Abstract
Vaccine-induced antibodies may wane more quickly in persons living with HIV than in healthy individuals. Here, we reviewed the literature on vaccines routinely recommended in HIV-infected patients to estimate how seroprotection decreases over time in those who initially responded to immunization. For each study retrieved from the literature, the decrease of seroprotection was modeled with a log binomial generalized linear model, and data were pooled in a meta-analysis in order to provide estimates of seroprotection two and five years after last vaccine administration. Our analyses confirmed that duration of seroprotection was shorter in HIV-infected patients, and that with current guidelines, a substantial proportion of patients would have lost protective antibodies before being proposed a booster. We therefore discuss the implications on the monitoring of antibody levels and timing of revaccination in these patients.
Introduction
Immune responses to most vaccines are known to be impaired in HIV patients [1,2]. However, besides primary response, long-term persistence of protection has been poorly documented. As of today, recommendations on the timing of booster injections were based on data collected in healthy persons although antibody decay patterns may be different. In this respect, an important question is to estimate, among patients who initially responded to immunization, how seroprotection decreases over time. Here, we reviewed data on long-term persistence of antibody concentrations after vaccination in HIV-infected patients. This choice was supported by three main reasons: (i) antibody concentrations are reported in most vaccine trials, providing enough data to allow meta-analysis, (ii) correlates of protections have been defined for most vaccines and (iii) antibody levels can be routinely assessed for most antigens with standardized methods. For some vaccines (i.e. measles, varicella, yellow fever), cell-mediated immunity is the critical determinant of protection, however methods of evaluation of cellular responses are not easily comparable between studies and correlates of protection not yet established. Our goal here was to provide a summary of available data to guide recommendations on revaccination in HIV-infected persons.
Methods
Search strategy and selection criteria
We searched the MEDLINE database for English-language articles up to January 2013 using Pubmed, without date restriction, using the terms “vaccine”, “antibodies”, “follow-up” “long-term”, “decline”, “duration”, and “HIV” (see search equation in the supplementary material). The review and meta-analysis were conducted according to the PRISMA guidelines. Studies were selected by one author (SK) according to the eligibility criteria: original experimental or observational studies on licensed vaccines in patients living with HIV, reporting measurements of antibody titers beyond 6 months after the last vaccine dose administration. Reports on influenza vaccines were excluded. The reference lists of all relevant articles were examined for additional data sources.
For each article, we abstracted the study design, vaccination scheme, sample size, follow-up duration and the percentage of ‘primary responders’ (patients who had mounted protective antibody titers after immunization) who remained seroprotected over time. Protective levels defining seroprotection were those reported by the authors and are detailed in Supplementary Information. Where relevant, the percentages of seroprotected patients were pooled in a meta-analysis. The meta-analysis was restricted to prospective studies and to vaccine antigens where at least two studies were available. No meta-analysis was undertook for pneumococcal vaccines since the specific antibody levels necessary for adequate protection against pneumococcal disease are not clearly defined, even in healthy persons [3].
Data analysis
To account for the great heterogeneity in follow-up times between the different studies, we first modelled for each study the decrease of seroprotection P(t), as a function of time since immunization, as P(t) = exp(−βt), where exp(−β) was the relative decrease in protection per additional time unit since immunization. Data were fit using log binomial generalized linear models. The predicted percentages of seroprotected individuals two and five years after immunization in each study were then pooled in a meta-analysis with random effects and presented in forest plots. All analyses were made using the R software version 2.13.2 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org).
Results
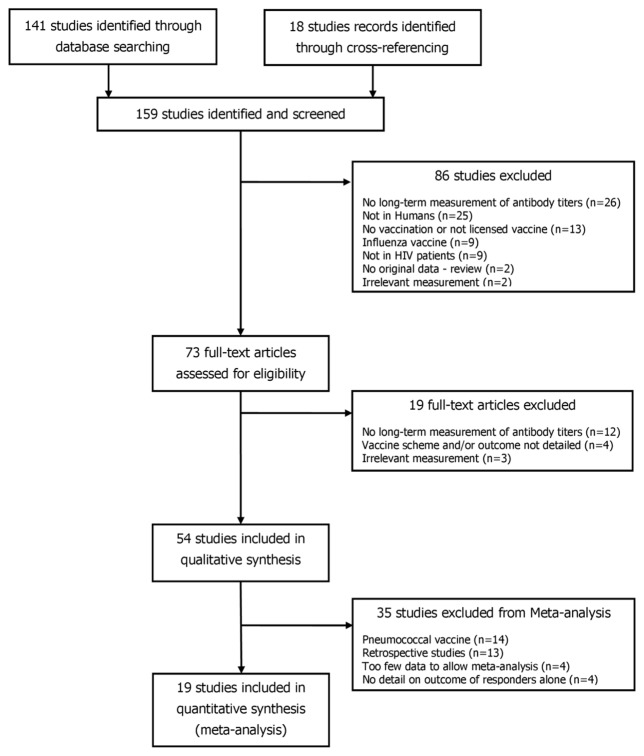
Of the 159 potentially relevant studies, 54 were selected for the qualitative review (figure 1).
Figure 1.
PRISMA flow diagram of the search strategy.
For each vaccine, percentages of seroprotection retrieved from the literature are presented in figure 2. Median follow-up ranged from nine months to nine years after the last vaccine dose. The median number of patients included per study was 40 (Interquartile range: 18; 63). Data were available for 13 vaccine antigens: Streptococcus pneumoniae (n=14), hepatitis B (n=12), measles (n=12), hepatitis A (n=5), tetanus (n=8), yellow fever (n=3), Haemophilus influenzae type b (n=3), rubella (n=2), varicella, (n=1), pertussis (n=1), polio virus (n=1), mumps (n=1), and Japanese encephalitis (n=1). Of the 54 studies included in the review, 19 fitted the eligibility criteria for meta-analysis. Others were excluded because they were on pneumococcal vaccine (n=14), were retrospective (n=13), did not differentiate outcome of primary responders and non-responders during follow-up (n=4), or because only one study was available for the vaccine (n=4: pertussis [4], Haemophilus influenzae type b [5], varicella [6], and Japanese encephalitis [7].
Figure 2. Data retrieved from the literature (2A–E) and graphical illustration of the statistical modeling for hepatitis B (2F).
Each symbol represents a percentage of individuals with protective antibody concentrations in relation with time (in years) elapsed since immunization, among those who initially responded to the vaccine, except for tetanus, where overall percentages of seroprotection are presented (responders and non-responders pooled together). The size of points is proportional to the sample sizes of studies. Symbols are colour-coded according to the vaccination scheme. Figure 2F shows the principle of modeling for hepatitis B vaccine: symbols represent the percentages of seroprotection retrieved from the literature and lines show extrapolations of the model at two and five years. Estimates of seroprotection (black symbols) are provided by the model at two and five years.
Hepatitis B
Twelve studies were included, with follow-up times ranging from 12 to 115 months [8–19]. As illustrated on figure 2A, seroprotection typically decreased over time: after 3 doses of 40μg HBsAg, 71% of primary responders maintained protective antibody titers at year one [8], 33%–61% at year two [8,10], and 40% at year five [10]. There was no clear trend of longer persistence of seroprotection with high-dose vaccine schemes [8,10]. Three retrospective studies reported data beyond five years after immunization [11,15,19] in HIV-infected children born to Ag HBs+ HIV-infected mothers, and maintenance of seroprotection was particularly poor: 24% after 5.5 years [11], 45% after 8 years [15], to only 1% after 9.6 years [19] after a three 10μg-doses scheme.
According to the meta-analysis, less than one half of primary responders would maintain protective antibody titers two years after immunization (38% (CI95% = 23%; 54%) in adults and 61% (27%; 90%) in children), and only 17% (CI95%: 3%; 36%) after five years (figures 3 and 4). Vaccine schemes with double-dosed schemes did not improve maintenance of seroprotection (compared to those with standard doses of vaccine (respectively 41% (CI 95% = 14%; 71%), and 50% (CI 95% = 24%; 77%) after two years, P-value=0.65).
Figure 3.
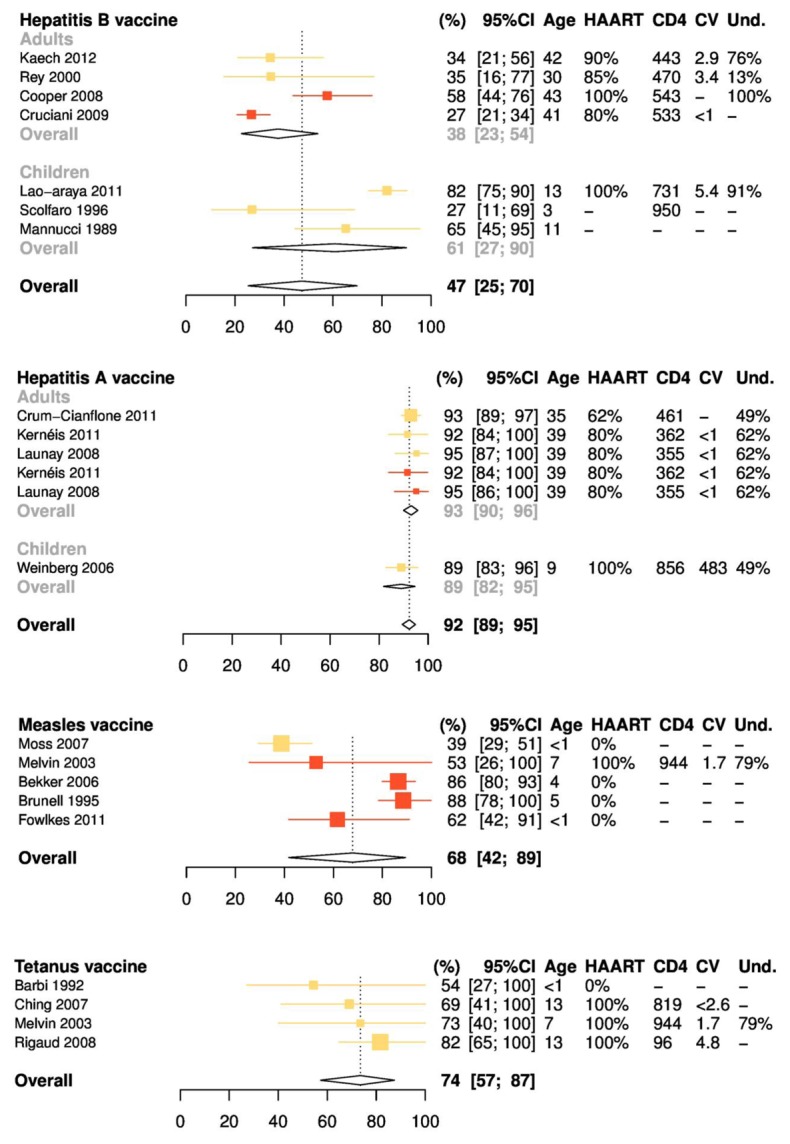
Meta-analysis of the percentages of seroprotection 2 years after the last vaccine dose, by vaccine antigen. Columns on right detail characteristics of study participants at the time of immunization, including mean or median age, percentage receiving highly active antiretroviral therapy (HAART), mean or median CD4 cell count (per microliter), mean or median human immunodeficiency virus (HIV) load (log copies per milliliter), and percentage with undetectable HIV loads. Colors represent vaccination protocols. For hepatitis B, yellow symbols represent 3 intramuscular 10-μg doses (Lao-Araya et al [13] and Scolfaro et al [18]) or 3 subcutaneous 5-μg doses (Mannucci et al [14]) in children or 3 intramuscular 20-μg doses in adults (Kaech et al [12] and Rey et al [17]). Red symbols represent 3 intramuscular 40-μg doses (Cooper et al [10] and Cruciani et al [8]). Heterogeneity: Q = 121.9; P < .001; I2 = 95% [92%–97%]. For hepatitis A, yellow symbols represent 1 dose (Weinberg et al [24], Crum-Cianflone et al [21], Kernéis et al [22], and Launay et al [23]); red symbols, 2 doses (Kernéis et al [22] and Launay et al [23]). Heterogeneity: Q = 2.9; P = .71; I2 = 0% [0%–57%]. For measles, yellow symbols represent 1 dose (Moss et al [31]); red symbols, 2 doses (Melvin and Mohan [30], Bekker et al [26], Brunell et al [27], and Fowlkes et al [29]). Heterogeneity: Q = 25.6; P < .001; I2 = 84% [65%–93%]. For tetanus, yellow symbols represent 2–5 doses plus boosters, according to local practices. Heterogeneity: Q = 2.5; P = .48; I2 = 0% [0%–81.3%].
Figure 4.
Meta-analysis of the percentages of seroprotection 5 years after the last vaccine dose, by vaccine antigen. Columns on right detail characteristics of study participants at the time of immunization, including mean or median age, percentage receiving highly active antiretroviral therapy (HAART), mean or median CD4 cell count (per microliter), mean or median human immunodeficiency virus (HIV) load (log copies per milliliter), and percentage with undetectable HIV loads. Colors represent vaccination protocols. For hepatitis B, yellow symbols represent 3 intramuscular 10-μg doses (Lao-Araya et al [13] and Scolfaro et al [18]) or 3 subcutaneous 5-μg doses (Mannucci et al [14]) in children or 3 intramuscular 20-μg doses in adults (Kaech et al [12] and Rey et al [17]). Red symbols represent 3 intramuscular 40-μg doses (Cooper et al [10] and Cruciani et al [8]). Heterogeneity: Q = 87.7; P < .001; I2 = 93% [88%–96%]. For hepatitis A, yellow symbols represent 1 dose (Weinberg et al [24], Crum-Cianflone et al [21], Kernéis et al [22], and Launay et al [23]); red symbols, 2 doses (Kernéis et al [22] and Launay et al [23]). Heterogeneity : Q = 6.7; P = .25; I2 = 25% [0%–69%]. For measles, yellow symbols represent 1 dose (Moss et al [31]); red symbols, 2 doses (Melvin and Mohan [30], Bekker et al [26], Brunell et al [27], and Fowlkes et al [29]). Heterogeneity: Q = 47.8; P < .001; I2 = 92% [84%–96%]. For tetanus, yellow symbols represent 2–5 doses plus boosters, according to local practices. Heterogeneity : Q = 4.5; P = .22; I2 = 33% [0%–76%].
Hepatitis A
One retrospective [20] and four prospective studies [21–24] were identified on hepatitis A. In adults, a slight decrease was observed over time (figure 2B): 100% of responders were still seroprotected after one year [23], 90% after three years [21], and 85% after four years [22]. No data were reported beyond four years. In children, persistence of responses was lower: 85% (n=84) after 9 months [20] and 92% after 18 months [24]. Percentages of seroprotection were not greater with three-dose schedules [22,23], as compared to standard two-dose schedule [21–24]. The pooled percentages of seroprotection against hepatitis A were of 92% (CI 95% = 89%; 95%) two years after vaccination, and 82% (CI 95% = 76%; 88%) after five years (figures 3 and 4).
Measles, Mumps and Rubella
Data on long-term persistence of measles immunity were reported in twelve studies, either in children (n=10) [25–34] or adults (n=2) [35,36]. As illustrated on figure 2C, percentages of seroprotection varied widely between studies, reflecting the great heterogeneity in the populations studied. In HIV vertically-infected Sub-saharian African children not on HAART and vaccinated in the first months of life, percentages of seroprotection rapidly dropped during follow-up: 62%–73% of primary responders were still protected after 15 months [29,31], 41%–49% after 28 months [25,31], and 30% after 50 months [28]. HAART improved seroprotection, either for children started on HAART after immunization (69% after six months of HAART [28] 40–60% after four to seven years of HAART [26, 34]) or those who were vaccinated or revaccinated after the start of HAART (73% after one year [30], 40–82% after four years [32]). In this last study, higher percentages of seroprotection were reported four years after vaccination in children started on HAART in their first year of life (82%) compared to others (40%) [32].
In adults, data were scarce and showed conflicting results. In adults vaccinated during childhood before HIV infection, at the median age of 35 years, 95% still had protective antibody titers (n=59) [36], whereas after revaccination of 26 HIV-infected adults at the age of 31 years, only 43% were still above the detection level after one year [35].
Because of great heterogeneity, the meta-analysis was restricted to five studies focusing on HIV vertically-infected children [26,27,29–31], immunized at the age of 6–42 months, with either one [31] or two doses [26,27,29,30] of the MMR vaccine. The pooled analysis estimated that in this particular population, protective antibody levels would persist in 68% (CI 95% = 45%; 88%) of primary responders after two years, and 40% (CI 95% = 10%; 73%) after five years (figure 3C).
Only three studies reported data on rubella [26,34] and mumps [26]. After four years on HAART, antibodies persisted in 62% of children against mumps and 27–89% against rubella, the highest seroprotection rates being reported for children with virological response.
Tetanus, Poliomyelitis and Pertussis
Studies on tetanus included 241 children followed from 12 to 53 months after immunization (figure 2E) [28,30,32–34,37–39]. Retrospective studies accounted for 76% of participants (n=182). Percentages of seroprotection were high: 67–90% within the first two years [30,33,34,37–39], 78% after 4 years [28]. Meta-analysis of the four prospective studies estimated overall percentages of seroprotection (figure 3D) of 74% (CI95% = 57%; 87%) and 43% (CI95% = 21%; 66%) two and five years after immunization, respectively. One study reported data on immunization against polio virus [37], one against pertussis [4], and one against diphtheria [34]. In all reports, duration of seroprotection was shorter than in healthy children.
Streptococcus pneumoniae
All 14 studies on persistence of antibodies against S. pneumoniae were prospective, either in children (n=5) [40–44] or adults (n=9) [45–53] and either on a conjugate vaccine (PCV, n=6) [40–44,49] or the 23-valent polysaccharide vaccine (PPSV23, n=9) [45–48,50–53]. Follow-up ranged from 8 to 60 months after immunization (median: 12). The median number of patients was 33. The definition of response varied greatly between studies: either a two-fold and greater increase in antibody titers [47,49], or an antibody concentration above either 0.35 μg/mL [42,43,47], 0.5, μg/mL [40], or 1 μg/mL [40,41,48,49,52]) or on the GMTs alone [44–47, 49, 50]. After PPSV23 in adults, rates of decrease of antibody concentrations were either similar [45,48,52,53] or more rapid [46,50] to that observed in healthy individuals. Beyond five years, [47,48], antibody concentrations had dropped below the cut-off values for most serotypes. This was reportedly more frequent in patients with low CD4 cell counts at vaccination or those who failed to achieve virological suppression [47]. Vaccination with PCV was not directly compared with PPSV23 in adults, but two doses of 7-valent PCV elicited more sustained responses than one during the one year-follow-up [49].
In HIV-infected children, the immunogenicity of PCV has been assessed from eight months to five years after primary immunization [40–44]. Here again, results were variable: HIV-infected children reportedly experienced a significantly greater decline of antibody levels during follow-up than HIV-uninfected children [42–44]; however with a vaccine scheme including two PCVs and one PPSV23 at 8-weeks intervals, Abzug et al. showed that 22 months after immunization, decline in antibody concentrations were similar to those in HIV-uninfected infants after PCV alone [40].
Other antigens
After immunization against Haemophilus influenzae b, estimates of seroprotection ranged from 16% to 78% [5,30,54] in vertically HIV-infected children at different time points after immunization. In the only report on varicella vaccine, less than 50% of 94 HIV-infected children (aged 1–8 years) still had detectable VZV IgG one year after the start of immunization [6]. For yellow fever, three retrospective studies [55–57] suggested that immunogenicity of YF immunization waned more quickly in HIV patients: the proportion of patients with non-reactive titers increasing from 17% to 23% over the ten years-period post-vaccination [57]. In the single study on an inactivated Japanese encephalitis vaccine in 50 HIV-infected children on HAART, only 4/38 primary responders lost protective antibodies during the three-year follow-up, suggesting long-term benefit of the vaccination in this population [7].
Discussion
Duration of seroprotection, as assessed by quantitative measurement of antibody responses, is shorter in HIV-infected patients than in otherwise healthy persons for most licensed vaccines. As a comparison, 65–95% of healthy children and 80% of adults maintain protective anti-HBs concentrations ten years after vaccination against hepatitis B. With inactivated hepatitis A vaccine, mathematical models estimate that protective levels of anti-HAV could be present for more than 25 years in adults and 14–20 years in children. Percentages of seroprotection reach 93–10% in healthy children ten years after tetanus vaccine and 95–100% after measles vaccine (see references in table 2 of the supplementary material).
Our analyses showed a rapid decrease in seroprotection after immunization in HIV-infected patients and have several implications:
Because of high prevalence and severity of chronic hepatitis B in HIV-infected patients, it appears that anti-HBs antibodies should be measured yearly in adults and every 2–5 years in children at risk (those in close contact with Ag HBs+ persons or living in a high endemicity country). Closer monitoring of anti-HBs titers could be considered in those with the lowest antibody titers at the end of the vaccination course (10 to 100 IU).
For persons at increased risk for hepatitis A (i.e. travelers, men who have sex with men, users of drugs, persons with occupational risk for HAV infection or chronic liver disease), anti-HAV antibodies should be monitored every five years since almost 20% of primary responders would have lost seroprotection by year five after immunization.
For tetanus, the meta-analysis of prospective studies found that around 75% of HIV-infected children would maintain protective concentrations after two years, and retrospective studies reported percentages of seroprotection around 70–80% after five years. In this respect, the interval of ten years between boosters seems reasonable.
To improve maintenance of seroprotection against measles, the initial vaccination scheme should include two doses, ideally administered after the start of HAART, in children with undetectable viral load. Children vaccinated before the start of HAART and/or with detectable HIV viral load at the time of immunization could be proposed a third dose 2–5 years after primary vaccination, when CD4-cell count is >200 or >15%.
The WHO recently stated that a single dose of yellow fever vaccine was sufficient to confer life-long immunity against the disease in immunocompetent persons. Data show that immunity wane more quickly in HIV-infected persons. In these patients, determination of antibody titers should be considered in case of potential exposure, and revaccination proposed to those with negative titers.
For now, data on immunogenicity of PCV or strategies combining PCV and PPSV23 are too preliminary to state on the optimal timing of booster injections in adults.
Several methodological issues warrant discussion. First, it is remarkable that the median number of patients per study was only 40. Data on immunogenicity of vaccines in the immunocompromised host are scarce and the small samples in each category gave little power for comparisons between age classes or vaccine schemes. Second, only prospective studies were included in the meta-analysis, which may have lead to a certain degree of underestimation of seroprotection. Indeed, as illustrated for tetanus, studies pooled in the meta-analysis were those with the shortest duration of follow-up and lower percentages of seroprotection. This choice was however essential to guarantee the quality of data analyzed. Conversely, relying on the exponential decrease could lead to overestimate the decrease in seroprotection. The choice of the model was supported by the good fit in studies where at least two follow-up points were available (see supplementary material). This model however leads by structure to an always decreasing trend in seroprotection with time, implying that all vaccinees will loose seroprotection at some point. An alternative assumption would be that some individuals would remain seroprotected permanently over time, as it has been hypothesized for example with hepatitis A vaccine. There was also great heterogeneity in some important variables that complicated comparisons across studies: immunologic status at the time of vaccination, use of antiretroviral therapy and whether treatment was started before or after vaccination, age, vaccine strain, assays used to measure antibody concentrations, antibody levels considered as protective, exposure to wild-type pathogens that could confound antibody measurements. The small samples gave little power for comparisons but several variables seemed to be associated with persistence of seroprotection. For example, like for immunocompetent persons, certain vaccine antigens such as rubella, tetanus and hepatitis A induce more sustained responses than others [58]. The viro-immunologic status and use of antiretroviral therapy at the time of immunization is also of critical importance in maintenance of seroprotection. As illustrated for measles, persistence of antibody concentrations was improved in children vaccinated or revaccinated while on HAART [30, 32], as compared to others [25, 26, 27, 28, 29, 31]. More generally, patients immunized while on HAART show prolonged seroprotection for most vaccine antigens, as illustrated in a recent review [59]. Similarly, HIV viral load at the time of immunization is an important independent predictor of persistence of seroprotection, as illustrated for hepatitis A [21, 22], yellow fever [55] and Pneumococcus vaccines [43]. On the other hand, whereas increasing the dose of antigen is an interesting strategy to improve responses in immuncompromised hosts [60], there was no clear trend in our meta-analysis indicating that this strategy would extend duration of seroprotection among primary responders.
Finally, antibody response is only one component of the immune response to vaccines. For some antigens (i.e. measles, varicella, yellow fever), cell-mediated immunity is the critical determinant of protection, and excluding quantification of CMI may lead to a mistaken conclusion that boosters are required for all vaccines. Indeed, loss of antibodies does not necessarily imply loss of clinical protection and immune memory can persist, even in the individuals with low antibody concentrations [61]. As recently underlined in a recent review: “a large variety of immune cells and functions are involved in controlling infections and any assignment of one as the mechanistic immune functions that contribute to protection (mCoP) must recognize that others may be involved as supplements or co-correlates of protection” [62]. In this respect, cellular responses seem to play a crucial role in the protection conferred by certain vaccines and our choice to exclude reports on CMI lead to a certain degree of imprecision in the evaluation of immune responses. Evaluation of CMI provide important data on the degree of protection at an individual level but methods of measurement are not routinely available and correlates of protection need to be established.
Clinical implications of this work need to be further explored on large prospective cohorts. In the future, the evaluation of new vaccines that specifically target persons with impaired immunity (inactivated or subunit zoster vaccine, cytomegalovirus vaccine) should confront clinical effectiveness with precise evaluation of the both humoral and cellular responses, in an attempt to establish reliable correlates of protection in these populations.
Key points.
This review and meta-analysis confirmed that duration of seroprotection conferred by most licensed vaccines was shorter in HIV-infected patients. Implications on the monitoring of antibody levels and timing of revaccination in these patients are therefore discussed.
Supplementary Material
Table 1: Data retrieved from studies included in the meta-analysis
Table 2: Selected reports on persistence of antibody levels after vaccination in healthy individuals
Acknowledgments
The authors thank Dr Béhazine Combadière for her very helpful comments on the manuscript, and the members of staff of the Saint Antoine-Axial University Library for their assistance on the literature search.
Funding
This work was supported by Sidaction [grant number BI20-1-01558] to S.K. The funding source had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributions
SK designed the study, performed the literature search, the statistical analysis, the interpretation of the data and drafted the manuscript. OL contributed to the design of the study, the interpretation of the data and to the drafting of the manuscript. CT contributed to the statistical analysis, the interpretation of the data and to the drafting of the manuscript. FB contributed to the interpretation of the data and to the drafting of the manuscript. TH contributed to the interpretation of the data and to the drafting of the manuscript. PYB designed the study, performed the statistical analysis, contributed to the interpretation of the data and the drafting of the manuscript.
Conflicts of interest
SK, CT, FB, and PYB declare no conflicts of interest.
OL, TH reported not having shares or paid employment with pharmaceutical companies, being investigators on studies sponsored by different pharmaceutical companies.
References
- 1.Geretti AM, Doyle T. Immunization for HIV-positive individuals. Curr Opin Infect Dis. 2010;23:32–38. doi: 10.1097/QCO.0b013e328334fec4. [DOI] [PubMed] [Google Scholar]
- 2.Abzug MJ. Vaccination in the immunocompromised child: a probe of immune reconstitution. Pediatr Infect Dis J. 2009;28:233–236. doi: 10.1097/INF.0b013e31819d31bc. [DOI] [PubMed] [Google Scholar]
- 3.Siber GR, Chang I, Baker S, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007;25:3816–3826. doi: 10.1016/j.vaccine.2007.01.119. [DOI] [PubMed] [Google Scholar]
- 4.Abzug MJ, Song L-Y, Fenton T, et al. Pertussis booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. Pediatrics. 2007;120:e1190–1202. doi: 10.1542/peds.2007-0729. [DOI] [PubMed] [Google Scholar]
- 5.Gibb D, Giacomelli A, Masters J, et al. Persistence of antibody responses to Haemophilus influenzae type b polysaccharide conjugate vaccine in children with vertically acquired human immunodeficiency virus infection. Pediatr Infect Dis J. 1996;15:1097–1101. doi: 10.1097/00006454-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Levin MJ, Gershon AA, Weinberg A, Song L-Y, Fentin T, Nowak B. Administration of live varicella vaccine to HIV-infected children with current or past significant depression of CD4(+) T cells. J Infect Dis. 2006;194:247–255. doi: 10.1086/505149. [DOI] [PubMed] [Google Scholar]
- 7.Puthanakit T, Aurpibul L, Yoksan S, Sirisanthana T, Sirisanthana V. A 3-year follow-up of antibody response in HIV-infected children with immune recovery vaccinated with inactivated Japanese encephalitis vaccine. Vaccine. 2010;28:5900–5902. doi: 10.1016/j.vaccine.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 8.Cruciani M, Mengoli C, Serpelloni G, et al. Serologic response to hepatitis B vaccine with high dose and increasing number of injections in HIV infected adult patients. Vaccine. 2009;27:17–22. doi: 10.1016/j.vaccine.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 9.Abzug MJ, Warshaw M, Rosenblatt HM, et al. Immunogenicity and immunologic memory after hepatitis B virus booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis. 2009;200:935–946. doi: 10.1086/605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper CL, Angel JB, Seguin I, Davis HL, Cameron DW. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin Infect Dis. 2008;46:1310–1314. doi: 10.1086/533467. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes SJ, Slhessarenko N, Souto FJD. Effects of vertical HIV infection on the persistence of anti-HBs after a schedule of three doses of recombinant hepatitis B vaccine. Vaccine. 2008;26:1032–1037. doi: 10.1016/j.vaccine.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Kaech C, Pache I, Bürgisser P, Elzi L, Darling KEA, Cavassini M. Immune response to hepatitis B vaccination in HIV-positive adults with isolated antibodies to HBV core antigen. J Infect. 2012;65:157–164. doi: 10.1016/j.jinf.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Lao-Araya M, Puthanakit T, Aurpibul L, Taecharoenkul S, Sirisanthana T, Sirisanthana V. Prevalence of protective level of hepatitis B antibody 3 years after revaccination in HIV-infected children on antiretroviral therapy. Vaccine. 2011;29:3977–3981. doi: 10.1016/j.vaccine.2011.03.077. [DOI] [PubMed] [Google Scholar]
- 14.Mannucci PM, Zanetti AR, Gringeri A, et al. Long-term immunogenicity of a plasma-derived hepatitis B vaccine in HIV seropositive and HIV seronegative hemophiliacs. Arch Intern Med. 1989;149:1333–1337. [PubMed] [Google Scholar]
- 15.Pessoa SD, Miyamoto M, Ono E, Gouvêa AFTB, de Moraes-Pinto MI, Succi RCM. Persistence of vaccine immunity against hepatitis B virus and response to revaccination in vertically HIV-infected adolescents on HAART. Vaccine. 2010;28:1606–1612. doi: 10.1016/j.vaccine.2009.11.045. [DOI] [PubMed] [Google Scholar]
- 16.Powis JE, Raboud J, Ostrowski M, Loutfy MR, Kovacs C, Walmsley SL. The recombinant hepatitis B surface antigen vaccine in persons with HIV: is seroconversion sufficient for long-term protection? J Infect Dis. 2012;205:1534–1538. doi: 10.1093/infdis/jis243. [DOI] [PubMed] [Google Scholar]
- 17.Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine. 2000;18:1161–1165. doi: 10.1016/s0264-410x(99)00389-8. [DOI] [PubMed] [Google Scholar]
- 18.Scolfaro C, Fiammengo P, Balbo L, Madon E, Tovo PA. Hepatitis B vaccination in HIV-1-infected children: double efficacy doubling the paediatric dose. AIDS. 1996;10:1169–1170. [PubMed] [Google Scholar]
- 19.Siriaksorn S, Puthanakit T, Sirisanthana T, Sirisanthana V. Prevalence of protective antibody against hepatitis B virus in HIV-infected children with immune recovery after highly active antiretroviral therapy. Vaccine. 2006;24:3095–3099. doi: 10.1016/j.vaccine.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 20.Siberry GK, Coller RJ, Henkle E, et al. Antibody response to hepatitis A immunization among human immunodeficiency virus-infected children and adolescents. Pediatr Infect Dis J. 2008;27:465–468. doi: 10.1097/INF.0b013e31816454a3. [DOI] [PubMed] [Google Scholar]
- 21.Crum-Cianflone NF, Wilkins K, Lee AW, et al. Long-term durability of immune responses after hepatitis A vaccination among HIV-infected adults. J Infect Dis. 2011;203:1815–1823. doi: 10.1093/infdis/jir180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kernéis S, Desaint C, Brichler S, et al. Long-term persistence of humoral immunity after hepatitis A vaccination in HIV-infected adults. J Acquir Immune Defic Syndr. 2011;57:e63–66. doi: 10.1097/QAI.0b013e31821fdec3. [DOI] [PubMed] [Google Scholar]
- 23.Launay O, Grabar S, Gordien E, et al. Immunological efficacy of a three-dose schedule of hepatitis A vaccine in HIV-infected adults: HEPAVAC study. J Acquir Immune Defic Syndr. 2008;49:272–275. doi: 10.1097/QAI.0b013e318183a9c0. [DOI] [PubMed] [Google Scholar]
- 24.Weinberg A, Gona P, Nachman SA, et al. Antibody responses to hepatitis A virus vaccine in HIV-infected children with evidence of immunologic reconstitution while receiving highly active antiretroviral therapy. J Infect Dis. 2006;193:302–311. doi: 10.1086/498979. [DOI] [PubMed] [Google Scholar]
- 25.al-Attar I, Reisman J, Muehlmann M, McIntosh K. Decline of measles antibody titers after immunization in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1995;14:149–151. [PubMed] [Google Scholar]
- 26.Bekker V, Scherpbier H, Pajkrt D, Jurriaans S, Zaaijer H, Kuijpers TW. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics. 2006;118:e315–322. doi: 10.1542/peds.2005-2616. [DOI] [PubMed] [Google Scholar]
- 27.Brunell PA, Vimal V, Sandu M, Courville TM, Daar E, Israele V. Abnormalities of measles antibody response in human immunodeficiency virus type 1 (HIV-1) infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:540–548. [PubMed] [Google Scholar]
- 28.Farquhar C, Wamalwa D, Selig S, et al. Immune responses to measles and tetanus vaccines among Kenyan human immunodeficiency virus type 1 (HIV-1)-infected children pre- and post-highly active antiretroviral therapy and revaccination. Pediatr Infect Dis J. 2009;28:295–299. doi: 10.1097/INF.0b013e3181903ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowlkes A, Witte D, Beeler J, et al. Persistence of vaccine-induced measles antibody beyond age 12 months: a comparison of response to one and two doses of Edmonston-Zagreb measles vaccine among HIV-infected and uninfected children in Malawi. J Infect Dis. 2011;204(Suppl 1):S149–157. doi: 10.1093/infdis/jir135. [DOI] [PubMed] [Google Scholar]
- 30.Melvin AJ, Mohan KM. Response to immunization with measles, tetanus, and Haemophilus influenzae type b vaccines in children who have human immunodeficiency virus type 1 infection and are treated with highly active antiretroviral therapy. Pediatrics. 2003;111:e641–644. doi: 10.1542/peds.111.6.e641. [DOI] [PubMed] [Google Scholar]
- 31.Moss WJ, Scott S, Mugala N, et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis. 2007;196:347–355. doi: 10.1086/519169. [DOI] [PubMed] [Google Scholar]
- 32.Pensieroso S, Cagigi A, Palma P, et al. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci US A. 2009;106:7939–7944. doi: 10.1073/pnas.0901702106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tejiokem MC, Gouandjika I, Béniguel L, et al. HIV-infected children living in Central Africa have low persistence of antibodies to vaccines used in the Expanded Program on Immunization. PLoS ONE. 2007;2:e1260. doi: 10.1371/journal.pone.0001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaccarelli-Filho CA, Ono E, Machado DM, et al. HIV-1-infected children on HAART: immunologic features of three different levels of viral suppression. Cytometry B Clin Cytom. 2007;72:14–21. doi: 10.1002/cyto.b.20152. [DOI] [PubMed] [Google Scholar]
- 35.Belaunzarán-Zamudio PF, García-León ML, Wong-Chew RM, et al. Early loss of measles antibodies after MMR vaccine among HIV-infected adults receiving HAART. Vaccine. 2009;27:7059–7064. doi: 10.1016/j.vaccine.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 36.Kemper CA, Zolopa AR, Hamilton JR, Fenstersheib M, Bhatia G, Deresinski SC. Prevalence of measles antibodies in adults with HIV infection: possible risk factors of measles seronegativity. AIDS. 1992;6:1321–1325. doi: 10.1097/00002030-199211000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Barbi M, Biffi MR, Binda S, et al. Immunization in children with HIV seropositivity at birth: antibody response to polio vaccine and tetanus toxoid. AIDS. 1992;6:1465–1469. [PubMed] [Google Scholar]
- 38.Ching N, Deville JG, Nielsen KA, et al. Cellular and humoral immune responses to a tetanus toxoid booster in perinatally HIV-1-infected children and adolescents receiving highly active antiretroviral therapy (HAART) Eur J Pediatr. 2007;166:51–56. doi: 10.1007/s00431-006-0184-2. [DOI] [PubMed] [Google Scholar]
- 39.Rigaud M, Borkowsky W, Muresan P, et al. Impaired immunity to recall antigens and neoantigens in severely immunocompromised children and adolescents during the first year of effective highly active antiretroviral therapy. J Infect Dis. 2008;198:1123–1130. doi: 10.1086/592050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abzug MJ, Pelton SI, Song L-Y, et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2006;25:920–929. doi: 10.1097/01.inf.0000237830.33228.c3. [DOI] [PubMed] [Google Scholar]
- 41.King JC, Jr, Vink PE, Chang I, et al. Antibody titers eight months after three doses of a five-valent pneumococcal conjugate vaccine in HIV and non-HIV-infected children less than two years of age. Vaccine. 1998;16:361–365. doi: 10.1016/s0264-410x(97)80914-0. [DOI] [PubMed] [Google Scholar]
- 42.Madhi SA, Adrian P, Kuwanda L, et al. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine. 2007;25:2451–2457. doi: 10.1016/j.vaccine.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Madhi SA, Klugman KP, Kuwanda L, Cutland C, Käyhty H, Adrian P. Quantitative and qualitative anamnestic immune responses to pneumococcal conjugate vaccine in HIV-infected and HIV-uninfected children 5 years after vaccination. J Infect Dis. 2009;199:1168–1176. doi: 10.1086/597388. [DOI] [PubMed] [Google Scholar]
- 44.Spoulou VI, Tsoumas DL, Papaevangelou VG, Mostrou GI, Theodoridou MC. Immunogenicity and immunological memory induced by a 7-valent pneumococcal CRM197 conjugate vaccine in symptomatic HIV-1 infected children. Vaccine. 2005;23:5289–5293. doi: 10.1016/j.vaccine.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Falcó V, Jordano Q, Cruz MJ, et al. Serological response to pneumococcal vaccination in HAART-treated HIV-infected patients: one year follow-up study. Vaccine. 2006;24:2567–2574. doi: 10.1016/j.vaccine.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 46.Glaser JB, Volpe S, Aguirre A, Simpkins H, Schiffman G. Zidovudine improves response to pneumococcal vaccine among persons with AIDS and AIDS-related complex. J Infect Dis. 1991;164:761–764. doi: 10.1093/infdis/164.4.761. [DOI] [PubMed] [Google Scholar]
- 47.Hung C-C, Chang S-Y, Su C-T, et al. A 5-year longitudinal follow-up study of serological responses to 23-valent pneumococcal polysaccharide vaccination among patients with HIV infection who received highly active antiretroviral therapy. HIV Med. 2010;11:54–63. doi: 10.1111/j.1468-1293.2009.00744.x. [DOI] [PubMed] [Google Scholar]
- 48.Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Enhanced antibody response to pneumococcal polysaccharide vaccine after prior immunization with conjugate pneumococcal vaccine in HIV-infected adults. Vaccine. 2000;19:886–894. doi: 10.1016/s0264-410x(00)00232-2. [DOI] [PubMed] [Google Scholar]
- 49.Lu C-L, Hung C-C, Chuang Y-C, et al. Comparison of serologic responses to vaccination with one dose or two doses of 7-valent pneumococcal conjugate vaccine in HIV-infected adult patients. Vaccine. 2012;30:3526–3533. doi: 10.1016/j.vaccine.2012.03.070. [DOI] [PubMed] [Google Scholar]
- 50.Mascart-Lemone F, Gérard M, Libin M, et al. Differential effect of human immunodeficiency virus infection on the IgA and IgG antibody responses to pneumococcal vaccine. J Infect Dis. 1995;172:1253–1260. doi: 10.1093/infdis/172.5.1253. [DOI] [PubMed] [Google Scholar]
- 51.Nielsen H, Kvinesdal B, Benfield TL, Lundgren JD, Konradsen HB. Rapid loss of specific antibodies after pneumococcal vaccination in patients with human immunodeficiency virus-1 infection. Scand J Infect Dis. 1998;30:597–601. doi: 10.1080/00365549850161160. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Barradas MC, Groover JE, Lacke CE, et al. IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response. J Infect Dis. 1996;173:1347–1353. doi: 10.1093/infdis/173.6.1347. [DOI] [PubMed] [Google Scholar]
- 53.Talesnik E, Vial PA, Labarca J, Méndez C, Soza X. Time course of antibody response to tetanus toxoid and pneumococcal capsular polysaccharides in patients infected with HIV. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:471–477. doi: 10.1097/00042560-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 54.Peters VB, Sood SK. Immunity to Haemophilus influenzae type b after reimmunization with oligosaccharide CRM197 conjugate vaccine in children with human immunodeficiency virus infection. Pediatr Infect Dis J. 1997;16:711–713. doi: 10.1097/00006454-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Pacanowski J, Lacombe K, Campa P, et al. Plasma HIV-RNA is the key determinant of long-term antibody persistence after Yellow fever immunization in a cohort of 364 HIV-infected patients. J Acquir Immune Defic Syndr. 2012;59:360–367. doi: 10.1097/QAI.0b013e318249de59. [DOI] [PubMed] [Google Scholar]
- 56.Tattevin P, Depatureaux AG, Chapplain JM, et al. Yellow fever vaccine is safe and effective in HIV-infected patients. AIDS. 2004;18:825–827. doi: 10.1097/00002030-200403260-00020. [DOI] [PubMed] [Google Scholar]
- 57.Veit O, Niedrig M, Chapuis-Taillard C, et al. Immunogenicity and safety of yellow fever vaccination for 102 HIV-infected patients. Clin Infect Dis. 2009;48:659–666. doi: 10.1086/597006. [DOI] [PubMed] [Google Scholar]
- 58.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 59.Sutcliffe CG, Moss WJ. Do children infected with HIV receiving HAART need to be revaccinated? The Lancet Infectious Diseases. 2010;10:630–642. doi: 10.1016/S1473-3099(10)70116-X. [DOI] [PubMed] [Google Scholar]
- 60.Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA. 2011;305:1432–40. doi: 10.1001/jama.2011.351. [DOI] [PubMed] [Google Scholar]
- 61.Wismans PJ, van Hattum J, de Gast GC, et al. A prospective study of in vitro anti-HBs producing B cells (spot-ELISA) following primary and supplementary vaccination with a recombinant hepatitis B vaccine in insulin dependent diabetic patients and matched controls. J Med Virol. 1991;35:216–22. doi: 10.1002/jmv.1890350313. [DOI] [PubMed] [Google Scholar]
- 62.Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis. 2013;56:1458–65. doi: 10.1093/cid/cit048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: Data retrieved from studies included in the meta-analysis
Table 2: Selected reports on persistence of antibody levels after vaccination in healthy individuals