive disorders. Front Neuroendocrinol. 2014;35(3):272–284. doi: 10.1016/j.yfrne.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinol. 2009;34:227–236. doi: 10.1016/j.psyneuen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Women’s Health. 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Brit J Pharmacol. 2007;152(5):795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Dep. 2012;121(3):240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179(3):662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav. 2013;114:70–81. doi: 10.1016/j.pbb.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AL, Peters RH. Development of substance abuse problems among drug-involved offenders: Evidence for the telescoping effect. J Subst Abuse. 2000;12(3):241–253. doi: 10.1016/s0899-3289(00)00053-5. [DOI] [PubMed] [Google Scholar]
- Hadaway PF, Alexander BK, Coambs RB, Beyerstein B. The effect of housing and gender on preference for morphine-sucrose solutions in rats. Psychopharmacology. 1979;66(1):87–91. doi: 10.1007/BF00431995. [DOI] [PubMed] [Google Scholar]
- Harris AC, Burroughs D, Pentel PR, LeSage MG. Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharmacol. 2008;16(1):86. doi: 10.1037/1064-1297.16.1.86. [DOI] [PubMed] [Google Scholar]
- Heppner CC, Kemble ED, Cox WM. Effects of food deprivation on caffeine consumption in male and female rats. Pharmacol Biochem Behav. 1986;24(6):1555–1559. doi: 10.1016/0091-3057(86)90484-3. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacol. 2004;29(1):81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacol. 2006;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Cohen JE, Delnevo CD, Giovino GA. Dispelling myths about gender differences in smoking cessation: population data from the USA, Canada and Britain. Tob Control. 2013;22(5):356–360. doi: 10.1136/tobaccocontrol-2011-050279. [DOI] [PubMed] [Google Scholar]
- Klein LC, Popke EJ, Grunberg NE. Sex differences in effects of predictable and unpredictable footshock on fentanyl self-administration in rats. Exp Clin Psychopharmacol. 1997;5(2):99. doi: 10.1037//1064-1297.5.2.99. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY. Sex differences in non-reinforced responding for cocaine. Am J Drug Alcohol Abuse. 2008;34(4):473–488. doi: 10.1080/00952990802082206. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Zhang XY, Kehoe P. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav Brain Res. 2004;151(1):137–149. doi: 10.1016/j.bbr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10(1):63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9(5):415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Lozano OM, Domingo-Salvany A, Martinez-Alonso M, Brugal MT, Alonso J, de la Fuente L ITINERE Investigators. Health-related quality of life in young cocaine users and associated factors. Qual Life Res. 2008;17(7):977–985. doi: 10.1007/s11136-008-9376-8. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197(2):237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152(2):132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers—implications for treatment and prognosis. Am J Addict. 1999;8(4):300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- Morse AC, Erwin VG, Jones BC. Strain and housing affect cocaine self-selection and open-field locomotor activity in mice. Pharmacol Biochem Behav. 1993;45(4):905–912. doi: 10.1016/0091-3057(93)90138-j. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the Care and Use of Laboratory Animals. 8th. Washington, DC: The National Academic Press; 2011. [Google Scholar]
- Perkins KA. The motivational impact of nicotine and its role in tobacco use. Springer US; 2009. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking; pp. 143–169. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. CNS Drugs. 2001;15(5):391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. Impact of pubertal and adult estradiol treatments on cocaine self-administration. Horm Beh. 2013;64(4):573–578. doi: 10.1016/j.yhbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology. 2014;31(13):2661–2670. doi: 10.1007/s00213-014-3437-1. [DOI] [PubMed] [Google Scholar]
- Pogun S, Yararbas G. Nicotine Psychopharmacology. Berlin Heidelberg: Springer; 2009. Sex differences in nicotine action; pp. 261–291. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Influence of sex differences and gonadal hormones on cocaine addiction. ILAR Journal. 2012;53(1):14–22. doi: 10.1093/ilar.53.1.14. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, Horton K, Johnson M, Levin ED. Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 2008;154(3):885–897. doi: 10.1016/j.neuroscience.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Bennett S, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98(3):408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology. 2004;172(4):443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Kranzler HR, Gelernter J. Rate of progression from first use to dependence on cocaine or opioids: A cross-substance examination of associated demographic, psychiatric, and childhood risk factors. Addict Behav. 2014;39(2):473–479. doi: 10.1016/j.addbeh.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Kasza KA, Hyland A, Fong GT, Borland R, Brady K, Carpenter MJ, Hartwell K, Cummings KM, McKee SA. Gender differences in medication use and cigarette smoking cessation: results from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2015;17(4):463–472. doi: 10.1093/ntr/ntu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron I. Patterns and causes of gender differences in smoking. Soc Sci Med. 1991;32(9):989–1005. doi: 10.1016/0277-9536(91)90157-8. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Perry AN, Becker JB. Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behav Brain Res. 2013;252:68–71. doi: 10.1016/j.bbr.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]